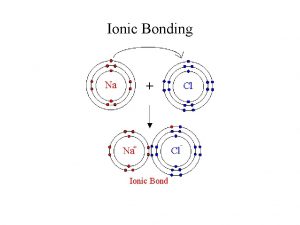
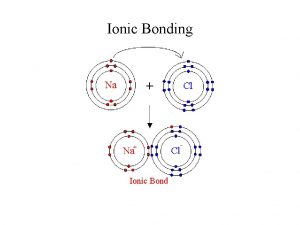
Electrolysis of molten ionic compounds To review electrolysis








- Slides: 24

Electrolysis of molten ionic compounds To review electrolysis and look at products in molten compounds (melts).

Instructions • On slides 3, 8, 9, 13 and 17 there activities to complete or watch • On slide 18 there is a suggested diagram to make a mind map to finish and bring the information together- this is optional. • Slide 19 onwards is the answers for slides 3, 8, 9 and 17, please review your work

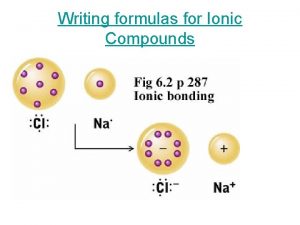
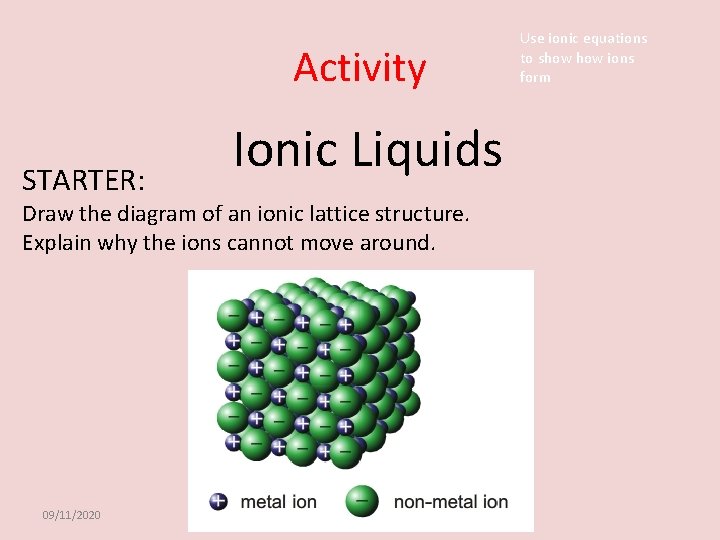
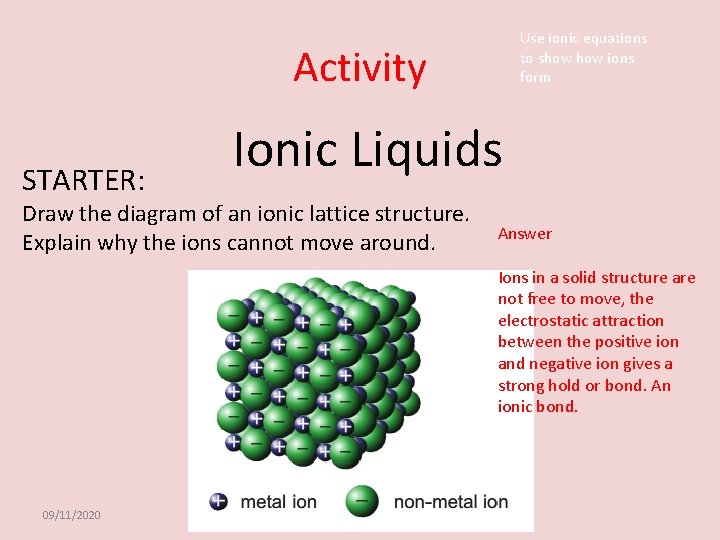
Activity STARTER: Ionic Liquids Draw the diagram of an ionic lattice structure. Explain why the ions cannot move around. 09/11/2020 Use ionic equations to show ions form


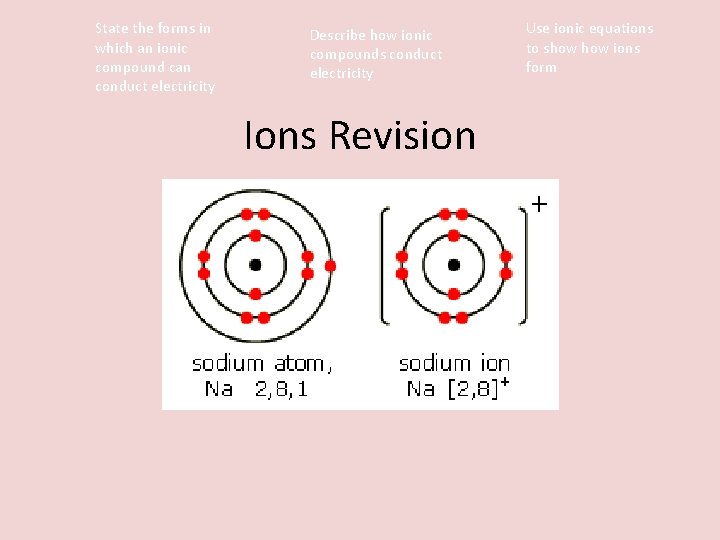
State the forms in which an ionic compound can conduct electricity Describe how ionic compounds conduct electricity Ions Revision Use ionic equations to show ions form

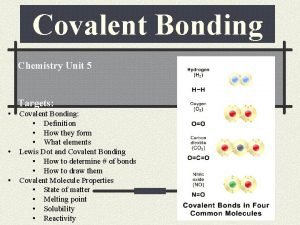
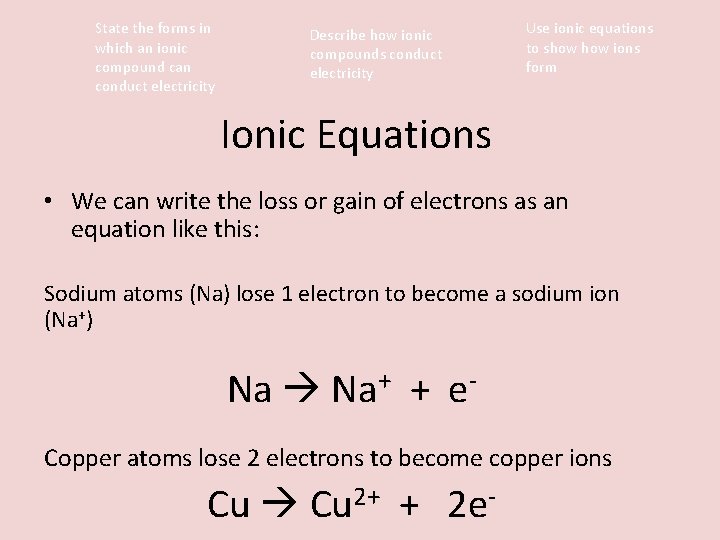
State the forms in which an ionic compound can conduct electricity Describe how ionic compounds conduct electricity Use ionic equations to show ions form Ionic Equations • We can write the loss or gain of electrons as an equation like this: Sodium atoms (Na) lose 1 electron to become a sodium ion (Na+) Na Na+ + e. Copper atoms lose 2 electrons to become copper ions Cu 2+ + 2 e-

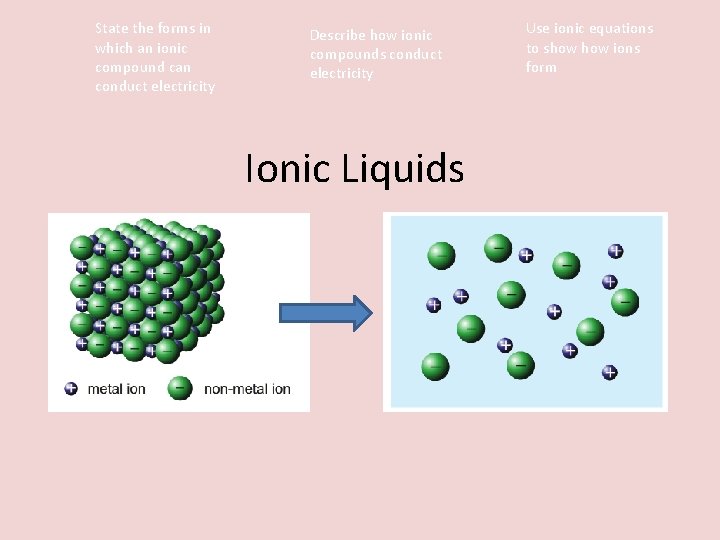
State the forms in which an ionic compound can conduct electricity Describe how ionic compounds conduct electricity Ionic Liquids Use ionic equations to show ions form

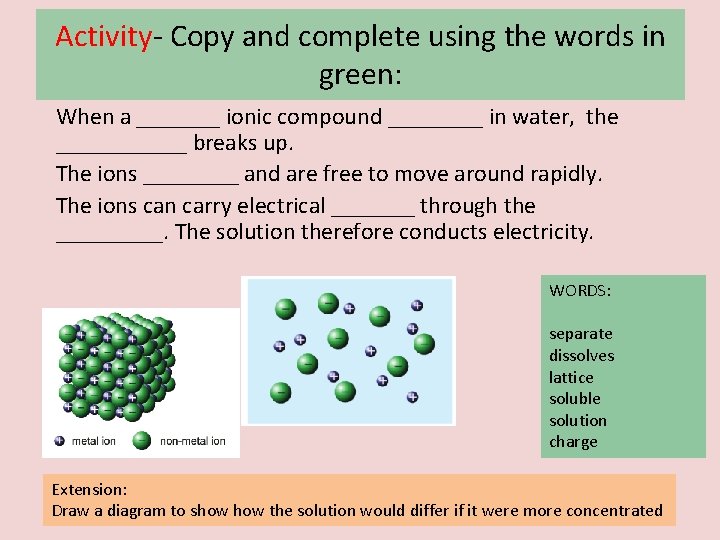
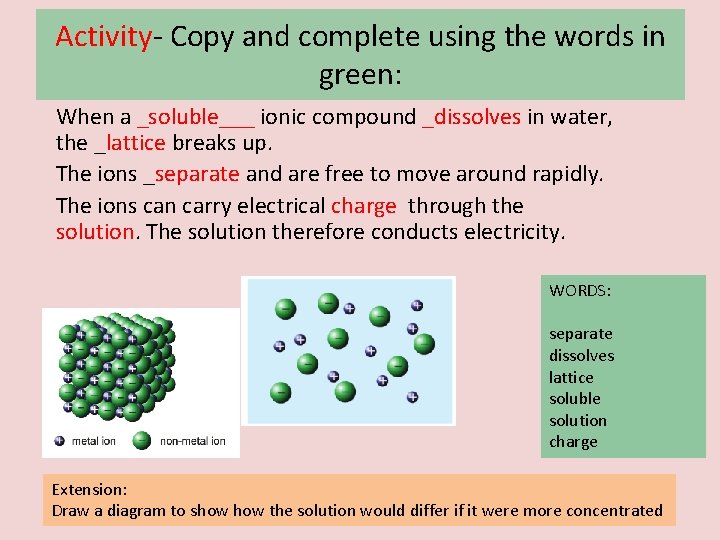
Activity- Copy and complete using the words in green: When a _______ ionic compound ____ in water, the ______ breaks up. The ions ____ and are free to move around rapidly. The ions can carry electrical _______ through the _____. The solution therefore conducts electricity. WORDS: separate dissolves lattice soluble solution charge Extension: Draw a diagram to show the solution would differ if it were more concentrated

State the forms in which an ionic compound can conduct electricity Describe how ionic compounds conduct electricity Use ionic equations to show ions form Activity-Progress check • Can you answer the following questions?

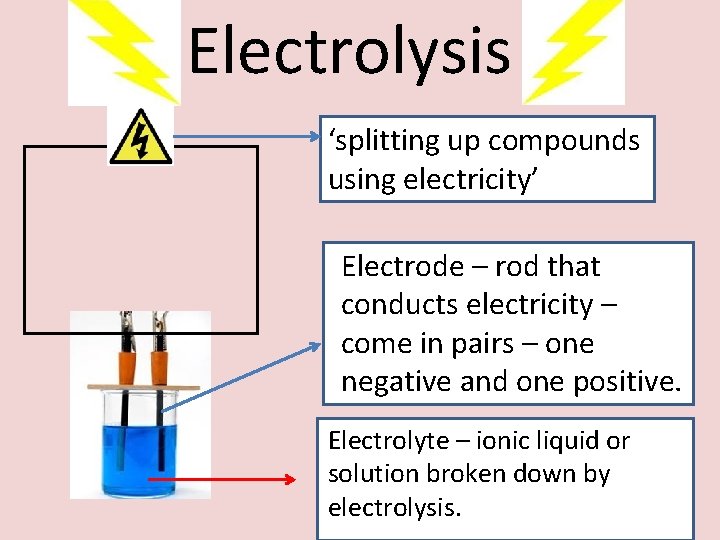
Electrolysis ‘splitting up compounds using electricity’ Electrode – rod that conducts electricity – come in pairs – one negative and one positive. Electrolyte – ionic liquid or solution broken down by electrolysis.

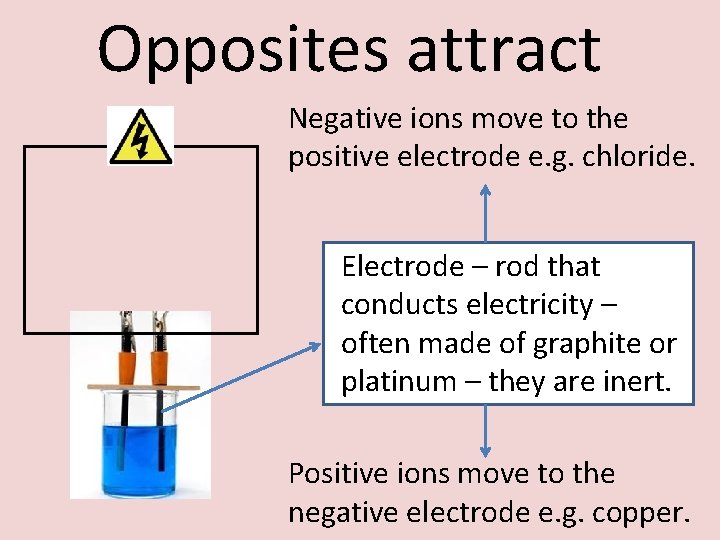
Opposites attract Negative ions move to the positive electrode e. g. chloride. Electrode – rod that conducts electricity – often made of graphite or platinum – they are inert. Positive ions move to the negative electrode e. g. copper.

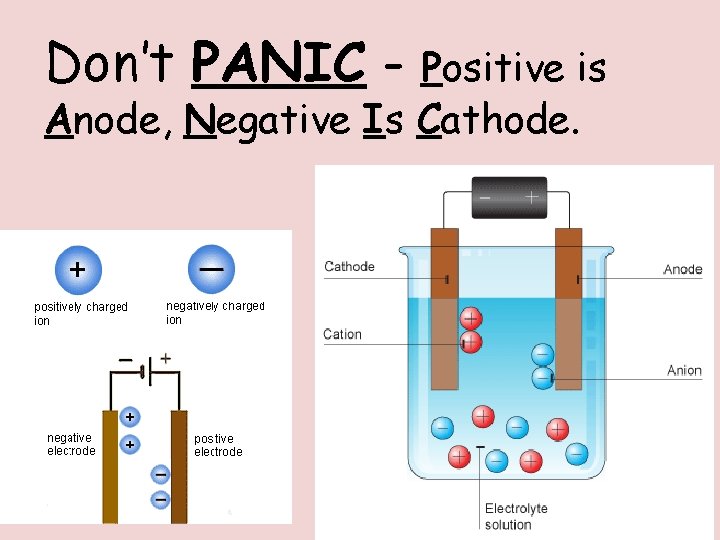
Don’t PANIC - Positive is Anode, Negative Is Cathode.

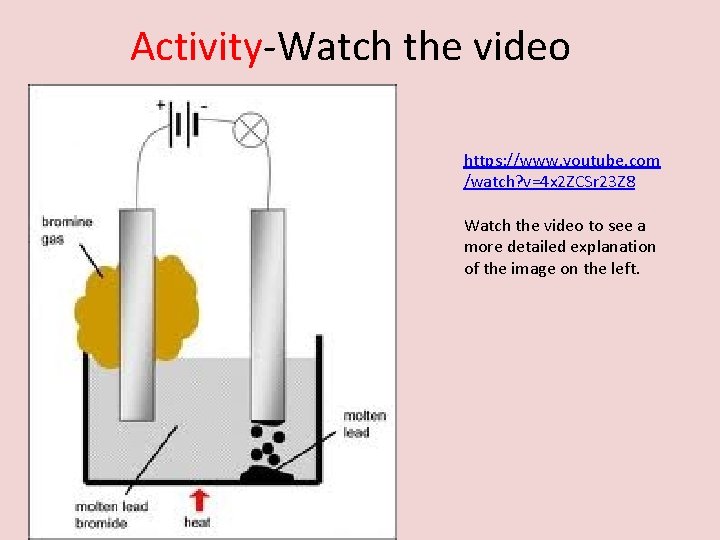
Activity-Watch the video https: //www. youtube. com /watch? v=4 x 2 ZCSr 23 Z 8 Watch the video to see a more detailed explanation of the image on the left.

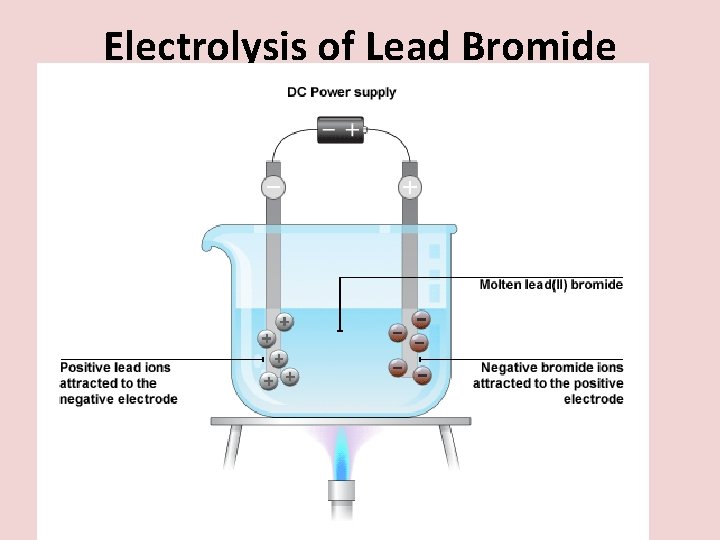
Electrolysis of Lead Bromide

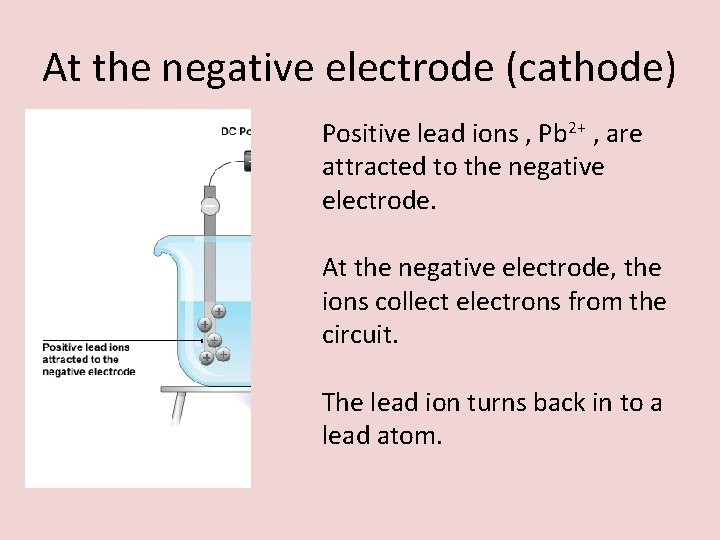
At the negative electrode (cathode) Positive lead ions , Pb 2+ , are attracted to the negative electrode. At the negative electrode, the ions collect electrons from the circuit. The lead ion turns back in to a lead atom.

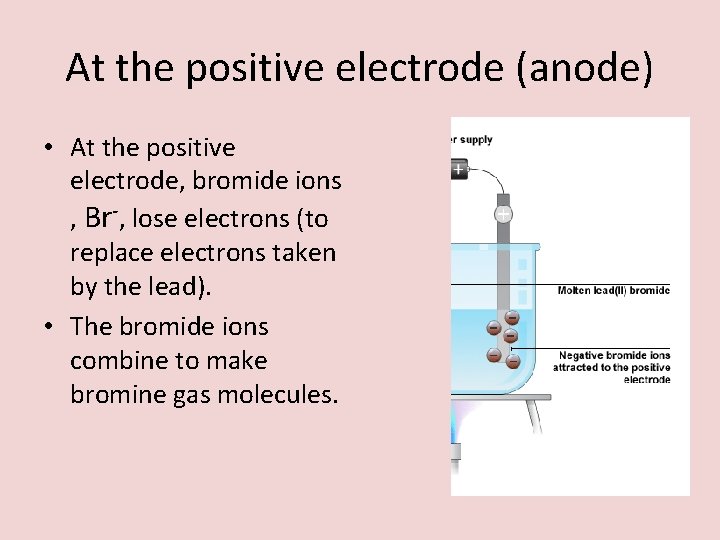
At the positive electrode (anode) • At the positive electrode, bromide ions , Br-, lose electrons (to replace electrons taken by the lead). • The bromide ions combine to make bromine gas molecules.

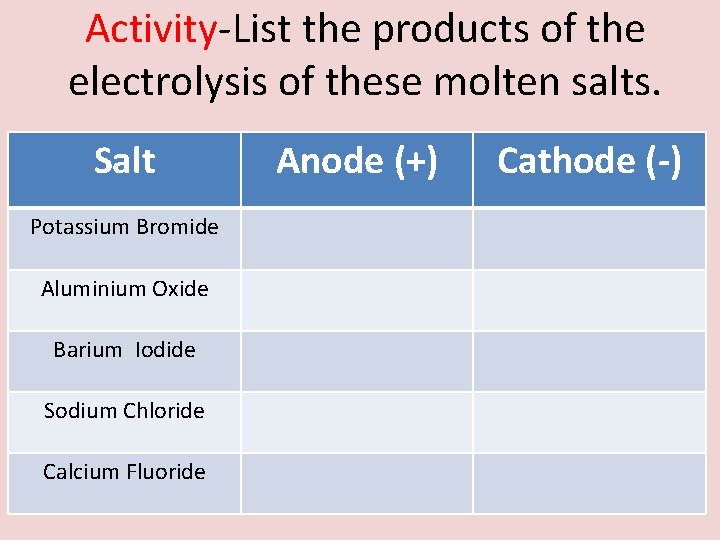
Activity-List the products of the electrolysis of these molten salts. Salt Potassium Bromide Aluminium Oxide Barium Iodide Sodium Chloride Calcium Fluoride Anode (+) Cathode (-)

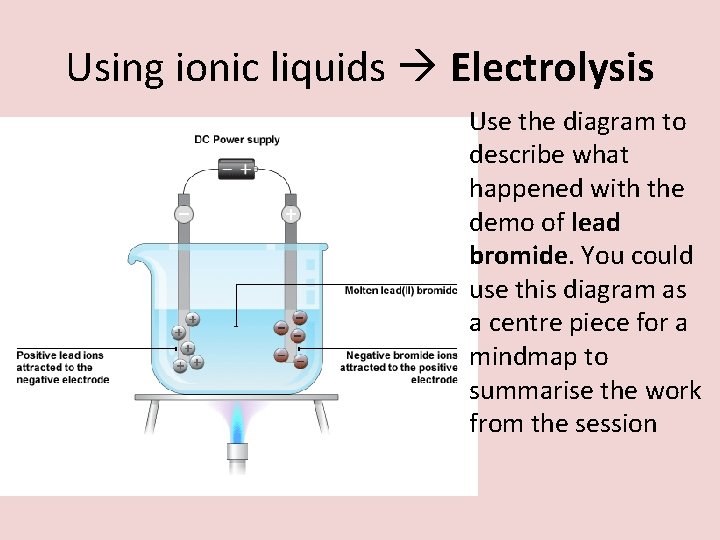
Using ionic liquids Electrolysis Use the diagram to describe what happened with the demo of lead bromide. You could use this diagram as a centre piece for a mindmap to summarise the work from the session

Answers to all slides follow

Use ionic equations to show ions form Activity STARTER: Ionic Liquids Draw the diagram of an ionic lattice structure. Explain why the ions cannot move around. Answer Ions in a solid structure are not free to move, the electrostatic attraction between the positive ion and negative ion gives a strong hold or bond. An ionic bond. 09/11/2020

Activity- Copy and complete using the words in green: When a _soluble___ ionic compound _dissolves in water, the _lattice breaks up. The ions _separate and are free to move around rapidly. The ions can carry electrical charge through the solution. The solution therefore conducts electricity. WORDS: separate dissolves lattice soluble solution charge Extension: Draw a diagram to show the solution would differ if it were more concentrated

State the forms in which an ionic compound can conduct electricity Describe how ionic compounds conduct electricity Use ionic equations to show ions form Activity-Progress check • Can you answer the following questions? Answers 1 - The ions are locked into a lattice structure and are not free to move 2 - Make it molten, a liquid or dissolve it in water, an aqueous solution 3 - No, it is not an ionic compound- it is a covalent molecule

Answer-List the products of the electrolysis of these molten salts. Salt Anode (+) Cathode (-) Potassium Bromide Bromine Potassium Aluminium Oxide Oxygen Aluminium Barium Iodide Iodine Barium Sodium Chloride Chlorine Sodium Calcium Fluoride Fluorine Calcium

To the summer • A well done from the science team, for always having a go, keeping up with the work and trying your best. • The odd sheet hasn’t worked, the odd answer in the wrong place and last week I managed to delete the video • Thank you for patience, enjoy the summer and we will see you in September.
 Electrolysis of molten ionic compounds
Electrolysis of molten ionic compounds Electrolysis of ionic compounds
Electrolysis of ionic compounds Electrolysis panic
Electrolysis panic Electrolysis of molten icl liberates i2 at
Electrolysis of molten icl liberates i2 at Half reaction
Half reaction Ionic bonding and covalent bonding venn diagram
Ionic bonding and covalent bonding venn diagram Review naming ionic compounds
Review naming ionic compounds Naming compounds review
Naming compounds review Covalent salt
Covalent salt Volatility of ionic compounds
Volatility of ionic compounds Ionic compound properties
Ionic compound properties Type ii binary ionic compounds
Type ii binary ionic compounds Molecular compounds def
Molecular compounds def Chapter 7 ionic compounds and metals assessment answer key
Chapter 7 ionic compounds and metals assessment answer key Solubility chart for ionic compounds
Solubility chart for ionic compounds Ionic binary compounds multiple charge cations
Ionic binary compounds multiple charge cations Uses of ionic compounds
Uses of ionic compounds Properties of ionic bond
Properties of ionic bond Naming binary compounds ionic
Naming binary compounds ionic Binary compounds chemistry
Binary compounds chemistry Naming binary ionic compounds
Naming binary ionic compounds Naming ionic and covalent compounds
Naming ionic and covalent compounds Nhmno4
Nhmno4 Monotomic ion
Monotomic ion Ionic compounds and metals chapter 7
Ionic compounds and metals chapter 7