RADIATION SAFETY Refresher Training Part 1 Attention This


















































































- Slides: 181

RADIATION SAFETY Refresher Training Part 1

Attention! This online training is intended to serve as an annual refresher training only! If you never had classroom Initial Radiation Safety Training, please contact Radiation Safety Office for the date of the next scheduled classroom training session: 656 3516, or kpovod@Clemson. edu Ø Tip: hover mouse pointer over words with hyperlinks to get definition or additional information

Course Part 1 Objectives • • Types of ionizing radiation Atomic structure; isotopes; notations Radioactive decay; activity; units of activity; half life Alpha, beta, gamma decay; x ray production Radiation interaction with matter Exposure, dose; units of dose Biological effects of radiation Acute and chronic exposures; stochastic and deterministic effects; dose response curve • Radiation in environment

Ionizing Radiation • Radiation that can cause ionization of the material through which it passes. Ionizing radiation may be in the form of • Electromagnetic Radiation • Particulate Radiation • Non ionizing radiation (radiowaves, cell phones, UV and IR light, microwaves are not covered in this presentation

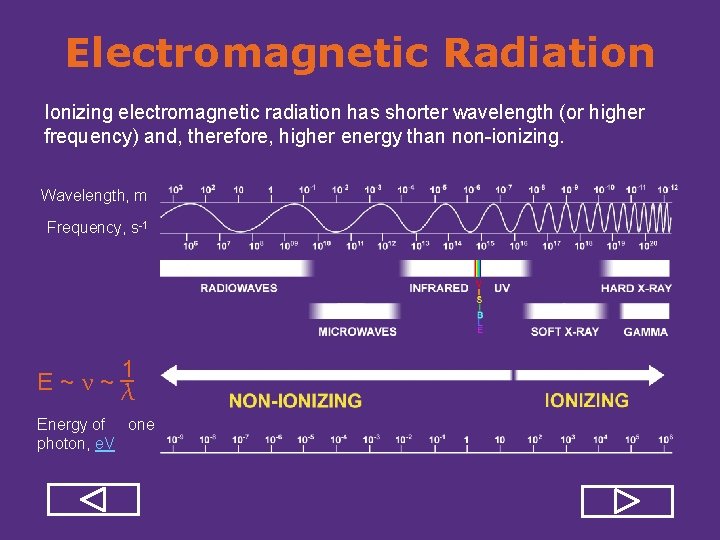
Electromagnetic Radiation Ionizing electromagnetic radiation has shorter wavelength (or higher frequency) and, therefore, higher energy than non-ionizing. Wavelength, m Frequency, s-1 1 _ E~ν~λ Energy of one photon, e. V

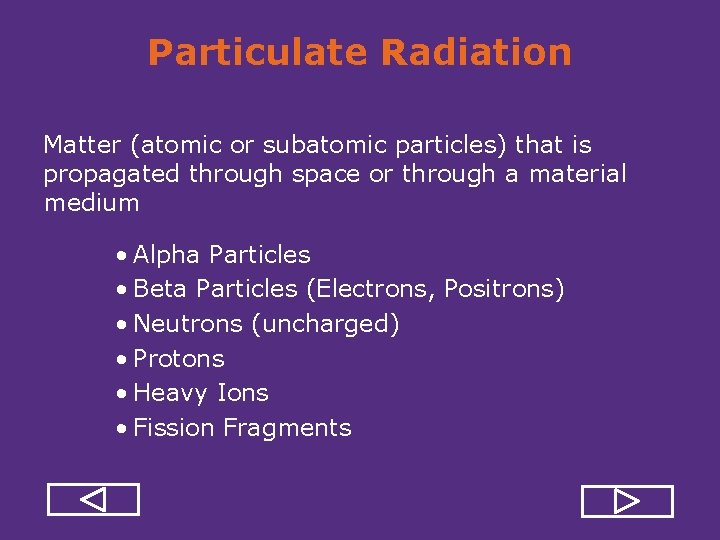
Particulate Radiation Matter (atomic or subatomic particles) that is propagated through space or through a material medium • Alpha Particles • Beta Particles (Electrons, Positrons) • Neutrons (uncharged) • Protons • Heavy Ions • Fission Fragments

Sources of Ionizing Radiation • Radioactive Materials • H 3, C 14, P 32, S 35, I 125, etc. • Radiation Producing Machines • X Ray equipment • Accelerators • Radiation producing machines do not produce radiation when not energized (switched off). Radioactive material always emits radiation: during work, in storage, after disposal.

Atomic Structure • Atom The smallest piece of a pure substance that still retains the characteristics of that substance • Composed of 3 Subatomic Particles – Protons, Positively Charged – Neutrons, No Charge – Electrons, Negative Charge • Protons and neutrons comprise nucleus of the atom, which is orbited by electrons.

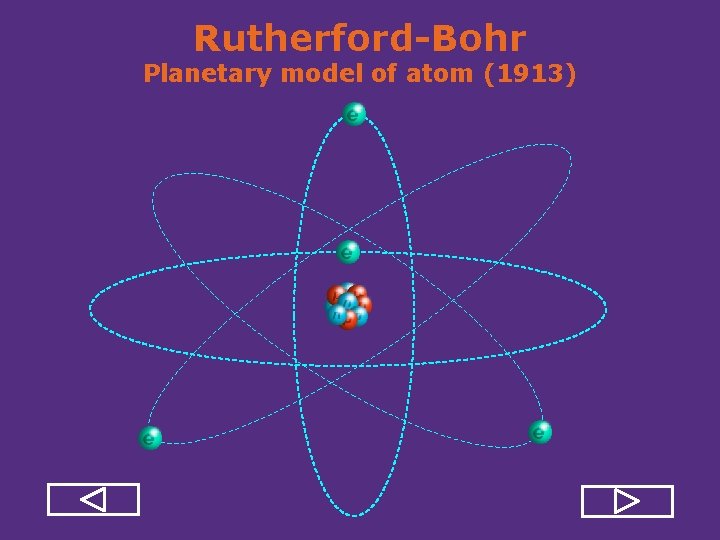
Rutherford-Bohr Planetary model of atom (1913)

Contemporary Model Electron orbits really are not well-defined lines but more like “clouds of probability” of finding electrons at any particular location. For the purposes of this presentation the shape of the electron orbits is not critical, though.

Size of the Atom • Diameter of Atom – (1 to 5) • 10 10 m • Diameter of the Nucleus – (1. 6 to 15) • 10 15 m • If the nucleus were the size of a golf ball: • First electron shell 1 km (0. 62 mi) • Second electron shell 4 km (2. 5 mi) • Third electron shell 9 km (5. 7 mi) • While diameter of nucleus is 4 to 5 orders of magnitude smaller than the size of an atom, almost all of the atom’s mass is concentrated in its nucleus.

Atomic Mass • Atomic Mass Unit (amu) – 1/12 the mass of a Carbon 12 atom – 1. 6592 x 10– 17 kg • Neutrons and Protons have a mass of about 1 amu each • Electrons have a mass of only 0. 00054 amu

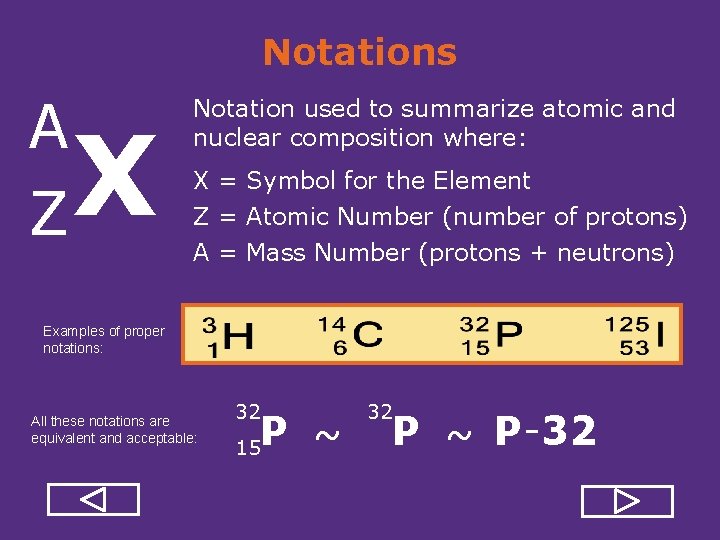
Notations A Z X Notation used to summarize atomic and nuclear composition where: X = Symbol for the Element Z = Atomic Number (number of protons) A = Mass Number (protons + neutrons) Examples of proper notations: All these notations are equivalent and acceptable: 32 P ~ 15 32 P ~ P 32

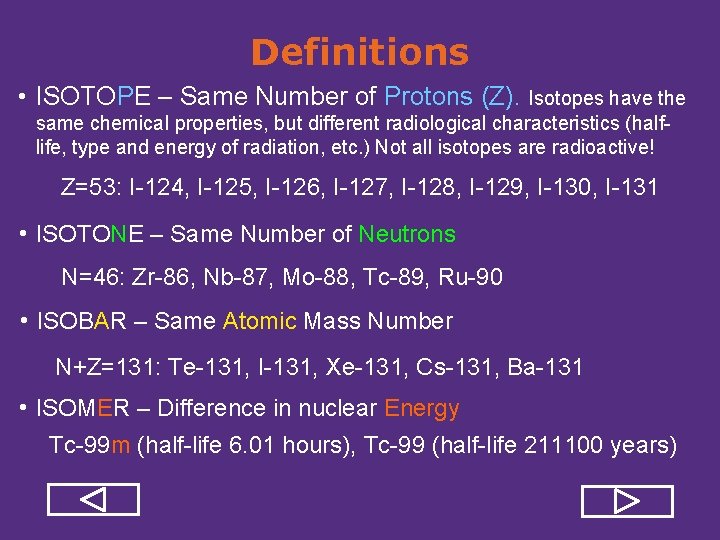
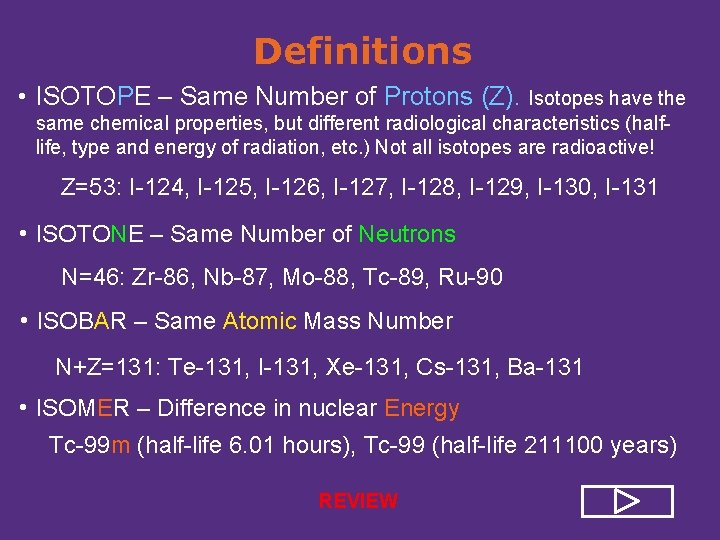
Definitions • ISOTOPE – Same Number of Protons (Z). Isotopes have the same chemical properties, but different radiological characteristics (halflife, type and energy of radiation, etc. ) Not all isotopes are radioactive! Z=53: I-124, I-125, I-126, I-127, I-128, I-129, I-130, I-131 • ISOTONE – Same Number of Neutrons N=46: Zr-86, Nb-87, Mo-88, Tc-89, Ru-90 • ISOBAR – Same Atomic Mass Number N+Z=131: Te-131, I-131, Xe-131, Cs-131, Ba-131 • ISOMER – Difference in nuclear Energy Tc-99 m (half-life 6. 01 hours), Tc-99 (half-life 211100 years)

Radioactive Decay • Atoms that have a neutron to proton ratio that is too high or too low to keep the nucleus stable undergo the process of radioactive decay • Radioactive decay is the spontaneous emission of matter and/or energy from the nucleus of the atom – Particles: Alpha and/or Beta Particles – Energy: Gamma Rays and X Rays • As a result of radioactive decay the atom(“parent”) transforms into a different (“daughter”) element, which can be either stable or also radioactive

Nature of Radioactive Decay • Decay is random, predicting when a given atom will decay is impossible • In sufficient numbers, the probability of decay becomes well defined • Decay Constant (λ) = The probability that any one atom will decay

Activity • Activity is the rate at which nuclear transformations occur in a radioactive material (rate of decay): A= λN • Number of radioactive atoms and, as a result, activity decreases exponentially with time: N = N 0 exp( λt) A = A 0 exp( λt)

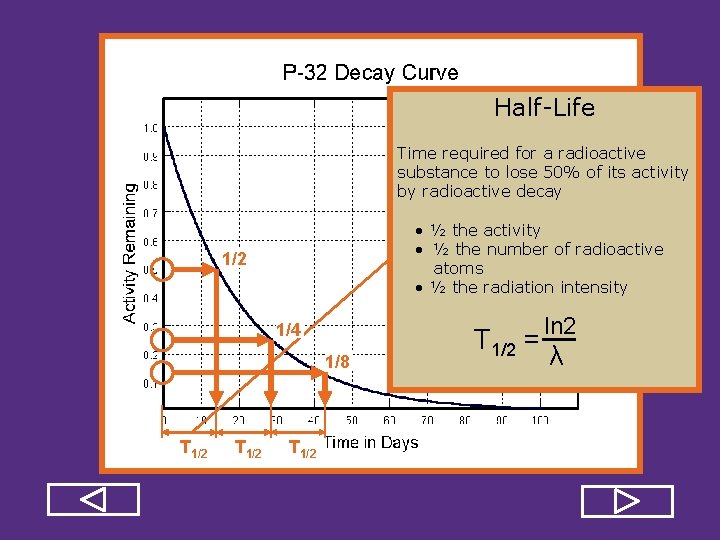
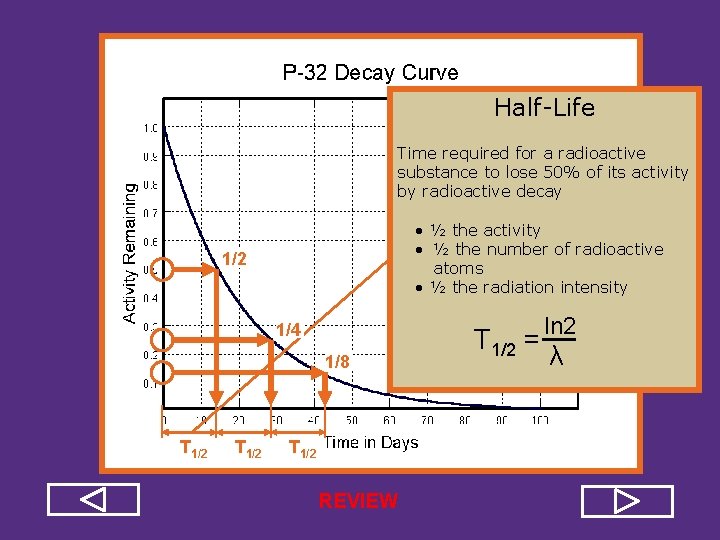
Half Life Time required for a radioactive substance to lose 50% of its activity by radioactive decay • ½ the activity • ½ the number of radioactive atoms • ½ the radiation intensity 1/2 1/4 1/8 T 1/2 = ln 2 λ

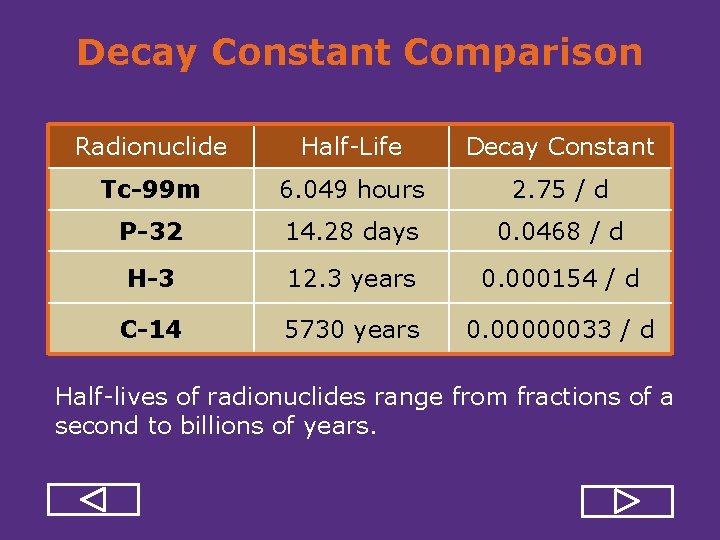
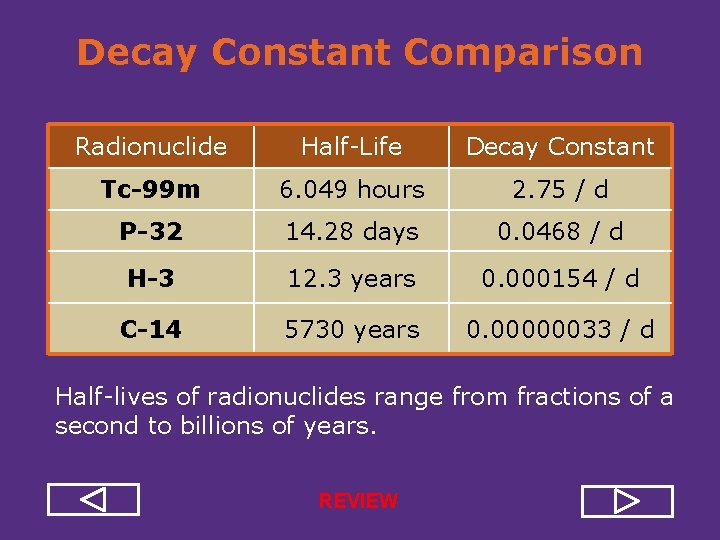
Decay Constant Comparison Radionuclide Half Life Decay Constant Tc-99 m 6. 049 hours 2. 75 / d P-32 14. 28 days 0. 0468 / d H-3 12. 3 years 0. 000154 / d C-14 5730 years 0. 00000033 / d Half lives of radionuclides range from fractions of a second to billions of years.

Units of Activity • Modern SI Unit – Becquerel (Bq) – 1 Bq = 1 decay per 1 second • Traditional Unit – Curie (Ci) – The number of radioactive decays occurring in one gram of pure Ra 226 1 Ci = 3. 7 x 1010 Bq = 37 GBq

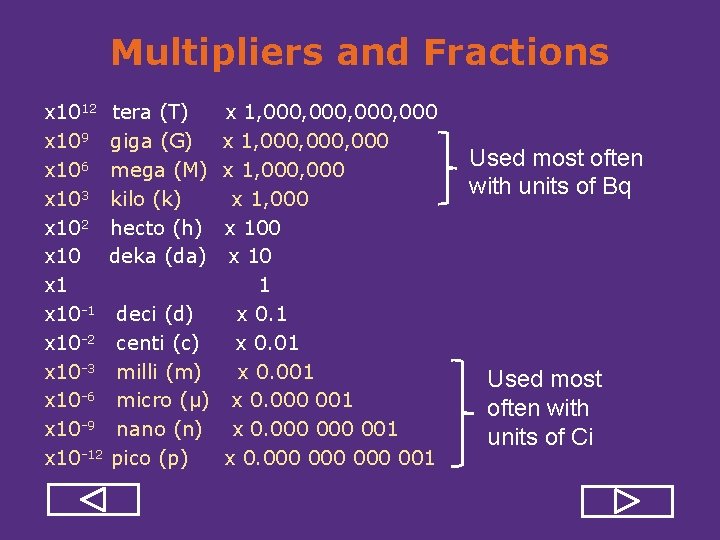
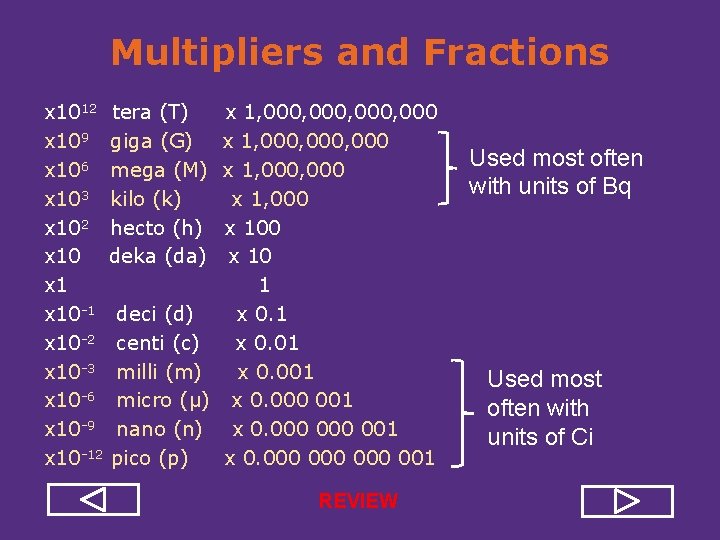
Multipliers and Fractions x 1012 tera (T) x 109 giga (G) x 106 mega (M) x 103 kilo (k) x 102 hecto (h) x 10 deka (da) x 10 1 deci (d) x 10 2 centi (c) x 10 3 milli (m) x 10 6 micro (μ) x 10 9 nano (n) x 10 12 pico (p) x 1, 000, 000 x 1, 000 x 100 x 10 1 x 0. 01 x 0. 000 001 x 0. 000 000 001 Used most often with units of Bq Used most often with units of Ci

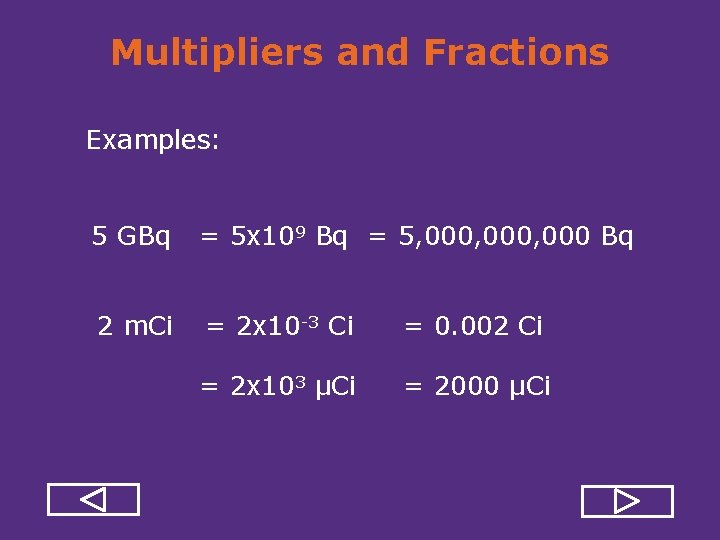
Multipliers and Fractions Examples: 5 GBq = 5 x 109 Bq = 5, 000, 000 Bq 2 m. Ci = 2 x 10 3 Ci = 0. 002 Ci = 2 x 103 μCi = 2000 μCi

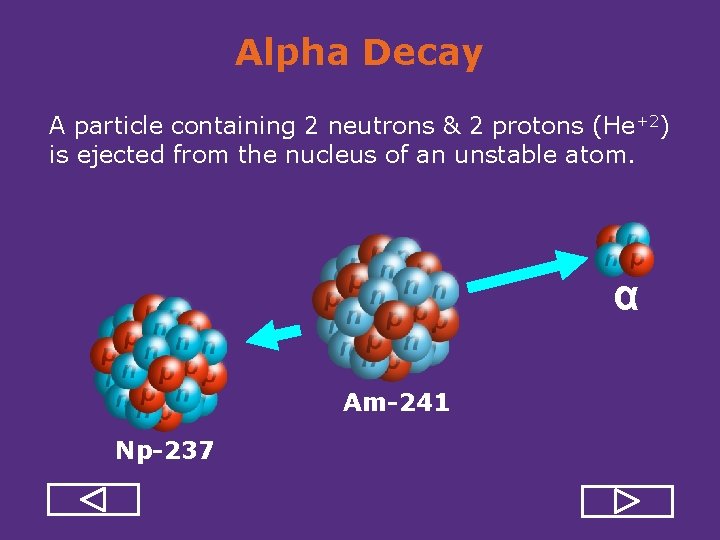
Alpha Decay A particle containing 2 neutrons & 2 protons (He+2) is ejected from the nucleus of an unstable atom. α Am-241 Np-237

Energy of Alpha-Radiation • During an alpha decay certain amount of energy is released. Majority of this energy is carried away by the alpha particle in the form of the kinetic energy. • All alpha particles produced by a certain isotope have the same kinetic energy (i. e. , are “monoenergetic”). For example, all alphas emitted by Am 241 have kinetic energy of 5. 486 Me. V. This property allows to identify isotope by measuring energy of the alpha particles using an instrument called “spectrometer”. • Usually, term “energy of the radiation” refers to the energy of the single particle (alpha, beta, x or gamma ray, neutron)

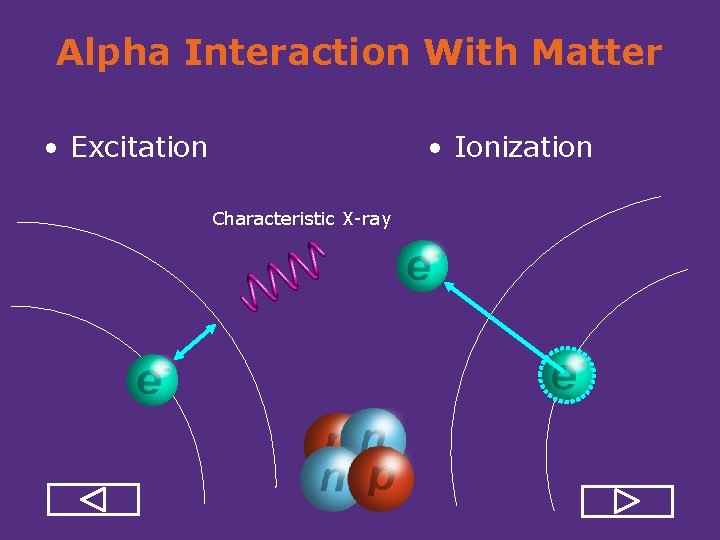
Alpha Interaction With Matter • Ionization • Excitation Characteristic X ray

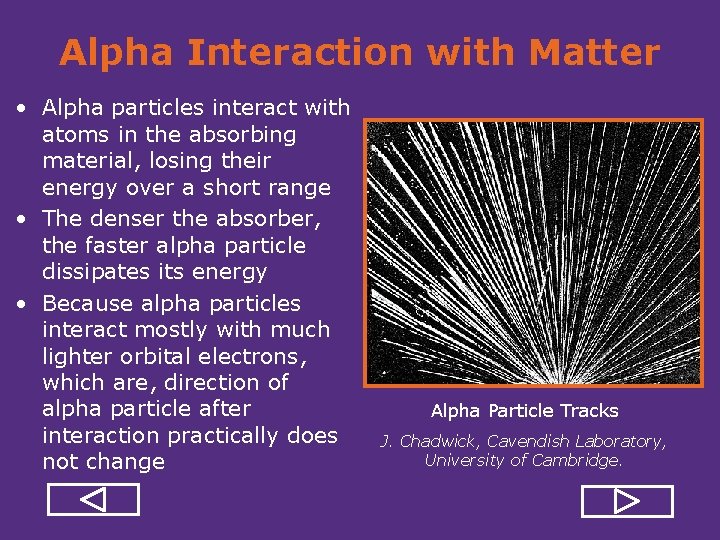
Alpha Interaction with Matter • Alpha particles interact with atoms in the absorbing material, losing their energy over a short range • The denser the absorber, the faster alpha particle dissipates its energy • Because alpha particles interact mostly with much lighter orbital electrons, which are, direction of alpha particle after interaction practically does not change Alpha Particle Tracks J. Chadwick, Cavendish Laboratory, University of Cambridge.

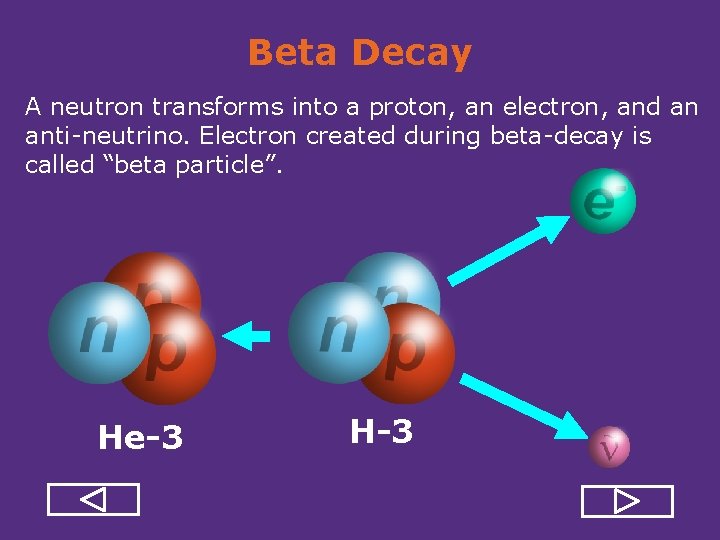
Beta Decay A neutron transforms into a proton, an electron, and an anti neutrino. Electron created during beta decay is called “beta particle”. He-3 H-3

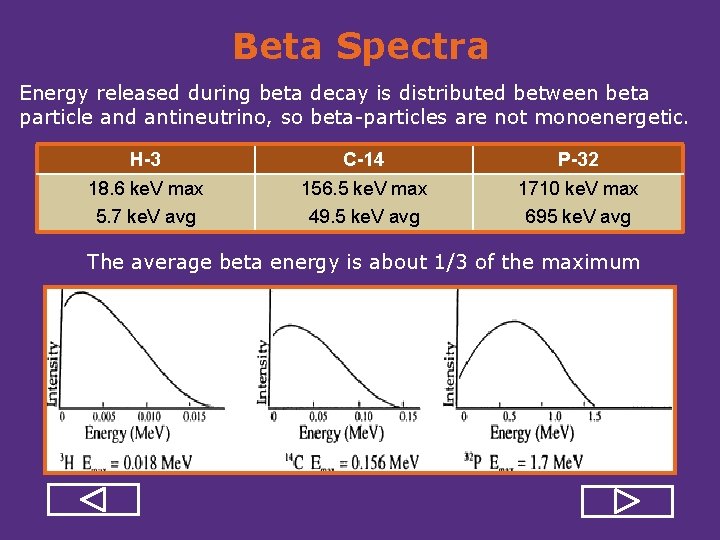
Beta Spectra Energy released during beta decay is distributed between beta particle and antineutrino, so beta particles are not monoenergetic. H-3 C-14 P-32 18. 6 ke. V max 5. 7 ke. V avg 156. 5 ke. V max 49. 5 ke. V avg 1710 ke. V max 695 ke. V avg The average beta energy is about 1/3 of the maximum

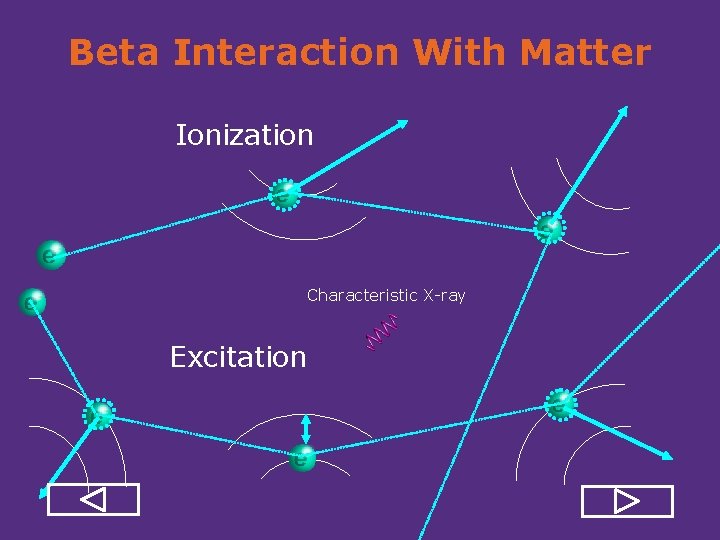
Beta Interaction With Matter Ionization Characteristic X ray Excitation

Interaction of Betas • Beta particles interact primarily with electrons in the absorbing material • Electron mass = 0. 00054 amu • Beta mass = 0. 00054 amu • Like a billiard ball hitting other billiard balls – Large angle deflections are possible – Relatively large energy loss possible per collision

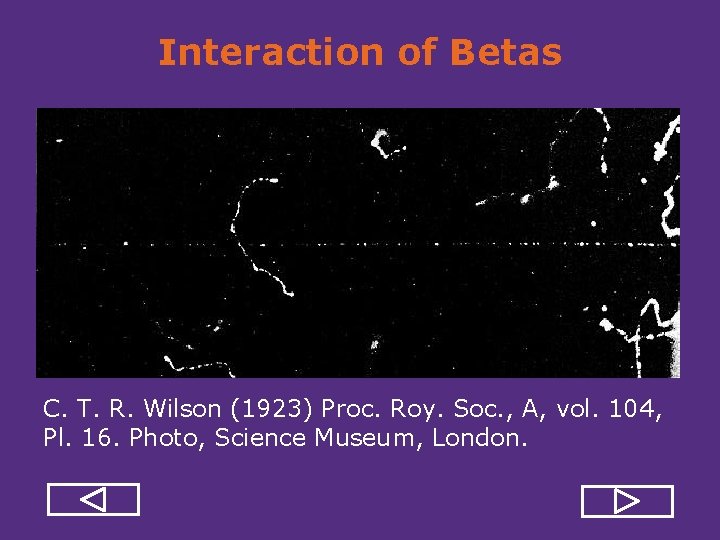
Interaction of Betas C. T. R. Wilson (1923) Proc. Roy. Soc. , A, vol. 104, Pl. 16. Photo, Science Museum, London.

Alpha Beta Comparison • For a given amount of energy, beta particles are faster than alpha particles – 1 Me. V alpha particles – 0. 05 c – 1 Me. V beta particles – 0. 95 c • Beta particles are less densely ionizing than alphas: they create less ions per unit of travel. This fact is important when considering biological effects of different types of radiation.

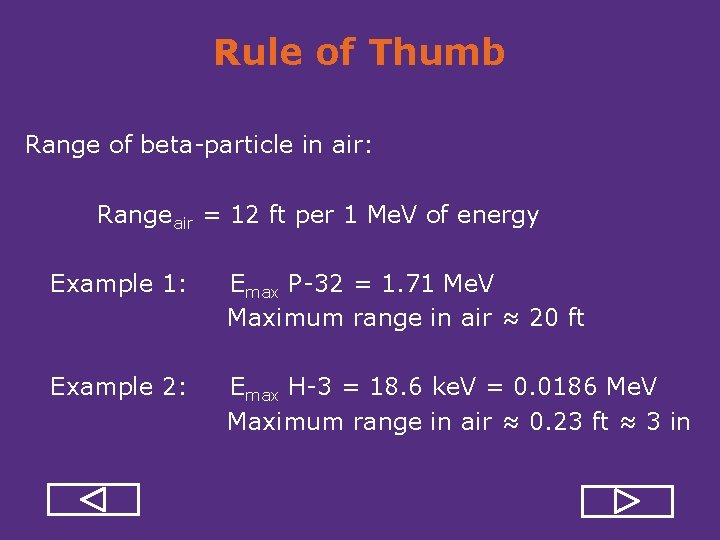
Rule of Thumb Range of beta particle in air: Rangeair = 12 ft per 1 Me. V of energy Example 1: Emax P 32 = 1. 71 Me. V Maximum range in air ≈ 20 ft Example 2: Emax H 3 = 18. 6 ke. V = 0. 0186 Me. V Maximum range in air ≈ 0. 23 ft ≈ 3 in

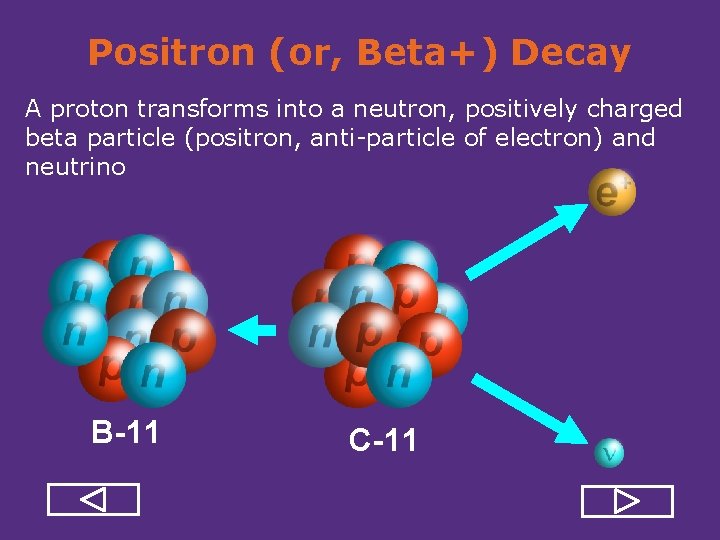
Positron (or, Beta+) Decay A proton transforms into a neutron, positively charged beta particle (positron, anti particle of electron) and neutrino B-11 C-11

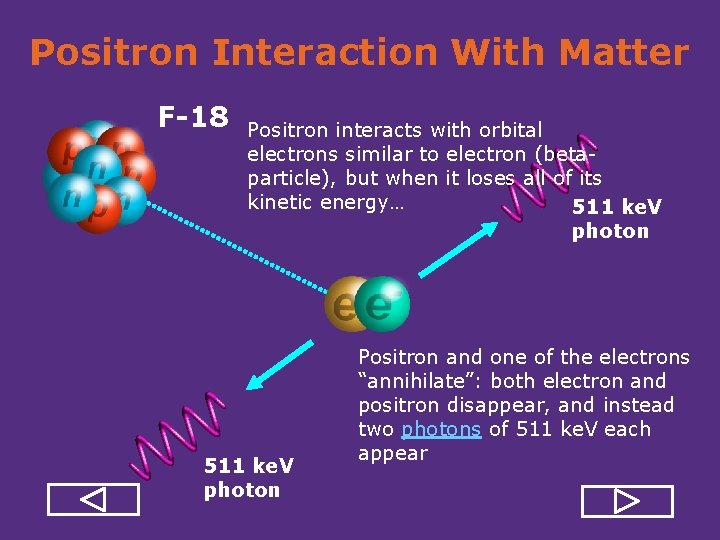
Positron Interaction With Matter F-18 Positron interacts with orbital electrons similar to electron (beta particle), but when it loses all of its kinetic energy… 511 ke. V photon Positron and one of the electrons “annihilate”: both electron and positron disappear, and instead two photons of 511 ke. V each appear

Mass Energy Equivalency • Process of annihilation illustrates Albert Einstein’s famous formula: E = mc 2 describing mass and energy interrelation

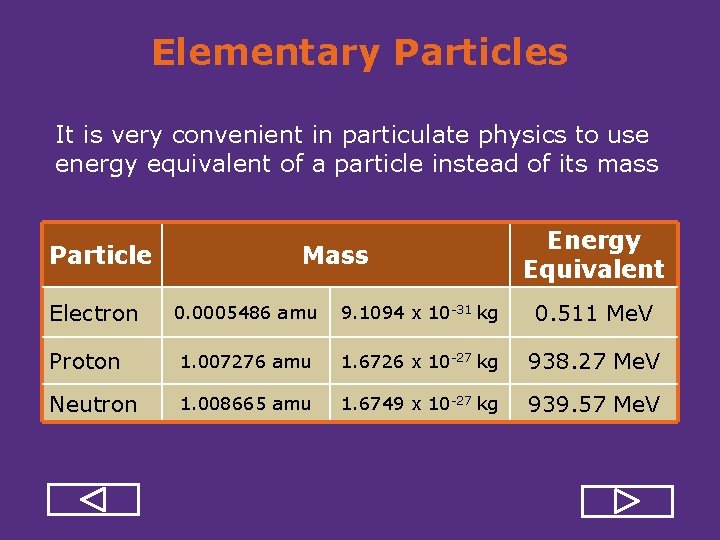
Elementary Particles It is very convenient in particulate physics to use energy equivalent of a particle instead of its mass Particle Electron Mass Energy Equivalent 0. 0005486 amu 9. 1094 x 10 31 kg 0. 511 Me. V Proton 1. 007276 amu 1. 6726 x 10 27 kg 938. 27 Me. V Neutron 1. 008665 amu 1. 6749 x 10 27 kg 939. 57 Me. V

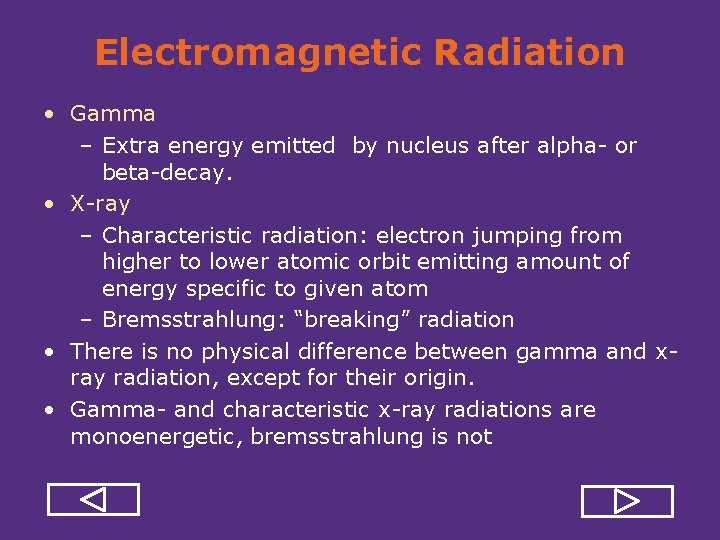
Electromagnetic Radiation • Gamma – Extra energy emitted by nucleus after alpha or beta decay. • X ray – Characteristic radiation: electron jumping from higher to lower atomic orbit emitting amount of energy specific to given atom – Bremsstrahlung: “breaking” radiation • There is no physical difference between gamma and x ray radiation, except for their origin. • Gamma and characteristic x ray radiations are monoenergetic, bremsstrahlung is not

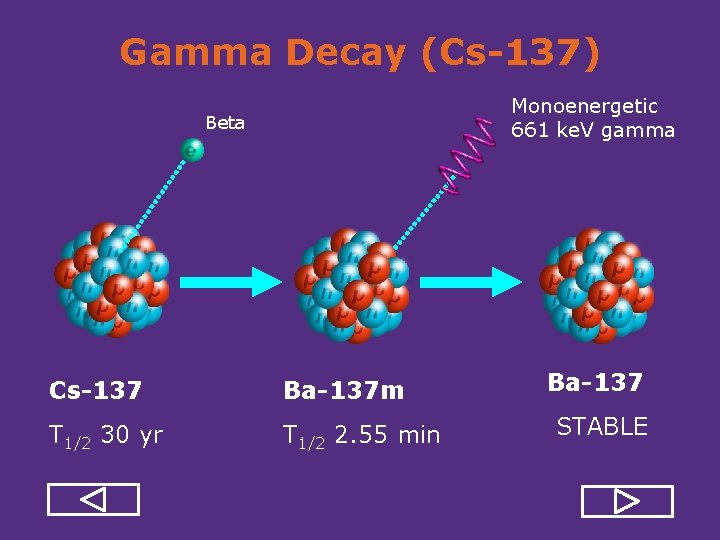
Gamma Decay (Cs-137) Monoenergetic 661 ke. V gamma Beta Cs-137 Ba-137 m T 1/2 30 yr T 1/2 2. 55 min Ba-137 STABLE

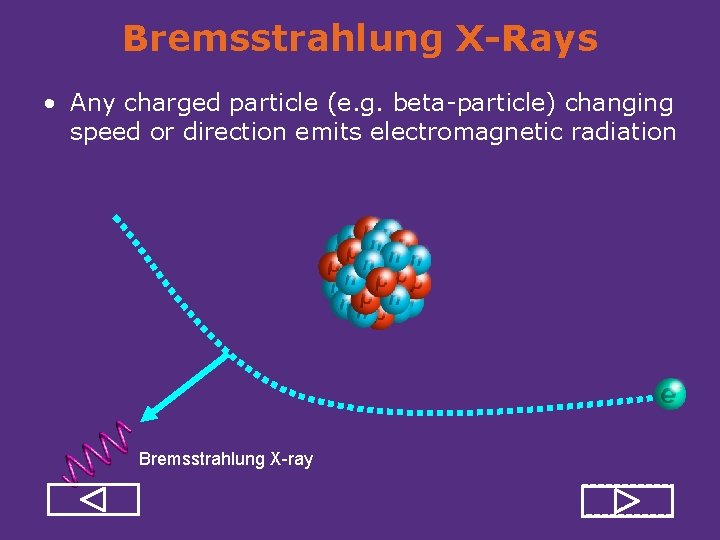
Bremsstrahlung X-Rays • Any charged particle (e. g. beta particle) changing speed or direction emits electromagnetic radiation Bremsstrahlung X-ray

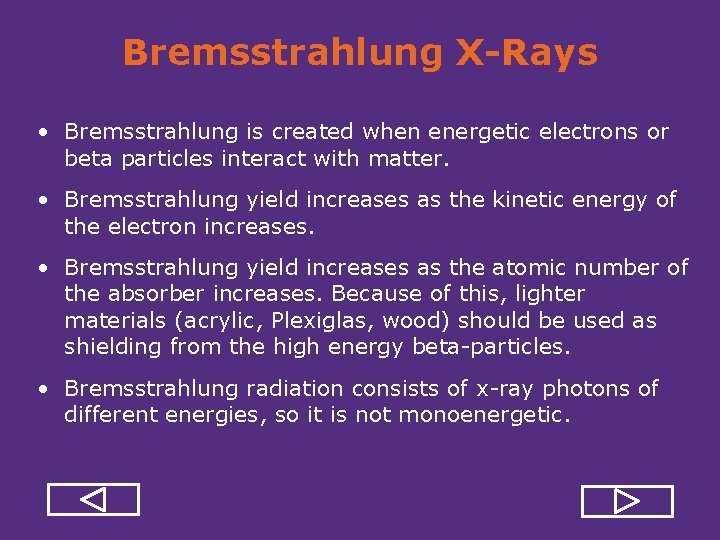
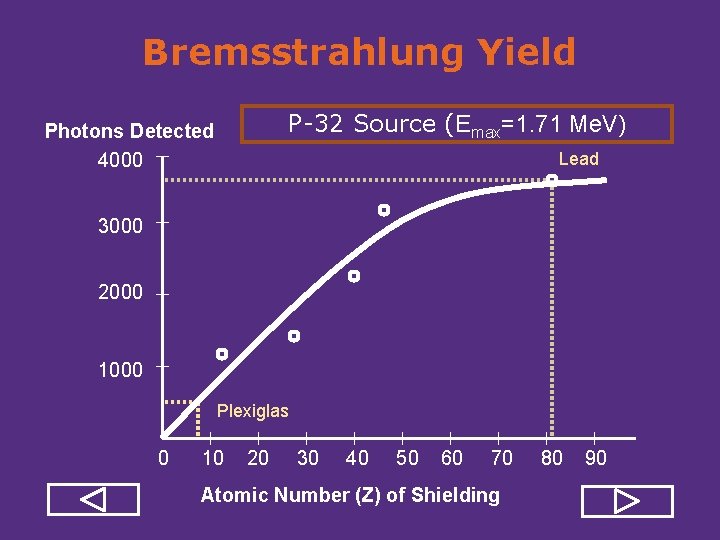
Bremsstrahlung X-Rays • Bremsstrahlung is created when energetic electrons or beta particles interact with matter. • Bremsstrahlung yield increases as the kinetic energy of the electron increases. • Bremsstrahlung yield increases as the atomic number of the absorber increases. Because of this, lighter materials (acrylic, Plexiglas, wood) should be used as shielding from the high energy beta particles. • Bremsstrahlung radiation consists of x ray photons of different energies, so it is not monoenergetic.

Bremsstrahlung Yield P 32 Source (Emax=1. 71 Me. V) Photons Detected 4000 Lead 3000 2000 1000 Plexiglas 0 10 20 30 40 50 60 70 Atomic Number (Z) of Shielding 80 90

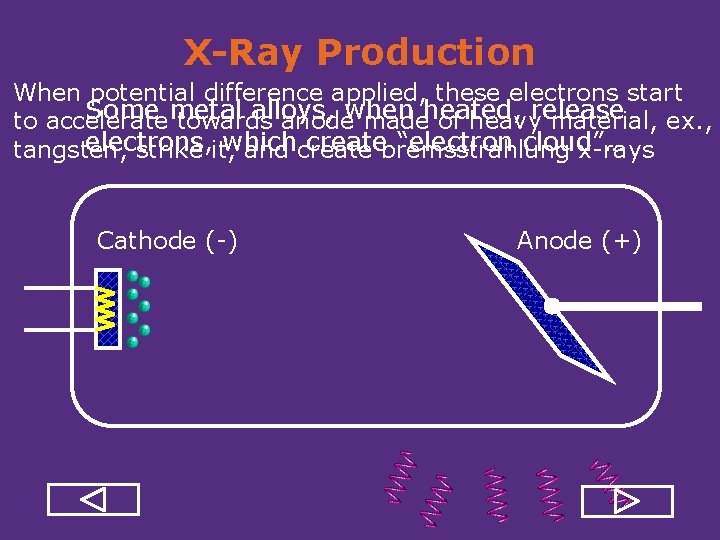
X-Ray Production When potential difference applied, these electrons start Some metal alloys, to accelerate towards anodewhen madeheated, of heavyrelease material, ex. , electrons, which createbremsstrahlung “electron cloud”… tangsten, strike it, and create x rays Cathode ( ) Anode (+)

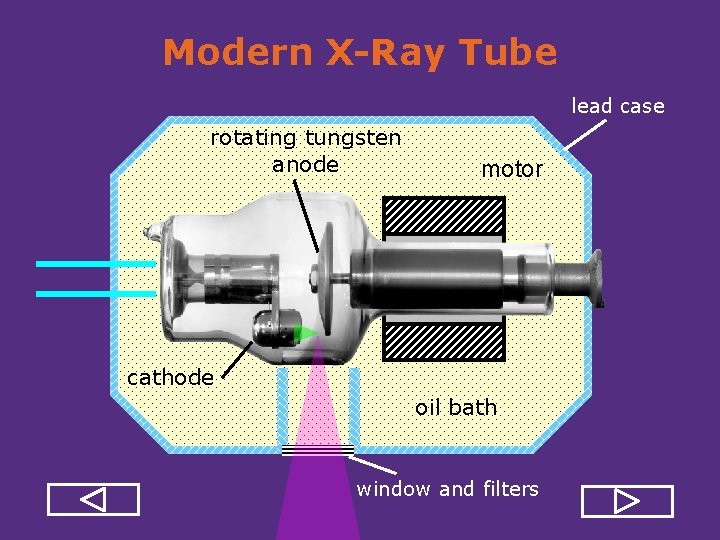
Modern X-Ray Tube lead case rotating tungsten anode motor cathode oil bath window and filters

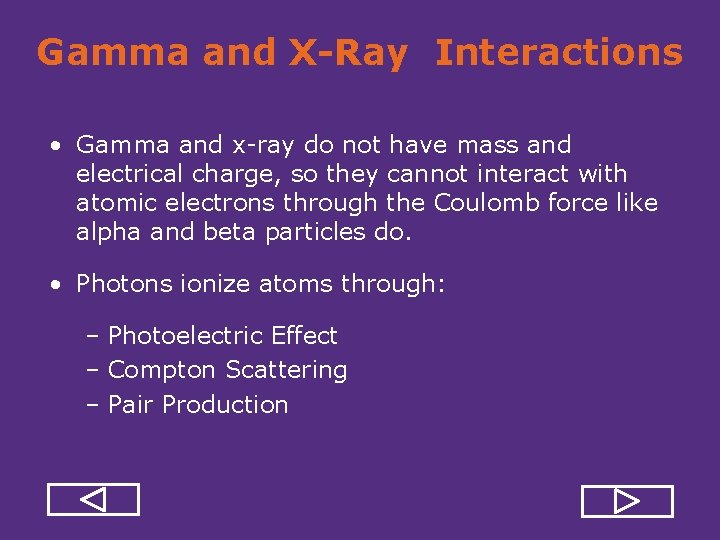
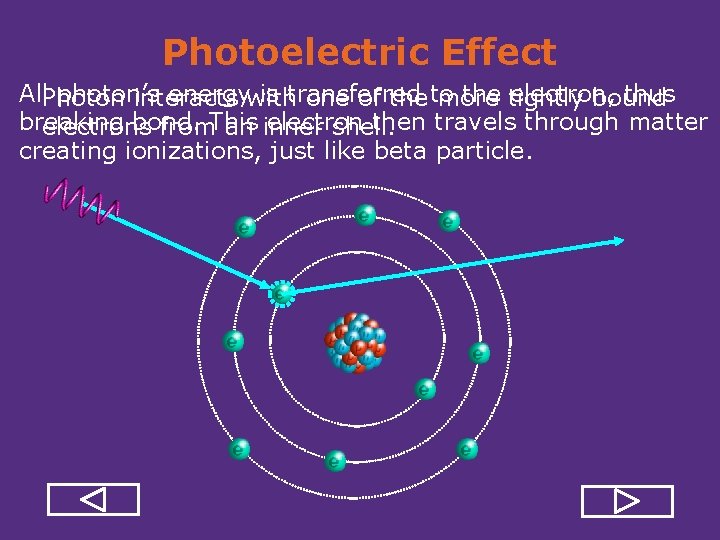
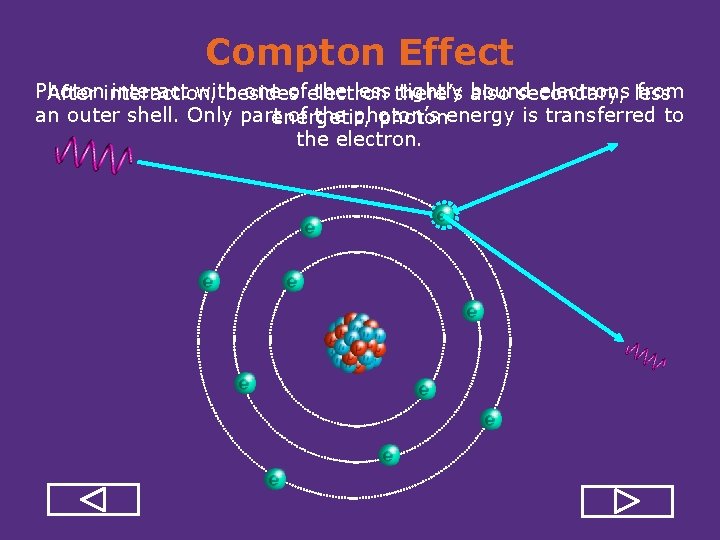
Gamma and X-Ray Interactions • Gamma and x ray do not have mass and electrical charge, so they cannot interact with atomic electrons through the Coulomb force like alpha and beta particles do. • Photons ionize atoms through: – Photoelectric Effect – Compton Scattering – Pair Production

Photoelectric Effect All. Photon photon’s energywith is transferred the tightly electron, thus interacts one of the to more bound breaking bond. electron then travels through matter electrons from. This an inner shell. creating ionizations, just like beta particle.

Compton Effect Photon interact with one ofelectron the less there’s tightly bound electrons less from After interaction, besides also secondary, an outer shell. Only partenergetic, of the photon’s photonenergy is transferred to the electron.

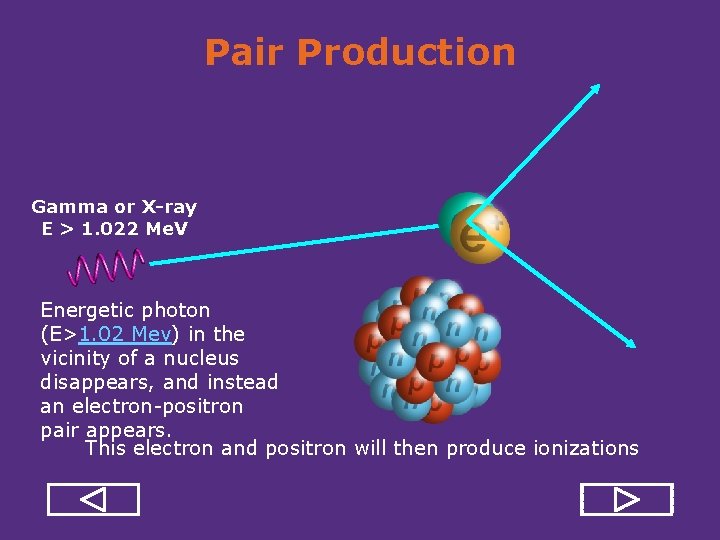
Pair Production Gamma or X-ray E > 1. 022 Me. V Energetic photon (E>1. 02 Mev) in the vicinity of a nucleus disappears, and instead an electron positron pair appears. This electron and positron will then produce ionizations

Absorbed Dose • Absorbed Dose - The total amount of energy imparted by all particles of the ionizing radiation per unit mass at a given location within irradiated material • rad - The traditional unit of absorbed dose, defined as the absorption of 100 ergs per gram • Gray (Gy) – The SI unit of absorbed dose, defined as the absorption of 1 Joule per 1 kilogram 1 Gy = 100 rad

Exposure • There two applications of the word “exposure” in radiation safety: – Common use, like in the sentence “exposure to radiation”; – And as a characteristic of the ionization created by radiation in air • Exposure – A measurement of the amount of ionization created by X rays or gamma rays in a volume of air

Units of Exposure • Roentgen (R) – amount of exposure creating 1 esu (electrostatic unit) of charge in 1 cubic cm of dry air at STP. • There is no special unit of exposure in SI 1 R = 2. 58 x 10 4 Coulombs / kg air • Amount of radiation creating 1 R of exposure in air delivers absorbed dose of 0. 96 rad to human tissue. This fact allows us by measuring exposure (with Geiger Mueller counter, for example) to have good estimate (almost one to one) of the absorbed dose to a human body.

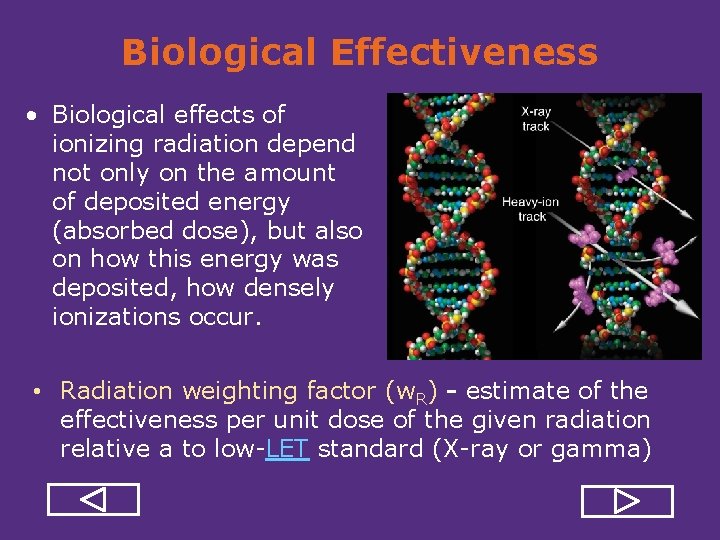
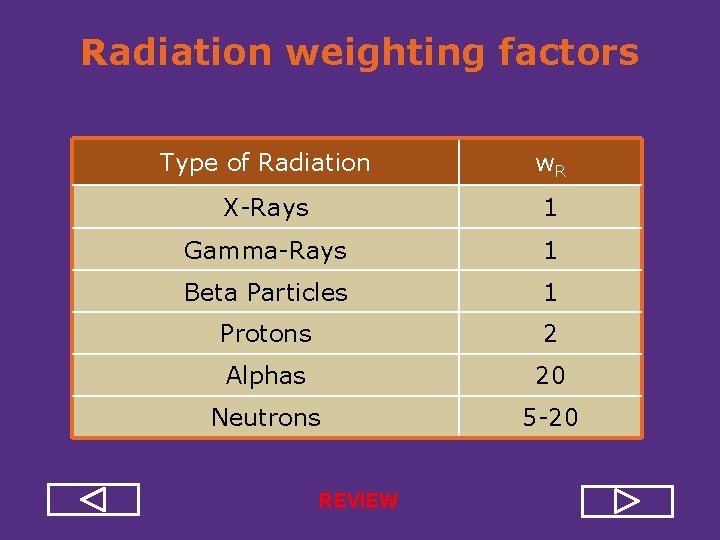
Biological Effectiveness • Biological effects of ionizing radiation depend not only on the amount of deposited energy (absorbed dose), but also on how this energy was deposited, how densely ionizations occur. • Radiation weighting factor (w. R) - estimate of the effectiveness per unit dose of the given radiation relative a to low LET standard (X ray or gamma)

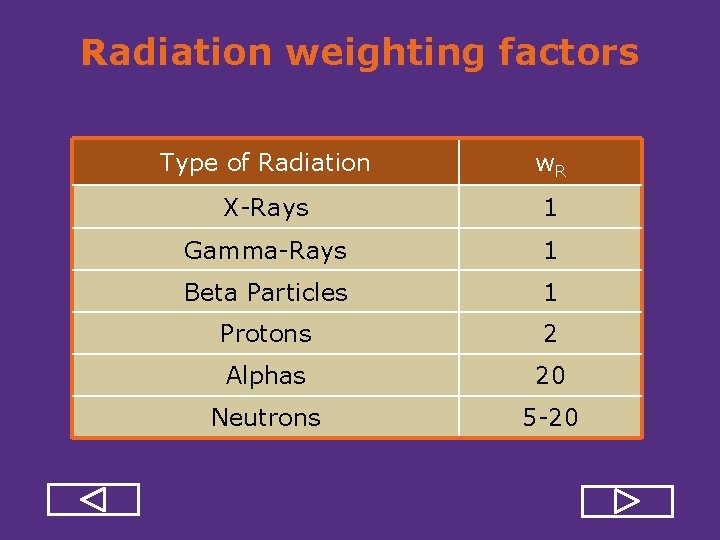
Radiation weighting factors Type of Radiation w. R X Rays 1 Gamma Rays 1 Beta Particles 1 Protons 2 Alphas 20 Neutrons 5 20

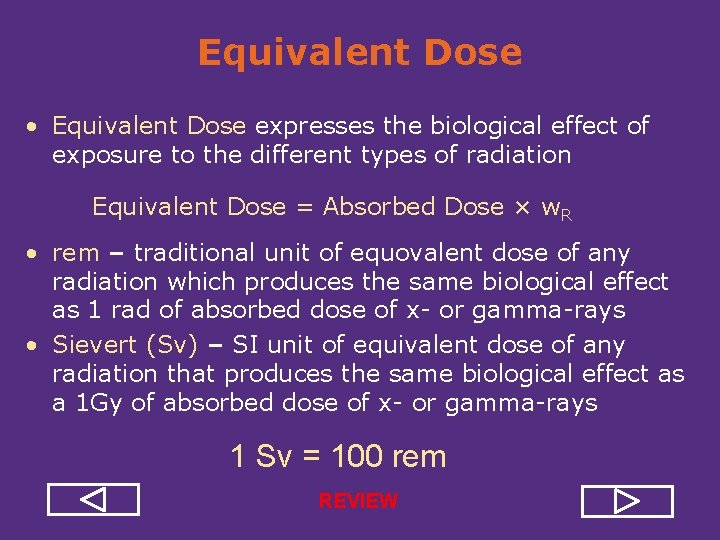
Equivalent Dose • Equivalent Dose expresses the biological effect of exposure to the different types of radiation Equivalent Dose = Absorbed Dose × w. R • rem – traditional unit of equivalent dose of any radiation which produces the same biological effect as 1 rad of absorbed dose of x or gamma rays • Sievert (Sv) – SI unit of equivalent dose of any radiation that produces the same biological effect as a 1 Gy of absorbed dose of x or gamma rays 1 Sv = 100 rem

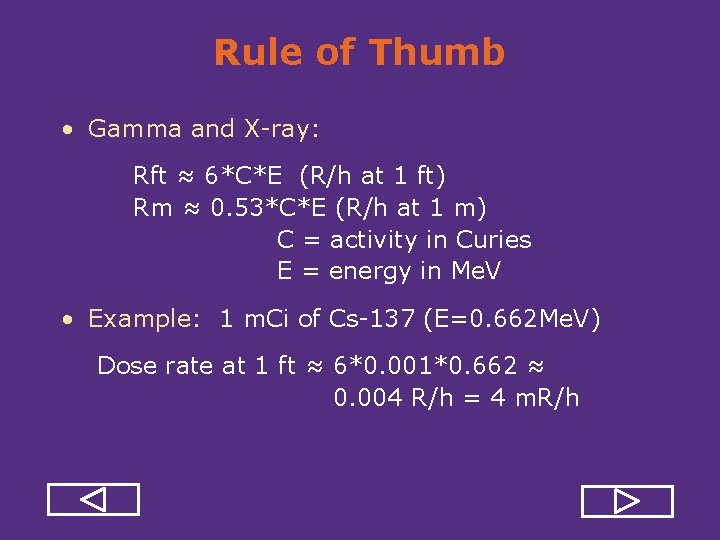
Rule of Thumb • Gamma and X ray: Rft ≈ 6*C*E (R/h at 1 ft) Rm ≈ 0. 53*C*E (R/h at 1 m) C = activity in Curies E = energy in Me. V • Example: 1 m. Ci of Cs 137 (E=0. 662 Me. V) Dose rate at 1 ft ≈ 6*0. 001*0. 662 ≈ 0. 004 R/h = 4 m. R/h

Biological Effects of Radiation • Direct action: radiation interacting directly with the atoms in the DNA molecules • Indirect action: ions and free radicals (H+, OH , H 2 O 2+) formed in the water in a cell, and these free radicals interact with DNA • Both direct and indirect action of radiation may produce damage to the DNA molecules: – Chemically alter bases; – Break sugar phosphate backbones; or – Break hydrogen bonds connecting base pairs

Biological Effects of Radiation • Biological effect of each individual interaction is a multi step process. Each step and, therefore, final outcome depends on many factors and is unpredictable. Only probability of an outcome (ex. , cancer) may be estimated. • Single strand DNA damage → repair, no harm • Double strand DNA damage → – Repair, no harm – Cell death, no harm (at low radiation levels) – Mutation → • Benign, no harm • Malignant → – Cell does not reproduce, no harm – Cell reproduces → CANCER

Radiation Sensitivity Different organs and tissues react differently to the radiation exposure. Cells that reproduce rapidly and are less specialized have greater radiosensitivity: • Blood and blood producing organs • • • Bone marrow Reproductive cells (egg, sperm) Digestive tract cells Immature white blood cells Fetus

Types of Exposures • Acute – Large exposures that occur in a relatively short period of time • Chronic – Small exposures that occur over a relatively long period of time

Acute Radiation Syndrome • Prodromal Period • Latent Period • Period of Illness • Recovery or Death

Latent Period • Initial symptoms (prodromal period) disappear in a day or two • A latent period varies in length depending on the amount of the radiation dose

Period of Illness • Infection, electrolyte imbalance, diarrhea, bleeding, cardiovascular collapse, and sometimes short periods of unconsciousness • Symptoms depend on dose

Period of Illness • Hematopoietic Syndrome 200 rad – Within a few hours – nausea, vomiting, malaise, fatigue – Within 2 to 3 weeks – epilation • Gastrointestinal Syndrome – 600 rad – Severe nausea, vomiting, diarrhea, dehydration – Death within 1 to 2 weeks is likely • CNS Syndrome – 2, 000 rad – Ataxia (partial inability to coordinate voluntary bodily movement), convulsions, coma – Unconsciousness within a few minutes followed by death within 1 to 2 weeks

Mortality • NCRP Value for LD 50/30 260 – 325 rads midline • UNSCEAR Value for LD 50/60 350 rads (500 rads with treatment) • UNSCEAR Value for 95% Mortality 700 rads

Delayed Effects Several hundred rads partial body exposure – Skin – Telangiectasia, atrophy, ulceration – Lymphopenia (lack of lymphocytes) – Agranulocytosis – Anemia – Thyroid suppression – Histological sterility – Complete absence of gametes – Artificial menopause – Temporary sterility

Deterministic Effects • The effect occurs after a THRESHOLD dose has been exceeded • The MAGNITUDE of the effect increases with increasing dose • The effect can be directly attributed to the radiation exposure Cataracts Epilation Lethality Cell Depletion in bone marrow Non malignant skin damage • Deterministic effects generally result from the acute exposures.

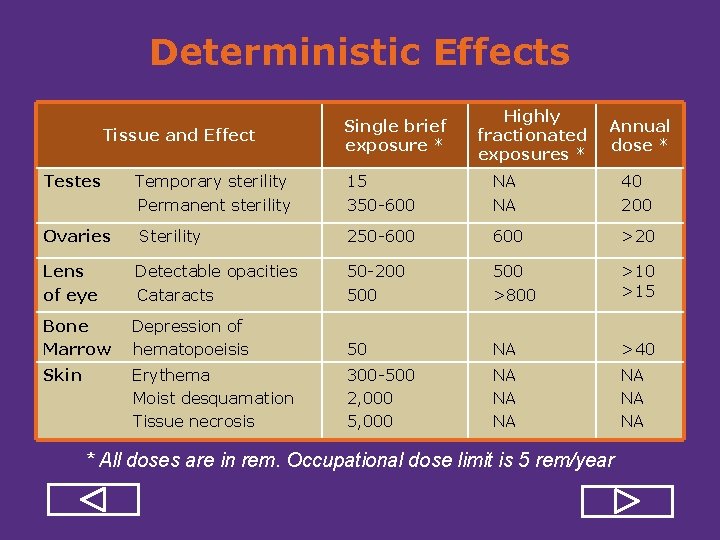
Deterministic Effects Tissue and Effect Single brief exposure * Highly fractionated exposures * Annual dose * Testes Temporary sterility Permanent sterility 15 350 600 NA NA 40 200 Ovaries Sterility 250 600 >20 Lens of eye Detectable opacities Cataracts 50 200 500 >800 >15 Bone Marrow Depression of hematopoeisis 50 NA >40 Skin Erythema Moist desquamation Tissue necrosis 300 500 2, 000 5, 000 NA NA NA * All doses are in rem. Occupational dose limit is 5 rem/year

Stochastic Effects • Chronic exposures to radiation are generally associated with stochastic effects. • The PROBABILITY that an effect occurs is related to the magnitude of the radiation dose • No relation between magnitude and severity of the effect – all or none response for an individual • Same effect can be seen in unexposed individuals, so it is impossible to attribute a particular case to the exposure to radiation

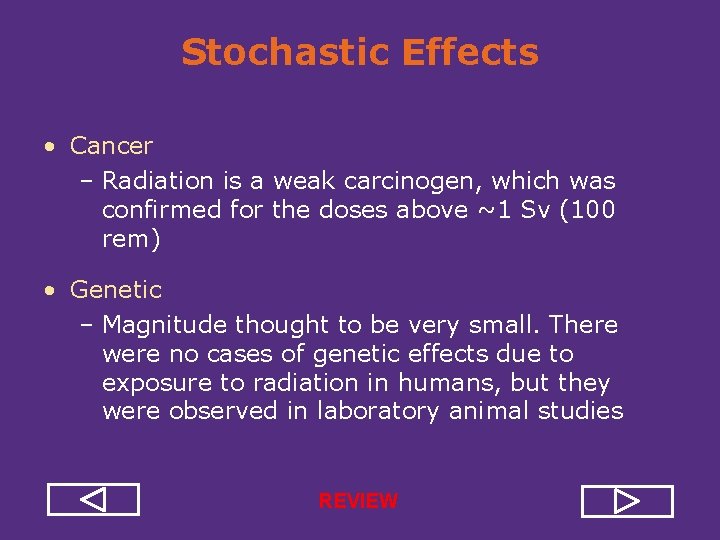
Stochastic Effects • Cancer – Radiation is a weak carcinogen, which was confirmed for the doses above ~1 Sv (100 rem) • Genetic – Magnitude thought to be very small. There were no cases of genetic effects due to exposure to radiation in humans, but they were observed in laboratory animal studies

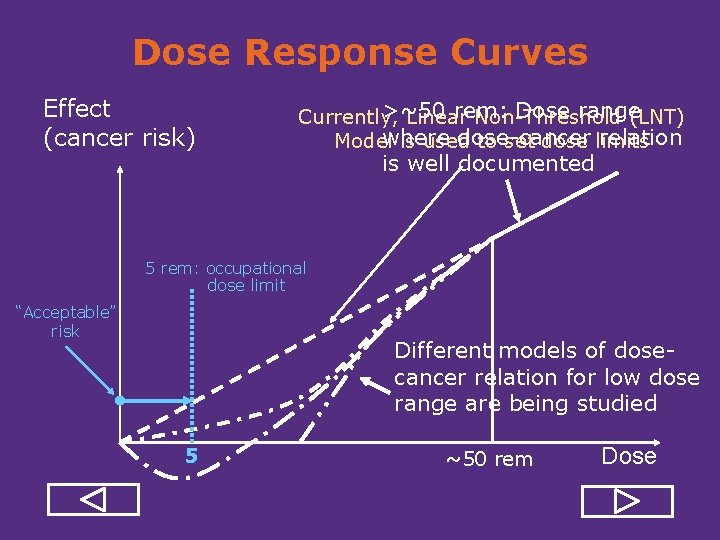
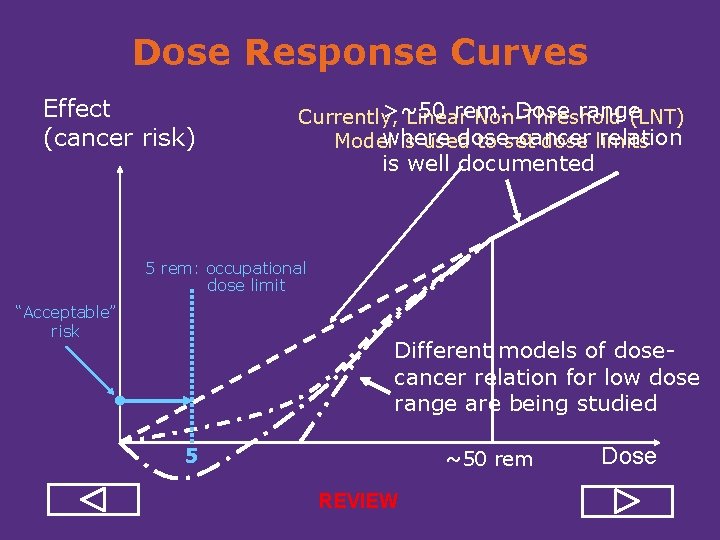
Dose Response Curves Effect (cancer risk) >~50 rem: Dose range Currently, Linear Non Threshold (LNT) where dose cancer relation Model is used to set dose limits is well documented 5 rem: occupational dose limit “Acceptable” risk Different models of dose cancer relation for low dose range are being studied 5 ~50 rem Dose

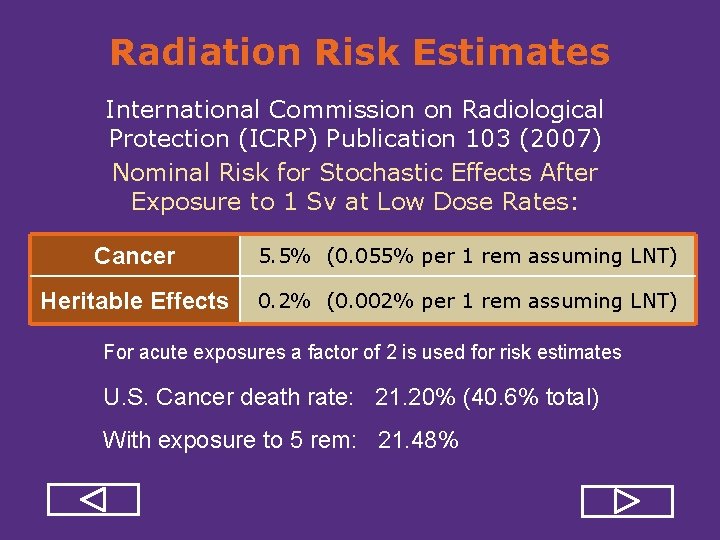
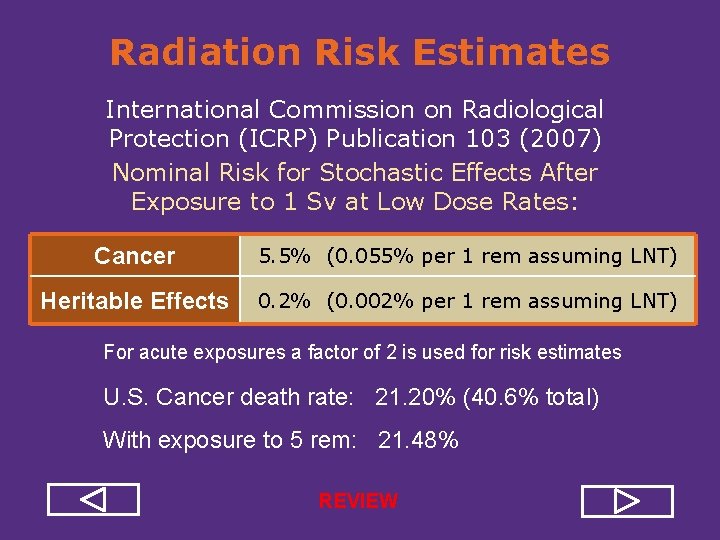
Radiation Risk Estimates International Commission on Radiological Protection (ICRP) Publication 103 (2007) Nominal Risk for Stochastic Effects After Exposure to 1 Sv at Low Dose Rates: Cancer 5. 5% (0. 055% per 1 rem assuming LNT) Heritable Effects 0. 2% (0. 002% per 1 rem assuming LNT) For acute exposures a factor of 2 is used for risk estimates U. S. Cancer death rate: 21. 20% (40. 6% total) With exposure to 5 rem: 21. 48%

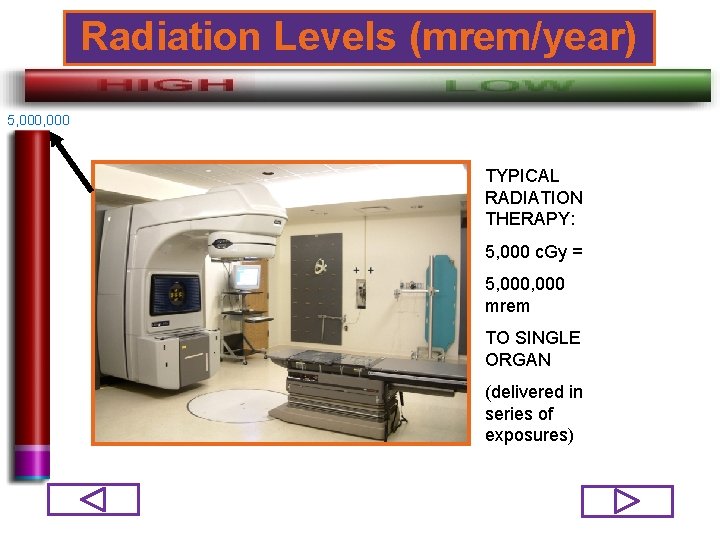
Radiation Levels (mrem/year) 5, 000 TYPICAL RADIATION THERAPY: 5, 000 c. Gy = 5, 000 mrem TO SINGLE ORGAN (delivered in series of exposures) 72/98

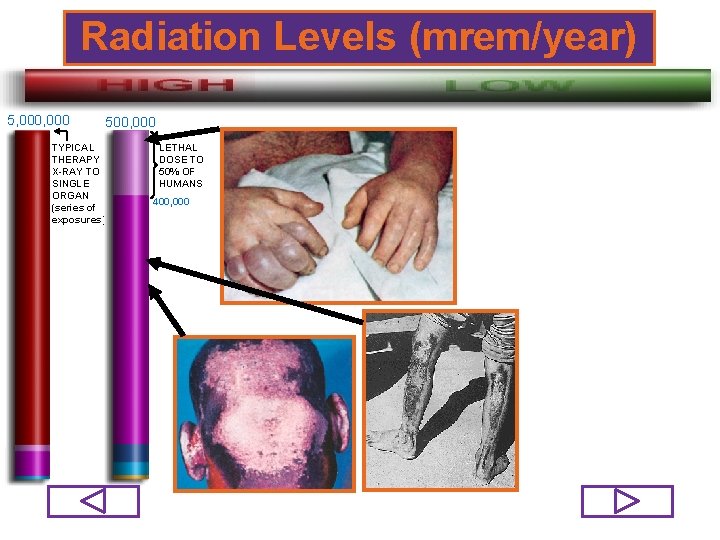
Radiation Levels (mrem/year) 5, 000 500, 000 TYPICAL THERAPY X-RAY TO SINGLE ORGAN (series of exposures) LETHAL DOSE TO 50% OF HUMANS 400, 000 73/98

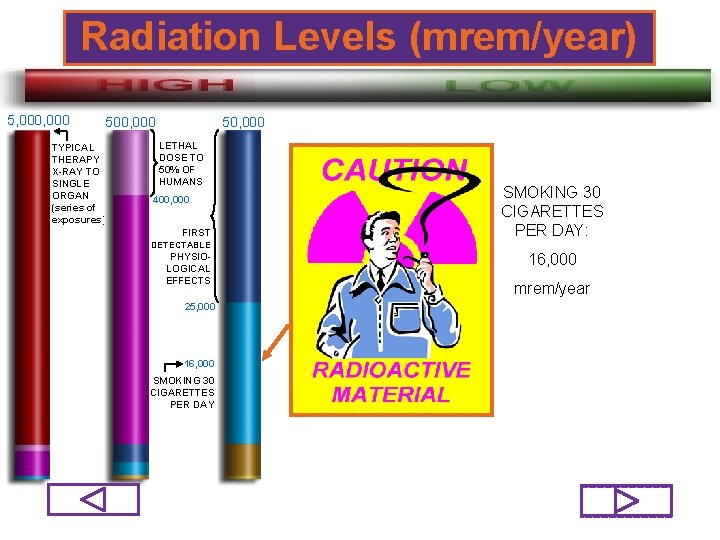
Radiation Levels (mrem/year) 5, 000 50, 000 500, 000 TYPICAL THERAPY X-RAY TO SINGLE ORGAN (series of exposures) LETHAL DOSE TO 50% OF HUMANS 400, 000 FIRST DETECTABLE PHYSIOLOGICAL EFFECTS SMOKING 30 CIGARETTES PER DAY: 16, 000 mrem/year 25, 000 16, 000 SMOKING 30 CIGARETTES PER DAY 74/98

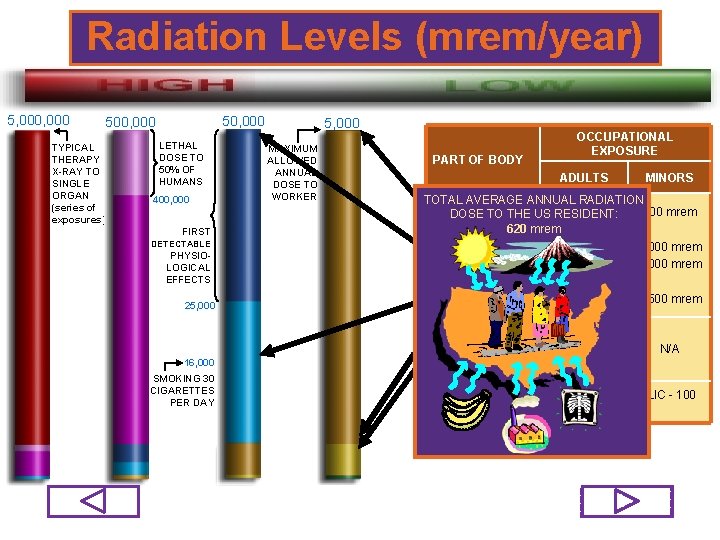
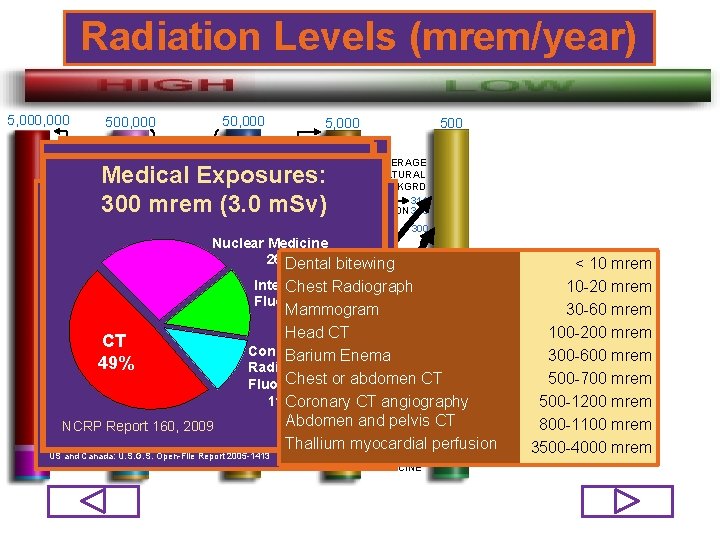
Radiation Levels (mrem/year) 5, 000 50, 000 500, 000 TYPICAL THERAPY X-RAY TO SINGLE ORGAN (series of exposures) LETHAL DOSE TO 50% OF HUMANS 400, 000 FIRST DETECTABLE PHYSIOLOGICAL EFFECTS 25, 000 16, 000 SMOKING 30 CIGARETTES PER DAY 5, 000 MAXIMUM ALLOWED ANNUAL DOSE TO WORKER PART OF BODY OCCUPATIONAL EXPOSURE ADULTS MINORS TOTAL AVERAGE ANNUAL RADIATION WHOLE mrem 500 mrem DOSEBODY TO THE US 5, 000 RESIDENT: 620 mrem SKIN EXTREMITIES 50, 000 mrem 5, 000 mrem LENS OF EYE 15, 000 mrem 1, 500 mrem N/A EMBRYO/FETUS (Declared Pregnancies) INDIVIDUAL MEMBERS OF THE PUBLIC - 100 mrem 75/98

Radiation Levels (mrem/year) 5, 000 50, 000 500, 000 TYPICAL THERAPY X-RAY TO SINGLE ORGAN (series of exposures) LETHAL DOSE TO 50% OF HUMANS 5, 000 28 mrem 500 MAXIMUM Occupational: 110 mrem (1. 1 m. Sv) AVERAGE ALLOWED Natural Background: ANNUAL NATURAL EPAMedical Map of Exposures: Radon Zones DOSE TO BACKGRD 311 Medical mrem (3. 11 m. Sv) Education, Research WORKER 400, 000 311 80 300 Gamma mrem (3. 0 m. Sv) Absorbed Dose 70 Rate in Air AVIATION 310 Industry 80 FIRST DETECTABLE Nuclear PHYSIOLOGICAL EFFECTS Cosmic Government, 11. 00% 300 Military Medicine MEDICAL 60 Terrestrial 26% Dental bitewing 7. 00% 223 X-RAY DIAGNOSTICS Interventional Chest Radiograph Radon-220 Fluoroscopy 25, 000 68% 5. 00% Mammogram RADON 14% 200 Nuclear Potassium-40 Head CT Aviation 190 CT 5. 00% Power Conventional 310 NUCLEAR Barium Enema AVERAGE 16, 000 190 49% Th & U Series Radiography and. POWER ANNUAL 4. 00%or abdomen SMOKING 30 Other <0. 01% Chest CT 147 CT RADIATION Fluoroscopy CIGARETTES EXPOSURE NCRP Report No. 160, 2009 OCCU 110 11% PER DAY Coronary angiography NCRP Report 160, 2009 CT TO U. S. Radon-222 PATIONAL Abdomen and pelvis CT 77 J. S. Duval et al, 2005, Terrestrial radioactivity and gamma-ray exposure in the Thallium myocardial perfusion NCRP Report 160, 2009 RESIDENT 620 US and Canada: U. S. G. S. Open-File Report 2005 -1413 NUCLEAR MEDICINE < 10 mrem 10 -20 mrem 30 -60 mrem 100 -200 mrem 300 -600 mrem 500 -700 mrem 500 -1200 mrem 800 -1100 mrem 3500 -4000 mrem 76/98

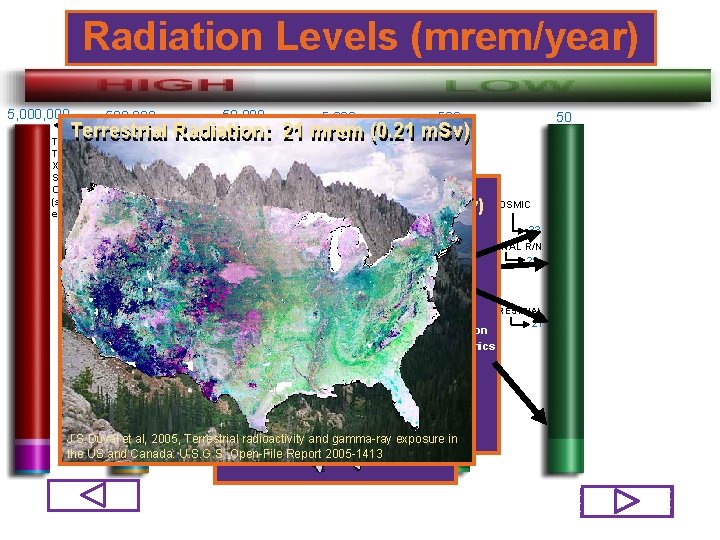
Radiation Levels (mrem/year) 5, 000 50, 000 500 5, 000 50 Terrestrial Radiation: 21 mrem Terrestrial Radiation: 21 Radionuclides mrem (0. 21 m. Sv) LETHAL Natural MAXIMUM TYPICAL THERAPY X-RAY TO SINGLE ORGAN (series of exposures) TO Cosmic. DOSE Radiation: 33 mrem m. Sv) ALLOWED 50% OF Contained In (0. 33 The. AVERAGE Body: HUMANS ANNUAL DOSE TO WORKER NATURAL BACKGRD 311 AVIATION 310 29 mrem (0. 29 m. Sv) Consumer Products: 13 mrem (0. 13 m. Sv) 400, 000 FIRST DETECTABLE PHYSIOLOGICAL EFFECTS 25, 000 27% Building 16, 000 Materials SMOKING 30 CIGARETTES PER DAY Po-210 26% Th-232 Commercial Air Travel Ra-228 Rb-87 COSMIC 300 MEDICAL K-40 33 INTERNAL R/N 29 223 6%X-RAY Mining and DIAGNOSTICS Agriculture RADON 2% Fossil Fuels Th-230 200 TERRESTRIAL 0. 6% Road Construction 190 <0. 03% Glass & Ceramics NUCLEAR POWER 3% Other CT 35% Rn-222 Tobacco 147 U-238 OCCU 110 PATIONAL J. S. Duval et al, 2005, Terrestrial radioactivity and gamma-ray exposure 77 in Ra-224 J. S. Duval al, Canada: 2005, Terrestrial radioactivity and 2005 -1413 gamma-ray exposure NCRP Report 160, in 2009 C-14 Report the US et and U. S. G. S. Open-File NUCLEAR the US and Canada: U. S. G. S. Open-File Report 2005 -1413 MEDICINE 21

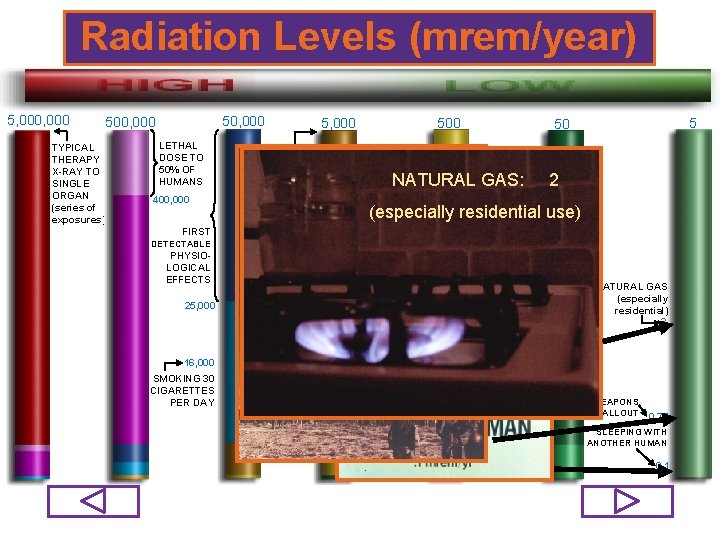
Radiation Levels (mrem/year) 5, 000 50, 000 500, 000 TYPICAL THERAPY X-RAY TO SINGLE ORGAN (series of exposures) LETHAL DOSE TO 50% OF HUMANS 400, 000 MAXIMUM ALLOWED ANNUAL DOSE TO WORKER 500 5, 000 AVERAGE NATURAL BACKGRD 311 AVIATION 310 NATURAL GAS: PHYSIOLOGICAL EFFECTS 2 COSMIC (especially residential use) 300 FIRST DETECTABLE MEDICAL 33 INTERNAL RN 29 223 X-RAY DIAGNOSTICS 25, 000 16, 000 SMOKING 30 CIGARETTES PER DAY 5 50 RADON FALLOUT FROM 200 190 WEAPONS TESTING: NUCLEAR POWER 0. 75 TERRESTRIAL 21 147 CONSUMER PRODUCTS OCCU- 110 PATIONAL 13 CT 77 NUCLEAR MEDICINE NATURAL GAS (especially residential) 2 WEAPONS FALLOUT 0. 75 SLEEPING WITH ANOTHER HUMAN 0. 1


Part I Quiz. Question 1 Isotopes have: q Same number of neutrons, but different number of protons q Same number of neutrons and protons combined q Same number of protons, but different number of neutrons q Extra energy stored inside nucleus q Different chemical properties Right! Wrong! Please proceed review to the and next try question again


Part I Quiz. Question 2 Half-life of a radionuclide is: q One half of the time required for complete decay q Time required for radioisotope to lose 50% of its activity q Time over which energy of emitted radiation reduces by half q Not well defined and depends on physical conditions q The same for all beta emitters Right! Wrong! Please proceed review to the and next try question again


Part I Quiz. Question 3 Unit of activity in SI: q Equals activity of 1 g of pure Ra 226 q Is called Curie (Ci) q Does not have a special name and is measured in decays/sec q Equals 3. 7 x 1010 decays per second q Is called Becquerel (Bq) and equals 1 decay per 1 second Right! Wrong! Please proceed review to the and next try question again


Part I Quiz. Question 4 SI units of exposure, absorbed dose and equivalent dose are: q Coulomb/kg (C/kg), Gray(Gy), Sievert(Sv) q Roentgen (R), rad, rem q Joule(J)/kg. SI does not have special units for these quantities q Coulomb/kg (C/kg), Gray/kg (G/kg), Sievert/kg (Sv/kg) q Used to express biological effect of different types of radiation Right! Wrong! Please proceed review to the and next try question again


Part I Quiz. Question 5 Linear non-threshold (LNT) model: q Is proven beyond doubt over the low dose range (0 25 rem) q Is used by regulators to establish occupational dose limits q Is being used only for high dose rate situations q Only accepted in USA and Canada q Can be used for stochastic and deterministic effects Right! Wrong! Please proceed review to the and next try question again

Definitions • ISOTOPE – Same Number of Protons (Z). Isotopes have the same chemical properties, but different radiological characteristics (halflife, type and energy of radiation, etc. ) Not all isotopes are radioactive! Z=53: I-124, I-125, I-126, I-127, I-128, I-129, I-130, I-131 • ISOTONE – Same Number of Neutrons N=46: Zr-86, Nb-87, Mo-88, Tc-89, Ru-90 • ISOBAR – Same Atomic Mass Number N+Z=131: Te-131, I-131, Xe-131, Cs-131, Ba-131 • ISOMER – Difference in nuclear Energy Tc-99 m (half-life 6. 01 hours), Tc-99 (half-life 211100 years) REVIEW

Nature of Radioactive Decay • Decay is random, predicting when a given atom will decay is impossible • In sufficient numbers, the probability of decay becomes well defined • Decay Constant (λ) = The probability that any one atom will decay REVIEW

Activity • Activity is the rate at which nuclear transformations occur in a radioactive material (rate of decay): A= λN • Number of radioactive atoms and, as a result, activity decreases exponentially with time: N = N 0 exp( λt) A = A 0 exp( λt) REVIEW

Half Life Time required for a radioactive substance to lose 50% of its activity by radioactive decay • ½ the activity • ½ the number of radioactive atoms • ½ the radiation intensity 1/2 1/4 1/8 T 1/2 REVIEW T 1/2 = ln 2 λ

Decay Constant Comparison Radionuclide Half Life Decay Constant Tc-99 m 6. 049 hours 2. 75 / d P-32 14. 28 days 0. 0468 / d H-3 12. 3 years 0. 000154 / d C-14 5730 years 0. 00000033 / d Half lives of radionuclides range from fractions of a second to billions of years. REVIEW

Units of Activity • Modern SI Unit – Becquerel (Bq) – 1 Bq = 1 decay per 1 second • Traditional Unit – Curie (Ci) – The number of radioactive decays occurring in one gram of pure Ra 226 1 Ci = 3. 7 x 1010 Bq = 37 GBq REVIEW

Multipliers and Fractions x 1012 tera (T) x 109 giga (G) x 106 mega (M) x 103 kilo (k) x 102 hecto (h) x 10 deka (da) x 10 1 deci (d) x 10 2 centi (c) x 10 3 milli (m) x 10 6 micro (μ) x 10 9 nano (n) x 10 12 pico (p) x 1, 000, 000 x 1, 000 x 100 x 10 1 x 0. 01 x 0. 000 001 x 0. 000 000 001 REVIEW Used most often with units of Bq Used most often with units of Ci

Absorbed Dose • Absorbed Dose - The total amount of energy imparted by all particles of the ionizing radiation per unit mass at a given location within irradiated material • rad - The traditional unit of absorbed dose, defined as the absorption of 100 ergs per gram • Gray (Gy) – The SI unit of absorbed dose, defined as the absorption of 1 Joule per 1 kilogram 1 Gy = 100 rad REVIEW

Exposure • There two applications of the word “exposure” in radiation safety: – Common use, like in the sentence “exposure to radiation”; – And as a characteristic of the ionization created by radiation in air • Exposure – A measurement of the amount of ionization created by X rays or gamma rays in a volume of air REVIEW

Units of Exposure • Roentgen (R) – amount of exposure creating 1 esu (electrostatic unit) of charge in 1 cubic cm of dry air at STP. • There is no special unit of exposure in SI 1 R = 2. 58 x 10 4 Coulombs / kg air • Amount of radiation creating 1 R of exposure in air delivers absorbed dose of 0. 96 rad to tissue. This fact allows us by measuring exposure (with Geiger Mueller counter, for example) to have good estimate (almost one to one) of the dose to a human body. REVIEW

Biological Effectiveness • Biological effects of ionizing radiation depend not only on the amount of deposited energy (absorbed dose), but also on how this energy was deposited, how densely ionizations occur. • Radiation weighting factor (w. R) - estimate of the effectiveness per unit dose of the given radiation relative a to low LET standard (X ray or gamma) REVIEW

Radiation weighting factors Type of Radiation w. R X Rays 1 Gamma Rays 1 Beta Particles 1 Protons 2 Alphas 20 Neutrons 5 20 REVIEW

Equivalent Dose • Equivalent Dose expresses the biological effect of exposure to the different types of radiation Equivalent Dose = Absorbed Dose × w. R • rem – traditional unit of equovalent dose of any radiation which produces the same biological effect as 1 rad of absorbed dose of x or gamma rays • Sievert (Sv) – SI unit of equivalent dose of any radiation that produces the same biological effect as a 1 Gy of absorbed dose of x or gamma rays 1 Sv = 100 rem REVIEW

Stochastic Effects • Chronic exposures to radiation are generally associated with stochastic effects. • The PROBABILITY that an effect occurs is related to the magnitude of the radiation dose • No relation between magnitude and severity of the effect – all or none response for an individual • Same effect can be seen in unexposed individuals, so it is impossible to attribute a particular case to the exposure to radiation REVIEW

Stochastic Effects • Cancer – Radiation is a weak carcinogen, which was confirmed for the doses above ~1 Sv (100 rem) • Genetic – Magnitude thought to be very small. There were no cases of genetic effects due to exposure to radiation in humans, but they were observed in laboratory animal studies REVIEW

Dose Response Curves Effect (cancer risk) >~50 rem: Dose range Currently, Linear Non Threshold (LNT) where dose cancer relation Model is used to set dose limits is well documented 5 rem: occupational dose limit “Acceptable” risk Different models of dose cancer relation for low dose range are being studied 5 ~50 rem REVIEW Dose

Radiation Risk Estimates International Commission on Radiological Protection (ICRP) Publication 103 (2007) Nominal Risk for Stochastic Effects After Exposure to 1 Sv at Low Dose Rates: Cancer 5. 5% (0. 055% per 1 rem assuming LNT) Heritable Effects 0. 2% (0. 002% per 1 rem assuming LNT) For acute exposures a factor of 2 is used for risk estimates U. S. Cancer death rate: 21. 20% (40. 6% total) With exposure to 5 rem: 21. 48% REVIEW

RADIATION SAFETY Refresher Training Part 2

COURSE PART 2 OBJECTIVES • • • Radiation dose limits; dose monitoring Basic principles of radiation protection Contamination; contamination control; contamination survey Radioactive material regulations; RAM “project” Signs, postings, labels RAM ordering, use and inventory Safe laboratory practices; PPE Radioactive material security Radioactive waste disposal Emergencies

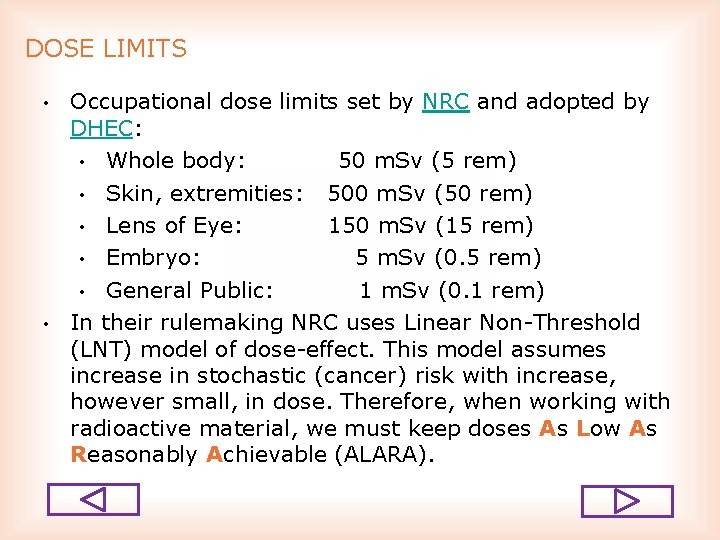
DOSE LIMITS • • Occupational dose limits set by NRC and adopted by DHEC: • Whole body: 50 m. Sv (5 rem) • Skin, extremities: 500 m. Sv (50 rem) • Lens of Eye: 150 m. Sv (15 rem) • Embryo: 5 m. Sv (0. 5 rem) • General Public: 1 m. Sv (0. 1 rem) In their rulemaking NRC uses Linear Non Threshold (LNT) model of dose effect. This model assumes increase in stochastic (cancer) risk with increase, however small, in dose. Therefore, when working with radioactive material, we must keep doses As Low As Reasonably Achievable (ALARA).

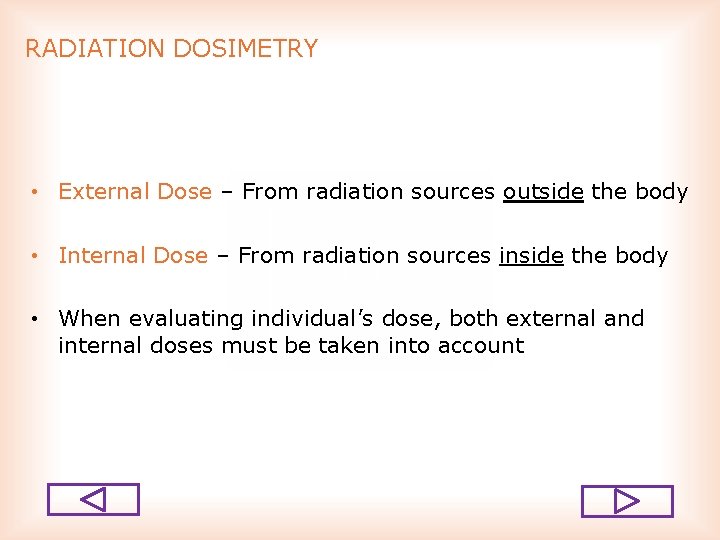
RADIATION DOSIMETRY • External Dose – From radiation sources outside the body • Internal Dose – From radiation sources inside the body • When evaluating individual’s dose, both external and internal doses must be taken into account

RADIATION DETECTION INSTRUMENTS There isn’t a single instrument that detects all kinds of radiations and in all situations. (hover instruments for more information)

MONITORING EQUIPMENT CHECK Every day before use: 1. Check calibration expiration date. Contact radiation safety office when calibration expiration date is close or passed. Do not use instruments with expired calibration for routine or recordable surveys. Only use them in emergency when no instruments with current calibration are available and only if all operational checks are OK.

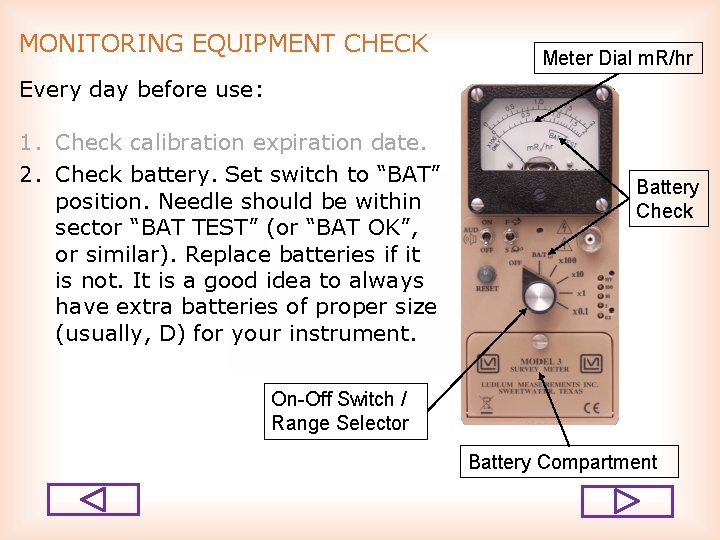
MONITORING EQUIPMENT CHECK Meter Dial m. R/hr Every day before use: 1. Check calibration expiration date. 2. Check battery. Set switch to “BAT” position. Needle should be within sector “BAT TEST” (or “BAT OK”, or similar). Replace batteries if it is not. It is a good idea to always have extra batteries of proper size (usually, D) for your instrument. Battery Check On-Off Switch / Range Selector Battery Compartment

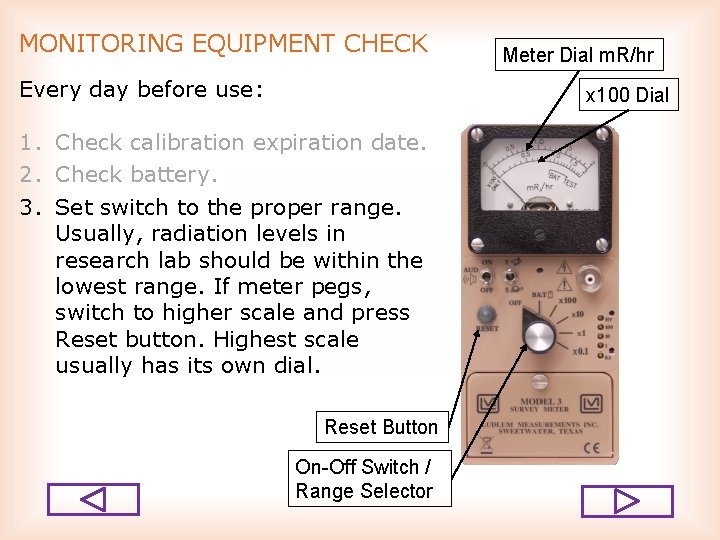
MONITORING EQUIPMENT CHECK Every day before use: Meter Dial m. R/hr x 100 Dial 1. Check calibration expiration date. 2. Check battery. 3. Set switch to the proper range. Usually, radiation levels in research lab should be within the lowest range. If meter pegs, switch to higher scale and press Reset button. Highest scale usually has its own dial. Reset Button On-Off Switch / Range Selector

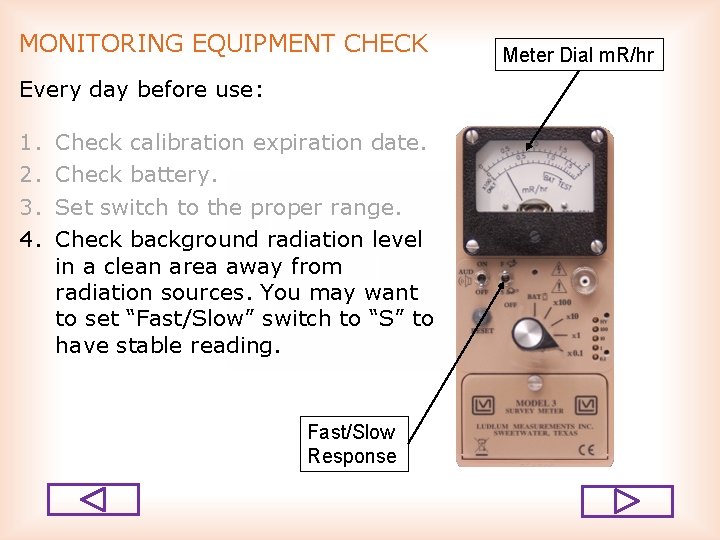
MONITORING EQUIPMENT CHECK Every day before use: 1. 2. 3. 4. Check calibration expiration date. Check battery. Set switch to the proper range. Check background radiation level in a clean area away from radiation sources. You may want to set “Fast/Slow” switch to “S” to have stable reading. Fast/Slow Response Meter Dial m. R/hr

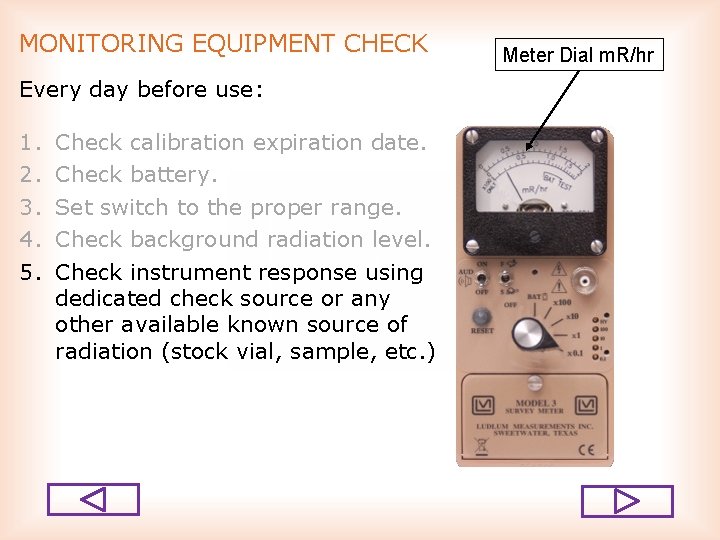
MONITORING EQUIPMENT CHECK Every day before use: 1. 2. 3. 4. 5. Check calibration expiration date. Check battery. Set switch to the proper range. Check background radiation level. Check instrument response using dedicated check source or any other available known source of radiation (stock vial, sample, etc. ) Meter Dial m. R/hr

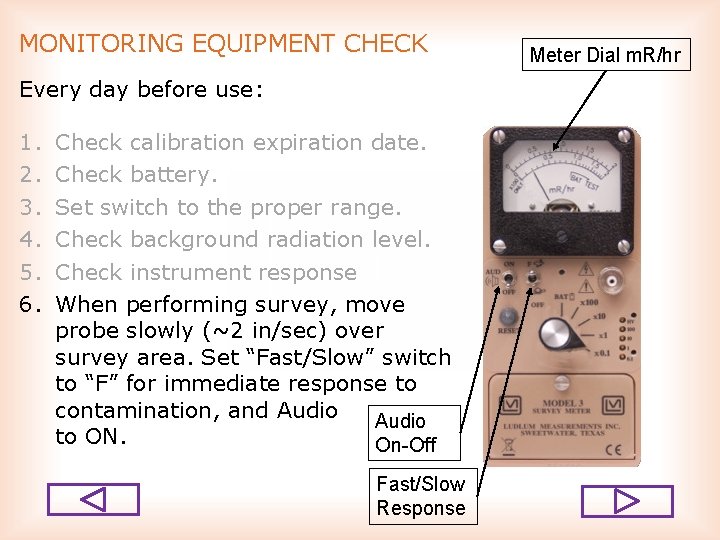
MONITORING EQUIPMENT CHECK Every day before use: 1. 2. 3. 4. 5. 6. Check calibration expiration date. Check battery. Set switch to the proper range. Check background radiation level. Check instrument response When performing survey, move probe slowly (~2 in/sec) over survey area. Set “Fast/Slow” switch to “F” for immediate response to contamination, and Audio to ON. On-Off Fast/Slow Response Meter Dial m. R/hr

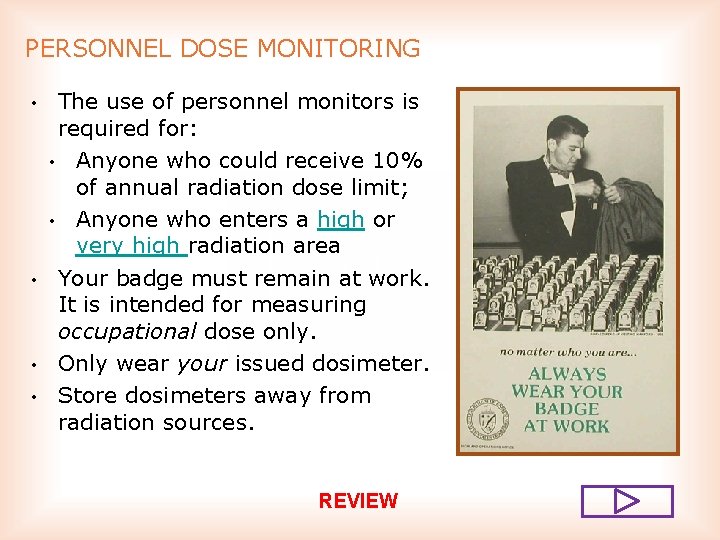
PERSONNEL DOSE MONITORING • • The use of personnel monitors is required for: • Anyone who may receive 10% of annual radiation dose limit; • Anyone who enters a high or very high radiation area Your badge must remain at work. It is intended for measuring occupational dose only. Only wear your issued dosimeter. Store dosimeters away from radiation sources.

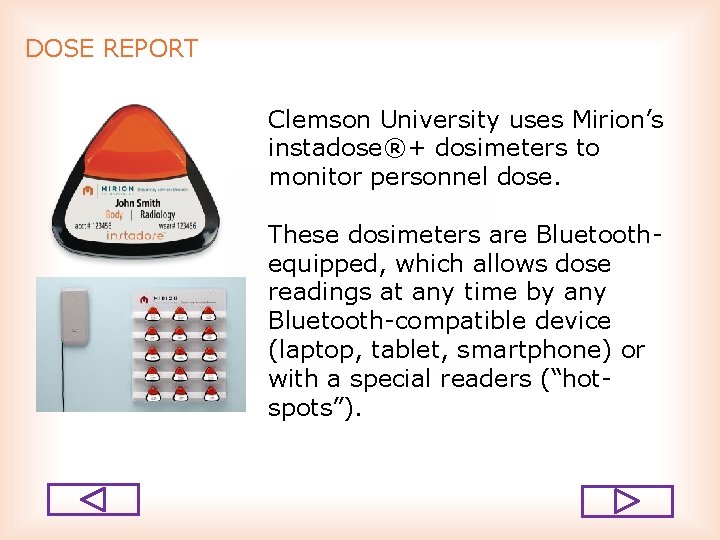
DOSE REPORT Clemson University uses Mirion’s instadose®+ dosimeters to monitor personnel dose. These dosimeters are Bluetooth equipped, which allows dose readings at any time by any Bluetooth compatible device (laptop, tablet, smartphone) or with a special readers (“hot spots”).

TLD RING DOSIMETER • Ring dosimeters are required for persons working with open beam x ray systems. • Radiation Safety Committee may require use of ring dosimeters for persons working with high activities of radioactive materials (eg. , 1 m. Ci of P 32).

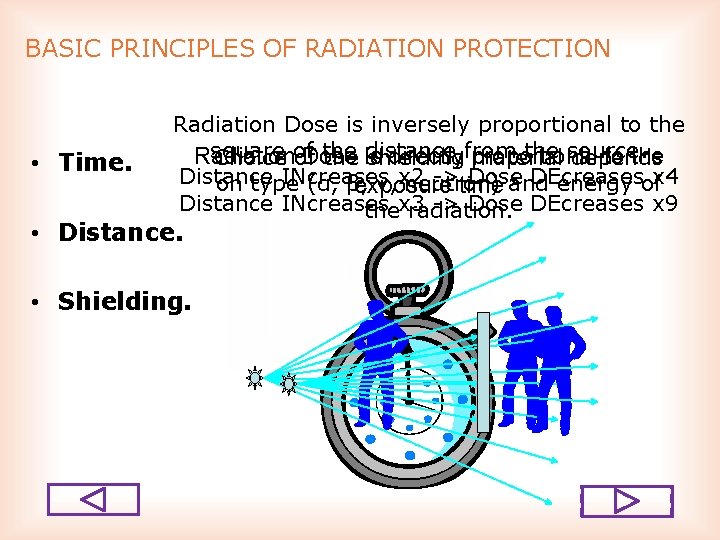
BASIC PRINCIPLES OF RADIATION PROTECTION • Time. Radiation Dose is inversely proportional to the square the distance the source. Radiation Dose directlyfrom proportional to the Choice of of the is shielding material depends Distance INcreases >time Dose DEcreases on type (α, β, γ, x 2 neutron) and energy ofx 4 exposure Distance INcreases > Dose DEcreases x 9 thex 3 radiation. • Distance. • Shielding.

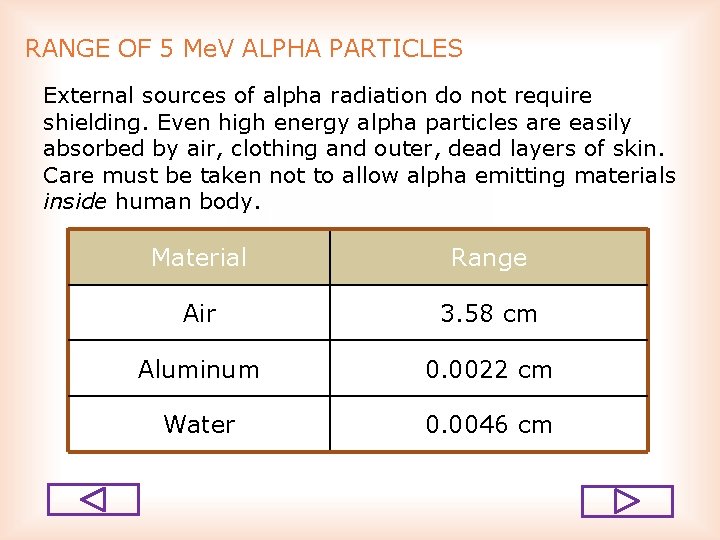
RANGE OF 5 Me. V ALPHA PARTICLES External sources of alpha radiation do not require shielding. Even high energy alpha particles are easily absorbed by air, clothing and outer, dead layers of skin. Care must be taken not to allow alpha emitting materials inside human body. Material Range Air 3. 58 cm Aluminum 0. 0022 cm Water 0. 0046 cm

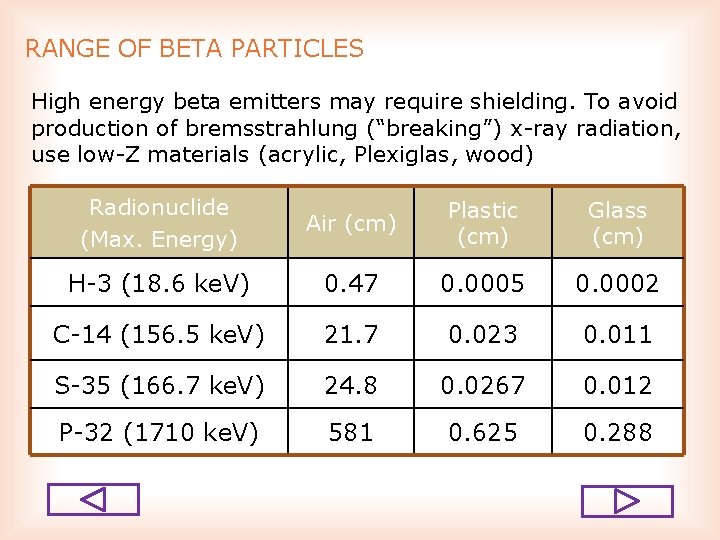
RANGE OF BETA PARTICLES High energy beta emitters may require shielding. To avoid production of bremsstrahlung (“breaking”) x ray radiation, use low Z materials (acrylic, Plexiglas, wood) Radionuclide (Max. Energy) Air (cm) Plastic (cm) Glass (cm) H 3 (18. 6 ke. V) 0. 47 0. 0005 0. 0002 C 14 (156. 5 ke. V) 21. 7 0. 023 0. 011 S 35 (166. 7 ke. V) 24. 8 0. 0267 0. 012 P 32 (1710 ke. V) 581 0. 625 0. 288

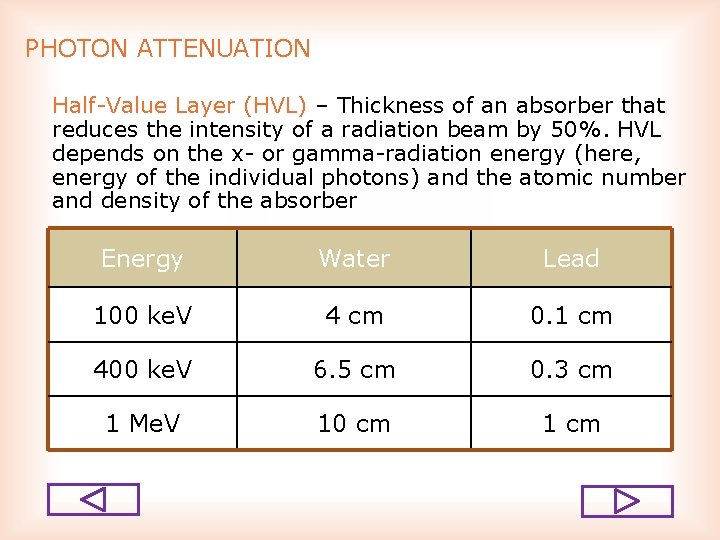
PHOTON ATTENUATION Half Value Layer (HVL) – Thickness of an absorber that reduces the intensity of a radiation beam by 50%. HVL depends on the x or gamma radiation energy (here, energy of the individual photons) and the atomic number and density of the absorber Energy Water Lead 100 ke. V 4 cm 0. 1 cm 400 ke. V 6. 5 cm 0. 3 cm 1 Me. V 10 cm 1 cm

BASIC PRINCIPLES OF RADIATION PROTECTION • Time. Contamination is a source of both external and internal contamination • Distance. • Shielding. • Contamination Control

SOURCES OF CONTAMINATION • Sloppy work practices, such as cross contamination of tools, equipment, or workers. • Not wearing gloves or removing them prematurely. • Poor housekeeping in potentially contaminated areas. • Opening radioactive materials without proper controls (hood, glove box, etc. ). • Leaking containers or tears in radiological containers such as barrels, plastic bags, boxes, or protective gear. • Spills, glass breakage, and animal fluids. • Airborne contamination depositing on surfaces. • Not adhering to standard laboratory procedures. • Emergencies including fire, earthquake, etc.

CONTAMINATION PREVENTION • Plastic backed absorbent paper should cover all surfaces where open radioactive sources are handled. Lab coats, gloves and safety glasses, goggles or face shield must be worn when working with radioactive material. • When wearing gloves, never touch anything that you will handle without gloves: pens, notebooks, phones, switches, door handles, computer keyboard, etc. • Secondary containment must be used for transfer and storage of all radioactive materials.

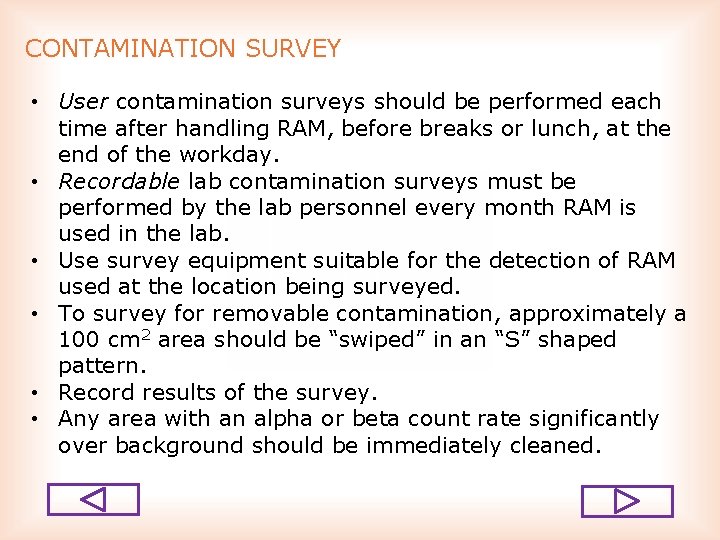
CONTAMINATION SURVEY • User contamination surveys should be performed each time after handling RAM, before breaks or lunch, at the end of the workday. • Recordable lab contamination surveys must be performed by the lab personnel every month RAM is used in the lab. • Use survey equipment suitable for the detection of RAM used at the location being surveyed. • To survey for removable contamination, approximately a 100 cm 2 area should be “swiped” in an “S” shaped pattern. • Record results of the survey. • Any area with an alpha or beta count rate significantly over background should be immediately cleaned.

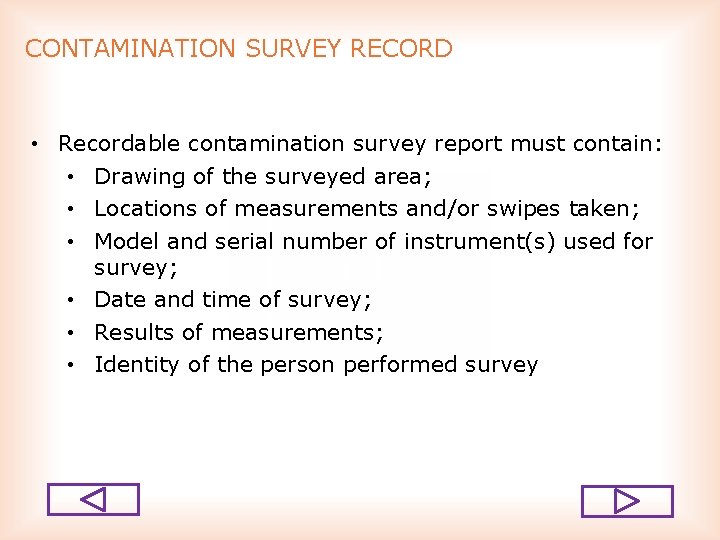
CONTAMINATION SURVEY RECORD • Recordable contamination survey report must contain: • Drawing of the surveyed area; • Locations of measurements and/or swipes taken; • Model and serial number of instrument(s) used for survey; • Date and time of survey; • Results of measurements; • Identity of the person performed survey

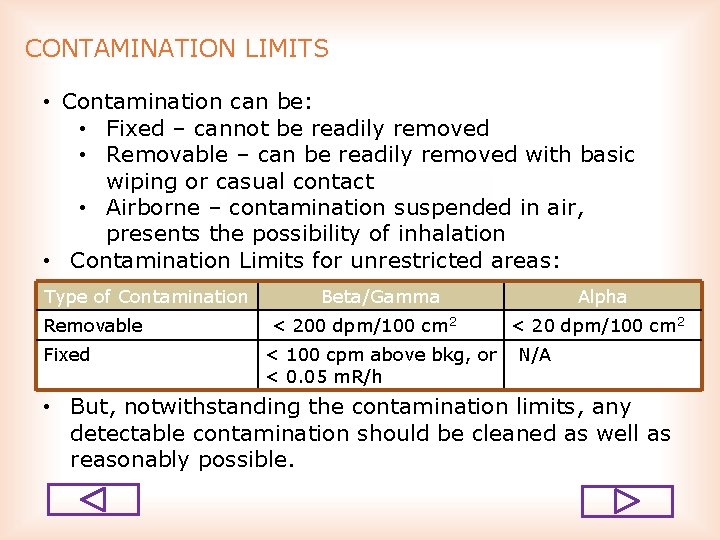
CONTAMINATION LIMITS • Contamination can be: • Fixed – cannot be readily removed • Removable – can be readily removed with basic wiping or casual contact • Airborne – contamination suspended in air, presents the possibility of inhalation • Contamination Limits for unrestricted areas: Type of Contamination Removable Fixed Beta/Gamma < 200 dpm/100 cm 2 < 100 cpm above bkg, or < 0. 05 m. R/h Alpha < 20 dpm/100 cm 2 N/A • But, notwithstanding the contamination limits, any detectable contamination should be cleaned as well as reasonably possible.

SPILLS OF RADIOACTIVE MATERIAL • All spills of RAM require immediate response. Initial response to a spill rests with the lab personnel. Untrained personnel is not allowed to examine or clean RAM spill. • Minor spills may be decontaminated by lab personnel. • If contamination widespread, includes unrestricted areas, unauthorized personnel, or involves high μCi or m. Ci amounts of RAM, notify RSO. • Notify all lab personnel. Have all personnel not involved with the spill to vacate the area but remain in one place to minimize possibility of the contamination spread. • Contain the spill from further spread. If the material is a liquid, place an absorbent material over the spill to prevent its spread. If the material spilled is a powdered solid, attempt to contain its spread by covering the area with a protective barrier (ex. , wet cloth or absorbent paper). If appropriate, close doors and windows, turn off room ven tilation fans.

SPILLS OF RADIOACTIVE MATERIAL (continued) • Monitor any personnel that were in the area at the time of the spill. Give special attention to the nose and mouth areas. Report any facial contamination to the RSO immediately. • Remove contaminated clothing at once; flush contaminated skin areas thoroughly. • Decontaminate the area: Plan ahead. Provide adequate protection and supplies for personnel involved in the cleanup. Besides required PPE, wear double gloves and shoe covers. Begin at the periphery and work toward the center of the contamination. Cover cleaned areas with plastic or paper to prevent its recontamination. Place all contaminated items in the proper waste containers. • Monitor the area: Survey area after each decontamination effort. If contamination persists after several decontamination efforts, notify RSO.

INTERNAL EXPOSURE PATHWAYS • Inhalation – Gases, aerosols, dusts, mists, fumes, smoke (including cigarettes) • Ingestion – Food, water, hand contamination • Absorption thru Skin – Thru intact skin, cuts, abrasions, etc. • Punctures – Needle sticks

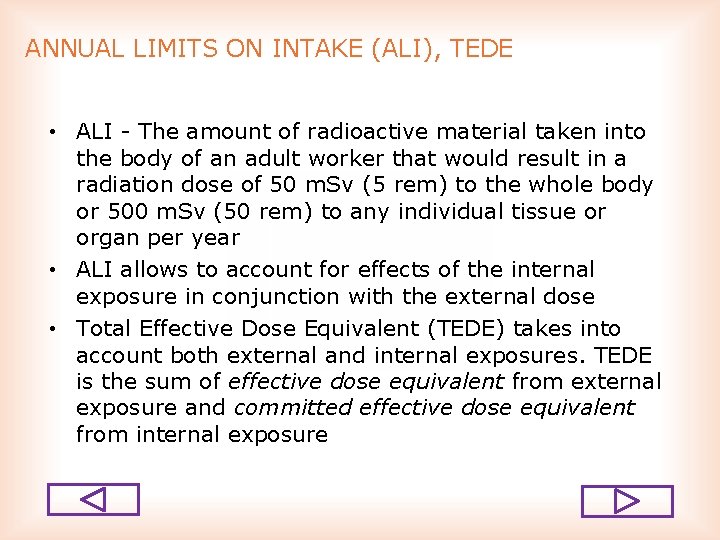
ANNUAL LIMITS ON INTAKE (ALI), TEDE • ALI The amount of radioactive material taken into the body of an adult worker that would result in a radiation dose of 50 m. Sv (5 rem) to the whole body or 500 m. Sv (50 rem) to any individual tissue or organ per year • ALI allows to account for effects of the internal exposure in conjunction with the external dose • Total Effective Dose Equivalent (TEDE) takes into account both external and internal exposures. TEDE is the sum of effective dose equivalent from external exposure and committed effective dose equivalent from internal exposure

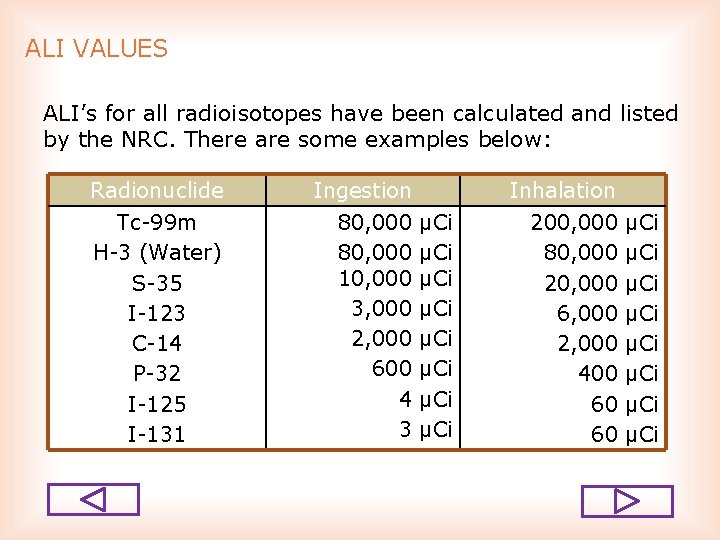
ALI VALUES ALI’s for all radioisotopes have been calculated and listed by the NRC. There are some examples below: Radionuclide Ingestion Tc 99 m H 3 (Water) S 35 I 123 C 14 P 32 I 125 I 131 80, 000 10, 000 3, 000 2, 000 600 4 3 Inhalation μCi μCi 200, 000 80, 000 20, 000 6, 000 2, 000 400 60 60 μCi μCi

TEDE CALCULATION EXAMPLE • • Nuclear medicine technician works with I 131 Personal dosimeter shows annual dose of 500 mrem Thyroid counting using Na. I(Tl) indicated I 131 uptake of 1. 5 µCi What is technician’s TEDE and how it compares to the occupational dose limit? I 131 ALI is 3 µCi, therefore, committed effective dose equivalent from the uptake of 1. 5 µCi equals (1. 5/3)*5 = 2. 5 rem Measured external dose equivalent is 500 mrem Adding external and internal doses, we have for TEDE: 2. 5 rem + 500 mrem = 3 rem, which is (3/5)*100 = 60% of the occupational dose limit

SKIN DECONTAMINATION Mechanical Remove with Sticky Tape Washing Soap or Detergent and Cool Water Surgical Scrub Brushes with Povidone Iodine Complexing Agents (1% Citric Acid) Chelating Agents (1% Versene) Biological Sweat it out Washing affected areas with soap and cool to lukewarm water is the first, and usually, the only required action for skin decontamination at a research lab of the Clemson University

RADIOACTIVE MATERIAL AUTHORIZATION • • The Nuclear Regulatory Commission (NRC) is the Federal agency authorized by the Congress to regulate radioactive material use. States may assume functions of the NRC within their borders. Such states are called Agreement States. South Carolina became an Agreement State in 1969 South Carolina’s Department of Health and Environmental Control (DHEC): • licenses radioactive material use; • regulates X ray registration and use; • performs inspections of licensed facilities; • regulates use of lasers.

RADIOACTIVE MATERIAL AUTHORIZATION • • Clemson University has a Broad Scope Radioactive Material License issued by DHEC Allows use of radioactive isotopes with atomic numbers 1 98 in any chemical or physical form All activities involving radioactive material (“projects”) within the Clemson University must be approved by the Radiation Safety Committee (RSC) RAM Project includes: • Responsible Investigator (RI); • list of authorized isotopes and possession limits; • list of personnel; • facilities where RAM use is authorized; • approved protocol( s).

PERIODIC INSPECTIONS • • • Clemson University’s Radioactive Material License requires semi annual inspections of RAM projects; Radiation safety personnel contacts project’s RI to set mutually acceptable date and time for inspection; RI does not have to be physically present during inspection, but (s)he has to assign AU to assist inspector. This AU has to be knowledgeable about project’s activities and capable to answer inspector’s questions.

WHO MAY WORK WITH RADIOACTIVE MATERIAL? • • • Only individuals listed on a Project may use RAM; Must receive instructions from the RI; May start working with RAM before attending Initial Training, but only under direct supervision of RI or AU (status called Radiation Worker (RW)) To become Authorized User (AU), must complete Initial Radiation Safety training; Must complete annual refresher training (online or classroom) to maintain AU status.

WHO MAY WORK WITH RADIOACTIVE MATERIAL? • • • Fill form R 003 Request To Add An Individual To A Radionuclide Project and submit to Radiation Safety. You may be listed on several Projects. Or, you may start your own project.

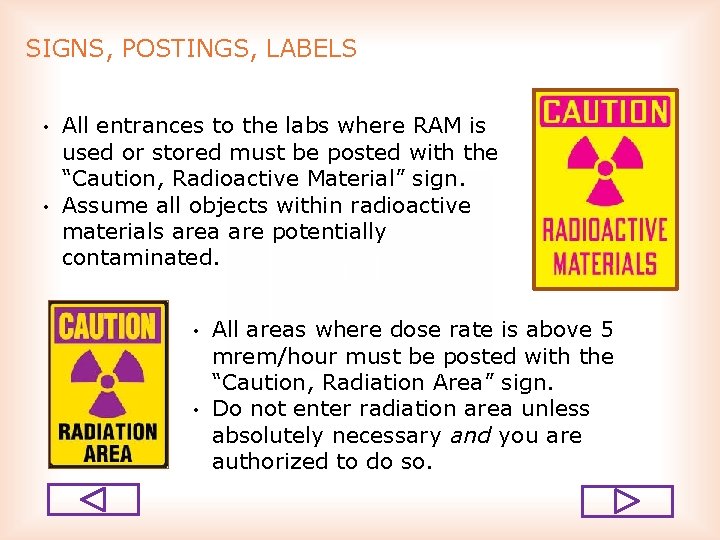
SIGNS, POSTINGS, LABELS • • All entrances to the labs where RAM is used or stored must be posted with the “Caution, Radioactive Material” sign. Assume all objects within radioactive materials area are potentially contaminated. • • All areas where dose rate is above 5 mrem/hour must be posted with the “Caution, Radiation Area” sign. Do not enter radiation area unless absolutely necessary and you are authorized to do so.

SIGNS, POSTINGS, LABELS • All equipment, containers, glassware, tools, etc. that may come into contact with RAM must be labelled with “Caution, Radioactive Material” Sign.

ORDERING RADIOACTIVE MATERIAL • You can only order radionuclides that the project is authorized for and within your possession limits • Use a licensed supplier (Amersham, MP Biomedicals, Perkin Elmer, etc. ) • All radioactive material shipments must be addressed to: Main Campus: Rich, CETL: 504 Lake Dr. Clemson, SC 29631 342 Computer Ct. Anderson, SC 29625 For: RN <your project>, <RI name>

RADIOACTIVE MATERIAL USE RECORD Upon RAM package receipt, radiation safety personnel: • checks radiation and contamination levels, package labelling and documentation; • assigns shipment number; • adds RAM to the project’s inventory and checks against possession limits; • produces RAM Use/ Inventory Record; • notifies project personnel and delivers shipment.

OPENING PACKAGE Follow these steps when opening RAM package in your lab: • Check paperwork to make sure that you are an intended recipient and you received what you ordered; • Open package, check integrity of the internal packaging. Notify Radiation Safety Office and manufacturer if internal package is broken and/or leaks; • Remove container with RAM from the packaging and mark it with the shipment number listed on the RAM Use form; • Survey inside of the packaging for residual contamination. Use appropriate survey equipment. • Remove or deface Radioactive Material labels from the packaging and dispose of it.

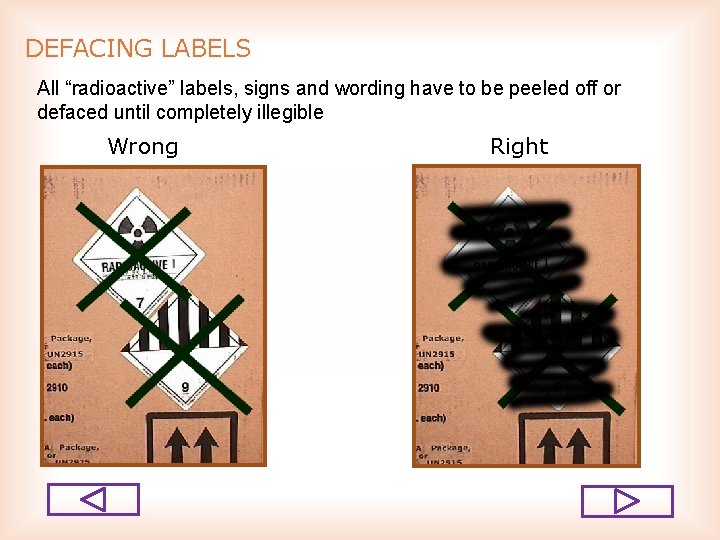
DEFACING LABELS All “radioactive” labels, signs and wording have to be peeled off or defaced until completely illegible Wrong Right

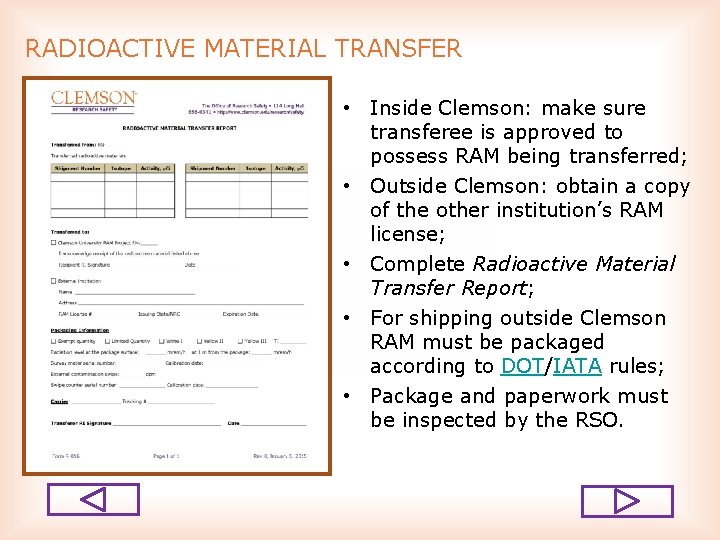
RADIOACTIVE MATERIAL TRANSFER • Inside Clemson: make sure transferee is approved to possess RAM being transferred; • Outside Clemson: obtain a copy of the other institution’s RAM license; • Complete Radioactive Material Transfer Report; • For shipping outside Clemson RAM must be packaged according to DOT/IATA rules; • Package and paperwork must be inspected by the RSO.

GENERAL LAB RULES • • Working with radioactive material in a research laboratory may involve exposure to many other hazards: chemical, biological, mechanical, electrical, etc. Following best lab practices described in the Laboratory Safety Manual is critical in avoiding these hazards. www. clemson. edu/research/safety/manuals/lab. Safety/index. html • Besides general lab safety, radiation safety requires additional considerations.

SAFE LAB PRACTICES • All work areas should be kept uncluttered to prevent accidents and minimize cross contamination. • There must be no eating, drinking, smoking, or storage of food in areas in which radioactive materials are used. • No mouth pipetting is allowed in a RAM work area. • Containers used in mixing, shaking, or centrifuging operations should be intact and sealed to prevent spillage. • Heating, drying, distilling, and other operations which could result in volatilization of the material should be performed in a fume hood or glove box. • Whenever possible, rehearse operations with non radioactive materials to ensure that the technique will be reasonably free of incidents. • Maintain accurate records of radioactive material use.

PERSONAL PROTECTIVE EQUIPMENT • When working with radioactive material minimum required PPE is lab coat, gloves and safety glasses or goggles. • No shorts, no open toe shoes, no short sleeves are allowed for any lab work. • Depending on the scope of activities and hazards involved, additional PPE requirements may be imposed on a project personnel by the Radiation Safety Committee.

RADIOACTIVE MATERIAL SECURITY • • Radioactive material must be secured against unauthorized use or removal • Entrances to unoccupied labs must be closed and locked, or • All radioactive material, including waste, must be kept in locked cabinets NRC commentary on 10 CFR PART 20: “The Commission believes that locking radiotracer laboratories when not being used is a small nuisance compared to the consequences of unauthorized access to or theft of radioactive materials, which could result in contamination of unrestricted areas or exposure of individuals, as well as having to report a loss of licensed material to the NRC. ”

ESCORTING VISITORS IN THE LABORATORY • Inform guests that they are entering a radioactive materials area. • Point out locations of any hazards or high radiation areas. • Do not allow guests to handle any materials from a radioactive materials area. • Demonstrate how to perform a personal scan prior to exiting the lab (or, survey them yourself). • If guests will be working with you in the lab, consult with RSO if personal dosimetry is required. But remember, only individuals listed on the RAM Project may work with radioactive material.

RADIOACTIVE WASTE DISPOSAL • Dry solid waste must be separated into Short (less than 65 day) and Long half life. Waste bags and containers are color coded: green for short, yellow for long half life.

RADIOACTIVE WASTE DISPOSAL • Deface all “Radioactive Material” labels before placing items into radioactive dry waste container. • Prohibited for disposal into radioactive dry waste: • sharp objects; • sealed sources; • biohazardous waste or bags; • lead containers • liquids • Sharp objects must be collected in a special sharps waste container labelled with “Radioactive Material” sign • Radioactive biohazard waste must be treated in the same manner as the regular biohazard waste (i. e. , autoclaving, bleach) before being placed into radioactive waste containers.

RADIOACTIVE WASTE DISPOSAL • For mixed (hazardous + radioactive) liquid waste follow hazardous waste compatibility guidelines. • If possible, use biodegradable LSC cocktail. • Do not wait for waste containers to overflow. Request waste pick up when containers are about 2/3 full. • Do not let housekeeping personnel to empty radioactive waste containers. • Keep reasonably accurate waste disposal record.

LIQUID RADIOACTIVE WASTE DISPOSAL • Some projects may be allowed to dispose liquid radioactive waste down the sewer. These disposals must be approved by the Radiation Safety Committee. • Only materials that are soluble or miscible in water or biological materials that are readily dispersible may be disposed via sink. • No hazardous chemicals! Mixed liquid waste containing short half-life RAM will be held by Radiation Safety Office for minimum of 10 half-lives, and then disposed as hazardous non-radioactive waste. Long half-life mixed waste has to be disposed through approved waste broker, and it is expensive! • It is very important to keep reasonably accurate record of all sewage disposals.

EMERGENCIES • Personal safety and health take precedence over radiological concerns. • Notify Radiation Safety in the event of radiological emergency: • accidental uptake; • lost/stolen RAM; • fire (call 911 first), flood, etc. • Konstantin Povod, RSO • 656 3516, • kpovod@clemson. edu • Ayman Seliman, ARSO • ayman@clemson. edu

SUMMARY • • • Follow general lab safety rules. Apply Time Distance Shielding to keep doses ALARA. Control contamination. Perform surveys at meaningful times. Maintain records of RAM use and disposal. If you cannot resolve a safety issue bring it to the attention of your peer/advisor/RSO. Remember: Your safety and safety of your peers is …. . . . primarily your responsibility!


Part II Quiz. Question 1 Dose limits for a) lens of eye; b) TEDE; c) embryo; d) general public are: q a) 15 Sv; b) 5 rem; c) 500 mrem; d) 100 mrem q a) 15 rem; b) 5 rem; c) 500 mrem; d) 100 m. Sv q a) 0. 15 Sv; b) 5000 mrem; c) 0. 5 rem; d) 1 m. Sv q a) 15 rem; b) 5 Sv; c) 500 mrem; d) 100 mrem q a) 1. 5 Sv; b) 5000 mrem; c) 500 mrem; d) 1 m. Sv Right! Wrong! Please proceed review to the and next try question again


Part II Quiz. Question 2 Personal dosimeter: q Provides adequate protection from the ionizing radiation q Required for anybody likely to receive dose >5 m. Sv/year and for persons entering High and Very High Radiation Areas q Should be used for the monitoring of medical exposures q Provides good estimate of the internal dose from alpha emitters q Must be worn by radiation worker 24/7 Right! Wrong! Please proceed review to the and next try question again


Part II Quiz. Question 3 All new RAM projects at Clemson must be approved by: q Radiation Safety Officer (RSO) q Nuclear Regulatory Commission (NRC) q SC Dep’t of Health and Environmental Control (DHEC) q Vice President for Research and RSO q Clemson University Radiation Safety Committee (RSC) Right! Wrong! Please proceed review to the and next try question again


Part II Quiz. Question 4 Recordable contamination surveys: q Required once every month RAM was used q Required every month in all posted RAM use areas q Must be performed after each RAM use q May be performed biweekly instead of personal use surveys q Only performed by the Radiation Safety personnel Right! Wrong! Please proceed review to the and next try question again


Part II Quiz. Question 5 A person can start working with RAM before attending Initial Radiation Safety training: q Under no conditions q If added to an approved project and works only under direct supervision of this project’s RI or AU q If this person has previous experience working with RAM q Under direct supervision of an RI or an AU q If an AU is readily available for a consultation Right! Wrong! Please proceed review to the and next try question again

DOSE LIMITS • • Occupational dose limits set by NRC and adopted by DHEC: • Whole body: 50 m. Sv (5 rem) • Skin, extremities: 500 m. Sv (50 rem) • Lens of Eye: 150 m. Sv (15 rem) • Embryo: 5 m. Sv (0. 5 rem) • General Public: 1 m. Sv (0. 1 rem) In their rulemaking NRC uses Linear Non Threshold (LNT) model of dose effect. This model assumes increase in stochastic (cancer) risk with increase, however small, in dose. Therefore, when working with radioactive material, we must keep doses As Low As Reasonably Achievable (ALARA). REVIEW

ANNUAL LIMITS ON INTAKE (ALI), TEDE • ALI The amount of radioactive material taken into the body of an adult worker that would result in a radiation dose of 50 m. Sv (5 rem) to the whole body or 500 m. Sv (50 rem) to any individual tissue or organ per year • ALI allows to account for effects of the internal exposure in conjunction with the external dose • Total Effective Dose Equivalent (TEDE) takes into account both external and internal exposures. TEDE is the sum of effective dose equivalent from external exposure and committed effective dose equivalent from internal exposure REVIEW

PERSONNEL DOSE MONITORING • • The use of personnel monitors is required for: • Anyone who could receive 10% of annual radiation dose limit; • Anyone who enters a high or very high radiation area Your badge must remain at work. It is intended for measuring occupational dose only. Only wear your issued dosimeter. Store dosimeters away from radiation sources. REVIEW

DOSE REPORT Clemson University uses Mirion’s instadose®+ dosimeters to monitor personnel dose. These dosimeters are Bluetooth equipped, which allows dose readings at any time by any Bluetooth compatible device (laptop, tablet, smartphone) or with a special readers (“hot spots”). REVIEW

TLD RING DOSIMETER • Ring dosimeters are required for persons working with open beam x ray systems. • Radiation Safety Committee may require use of ring dosimeters for persons working with high activities of radioactive materials (eg. , 1 m. Ci of P 32). REVIEW

RADIOACTIVE MATERIAL AUTHORIZATION • • The Nuclear Regulatory Commission (NRC) is the Federal agency authorized by the Congress to regulate radioactive material use States may assume functions of the NRC within their borders. Such states are called Agreement States South Carolina became an Agreement State in 1969 South Carolina’s Department of Health and Environmental Control (DHEC): • licenses radioactive material use; • regulates X ray registration and use; • performs inspections of licensed facilities; • regulates use of lasers. REVIEW

RADIOACTIVE MATERIAL AUTHORIZATION • • Clemson University has a Broad Scope Radioactive Material License issued by DHEC Allows use of radioactive isotopes with atomic numbers 1 98 in any chemical or physical form All activities involving radioactive material (“projects”) within the Clemson University must be approved by the Radiation Safety Committee (RSC) RAM Project includes: • Responsible Investigator (RI); • list of authorized isotopes and possession limits; • list of personnel; • facilities where RAM use is authorized; • approved protocol( s). REVIEW

CONTAMINATION SURVEY • User contamination surveys should be performed each time after handling RAM, before breaks or lunch, at the end of the workday. • Recordable lab contamination surveys must be performed by the lab personnel every month RAM is used in the lab. • Use survey equipment suitable for the detection of RAM used at the location being surveyed. • To survey for removable contamination, approximately a 100 cm 2 area should be “swiped” in an “S” shaped pattern. • Record results of the survey. • Any area with an alpha or beta count rate significantly over background should be immediately cleaned. REVIEW

CONTAMINATION SURVEY RECORD • Recordable contamination survey report must contain: • Drawing of the surveyed area; • Locations of measurements and/or swipes taken; • Model and serial number of instrument(s) used for survey; • Date and time of survey; • Results of measurements; • Identity of the person performed survey REVIEW

WHO MAY WORK WITH RADIOACTIVE MATERIAL? • • • Only individuals listed on a Project may use RAM; Must receive instructions from the RI; May start working with RAM before attending Initial Training, but only under direct supervision of RI or AU (status called Radiation Worker (RW)) To become Authorized User (AU), must complete Initial Radiation Safety training; Must complete annual refresher training (online or classroom) to maintain AU status. REVIEW

WHO MAY WORK WITH RADIOACTIVE MATERIAL? • • • Fill form R 003 Request To Add An Individual To A Radionuclide Project and submit to Radiation Safety. You may be listed on several Projects. Or, you may start your own project. REVIEW

REGISTRATION Good job! You successfully completed required Annual Radiation Safety Refresher Training Please register your achievement by clicking the button below. Enter your name and Clemson email address in the body of the email : SUBMIT (*) "Submit" button uses your default email app. If, after clicking on "Submit" button, unexpected email service (ex. , Mail for Windows) opens, please do the following (instructions are for Windows 10): 1. Go to your computer's Settings (gear icon) 2. Select "Apps" 3. Select "Default Apps" from the menu on the left 4. Set the app you usually use for email (ex. , Outlook, Gmail, Yahoo, etc. )