Electrochemistry Electrolysis using energy to drive a nonspontaneous
















































- Slides: 79

Electrochemistry Electrolysis using energy to drive a nonspontaneous reaction 1

Voltaic cells work because of spontaneous redox reactions. Electricity can be used to force a nonspontaneous reaction to run. • We call this electrolysis • We say it takes place in an electrolytic cell. 2

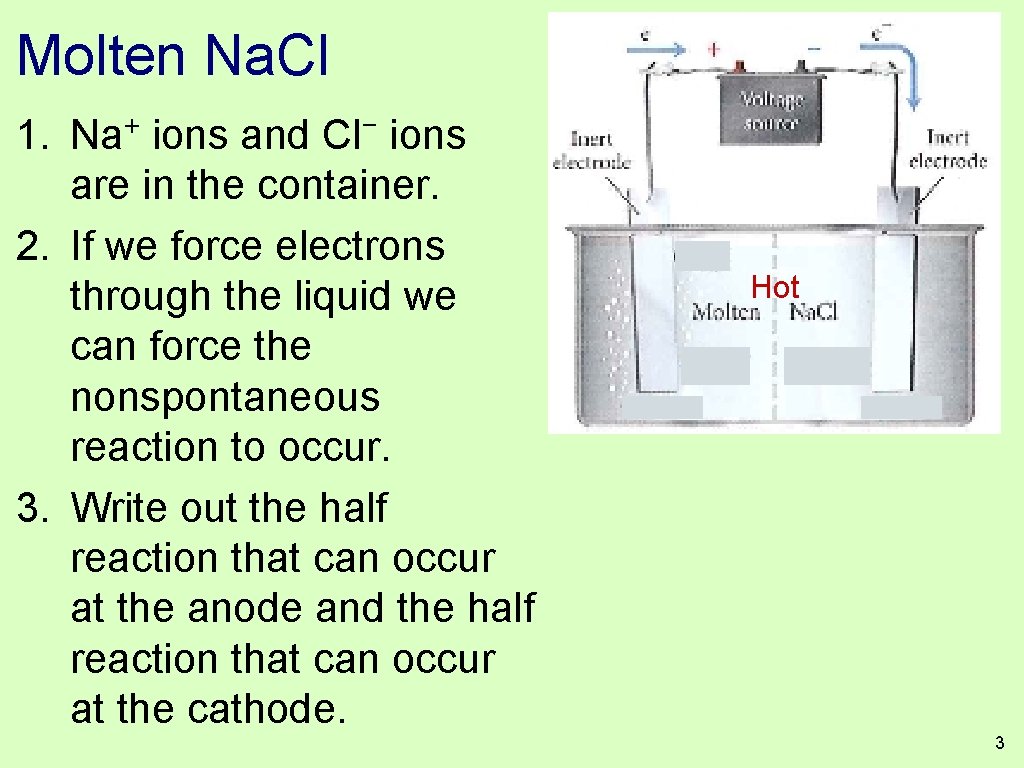
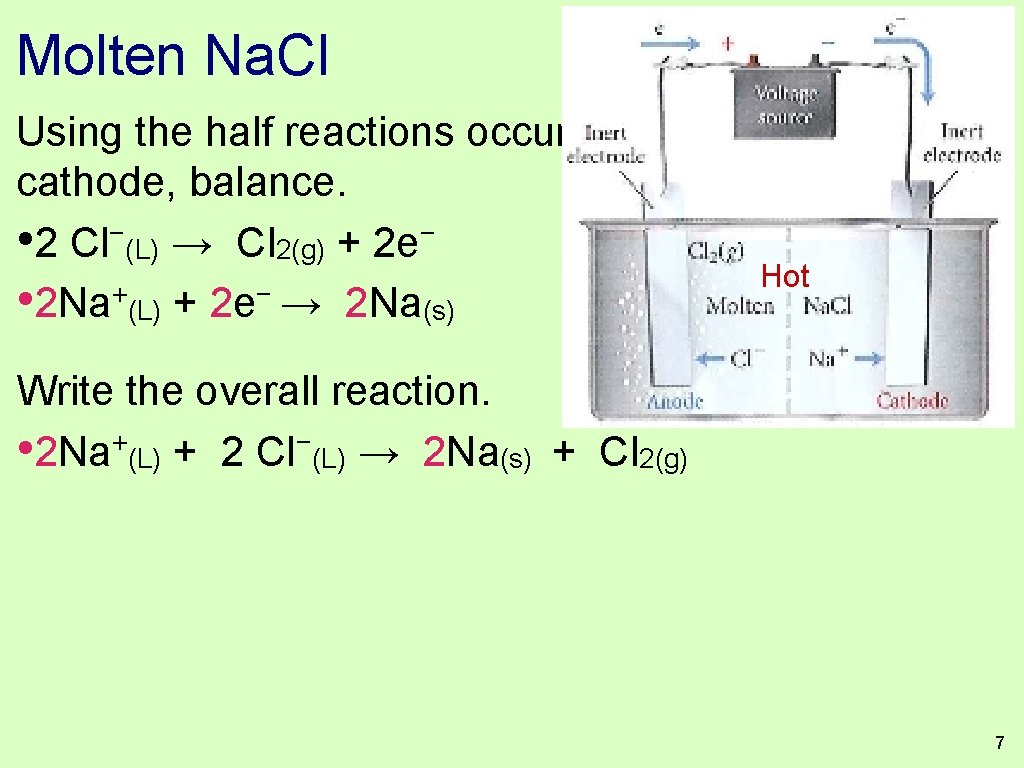
Molten Na. Cl 1. + Na − Cl ions and ions are in the container. 2. If we force electrons through the liquid we can force the nonspontaneous reaction to occur. 3. Write out the half reaction that can occur at the anode and the half reaction that can occur at the cathode. Hot 3

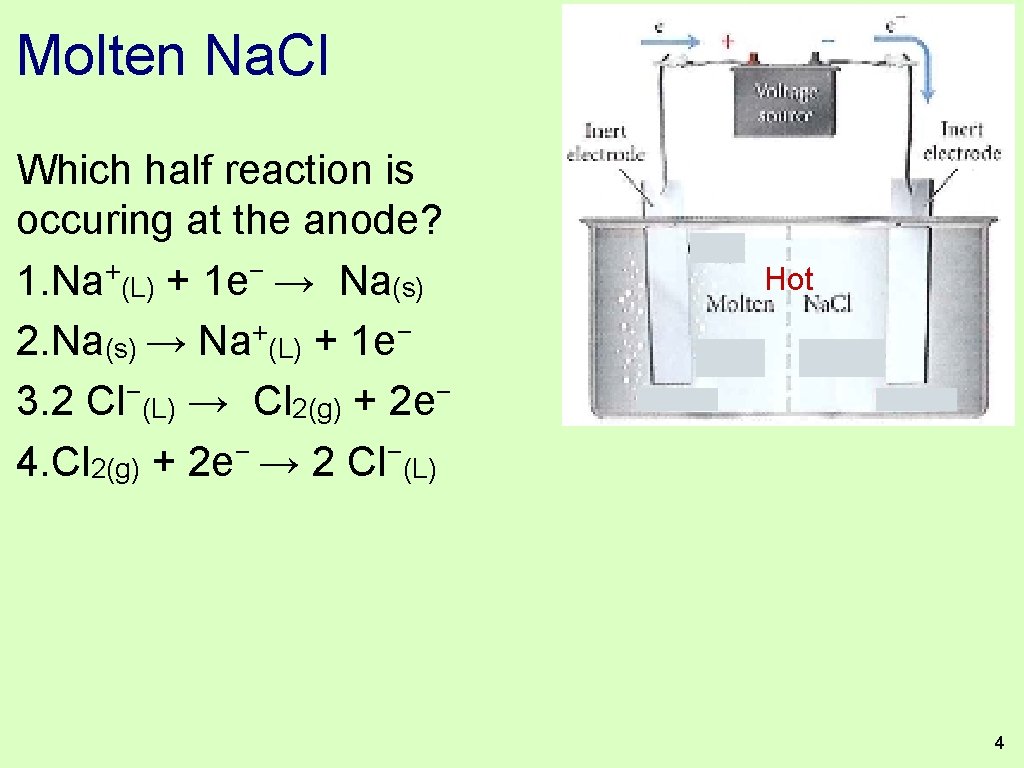
Molten Na. Cl Which half reaction is occuring at the anode? 1. Na+(L) + 1 e− → Na(s) 2. Na(s) → Na+(L) + 1 e− 3. 2 Cl−(L) → Cl 2(g) + 2 e− 4. Cl 2(g) + 2 e− → 2 Cl−(L) Hot 4

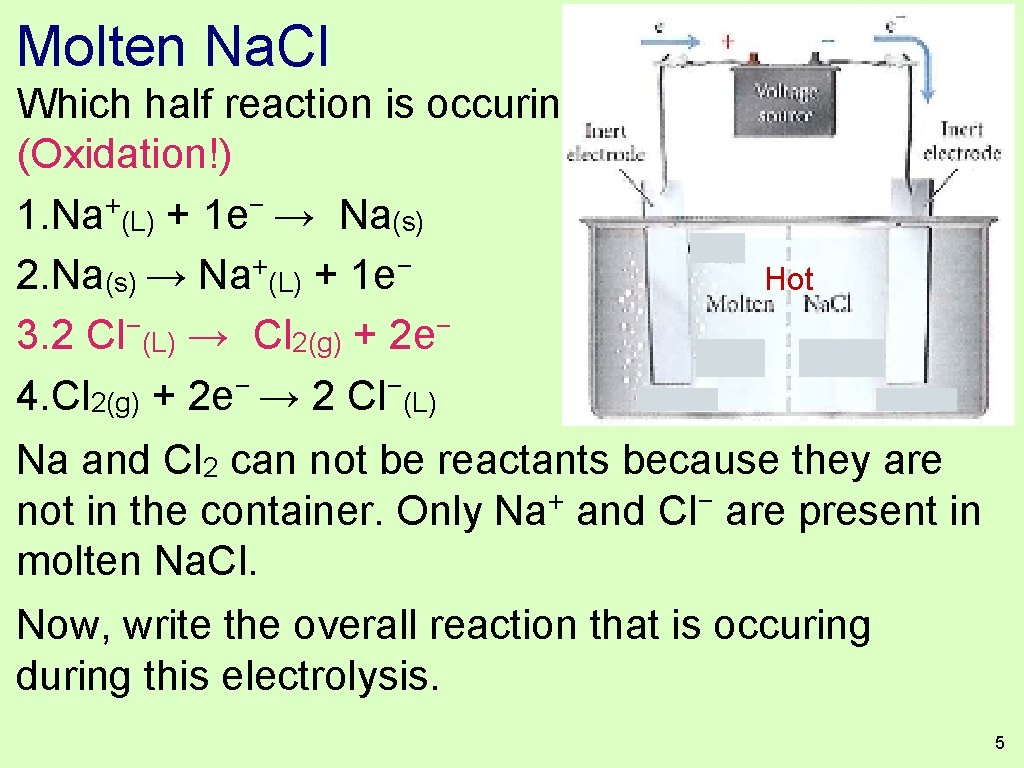
Molten Na. Cl Which half reaction is occuring at the anode? (Oxidation!) + − 1. Na (L) + 1 e → Na(s) + − 2. Na(s) → Na (L) + 1 e Hot − − 3. 2 Cl (L) → Cl 2(g) + 2 e 4. Cl 2(g) + 2 e− → 2 Cl−(L) Na and Cl 2 can not be reactants because they are + − not in the container. Only Na and Cl are present in molten Na. Cl. Now, write the overall reaction that is occuring during this electrolysis. 5

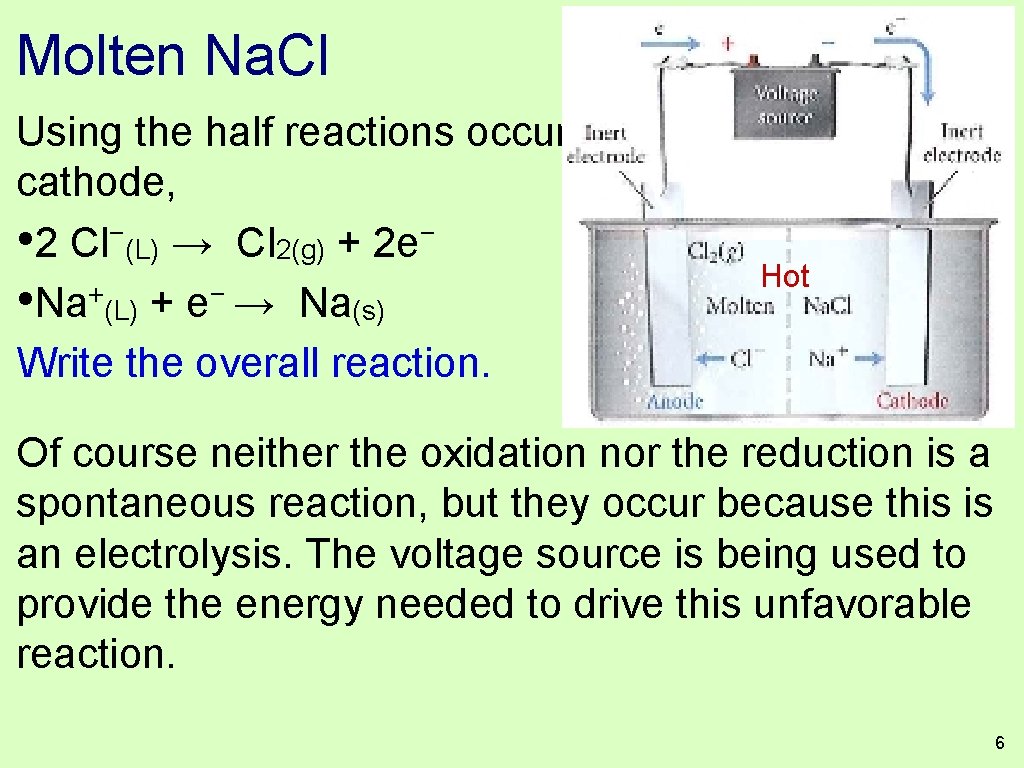
Molten Na. Cl Using the half reactions occuring at the anode and cathode, − − • 2 Cl (L) → Cl 2(g) + 2 e Hot + − • Na (L) + e → Na(s) Write the overall reaction. Of course neither the oxidation nor the reduction is a spontaneous reaction, but they occur because this is an electrolysis. The voltage source is being used to provide the energy needed to drive this unfavorable reaction. 6

Molten Na. Cl Using the half reactions occuring at the anode and cathode, balance. − − • 2 Cl (L) → Cl 2(g) + 2 e Hot + − • 2 Na (L) + 2 e → 2 Na(s) Write the overall reaction. • 2 Na+(L) + 2 Cl−(L) → 2 Na(s) + Cl 2(g) 7

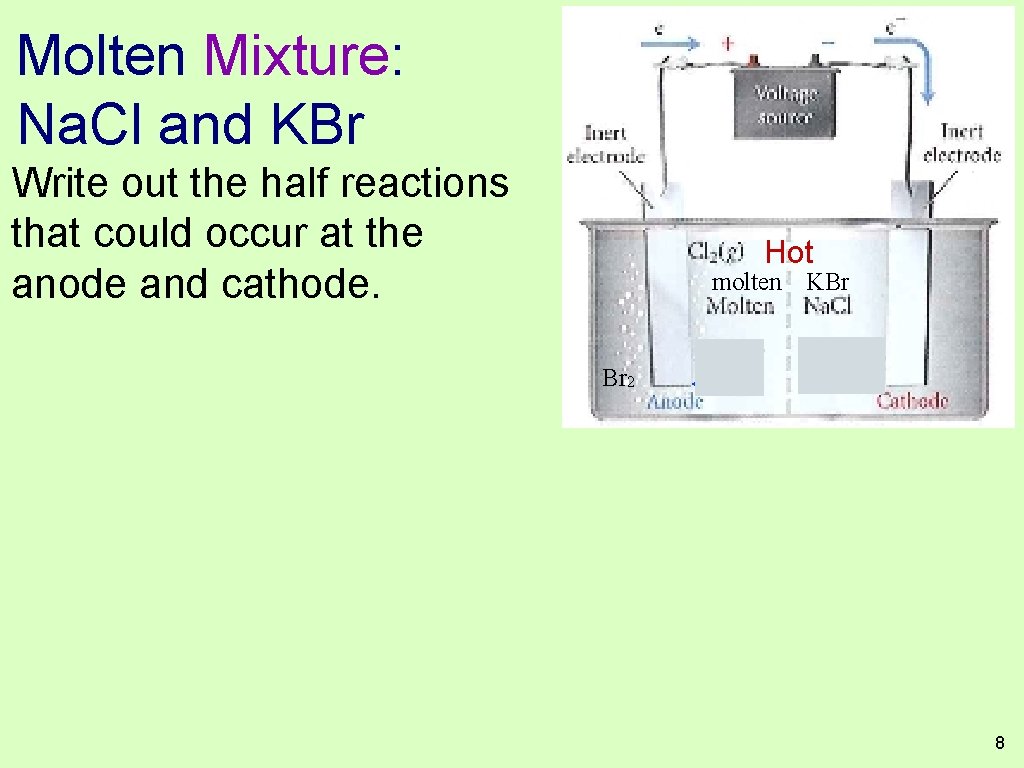
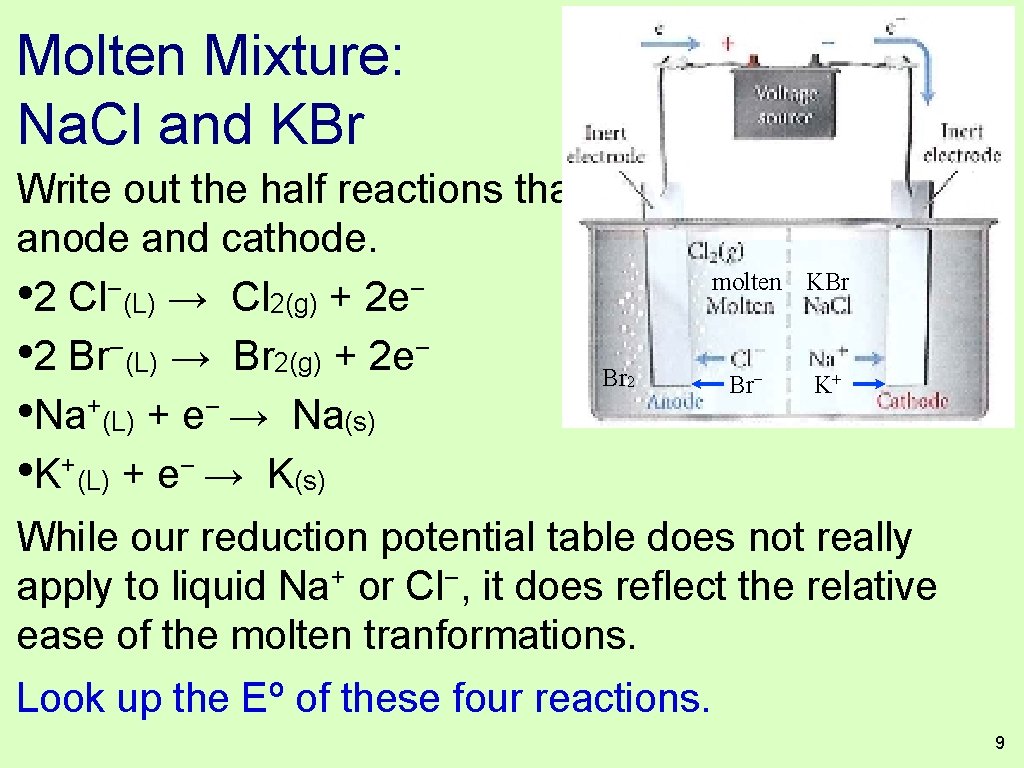
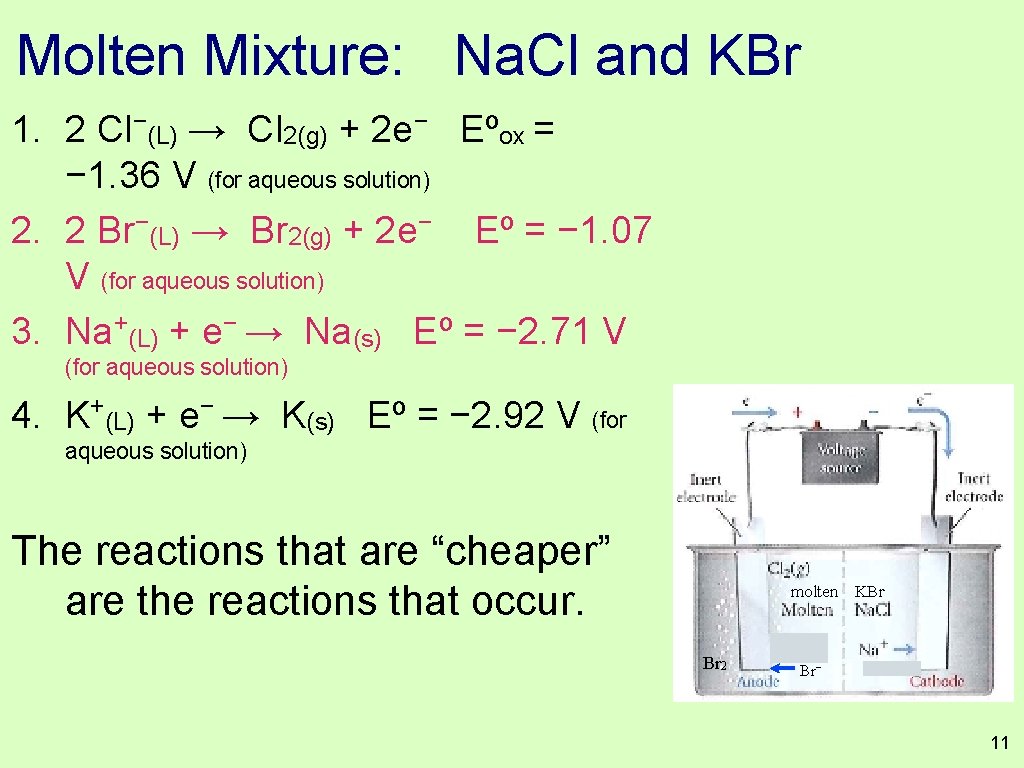
Molten Mixture: Na. Cl and KBr Write out the half reactions that could occur at the anode and cathode. Hot Br 2 molten KBr Br− K+ 8

Molten Mixture: Na. Cl and KBr Write out the half reactions that could occur at the anode and cathode. molten KBr − − • 2 Cl (L) → Cl 2(g) + 2 e • 2 Br−(L) → Br 2(g) + 2 e− Br 2 Br− K+ + − • Na (L) + e → Na(s) • K+(L) + e− → K(s) While our reduction potential table does not really apply to liquid Na+ or Cl−, it does reflect the relative ease of the molten tranformations. Look up the Eº of these four reactions. 9

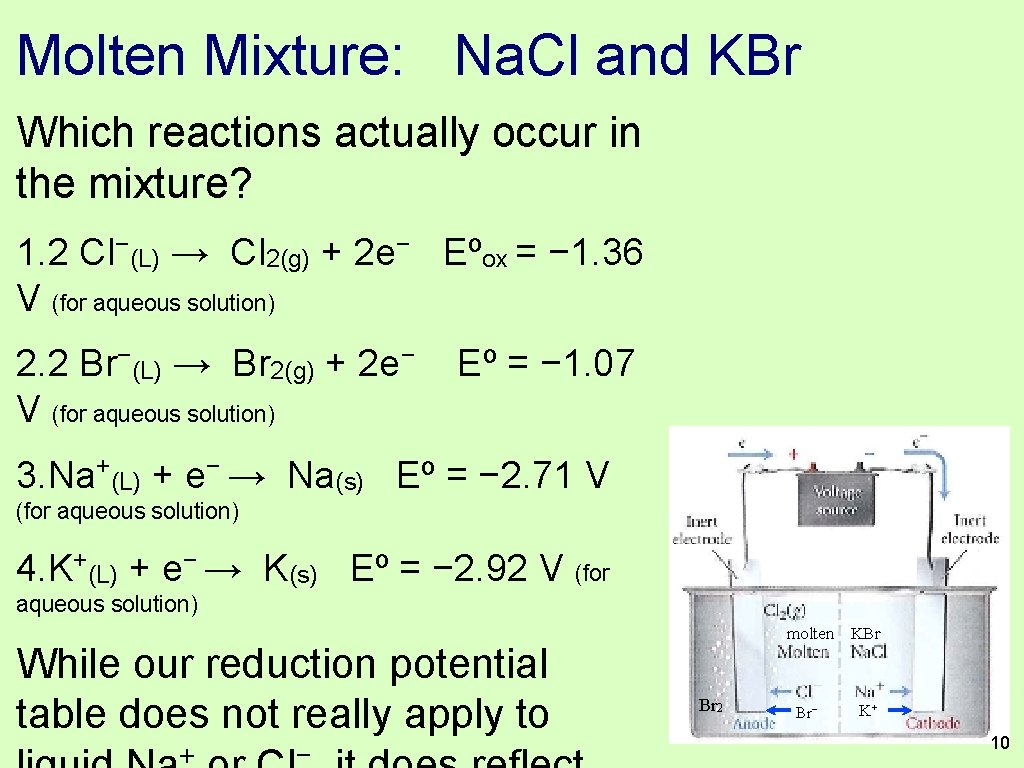
Molten Mixture: Na. Cl and KBr Which reactions actually occur in the mixture? 1. 2 Cl−(L) → Cl 2(g) + 2 e− Eºox = − 1. 36 V (for aqueous solution) 2. 2 Br−(L) → Br 2(g) + 2 e− V (for aqueous solution) Eº = − 1. 07 3. Na+(L) + e− → Na(s) Eº = − 2. 71 V (for aqueous solution) 4. K+(L) + e− → K(s) Eº = − 2. 92 V (for aqueous solution) While our reduction potential table does not really apply to + − Br 2 molten KBr Br− K+ 10

Molten Mixture: Na. Cl and KBr 1. 2 Cl−(L) → Cl 2(g) + 2 e− Eºox = − 1. 36 V (for aqueous solution) 2. 2 Br−(L) → Br 2(g) + 2 e− Eº = − 1. 07 V (for aqueous solution) 3. Na+(L) + e− → Na(s) Eº = − 2. 71 V (for aqueous solution) 4. K+(L) + e− → K(s) Eº = − 2. 92 V (for aqueous solution) The reactions that are “cheaper” are the reactions that occur. Br 2 molten KBr Br− K+ 11

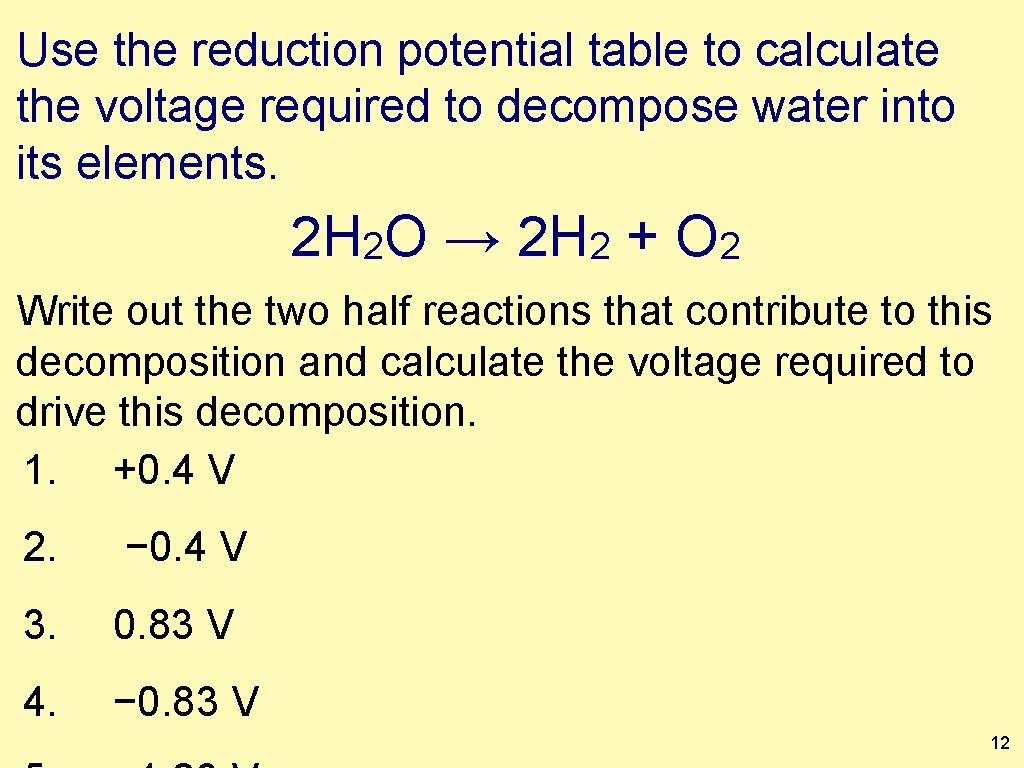
Use the reduction potential table to calculate the voltage required to decompose water into its elements. 2 H 2 O → 2 H 2 + O 2 Write out the two half reactions that contribute to this decomposition and calculate the voltage required to drive this decomposition. 1. +0. 4 V 2. − 0. 4 V 3. 0. 83 V 4. − 0. 83 V 12

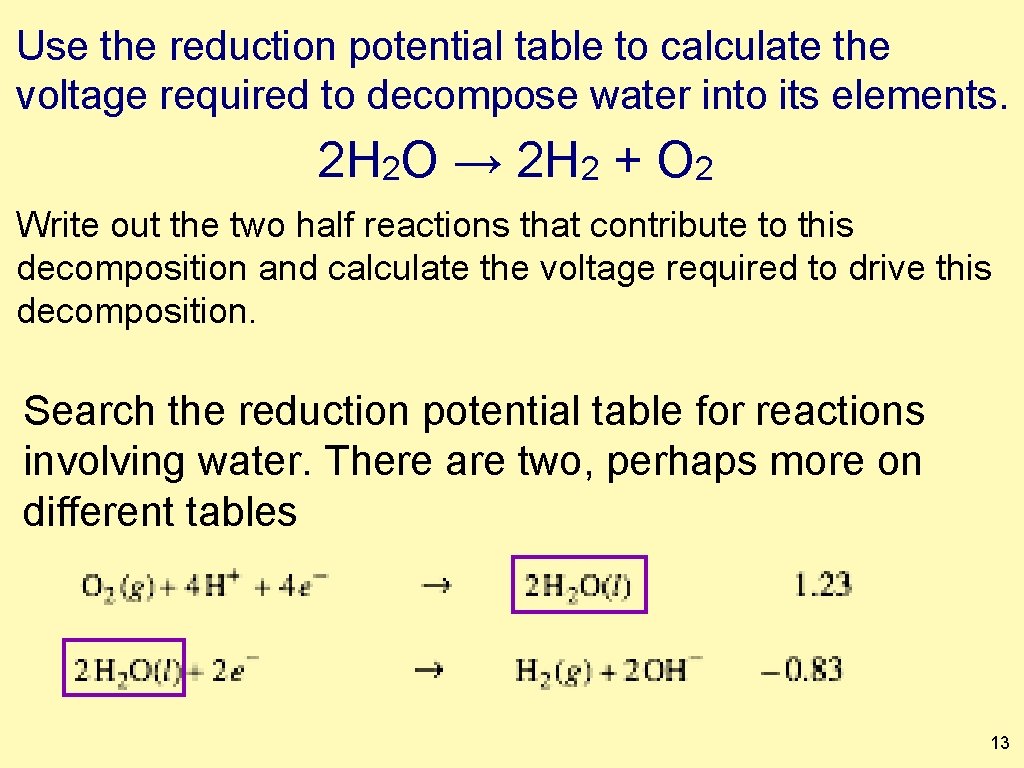
Use the reduction potential table to calculate the voltage required to decompose water into its elements. 2 H 2 O → 2 H 2 + O 2 Write out the two half reactions that contribute to this decomposition and calculate the voltage required to drive this decomposition. Search the reduction potential table for reactions involving water. There are two, perhaps more on different tables 13

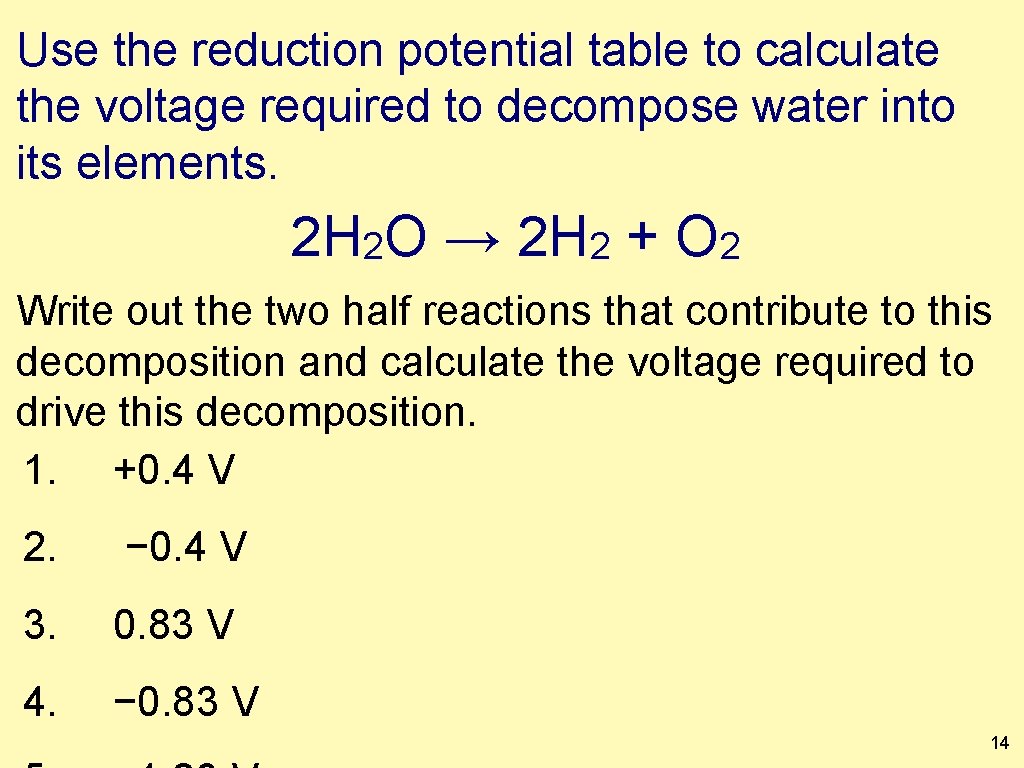
Use the reduction potential table to calculate the voltage required to decompose water into its elements. 2 H 2 O → 2 H 2 + O 2 Write out the two half reactions that contribute to this decomposition and calculate the voltage required to drive this decomposition. 1. +0. 4 V 2. − 0. 4 V 3. 0. 83 V 4. − 0. 83 V 14

Use the reduction table to calculate the voltage required to decompose water into its elements. 2 H 2 O → 2 H 2 + O 2 Write out the two half reactions that contribute to this decomposition. 1. Eºcell = -2. 06 V Let’s look at what’s going on in more detail…. slide show 15

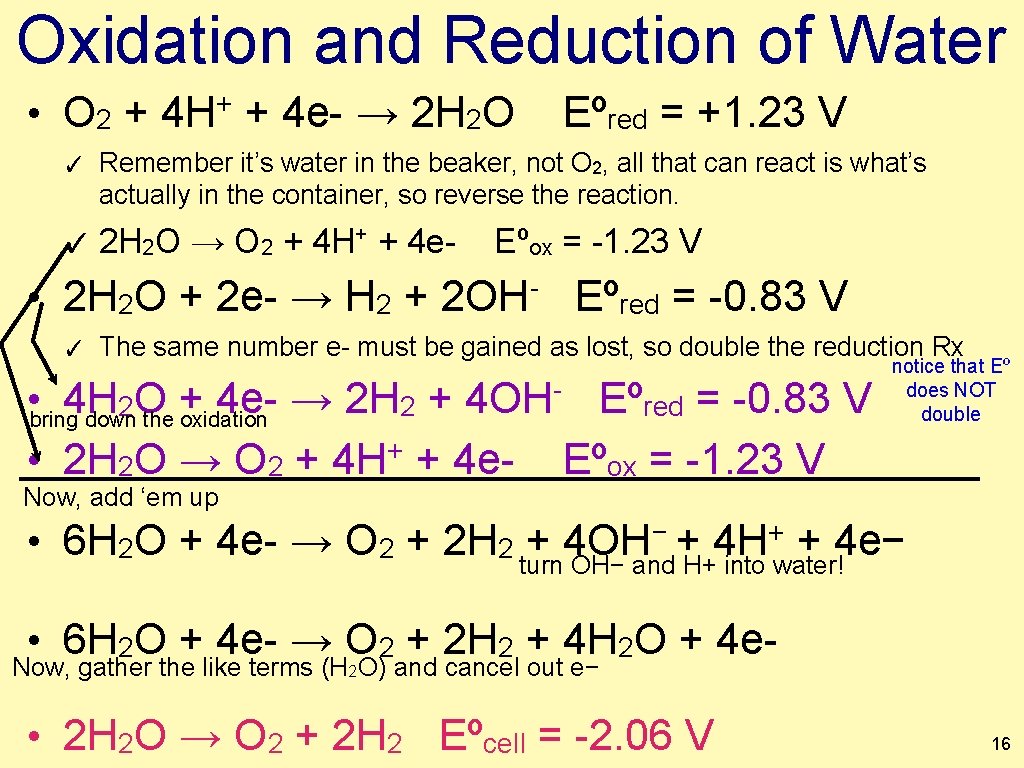
Oxidation and Reduction of Water • O 2 + 4 H+ + 4 e- → 2 H 2 O ✓ Eºred = +1. 23 V Remember it’s water in the beaker, not O 2, all that can react is what’s actually in the container, so reverse the reaction. ✓ 2 H 2 O → O 2 + 4 H+ + 4 e- Eºox = -1. 23 V • 2 H 2 O + 2 e- → H 2 + 2 OH- Eºred = -0. 83 V ✓ The same number e- must be gained as lost, so double the reduction Rx • bring 4 H + 4 e- → 2 H 2 + 2 O down the oxidation 4 OH • 2 H 2 O → O 2 + 4 H+ + 4 e- Now, add ‘em up Eºred = -0. 83 V Eºox = -1. 23 V notice that Eº does NOT double − + 4 H+ + 4 e− • 6 H 2 O + 4 e- → O 2 + 2 H 2 turn + 4 OH OH− and H+ into water! • 6 H 2 O + 4 e- → O 2 + 2 H 2 + 4 H 2 O + 4 e- Now, gather the like terms (H 2 O) and cancel out e− • 2 H 2 O → O 2 + 2 H 2 Eºcell = -2. 06 V 16

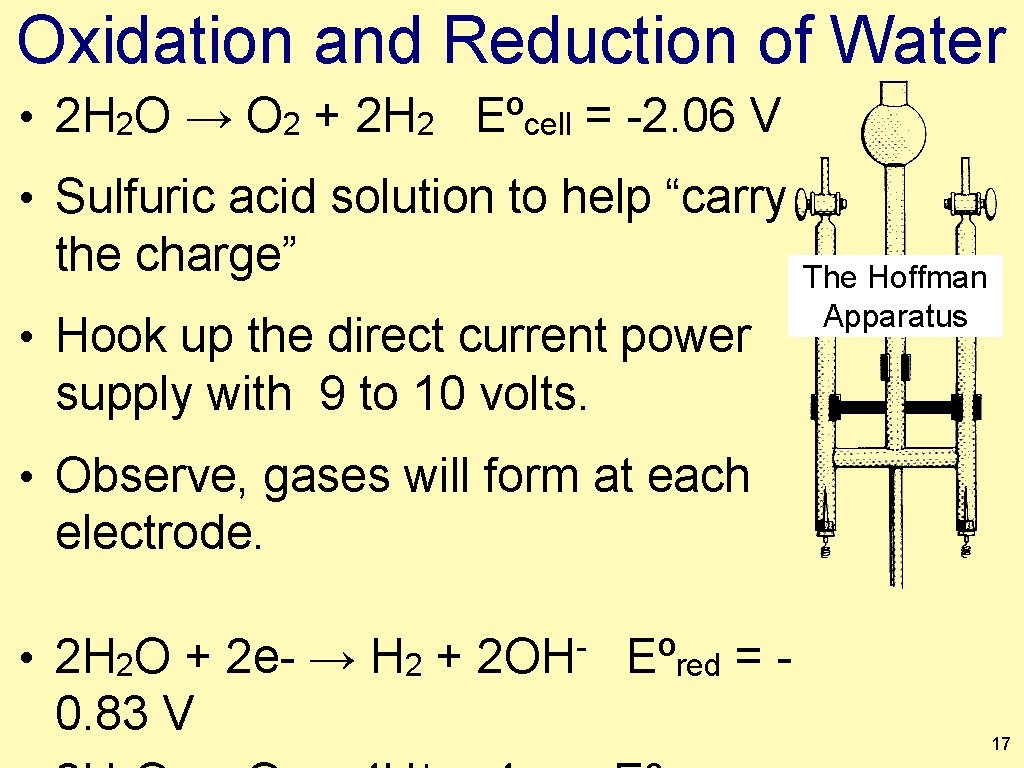
Oxidation and Reduction of Water • 2 H 2 O → O 2 + 2 H 2 Eºcell = -2. 06 V • Sulfuric acid solution to help “carry the charge” • Hook up the direct current power The Hoffman Apparatus supply with 9 to 10 volts. • Observe, gases will form at each electrode. • 2 H 2 O + 2 e- → H 2 + 0. 83 V 2 OH Eºred = 17

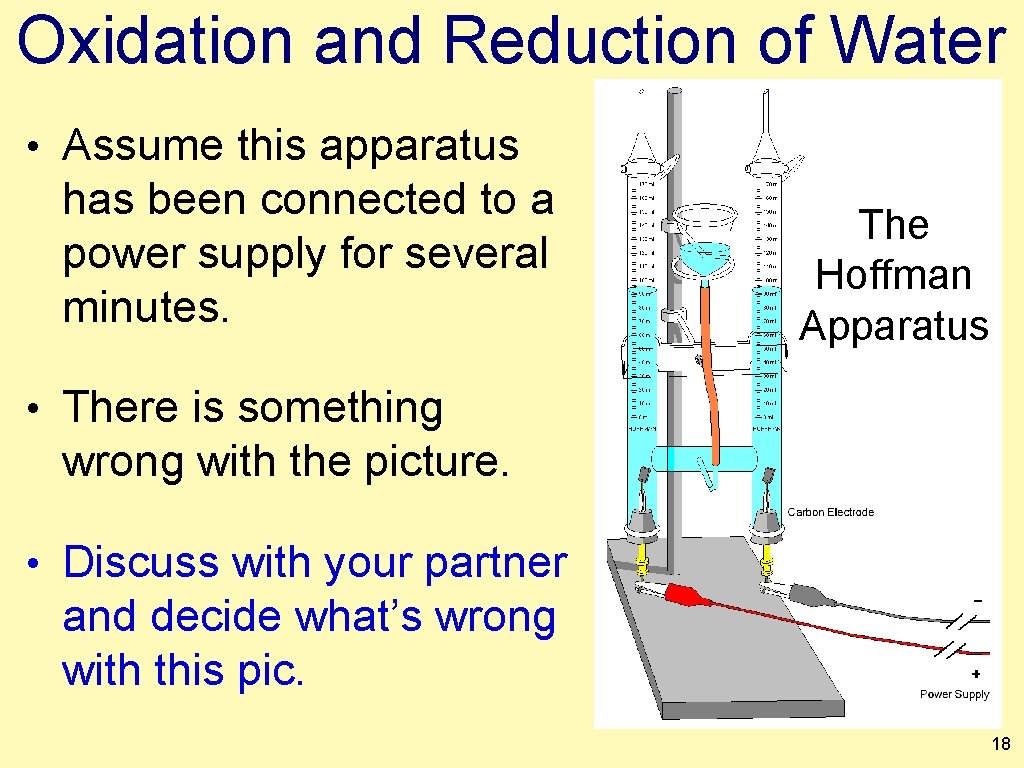
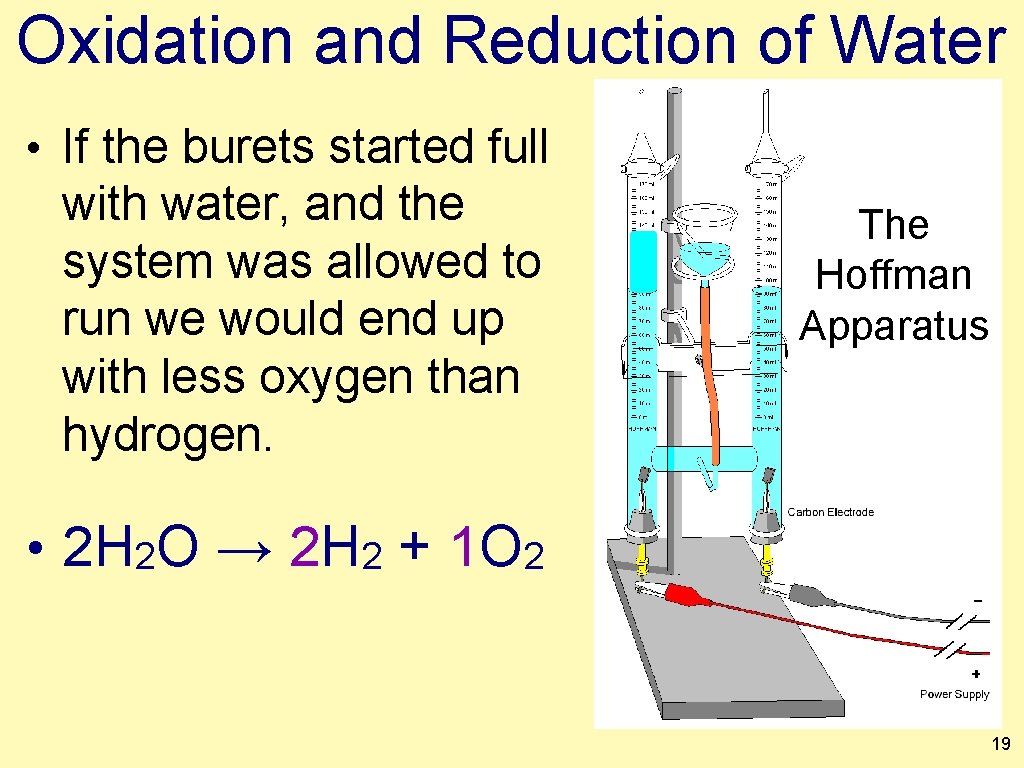
Oxidation and Reduction of Water • Assume this apparatus has been connected to a power supply for several minutes. The Hoffman Apparatus • There is something wrong with the picture. • Discuss with your partner and decide what’s wrong with this pic. 18

Oxidation and Reduction of Water • If the burets started full with water, and the system was allowed to run we would end up with less oxygen than hydrogen. The Hoffman Apparatus • 2 H 2 O → 2 H 2 + 1 O 2 19

Let’s consider a potassium iodide solution that we will run electricity through. Write out all the oxidation and reduction halfreactions that could possibly occur in this solution. Don’t worry about Eº yet…. just consider which reactions are possibilities. 20

Let’s consider a potassium iodide solution that we will run electricity through. Write out all the oxidation and reduction halfreactions that could possibly occur in this solution. Don’t worry about Eº yet…. just consider which reactions are possibilities. Think about what species are actually in + − the beaker… K and I and H 2 O ! 21

Remember to consider all the species that are actually present in this KI(aq) solution. After writing out all the oxidation and reduction half-reactions that could possibly occur in this solution, now let’s look up the potential of these reactions. 22

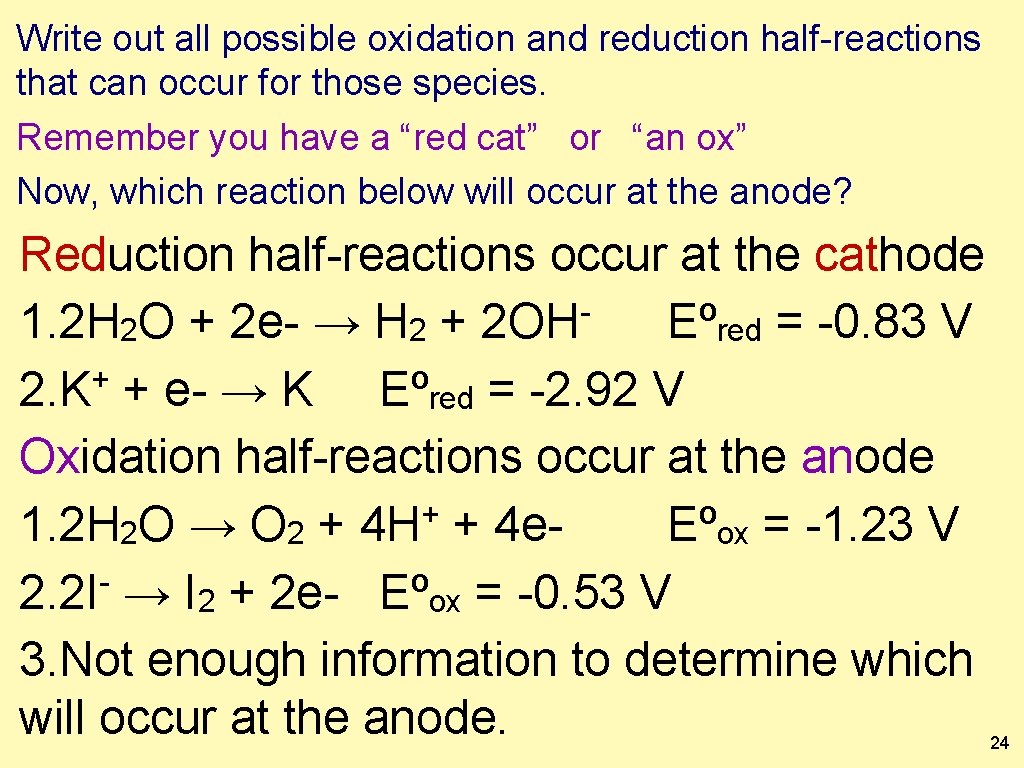
Of the four reactions you should have considered, only one of each will occur. Which reaction will occur at the anode? 1. 2. 3. 4. 5. − 2 OH 2 H 2 O + 2 e- → H 2 + + K + e− → K + 2 H 2 O → O 2 + 4 H + 4 e− 2 I → I 2 + 2 e− Not enough information to determine which will occur at the anode. 23

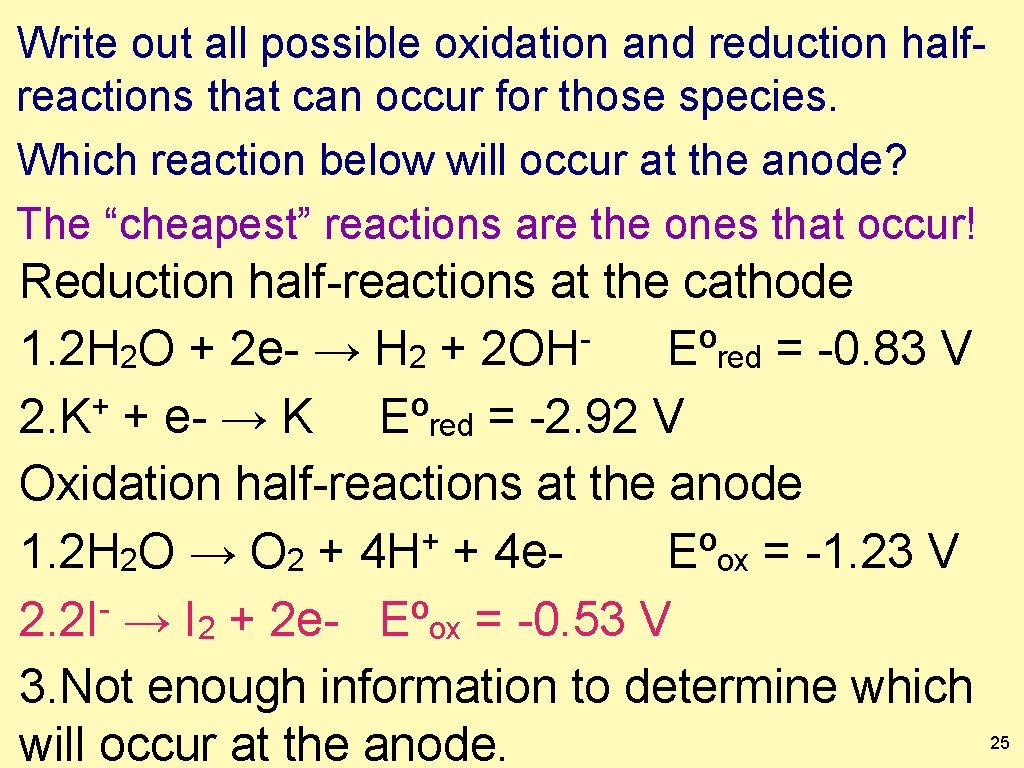
Write out all possible oxidation and reduction half-reactions that can occur for those species. Remember you have a “red cat” or “an ox” Now, which reaction below will occur at the anode? Reduction half-reactions occur at the cathode 1. 2 H 2 O + 2 e- → H 2 + 2 OH Eºred = -0. 83 V + 2. K + e- → K Eºred = -2. 92 V Oxidation half-reactions occur at the anode + 1. 2 H 2 O → O 2 + 4 H + 4 e. Eºox = -1. 23 V 2. 2 I → I 2 + 2 e- Eºox = -0. 53 V 3. Not enough information to determine which will occur at the anode. 24

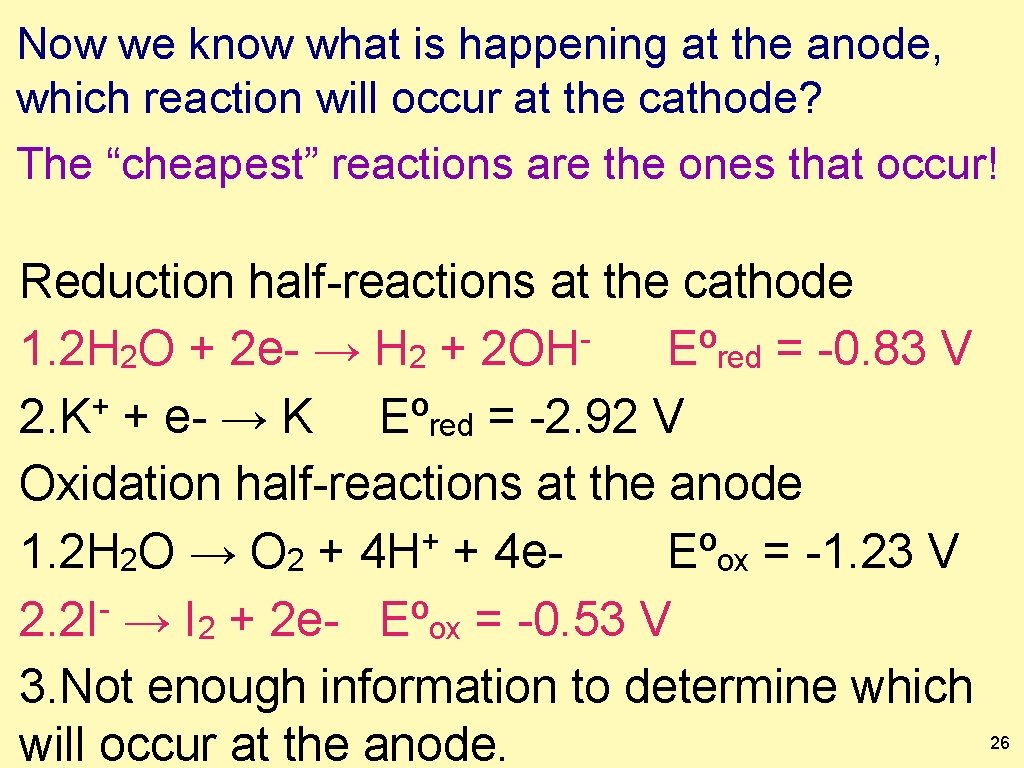
Write out all possible oxidation and reduction halfreactions that can occur for those species. Which reaction below will occur at the anode? The “cheapest” reactions are the ones that occur! Reduction half-reactions at the cathode 1. 2 H 2 O + 2 e- → H 2 + 2 OH Eºred = -0. 83 V + 2. K + e- → K Eºred = -2. 92 V Oxidation half-reactions at the anode + 1. 2 H 2 O → O 2 + 4 H + 4 e. Eºox = -1. 23 V 2. 2 I- → I 2 + 2 e- Eºox = -0. 53 V 3. Not enough information to determine which will occur at the anode. 25

Now we know what is happening at the anode, which reaction will occur at the cathode? The “cheapest” reactions are the ones that occur! Reduction half-reactions at the cathode 1. 2 H 2 O + 2 e- → H 2 + 2 OH Eºred = -0. 83 V + 2. K + e- → K Eºred = -2. 92 V Oxidation half-reactions at the anode + 1. 2 H 2 O → O 2 + 4 H + 4 e. Eºox = -1. 23 V 2. 2 I- → I 2 + 2 e- Eºox = -0. 53 V 3. Not enough information to determine which will occur at the anode. 26

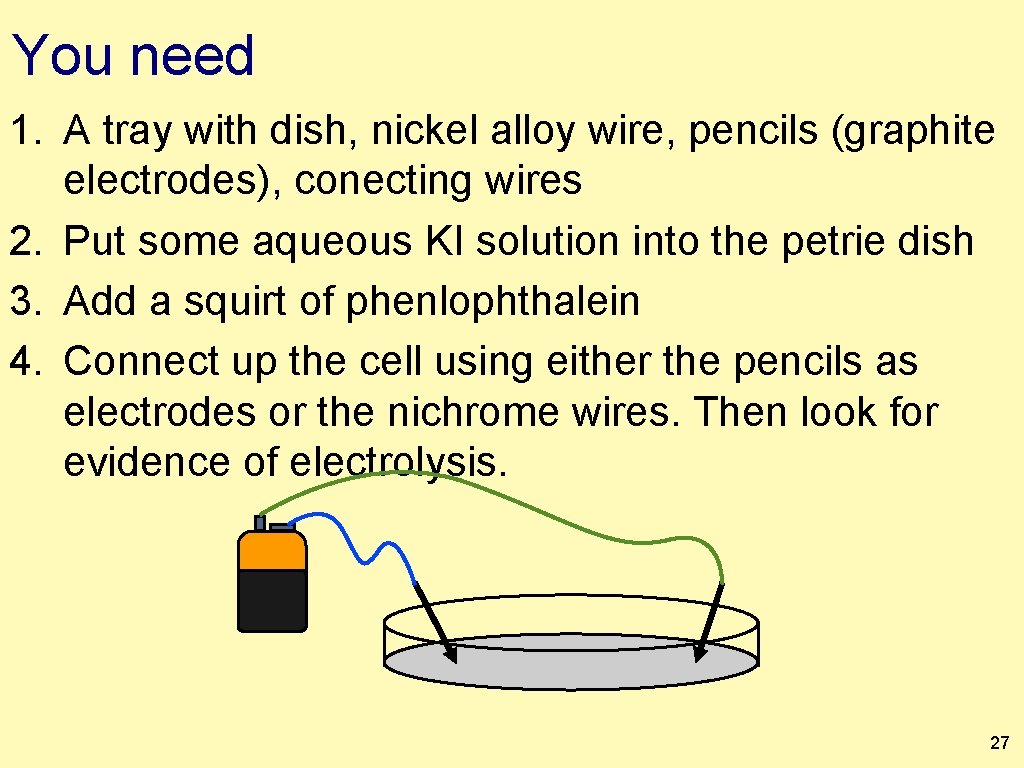
You need 1. A tray with dish, nickel alloy wire, pencils (graphite electrodes), conecting wires 2. Put some aqueous KI solution into the petrie dish 3. Add a squirt of phenlophthalein 4. Connect up the cell using either the pencils as electrodes or the nichrome wires. Then look for evidence of electrolysis. 27

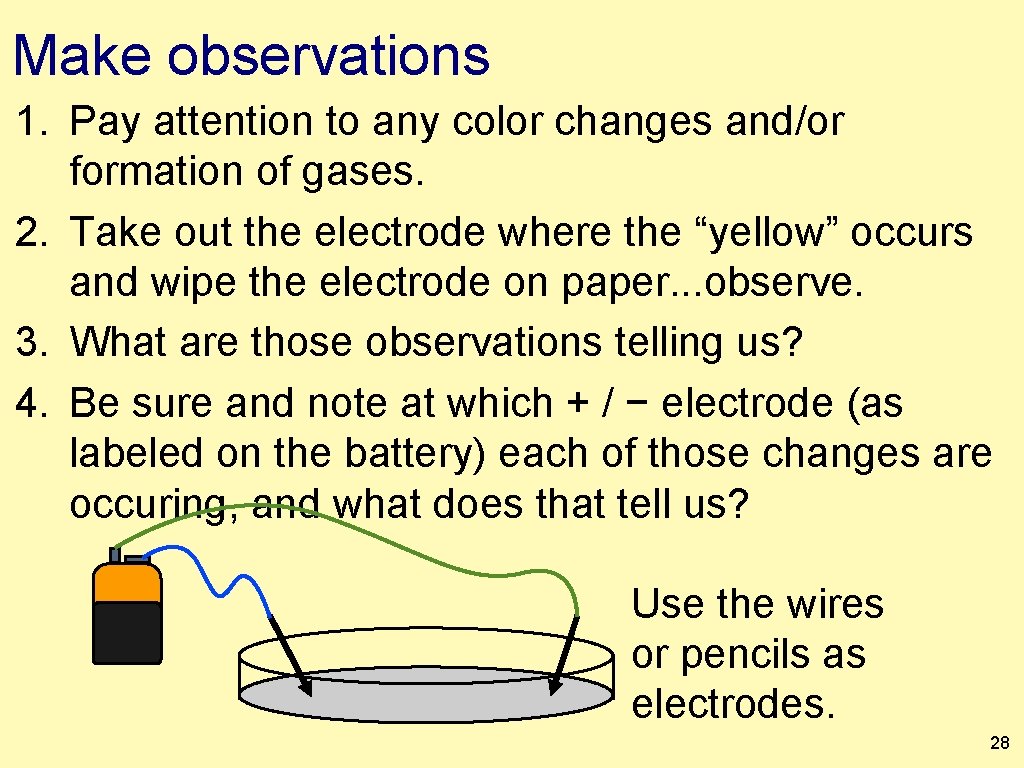
Make observations 1. Pay attention to any color changes and/or formation of gases. 2. Take out the electrode where the “yellow” occurs and wipe the electrode on paper. . . observe. 3. What are those observations telling us? 4. Be sure and note at which + / − electrode (as labeled on the battery) each of those changes are occuring, and what does that tell us? Use the wires or pencils as electrodes. 28

Consider the electrolysis done in class. − − 2 I + 2 H 2 O → H 2 + I 2 + 2 OH If the + and − markings were worn off the battery, the yellow/brown substance would form at the pencil attached to the which end of the battery? 1. 2. 3. + no way of knowing 29

Consider the electrolysis done in class. 2 I− + 2 H 2 O → H 2 + I 2 + 2 OH− If the + and - markings were worn off the battery, the yellow solution would form at the pencil attached to which end of the battery? 1. • • 2. 3. + The negatively labeled (−) external electrode of the battery supplies the electrons and the positive (+) external electrode receives electrons to complete the circuit. − Since I is reduced, at the cathode, those electrons will return to the battery at its + end. − no way of knowing 30

Electrochemistry Quantifying electrolysis watching the amount of charge flowing during the electrolysis 31

To quantify electrolysis, we should know. . . 1. The charge on 1 electron • this is on the formula sheet in the atomic theory section. 2. The “flow” of electrons is measured in amps • this is not on the formula sheet, but that’s ok, you won’t need it. • The Faraday Constant (F ) (aka the Faraday) is the amount of charge per mole of electrons 32

Consider the electrolysis done in class. − − 2 I + 2 H 2 O → H 2 + I 2 + 2 OH If you ran the system for 2. 0 minutes at 0. 50 amps, the amount of electric charge in coulombs, that passed is 1. 2. 3. 4. 5. 0. 25 coulomb 1. 0 coulomb 4. 0 coulombs 60. coulombs 120 coulombs 33

Consider the electrolysis done in class. − − 2 I + 2 H 2 O → H 2 + I 2 + 2 OH If you ran the system for 2 minutes at 0. 5 amps, the amount of electric charge in coulombs, that passed is 1. 2. 3. 4. 5. 0. 25 coulomb 1. 0 coulomb 4. 0 coulombs 60. coulombs 120 coulombs 34

Consider the electrolysis done in class. − − 2 I + 2 H 2 O → H 2 + I 2 + 2 OH If you ran the system for 2. 0 minutes at 0. 50 amps, the moles of hydrogen gas formed is 1. 2. 3. 4. 5. 6. 60. moles 120 moles 0. 00062 mole 0. 0012 mole 0. 00031 mole cannot be determined without more information. 35

Consider the electrolysis done in class. − − 2 I + 2 H 2 O → H 2 + I 2 + 2 OH If you ran the system for 2 minutes at 0. 5 amps, the moles of hydrogen gas formed is 1. 2. 3. 4. 5. 6. 60. moles 120 moles 0. 00062 mole 0. 0012 mole 0. 00031 mole cannot be determined without more information. 36

Consider the electrolysis done in class. − − 2 I + 2 H 2 O → H 2 + I 2 + 2 OH If you ran the system for 2 minutes at 0. 5 amps, the volume of hydrogen gas you could collect would be closest to. Assume room temp, 25ºC and 1 atm of pressure. 1. 2. 3. 4. 5. 100 ml 2. 5 ml 7. 5 ml 15 ml 22. 4 ml 37

Consider the electrolysis done in class. − − 2 I + 2 H 2 O → H 2 + I 2 + 2 OH If you ran the system for 2 minutes at 0. 5 amps, the volume of hydrogen gas you could collect would be closest to Assume room temp, 25º and 1 atm of pressure. 1. 2. 3. 4. 5. 100 ml 2. 5 ml 7. 5 ml 15 ml 22. 4 ml 38

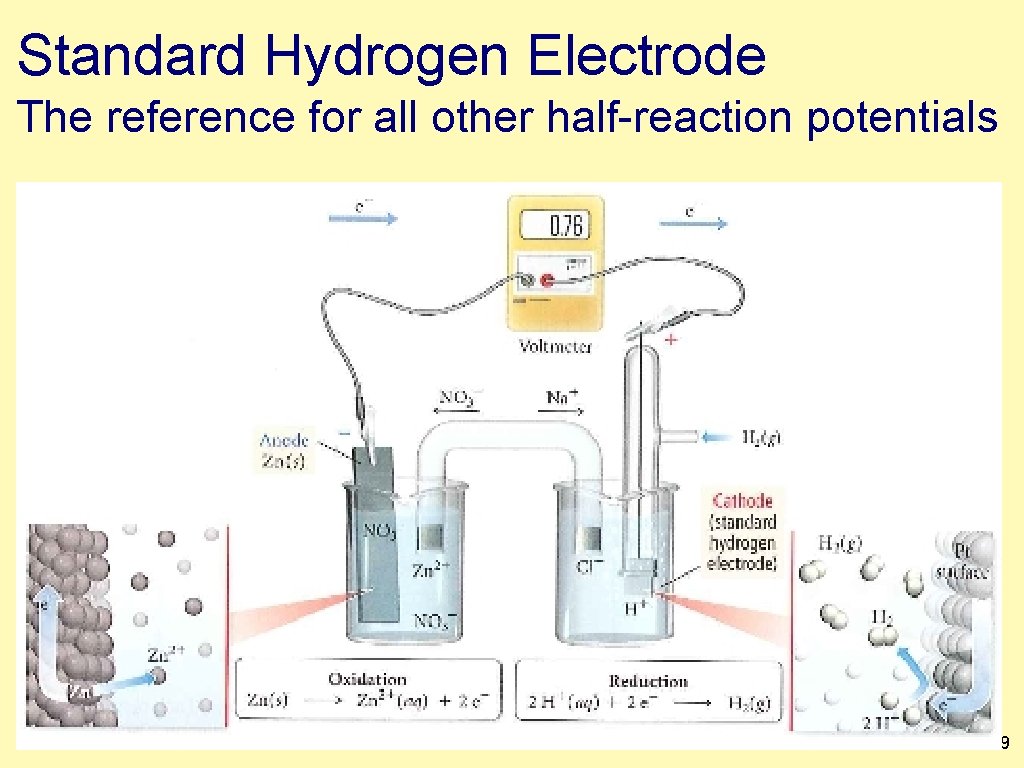
Standard Hydrogen Electrode The reference for all other half-reaction potentials 1. 39

If an electrolytic cell was allowed to run at 1. 5 amps for 2 hours, what mass of zinc could be deposited onto an electrode from a zinc nitrate solution? 40

If an electrolytic cell was allowed to run at 1. 5 amps for 2 hours, what mass of zinc could be deposited onto an electrode from a zinc nitrate solution? 3. 7 g Zn deposited Let’s look at how to calculate…. 41

If an electrolytic cell was allowed to run at 1. 5 amps for 2 hours, what mass of zinc could be deposited onto an electrode from a zinc nitrate solution? 3. 7 g Zn deposited 42

Calculate the molar mass of a metal which was deposited from a 3+ ion in solution in an electrolytic cell that ran at 4. 5 amps for 3. 0 hours, and 11. 7 g of metal was deposited. 43

Calculate the molar mass of a metal which was deposited from a 3+ ion in solution in an electrolytic cell that ran at 4. 5 amps for 3. 0 hours, and 11. 7 g of metal was deposited. 69. 7 g/mol maybe gallium Let’s look at how to calculate…. 44

Calculate the molar mass of a metal which was deposited from a 3+ ion in solution in an electrolytic cell that ran at 4. 5 amps for 3. 0 hours, and 11. 7 g of metal was deposited. MM = 69. 7 g/mol, maybe the metal is Gallium OR, if you prefer. . . 45

0. 937 g of chromium was deposited in an electrolytic cell after it was run for 1. 55 hours at 1. 87 amps. What was the charge of chromium in solution? 46

0. 937 g of chromium was deposited in an electrolytic cell after it was run for 1. 55 hours at 1. 87 amps. What was the charge of chromium in solution? 6+ charge Chromium has 4 oxidation states: 0, 2+, 3+, 6+ Let’s look at how to calculate…. 47

0. 937 g of chromium was deposited in an electrolytic cell after it was run for 1. 55 hours at 1. 87 amps. What was the charge of chromium in solution? 6+ charge The charge is mole of electrons transferred per mole of the metal. OR, if you prefer. . . 48

Electro related to Thermo Perhaps this section should be saved until after unit C, Thermochemistry and Thermodynamics 49

+2 Ni/Ni A voltaic cell is set up using and +3 Al/Al as the two half-cells. Calculate Eº for the reaction of this cell. 50

+2 Ni/Ni A voltaic cell is set up using and +3 Al/Al as the two half-cells. Calculate Eº for the reaction of this cell. • • • Ni+2 → Ni Eºred = -0. 25 +23 Al → Al Eºox = +1. 66 Eº = +1. 41 V 51

+2 Ni/Ni A voltaic cell is set up using and +3 Al/Al as the two half-cells. ∆G for the reaction of of this cell is 1. positive Do not use your calculator 2. negative 3. too difficult to figure out without a calculator. 52

+2 Ni/Ni A voltaic cell is set up using and +3 Al/Al as the two half-cells. ∆G for the reaction of of this cell is 1. positive Do not use your calculator 2. negative • We know that a positive Eº must mean a negative ∆G. 3. too difficult to figure out without a calculator. 53

+2 Ni/Ni A voltaic cell is set up using and +3 Al/Al as the two half-cells. Calculate ∆G for the reaction of of this cell. input a value You may use your calculator 54

+2 Ni/Ni A voltaic cell is set up using and +3 Al/Al as the two half-cells. Calculate ∆G for the reaction of of this cell. • ∆G = - n. FE • ∆G = - 6 mole e-/3 mole Al * 96, 500 coul/mole e- * 1. 41 joule/coul • • ∆G = -816 k. J which is, ∆G = -816 k. J/ 3 mol Ni or 2 mol Al 55

+2 Ni/Ni A voltaic cell is set up using and +3 Al/Al as the two half-cells. Calculate Keq for the reaction of of this cell. 56

+2 Ni/Ni A voltaic cell is set up using and +3 Al/Al as the two half-cells. Calculate Keq for the reaction of of this cell. • • • log. Keq = = n. E/0. 0592 log. Keq = = (6*1. 41)/0. 0592 log. Keq = = 142 Keq = = 10142 You can not do this on your calculator, because your calculator can only handle 1099 57

Calculate Keq for some voltaic cell in which one electron is transferred and the voltage is only 0. 1 V Report your answer. 58

Calculate Keq for some voltaic cell in which one electron is transferred and the voltage is only 0. 1 V • log K = n. Eº/0. 0592 • log K = 1(. 1)/0. 0592 • log K = 1. 69 • K = 49 59

Calculate Keq for some voltaic cell in which one mole electron is transferred and the voltage is only 0. 2 V Report your answer. 60

Calculate Keq for some voltaic cell in which one electron is transferred and the voltage is only 0. 2 V • • • log K = n. Eº/0. 0592 log K = 1(. 1)/0. 0592 log K = 3. 38 K = 2390 Many voltaic cells transfer more than one electron and have voltages larger than 0. 1 or 0. 2, thus K values are very large for these spontaneous cells. 61

The Keq for all spontaneous voltaic cell reactions is likely to be in the order of magnitude of -10 1. 10 2. 10 7 3. 10 15 4. 10 5. I have no idea of how to even consider this. 62

The Keq for all spontaneous voltaic cell reactions is likely to be in the order of magnitude of -10 1. 10 2. 10 3 3. 10 15 4. 10 15 • Either is possible, however, 10 is most 3 likely, even 10 would be for very small voltages. 1. I have no idea of how to even consider this. 63

+2 3 X +3 2 Al + → + 3 X The total number of electrons transfered is 1. 2. 3. 4. 5. 2 3 5 6 no way of knowing without the identity of X 64

+2 3 X +3 2 Al + → + 3 X The total number of electrons transfered is 1. 2. 3. 4. 2 3 5 6 -1 • Since Al indicates that 6 e are transfered, the X element must also gain 6 e-1 5. no way of knowing without the identity of X 65

+2 3 X +3 2 Al + → + 3 X Using only your blue sheets, which element of the choices below is X most likely to be for the reaction to occur? 1. 2. 3. 4. 5. Cr Ag Co Mg no way of knowing the identity of X without more info 66

+2 3 X +3 2 Al + → + 3 X This reaction does occur, which element of the choices below is X most likely to be? 1. Cr 2. Ag 3. Cd • This you can know because Cd can gain 2 e • You might think it could be Mg, however, the reduction of Mg would give a negative Eº and would not be spontaneous as is implied in the question. 4. Mg 5. no way of knowing without the identity of X 67

+2 3 X +3 2 Al + → + 3 X Eº = 1. 41 The identity of X is likely to be 1. 2. 3. 4. 5. 6. Cu Sn Ni Cd Cr no way of knowing 68

+2 3 X +3 2 Al + → + 3 X Eº = 1. 41 The identity of X is likely to be 1. Cu 2. Sn 3. Ni+2 → Ni Eºred = -0. 25 +3 • Al → Al Eºox = +1. 66 together Eºcell = 1. 41 4. Cd 5. Cr 6. no way of knowing 69

A voltaic cell based on the reaction below was found to have a voltage of 0. 84 V Which of the following could account for the +1 +2 observed voltage? Zn + 2 H → Zn + H 2 increase in [Zn+2] anode compartment +1 increase in [H ] in the cathode compartment decrease in H 2 pressure increase in the size of the Zn electrode addition of sodium nitrate to the anode compartment 6. replacement of the salt bridge with a platinum wire. 7. the voltage was allowed to run for several minutes 1. 2. 3. 4. 5. 70

A voltaic cell based on the reaction below was found to have a voltage of 0. 84 V Which of the following could account for the observed voltage? Zn + 2 H+1 → Zn+2 + H 2 • • 1. 2. 3. • • 1. 2. First it’s important to realize that 0. 84 is greater voltage than Eº, thus you are looking for factors that will increase voltage. Use Le. Chatelier’s to help. increase in [Zn+2] anode compartment increase in [H+1] in the cathode compartment decrease in H 2 pressure increase in the size of the Zn electrode addition of sodium nitrate to the anode compartment replacement of the salt bridge with a platinum wire. the voltage was allowed to run for several minutes 71

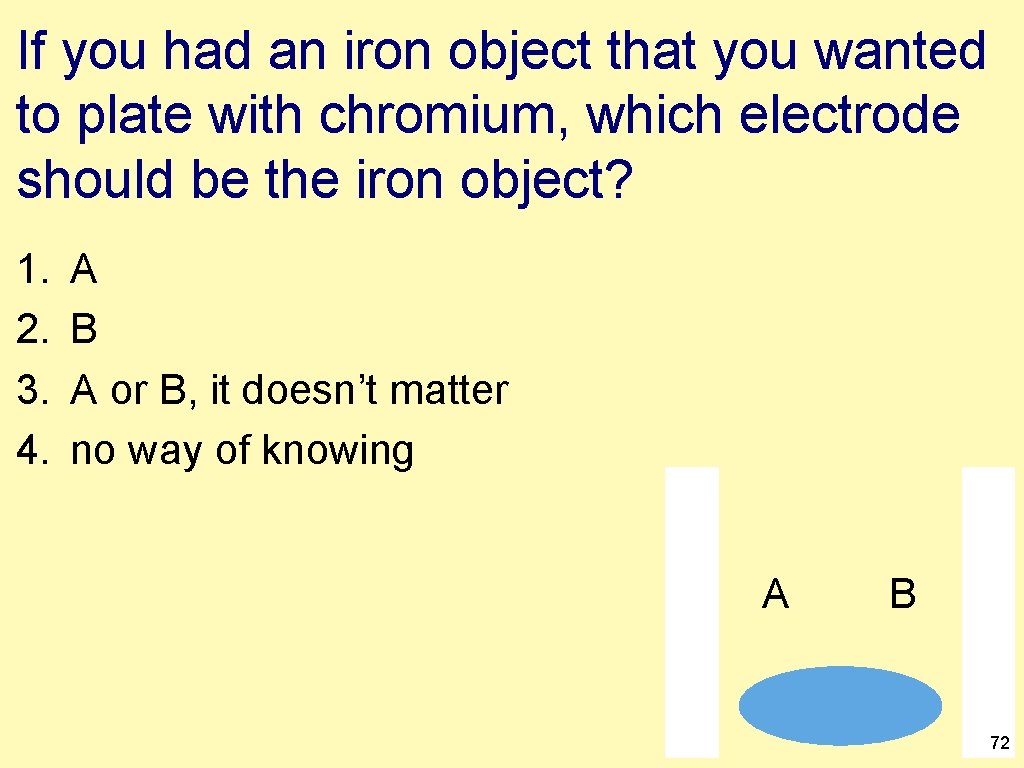
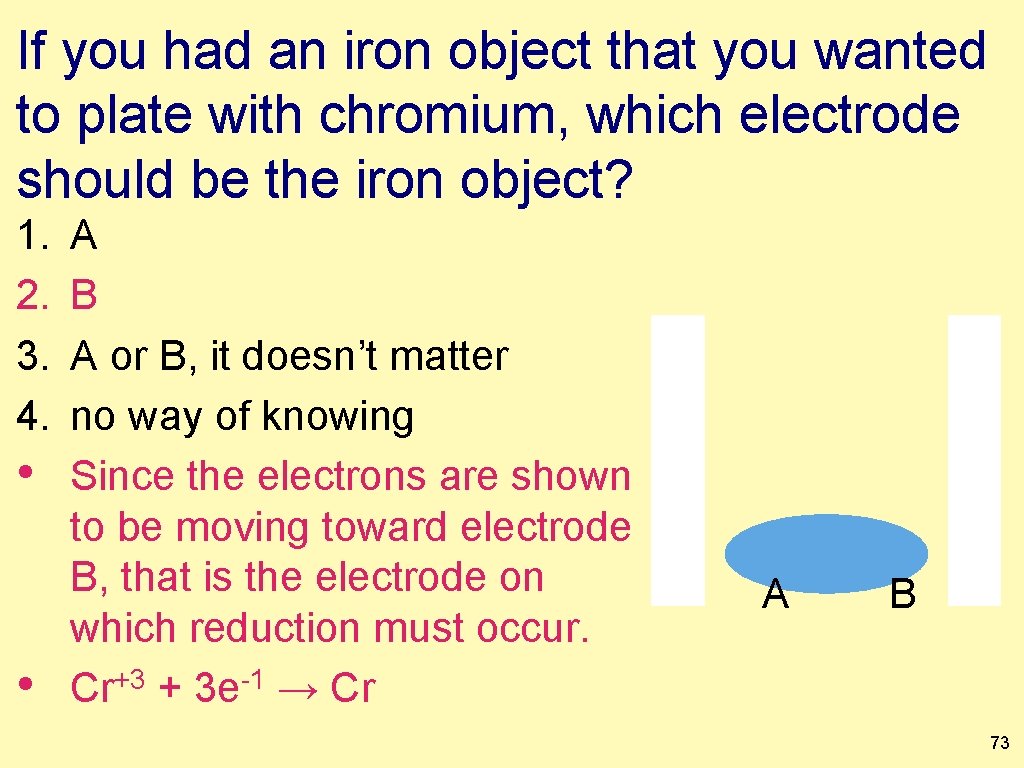
If you had an iron object that you wanted to plate with chromium, which electrode should be the iron object? 1. 2. 3. 4. A B A or B, it doesn’t matter no way of knowing A B 72

If you had an iron object that you wanted to plate with chromium, which electrode should be the iron object? 1. 2. 3. 4. • • A B A or B, it doesn’t matter no way of knowing Since the electrons are shown to be moving toward electrode B, that is the electrode on which reduction must occur. Cr+3 + 3 e-1 → Cr A B 73

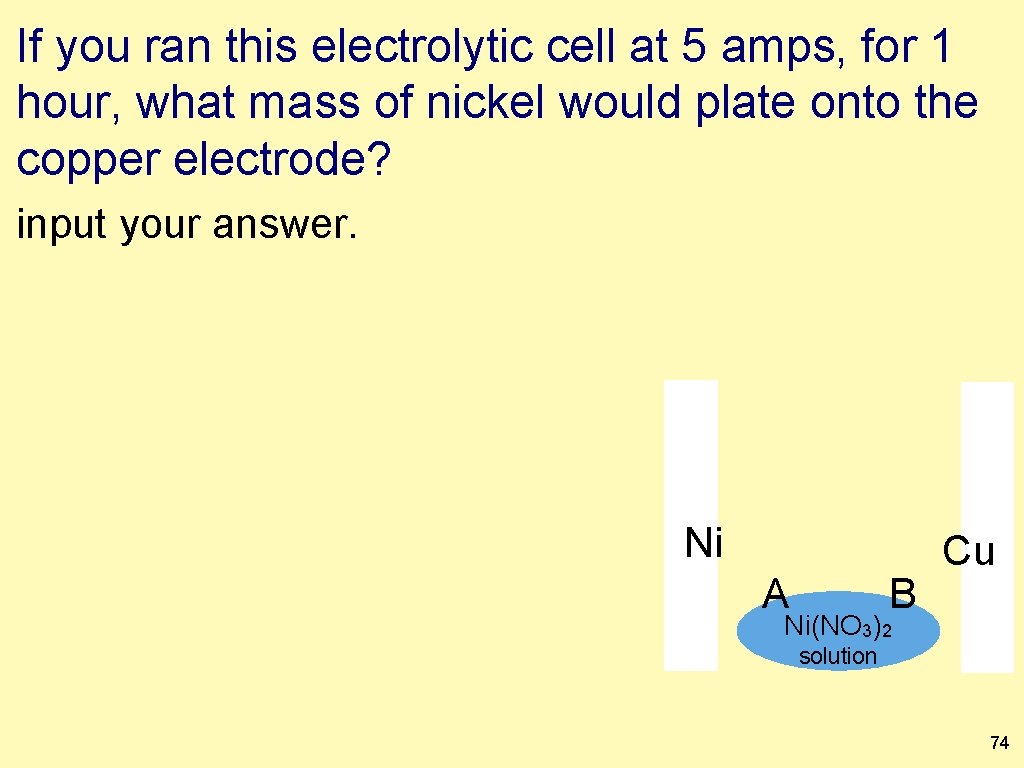
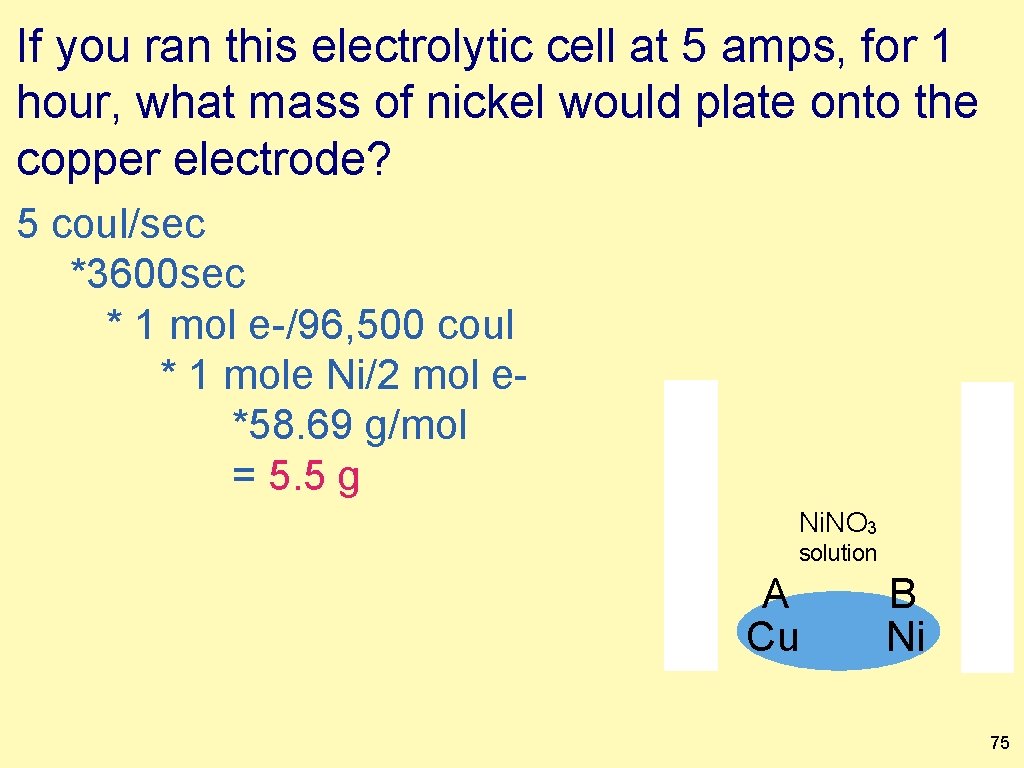
If you ran this electrolytic cell at 5 amps, for 1 hour, what mass of nickel would plate onto the copper electrode? input your answer. Ni A B Cu Ni(NO 3)2 solution 74

If you ran this electrolytic cell at 5 amps, for 1 hour, what mass of nickel would plate onto the copper electrode? 5 coul/sec *3600 sec * 1 mol e-/96, 500 coul * 1 mole Ni/2 mol e*58. 69 g/mol = 5. 5 g Ni. NO 3 solution A Cu B Ni 75

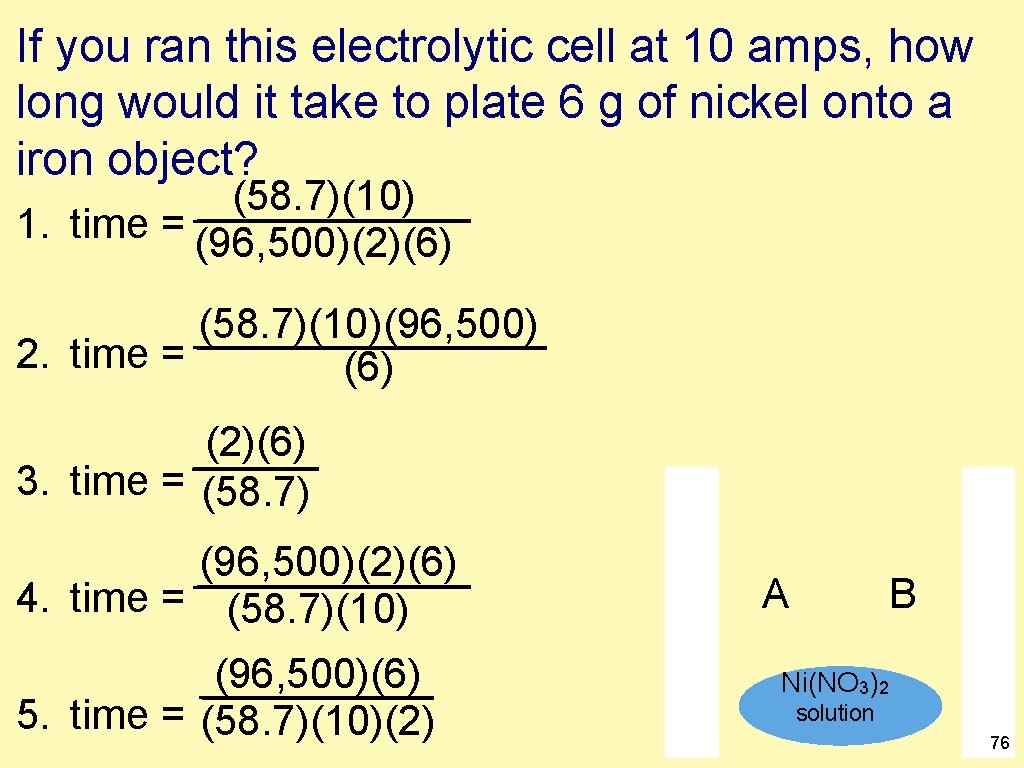
If you ran this electrolytic cell at 10 amps, how long would it take to plate 6 g of nickel onto a iron object? (58. 7)(10) 1. time = (96, 500)(2)(6) (58. 7)(10)(96, 500) 2. time = (6) (2)(6) 3. time = (58. 7) (96, 500)(2)(6) 4. time = (58. 7)(10) (96, 500)(6) 5. time = (58. 7)(10)(2) A B Ni(NO 3)2 solution 76

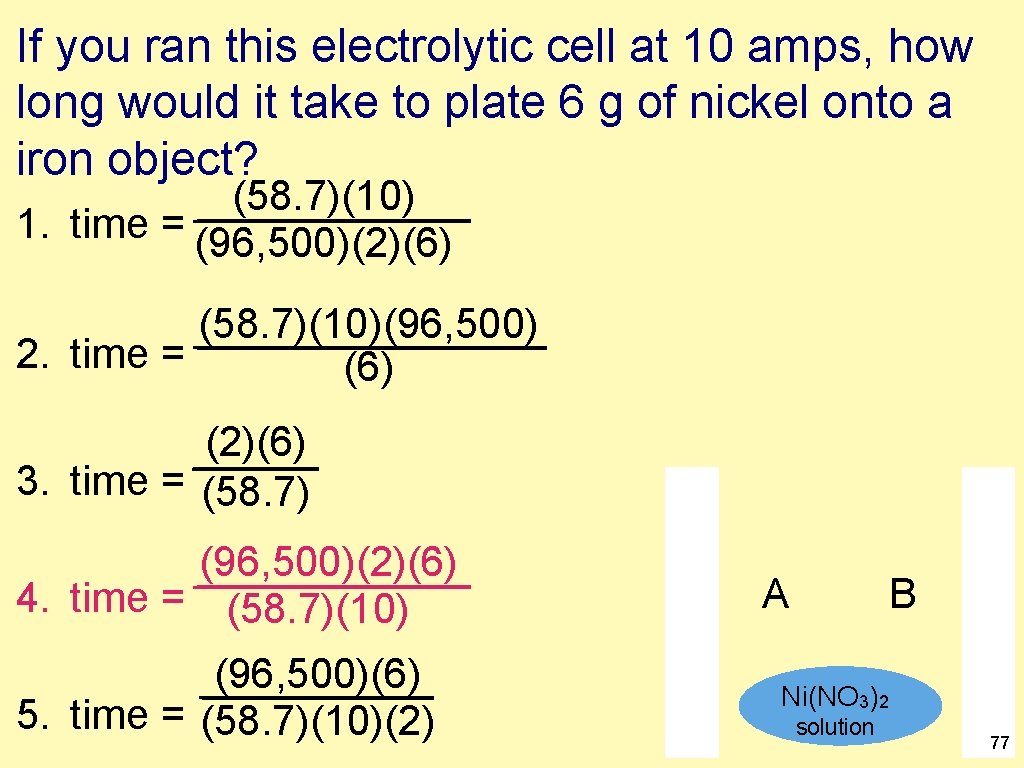
If you ran this electrolytic cell at 10 amps, how long would it take to plate 6 g of nickel onto a iron object? (58. 7)(10) 1. time = (96, 500)(2)(6) (58. 7)(10)(96, 500) 2. time = (6) (2)(6) 3. time = (58. 7) (96, 500)(2)(6) 4. time = (58. 7)(10) (96, 500)(6) 5. time = (58. 7)(10)(2) A B Ni(NO 3)2 solution 77

If you were electroplating aluminum, and. 60 faraday was passed through the aluminum sulfate solution, what mass of aluminum would plate out? 1. 2. 3. 4. 5. 6 x 10 -5 g -4 1. 7 x 10 g 0. 60 g 5. 4 g 16. 2 g 48. 6 g 78

If you were electroplating aluminum, and. 60 faraday was passed through the aluminum sulfate solution, what mass of aluminum would plate out? 1. 2. 3. 4. 5. 6 x 10 -5 g 1. 7 x 10 -4 g 0. 60 g 5. 4 g By definition, 1 faraday = 96, 500 coulombs • (0. 6 faraday)(96, 500 coul/1 faraday)(1 mole e-/96, 500 coul)(1 mole Al/3 mole e-)(27 g/1 mole) • Alternatively: (0. 6 faraday)(1 mole e/1 faraday)((1 mole Al/3 mole e-)(27 g/1 mole) 1. 16. 2 g 2. 48. 6 g 79