MedicationAssisted Treatment for Opiate Use DisordersYouth and Young






































- Slides: 77

Medication-Assisted Treatment for Opiate Use Disorders-Youth and Young Adults Gary M. Henschen, MD, LFAPA, Chief Medical Officer-Behavioral Health, Magellan Healthcare

About the speaker Gary M. Henschen, M. D. is Chief Behavioral Health Officer for Magellan Healthcare, Inc. He has been employed by Magellan since 2001, and has been in his current position since 2008. In his current role, he directs a team that develops medical necessity criteria, new technology assessments, and clinical practice guidelines for behavioral health. He provides clinical expertise in new product development and the quality improvement program of Magellan. He oversees medical management for Magellan’s behavioral health programs. Prior to joining Magellan, Dr. Henschen was Chief Medical Officer of Charter Behavioral Health Systems, LLC. He was previously in private practice for psychiatry and psychoanalysis in Greensboro, North Carolina for 15 years. Dr. Henschen is a graduate of Davidson College. He received the M. D. degree from the University of North Carolina at Chapel Hill. He completed his internship in medicine at Letterman Army Medical Center, San Francisco, and completed military service with the U. S. Army in Germany where he was flight surgeon and commander of the 536 th General Dispensary. Dr. Henschen completed his residency and chief residency in psychiatry at Duke Medical Center, and completed psychoanalytic training at the UNC-Duke Psychoanalytic Institute. His research interests have included the assessment and prevention of suicide; psychiatric consultation-liaison with primary care physicians; the development of quality metrics; addressing the needs of individuals diagnosed with both serious mental illness and substance use disorders; and providing consultation to behavioral special investigation units. Dr. Henschen is licensed to practice medicine in Georgia, North Carolina, Tennessee, New Jersey, Pennsylvania and Iowa. 2 Copyright 2016 Magellan Health, Inc.

Disclosure Gary Henschen, M. D. has no relevant financial relationship or commercial interest that could be reasonably construed as a conflict of interest. 3 Copyright 2016 Magellan Health, Inc.

Learning objectives Upon completion of this activity, participants should be able to: • Demonstrate the effective use of naltrexone in opiate use disorders • Demonstrate the effective use of buprenorphine in opiate use disorders • Report theories explaining the need for psychosocial interventions in SUD treatment • Explain the role of medication-assisted treatment in the continuum of care for substance use disorder patients • Demonstrate understanding of techniques to improve youth patient medication compliance 4 Copyright 2016 Magellan Health, Inc.

Opiate abuse in the headlines 5 Copyright 2016 Magellan Health, Inc.

Background The United States is in the midst of an unprecedented drug abuse epidemic, with prescription drug abuse quickly becoming a top public health concern There an estimated 110 million chronic pain sufferers in the United States, and the numbers are expected to further increase as Americans age and live longer Chronic pain sufferers taking opioid pain medications are not the only users of pain killers In 2010, approximately five million Americans used pain relievers for nonmedical reasons Source: Link: http: //www. drugabuse. gov/related-topics/trends-statistics/infographics/popping-pills-prescription-drug-abuse-in-america, 2011 6 Copyright 2016 Magellan Health, Inc.

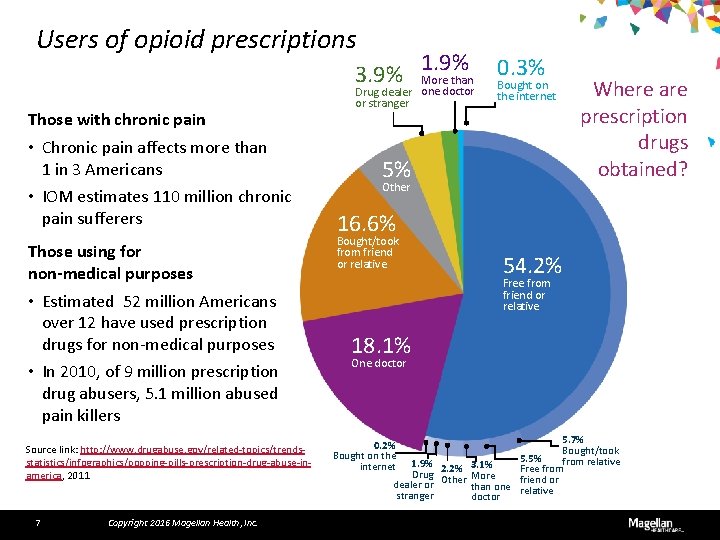
Users of opioid prescriptions 3. 9% Those with chronic pain • Chronic pain affects more than 1 in 3 Americans • IOM estimates 110 million chronic pain sufferers Those using for non-medical purposes • Estimated 52 million Americans over 12 have used prescription drugs for non-medical purposes • In 2010, of 9 million prescription drug abusers, 5. 1 million abused pain killers Source link: http: //www. drugabuse. gov/related-topics/trendsstatistics/infographics/popping-pills-prescription-drug-abuse-inamerica, 2011 7 Copyright 2016 Magellan Health, Inc. 1. 9% More than Drug dealer one doctor or stranger 0. 3% Bought on the internet 5% Other Where are prescription drugs obtained? 16. 6% Bought/took from friend or relative 54. 2% Free from friend or relative 18. 1% One doctor 5. 7% Bought/took 5. 5% from relative 1. 9% 2. 2% 3. 1% Free from Drug Other More friend or dealer or than one relative stranger doctor 0. 2% Bought on the internet

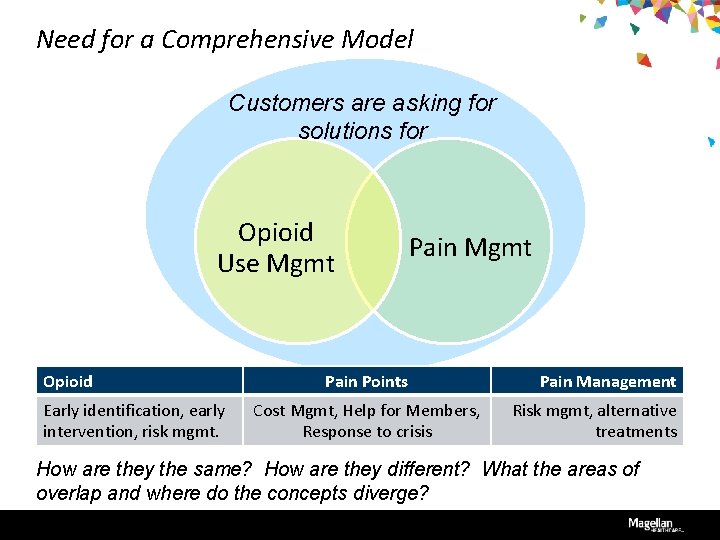
Need for a Comprehensive Model Customers are asking for solutions for Opioid Use Mgmt Opioid Early identification, early intervention, risk mgmt. Pain Mgmt Pain Points Cost Mgmt, Help for Members, Response to crisis Pain Management Risk mgmt, alternative treatments How are they the same? How are they different? What the areas of overlap and where do the concepts diverge?

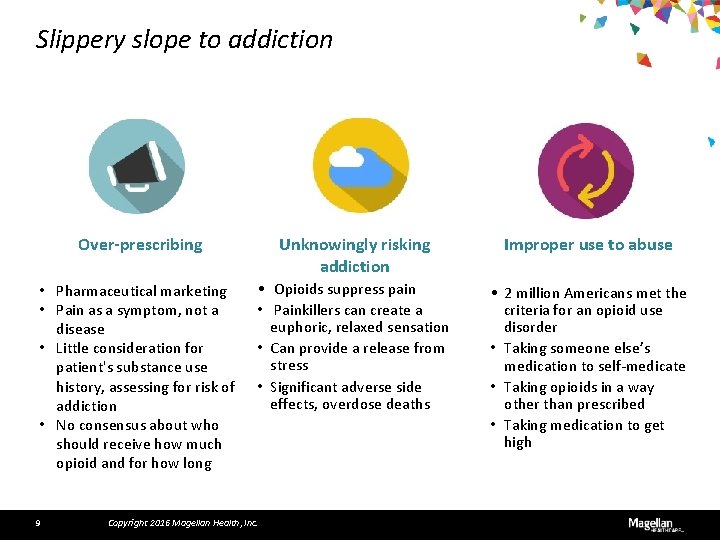
Slippery slope to addiction Over-prescribing • Pharmaceutical marketing • Pain as a symptom, not a disease • Little consideration for patient's substance use history, assessing for risk of addiction • No consensus about who should receive how much opioid and for how long 9 Unknowingly risking addiction Improper use to abuse • Opioids suppress pain • Painkillers can create a euphoric, relaxed sensation • Can provide a release from stress • Significant adverse side effects, overdose deaths • 2 million Americans met the criteria for an opioid use disorder • Taking someone else’s medication to self-medicate • Taking opioids in a way other than prescribed • Taking medication to get high Copyright 2016 Magellan Health, Inc.

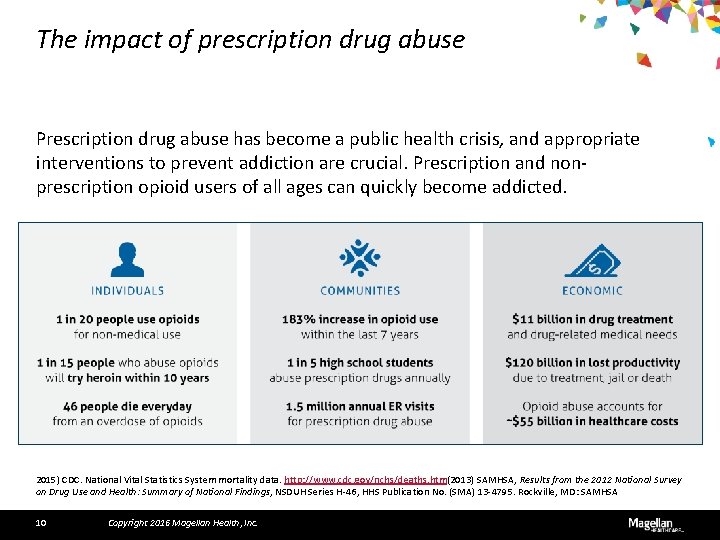
The impact of prescription drug abuse Prescription drug abuse has become a public health crisis, and appropriate interventions to prevent addiction are crucial. Prescription and nonprescription opioid users of all ages can quickly become addicted. 2015) CDC. National Vital Statistics System mortality data. http: //www. cdc. gov/nchs/deaths. htm(2013) SAMHSA, Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13 -4795. Rockville, MD: SAMHSA 10 Copyright 2016 Magellan Health, Inc.

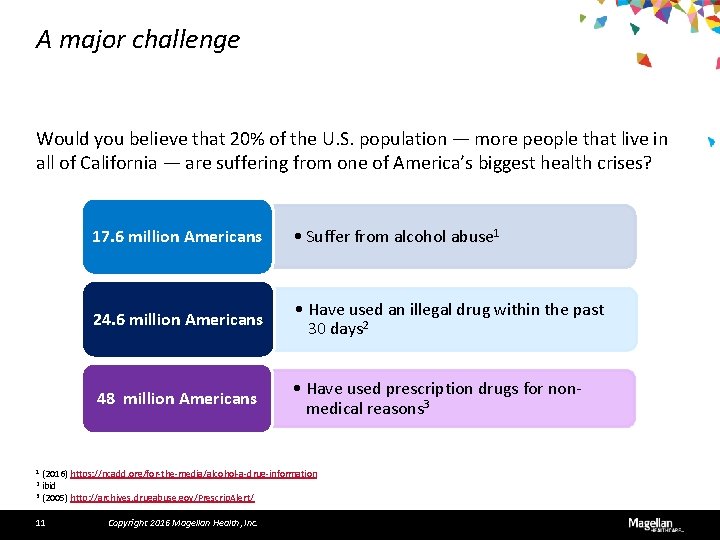
A major challenge Would you believe that 20% of the U. S. population — more people that live in all of California — are suffering from one of America’s biggest health crises? 17. 6 million Americans • Suffer from alcohol abuse 1 24. 6 million Americans • Have used an illegal drug within the past 30 days 2 48 million Americans • Have used prescription drugs for nonmedical reasons 3 1 (2016) https: //ncadd. org/for-the-media/alcohol-a-drug-information 2 ibid 3 (2005) http: //archives. drugabuse. gov/Prescrip. Alert/ 11 Copyright 2016 Magellan Health, Inc.

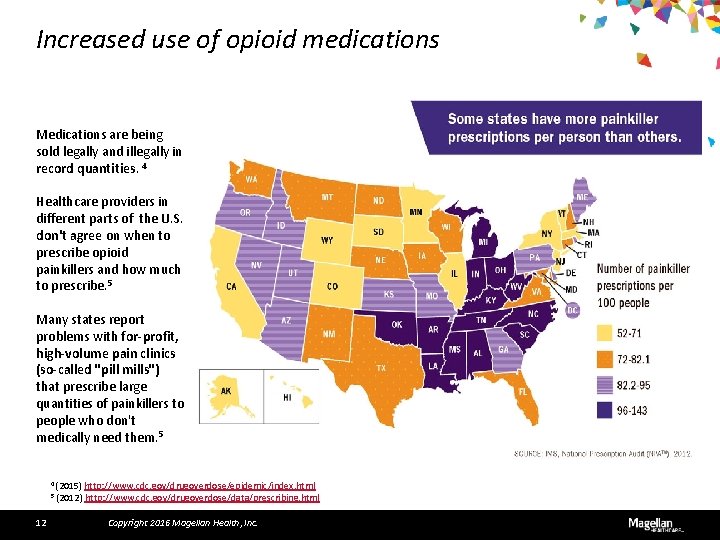
Increased use of opioid medications Medications are being sold legally and illegally in record quantities. 4 Healthcare providers in different parts of the U. S. don't agree on when to prescribe opioid painkillers and how much to prescribe. 5 Many states report problems with for-profit, high-volume pain clinics (so-called "pill mills") that prescribe large quantities of painkillers to people who don't medically need them. 5 4 (2015) http: //www. cdc. gov/drugoverdose/epidemic/index. html 5 (2012) http: //www. cdc. gov/drugoverdose/data/prescribing. html 12 Copyright 2016 Magellan Health, Inc.

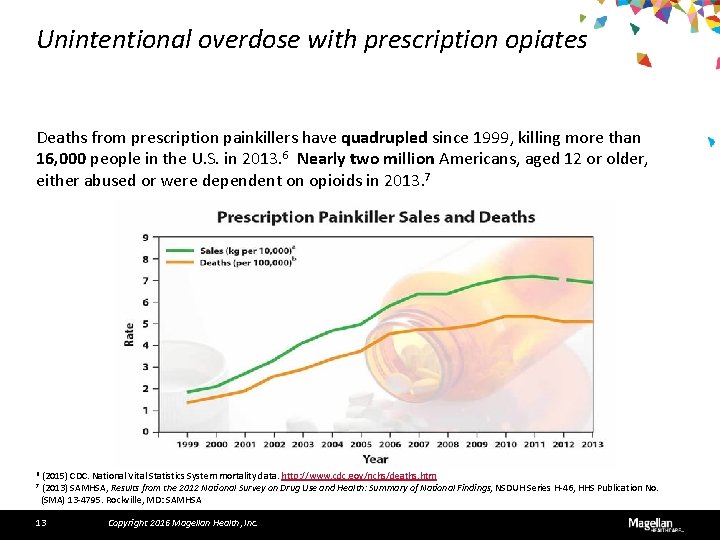
Unintentional overdose with prescription opiates Deaths from prescription painkillers have quadrupled since 1999, killing more than 16, 000 people in the U. S. in 2013. 6 Nearly two million Americans, aged 12 or older, either abused or were dependent on opioids in 2013. 7 6 (2015) CDC. National Vital Statistics System mortality data. http: //www. cdc. gov/nchs/deaths. htm 7 (2013) SAMHSA, Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13 -4795. Rockville, MD: SAMHSA 13 Copyright 2016 Magellan Health, Inc.

Adolescent substance abuse

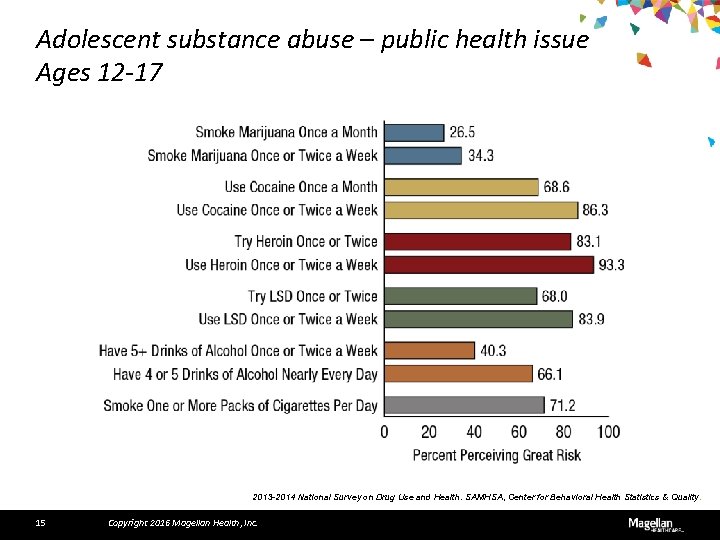
Adolescent substance abuse – public health issue Ages 12 -17 2013 -2014 National Survey on Drug Use and Health. SAMHSA, Center for Behavioral Health Statistics & Quality. 15 Copyright 2016 Magellan Health, Inc.

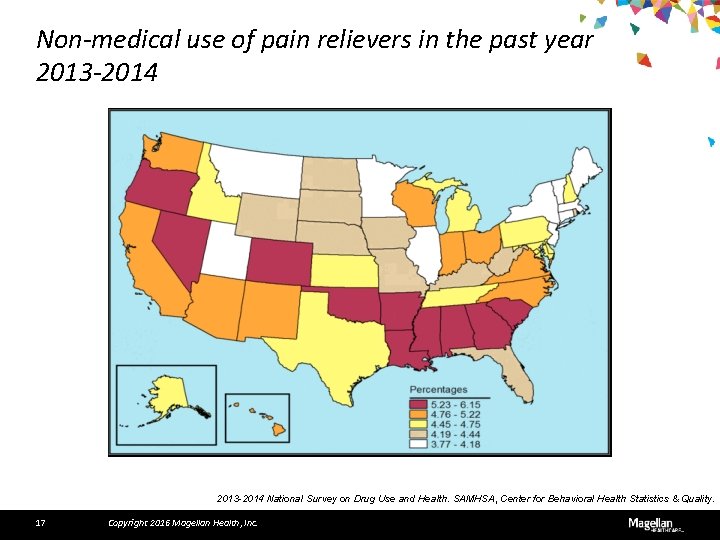
Non-medical use of pain relievers in the past year 2013 -2014 National Survey on Drug Use and Health. SAMHSA, Center for Behavioral Health Statistics & Quality. 17 Copyright 2016 Magellan Health, Inc.

Adolescent-specific interventions

Adolescent substance abuse Continues to be persistent, widespread public health issue New drugs, rediscovery of older drugs - cyclic pattern of substance abuse Age of onset use highly correlated with future use as an adult Adolescence is a period of experimentation Practitioners have an important role in screening adolescents 19 Copyright 2016 Magellan Health, Inc.

Risk factors Biological vulnerability Early exposure to substance abuse Pre-existing psychological/psychiatric problems Neurobiological developmental factors Risk factors change with maturity and changes in the environment Key risk periods during life transitions 20 Copyright 2016 Magellan Health, Inc.

The importance of screening American Academy of Pediatrics: • Screening should occur at all routine clinical visits with youth Screening, brief intervention, and referral to treatment (SBIRT) CRAFFT (acronym based on questions in part B of tool) www. ceasar. org Easily administered by interview Better specificity than the CAGE questionnaire for adolescents www. sbirttraining. com/node/524 21 Copyright 2016 Magellan Health, Inc.

The CRAFFT Screen for Youth-Part A During the PAST 12 MONTHS, did you: 1. Drink any alcohol (more than a few sips)? (Do not count sips of alcohol taken during family or religious events. ) 2. Smoke any marijuana or hashish? 3. Use anything else to get high? (“anything else” includes illegal drugs, over the counter and prescription drugs, and things that you sniff or “huff”) If the answer is no to all questions, only ask the C question in Part B If the answer is yes to any Part A questions, complete Part B Knight JR, Shrier LA, Bravender TD, Farrell M, Vander Bilt J, Shaffer HJ. A new brief screen for adolescent substance abuse. Arch Pediatr Adolesc Med 1999; 153(6): 591 -6.

The CRAFFT screen for youth-Part B C - Have you ever ridden in a CAR driven by someone (including yourself) who was "high" or had been using alcohol or drugs? R - Do you ever use alcohol or drugs to RELAX, feel better about yourself, or fit in? A - Do you ever use alcohol/drugs while you are by yourself, ALONE? F - Do you ever FORGET things you did while using alcohol or drugs? F - Do your family or FRIENDS ever tell you that you should cut down on your drinking or drug use? T - Have you gotten into TROUBLE while you were using alcohol or drugs? CRAFFT Scoring: Each “yes” response in Part B scores 1 point. A total score of 2 or higher is a positive screen, indicating a need for additional assessment. Knight JR, Shrier LA, Bravender TD, Farrell M, Vander Bilt J, Shaffer HJ. A new brief screen for adolescent substance abuse. Arch Pediatr Adolesc Med 1999; 153(6): 591 -6.

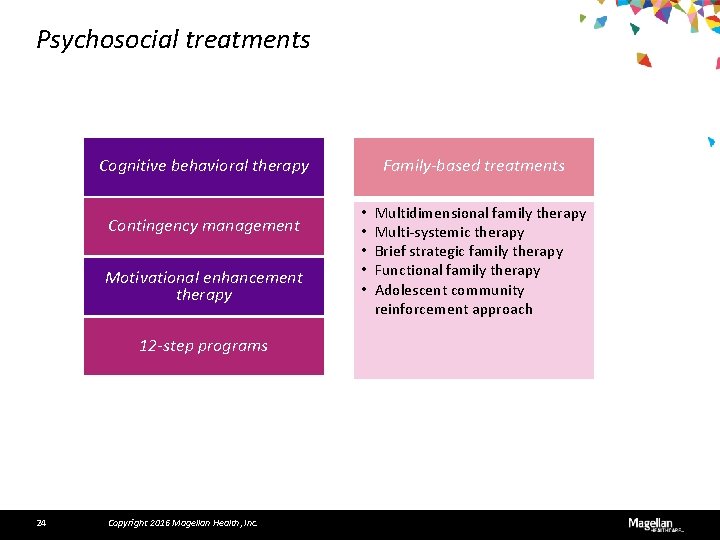
Psychosocial treatments Family-based treatments Cognitive behavioral therapy Contingency management Motivational enhancement therapy 12 -step programs 24 Copyright 2016 Magellan Health, Inc. • • • Multidimensional family therapy Multi-systemic therapy Brief strategic family therapy Functional family therapy Adolescent community reinforcement approach

Transition-age drug & alcohol treatment survey Voice & Vision, Inc. in collaboration with Bucks County departments and Magellan conducted face-to-face interviews with 74 transition-age youth (TAY) age 26 and younger who received D&A treatment in Bucks County (inpatient, outpatient, and non-treatment settings were included) • Almost 80% of the substance users came to treatment because of opioid use • Almost half were JPO/court-ordered for treatment • Most common reason for starting opioid use was “emotional pain” • About one-third were receiving or had received medication-assisted treatment 25 Copyright 2016 Magellan Health, Inc.

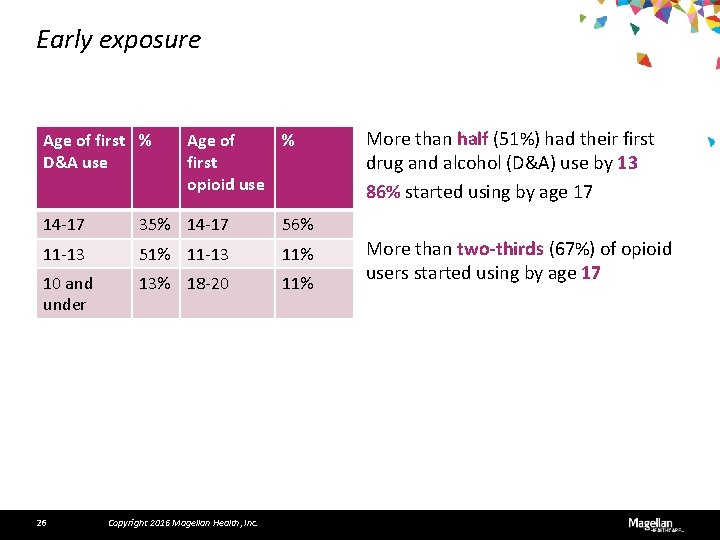
Early exposure Age of first % D&A use Age of % first opioid use 14 -17 35% 14 -17 56% 11 -13 51% 11 -13 11% 10 and under 13% 18 -20 11% 26 Copyright 2016 Magellan Health, Inc. More than half (51%) had their first drug and alcohol (D&A) use by 13 86% started using by age 17 More than two-thirds (67%) of opioid users started using by age 17

What helps, what doesn’t in treatment programs Helps Hurts • Family involvement in treatment (75% endorsed this) • Support/learning from others in group therapy • Education on drug use - better life without use • Individual therapy • AA/NA programs/sponsor • Having structure in a drug-free environment • Talking with staff 27 Copyright 2016 Magellan Health, Inc. • Unprofessional staff • Unnecessary rules • Groups that were not helpful (e. g. , groups that just asked how their week was going instead of helping with strategies to improve their lives) • Groups that mixed heavy users with light users

“Ideal treatment program” The respondents in this survey were asked what would they include if they were to “create their own treatment program”: • • 28 More activities like music, art and nature More groups—inside and outside the treatment program Sports and gym Training/preparation for independent living Individualized treatment Relaxed atmosphere Daily routine and responsibilities Longer treatment periods Copyright 2016 Magellan Health, Inc.

Medication-assisted treatment (MAT)

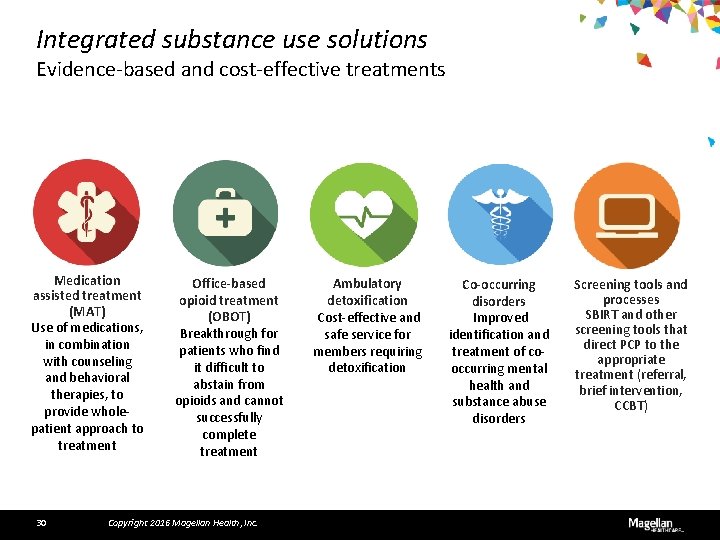
Integrated substance use solutions Evidence-based and cost-effective treatments Medication assisted treatment (MAT) Use of medications, in combination with counseling and behavioral therapies, to provide wholepatient approach to treatment 30 Office-based opioid treatment (OBOT) Breakthrough for patients who find it difficult to abstain from opioids and cannot successfully complete treatment Copyright 2016 Magellan Health, Inc. Ambulatory detoxification Cost-effective and safe service for members requiring detoxification Co-occurring disorders Improved identification and treatment of cooccurring mental health and substance abuse disorders Screening tools and processes SBIRT and other screening tools that direct PCP to the appropriate treatment (referral, brief intervention, CCBT)

Increasing recognition of role of medications in treatment of SUD Medications highly underutilized APA and NCQA performance measures • Psychosocial interventions • Pharmacologic interventions Psychosocial interventions must accompany MAT Medications mandated in VA Magellan’s OBOT initiative 31 Copyright 2016 Magellan Health, Inc.

Increasing recognition of the role of medications in treatment of SUD Barriers to utilization of these medications • • • 32 Lack of education of clinicians Perceived ineffectiveness Medications will reduce motivation for psychosocial treatment Side effects Cost Lack of time in patient management Reluctance to take medications Formulary status in health plan Lower level of education in SUD facility staff Copyright 2016 Magellan Health, Inc.

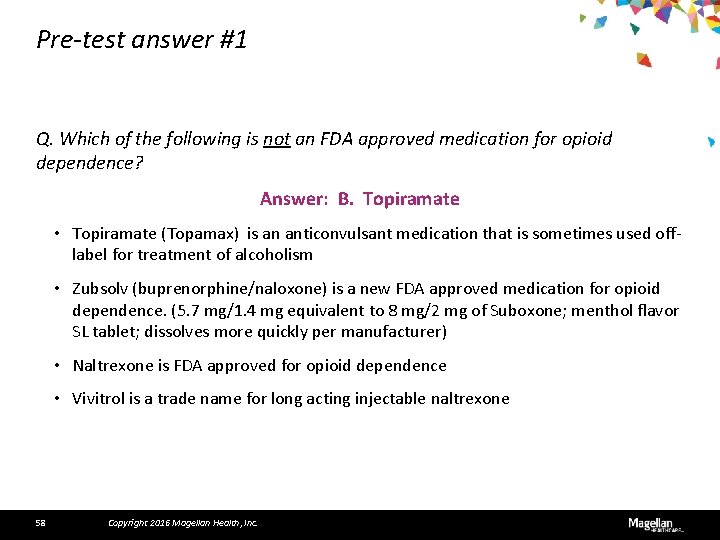
Pre-test question #1 Q. Which of the following is not an FDA approved medication for opioid dependence? A. Zubsolv B. Topiramate C. Naltrexone D. Vivitrol 34 Copyright 2016 Magellan Health, Inc.

Pre-test question #2 Q. Which of the following are used to treat alcohol dependence? A. Acamprosate B. Oral naltrexone C. Long-acting injectable naltrexone D. All of the above 35 Copyright 2016 Magellan Health, Inc.

Medication-assisted treatment (MAT) introduction Medication-assisted treatment is most successful as part of a multi-pronged approach to substance use treatment Medications can address the neurobiological aspect of addiction but the psychological and social aspects should also be addressed • Counseling and mutual help groups are also important to give the individual the best chance of success The selection of medication should take into account individual preference, past treatment history, setting in which the individual will receive the medication and psychosocial supports • Opioid treatment programs (OTPs) offer methadone and often buprenorphine • Office-based opioid treatment (OBOT) is limited to buprenorphine 37 Copyright 2016 Magellan Health, Inc.

Medication-assisted treatment (MAT) introduction Buprenorphine is the only FDA-approved MAT in those under age 18, and even this is only approved for those 16 and older There are no FDA-approved MAT during pregnancy. All FDA-approved medications are pregnancy category “C”—use when benefits outweigh risks. Animal studies indicate some risk or no animal or human studies available Methadone and buprenorphine have been used in pregnancy but risks to the fetus are not certain 38 Copyright 2016 Magellan Health, Inc.

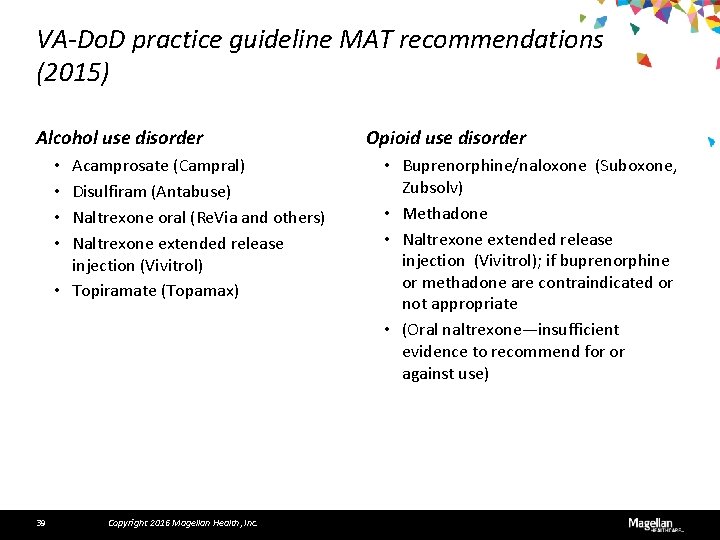
VA-Do. D practice guideline MAT recommendations (2015) Alcohol use disorder Acamprosate (Campral) Disulfiram (Antabuse) Naltrexone oral (Re. Via and others) Naltrexone extended release injection (Vivitrol) • Topiramate (Topamax) • • 39 Copyright 2016 Magellan Health, Inc. Opioid use disorder • Buprenorphine/naloxone (Suboxone, Zubsolv) • Methadone • Naltrexone extended release injection (Vivitrol); if buprenorphine or methadone are contraindicated or not appropriate • (Oral naltrexone—insufficient evidence to recommend for or against use)

Disulfiram (Antabuse) FDA-approved for alcohol dependence Blocks alcohol dehydrogenase, which increases acetaldehyde when an individual drinks Side effects: Alcohol-disulfiram reaction • • Nausea Vomiting Flushing Weakness Dosage: 125 -500 mg/day No risk of abuse; not a controlled substance 40 Copyright 2016 Magellan Health, Inc.

Acamprosate (Campral) FDA-approved for alcohol dependence Exact mechanism of action unknown but appears to stabilize the glutamate system More than 17 clinical trials Results have generally indicated 50% increase in abstinence rates 41 Copyright 2016 Magellan Health, Inc.

Acamprosate (Campral) Does not metabolize through the liver; generally safer for alcoholics with liver impairment Side effects: Minimal (e. g. , diarrhea) Drug interactions: Few; increases naltrexone blood levels Dosage: 666 mg three times a day No risk of abuse; not a controlled substance 42 Copyright 2016 Magellan Health, Inc.

Oral naltrexone (Re. Via) FDA-approved for both alcohol and opioid dependence Classified as an opioid antagonist More than 29 clinical trials Lengthens time to relapse to drinking and amount of drinking Diminishes the pleasurable effects of drinking and high-risk drinking Blocks “high” of endogenous opioids In alcoholics, those with high levels of craving responded better to NTX Better response in alcoholics with family history of alcoholism 43 Copyright 2016 Magellan Health, Inc.

Oral naltrexone (Re. Via) Poor medication adherence and high dropout rate for general opioid dependent users It should be reserved for highly motivated individuals or certain populations, such as court-mandated individuals, individuals on probation, impaired professionals Must be opioid-free for 7 -10 days. Consider naltrexone challenge if not sure - 25 mg po x 1, repeat in 1 hour if no withdrawal symptoms Side effects: Metabolized through the liver; may cause hepatotoxicity at doses 5 x therapeutic Drug interactions: Do not use with disulfiram (Antabuse); antagonizes opioid analgesics, opioid containing cough/cold medications and antidiarrheal agents Dosage: 50 mg/day No risk of abuse; not a controlled substance 44 Copyright 2016 Magellan Health, Inc.

Long-acting injectable naltrexone (Vivitrol) FDA-approved in 2006 for alcohol dependence in individuals who are not actively using alcohol at the time of initiation FDA-approved in 2010 for opioid dependence for the prevention of relapse to opioid misuse following opioid detoxification Competitive antagonist at opioid receptor sites within the brain Efficacy noted in maintaining abstinence, reducing cravings for opioids Side effects: Same cautions and side effects as oral naltrexone, except injection -site reactions • Cold-like symptoms, fatigue, abdominal cramping, diarrhea • Injection-site reactions such as bruising, swelling, tenderness, pain—generally due to poor injection technique and giving subcutaneous instead of IM injection 45 Copyright 2016 Magellan Health, Inc.

Long-acting injectable naltrexone (Vivitrol) Dosage: 380 mg IM in gluteus every four weeks; given once monthly as an intramuscular injection No risk of abuse; not a controlled substance Metabolized in the liver; no renal clearance May cause liver enzyme elevation and hepatitis; baseline liver enzymes are recommended; can continue naltrexone with mild liver enzyme elevation Post-marketing reports of suicidality, depression, anxiety and mood symptoms. Whether these were directly related to naltrexone unclear Individual should be opioid-free for 7 -10 days prior to giving injection to avoid opioid withdrawal 46 Copyright 2016 Magellan Health, Inc.

Methadone FDA-approved for opioid dependence; can only be used in a registered clinic Controlled schedule II substance Full opioid agonist at mu receptor Cautions: may cause respiratory depression, QT prolongation and cardiac arrhythmias Drug interactions: contraindicated with partial/mixed opioid agonists (e. g. , buprenorphine), opioid antagonists (e. g. , naltrexone) and tramadol (Ultram) Dosage: initially 10 mg/day (first dosage no greater than 30 mg by regulation, and no more than 40 mg on the first day); titrated up to 80 -120 mg Better treatment retention with daily maintenance dosage of 60 mg or more 47 Copyright 2016 Magellan Health, Inc.

Buprenorphine (Subutex) and Buprenorphine/naloxone (Suboxone, Zubsolv) FDA-approved; only a licensed and registered “qualifying physician” with additional training may prescribe Can be used for office-based treatment Partial opioid agonist at mu receptor; opioid antagonist at kappa receptor Suboxone or Zubsolv is the medication that is used clinically for maintenance treatment since it has naloxone added to it to prevent abuse. Naloxone precipitates opioid withdrawal if an opioid-dependent individual dissolves and injects Suboxone tablet Metabolized in the liver; generally only mild elevations in liver enzymes 48 Copyright 2016 Magellan Health, Inc.

Buprenorphine and buprenorphine/naloxone Side effects: Mainly nausea and constipation Cautions: Respiratory depression when combined with other CNS depressants Dosage: 4 -24 mg/day sublingual (Suboxone) film. Induction: 8 mg on day 1; 16 mg on day 2; Target dose = 16 mg daily (range 4 -24 mg daily); Give first dose at least 4 hrs after last use of opioids • Zubsolv 2. 8 -17. 1 mg/day; sublingual tablet; 5. 7 mg of Zubsolv=8 mg of Suboxone Better treatment retention with dosage of 16 mg or more New generic formulation in tablet form but cost is still high Some abuse potential Controlled schedule III substance 49 Copyright 2016 Magellan Health, Inc.

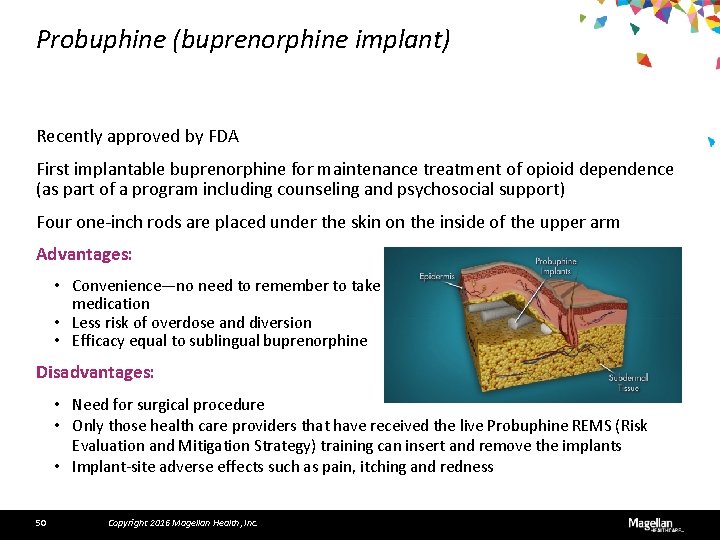
Probuphine (buprenorphine implant) Recently approved by FDA First implantable buprenorphine for maintenance treatment of opioid dependence (as part of a program including counseling and psychosocial support) Four one-inch rods are placed under the skin on the inside of the upper arm Advantages: • Convenience—no need to remember to take medication • Less risk of overdose and diversion • Efficacy equal to sublingual buprenorphine Disadvantages: • Need for surgical procedure • Only those health care providers that have received the live Probuphine REMS (Risk Evaluation and Mitigation Strategy) training can insert and remove the implants • Implant-site adverse effects such as pain, itching and redness 50 Copyright 2016 Magellan Health, Inc.

Off-label medications Topiramate (Topamax)– Four trials have shown to decrease alcohol use (percent drinking days, days abstinent, number of drinks/day). It is hypothesized to increase GABA activity and reduce glutamate activity. No head-to-head trials comparing it with naltrexone or acamprosate. Dosage: 50 -150 mg/day Gabapentin (Neurontin)— A couple of RCTs have demonstrated efficacy in reducing drinking and improving sleep in individuals with alcohol dependence. Insomnia is common among those with alcohol dependence and the hypothesis is that gabapentin improves drinking outcomes by improving sleep. Dosage: 900 -1800 mg/day Baclofen (Lioresal)—Two of three trials demonstrated greater abstinence rates. Generally well-tolerated with few side effects. Dosage: 10 mg tid (SSRIs—no benefit seen without co-morbid mental disorder. May reduce alcohol intake when depression and alcoholism co-exist) 51 Copyright 2016 Magellan Health, Inc.

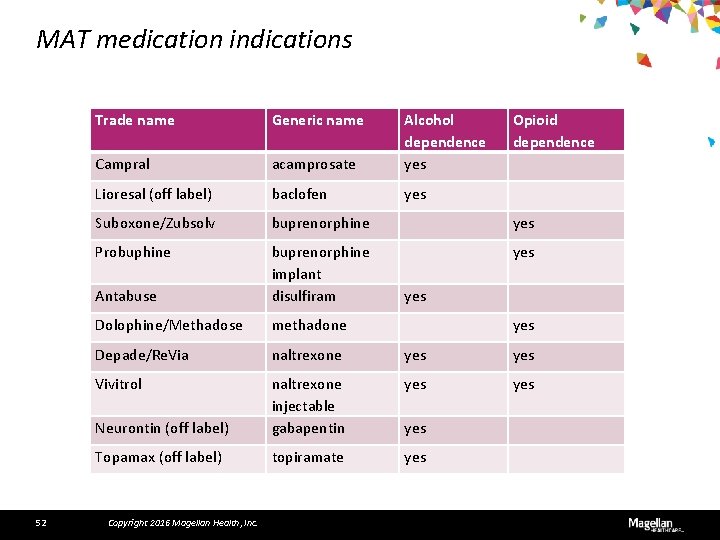
MAT medication indications 52 Trade name Generic name Campral acamprosate Alcohol dependence yes Lioresal (off label) baclofen yes Suboxone/Zubsolv buprenorphine yes Probuphine yes Antabuse buprenorphine implant disulfiram Dolophine/Methadose methadone Depade/Re. Via naltrexone yes Vivitrol yes Neurontin (off label) naltrexone injectable gabapentin Topamax (off label) topiramate yes Copyright 2016 Magellan Health, Inc. Opioid dependence yes yes

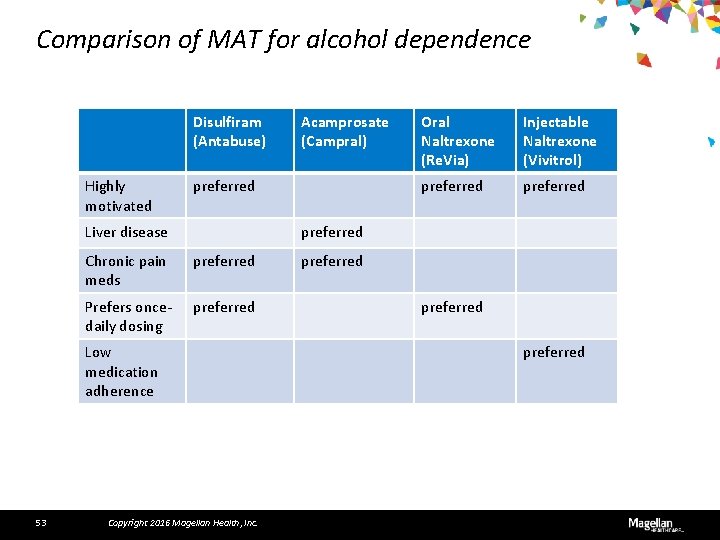
Comparison of MAT for alcohol dependence Disulfiram (Antabuse) Highly motivated preferred Liver disease Oral Naltrexone (Re. Via) Injectable Naltrexone (Vivitrol) preferred Chronic pain meds preferred Prefers oncedaily dosing preferred Low medication adherence 53 Acamprosate (Campral) Copyright 2016 Magellan Health, Inc. preferred

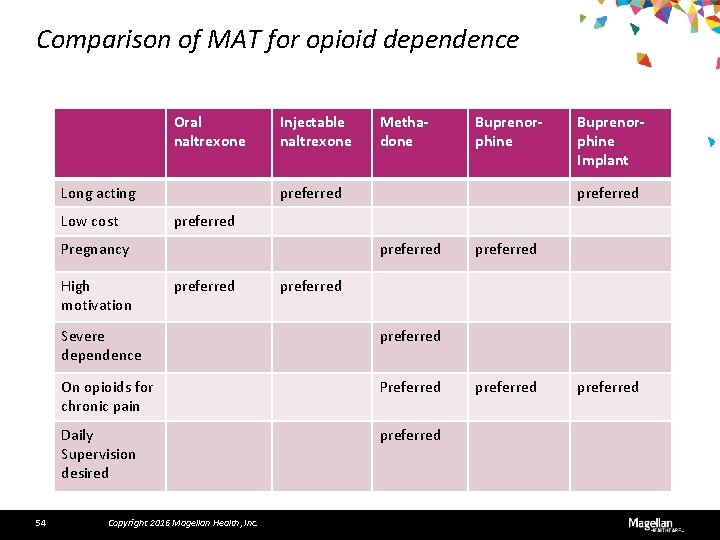
Comparison of MAT for opioid dependence Oral naltrexone Long acting Low cost Injectable naltrexone 54 Buprenorphine preferred Buprenorphine Implant preferred Pregnancy High motivation Methadone preferred Severe dependence preferred On opioids for chronic pain Preferred Daily Supervision desired preferred Copyright 2016 Magellan Health, Inc. preferred

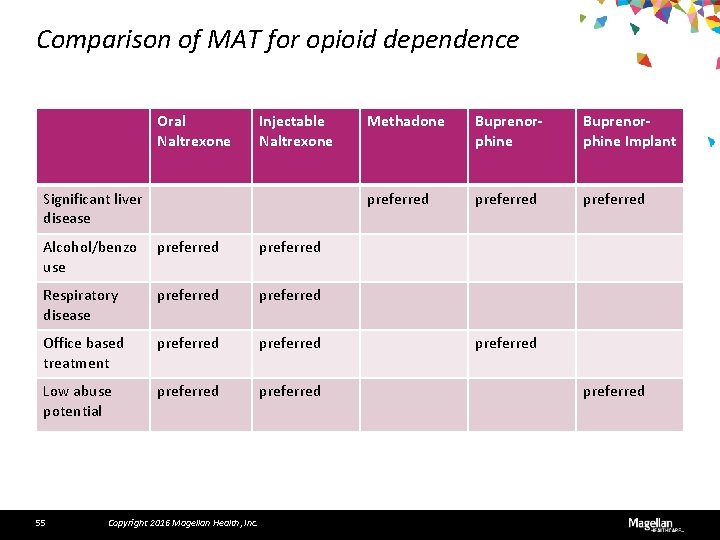
Comparison of MAT for opioid dependence Oral Naltrexone Injectable Naltrexone Significant liver disease Alcohol/benzo use preferred Respiratory disease preferred Office based treatment preferred Low abuse potential preferred 55 Copyright 2016 Magellan Health, Inc. Methadone Buprenorphine Implant preferred preferred

MAT in youth There are limited studies for MAT in adolescents. Again, buprenorphine is the only approved medication for under age 18, and this only for 16 yrs and older Randomized trial of detox v. 12 weeks of buprenorphine (Woody et al, 2008). While both groups had high rates of positive urines: • There were lower rates of positive urines up to week 8 in the buprenorphine group • At week 12 positive urines were 51% in detox group v. 43% in buprenorphine group • Retention in treatment in the buprenorphine group was significantly higher (70% v. 20. 5%) 56 Copyright 2016 Magellan Health, Inc.

MAT in youth Another trial (Marsh et al, 2005) for buprenorphine v. clonidine for detox demonstrated: • Significantly improved retention (72% v. 39%) • Opiate-negative urines (64% v. 32%) • 61% of those in the buprenorphine detox group initiated trial with naltrexone compared to only 5% in the clonidine group A small non-controlled case series of 16 youth (avg. age 18. 5) placed on extended release naltrexone (Vivitrol) showed: • 10 of 16 (63%) were retained in treatment for at least 4 months • Nine of 16 (56%) had decreased opioid use 57 Copyright 2016 Magellan Health, Inc.

Pre-test answer #1 Q. Which of the following is not an FDA approved medication for opioid dependence? Answer: B. Topiramate • Topiramate (Topamax) is an anticonvulsant medication that is sometimes used offlabel for treatment of alcoholism • Zubsolv (buprenorphine/naloxone) is a new FDA approved medication for opioid dependence. (5. 7 mg/1. 4 mg equivalent to 8 mg/2 mg of Suboxone; menthol flavor SL tablet; dissolves more quickly per manufacturer) • Naltrexone is FDA approved for opioid dependence • Vivitrol is a trade name for long acting injectable naltrexone 58 Copyright 2016 Magellan Health, Inc.

Pre-test answer #2 Q. Which of the following are used to treat alcohol dependence? Answer: D. All of the above. FDA-approved medications for alcohol dependence are: • • 59 Disulfiram (Antabuse) Acamprosate (Campral) Oral naltrexone (Re. Via) Long acting injectable naltrexone (Vivitrol) Copyright 2016 Magellan Health, Inc.

Office-based opioid treatment (OBOT) Ideal for patients who find it difficult to attend daily outpatient program and are not able to travel long distances to obtain treatment • Occurs in provider’s office • Prescriptions can be filled at local pharmacy • Buprenorphine (Suboxone®, Subutex®, Zubsolv®) • Naltrexone (Vivitrol®, Re. Via®, Depade®) have better overdosing safety profile than methadone • Suboxone must begin post-detox • Induction can take several days • Extended induction with Suboxone can take 2 -12 weeks on outpatient basis • Patient must be engaged in psychosocial treatments as well • Barrier is lack of sufficient buprenorphine prescribers 63 Copyright 2016 Magellan Health, Inc.

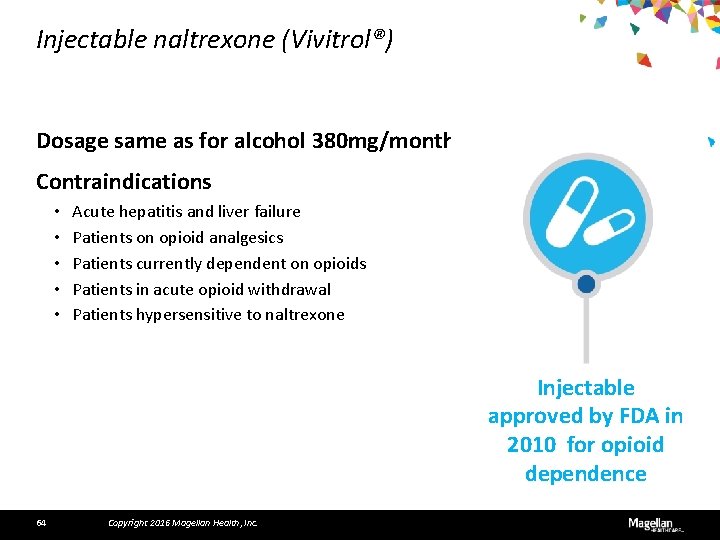
Injectable naltrexone (Vivitrol®) Dosage same as for alcohol 380 mg/month Contraindications • • • Acute hepatitis and liver failure Patients on opioid analgesics Patients currently dependent on opioids Patients in acute opioid withdrawal Patients hypersensitive to naltrexone Injectable approved by FDA in 2010 for opioid dependence 64 Copyright 2016 Magellan Health, Inc.

Vivitrol utilization among Bucks County (PA) Medicaid members In 2015, Magellan did a comparative study of individuals receiving Vivitrol (naltrexone extended-release injection) in outpatient drug and alcohol providers in Bucks County • 82 unduplicated members were prescribed Vivitrol from Jan to Sept 2015 • Compared four age groups: 18 -27; 2844; 45 -64; 65+ 65 Copyright 2016 Magellan Health, Inc.

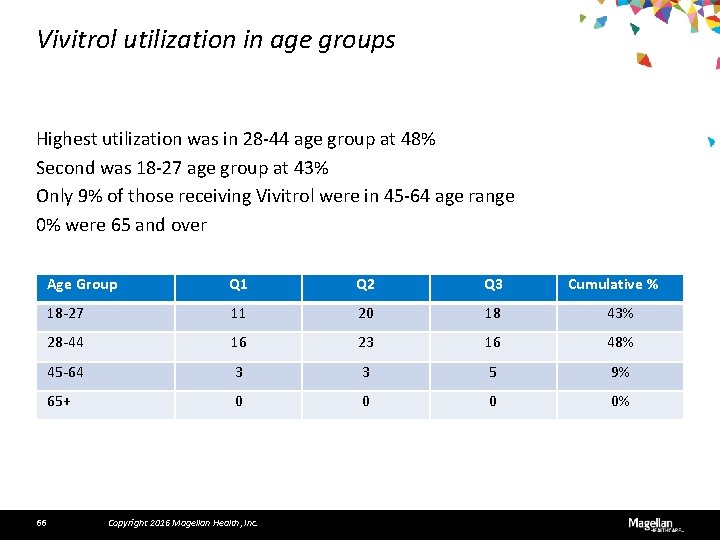
Vivitrol utilization in age groups Highest utilization was in 28 -44 age group at 48% Second was 18 -27 age group at 43% Only 9% of those receiving Vivitrol were in 45 -64 age range 0% were 65 and over 66 Age Group Q 1 Q 2 Q 3 18 -27 11 20 18 43% 28 -44 16 23 16 48% 45 -64 3 3 5 9% 65+ 0 0% Copyright 2016 Magellan Health, Inc. Cumulative %

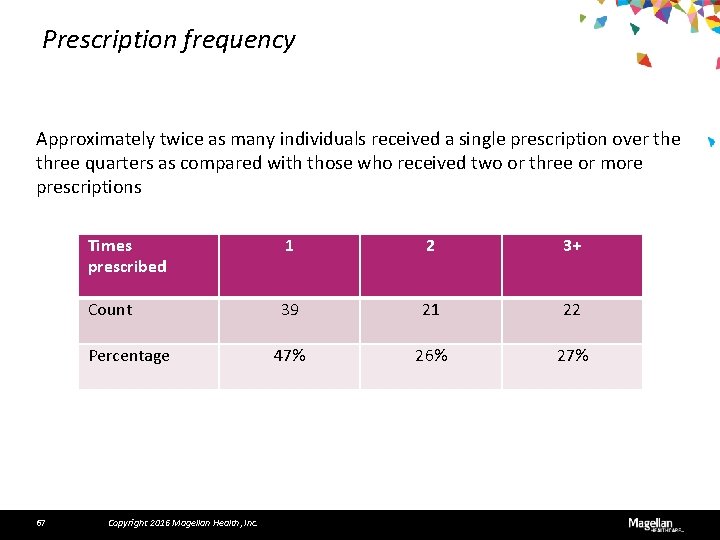
Prescription frequency Approximately twice as many individuals received a single prescription over the three quarters as compared with those who received two or three or more prescriptions Times prescribed 1 2 3+ Count 39 21 22 47% 26% 27% Percentage 67 Copyright 2016 Magellan Health, Inc.

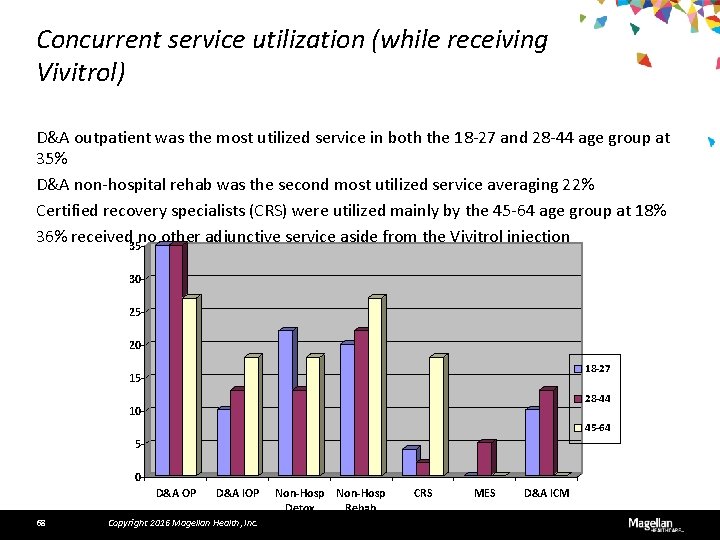
Concurrent service utilization (while receiving Vivitrol) D&A outpatient was the most utilized service in both the 18 -27 and 28 -44 age group at 35% D&A non-hospital rehab was the second most utilized service averaging 22% Certified recovery specialists (CRS) were utilized mainly by the 45 -64 age group at 18% 36% received no other adjunctive service aside from the Vivitrol injection 35 30 25 20 18 -27 15 28 -44 10 45 -64 5 0 D&A OP 68 D&A IOP Copyright 2016 Magellan Health, Inc. Non-Hosp Detox Rehab CRS MES D&A ICM

Improvement opportunities Outcome: Most of the individuals who did not receive adjunctive psychosocial interventions besides the Vivitrol injection were those who received their prescription from a stand-alone prescriber Opportunity: Greater outreach to standalone providers of Vivitrol about other available substance abuse services Outcome: Low utilization of certified recovery specialists (CRS) and mobile engagement specialists (MES) Opportunity: Create greater awareness about CRS and MES to support individuals in their recovery Outcome: Only about half of the individuals who received their first injection did not come for a second or more injections Opportunity: Identify barriers to continuation of Vivitrol after the first injection 69 Copyright 2016 Magellan Health, Inc.

Magellan MAT initiative Expand network of MAT prescribers by actively promoting and campaigning to healthcare providers • Provider and member communications through webinars, newsletters, emails and web • Include MAT expectations in provider handbook and Medical Necessity Criteria • Share research and other educational material postings with providers Intervene early in the treatment process and work closely with both providers and patients Initiate procedures to improve care coordination activities in support of transition of care • Capture use of MAT medications in all systems • Create internal benchmarks for use of MAT and medication guideline for staff • Train clinical and medical staff for peer to peer discussions to increase use of MAT • Develop outcome measures (increase in use of MAT medications and re-admission data) National quality improvement study for NCQA 71 Copyright 2016 Magellan Health, Inc.

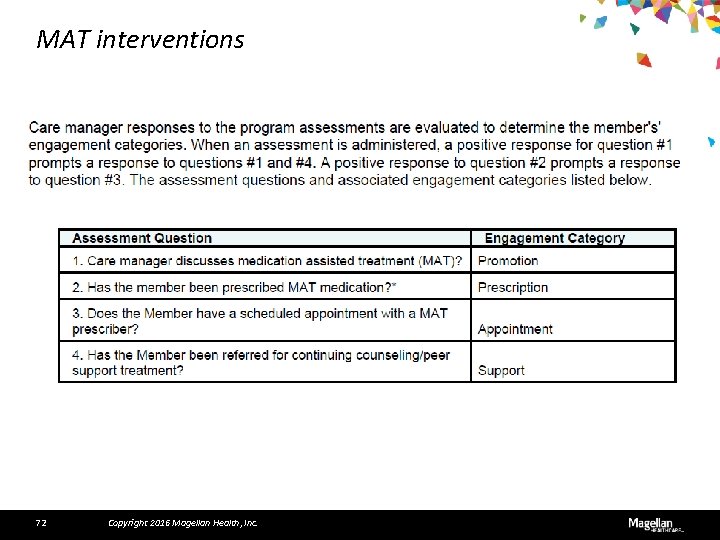
MAT interventions 72 Copyright 2016 Magellan Health, Inc.

Use of MAT still needs improvement During the first quarter of 2016, our care managers reached out to almost 13, 000 members and their care providers nationally to encourage the use of MAT. Of these, 8, 343 had an opiate use disorder, or opiate use disorder combined with other drugs or alcohol, or were also diagnosed with a mental health condition. Only 1, 154 or 13. 8 percent received prescriptions for MAT medications at discharge.

Key characteristics, predictors and behaviors associated with short-term and persistent prescription opioid use A three-year study of a commercial health plan population

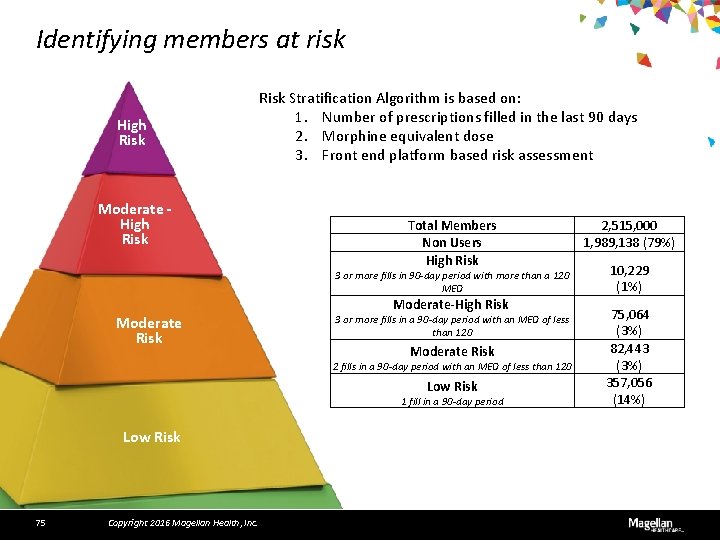
Identifying members at risk High Risk Moderate - High Risk Stratification Algorithm is based on: 1. Number of prescriptions filled in the last 90 days 2. Morphine equivalent dose 3. Front end platform based risk assessment Total Members Non Users High Risk 3 or more fills in 90 -day period with more than a 120 MED Moderate Risk Moderate-High Risk 3 or more fills in a 90 -day period with an MED of less than 120 Moderate Risk 2 fills in a 90 -day period with an MED of less than 120 Low Risk 1 fill in a 90 -day period Low Risk 75 Copyright 2016 Magellan Health, Inc. 2, 515, 000 1, 989, 138 (79%) 10, 229 (1%) 75, 064 (3%) 82, 443 (3%) 357, 056 (14%)

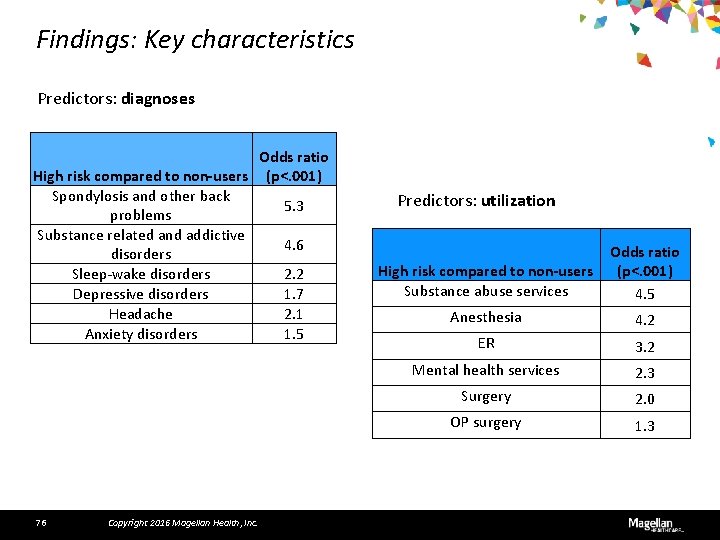
Findings: Key characteristics Predictors: diagnoses Odds ratio High risk compared to non-users (p<. 001) Spondylosis and other back 5. 3 problems Substance related and addictive 4. 6 disorders Sleep-wake disorders 2. 2 Depressive disorders 1. 7 Headache 2. 1 Anxiety disorders 1. 5 76 Copyright 2016 Magellan Health, Inc. Predictors: utilization High risk compared to non-users Substance abuse services Odds ratio (p<. 001) 4. 5 Anesthesia 4. 2 ER 3. 2 Mental health services 2. 3 Surgery 2. 0 OP surgery 1. 3

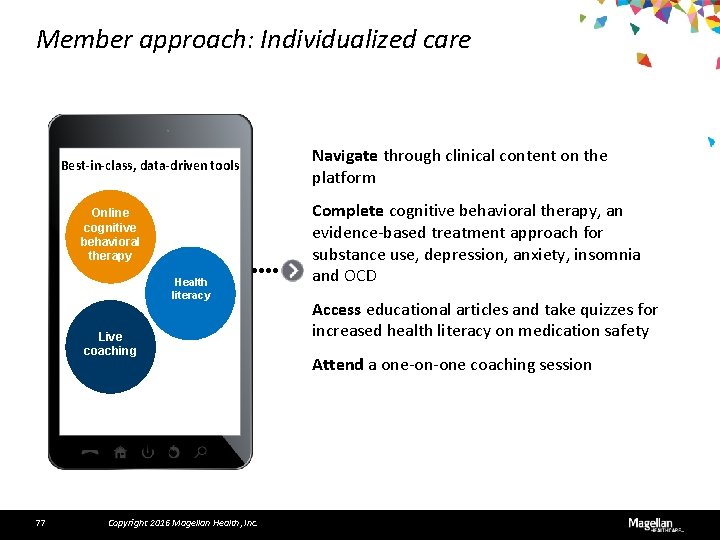
Member approach: Individualized care Best-in-class, data-driven tools Online cognitive behavioral therapy Health literacy Live coaching 77 Copyright 2016 Magellan Health, Inc. Navigate through clinical content on the platform Complete cognitive behavioral therapy, an evidence-based treatment approach for substance use, depression, anxiety, insomnia and OCD Access educational articles and take quizzes for increased health literacy on medication safety Attend a one-on-one coaching session

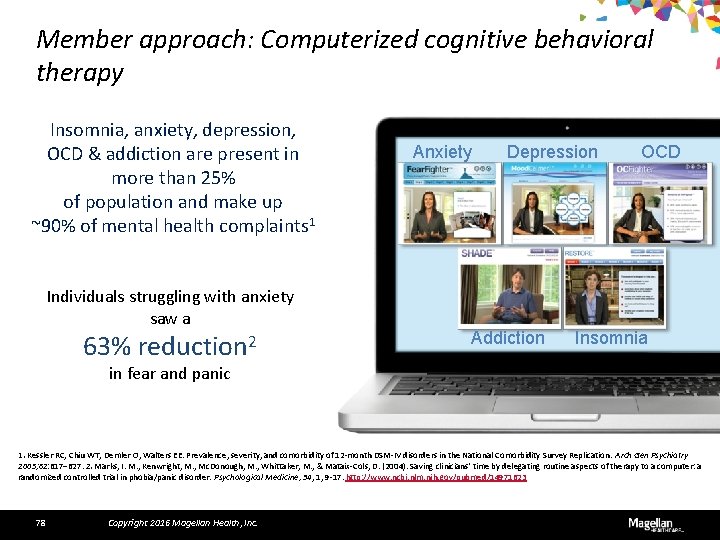
Member approach: Computerized cognitive behavioral therapy Insomnia, anxiety, depression, OCD & addiction are present in more than 25% of population and make up ~90% of mental health complaints 1 Individuals struggling with anxiety saw a 63% reduction 2 Anxiety Depression Addiction OCD Insomnia in fear and panic 1. Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12 -month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62: 617– 627. 2. Marks, I. M. , Kenwright, M. , Mc. Donough, M. , Whittaker, M. , & Mataix-Cols, D. (2004). Saving clinicians' time by delegating routine aspects of therapy to a computer: a randomized controlled trial in phobia/panic disorder. Psychological Medicine, 34, 1, 9 -17. http: //www. ncbi. nlm. nih. gov/pubmed/14971623 78 Copyright 2016 Magellan Health, Inc.

Member approach: Health coaching Key services • Coaching for specialty case management • Integration of behavioral and physical health methodologies • Strengths-based motivational interviewing approach • Expert approach to case management vs. generalist approach • Case managers and health coaches specially trained in prescription drug abuse and pain conditions Key features • Ability to service multiple time zones 79 Copyright 2016 Magellan Health, Inc.

Provider approach Provide a high-level program overview, including specific member case and identifying triggers Review and focus on best practices and recommendations to help minimize risk • Utilize state PMP program o May authorize delegate access to members of staff if provider is unable to check due to time constraints o State PMP data contains schedules II, III, and IV controlled substances, and opioids account for more than half of all prescriptions • Recurring random urine drugs tests including ‘no threshold’ testing • Utilize “do not fill until” dates on all opioid prescriptions • Utilize pain management agreements with an emphasis on o Using one pharmacy for all medications o Notification to treating provider if/when prescribed opioids from other providers • Underscore importance of safe storage of medications and disposal when no longer needed • Tamper-resistant formulations (if appropriate based on formulary) • Non-drug or non-opioid interventions when feasible (i. e. physical therapy) • Patient medication diary and/or pill counts Determine additional resources needed, including referrals (if appropriate) • Psychiatrist, pain management specialist, case management • Locking patients into one pharmacy Contact dispensing pharmacies (if appropriate) 80 Copyright 2016 Magellan Health, Inc.

Bibliography Drugs, Brain and Behavior: The Science of Addiction. National Institute on Drug Abuse. Washington, 2014 Howlett, KD, Williams, T et al. Understanding and Treating Adolescent Substance Abuse: A Preliminary Review. FOCUS. Summer 2012, vol X, No 3. 293 -299. Roten, AT, Gray, KM. Adolescent Substance Use Disroders: Epidemiology, Neurobiology, and Screening in The American Psychiatric Publishing Textbook of Substance Abuse Treatment. Washington, DC. 2015. 745751. American Psychiatric Association: manual of Clinical Psychopharmacology. American Psychiatric Publishing, Inc. 2010 Practice Guideline for the treatment of patients with substance use disorders, 2 nd edition. American Journal of Psychiatry 2006; 164 -suppl A 5 -A 124. Sigmon SC, Dunn KE, et al. A randomized, double-blind evaluation of buprenorphine taper duration in primary prescription opioid abusers. JAMA Psychiatry. 2013; 70(12): 1347 -1354. Albright J, Ciaverelli R. , et al. Psychiatrist characteristics that influence use of buprenorphine medicationassisted treatment. J Addict Med. 2010; 4(4); 197 -203. Reutsch, C. Treating Presciription Opiate Dependence. JAMA Psychiatry. 2013; 70(12); 1347 -1354 81 Copyright 2016 Magellan Health, Inc.

Bibliography Magellan Vivitrol Study for Bucks County, 2015 FDA News Release, May 26, 2016: FDA approves first buprenorphine implant for treatment of opioid dependence Bucks County Transition-Age Drug & Alcohol Treatment Survey, 2016, Voice & Vision, Inc. Clinical Use of Extended-Release Injectable Naltrexone in the Treatment of Opioid Use Disorder: A Brief Guide, SAMSHA National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use, ASAM, 2015 VA-Do. D Clinical Practice Guideline for Management of Substance Use Disorders (CPG-SUD), 2015 Extended vs. Short-term Buprenorphine-Naloxone for Treatment of Opioid-Addicted Youth: A Randomized Trial; Woody, GE et al, JAMA. 2008; 300(17): 2003 -2011 Comparison of Pharmacological Treatments for Opioid-Dependent Adolescents, A Randomized Controlled Trial, Marsh, Lisa et al, ARCH GEN PSYCHIATRY/VOL 62, OCT 2005 Treatment of opioid dependence in adolescents and young adults with extended release naltrexone: preliminary case-series and feasibility; Fishman, MJ; Addiction, Volume 105, Issue 9, September 2010 82 Copyright 2016 Magellan Health, Inc.

Confidential information By receipt of this presentation, each recipient agrees that the information contained herein will be kept confidential and that the information will not be photocopied, reproduced, or distributed to or disclosed to others at any time without the prior written consent of Magellan Health, Inc. The information contained in this presentation is intended for educational purposes only and is not intended to define a standard of care or exclusive course of treatment, nor be a substitute for treatment. 83 Copyright 2016 Magellan Health, Inc.

Questions?
 Medicationassisted treatment market
Medicationassisted treatment market Herzreizleitungsstörung
Herzreizleitungsstörung Treatment of substance use disorder
Treatment of substance use disorder Kontinuitetshantering i praktiken
Kontinuitetshantering i praktiken Novell typiska drag
Novell typiska drag Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Returpilarna
Returpilarna Varför kallas perioden 1918-1939 för mellankrigstiden?
Varför kallas perioden 1918-1939 för mellankrigstiden? En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Adressändring ideell förening
Adressändring ideell förening Vilotidsbok
Vilotidsbok Anatomi organ reproduksi
Anatomi organ reproduksi Förklara densitet för barn
Förklara densitet för barn Datorkunskap för nybörjare
Datorkunskap för nybörjare Stig kerman
Stig kerman Debattartikel struktur
Debattartikel struktur Magnetsjukhus
Magnetsjukhus Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Kraft per area
Kraft per area Svenskt ramverk för digital samverkan
Svenskt ramverk för digital samverkan Urban torhamn
Urban torhamn Presentera för publik crossboss
Presentera för publik crossboss Vad är ett minoritetsspråk
Vad är ett minoritetsspråk Plats för toran ark
Plats för toran ark Klassificeringsstruktur för kommunala verksamheter
Klassificeringsstruktur för kommunala verksamheter Luftstrupen för medicinare
Luftstrupen för medicinare Bästa kameran för astrofoto
Bästa kameran för astrofoto Cks
Cks Lågenergihus nyproduktion
Lågenergihus nyproduktion Mat för idrottare
Mat för idrottare Verktyg för automatisering av utbetalningar
Verktyg för automatisering av utbetalningar Rutin för avvikelsehantering
Rutin för avvikelsehantering Smärtskolan kunskap för livet
Smärtskolan kunskap för livet Ministerstyre för och nackdelar
Ministerstyre för och nackdelar Tack för att ni har lyssnat
Tack för att ni har lyssnat Vad är referatmarkeringar
Vad är referatmarkeringar Redogör för vad psykologi är
Redogör för vad psykologi är Stål för stötfångarsystem
Stål för stötfångarsystem Atmosfr
Atmosfr Borra hål för knoppar
Borra hål för knoppar Vilken grundregel finns det för tronföljden i sverige?
Vilken grundregel finns det för tronföljden i sverige? Fr formel
Fr formel Tack för att ni har lyssnat
Tack för att ni har lyssnat Rita perspektiv
Rita perspektiv Verksamhetsanalys exempel
Verksamhetsanalys exempel Tobinskatten för och nackdelar
Tobinskatten för och nackdelar Toppslätskivling dos
Toppslätskivling dos Gibbs reflekterande cykel
Gibbs reflekterande cykel Egg för emanuel
Egg för emanuel Elektronik för barn
Elektronik för barn Plagg i rom
Plagg i rom Strategi för svensk viltförvaltning
Strategi för svensk viltförvaltning Kung som dog 1611
Kung som dog 1611 Ellika andolf
Ellika andolf Sju för caesar
Sju för caesar Tack för att ni lyssnade
Tack för att ni lyssnade Samlade siffror för tryck
Samlade siffror för tryck Korta dikter som rimmar
Korta dikter som rimmar Inköpsprocessen steg för steg
Inköpsprocessen steg för steg Fuktmätningar i betong enlig rbk
Fuktmätningar i betong enlig rbk Ledarskapsteorier
Ledarskapsteorier Expektans
Expektans Myndigheten för delaktighet
Myndigheten för delaktighet Frgar
Frgar Sju principer för tillitsbaserad styrning
Sju principer för tillitsbaserad styrning Läkarutlåtande för livränta
Läkarutlåtande för livränta Karttecken brun triangel
Karttecken brun triangel Gumman cirkel
Gumman cirkel Shaktismen
Shaktismen Biologiska arvet
Biologiska arvet Bris för vuxna
Bris för vuxna Jätte råtta
Jätte råtta How you use ict today and how you will use it tomorrow
How you use ict today and how you will use it tomorrow Young and dyslexic analysis
Young and dyslexic analysis Us prior to brown map key
Us prior to brown map key Trauma awareness and treatment center utah
Trauma awareness and treatment center utah Julie woodside
Julie woodside