ELECTROLYSIS A guide for GCSE students 2010 KNOCKHARDY
































- Slides: 98

ELECTROLYSIS A guide for GCSE students 2010 KNOCKHARDY PUBLISHING SPECIFICATIONS

ELECTROLYSIS INTRODUCTION This Powerpoint show is one of several produced to help students understand selected GCSE Chemistry topics. It is based on the requirements of the AQA specification but is suitable for other examination boards. Individual students may use the material at home for revision purposes and it can also prove useful for classroom teaching with an interactive white board. Accompanying notes on this, and the full range of AS and A 2 Chemistry topics, are available from the KNOCKHARDY WEBSITE at. . . www. knockhardy. org. uk All diagrams, photographs and any animations in this Powerpoint are original and created by Jonathan Hopton. Permission must be obtained for their use in any work that is distributed for financial gain.

ELECTROLYSIS CONTENTS • What is electrolysis? • Electrolysis of ionic compounds • Electrolysis of molten sodium chloride • Electrolysis of aqueous sodium chloride (brine) • Diaphragm cell • Electrolysis of aluminium oxide • Other electrolytic systems • Electrolysis of water

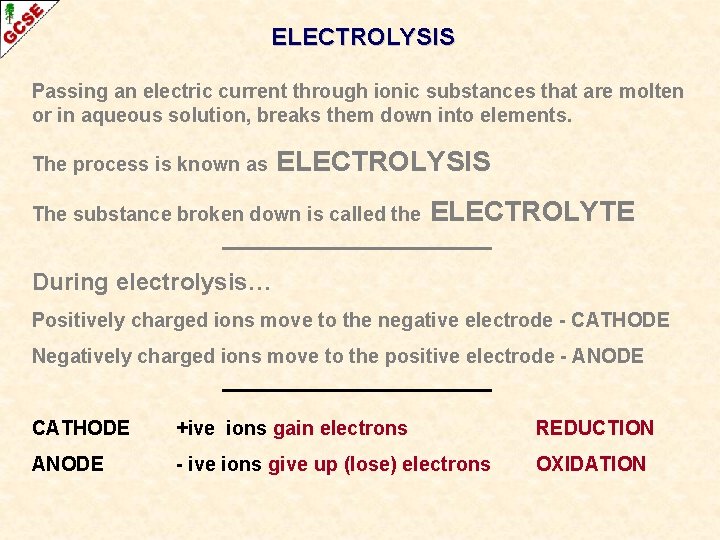
ELECTROLYSIS Passing an electric current through ionic substances that are molten or in aqueous solution, breaks them down into elements.

ELECTROLYSIS Passing an electric current through ionic substances that are molten or in aqueous solution, breaks them down into elements. The process is known as ELECTROLYSIS

ELECTROLYSIS Passing an electric current through ionic substances that are molten or in aqueous solution, breaks them down into elements. The process is known as ELECTROLYSIS The substance broken down is called the ELECTROLYTE

ELECTROLYSIS Passing an electric current through ionic substances that are molten or in aqueous solution, breaks them down into elements. The process is known as ELECTROLYSIS The substance broken down is called the ELECTROLYTE During electrolysis… Positively charged ions move to the negative electrode - CATHODE Negatively charged ions move to the positive electrode - ANODE

ELECTROLYSIS Passing an electric current through ionic substances that are molten or in aqueous solution, breaks them down into elements. The process is known as ELECTROLYSIS The substance broken down is called the ELECTROLYTE During electrolysis… Positively charged ions move to the negative electrode - CATHODE Negatively charged ions move to the positive electrode - ANODE CATHODE +ive ions gain electrons REDUCTION ANODE - ive ions give up (lose) electrons OXIDATION

ELECTROLYSIS OF IONIC COMPOUNDS

CONDUCTING ELECTRICITY

CONDUCTING ELECTRICITY For a substance to conduct electricity it must have either…

CONDUCTING ELECTRICITY For a substance to conduct electricity it must have either… electrons which are free to move about (metals / graphite)

CONDUCTING ELECTRICITY For a substance to conduct electricity it must have either… electrons which are free to move about or ions which are free to move about (metals / graphite)

CONDUCTING ELECTRICITY For a substance to conduct electricity it must have either… electrons which are free to move about or (metals / graphite) ions which are free to move about When an ionic substance is melted or dissolved in water, the ions are free to move about within the liquid or solution.

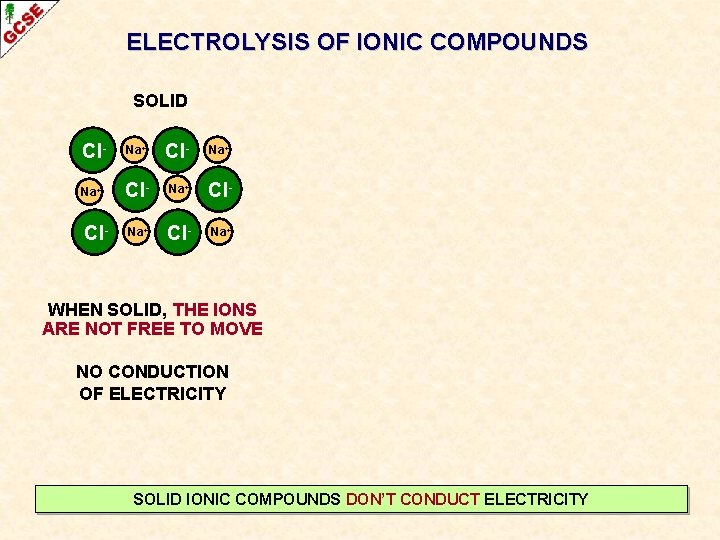
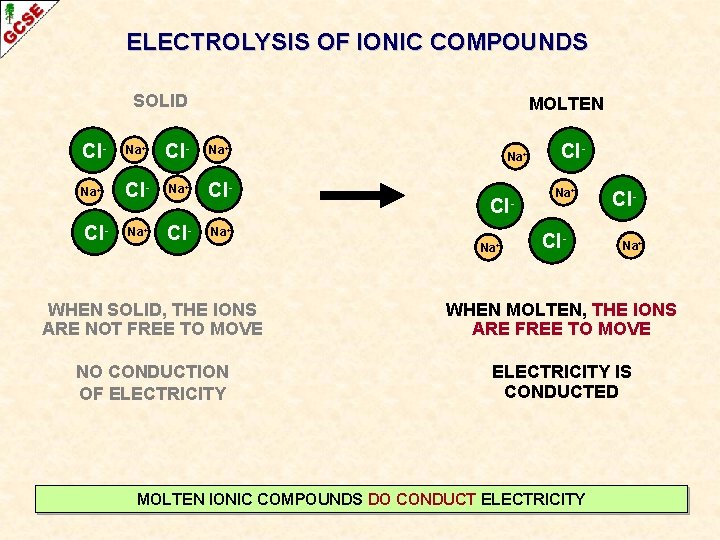
ELECTROLYSIS OF IONIC COMPOUNDS SOLID Cl- Na+ Na+ Cl- Cl- Na+ WHEN SOLID, THE IONS ARE NOT FREE TO MOVE NO CONDUCTION OF ELECTRICITY SOLID IONIC COMPOUNDS DON’T CONDUCT ELECTRICITY

ELECTROLYSIS OF IONIC COMPOUNDS SOLID MOLTEN Cl- Na+ Na+ Cl- Cl. Na+ WHEN SOLID, THE IONS ARE NOT FREE TO MOVE WHEN MOLTEN, THE IONS ARE FREE TO MOVE NO CONDUCTION OF ELECTRICITY IS CONDUCTED MOLTEN IONIC COMPOUNDS DO CONDUCT ELECTRICITY

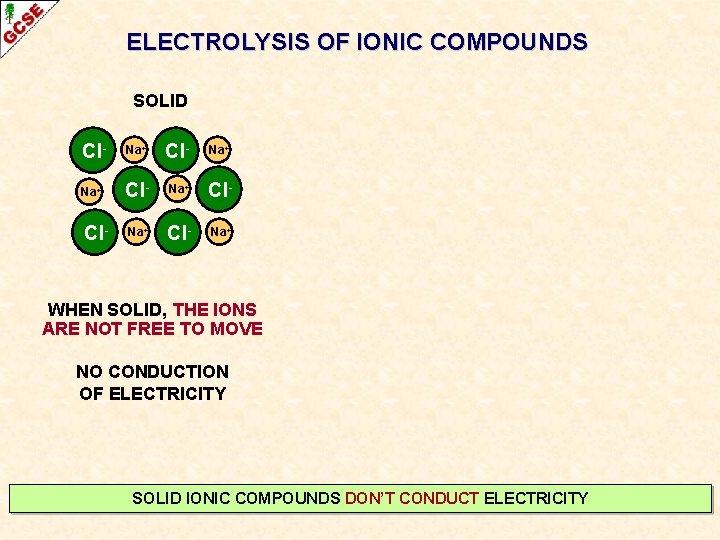
ELECTROLYSIS OF IONIC COMPOUNDS SOLID Cl- Na+ Na+ Cl- Cl- Na+ WHEN SOLID, THE IONS ARE NOT FREE TO MOVE NO CONDUCTION OF ELECTRICITY SOLID IONIC COMPOUNDS DON’T CONDUCT ELECTRICITY

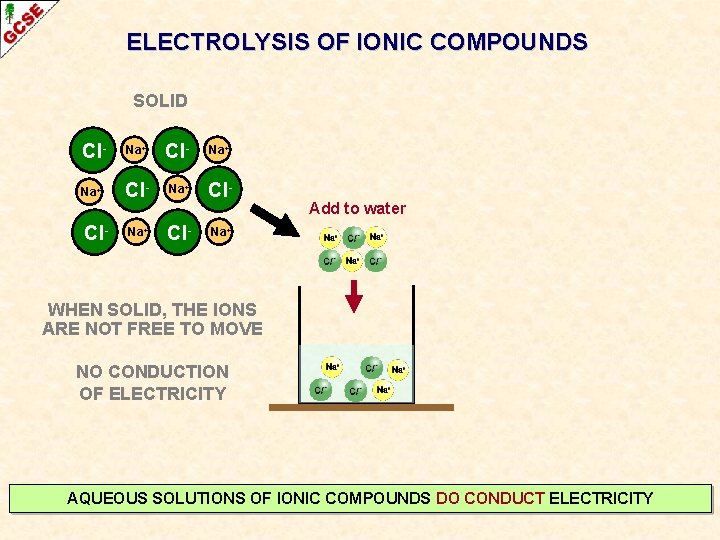
ELECTROLYSIS OF IONIC COMPOUNDS SOLID Cl- Na+ Na+ Cl- Add to water Na+ WHEN SOLID, THE IONS ARE NOT FREE TO MOVE NO CONDUCTION OF ELECTRICITY AQUEOUS SOLUTIONS OF IONIC COMPOUNDS DO CONDUCT ELECTRICITY

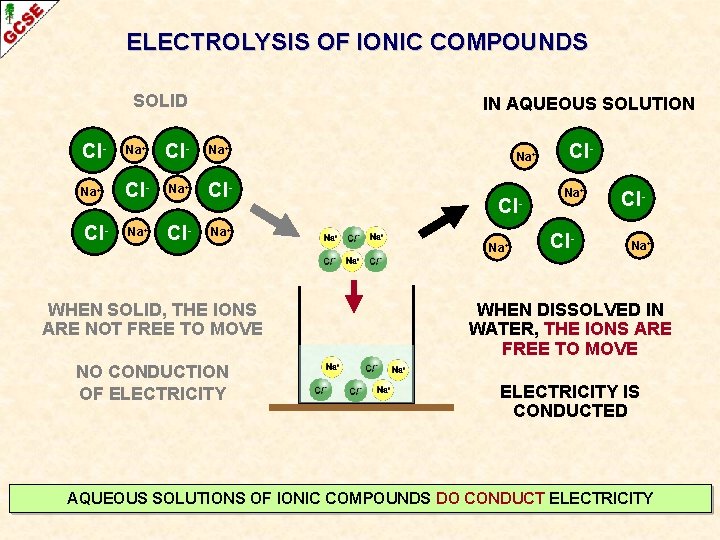
ELECTROLYSIS OF IONIC COMPOUNDS SOLID IN AQUEOUS SOLUTION Cl- Na+ Na+ Cl- Cl- Na+ WHEN SOLID, THE IONS ARE NOT FREE TO MOVE NO CONDUCTION OF ELECTRICITY Na+ Cl- Cl. Na+ WHEN DISSOLVED IN WATER, THE IONS ARE FREE TO MOVE ELECTRICITY IS CONDUCTED AQUEOUS SOLUTIONS OF IONIC COMPOUNDS DO CONDUCT ELECTRICITY

ELECTROLYSIS OF MOLTEN SODIUM CHLORIDE

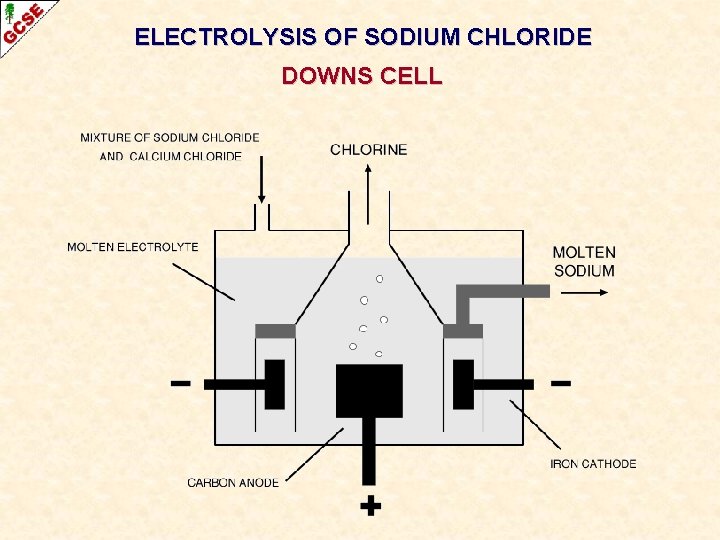
ELECTROLYSIS OF SODIUM CHLORIDE DOWNS CELL

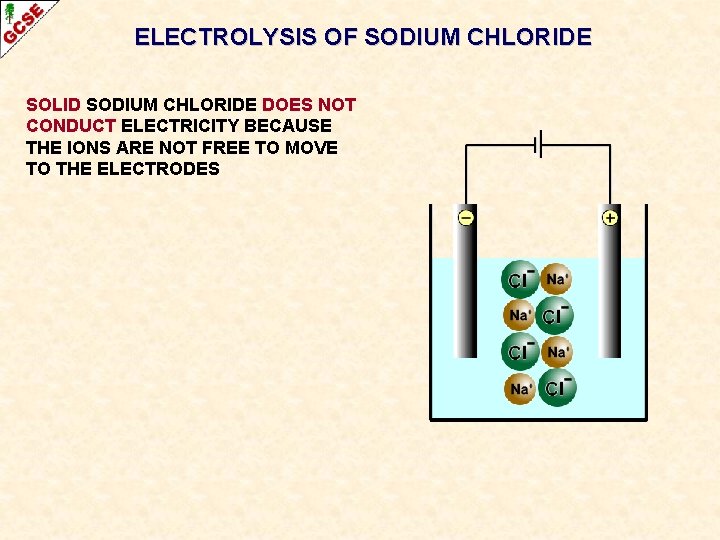
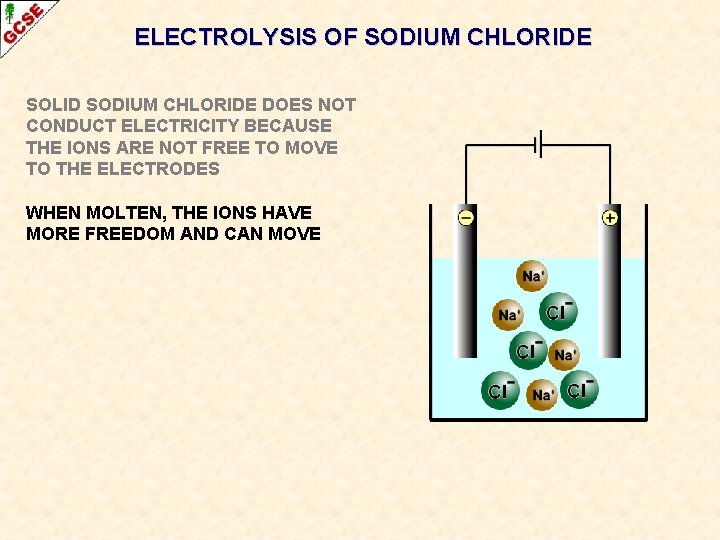
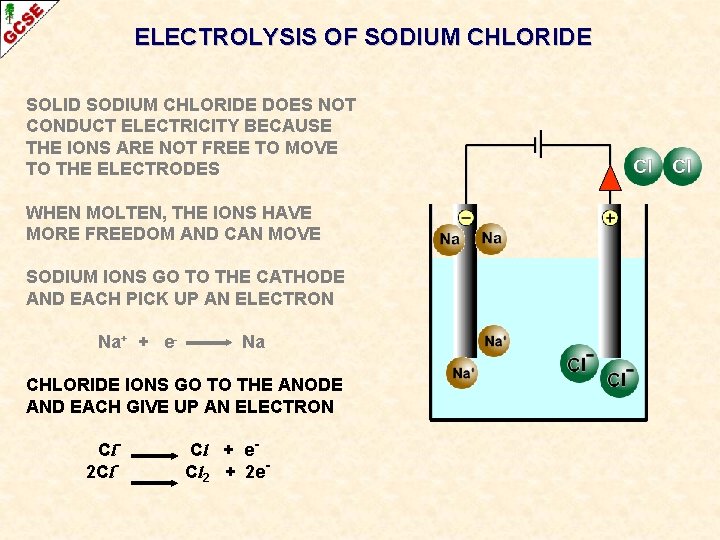
ELECTROLYSIS OF SODIUM CHLORIDE SOLID SODIUM CHLORIDE DOES NOT CONDUCT ELECTRICITY BECAUSE THE IONS ARE NOT FREE TO MOVE TO THE ELECTRODES

ELECTROLYSIS OF SODIUM CHLORIDE SOLID SODIUM CHLORIDE DOES NOT CONDUCT ELECTRICITY BECAUSE THE IONS ARE NOT FREE TO MOVE TO THE ELECTRODES WHEN MOLTEN, THE IONS HAVE MORE FREEDOM AND CAN MOVE

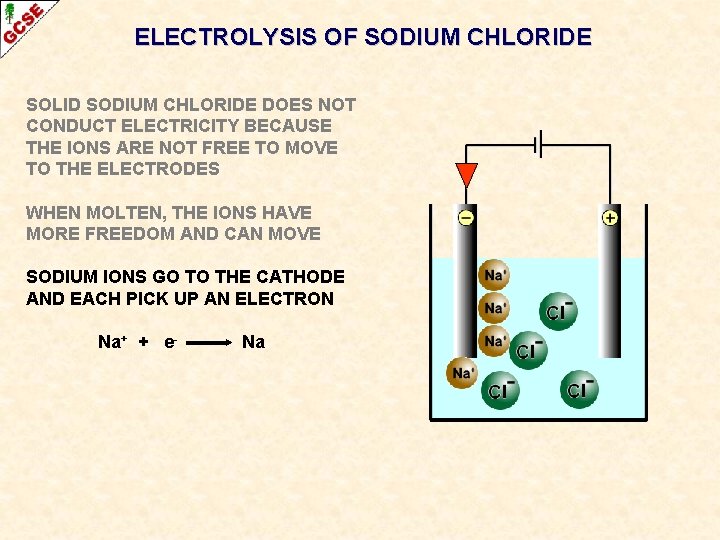
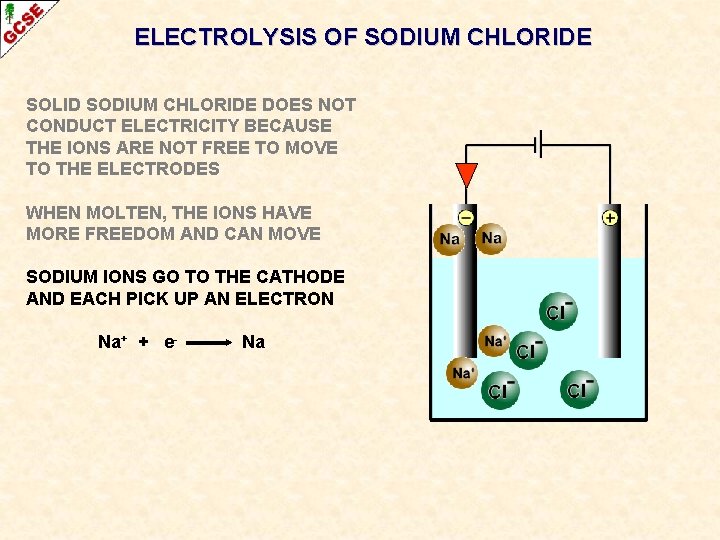
ELECTROLYSIS OF SODIUM CHLORIDE SOLID SODIUM CHLORIDE DOES NOT CONDUCT ELECTRICITY BECAUSE THE IONS ARE NOT FREE TO MOVE TO THE ELECTRODES WHEN MOLTEN, THE IONS HAVE MORE FREEDOM AND CAN MOVE SODIUM IONS GO TO THE CATHODE AND EACH PICK UP AN ELECTRON Na+ + e- Na

ELECTROLYSIS OF SODIUM CHLORIDE SOLID SODIUM CHLORIDE DOES NOT CONDUCT ELECTRICITY BECAUSE THE IONS ARE NOT FREE TO MOVE TO THE ELECTRODES WHEN MOLTEN, THE IONS HAVE MORE FREEDOM AND CAN MOVE SODIUM IONS GO TO THE CATHODE AND EACH PICK UP AN ELECTRON Na+ + e- Na

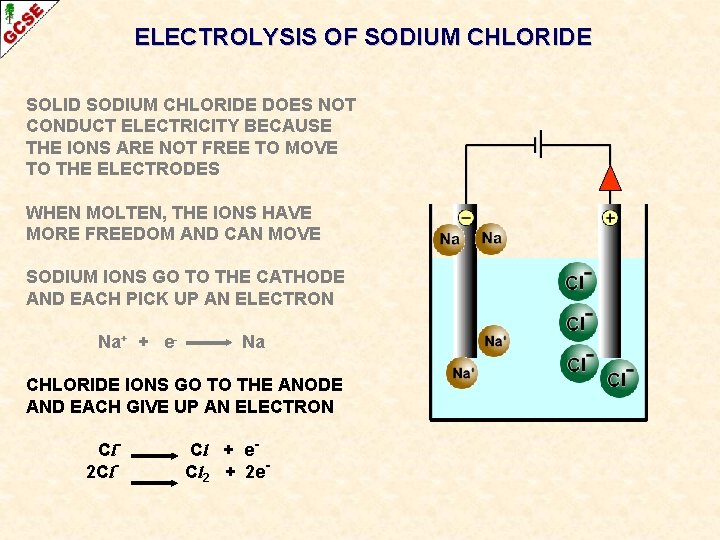
ELECTROLYSIS OF SODIUM CHLORIDE SOLID SODIUM CHLORIDE DOES NOT CONDUCT ELECTRICITY BECAUSE THE IONS ARE NOT FREE TO MOVE TO THE ELECTRODES WHEN MOLTEN, THE IONS HAVE MORE FREEDOM AND CAN MOVE SODIUM IONS GO TO THE CATHODE AND EACH PICK UP AN ELECTRON Na+ + e- Na CHLORIDE IONS GO TO THE ANODE AND EACH GIVE UP AN ELECTRON Cl 2 Cl- Cl + e. Cl 2 + 2 e-

ELECTROLYSIS OF SODIUM CHLORIDE SOLID SODIUM CHLORIDE DOES NOT CONDUCT ELECTRICITY BECAUSE THE IONS ARE NOT FREE TO MOVE TO THE ELECTRODES WHEN MOLTEN, THE IONS HAVE MORE FREEDOM AND CAN MOVE SODIUM IONS GO TO THE CATHODE AND EACH PICK UP AN ELECTRON Na+ + e- Na CHLORIDE IONS GO TO THE ANODE AND EACH GIVE UP AN ELECTRON Cl 2 Cl- Cl + e. Cl 2 + 2 e-

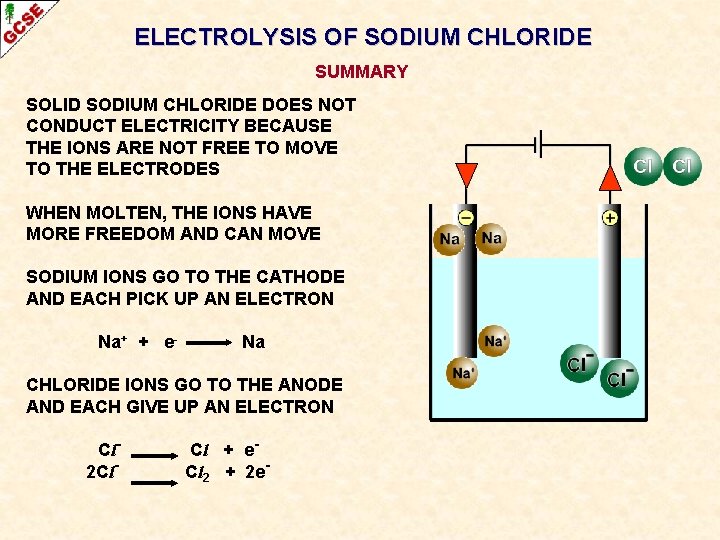
ELECTROLYSIS OF SODIUM CHLORIDE SUMMARY SOLID SODIUM CHLORIDE DOES NOT CONDUCT ELECTRICITY BECAUSE THE IONS ARE NOT FREE TO MOVE TO THE ELECTRODES WHEN MOLTEN, THE IONS HAVE MORE FREEDOM AND CAN MOVE SODIUM IONS GO TO THE CATHODE AND EACH PICK UP AN ELECTRON Na+ + e- Na CHLORIDE IONS GO TO THE ANODE AND EACH GIVE UP AN ELECTRON Cl 2 Cl- Cl + e. Cl 2 + 2 e-

ELECTROLYSIS OF AQUEOUS SODIUM CHLORIDE (BRINE)

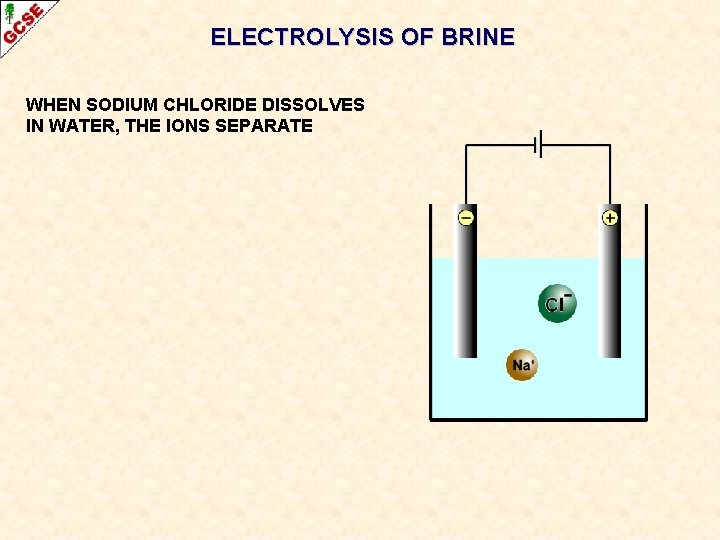
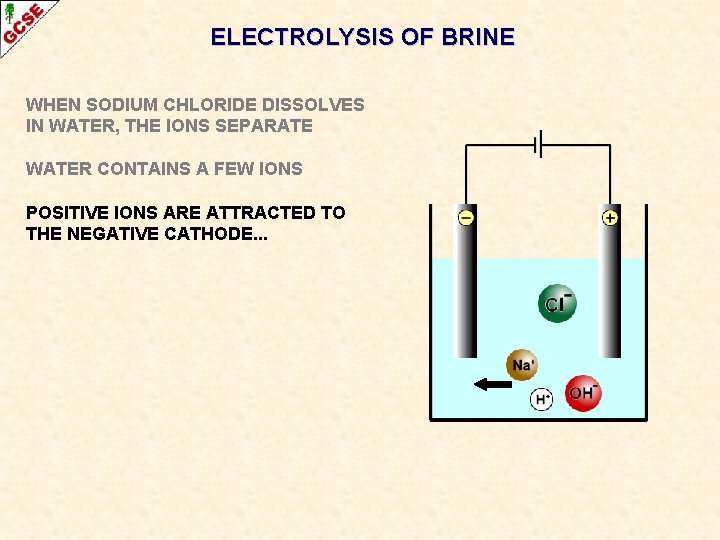
ELECTROLYSIS OF BRINE WHEN SODIUM CHLORIDE DISSOLVES IN WATER, THE IONS SEPARATE

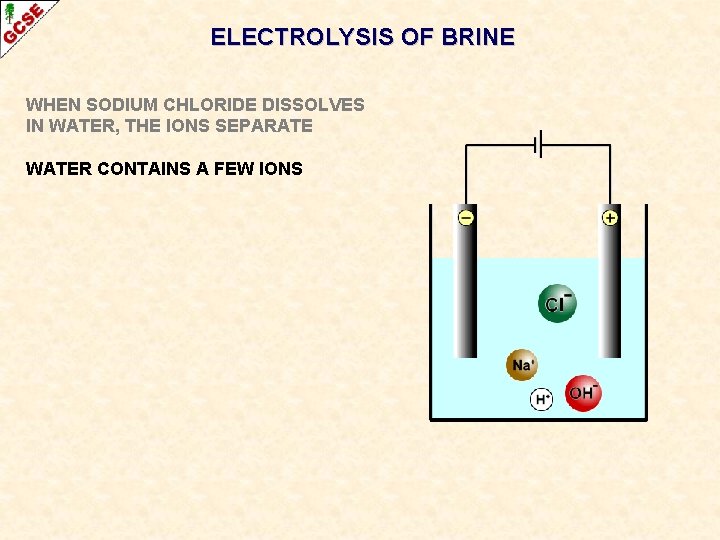
ELECTROLYSIS OF BRINE WHEN SODIUM CHLORIDE DISSOLVES IN WATER, THE IONS SEPARATE WATER CONTAINS A FEW IONS

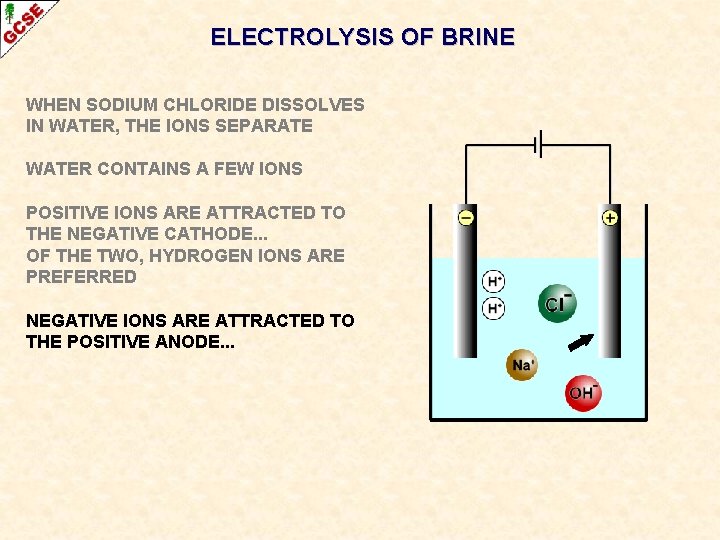
ELECTROLYSIS OF BRINE WHEN SODIUM CHLORIDE DISSOLVES IN WATER, THE IONS SEPARATE WATER CONTAINS A FEW IONS POSITIVE IONS ARE ATTRACTED TO THE NEGATIVE CATHODE. . .

ELECTROLYSIS OF BRINE WHEN SODIUM CHLORIDE DISSOLVES IN WATER, THE IONS SEPARATE WATER CONTAINS A FEW IONS POSITIVE IONS ARE ATTRACTED TO THE NEGATIVE CATHODE. . . OF THE TWO, HYDROGEN IONS ARE PREFERRED

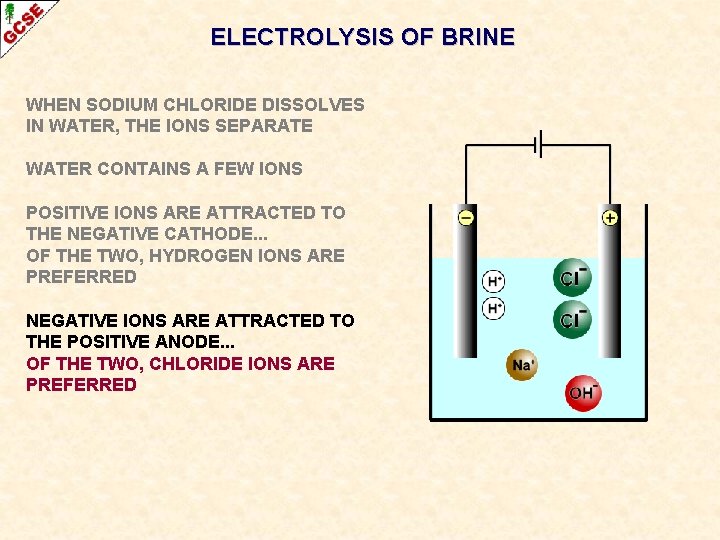
ELECTROLYSIS OF BRINE WHEN SODIUM CHLORIDE DISSOLVES IN WATER, THE IONS SEPARATE WATER CONTAINS A FEW IONS POSITIVE IONS ARE ATTRACTED TO THE NEGATIVE CATHODE. . . OF THE TWO, HYDROGEN IONS ARE PREFERRED NEGATIVE IONS ARE ATTRACTED TO THE POSITIVE ANODE. . .

ELECTROLYSIS OF BRINE WHEN SODIUM CHLORIDE DISSOLVES IN WATER, THE IONS SEPARATE WATER CONTAINS A FEW IONS POSITIVE IONS ARE ATTRACTED TO THE NEGATIVE CATHODE. . . OF THE TWO, HYDROGEN IONS ARE PREFERRED NEGATIVE IONS ARE ATTRACTED TO THE POSITIVE ANODE. . . OF THE TWO, CHLORIDE IONS ARE PREFERRED

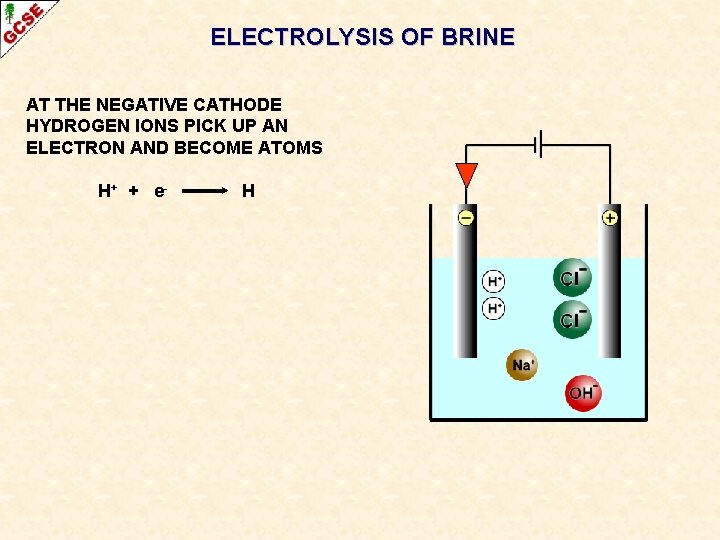
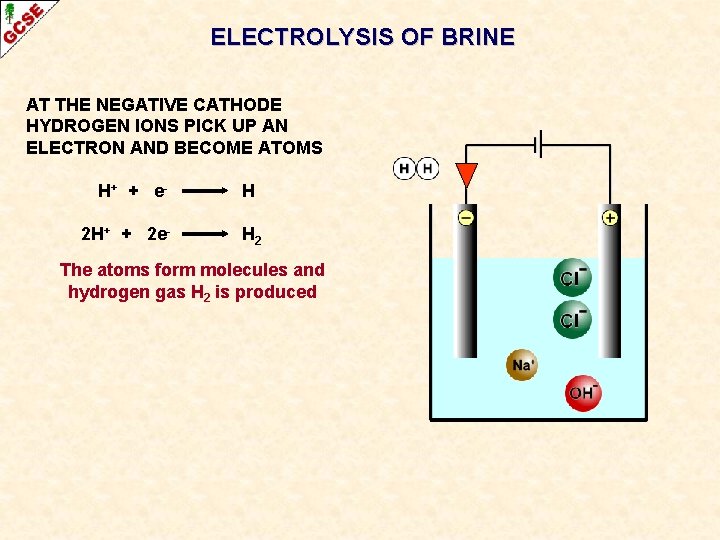
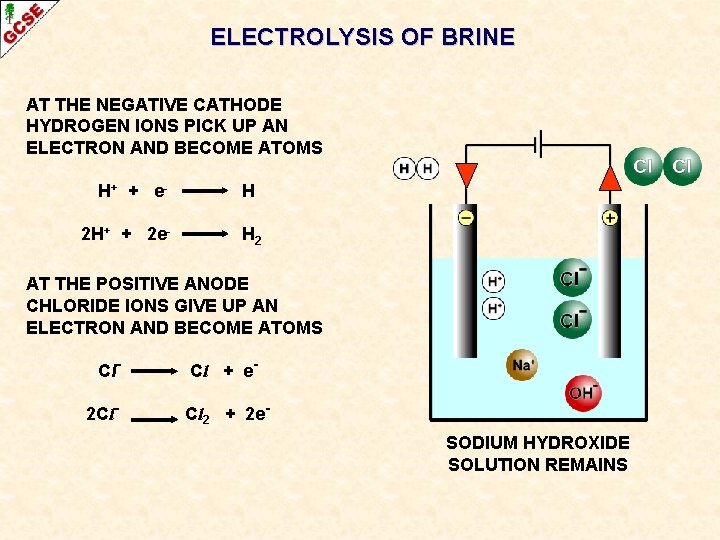
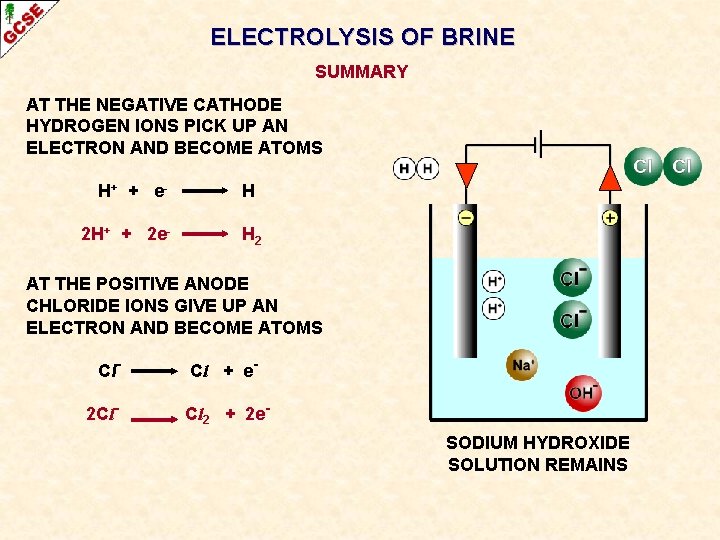
ELECTROLYSIS OF BRINE AT THE NEGATIVE CATHODE HYDROGEN IONS PICK UP AN ELECTRON AND BECOME ATOMS H+ + e - H

ELECTROLYSIS OF BRINE AT THE NEGATIVE CATHODE HYDROGEN IONS PICK UP AN ELECTRON AND BECOME ATOMS H+ + e 2 H+ + 2 e- H H 2 The atoms form molecules and hydrogen gas H 2 is produced

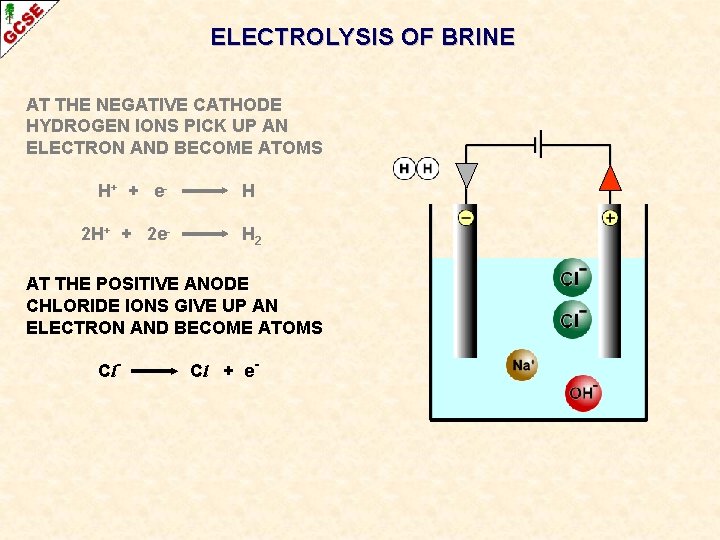
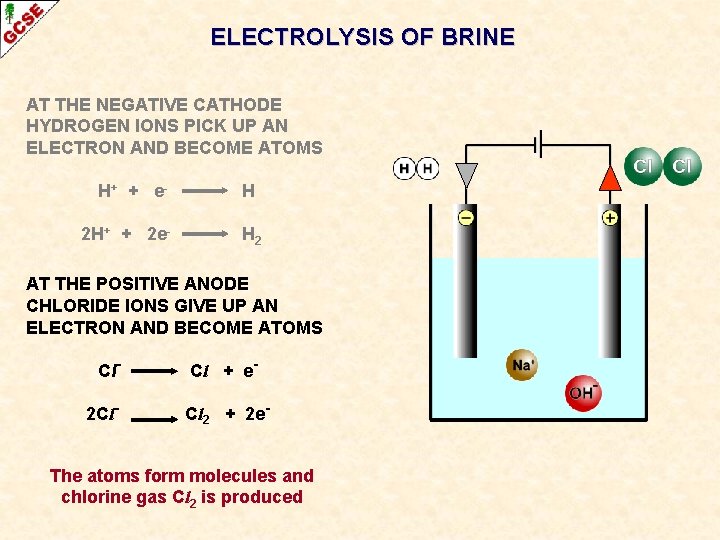
ELECTROLYSIS OF BRINE AT THE NEGATIVE CATHODE HYDROGEN IONS PICK UP AN ELECTRON AND BECOME ATOMS H+ + e 2 H+ + 2 e- H H 2 AT THE POSITIVE ANODE CHLORIDE IONS GIVE UP AN ELECTRON AND BECOME ATOMS Cl- Cl + e-

ELECTROLYSIS OF BRINE AT THE NEGATIVE CATHODE HYDROGEN IONS PICK UP AN ELECTRON AND BECOME ATOMS H+ + e 2 H+ + 2 e- H H 2 AT THE POSITIVE ANODE CHLORIDE IONS GIVE UP AN ELECTRON AND BECOME ATOMS Cl 2 Cl- Cl + e. Cl 2 + 2 e- The atoms form molecules and chlorine gas Cl 2 is produced

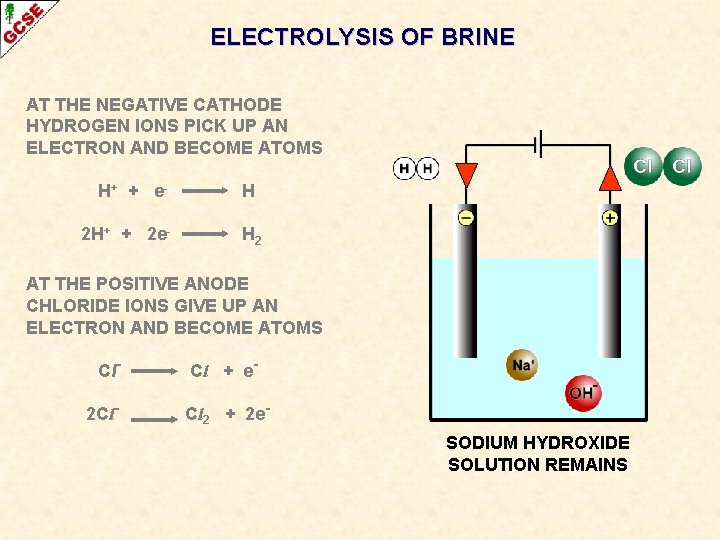
ELECTROLYSIS OF BRINE AT THE NEGATIVE CATHODE HYDROGEN IONS PICK UP AN ELECTRON AND BECOME ATOMS H+ + e 2 H+ + 2 e- H H 2 AT THE POSITIVE ANODE CHLORIDE IONS GIVE UP AN ELECTRON AND BECOME ATOMS Cl 2 Cl- Cl + e. Cl 2 + 2 e. SODIUM HYDROXIDE SOLUTION REMAINS

ELECTROLYSIS OF BRINE AT THE NEGATIVE CATHODE HYDROGEN IONS PICK UP AN ELECTRON AND BECOME ATOMS H+ + e 2 H+ + 2 e- H H 2 AT THE POSITIVE ANODE CHLORIDE IONS GIVE UP AN ELECTRON AND BECOME ATOMS Cl 2 Cl- Cl + e. Cl 2 + 2 e. SODIUM HYDROXIDE SOLUTION REMAINS

ELECTROLYSIS OF BRINE SUMMARY AT THE NEGATIVE CATHODE HYDROGEN IONS PICK UP AN ELECTRON AND BECOME ATOMS H+ + e 2 H+ + 2 e- H H 2 AT THE POSITIVE ANODE CHLORIDE IONS GIVE UP AN ELECTRON AND BECOME ATOMS Cl 2 Cl- Cl + e. Cl 2 + 2 e. SODIUM HYDROXIDE SOLUTION REMAINS

INDUSTRIAL ELECTROLYSIS OF BRINE

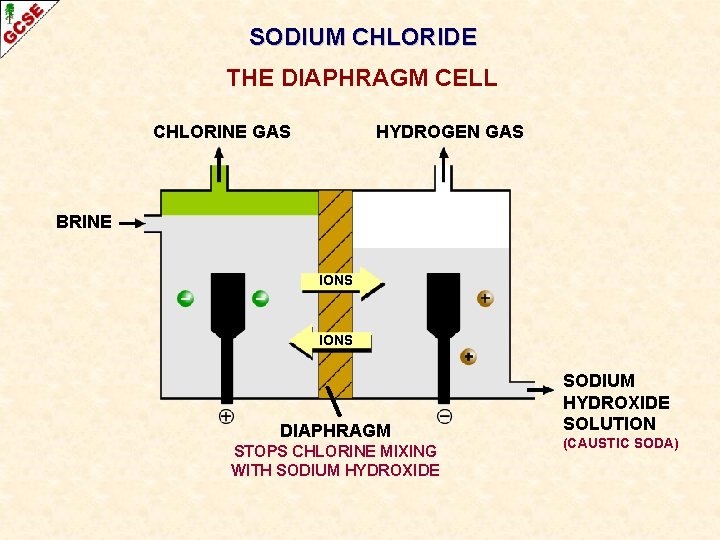
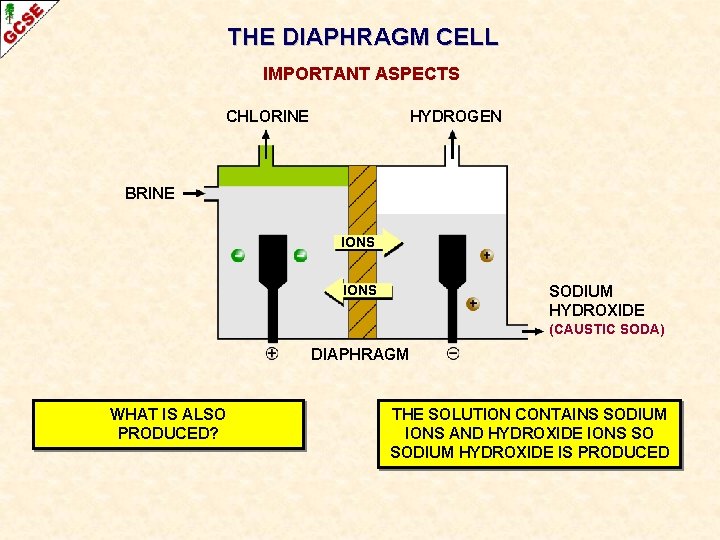
SODIUM CHLORIDE THE DIAPHRAGM CELL CHLORINE GAS HYDROGEN GAS BRINE IONS DIAPHRAGM STOPS CHLORINE MIXING WITH SODIUM HYDROXIDE SOLUTION (CAUSTIC SODA)

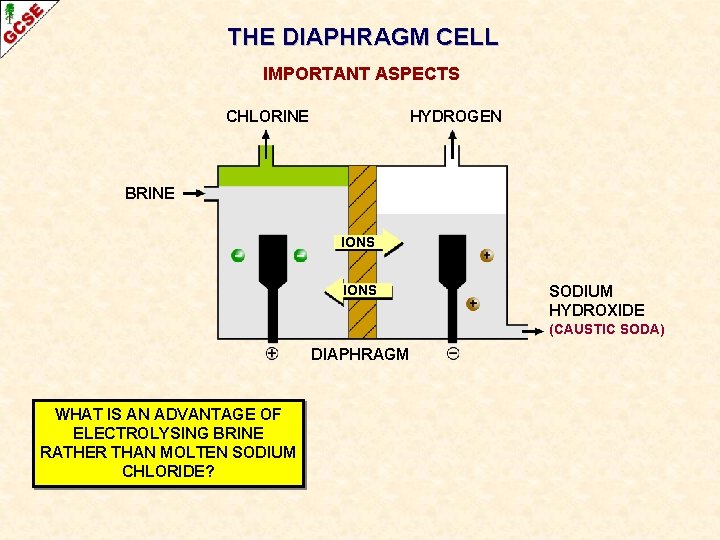
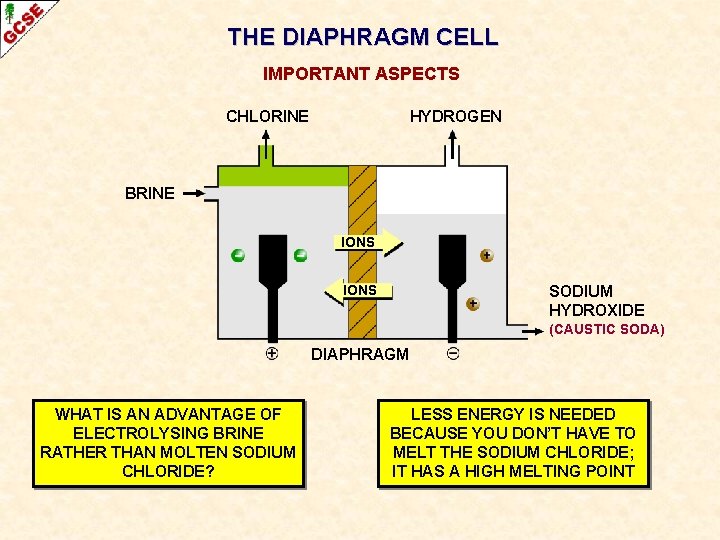
THE DIAPHRAGM CELL IMPORTANT ASPECTS CHLORINE HYDROGEN BRINE IONS SODIUM HYDROXIDE (CAUSTIC SODA) DIAPHRAGM WHAT IS AN ADVANTAGE OF ELECTROLYSING BRINE RATHER THAN MOLTEN SODIUM CHLORIDE?

THE DIAPHRAGM CELL IMPORTANT ASPECTS CHLORINE HYDROGEN BRINE IONS SODIUM HYDROXIDE (CAUSTIC SODA) DIAPHRAGM WHAT IS AN ADVANTAGE OF ELECTROLYSING BRINE RATHER THAN MOLTEN SODIUM CHLORIDE? LESS ENERGY IS NEEDED BECAUSE YOU DON’T HAVE TO MELT THE SODIUM CHLORIDE; IT HAS A HIGH MELTING POINT

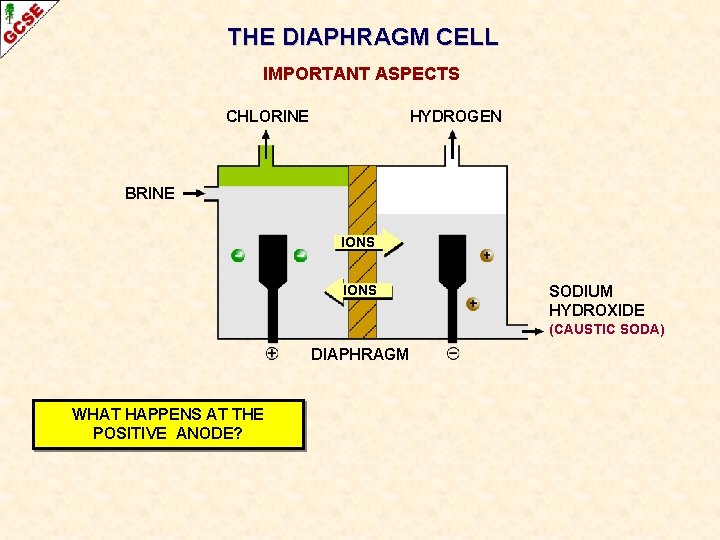
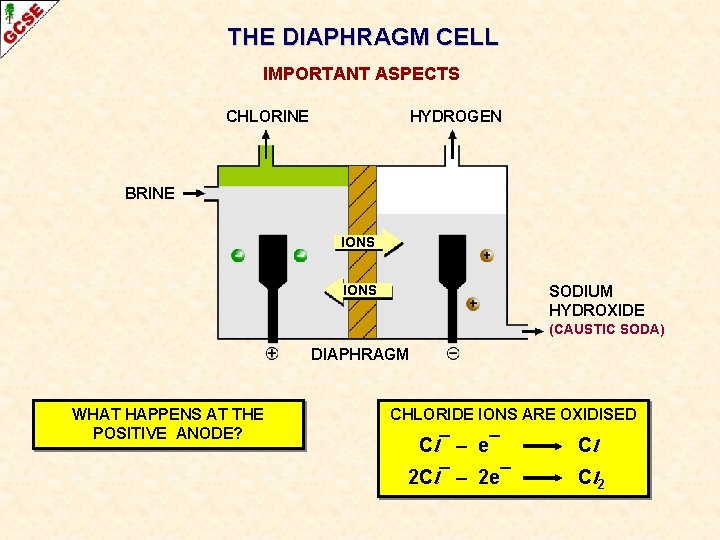
THE DIAPHRAGM CELL IMPORTANT ASPECTS CHLORINE HYDROGEN BRINE IONS SODIUM HYDROXIDE (CAUSTIC SODA) DIAPHRAGM WHAT HAPPENS AT THE POSITIVE ANODE?

THE DIAPHRAGM CELL IMPORTANT ASPECTS CHLORINE HYDROGEN BRINE IONS SODIUM HYDROXIDE (CAUSTIC SODA) DIAPHRAGM WHAT HAPPENS AT THE POSITIVE ANODE? CHLORIDE IONS ARE OXIDISED Cl¯ – e¯ Cl 2 Cl¯ – 2 e¯ Cl 2

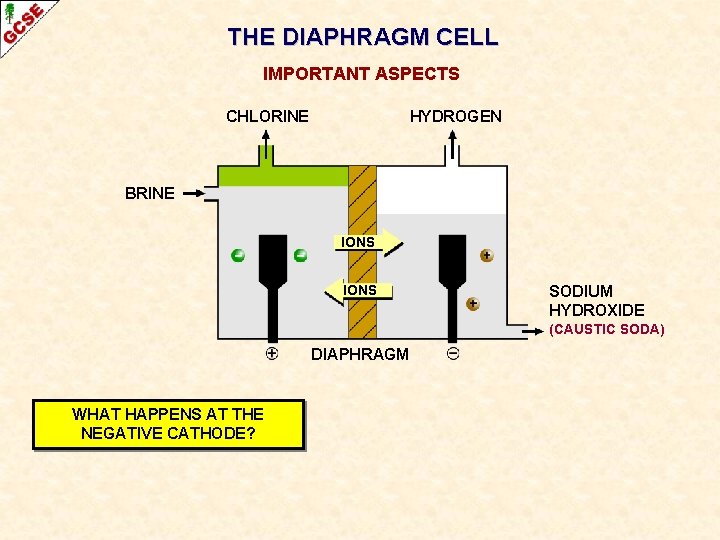
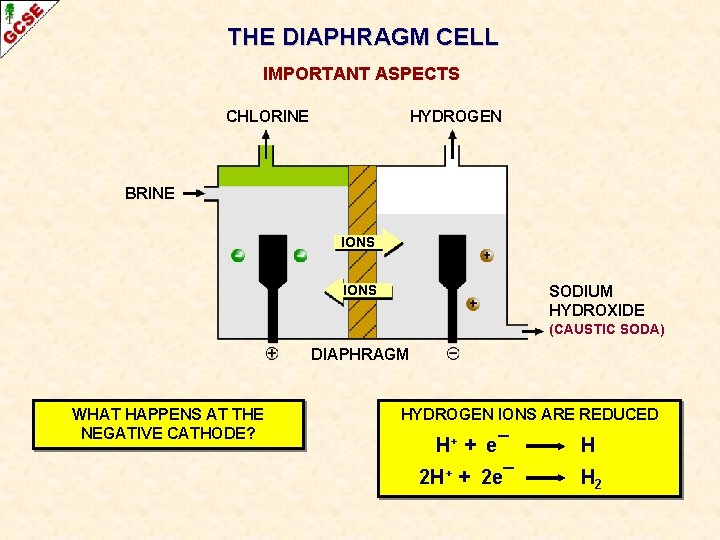
THE DIAPHRAGM CELL IMPORTANT ASPECTS CHLORINE HYDROGEN BRINE IONS SODIUM HYDROXIDE (CAUSTIC SODA) DIAPHRAGM WHAT HAPPENS AT THE NEGATIVE CATHODE?

THE DIAPHRAGM CELL IMPORTANT ASPECTS CHLORINE HYDROGEN BRINE IONS SODIUM HYDROXIDE (CAUSTIC SODA) DIAPHRAGM WHAT HAPPENS AT THE NEGATIVE CATHODE? HYDROGEN IONS ARE REDUCED H+ + e¯ H 2 H+ + 2 e¯ H 2

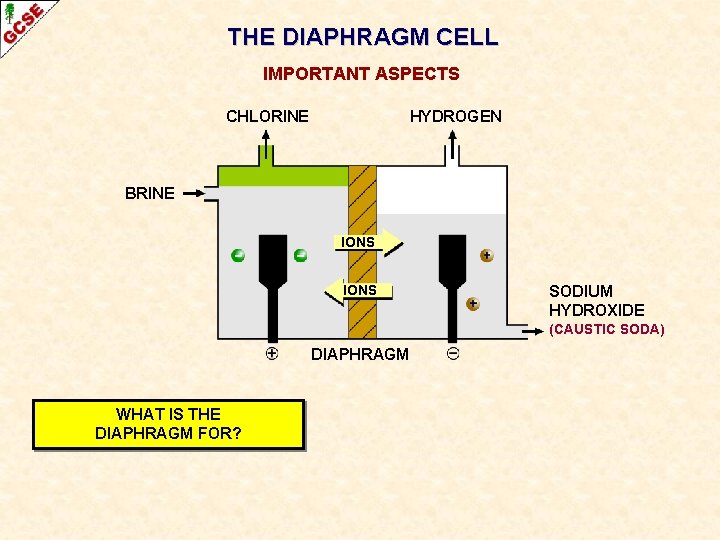
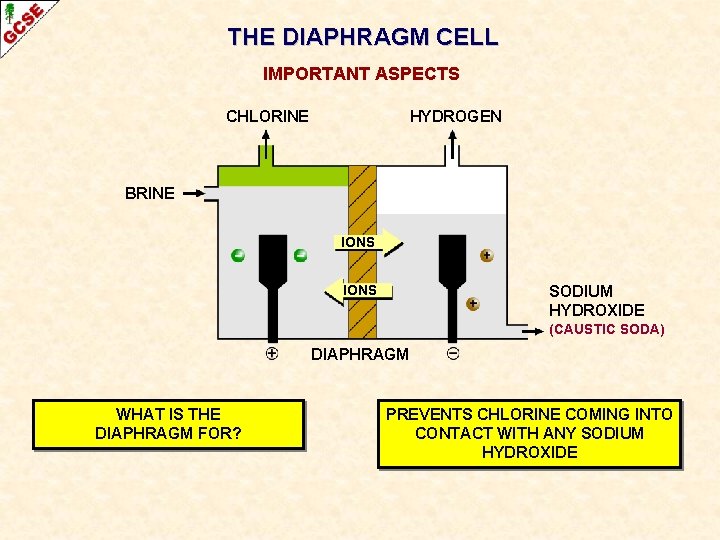
THE DIAPHRAGM CELL IMPORTANT ASPECTS CHLORINE HYDROGEN BRINE IONS SODIUM HYDROXIDE (CAUSTIC SODA) DIAPHRAGM WHAT IS THE DIAPHRAGM FOR?

THE DIAPHRAGM CELL IMPORTANT ASPECTS CHLORINE HYDROGEN BRINE IONS SODIUM HYDROXIDE (CAUSTIC SODA) DIAPHRAGM WHAT IS THE DIAPHRAGM FOR? PREVENTS CHLORINE COMING INTO CONTACT WITH ANY SODIUM HYDROXIDE

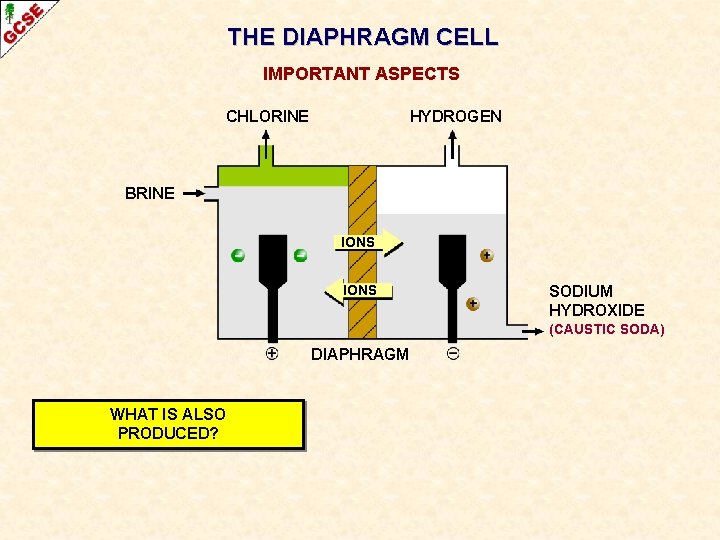
THE DIAPHRAGM CELL IMPORTANT ASPECTS CHLORINE HYDROGEN BRINE IONS SODIUM HYDROXIDE (CAUSTIC SODA) DIAPHRAGM WHAT IS ALSO PRODUCED?

THE DIAPHRAGM CELL IMPORTANT ASPECTS CHLORINE HYDROGEN BRINE IONS SODIUM HYDROXIDE (CAUSTIC SODA) DIAPHRAGM WHAT IS ALSO PRODUCED? THE SOLUTION CONTAINS SODIUM IONS AND HYDROXIDE IONS SO SODIUM HYDROXIDE IS PRODUCED

THE DIAPHRAGM CELL INDUSTRIAL IMPORTANCE The products of the electrolysis of brine are of great industrial importance… CHLORINE making bleach making plastics such as pvc HYDROGEN used as fuel used in margarine production SODIUM HYDROXIDE production of soaps and detergents used in the paper industry

EXTRACTION OF ALUMINIUM BY ELECTROLYSIS

EXTRACTION OF ALUMINIUM ELECTROLYSIS Unlike iron, aluminium cannot be extracted using carbon. (Aluminium is above carbon in the reactivity series)

EXTRACTION OF ALUMINIUM ELECTROLYSIS Unlike iron, aluminium cannot be extracted using carbon. (Aluminium is above carbon in the reactivity series) Reactive metals are extracted using electrolysis

EXTRACTION OF ALUMINIUM ELECTROLYSIS Unlike iron, aluminium cannot be extracted using carbon. (Aluminium is above carbon in the reactivity series) Reactive metals are extracted using electrolysis Electrolysis is expensive - it requires a lot of energy… - ore must be molten (ores have high melting points) - electricity is needed for the electrolysis process

EXTRACTION OF ALUMINIUM RAW MATERIALS BAUXITE aluminium ore Bauxite contains alumina (Al 2 O 3 aluminium oxide) plus impurities such as iron oxide – it is purified before use.

EXTRACTION OF ALUMINIUM RAW MATERIALS BAUXITE aluminium ore Bauxite contains alumina (Al 2 O 3 aluminium oxide) plus impurities such as iron oxide – it is purified before use. CRYOLITE Aluminium oxide has a very high melting point. Adding cryolite lowers the melting point and saves energy.

EXTRACTION OF ALUMINIUM

EXTRACTION OF ALUMINIUM THE CELL CONSISTS OF A

EXTRACTION OF ALUMINIUM CARBON ANODE THE CELL CONSISTS OF A CARBON ANODE

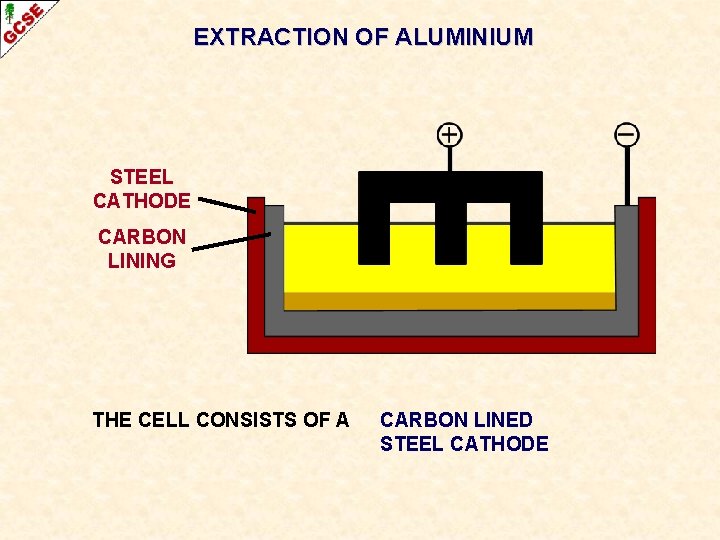
EXTRACTION OF ALUMINIUM STEEL CATHODE CARBON LINING THE CELL CONSISTS OF A CARBON LINED STEEL CATHODE

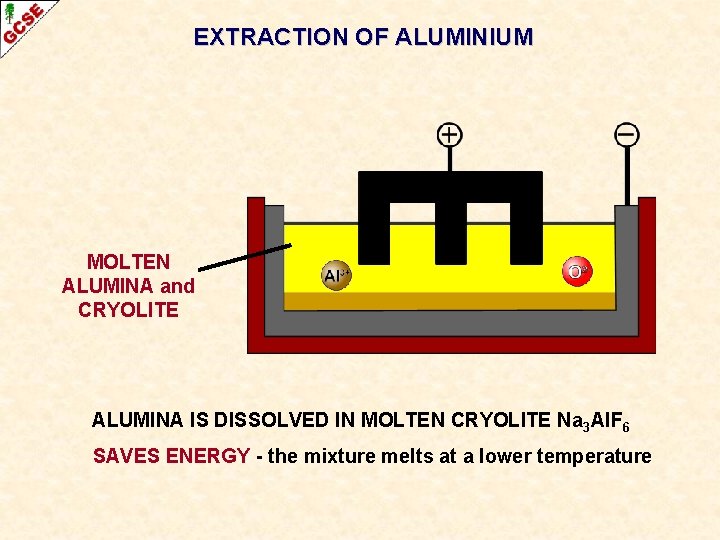
EXTRACTION OF ALUMINIUM MOLTEN ALUMINA and CRYOLITE ALUMINA IS DISSOLVED IN MOLTEN CRYOLITE Na 3 Al. F 6 SAVES ENERGY - the mixture melts at a lower temperature

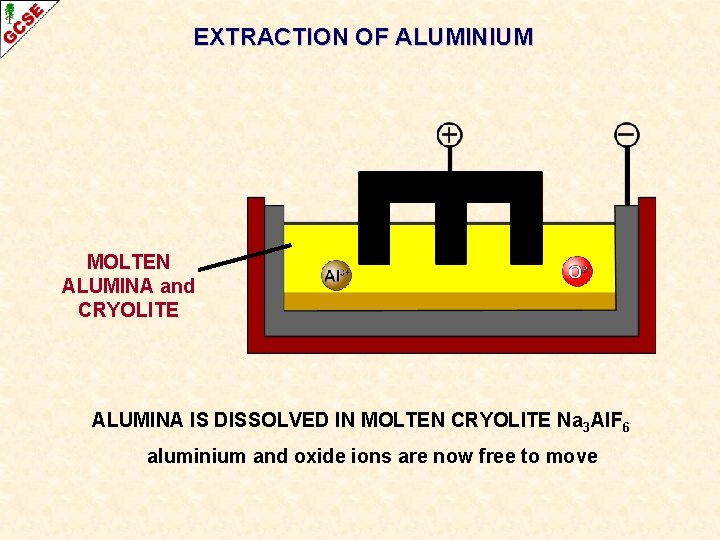
EXTRACTION OF ALUMINIUM MOLTEN ALUMINA and CRYOLITE ALUMINA IS DISSOLVED IN MOLTEN CRYOLITE Na 3 Al. F 6 aluminium and oxide ions are now free to move

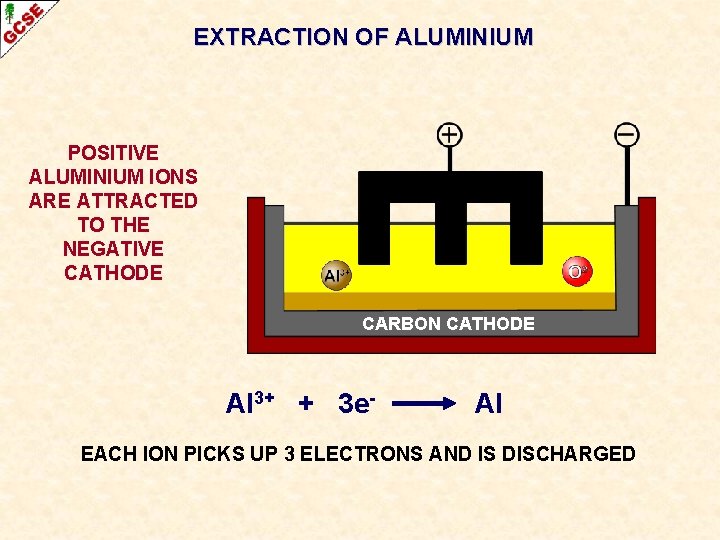
EXTRACTION OF ALUMINIUM POSITIVE ALUMINIUM IONS ARE ATTRACTED TO THE NEGATIVE CATHODE CARBON CATHODE Al 3+ + 3 e- Al EACH ION PICKS UP 3 ELECTRONS AND IS DISCHARGED

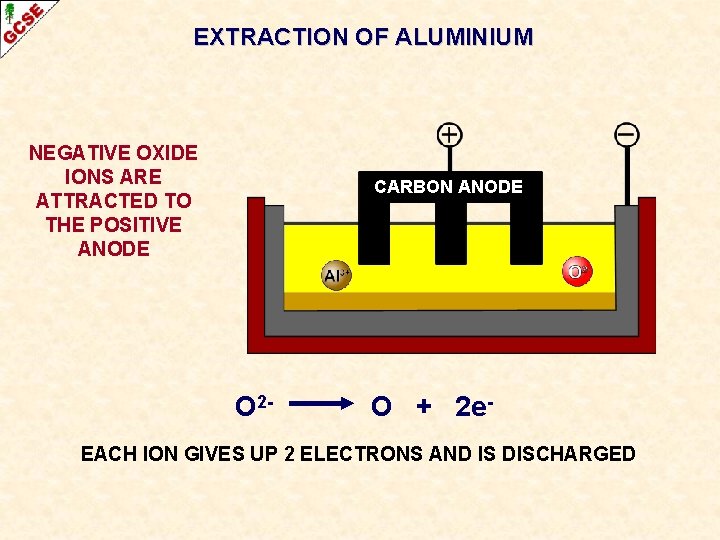
EXTRACTION OF ALUMINIUM NEGATIVE OXIDE IONS ARE ATTRACTED TO THE POSITIVE ANODE CARBON ANODE O 2 - O + 2 e- EACH ION GIVES UP 2 ELECTRONS AND IS DISCHARGED

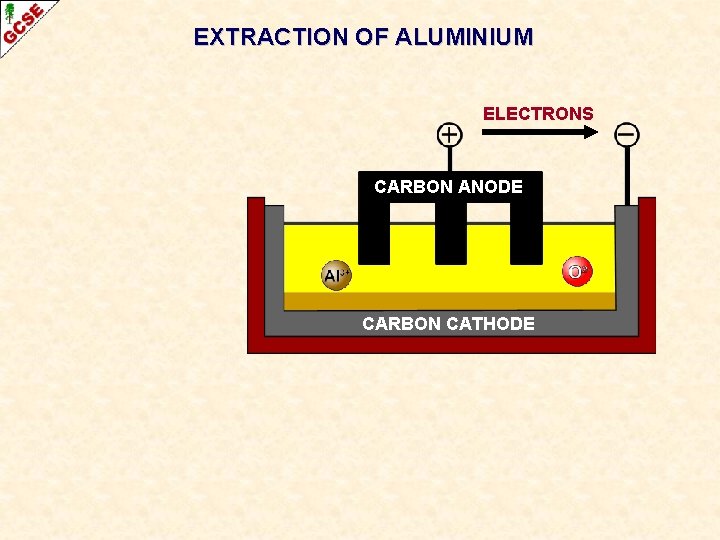
EXTRACTION OF ALUMINIUM ELECTRONS CARBON ANODE CARBON CATHODE

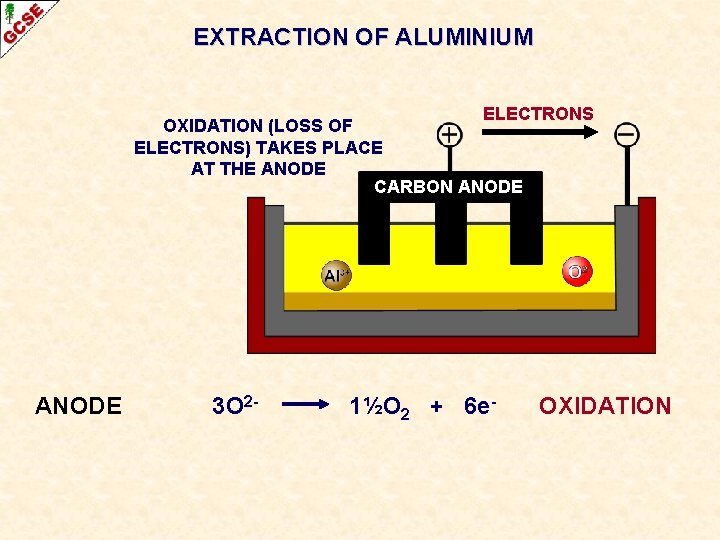
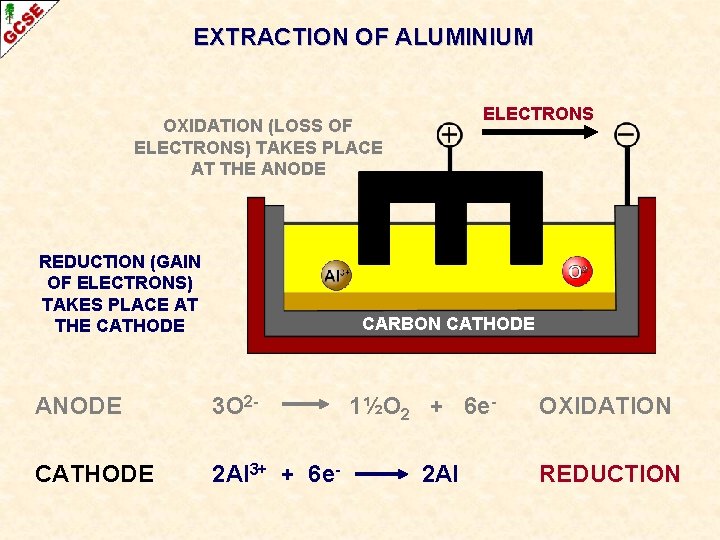
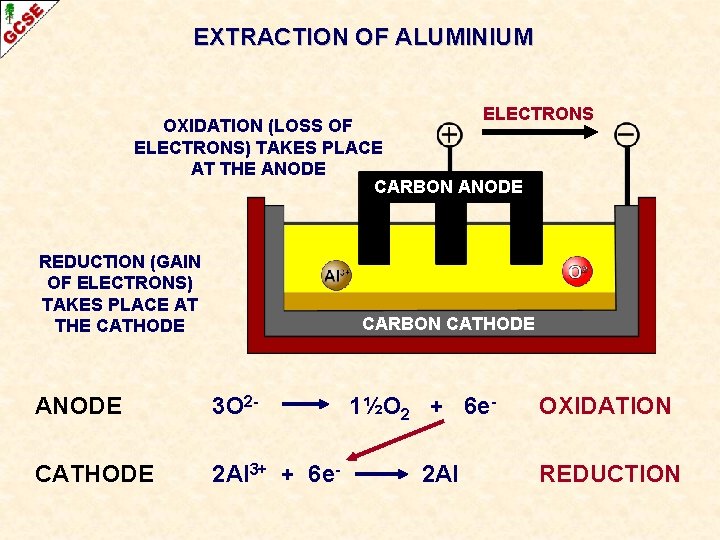
EXTRACTION OF ALUMINIUM ELECTRONS OXIDATION (LOSS OF ELECTRONS) TAKES PLACE AT THE ANODE CARBON ANODE 3 O 2 - 1½O 2 + 6 e- OXIDATION

EXTRACTION OF ALUMINIUM ELECTRONS OXIDATION (LOSS OF ELECTRONS) TAKES PLACE AT THE ANODE REDUCTION (GAIN OF ELECTRONS) TAKES PLACE AT THE CATHODE CARBON CATHODE ANODE 3 O 2 - CATHODE 2 Al 3+ + 6 e- 1½O 2 + 6 e 2 Al OXIDATION REDUCTION

EXTRACTION OF ALUMINIUM ELECTRONS OXIDATION (LOSS OF ELECTRONS) TAKES PLACE AT THE ANODE CARBON ANODE REDUCTION (GAIN OF ELECTRONS) TAKES PLACE AT THE CATHODE CARBON CATHODE ANODE 3 O 2 - CATHODE 2 Al 3+ + 6 e- 1½O 2 + 6 e 2 Al OXIDATION REDUCTION

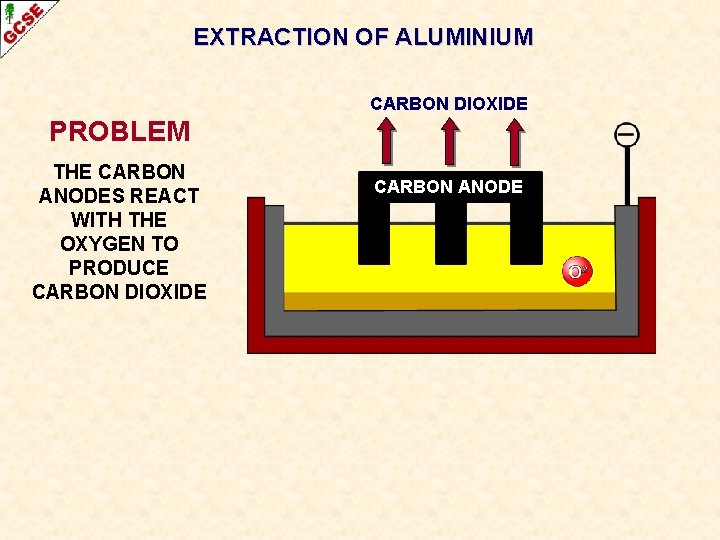
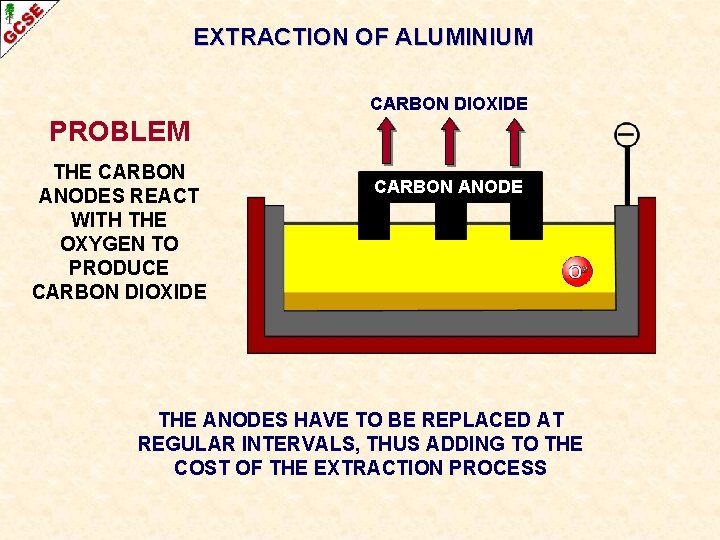
EXTRACTION OF ALUMINIUM CARBON DIOXIDE PROBLEM THE CARBON ANODES REACT WITH THE OXYGEN TO PRODUCE CARBON DIOXIDE CARBON ANODE

EXTRACTION OF ALUMINIUM CARBON DIOXIDE PROBLEM THE CARBON ANODES REACT WITH THE OXYGEN TO PRODUCE CARBON DIOXIDE CARBON ANODE THE ANODES HAVE TO BE REPLACED AT REGULAR INTERVALS, THUS ADDING TO THE COST OF THE EXTRACTION PROCESS

USES OF ALUMINIUM THE IMPORTANCE OF ALUMINIUM LOW DENSITY AND ELECTRICAL CONDUCTIVITY OVERHEAD CABLES LOW DENSITY needs to be an alloyed with other metals for extra strength AIRCRAFT BODIES GOOD HEAT CONDUCTIVITY SAUCEPANS

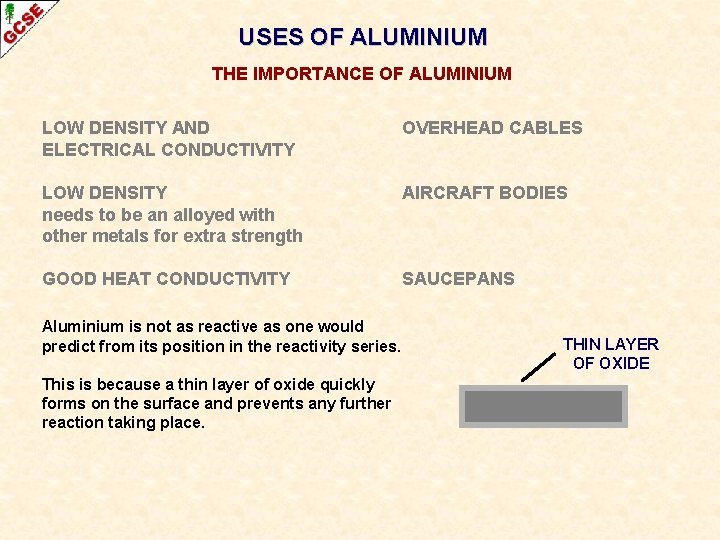
USES OF ALUMINIUM THE IMPORTANCE OF ALUMINIUM LOW DENSITY AND ELECTRICAL CONDUCTIVITY OVERHEAD CABLES LOW DENSITY needs to be an alloyed with other metals for extra strength AIRCRAFT BODIES GOOD HEAT CONDUCTIVITY SAUCEPANS Aluminium is not as reactive as one would predict from its position in the reactivity series. This is because a thin layer of oxide quickly forms on the surface and prevents any further reaction taking place. THIN LAYER OF OXIDE

USES OF ALUMINIUM THE IMPORTANCE OF ALUMINIUM LOW DENSITY AND ELECTRICAL CONDUCTIVITY OVERHEAD CABLES LOW DENSITY needs to be an alloyed with other metals for extra strength AIRCRAFT BODIES GOOD HEAT CONDUCTIVITY SAUCEPANS Aluminium is not as reactive as one would predict from its position in the reactivity series. THIN LAYER OF OXIDE This is because a thin layer of oxide quickly forms on the surface and prevents any further reaction taking place. ANODISING puts on a controlled layer of oxide so the metal can be used for household items such as pans and electrical goods.

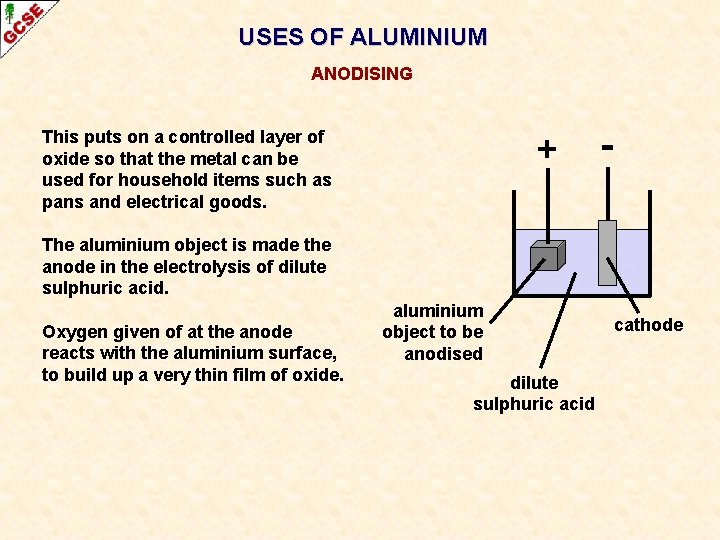
USES OF ALUMINIUM ANODISING This puts on a controlled layer of oxide so that the metal can be used for household items such as pans and electrical goods. + - The aluminium object is made the anode in the electrolysis of dilute sulphuric acid. Oxygen given of at the anode reacts with the aluminium surface, to build up a very thin film of oxide. aluminium object to be anodised dilute sulphuric acid cathode

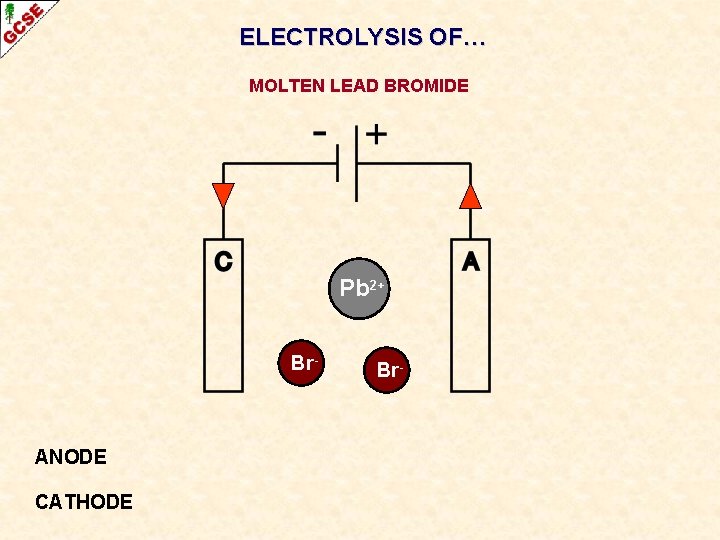
ELECTROLYSIS OF… MOLTEN LEAD BROMIDE

ELECTROLYSIS OF… MOLTEN LEAD BROMIDE Pb 2+ Br- ANODE CATHODE Br-

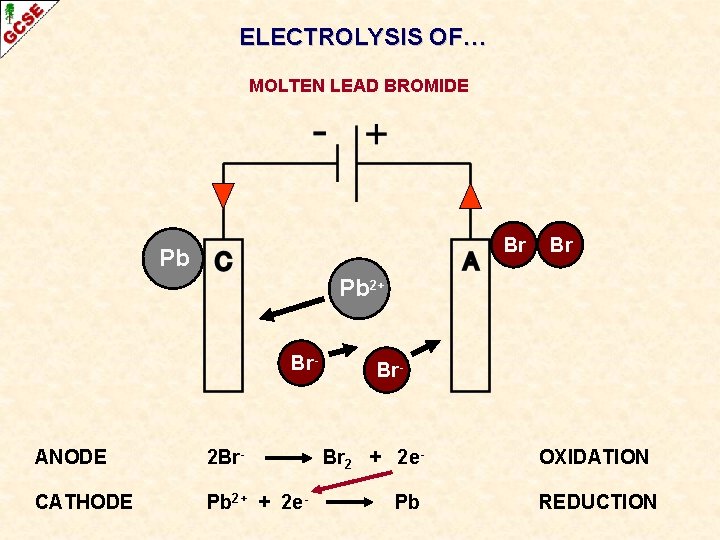
ELECTROLYSIS OF… MOLTEN LEAD BROMIDE Br Pb 2+ Br- ANODE 2 Br- CATHODE Pb 2+ + 2 e- Br 2 + 2 e. Pb OXIDATION REDUCTION

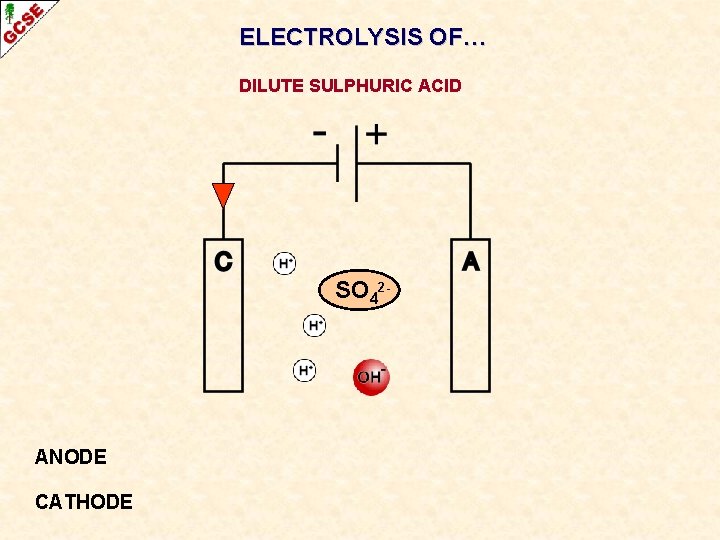
ELECTROLYSIS OF… DILUTE SULPHURIC ACID

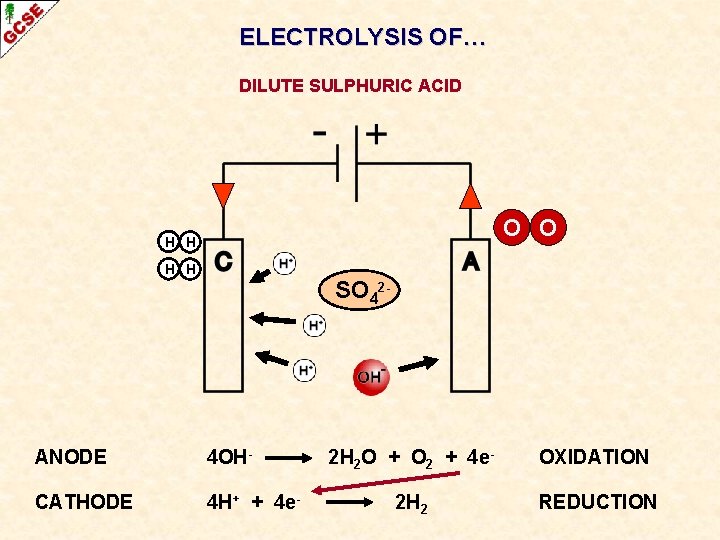
ELECTROLYSIS OF… DILUTE SULPHURIC ACID SO 42 - ANODE CATHODE

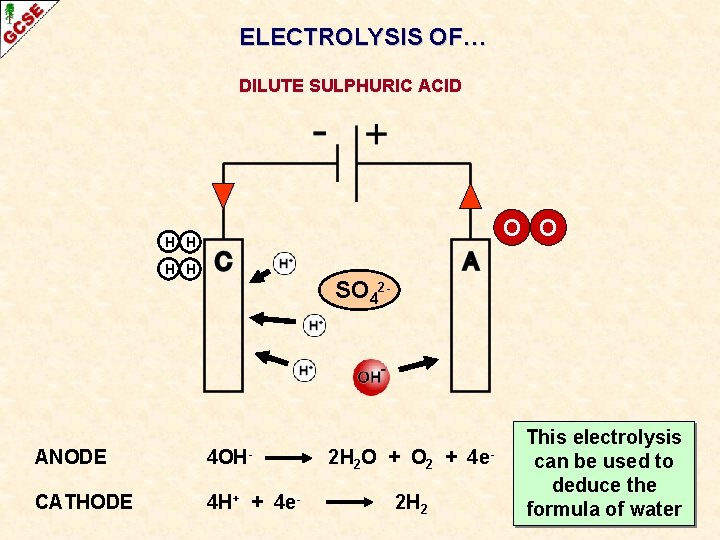
ELECTROLYSIS OF… DILUTE SULPHURIC ACID O O H H SO 42 - ANODE 4 OH- CATHODE 4 H+ + 4 e- 2 H 2 O + O 2 + 4 e- OXIDATION 2 H 2 REDUCTION

ELECTROLYSIS OF… DILUTE SULPHURIC ACID O O H H SO 42 - ANODE 4 OH- CATHODE 4 H+ + 4 e- 2 H 2 O + O 2 + 2 H 2 4 e- This electrolysis OXIDATION can be used to deduce the REDUCTION formula of water

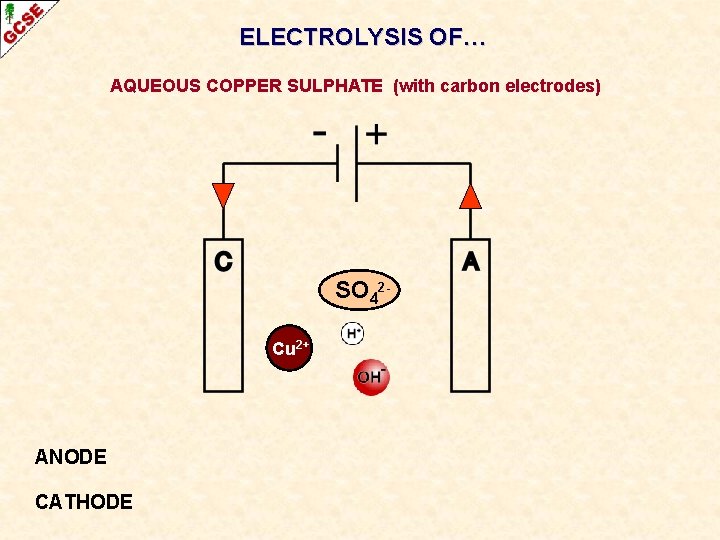
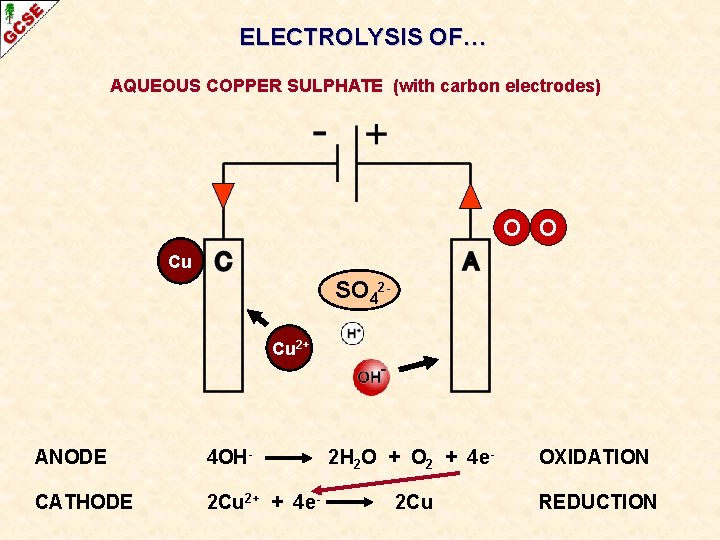
ELECTROLYSIS OF… AQUEOUS COPPER SULPHATE (with carbon electrodes)

ELECTROLYSIS OF… AQUEOUS COPPER SULPHATE (with carbon electrodes) SO 42 Cu 2+ ANODE CATHODE

ELECTROLYSIS OF… AQUEOUS COPPER SULPHATE (with carbon electrodes) O O Cu SO 42 Cu 2+ ANODE 4 OH- CATHODE 2 Cu 2+ + 4 e- 2 H 2 O + O 2 + 4 e- OXIDATION 2 Cu REDUCTION

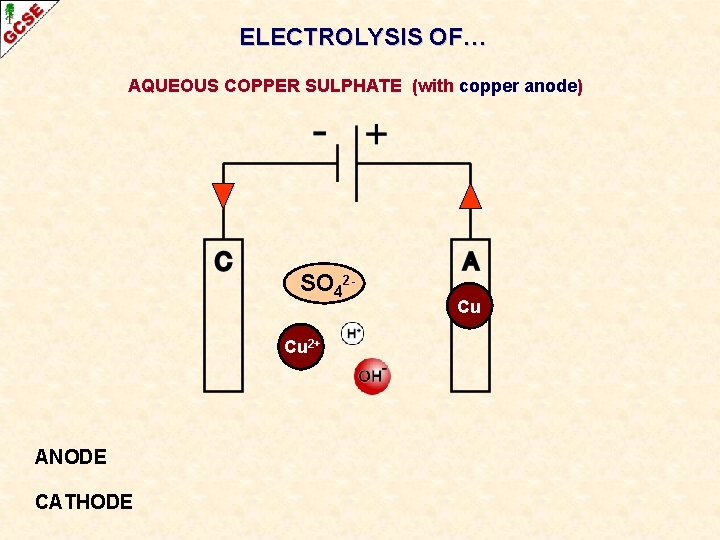
ELECTROLYSIS OF… AQUEOUS COPPER SULPHATE (with copper anode) A different reaction takes place if the anode is made of copper

ELECTROLYSIS OF… AQUEOUS COPPER SULPHATE (with copper anode) SO 42 Cu 2+ ANODE CATHODE Cu

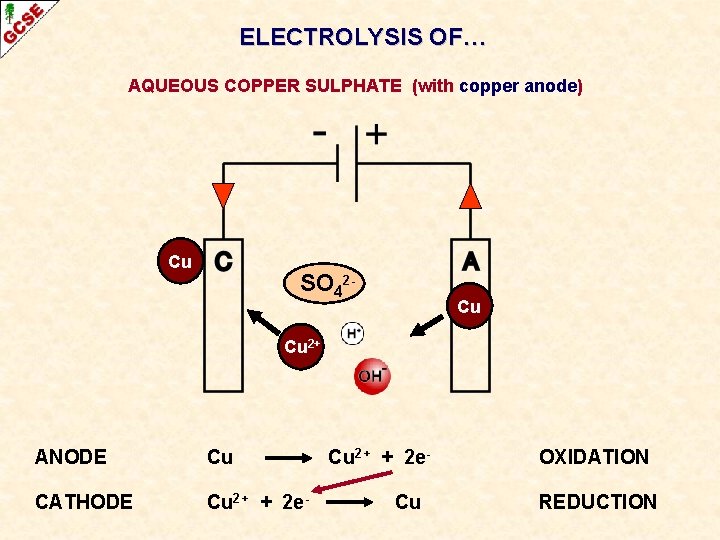
ELECTROLYSIS OF… AQUEOUS COPPER SULPHATE (with copper anode) Cu SO 42 - Cu Cu 2+ ANODE Cu CATHODE Cu 2+ + 2 e- Cu 2+ + 2 e. Cu OXIDATION REDUCTION

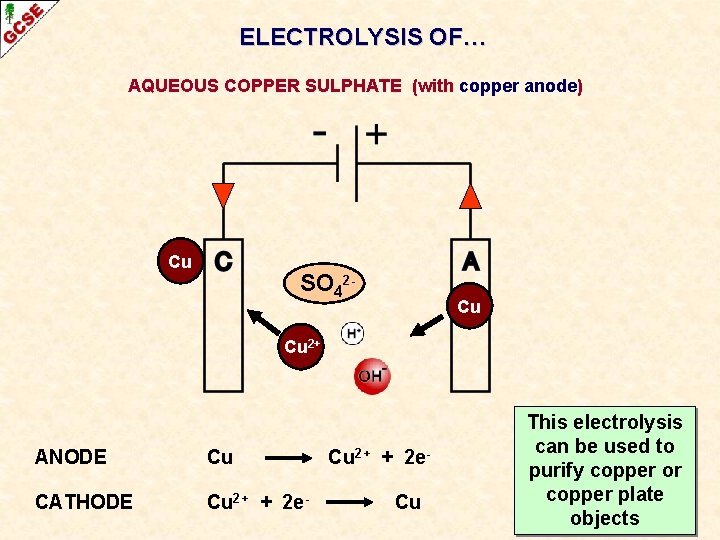
ELECTROLYSIS OF… AQUEOUS COPPER SULPHATE (with copper anode) Cu SO 42 - Cu Cu 2+ ANODE Cu CATHODE Cu 2+ + 2 e- Cu 2+ + 2 e. Cu This electrolysis can be used to OXIDATION purify copper or copper plate REDUCTION objects

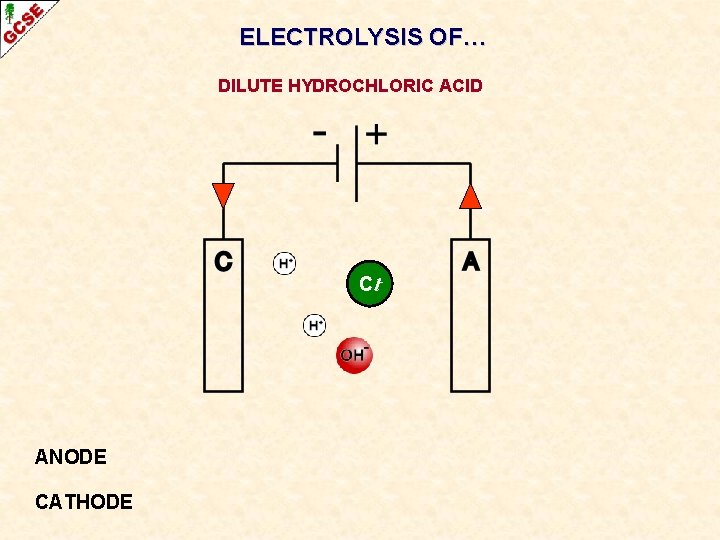
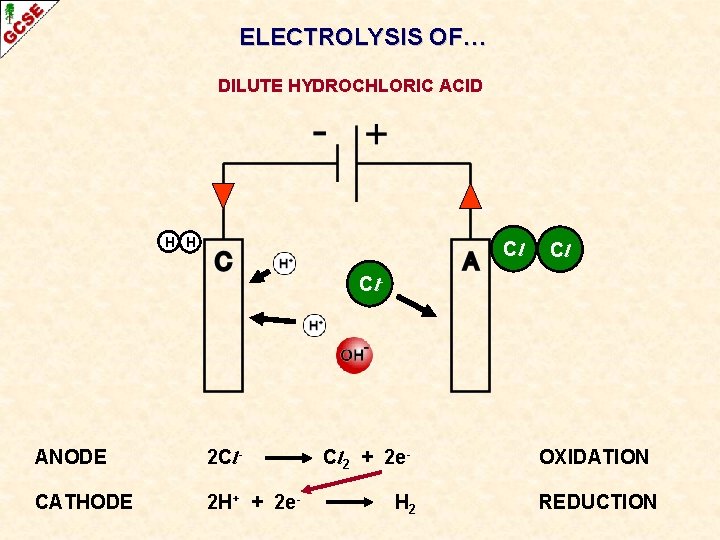
ELECTROLYSIS OF… DILUTE HYDROCHLORIC ACID

ELECTROLYSIS OF… DILUTE HYDROCHLORIC ACID Cl- ANODE CATHODE

ELECTROLYSIS OF… DILUTE HYDROCHLORIC ACID H H Cl Cl Cl- ANODE 2 Cl- CATHODE 2 H+ + 2 e- Cl 2 + 2 e. H 2 OXIDATION REDUCTION

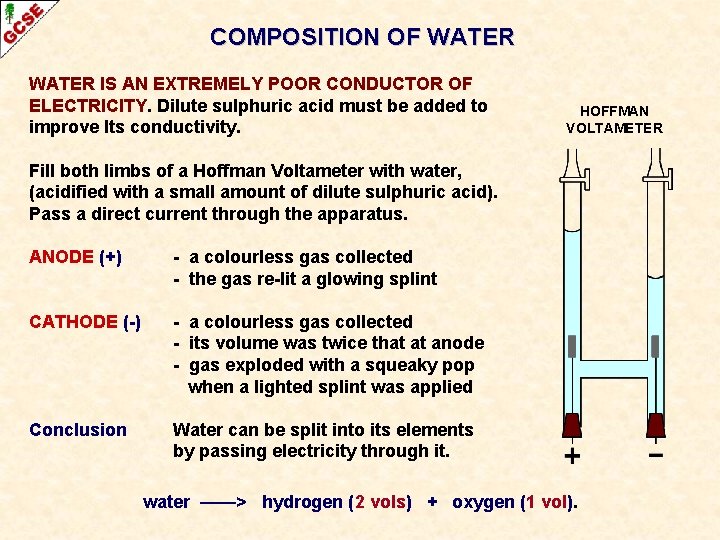
COMPOSITION OF WATER IS AN EXTREMELY POOR CONDUCTOR OF ELECTRICITY. Dilute sulphuric acid must be added to improve Its conductivity. HOFFMAN VOLTAMETER Fill both limbs of a Hoffman Voltameter with water, (acidified with a small amount of dilute sulphuric acid). Pass a direct current through the apparatus. ANODE (+) - a colourless gas collected - the gas re-lit a glowing splint CATHODE (-) - a colourless gas collected - its volume was twice that at anode - gas exploded with a squeaky pop when a lighted splint was applied Conclusion Water can be split into its elements by passing electricity through it. water ——> hydrogen (2 vols) + oxygen (1 vol).

ELECTROLYSIS THE END © 2011 JONATHAN HOPTON & KNOCKHARDY PUBLISHING