Neuropsychology Cognitive Neuroscience of ADHD Michelle Benjamin 41509

Neuropsychology & Cognitive Neuroscience of ADHD Michelle Benjamin 4/15/09

Cognitive Neuroscience of ADHD • • Neuropsychological assessment of attention Theories from neuropsychology Neuroanatomy overview Neuroimaging – Structural imaging findings – Functional imaging results • Genetics • Future directions

Neuropsychological Perspective BRAIN—BEHAVIOR relationships • Neuropsychology is the “applied science concerned with the behavioral expression of brain dysfunction” (Lezak, 1995) • Questions to think about in neuropsychology: – What brain regions are implicated in the behavioral manifestations of attention problems and ADHD? – How do we measure the functioning of those brain regions? • Inattention • Impulsivity • Hyperactivity

Attention Assessment

Attention: Basic Definitions • Selective or Focused Attention: vigilance in monitoring information • Divided Attention: ability to respond to more than one task simultaneously • Sustained Attention: ability to maintain attention and respond consistently during a repetitive or continuous activity • Alternating Attention/Mental Shifting: mental flexibility to shift from one task to another as required Baron, 2004

Attention: Measures • Attention batteries: – Test of Everyday Attention (TEA) • adult battery – Test of Everyday Attention for Children (TEA-Ch) • Child extension of adult TEA

Attention: Measures • Single instruments: – Wecshler test examples: WISC-IV Letter-Number Sequencing, Digit Span, Cancellation, WISC-III Arithmetic – Spatial Span tasks: e. g. , Corsi blocks/WMS-III Spatial Span – Other span tests: Knox Cube (visual span), alpha-, pattern- & word-span measures (discussed in Baron, 2004) – Continuous Performance Tests (e. g. , CPT-II) – Auditory Consonant Trigrams

Attention Measures Single Instruments, continued… – CHIPASAT (Children’s Paced Auditory Serial Addition Test) – Trail Making Tests: D-KEFS Trails, TMT, Color Trails – Symbol Digit Modalities Test – Visual Search Cancellation Tests – Underlining Test – Progressive Figure Test & Color Form Test

Attention: Measures • Behavioral report (covered already in class). Examples include: – Connor’s Rating Scales-Revised (CPRS) – Specific subscales from parent/self report (e. g. , BASC Attention scale) – BRIEF (2000) – Brown Attention-Deficit Disorders Scales (2001) – Diagnostic Rating Scale – Attention Deficit Hyperactivity Disorder Rating Scale. IV – Home Version

Test of Everyday Attention for Children (TEA-Ch) • For children ages 6 -16 years. • Extension of Test of Everyday Attention (adult attention battery). • Published in 1999 • 2 forms: useful for re-testing situations • Normed on 293 Australian children & adolescents 6 -16 years.

TEA-Ch Subtests • Sustained Attention: – – – Score! Sky Search DT Score DT Walk, Don’t Walk Code Transmission • Selective Attention: – Sky Search – Map Mission • Attentional Control/Switching: – Creature Counting – Opposite Worlds Note. See handout for individual subtest descriptions

TEA-Ch • Clinical populations reported in manual – ADHD (N=24 boys) • Out of 6 subtests, the ADHD group was worse that age-matched controls for the following: Score!, Score DT, Walk, Don’t Walk, Opposite Worlds – Note: Creature Counting, Code Transmission and Map Mission were not given – TBI (N=18) • Out of 8 subtests, the TBI group was worse that agematched controls for ALL but the following: Creature Counting (timing) and Score! – Note: Walk, Don’t’ Walk was not given

Continuous Performance Tests • Many different versions: (Riccio et al 2001 has review & comparison) • Handout explaining CPTs E. g. , CPT-II • Visual vigilance measure. • Individuals 4 years to adulthood. • Press for all letters EXCEPT ‘X’ • Non-X and X stimuli are presented in blocks varying from 1 -, 2 - and 4 -second ISIs across blocks. • Task lasts for approximately 14 minutes.

CPT-II • Normative data: – N=1483 children 6 -17 years. The smallest sample is 67 years (N=88). – Total normative sample: N=1920; through adulthood. • Oldest normative group is 55+, also small sample in this subgroup (N=54). – Total ADHD clinical sample: N=378. • N=271 for ADHD children/adolescents 6 -17 years.
Continuous Performance Tests • CPT indices (see handout): – Omissions: suggestive of inattentiveness. Measures nonresponding – Comissions: may represent an inability to withhold motor responses (suggests impulsivity) – Overall hit reaction time: average speed of correct responses for entire test. Slowed RT and nonresponding suggestive of inattention to task – Overall standard error: attentional variability overall (e. g. , high levels suggest inconsistency of speed of responses (fluctuating attention from trial to trial)
Continuous Performance Tests • Other CPT indices: – Perceptual sensitivity (d’): whether difficulty in discriminating perceptual features of targets vs. nontargets – Response bias (B): individual’s response tendency. e. g. , cautious vs. risk-taking Other CPT output (e. g. , CPT-II) includes hit RT block rate change, hit SE block change, hit RT ISI, hit SE ISI change.

Other Attention Single Measures • Trail Making Tests: • Extension from adult measures (e. g. , Army Individual Test Battery (1944), Halstead-Reitan battery) • Trail A: number sequencing (1 -2 -3 -4…) • Trail B: Number-letter sequencing (1 -A-2 -B. . ) • Children 9 -14 use versions with 15 items for each of the above (some norms available from research extend lower) • Ages 15 -adult use versions A & B with 25 items each

Other Attention Single Measures • DKEFS Trail Making Test: – normed for 8 to 89 years – Better for teasing apart problems with TMT: e. g. , if problem is basic motor &/or visual problem vs. cognitive shifting – 5 separate conditions: • • • 1) Visual scanning 2) Number sequencing (similar to typ. Trails A) 3) Letter sequencing 4 Number-letter switching (similar to typ. Trails B) 5) Motor speed – Maximum times: 150 sec for Conditions 1, 2, 3, 5; 240 sec for Condition 4

Other Attention Single Measures • Color Trails Test: – Instead of letters, colors are substituted to minimize knowledge of English alphabet. – Alternate between colors and 25 numbers.

Other Attention Single Measures • Auditory Consonant Trigrams Test: – Test of divided attention and rapid information processing – Normative data for ages 9 -15 are reported in Baron, 2004 – Interval length for children: 0, 3, 9, and 18 seconds – Person is given 3 letters to remember in any order. – S/he is then told to count backwards from a certain number until told to stop. (Children count backward by 1 s; adults by 3 s. )

Other Attention Single Measures • Children’s PASAT (CHIPASAT): – Test of divided attention, sustained auditory attention and information processing speed – Requires math calculation skills – Normative data for ages 8 -15 are reported in Baron, 2004. (Note: very small N for 14 -15 y. o. ) – Presentation is 1 digit every 2. 8 (suggested for practice), 2. 4, 2. 0, 1. 6 or 1. 2 seconds. 61 digits per trial on tape. – Person must add each new number presented to the one heard immediately prior and say the sum aloud, continuing to do this with each new number. – Baron cautions against interpreting scores below 9. 5 years with this measure.

ADHD: Neuropsych Assessment • NP assessment also traditionally includes measures of executive functions, such as response inhibition, task organization, planning abilities, reasoning, and working memory. Example measures include: – – – Stroop Inteference Verbal fluency Tower tasks WCST Go/No. Go tasks

ADHD: Neuropsychological theories • Executive Function Theory of ADHD – ADHD symptoms arise from a primary deficit in executive functions (neurocognitive processes for maintaining an appropriate problem-solving set to attain a later goal). – Executive functions involves the prefrontal cortex, basal ganglia & thalamus – 4 factors of executive function tasks 1) Response inhibition and execution 2) Working memory and updating 3) Set-shifting and task-switching 4) Interference control

ADHD: Neuropsychological findings • Meta-analysis on executive function Willcutt et al 2005 – – 83 studies (N=3734 ADHD; N=2969 no ADHD) Significant group differences in 109: 168 (65%) comparisons Mean weighted effect size=. 54 (range. 43 -. 69; medium effect) ADHD vs. control differences most consistently seen in • stop-signal reaction time (SSRT) (82% of 27 studies) • CPT omission errors (77% of 30 studies) – Less studies in working memory but promising • 75% of spatial WM w/sign group differences • 55% of verbal WM w/sign group differences – WCST more weakly related to ADHD than other EF measures
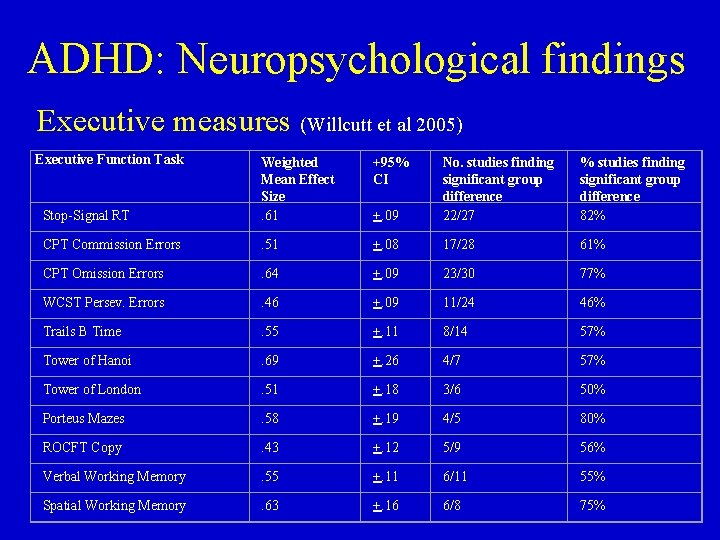
ADHD: Neuropsychological findings Executive measures (Willcutt et al 2005) Executive Function Task +95% CI Stop-Signal RT Weighted Mean Effect Size. 61 +. 09 No. studies finding significant group difference 22/27 % studies finding significant group difference 82% CPT Commission Errors . 51 +. 08 17/28 61% CPT Omission Errors . 64 +. 09 23/30 77% WCST Persev. Errors . 46 +. 09 11/24 46% Trails B Time . 55 +. 11 8/14 57% Tower of Hanoi . 69 +. 26 4/7 57% Tower of London . 51 +. 18 3/6 50% Porteus Mazes . 58 +. 19 4/5 80% ROCFT Copy . 43 +. 12 5/9 56% Verbal Working Memory . 55 +. 11 6/11 55% Spatial Working Memory . 63 +. 16 6/8 75%

ADHD: Neuropsychological findings Nigg et al 2005 Nigg 2005

ADHD: Neuropsychological theories • Problem with executive function theory – Sensitivity and specificity of any single executive deficit is not high enough to support EF as cause of all ADHD cases. • While 80% ADHD have deficit on at least 1 EF measure, so do 50% of controls. • Only 50% of ADHD children have deficit on most sensitive measure (SSRT) compared to 10% of controls. – Argue that an executive dysfunction subtype may be distinguishable from other subtypes. Nigg et al (2005)
ADHD: Neuropsychological theories • Motivational dysfunction model Sonuga-Barke (2005) – Disruption in signaling of delayed reward – Delayed aversion model of ADHD supported by human and animal data – Sonuga-Barke (2005) • Executive dysfunction model: frontostriatal circuit prefrontal—dorsal striatum • Delay aversion model: orbitofrontal—ventral striatum • Both circuits modulated by dopamine • Dual or multiple deficits (versus single-deficit model)
ADHD: Neuropsychological theories • Cognitive-Energetic Model Sergeant 1999, 2005 – Overall efficiency of information processing is determined by interplay between computation mechanisms of attention, state factors & management/executive function. – Encompasses both bottom-up and top-down process and approaches in ADHD at 3 levels. – Attention to fact that ADHD causes defects at 3 levels: • Cognitive mechanisms (e. g. , response output) • Energetic mechanisms (e. g. , activation; effort) • Management systems
ADHD: Neuropsychological theories • Cognitive-Energetic model levels • 1) 4 stages of the computational mechanisms of attention • 2) 3 distinct energetic pools • 3) Overriding management or executive system
ADHD: Neuropsychological theories • Cognitive-Energetic model levels • 1) 4 stages of the computational mechanisms of attention – Encoding – Search – Decision – Motor organization – Stages all associated with experimental task variables
ADHD: Neuropsychological theories • Cognitive-Energetic model levels • 2) 3 distinct energetic pools: – Effort, arousal & activation – A) EFFORT: • Defined as energy necessary to meet task demands. • Affected by cognitive load. • Required when current organism state does not match task demand. • Encompasses motivation & response to contingencies. • Associated with hippocampus. • Functions to excite & inhibit arousal & activation.
ADHD: Neuropsychological theories • Cognitive-Energetic model levels • 2) 3 distinct energetic pools: – B) AROUSAL: – Defined as phasic responding to that is time locked to stimulus processing. – Typically influenced by signal intensity and novelty. – Associated with mesencephalic reticular formation & amygdala.
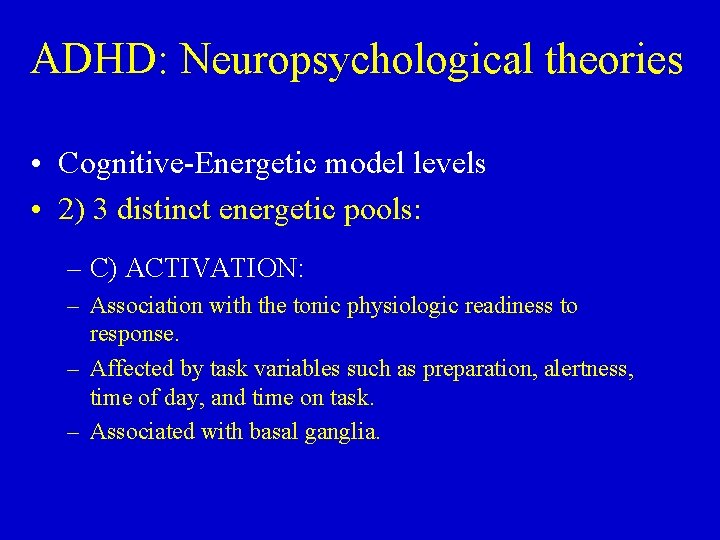
ADHD: Neuropsychological theories • Cognitive-Energetic model levels • 2) 3 distinct energetic pools: – C) ACTIVATION: – Association with the tonic physiologic readiness to response. – Affected by task variables such as preparation, alertness, time of day, and time on task. – Associated with basal ganglia.

ADHD: Neuropsychological theories • Cognitive-Energetic model levels • 3) Overriding management or executive system – Associated with planning, monitoring, error detection & correction – Associated with prefrontal cortex
ADHD: Neuropsychological theories
ADHD: Neuropsychological theories • Cognitive-Energetic model levels • Supported by the following: – ADHD individuals are slower and more variable in RTs than controls – ADHD performance varies more than controls as a function of event rate (e. g. , faster rate normalizes performance of ADHD groups)

ADHD: Neuroanatomy review LATERAL VIEW MEDIAL VIEW Nadeau et al 2004
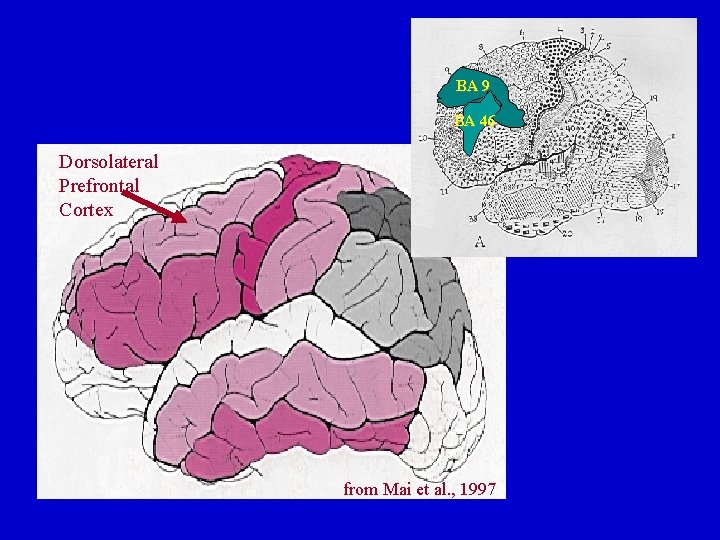
BA BA 99 BA 46 Dorsolateral Prefrontal Cortex from Mai et al. , 1997
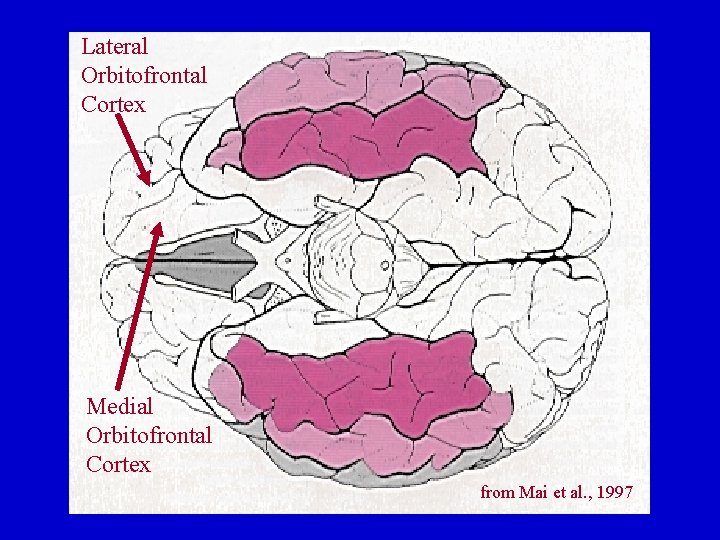
Lateral Orbitofrontal Cortex Medial Orbitofrontal Cortex from Mai et al. , 1997
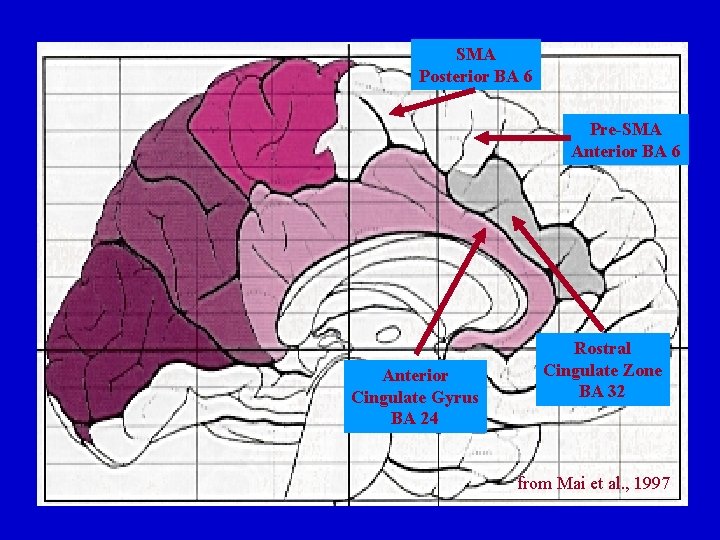
SMA Posterior BA 6 Pre-SMA Anterior BA 6 Anterior Cingulate Gyrus BA 24 Rostral Cingulate Zone BA 32 from Mai et al. , 1997
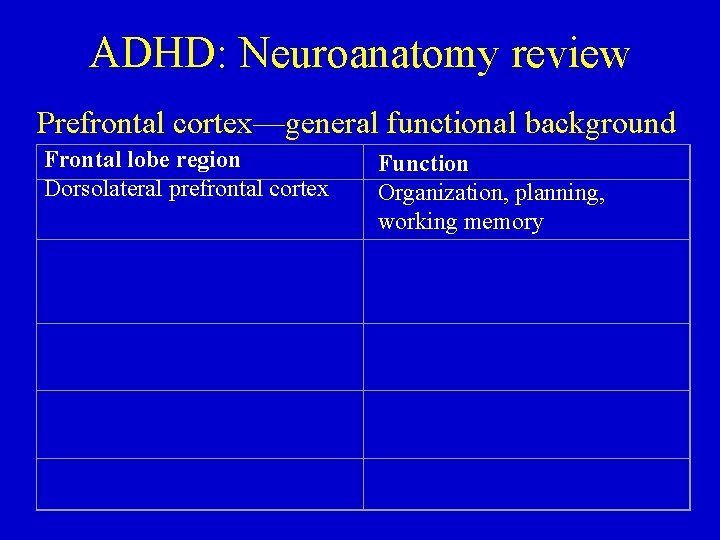
ADHD: Neuroanatomy review Prefrontal cortex—general functional background Frontal lobe region Dorsolateral prefrontal cortex Function Organization, planning, working memory
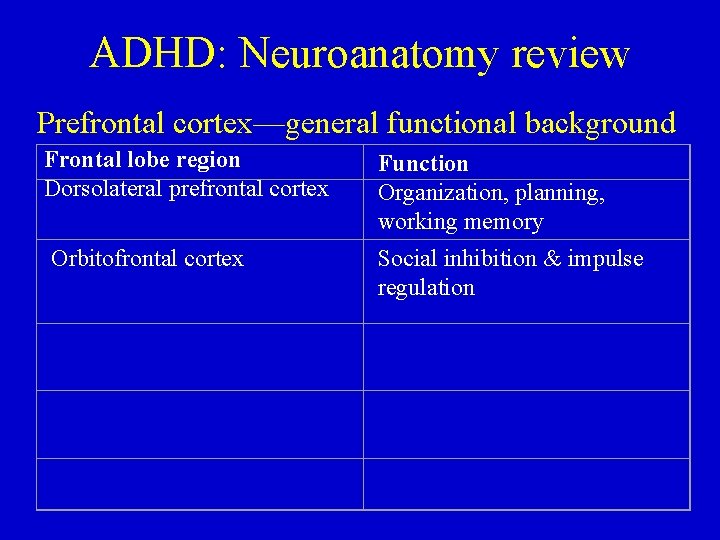
ADHD: Neuroanatomy review Prefrontal cortex—general functional background Frontal lobe region Dorsolateral prefrontal cortex Function Organization, planning, working memory Orbitofrontal cortex Social inhibition & impulse regulation
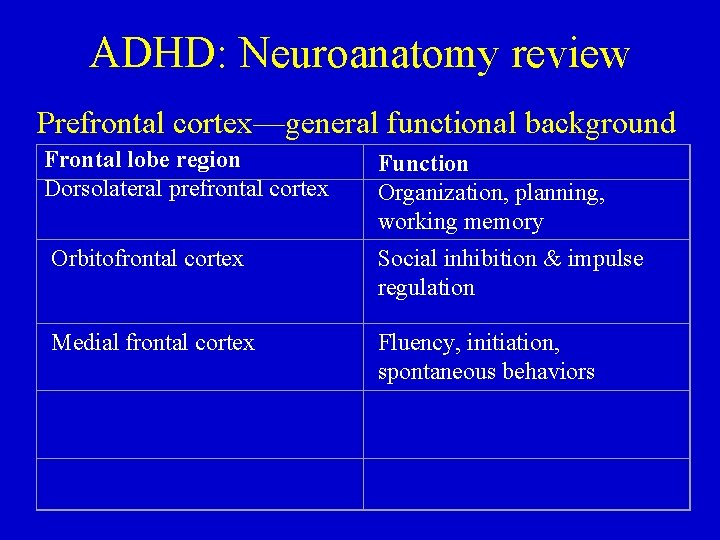
ADHD: Neuroanatomy review Prefrontal cortex—general functional background Frontal lobe region Dorsolateral prefrontal cortex Function Organization, planning, working memory Orbitofrontal cortex Social inhibition & impulse regulation Medial frontal cortex Fluency, initiation, spontaneous behaviors
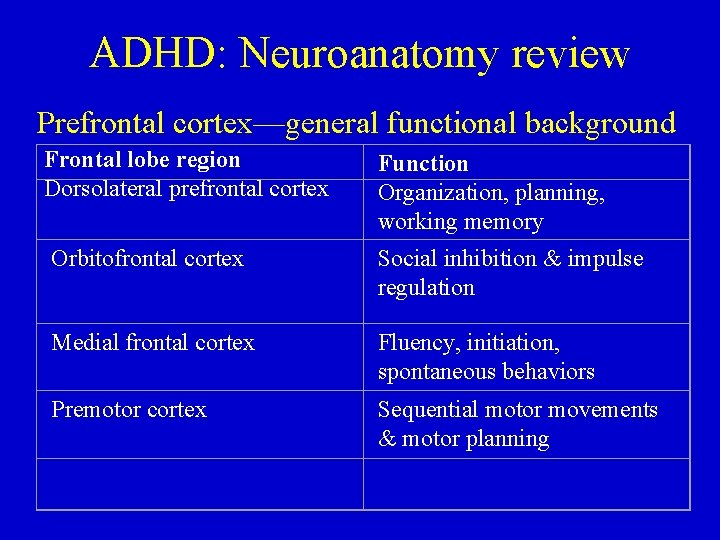
ADHD: Neuroanatomy review Prefrontal cortex—general functional background Frontal lobe region Dorsolateral prefrontal cortex Function Organization, planning, working memory Orbitofrontal cortex Social inhibition & impulse regulation Medial frontal cortex Fluency, initiation, spontaneous behaviors Premotor cortex Sequential motor movements & motor planning
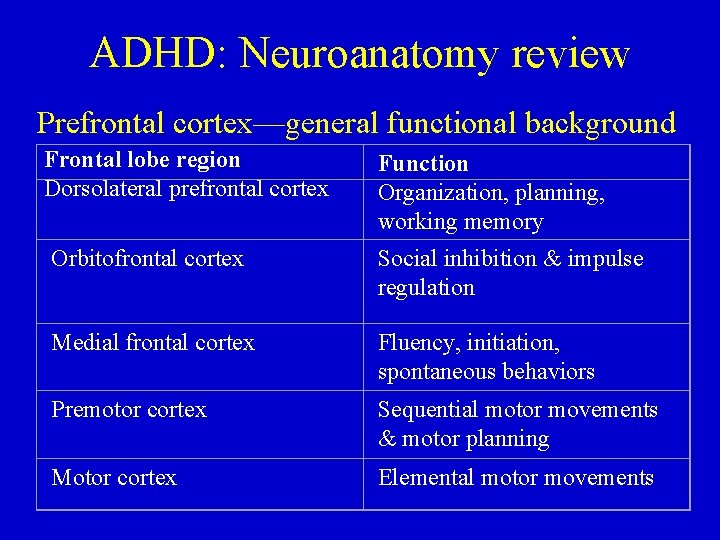
ADHD: Neuroanatomy review Prefrontal cortex—general functional background Frontal lobe region Dorsolateral prefrontal cortex Function Organization, planning, working memory Orbitofrontal cortex Social inhibition & impulse regulation Medial frontal cortex Fluency, initiation, spontaneous behaviors Premotor cortex Sequential motor movements & motor planning Motor cortex Elemental motor movements

ADHD: Neuroanatomy review Basal Ganglia Striatum Ventral Striatum (Archistriatum) Nucleus Accumbens Olfactory Tubercle Neostriatum Caudate Nucleus Putamen In most cognitive neuroscience articles, the striatum refers to the caudate and putamen.

ADHD: Neuroanatomy review Basal Ganglia (cont): Globus Pallidus Medial Globus Pallidus Lateral Globus Pallidus Ventral Pallidum (Archipallidum) Subthalamic Nucleus In cognitive neuroscience articles, the lentiform nucleus refers to the putamen and globus pallidus together.
ADHD: Neuroanatomy review SUBCORTICAL STRUCTURES Coronal view Axial view Nadeau et al 2004

Amygdala from Crosson, 1992
ADHD: Neuroanatomy review SUBCORTICAL STRUCTURES NEUROREGULATORY FUNCTIONS (Enhancement & Suppression)

BASAL GANGLIA REGULATORY BEHAVIOR + + Cortex + - + Neostriatum - +/- + - SNpc LGP MGP - Ventral Ant. Thalamus enhance Direct Ventral Ant. Thalamus suppress Indirect suppress Hyperdirect + All 3 Modulate
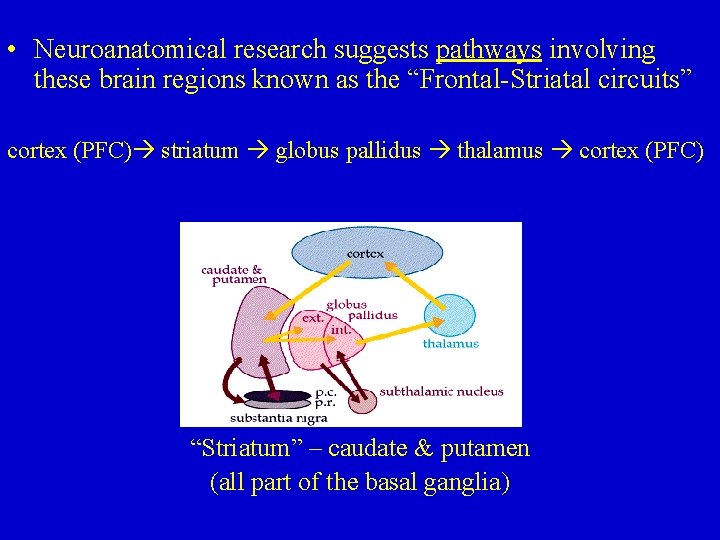
• Neuroanatomical research suggests pathways involving these brain regions known as the “Frontal-Striatal circuits” cortex (PFC) striatum globus pallidus thalamus cortex (PFC) “Striatum” – caudate & putamen (all part of the basal ganglia)
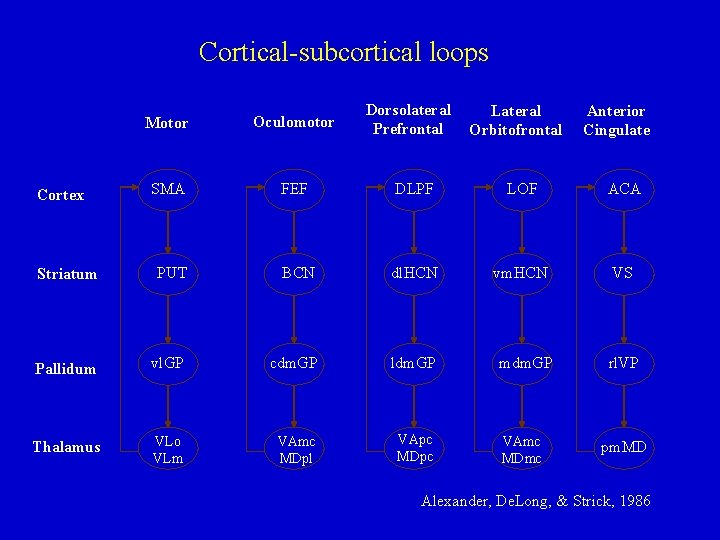
Cortical-subcortical loops Dorsolateral Prefrontal Lateral Orbitofrontal Anterior Cingulate Motor Oculomotor SMA FEF DLPF LOF ACA Striatum PUT BCN dl. HCN vm. HCN VS Pallidum vl. GP cdm. GP ldm. GP mdm. GP rl. VP Thalamus VLo VLm VAmc MDpl VApc MDpc VAmc MDmc pm. MD Cortex Alexander, De. Long, & Strick, 1986
BA BA 99 BA 46 Dorsolateral Prefrontal Cortex from Mai et al. , 1997

Dorsolateral Prefrontal Cortex: Basal Ganglia Connections Dorsolateral Prefrontal (BA 9) Loop Dorsolateral Prefrontal (BA 46) Loop Dorsolateral PFC Dorsolateral Caudate Head rd Globus Pallidus dm Globus Pallidus VA Thalamus VA & DM Thalamus

Lateral Orbitofrontal Cortex Medial Orbitofrontal Cortex from Mai et al. , 1997
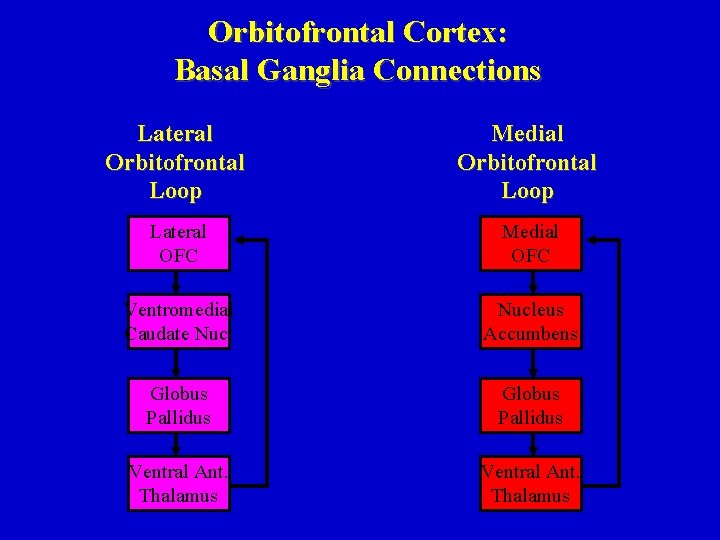
Orbitofrontal Cortex: Basal Ganglia Connections Lateral Orbitofrontal Loop Medial Orbitofrontal Loop Lateral OFC Medial OFC Ventromedial Caudate Nucleus Accumbens Globus Pallidus Ventral Ant. Thalamus

SMA Posterior BA 6 Pre-SMA Anterior BA 6 Anterior Cingulate Gyrus BA 24 Rostral Cingulate Zone BA 32 from Mai et al. , 1997

Medial Frontal Cortex: Basal Ganglia Connections SMA Loop Pre-SMA Loop Anterior Cingulate Loop Rostral Cingulate Loops SMA Pre-SMA Anterior Cingualte Gyrus Rostral Cingulate Zone Putamen Striatal Bridges Nucleus Accumbens ? Globus Pallidus Ventral Pallidum ? Ventral Lat. Thalamus Ventral Ant. Thalamus? Dorsomedial Thalamus ?

ADHD: Neuroanatomy & NTs Curtolo et al 2008 Nigg 2005

ADHD: Neuroimaging
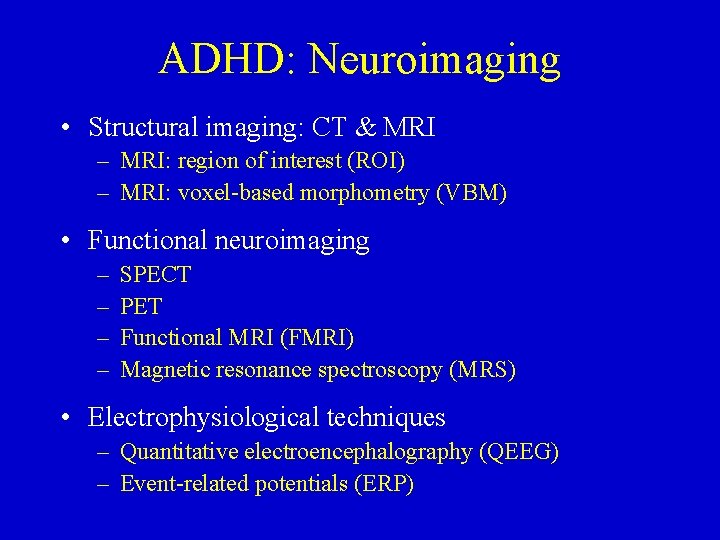
ADHD: Neuroimaging • Structural imaging: CT & MRI – MRI: region of interest (ROI) – MRI: voxel-based morphometry (VBM) • Functional neuroimaging – – SPECT PET Functional MRI (FMRI) Magnetic resonance spectroscopy (MRS) • Electrophysiological techniques – Quantitative electroencephalography (QEEG) – Event-related potentials (ERP)

ADHD: Structural neuroimaging • CT & structural MRI – Both methods provide information on the macroscopically visible brain • Neuroanatomy of the skull, brain tissue and blood vessels – Structural imaging does not assess function, e. g. , cerebral metabolic rate and cerebral blood flow
ADHD: Structural neuroimaging Computed tomography (CT) or computed axial tomography (CAT) – axially acquired series of x-rays of the head to determine brain structure – Preferred radiological test in ER: quick, accurate, widely available – Used primarily to assess swelling, fractures, blood products and ventricle size – Poorer spatial resolution than MRI
ADHD: Structural neuroimaging – Structural magnetic resonance imaging (MRI): • Uses magnetic fields and radio waves to image brain structures without the use of ionizing radiation (e. g, x-rays, CT, SPECT, PET) • Superior resolution and better anatomic fidelity than CT • Different planes & types of MRI sequences can be done to detect abnormalities
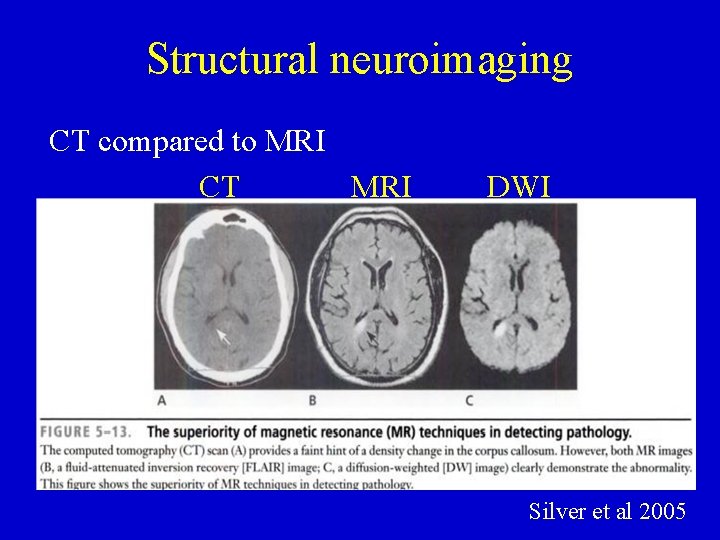
Structural neuroimaging CT compared to MRI CT MRI DWI Silver et al 2005
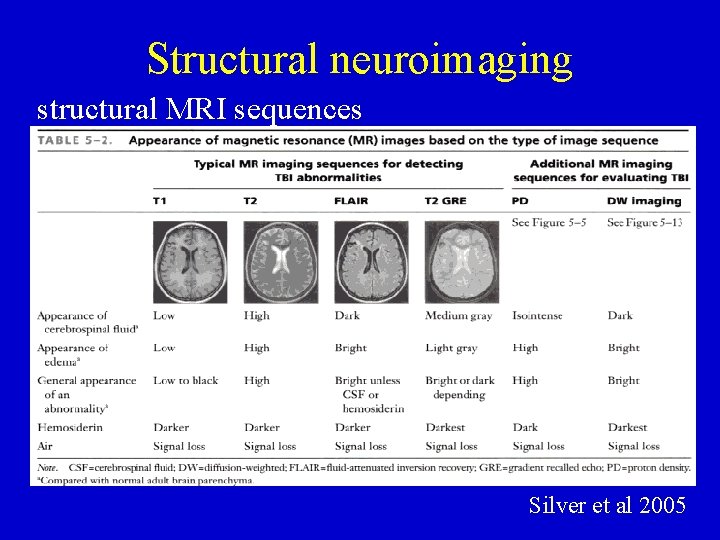
Structural neuroimaging structural MRI sequences Silver et al 2005
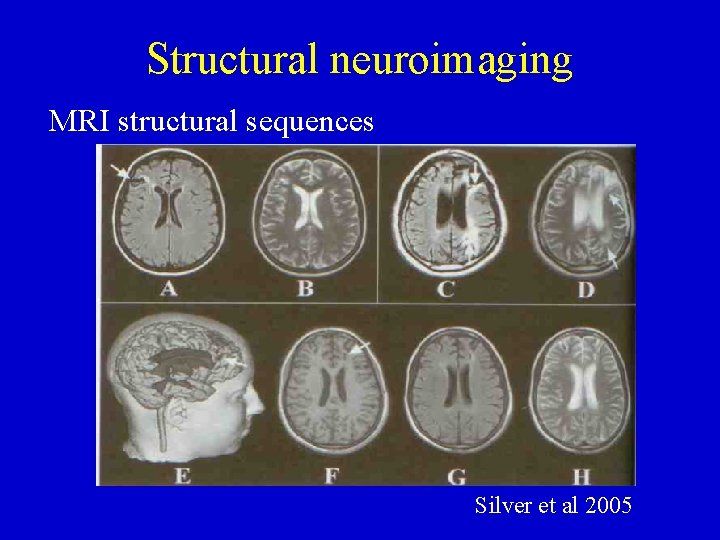
Structural neuroimaging MRI structural sequences Silver et al 2005
ADHD: Structural neuroimaging STRUCTURAL MRI METHODS 1) MRI region of interest (ROI) – Method of measures brain volumes for particular areas of study and comparing size between subject groups. – Manual (or now semi- or fully automated) tracings of particular cortical and subcortical areas in the brain. – Uses structural brain landmarks to define ROIs. – Most studies in ADHD (or patient populations) have used this technique.

ADHD: Structural neuroimaging STRUCTURAL MRI METHODS 2) Voxel-based morphometry (VBM) – Method for simultaneously comparing, voxelby-voxel, where the major differences occur in subject patient populations and healthy controls. – Differences between subject groups (regions of reduced voxel density of either gray or white matter) are plotted on a standard 3 D surface place of the brain.

ADHD: Structural neuroimaging • Structural imaging findings in ADHD – Total cerebral volume – Cortical areas – White matter (corpus callosum) – Subcortical regions – Cerebellum
ADHD: Structural neuroimaging • Structural imaging findings in ADHD – Total cerebral volume – Cortical areas – White matter (corpus callosum) – Subcortical regions – Cerebellum
ADHD: Structural neuroimaging • Total & lateralized cerebral volume reductions – 7 of 12 studies with children thru age 19 • Total cerebrum, particularly the right hemisphere is 35% smaller. • 1 study reported reduced intracranial volume. – Smaller total gray and white matter also reported. – NO studies report significantly larger volumes. Seidman et al 2005 review
ADHD: Structural neuroimaging • Structural imaging findings in ADHD – Total cerebral volume – Cortical areas – White matter (corpus callosum) – Subcortical regions – Cerebellum

ADHD: Structural neuroimaging • Cortical regions – Frontal hypothesis of ADHD focus on dorsolateral (DLPFC) and orbitofrontal (OFC) areas. • Ability to maintain context relies on dopaminergic tone in DLPFC. • Behavioral inhibition may be associated with OFC function.
ADHD: Structural neuroimaging • ADHD prefrontal cortex findings: – All studies measuring at least 1 part of PFC reported smaller volumes in ADHD. • 9 ROI studies in child ADHD reported smaller DLPFC volumes in either right or left hemisphere. • VBM study showed right superior frontal gyrus reduction. • Reduced brain surface extent in inferior DLPFC bilaterally, using automated, computational image analysis. Seidman et al 2005 review

ADHD: Structural neuroimaging • Dorsal anterior cingulate cortex (d. ACC): – On medial surface of frontal lobe – Strong connections to DLPFC – Considered to play critical role in complex cognitive processing: • • target detection response selection error detection reward-based decision making
ADHD: Structural neuroimaging • Dorsal anterior cingulate cortex (d. ACC) – Functional imaging in normals: d. ACC active in number of cognitive tasks, particularly Stroop and similar cognitive interference tasks. – No structural ROI studies of d. ACC in ADHD. – VBM study in ADHD children: significant cingulate abnormalities. • Reduction in right posterior cingulate volume. Seidman et al 2005 review
ADHD: Structural neuroimaging • Structural imaging findings in ADHD – Total cerebral volume – Cortical areas – White matter (corpus callosum) – Subcortical regions – Cerebellum

ADHD: Structural neuroimaging • Corpus callosum – Composed of mostly myelinated axons – Connect homotypic regions of the 2 cerebral hemispheres – Essential for communication between the 2 hemispheres – Size variations in the corpus callosum reflects differences in number or size of axons that connects regions (relates to extent of myelination)

ADHD: Structural neuroimaging • Corpus callosum in ADHD – Important role in attentional control in neurologically intact individuals – Critical role in distributing processing load across hemispheres under high attention demands, so that demands can be met. Banich 2003 review – Research in split brain patients suggests CC important for sustaining attention & dividing attention between tasks

ADHD: Structural neuroimaging • Corpus callosum: – Abnormalities in ADHD consistently found in posterior regions linked to temporal and parietal cortices • Splenium Seidman et al 2005 review Valera et al 2006 meta-analysis – By gender (meta-analysis of N=595): • Splenium findings likely driven by splenium in ADHD girls. • ADHD boys exhibit smaller rostral body. Hutchinson et al 2008 meta-analysis
ADHD: Structural neuroimaging • Structural imaging findings in ADHD – Total cerebral volume – Cortical areas – White matter (corpus callosum) – Subcortical regions – Cerebellum

ADHD: Structural neuroimaging • Basal ganglia: caudate, putamen, globus pallidus – Part of discrete, somatotopically distributed corticalsubcortical circuits essential for executive functions – Vulnerable to hypoxic complications (which occurs at higher rates in ADHD) – Animal models of striatal lesions suggest hyperactivity and poor performance on working memory and response inhibition tasks – Striatum is one of the richest sources of dopaminergic synapses – Stimulant medications have effects on striatum
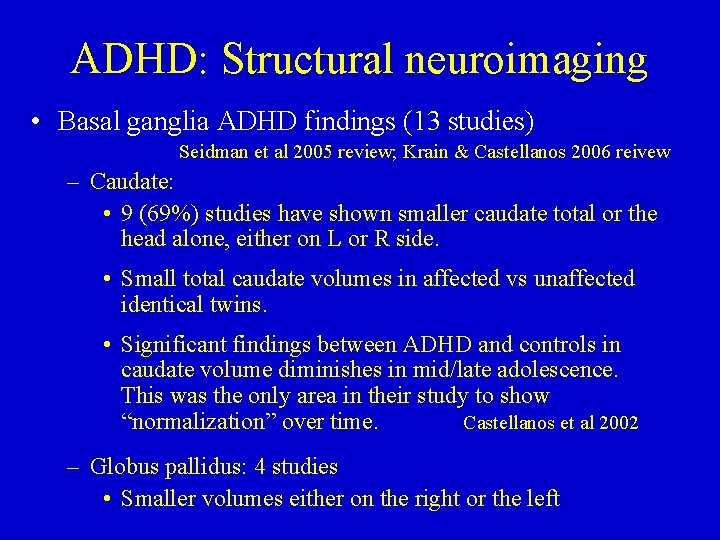
ADHD: Structural neuroimaging • Basal ganglia ADHD findings (13 studies) Seidman et al 2005 review; Krain & Castellanos 2006 reivew – Caudate: • 9 (69%) studies have shown smaller caudate total or the head alone, either on L or R side. • Small total caudate volumes in affected vs unaffected identical twins. • Significant findings between ADHD and controls in caudate volume diminishes in mid/late adolescence. This was the only area in their study to show “normalization” over time. Castellanos et al 2002 – Globus pallidus: 4 studies • Smaller volumes either on the right or the left
ADHD: Structural neuroimaging • Basal ganglia ADHD findings (cont) – Putamen: no structural findings in ADHD versus controls – TBI & secondary ADHD • 2 severe ADHD cases with traumatic amniocentisis at 17 wks gestation with completed elimination of basal ganglia. • Lesions of right putamen & posterior ventral putamen associated with higher incidence of S-ADHD & ADHD respectively. • Odds of developing S-ADHD 3. 6 times higher for TBI children with thalamic damage & 3. 2 higher for children with basal ganglia damage. Krain & Castellanos 2006 review
ADHD: Structural neuroimaging • Structural imaging findings in ADHD – Total cerebral volume – Cortical areas – White matter (corpus callosum) – Subcortical regions – Cerebellum
ADHD: Structural neuroimaging • Cerebellum – Associated with coordination of motor movements – Also involved in non-motor functions such as timing and attentional shifting – Posterior-inferior lobules of cerebellar vermis appear to differ from remaining cerebellar hemispheres and vermis in selectively containing dopamine-transporterlike immunoreactive axons.
ADHD: Structural neuroimaging • Cerebellum ADHD findings Seidman et al 2005 & Krain & Castellanos 2006 reviews – Smaller cerebellar hemispheres (up to 6%) in ADHD, sustained through adolescence. – Vermal volume smaller in ADHD. – Decreased size of posterior inferior lobe of cerebellum (specific to lobules VIII-X) in ADHD. – Cerebellar volumes significantly negatively correlated with rating of attention problems. (Castellanos et al 2002) – Small right cerebellar volumes. (Durston et al 2004)
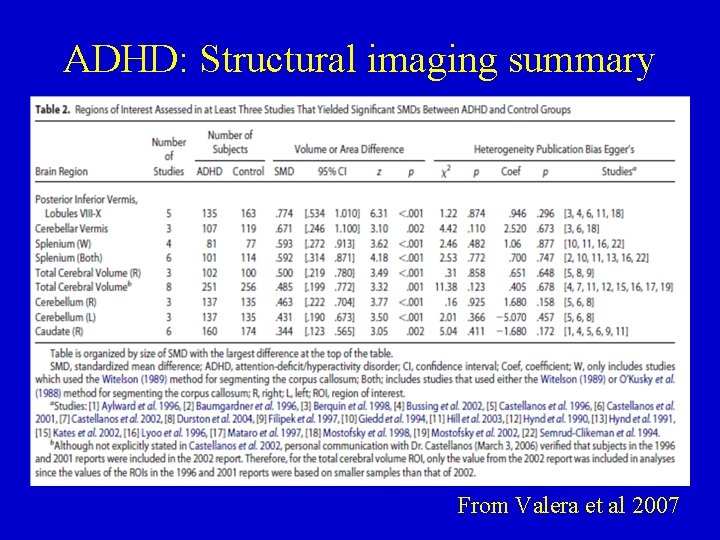
ADHD: Structural imaging summary From Valera et al 2007

ADHD: Structural neuroimaging New applications Segmentation methods: Cerebellum example From Seidman et al 2005 review
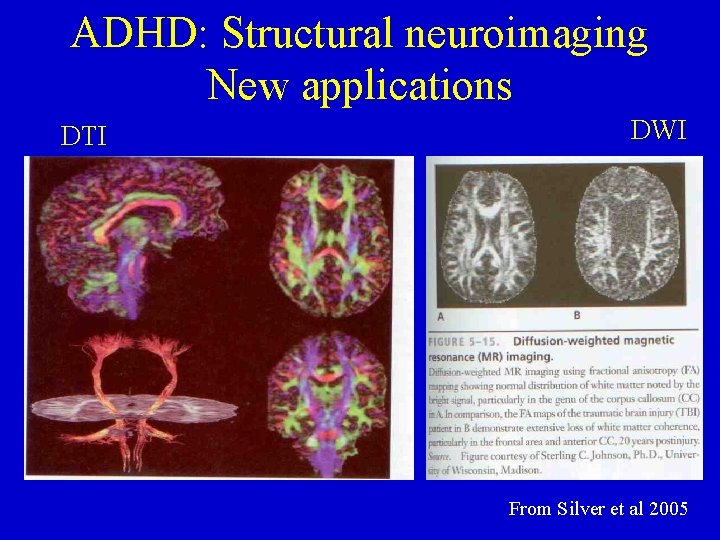
ADHD: Structural neuroimaging New applications DTI DWI From Silver et al 2005
ADHD: Structural neuroimaging Newer applications in structural imaging: 1) Diffusion-weighted MR – Capitalizes on the molecular motion of water – White matter pathologically altered in certain injury conditions (e. g. , TBI) which have attentional impairments
ADHD: Structural neuroimaging 2) Diffusion tensor imaging (DTI) – Another technique for evaluating integrity of white matter (WM) in the brain – Allows for more refined image analysis of subtle brain differences associated in certain conditions – Tracks aggregate groups of axons & their projections within the brain

ADHD: Structural neuroimaging DTI (cont. ): – Capitalizes on 2 biological principles of brain organization • WM projections in brain follow orderly projection routes (anterior-posterior, lateral, and inferior-superior projects) • WM integrity can be assessed by applying the principle of anisotropy: water molecule diffusion rates are dependent on direction of WM pathway, which can be determined by physics & mathematics of vectors (or tensors) – FA (fractional anisotropy) maps & tractography • FA maps: Created in which brighter voxels indicate greater anisotrophy (greater directionality, integrity, or coherence)

ADHD: Functional Neuroimaging
ADHD: Functional neuroimaging • Functional neuroimaging – SPECT: single photon emission computed tomography – PET: Positron emission tomography – Functional magnetic resonance imaging (FMRI) – Magnetic resonance spectroscopy (MRS)

ADHD: Functional neuroimaging SPECT: single photon emission computed tomography – 1 st emerged in 1950 s as a technique. – Requires the injection or inhalation of radiopharmacueticals – These radioactive compounds distribute throughout the body, including the brain, and emit single photon radiation (typically gamma rays) as they decay. – More highly active brain areas receive greater blood flow (and therefore more of the tracer), which is then quantified with SPECT imaging. – Poor spatial and temporal resolution compared to FMRI.

ADHD: Functional neuroimaging • SPECT – In ADHD, numerous methodological concerns with most studies done • Qualitative analysis, minimal or no control groups, comorbid conditions, poor subject matching, very crude techniques (e. g. , 17 mm slices) – Ethical concerns: radioactive tracers (particularly for use in children & healthy subjects)

ADHD: Functional neuroimaging Current SPECT capabilities Silver et al 2005

ADHD: Functional neuroimaging SPECT vs structural imaging comparison Silver et al 2005

ADHD: Functional neuroimaging • SPECT ADHD findings: – Decreased striatal perfusion Lou et al 1998 – Decreased right DLPFC, middle temporal, and cerebellar cortex activity at rest in medication naïve subjects children & adolescent. Increased rest activity in angular/postcentral and occipital gyri. Kim et al 2002 – Higher activity in d. ACC, motor and premotor cortices while off methylphenidate. Langleben et al 2002 – Methylphenidate increased regional cerebral blood flow in DLPFC, caudate, and thalamus bilaterally in previous treatment naïve ADHD children & adolescents. Kim et al 2001

ADHD: Functional neuroimaging • PET: positron emission tomography – Introduced in the 1970 s – Also requires the injection or inhalation of radiopharmacueticals – As the radioactive isotope decay (typically oxygen-15, carbon-11, flourine-18) they emit positrons which are detected by the PET camera. – Some PET methods are flow dependent but others measure cerebral metabolism rates.

ADHD: Functional neuroimaging Silver et al 2005
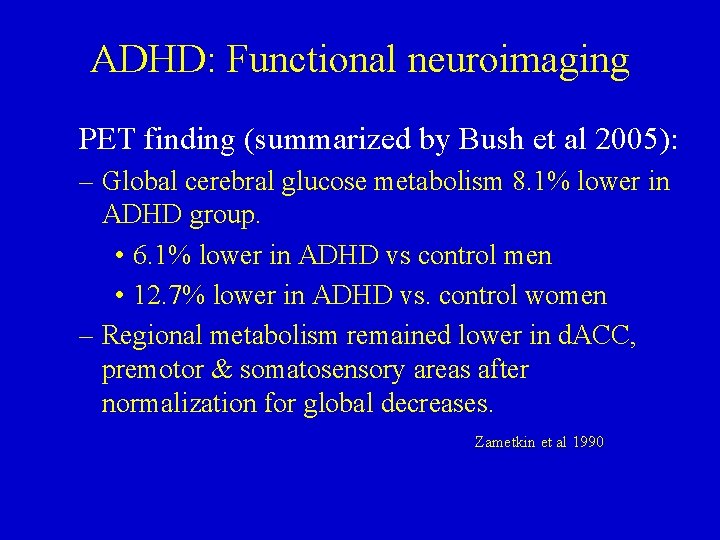
ADHD: Functional neuroimaging PET finding (summarized by Bush et al 2005): – Global cerebral glucose metabolism 8. 1% lower in ADHD group. • 6. 1% lower in ADHD vs control men • 12. 7% lower in ADHD vs. control women – Regional metabolism remained lower in d. ACC, premotor & somatosensory areas after normalization for global decreases. Zametkin et al 1990

ADHD: Functional neuroimaging PET findings (cont. ): • Series of drug studies of acute stimulant use in ADHD: – No consistent acute or chronic stimulant effects on the brain – Caveat: more recent data suggests full stimulant effects may take up to 4 wks to manifest • FDG-PET: with CPT tasks, may not have enough power to detect drug-related differences detected with SPECT & FMRI. • PET: working memory & gambling tasks – Consistent for frontal-striatal conclusions in ADHD.
ADHD: Functional neuroimaging – While both SPECT and PET are still useful in certain areas (e. g. , measuring dopamine transporter level), FMRI has become choice for functional studies. – Functional MRI (FMRI): uses magnetic fields and radio waves to image brain structures • Noninvasive; no exposure to ionizing radiation. – Repeated scanning • Better spatial and temporal resolution • Tasks can be either block or event-related, allowing more flexibility in task design.
ADHD: Functional neuroimaging • FMRI: d. ACC findings – d. ACC: important role in attention, cognition (e. g. , error detection), motor control & reward-based decision making. – Hypofunctional in ADHD adults: Counting Stroop task. Bush 1999 – Medial prefrontal hypoactivity in d. ACC during stop-signal & motor timing tasks. Rubia et al 1999 – No activity or hypoactivity in d. ACC of ADHD versus control child/adolescent subjects on Go-No. Go tasks. Durston et al 2003; Tamm et al 2004
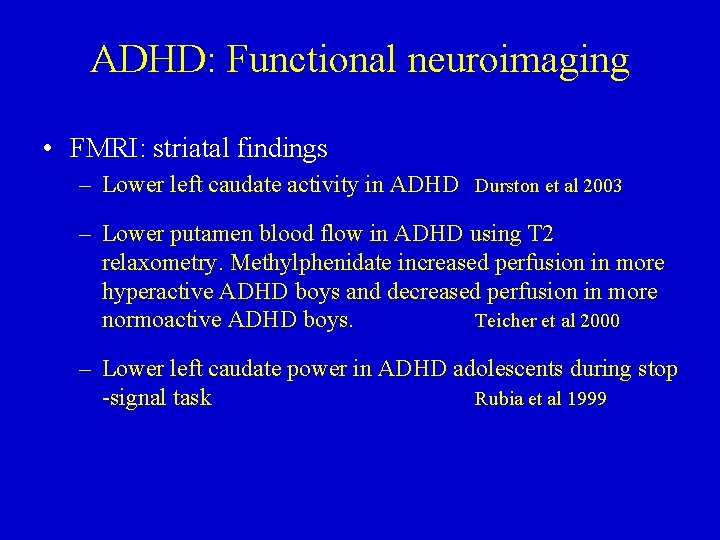
ADHD: Functional neuroimaging • FMRI: striatal findings – Lower left caudate activity in ADHD Durston et al 2003 – Lower putamen blood flow in ADHD using T 2 relaxometry. Methylphenidate increased perfusion in more hyperactive ADHD boys and decreased perfusion in more normoactive ADHD boys. Teicher et al 2000 – Lower left caudate power in ADHD adolescents during stop -signal task Rubia et al 1999
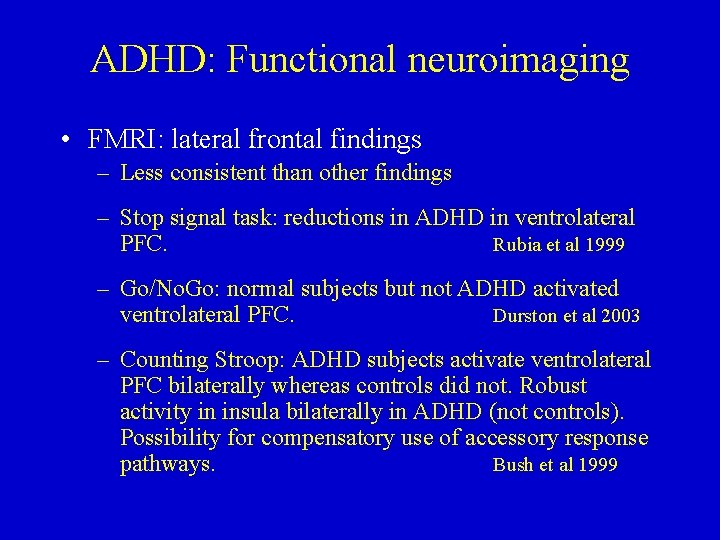
ADHD: Functional neuroimaging • FMRI: lateral frontal findings – Less consistent than other findings – Stop signal task: reductions in ADHD in ventrolateral PFC. Rubia et al 1999 – Go/No. Go: normal subjects but not ADHD activated ventrolateral PFC. Durston et al 2003 – Counting Stroop: ADHD subjects activate ventrolateral PFC bilaterally whereas controls did not. Robust activity in insula bilaterally in ADHD (not controls). Possibility for compensatory use of accessory response pathways. Bush et al 1999
ADHD: Functional neuroimaging Magnetic resonance spectroscopy (MRS): • Noninvasive, MRI-based method for quantifying various chemicals • Not all chemicals are visible with MRS • Drawback: only able to study a few restricted ROIs during a session – Focus on 1 or 2 sites during a single study – E. g. , unilateral DLPFC, cingulate or caudate.
ADHD: Functional neuroimaging • Compounds commonly studied with MRS: – N-acelyspartate (NAA): marker for neuronal integrity • Low values indicate neuronal dysfunction or death • May also be marker for myelination – Glutamine/glutamate/-butyric acid • Elevations associated with neuronal destruction – Choline • Increases associated with myelin breakdown – Creatine/phosphocreatine • Creatine most often used as an internal control
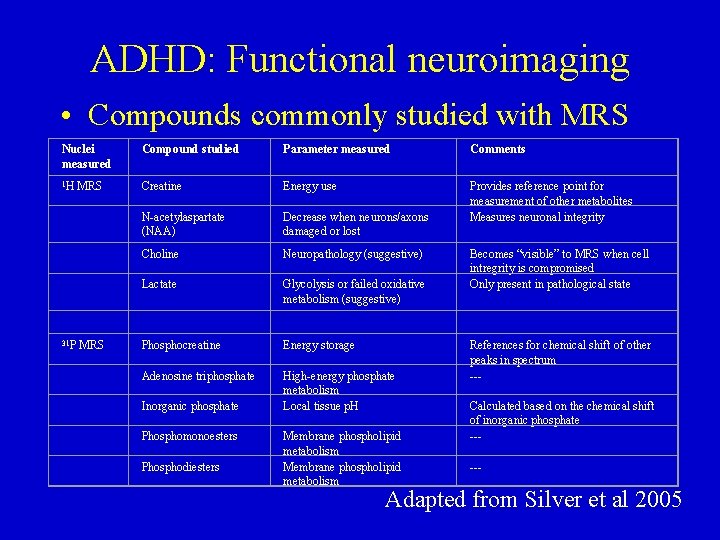
ADHD: Functional neuroimaging • Compounds commonly studied with MRS Nuclei measured Compound studied Parameter measured Comments 1 H MRS Creatine Energy use N-acetylaspartate (NAA) Decrease when neurons/axons damaged or lost Provides reference point for measurement of other metabolites Measures neuronal integrity Choline Neuropathology (suggestive) Lactate Glycolysis or failed oxidative metabolism (suggestive) 31 P MRS Phosphocreatine Energy storage Adenosine triphosphate Inorganic phosphate High-energy phosphate metabolism Local tissue p. H References for chemical shift of other peaks in spectrum --- Phosphomonoesters Phosphodiesters Membrane phospholipid metabolism Becomes “visible” to MRS when cell intregrity is compromised Only present in pathological state Calculated based on the chemical shift of inorganic phosphate ----- Adapted from Silver et al 2005
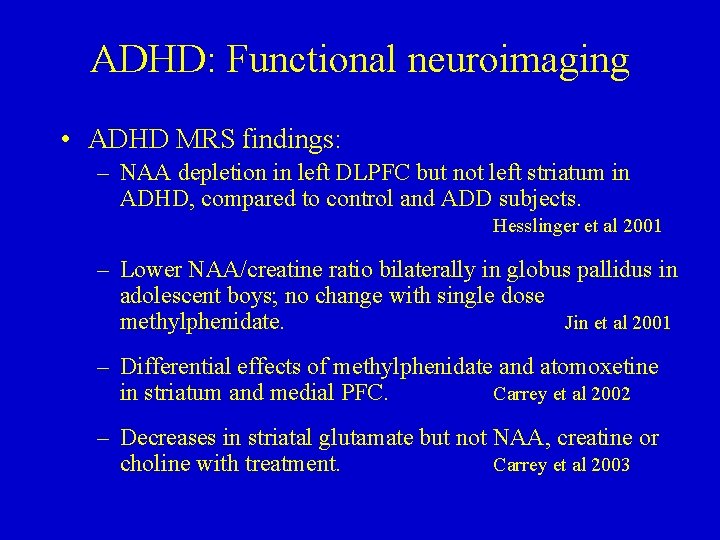
ADHD: Functional neuroimaging • ADHD MRS findings: – NAA depletion in left DLPFC but not left striatum in ADHD, compared to control and ADD subjects. Hesslinger et al 2001 – Lower NAA/creatine ratio bilaterally in globus pallidus in adolescent boys; no change with single dose methylphenidate. Jin et al 2001 – Differential effects of methylphenidate and atomoxetine in striatum and medial PFC. Carrey et al 2002 – Decreases in striatal glutamate but not NAA, creatine or choline with treatment. Carrey et al 2003
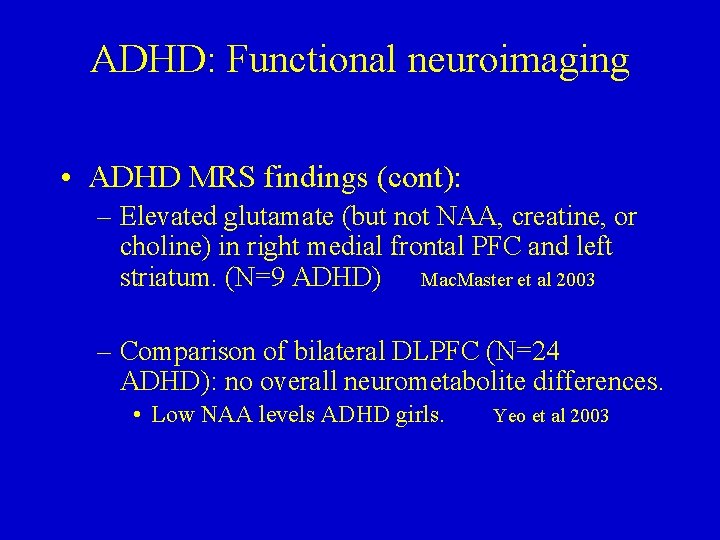
ADHD: Functional neuroimaging • ADHD MRS findings (cont): – Elevated glutamate (but not NAA, creatine, or choline) in right medial frontal PFC and left striatum. (N=9 ADHD) Mac. Master et al 2003 – Comparison of bilateral DLPFC (N=24 ADHD): no overall neurometabolite differences. • Low NAA levels ADHD girls. Yeo et al 2003

ADHD: Neuroimaging • Electrophysiological techniques – Quantitative electroencephalography (QEEG) – Event-related potentials (ERP)

ADHD: Electrophysiology studies • Quantitative EEG: – Computer-assisted spectral analysis of the EEG signal. – Quantification of the alpha, beta, theta and delta frequencies. – Signal generators are not localized to specific neural structures with precision. – ADHD characterized by “theta excess” and “alpha slowing” as compare to controls. • Caveat: Patterns of “theta excess” and/or “abnormal alpha” can be indicative of other disorders (e. g. , dementia, schizophrenia, mood disorders, OCD, learning disorders, and TBI, to name a few).
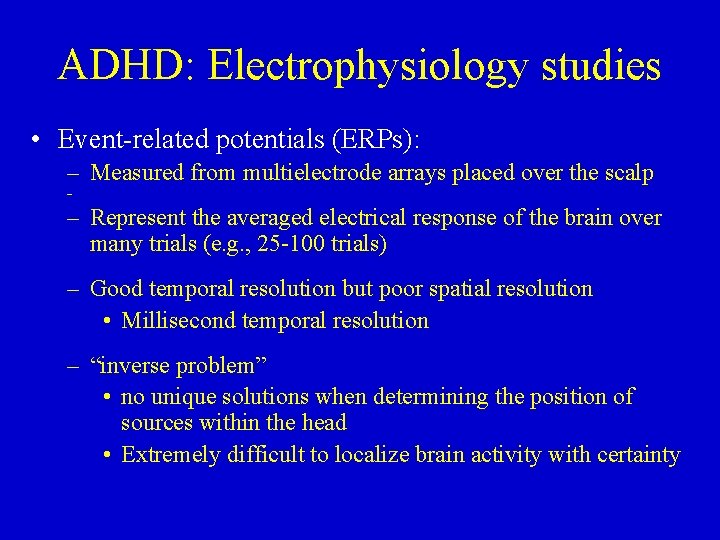
ADHD: Electrophysiology studies • Event-related potentials (ERPs): – Measured from multielectrode arrays placed over the scalp – – Represent the averaged electrical response of the brain over many trials (e. g. , 25 -100 trials) – Good temporal resolution but poor spatial resolution • Millisecond temporal resolution – “inverse problem” • no unique solutions when determining the position of sources within the head • Extremely difficult to localize brain activity with certainty
Genetics Research 1) Family, twin & adoption studies 2) Molecular genetic studies
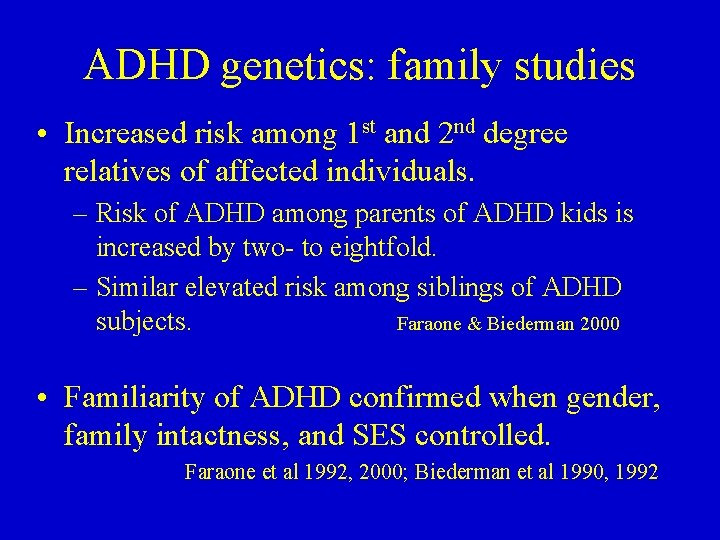
ADHD genetics: family studies • Increased risk among 1 st and 2 nd degree relatives of affected individuals. – Risk of ADHD among parents of ADHD kids is increased by two- to eightfold. – Similar elevated risk among siblings of ADHD subjects. Faraone & Biederman 2000 • Familiarity of ADHD confirmed when gender, family intactness, and SES controlled. Faraone et al 1992, 2000; Biederman et al 1990, 1992
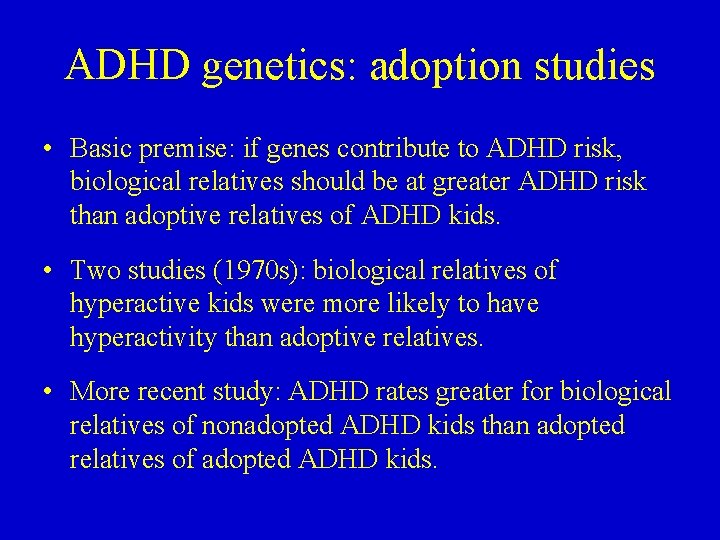
ADHD genetics: adoption studies • Basic premise: if genes contribute to ADHD risk, biological relatives should be at greater ADHD risk than adoptive relatives of ADHD kids. • Two studies (1970 s): biological relatives of hyperactive kids were more likely to have hyperactivity than adoptive relatives. • More recent study: ADHD rates greater for biological relatives of nonadopted ADHD kids than adopted relatives of adopted ADHD kids.
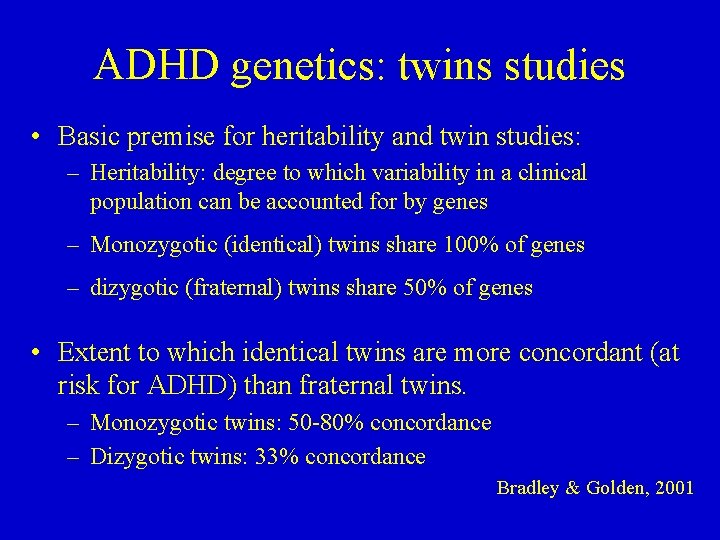
ADHD genetics: twins studies • Basic premise for heritability and twin studies: – Heritability: degree to which variability in a clinical population can be accounted for by genes – Monozygotic (identical) twins share 100% of genes – dizygotic (fraternal) twins share 50% of genes • Extent to which identical twins are more concordant (at risk for ADHD) than fraternal twins. – Monozygotic twins: 50 -80% concordance – Dizygotic twins: 33% concordance Bradley & Golden, 2001

ADHD genetics: twin studies • Faraone et al 2005: – Estimated heritability of ADHD based on pooled results from 20 twin studies. – 76% mean heritability estimate
ADHD genetics: molecular studies 1) Genome-wide linkage scans: – Many DNA acid markers across the genome are examined to determine whether any chromosomal regions are shared more often than expected among ADHD family members. • 4 studies: some evidence of genomic region linkage, but inconsistent across studies. – 17 p 11 has been the one replicated risk locus
ADHD genetics: molecular studies 2) Candidate gene studies: Method of association to determine whether biologically relevant genes influence ADHD susceptibility. – Case-control & family-based designs. • Case-control: compare allele frequencies between ADHD pts & non. ADHD controls. (ADHD allele > in ADHD pt) • Family-based: compares alleles that parents transmit to ADHD children with those they do not transmit. (ADHD allele more common in transmitted versus nontransmitted alleles) – Both designs can derive odds ratios (OR) or relative risk (RR) statistics. • OR or RR > 1. 0 indicates allele increases ADHD risk • OR or RR of 1. 0 = no association; OR or RR <1. 0 indicates decreased ADHD risk.
ADHD genetics: molecular studies • Candidate genes: – Catecholiminergic genes (N=4) • Dopamine-4 and dopamine-5 receptors (DRD 4 & DRD 5) • Dopamine transporter (DAT 1) • Dopamine-b-hydoylase (DBH) – SNAP-25 gene – Serotonergic genes (N=2) • Serotonin transporter (5 -HTT) • Serotonin 1 B-receptor (HTR 1 B) Faraone et al 2005; Durston & Konrad, 2007 – Norepinephrine receptors (N=2) • ADRA 2 A • ADRA 2 C Pennington et al 2009
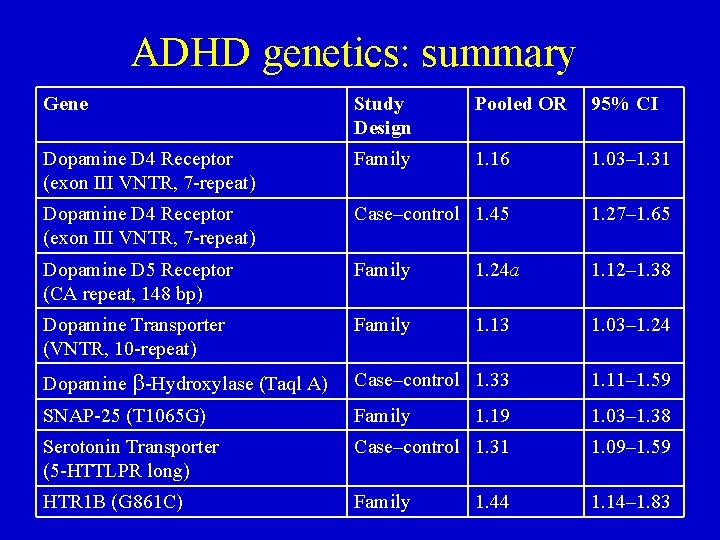
ADHD genetics: summary Gene Study Design Pooled OR 95% CI Dopamine D 4 Receptor (exon III VNTR, 7 -repeat) Family 1. 16 1. 03– 1. 31 Dopamine D 4 Receptor (exon III VNTR, 7 -repeat) Case–control 1. 45 1. 27– 1. 65 Dopamine D 5 Receptor (CA repeat, 148 bp) Family 1. 24 a 1. 12– 1. 38 Dopamine Transporter (VNTR, 10 -repeat) Family 1. 13 1. 03– 1. 24 Dopamine b-Hydroxylase (Taql A) Case–control 1. 33 1. 11– 1. 59 SNAP-25 (T 1065 G) Family 1. 19 1. 03– 1. 38 Serotonin Transporter (5 -HTTLPR long) Case–control 1. 31 1. 09– 1. 59 HTR 1 B (G 861 C) Family 1. 14– 1. 83 1. 44
ADHD genetics: molecular studies • Catecholiminergic genes – Dopamine-4 (DRD 4): • Gene variance may be associated with post-synaptic receptor sensitivity to dopamine. • Both noradrenaline and dopamine are potent agonists of the D 4 receptor. • D 4 is prevalent in frontal-subcortical networks. • Research predominantly focused on tandem repeat polymorphism in exon III of D 4 given that one variant (the 7 -repeat allele) produces a blunted response to dopamine. • ADHD association between 7 -repeat allele and ADHD in case-control and family studies (OR=1. 9 and OR=1. 4). • Studies using symptom dimensions rather than categorical dx suggest DRD 4 is particularly relevant to symptoms of inattention.
ADHD genetics: molecular studies Catecholiminergic genes – Dopamine transporter (DAT 1): • gene variance may be associated with a dopamine transporter abnormally inefficient at the reuptake process. • Stimulant medications block the dopamine transporter as one mechanism of action for achieving therapeutic effect. • In mice, eliminating SLC 6 A 3 gene function leads to hyperactivity and inhibitory behavior; stimulants reduces hyperactivity. Mice show extracellular dopamine, a doubling of dopamine synthesis rate, and tyrosine hydroxylase in the striatum. • SPECT ADHD adult findings: DAT activity elevated by 70%
ADHD genetics: molecular studies • Catecholiminergic genes – Dopamine-5 (DRD 5): little is known about functional relevance of allelic variants. • 148 -bp allele found in family studies (OR=1. 2) as well as a strong effect in one study without parental ADHD hx. – Dopamine-b-hydoylase (DBH): • involved in regulation of dopamine metabolism; • enzyme responsible for converting dopamine to noradrenaline.

ADHD genetics: molecular studies – Serotonergic genes • Serotonin 1 B-receptor (HTR 1 B): – Mutation in HTR 1 B-gene which encosed the 5 HT 1 B receptor is thought to lead to an activity decrease in the enzyme converting tryptophan (the precursor) into serotonin. – Low serotonin activity has been associated with impulsivity, aggression, and disinhibited behavior in animal and human studies. • Serotonin transporter (5 -HTT): – Polymorphisms in the serotonin transportor (5 -HTT) may similarly result in reduced transcription and lower transporter protein levels.
ADHD genetics: molecular studies • SNAP-25: • Implications from mouse mutant strain coloboma. • Mutations within the gene are thought to affect the functions of synaptic vesicle fusion and neurotransmitter release. • Noradrenergic receptors: – ADRA 2 A: • G allele associated with ADHD, ODD, and CD symptoms. • 2 studies failed to show association between ADRA 2 A and ADHD dx but one showed significant association of the G allele with elevated attention and combined symptom scores.

ADHD: Future directions in genetics • Endophenotypes: – Phenotype more proximal to the biological etiology of a clinical disorder than its symptoms and influenced by 1 or more of the same susceptibility genes as the condition. – Growing interest in molecular genetics for these (including in ADHD). • Candidate gene studies show inconsistent replication patterns, with significant but not overwhelming odds ratios (OR=1. 2 -1. 5 in ADHD). • Genome scans so far have found largely non-overlapping chromosomal regions for potentially harboring susceptibility genes.

ADHD: Future directions in genetics • Useful endophenotypes should: – Co-occur with the condition of interest – Be measured by tools with good psychometric properties – Show evidence of heritability – Show familial-genetic overlap with disorder of study. (should appear in unaffected family members)
ADHD: Cognitive Neuroscience Future directions: • Combining neurobiological approaches – functional imaging & electrophysiological methods – Structural & functional imaging methods – Imaging methods paired with neuropsychological testing – Combining genetics with above – Better defining & delineating cognitive aspects of function

ADHD: Cognitive Neuroscience Future directions: – Direct comparisons of neuropsychological theories – Better defining & delineating cognitive aspects of function
- Slides: 137