WATER A guide for GCSE students 2010 KNOCKHARDY













































- Slides: 67

WATER A guide for GCSE students 2010 KNOCKHARDY PUBLISHING SPECIFICATIONS

WATER INTRODUCTION This Powerpoint show is one of several produced to help students understand selected GCSE Chemistry topics. It is based on the requirements of the AQA specification but is suitable for other examination boards. Individual students may use the material at home for revision purposes and it can also prove useful for classroom teaching with an interactive white board. Accompanying notes on this, and the full range of AS and A 2 Chemistry topics, are available from the KNOCKHARDY WEBSITE at. . . www. knockhardy. org. uk All diagrams, photographs and any animations in this Powerpoint are original and created by Jonathan Hopton. Permission must be obtained for their use in any work that is distributed for financial gain.

WATER CONTENTS • Properties of water • Occurrence of water – The water cycle • Hardness of water – the causes • Temporary hardness • Permanent hardness • Removing permanent hardness in water • Hard water – advantages and disadvantages • Water pollution and its treatment

PROPERTIES OF WATER GENERAL INFORMATION Structure Water consists of covalent molecules of formula H 2 O. Physical properties colourless, odourless liquid. boils at 100°C freezes at 0°C (if pure and at atmospheric pressure) Chemical properties Reacts with some metals to produce hydrogen. Uses Essential for life. An important solvent. A coolant for many industrial processes (e. g. power stations) Raw material in the manufacture of ammonia. Raw material in the conversion of ethene to ethanol. Test Turns blue cobalt chloride pink. . . or Turns white anhydrous copper(II) sulphate blue.

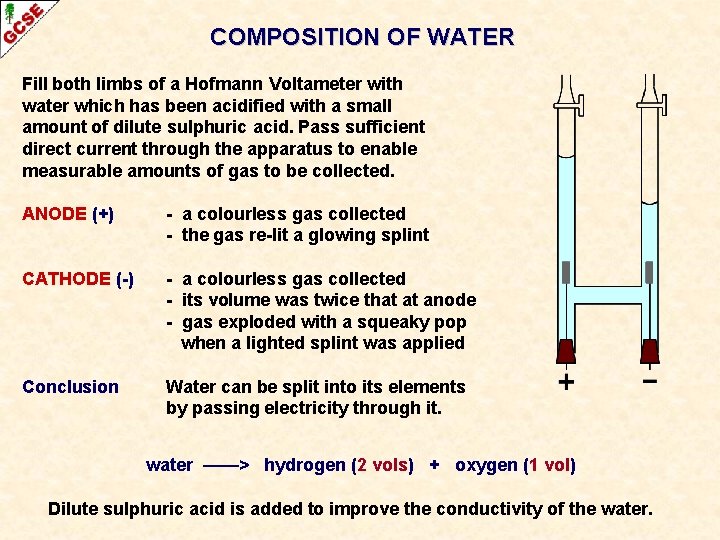
COMPOSITION OF WATER Fill both limbs of a Hofmann Voltameter with water which has been acidified with a small amount of dilute sulphuric acid. Pass sufficient direct current through the apparatus to enable measurable amounts of gas to be collected. ANODE (+) - a colourless gas collected - the gas re-lit a glowing splint CATHODE (-) - a colourless gas collected - its volume was twice that at anode - gas exploded with a squeaky pop when a lighted splint was applied Conclusion Water can be split into its elements by passing electricity through it. water ——> hydrogen (2 vols) + oxygen (1 vol) Dilute sulphuric acid is added to improve the conductivity of the water.

OCCURRENCE Water is the most abundant substance on the surface of our planet.

OCCURRENCE Water is the most abundant substance on the surface of our planet. Importance LIVING SYSTEMS RESOURCE FOR INDUSTRIAL PROCESSES

OCCURRENCE Water is the most abundant substance on the surface of our planet. Importance LIVING SYSTEMS RESOURCE FOR INDUSTRIAL PROCESSES Occurrence OCEANS LAKES RIVERS ATMOSPHERE - clouds and water vapour

OCCURRENCE Water is the most abundant substance on the surface of our planet. Importance LIVING SYSTEMS RESOURCE FOR INDUSTRIAL PROCESSES Occurrence OCEANS LAKES RIVERS ATMOSPHERE - clouds and water vapour Water Cycle Shows the inter- relationship between water in different environments.

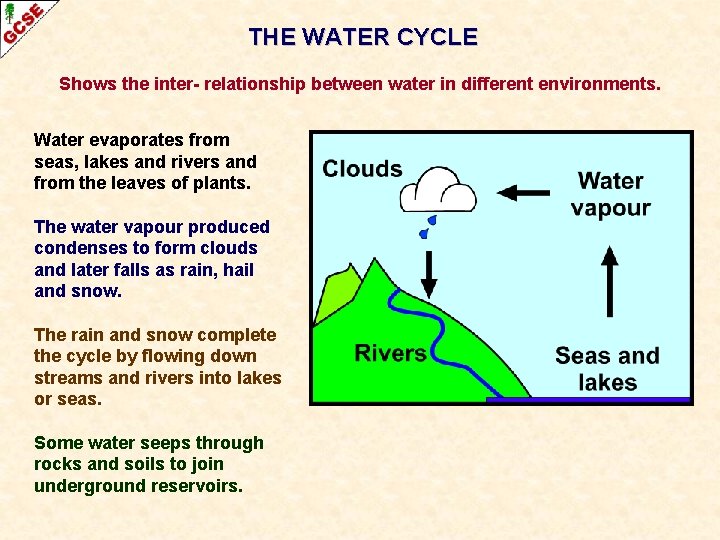
THE WATER CYCLE Shows the inter- relationship between water in different environments. Water evaporates from seas, lakes and rivers and from the leaves of plants. The water vapour produced condenses to form clouds and later falls as rain, hail and snow. The rain and snow complete the cycle by flowing down streams and rivers into lakes or seas. Some water seeps through rocks and soils to join underground reservoirs.

PURITY OF WATER Natural water is never pure. It is such a good solvent that it contains dissolved substances whatever its origin. RAIN dissolved gases from the air e. g. CO 2, SO 2 RIVERS dissolved salts from rocks and soils; also oxygen. SEA dissolved sodium and magnesium salts (e. g. Na. Cl) and CO 2 water also contains man-made chemicals such as detergents, acids, fertilizers and other pollutants.

WHAT IS HARDNESS? “Hard water is water which does not readily form a lather with soap”

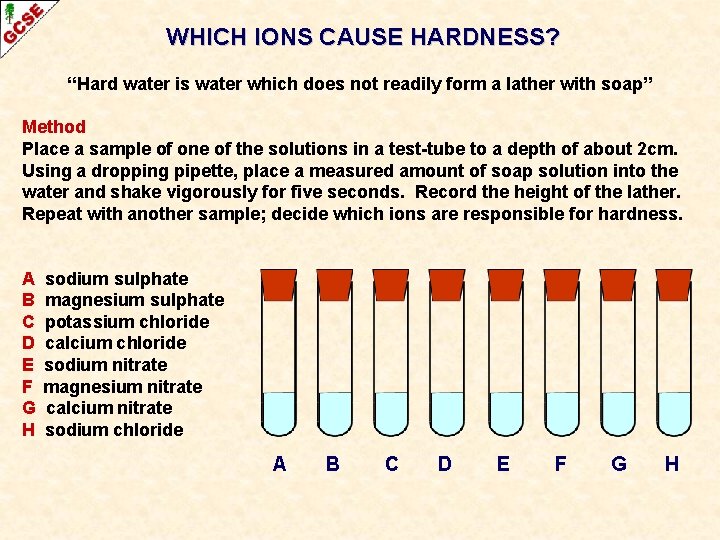
WHICH IONS CAUSE HARDNESS? “Hard water is water which does not readily form a lather with soap” Method Place a sample of one of the solutions in a test-tube to a depth of about 2 cm. Using a dropping pipette, place a measured amount of soap solution into the water and shake vigorously for five seconds. Record the height of the lather. Repeat with another sample; decide which ions are responsible for hardness.

WHICH IONS CAUSE HARDNESS? “Hard water is water which does not readily form a lather with soap” Method Place a sample of one of the solutions in a test-tube to a depth of about 2 cm. Using a dropping pipette, place a measured amount of soap solution into the water and shake vigorously for five seconds. Record the height of the lather. Repeat with another sample; decide which ions are responsible for hardness. A B C D E F G H sodium sulphate magnesium sulphate potassium chloride calcium chloride sodium nitrate magnesium nitrate calcium nitrate sodium chloride A B C D E F G H

WHICH IONS CAUSE HARDNESS? “Hard water is water which does not readily form a lather with soap” Method Place a sample of one of the solutions in a test-tube to a depth of about 2 cm. Using a dropping pipette, place a measured amount of soap solution into the water and shake vigorously for five seconds. Record the height of the lather. Repeat with another sample; decide which ions are responsible for hardness. A B C D E F G H sodium sulphate magnesium sulphate potassium chloride calcium chloride sodium nitrate magnesium nitrate calcium nitrate sodium chloride A B C D E F G H

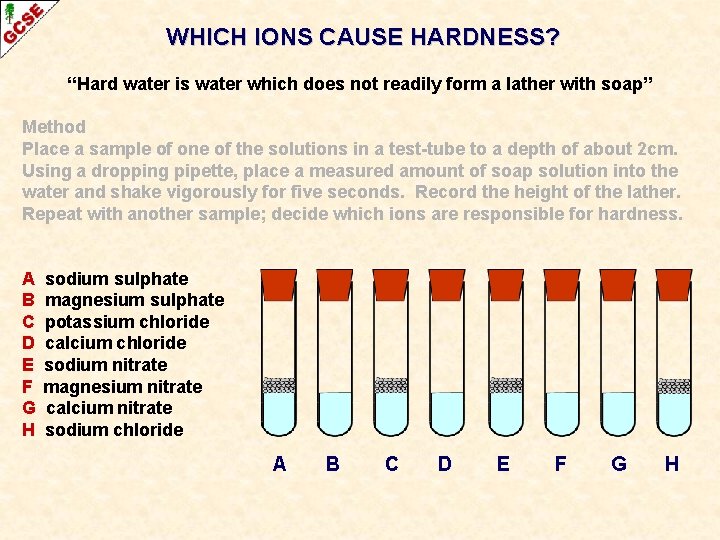
WHICH IONS CAUSE HARDNESS? “Hard water is water which does not readily form a lather with soap” Method Place a sample of one of the solutions in a test-tube to a depth of about 2 cm. Using a dropping pipette, place a measured amount of soap solution into the water and shake vigorously for five seconds. Record the height of the lather. Repeat with another sample; decide which ions are responsible for hardness. Solution used A B C D E F G H sodium sulphate magnesium sulphate potassium chloride calcium chloride sodium nitrate magnesium nitrate calcium nitrate sodium chloride Conclusions present + ive - ive Na+ SO 42 Mg 2+ SO 42 K+ Cl. Ca 2+ Cl. Na+ NO 3 Mg 2+ NO 3 Ca 2+ NO 3 Na+ Cl- lather YES NO NO YES The ion(s) responsible for hardness is / are. . .

WHICH IONS CAUSE HARDNESS? “Hard water is water which does not readily form a lather with soap” Method Place a sample of one of the solutions in a test-tube to a depth of about 2 cm. Using a dropping pipette, place a measured amount of soap solution into the water and shake vigorously for five seconds. Record the height of the lather. Repeat with another sample; decide which ions are responsible for hardness. Solution used A B C D E F G H sodium sulphate magnesium sulphate potassium chloride calcium chloride sodium nitrate magnesium nitrate calcium nitrate sodium chloride Conclusions present + ive - ive Na+ SO 42 Mg 2+ SO 42 K+ Cl. Ca 2+ Cl. Na+ NO 3 Mg 2+ NO 3 Ca 2+ NO 3 Na+ Cl- lather YES NO NO YES The ion(s) responsible for hardness is / are. . . Ca 2+ and Mg 2+

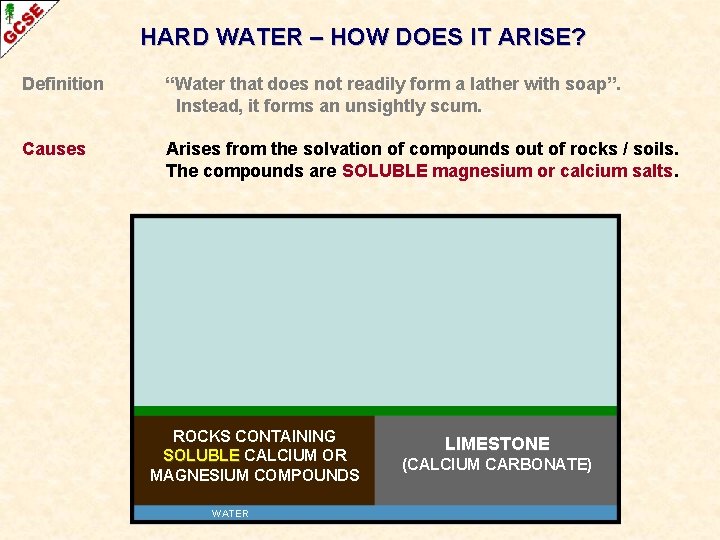
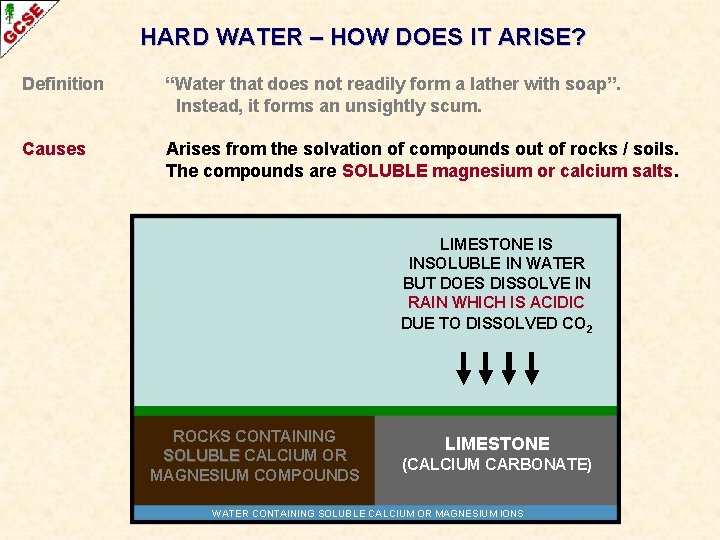
HARD WATER – HOW DOES IT ARISE? Definition “Water that does not readily form a lather with soap”. Instead, it forms an unsightly scum. Causes Arises from the solvation of compounds out of rocks / soils. The compounds are SOLUBLE magnesium or calcium salts.

HARD WATER – HOW DOES IT ARISE? Definition “Water that does not readily form a lather with soap”. Instead, it forms an unsightly scum. Causes Arises from the solvation of compounds out of rocks / soils. The compounds are SOLUBLE magnesium or calcium salts. ROCKS CONTAINING SOLUBLE CALCIUM OR MAGNESIUM COMPOUNDS WATER LIMESTONE (CALCIUM CARBONATE)

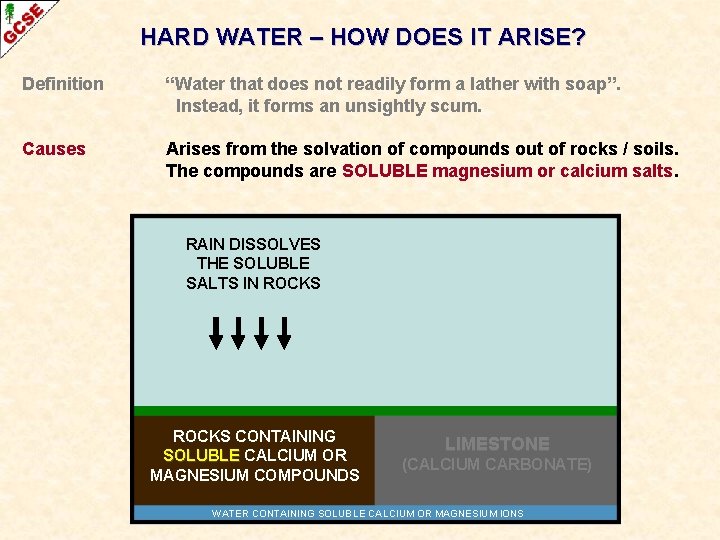
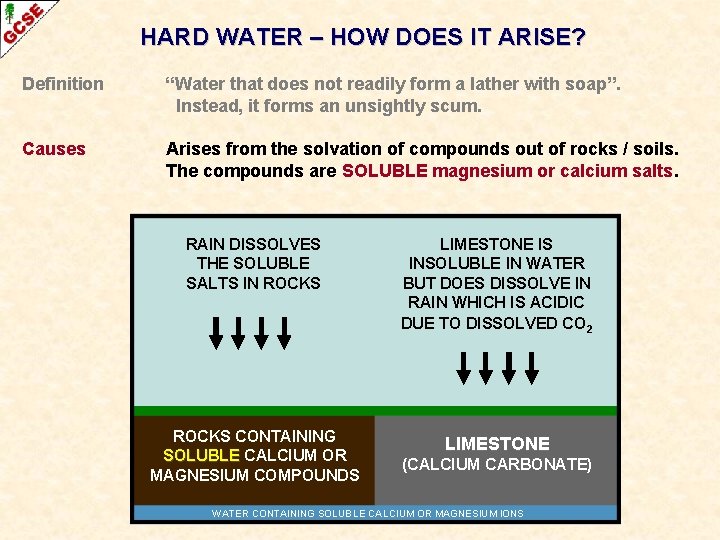
HARD WATER – HOW DOES IT ARISE? Definition “Water that does not readily form a lather with soap”. Instead, it forms an unsightly scum. Causes Arises from the solvation of compounds out of rocks / soils. The compounds are SOLUBLE magnesium or calcium salts. RAIN DISSOLVES THE SOLUBLE SALTS IN ROCKS CONTAINING SOLUBLE CALCIUM OR MAGNESIUM COMPOUNDS LIMESTONE (CALCIUM CARBONATE) WATER CONTAINING SOLUBLE CALCIUM OR MAGNESIUM IONS

HARD WATER – HOW DOES IT ARISE? Definition “Water that does not readily form a lather with soap”. Instead, it forms an unsightly scum. Causes Arises from the solvation of compounds out of rocks / soils. The compounds are SOLUBLE magnesium or calcium salts. LIMESTONE IS INSOLUBLE IN WATER BUT DOES DISSOLVE IN RAIN WHICH IS ACIDIC DUE TO DISSOLVED CO 2 ROCKS CONTAINING SOLUBLE CALCIUM OR MAGNESIUM COMPOUNDS LIMESTONE (CALCIUM CARBONATE) WATER CONTAINING SOLUBLE CALCIUM OR MAGNESIUM IONS

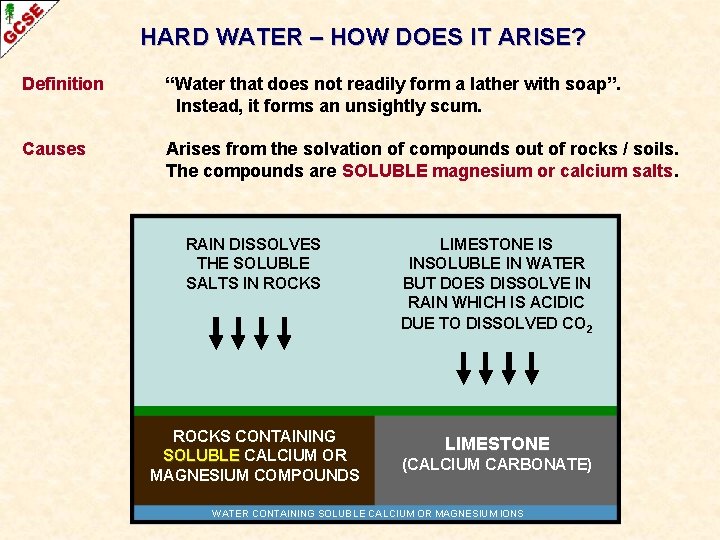
HARD WATER – HOW DOES IT ARISE? Definition “Water that does not readily form a lather with soap”. Instead, it forms an unsightly scum. Causes Arises from the solvation of compounds out of rocks / soils. The compounds are SOLUBLE magnesium or calcium salts. RAIN DISSOLVES THE SOLUBLE SALTS IN ROCKS LIMESTONE IS INSOLUBLE IN WATER BUT DOES DISSOLVE IN RAIN WHICH IS ACIDIC DUE TO DISSOLVED CO 2 ROCKS CONTAINING SOLUBLE CALCIUM OR MAGNESIUM COMPOUNDS LIMESTONE (CALCIUM CARBONATE) WATER CONTAINING SOLUBLE CALCIUM OR MAGNESIUM IONS

HARD WATER – HOW DOES IT ARISE? Definition “Water that does not readily form a lather with soap”. Instead, it forms an unsightly scum. Causes Arises from the solvation of compounds out of rocks / soils. The compounds are SOLUBLE magnesium or calcium salts. RAIN DISSOLVES THE SOLUBLE SALTS IN ROCKS LIMESTONE IS INSOLUBLE IN WATER BUT DOES DISSOLVE IN RAIN WHICH IS ACIDIC DUE TO DISSOLVED CO 2 ROCKS CONTAINING SOLUBLE CALCIUM OR MAGNESIUM COMPOUNDS LIMESTONE (CALCIUM CARBONATE) WATER CONTAINING SOLUBLE CALCIUM OR MAGNESIUM IONS

HARD WATER – HOW DOES IT ARISE? Definition “Water that does not readily form a lather with soap”. Instead, it forms an unsightly scum. Causes Arises from the solvation of compounds out of rocks / soils. The compounds are SOLUBLE magnesium or calcium salts. There are TWO main types of hardness…

HARD WATER – HOW DOES IT ARISE? Definition “Water that does not readily form a lather with soap”. Instead, it forms an unsightly scum. Causes Arises from the solvation of compounds out of rocks / soils. The compounds are SOLUBLE magnesium or calcium salts. There are TWO main types of hardness… PERMANENT HARDNESS TEMPORARY HARDNESS

TEMPORARY HARDNESS

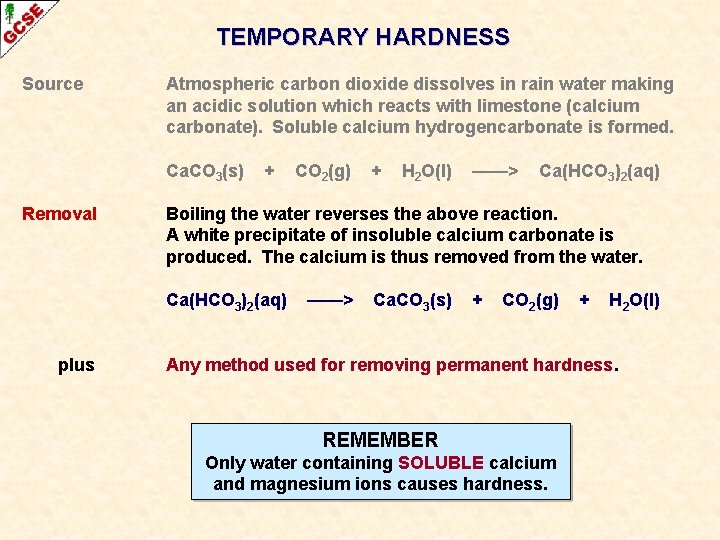
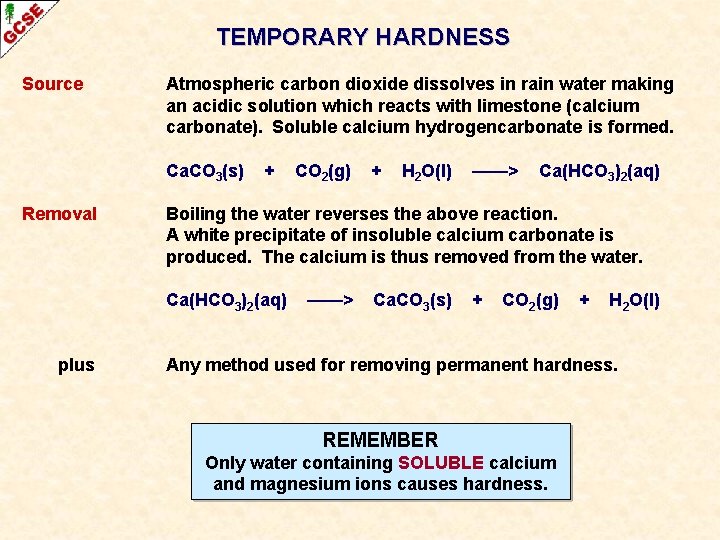
TEMPORARY HARDNESS Source Atmospheric carbon dioxide dissolves in rain water making an acidic solution which reacts with limestone (calcium carbonate). Soluble calcium hydrogencarbonate is formed. Ca. CO 3(s) + CO 2(g) + H 2 O(l) ——> Ca(HCO 3)2(aq) LIMESTONE IS INSOLUBLE IN WATER BUT DOES DISSOLVE IN RAIN WHICH IS ACIDIC DUE TO DISSOLVED CO 2 ROCKS CONTAINING SOLUBLE CALCIUM OR MAGNESIUM COMPOUNDS LIMESTONE (CALCIUM CARBONATE) WATER CONTAINING SOLUBLE CALCIUM OR MAGNESIUM IONS

TEMPORARY HARDNESS Source Atmospheric carbon dioxide dissolves in rain water making an acidic solution which reacts with limestone (calcium carbonate). Soluble calcium hydrogencarbonate is formed. Ca. CO 3(s) Removal + CO 2(g) + H 2 O(l) ——> Ca(HCO 3)2(aq) Boiling the water reverses the above reaction. A white precipitate of insoluble calcium carbonate is produced. The calcium is thus removed from the water. Ca(HCO 3)2(aq) ——> Ca. CO 3(s) + CO 2(g) + H 2 O(l)

TEMPORARY HARDNESS Source Atmospheric carbon dioxide dissolves in rain water making an acidic solution which reacts with limestone (calcium carbonate). Soluble calcium hydrogencarbonate is formed. Ca. CO 3(s) Removal + + H 2 O(l) ——> Ca(HCO 3)2(aq) Boiling the water reverses the above reaction. A white precipitate of insoluble calcium carbonate is produced. The calcium is thus removed from the water. Ca(HCO 3)2(aq) plus CO 2(g) ——> Ca. CO 3(s) + CO 2(g) + H 2 O(l) Any method used for removing permanent hardness. REMEMBER Only water containing SOLUBLE calcium and magnesium ions causes hardness.

TEMPORARY HARDNESS Source Atmospheric carbon dioxide dissolves in rain water making an acidic solution which reacts with limestone (calcium carbonate). Soluble calcium hydrogencarbonate is formed. Ca. CO 3(s) Removal + + H 2 O(l) ——> Ca(HCO 3)2(aq) Boiling the water reverses the above reaction. A white precipitate of insoluble calcium carbonate is produced. The calcium is thus removed from the water. Ca(HCO 3)2(aq) plus CO 2(g) ——> Ca. CO 3(s) + CO 2(g) + H 2 O(l) Any method used for removing permanent hardness. REMEMBER Only water containing SOLUBLE calcium and magnesium ions causes hardness.

PERMANENT HARDNESS

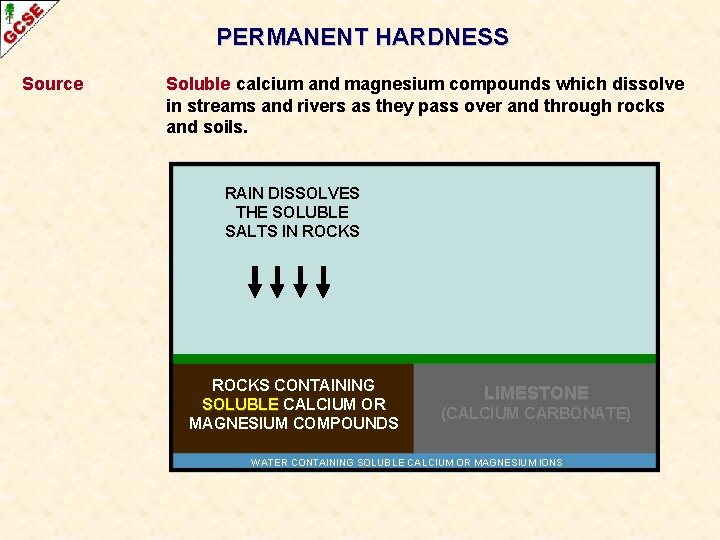
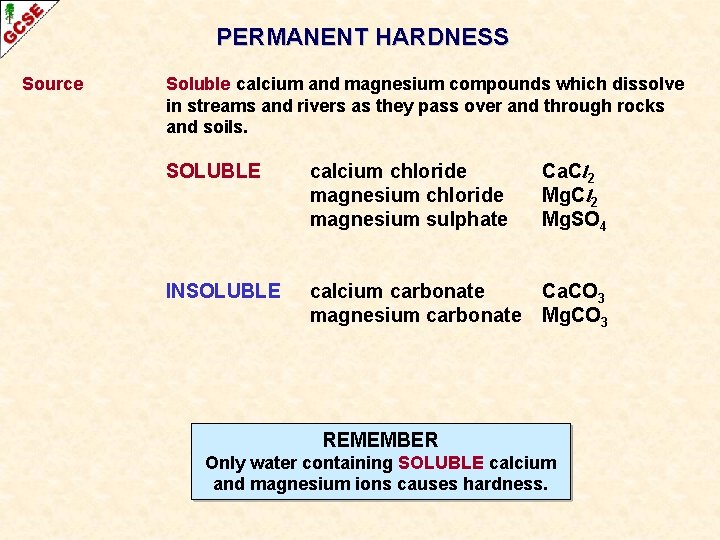
PERMANENT HARDNESS Source Soluble calcium and magnesium compounds which dissolve in streams and rivers as they pass over and through rocks and soils. RAIN DISSOLVES THE SOLUBLE SALTS IN ROCKS CONTAINING SOLUBLE CALCIUM OR MAGNESIUM COMPOUNDS LIMESTONE (CALCIUM CARBONATE) WATER CONTAINING SOLUBLE CALCIUM OR MAGNESIUM IONS

PERMANENT HARDNESS Source Soluble calcium and magnesium compounds which dissolve in streams and rivers as they pass over and through rocks and soils. SOLUBLE calcium chloride magnesium sulphate Ca. Cl 2 Mg. SO 4 INSOLUBLE calcium carbonate magnesium carbonate Ca. CO 3 Mg. CO 3

PERMANENT HARDNESS Source Soluble calcium and magnesium compounds which dissolve in streams and rivers as they pass over and through rocks and soils. SOLUBLE calcium chloride magnesium sulphate Ca. Cl 2 Mg. SO 4 INSOLUBLE calcium carbonate magnesium carbonate Ca. CO 3 Mg. CO 3 REMEMBER Only water containing SOLUBLE calcium and magnesium ions causes hardness.

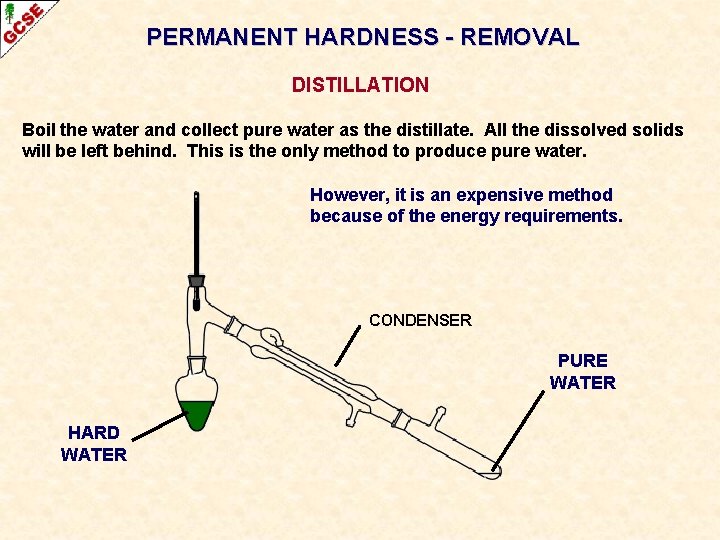
PERMANENT HARDNESS - REMOVAL DISTILLATION

PERMANENT HARDNESS - REMOVAL DISTILLATION Boil the water and collect pure water as the distillate. All the dissolved solids will be left behind. This is the only method to produce pure water. However, it is an expensive method because of the energy requirements. CONDENSER PURE WATER HARD WATER

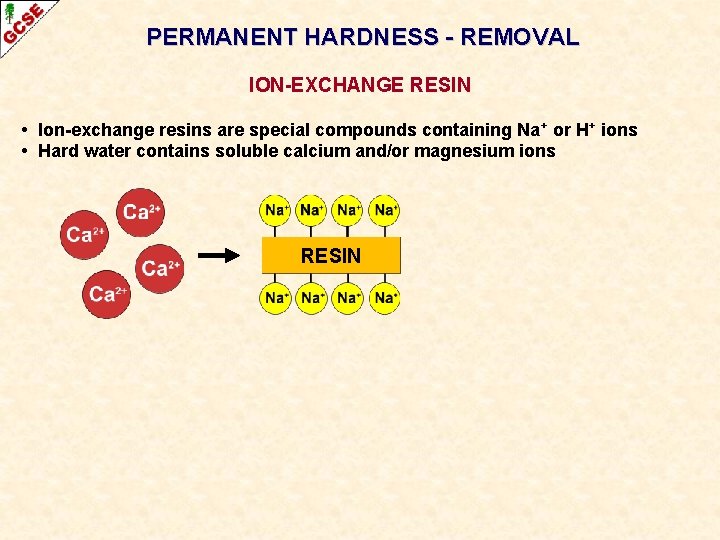
PERMANENT HARDNESS - REMOVAL ION-EXCHANGE RESIN

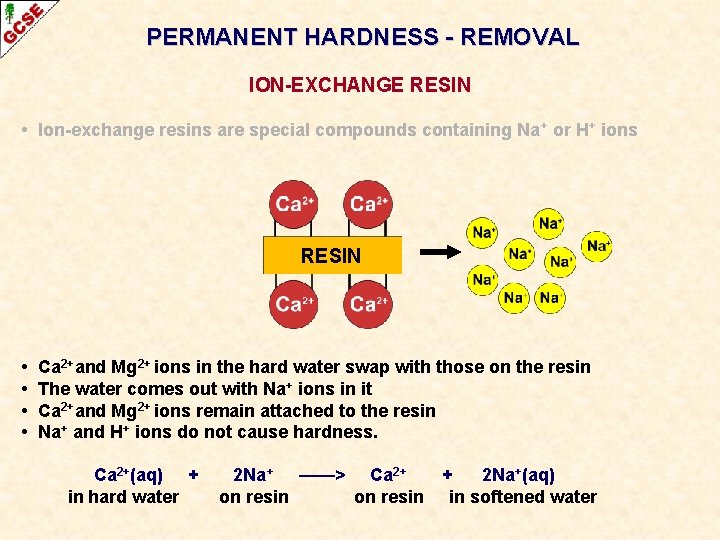
PERMANENT HARDNESS - REMOVAL ION-EXCHANGE RESIN • Ion-exchange resins are special compounds containing Na + or H+ ions • Hard water contains soluble calcium and/or magnesium ions RESIN

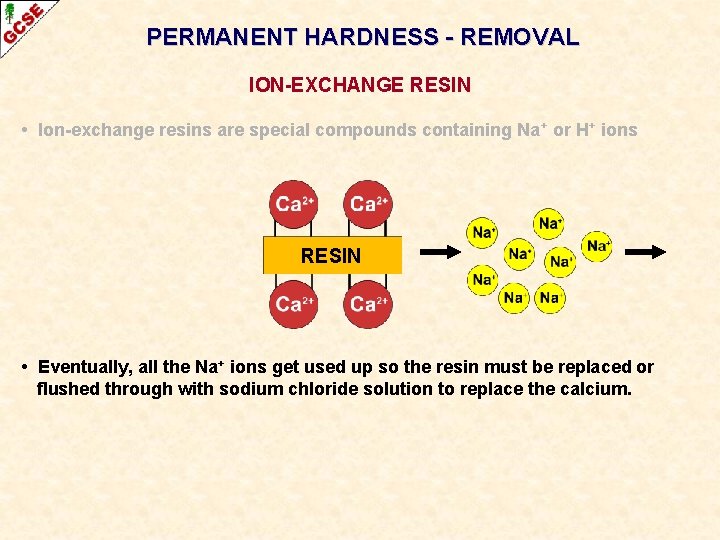
PERMANENT HARDNESS - REMOVAL ION-EXCHANGE RESIN • Ion-exchange resins are special compounds containing Na + or H+ ions RESIN • • Ca 2+and Mg 2+ ions in the hard water swap with those on the resin The water comes out with Na+ ions in it Ca 2+and Mg 2+ ions remain attached to the resin Na+ and H+ ions do not cause hardness. Ca 2+(aq) + in hard water 2 Na+ ——> Ca 2+ + 2 Na+(aq) on resin in softened water

PERMANENT HARDNESS - REMOVAL ION-EXCHANGE RESIN • Ion-exchange resins are special compounds containing Na + or H+ ions RESIN • Eventually, all the Na+ ions get used up so the resin must be replaced or flushed through with sodium chloride solution to replace the calcium.

PERMANENT HARDNESS - REMOVAL SOAP

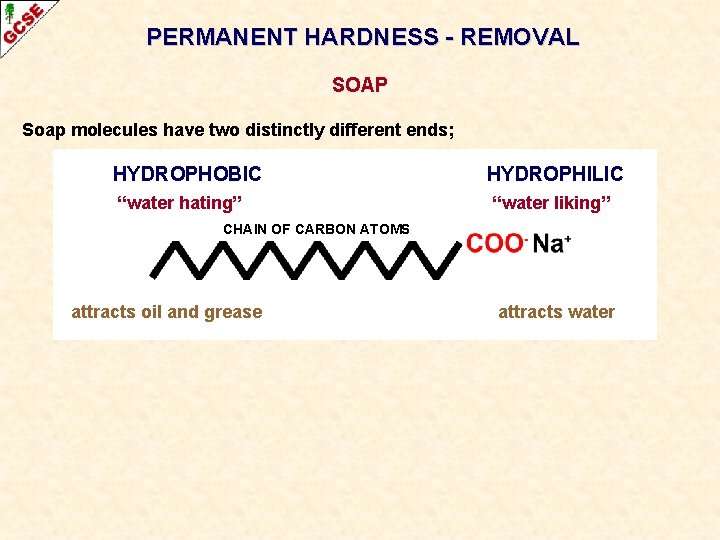
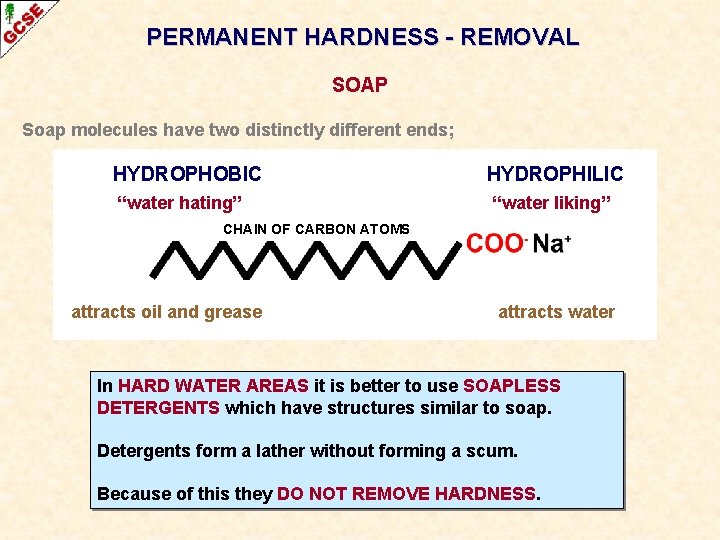
PERMANENT HARDNESS - REMOVAL SOAP Soap molecules have two distinctly different ends; HYDROPHOBIC “water hating” HYDROPHILIC “water liking” CHAIN OF CARBON ATOMS attracts oil and grease attracts water

PERMANENT HARDNESS - REMOVAL SOAP Soap molecules have two distinctly different ends; HYDROPHOBIC “water hating” HYDROPHILIC “water liking” CHAIN OF CARBON ATOMS attracts oil and grease attracts water When soap is placed in hard water, it reacts with the calcium and magnesium ions to produce an unsightly, insoluble grey scum.

PERMANENT HARDNESS - REMOVAL SOAP Soap molecules have two distinctly different ends; HYDROPHOBIC “water hating” HYDROPHILIC “water liking” CHAIN OF CARBON ATOMS attracts oil and grease attracts water When soap is placed in hard water, it reacts with the calcium and magnesium ions to produce an unsightly, insoluble grey scum. The scum is a calcium compound and is thus removed from the water. When all the hardness has been removed, the soap can act in the normal way.

PERMANENT HARDNESS - REMOVAL SOAP Soap molecules have two distinctly different ends; HYDROPHOBIC “water hating” HYDROPHILIC “water liking” CHAIN OF CARBON ATOMS attracts oil and grease attracts water In HARD WATER AREAS it is better to use SOAPLESS DETERGENTS which have structures similar to soap. Detergents form a lather without forming a scum. Because of this they DO NOT REMOVE HARDNESS.

PERMANENT HARDNESS - REMOVAL WASHING SODA (sodium carbonate)

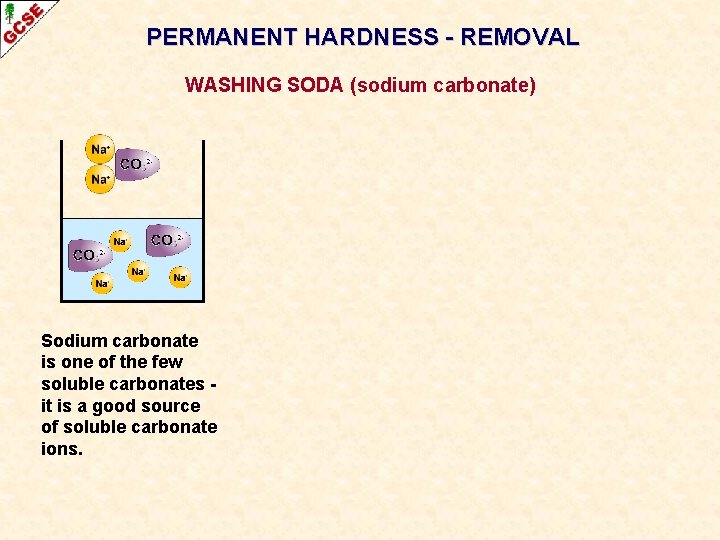
PERMANENT HARDNESS - REMOVAL WASHING SODA (sodium carbonate) Sodium carbonate is one of the few soluble carbonates it is a good source of soluble carbonate ions.

PERMANENT HARDNESS - REMOVAL WASHING SODA (sodium carbonate) Sodium carbonate is one of the few soluble carbonates it is a good source of soluble carbonate ions. Hard water contains soluble calcium (or magnesium) ions.

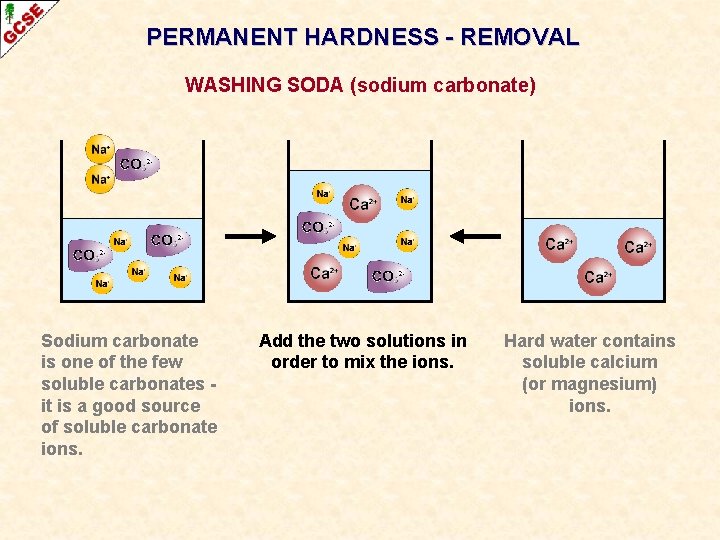
PERMANENT HARDNESS - REMOVAL WASHING SODA (sodium carbonate) Sodium carbonate is one of the few soluble carbonates it is a good source of soluble carbonate ions. Add the two solutions in order to mix the ions. Hard water contains soluble calcium (or magnesium) ions.

PERMANENT HARDNESS - REMOVAL WASHING SODA (sodium carbonate) The Ca 2+ and CO 32 - ions come together to form a precipitate of insoluble calcium carbonate. The calcium ions are removed from the water so it is now soft.

PERMANENT HARDNESS - REMOVAL WASHING SODA (sodium carbonate) - SUMMARY Sodium carbonate is one of the few soluble carbonates - it is a good source of soluble carbonate ions. Addition of washing soda to a solution containing magnesium ions or calcium ions results in the precipitation of insoluble carbonates. Once the magnesium, or calcium, has been removed from the water, the water is no longer hard - it is soft. equation ionic equation Ca. Cl 2(aq) + Na 2 CO 3(aq) ——> Ca 2+(aq) + 2 Na. Cl(aq) CO 32 -(aq) ——> Ca 2+CO 32 -(s) + Ca. CO 3(s)

HARD WATER Disadvantages Furring up of pipes and boilers; the heating is not as effective blockages may lead to an explosion

HARD WATER Disadvantages Furring up of pipes and boilers; the heating is not as effective blockages may lead to an explosion Furring up of kettle elements; wastes electricity

HARD WATER Disadvantages Furring up of pipes and boilers; the heating is not as effective blockages may lead to an explosion Furring up of kettle elements; wastes electricity Wastes soap; as some is needed to remove the hardness unsightly scum is formed during washing

HARD WATER Disadvantages Furring up of pipes and boilers; the heating is not as effective blockages may lead to an explosion Furring up of kettle elements; wastes electricity Wastes soap; as some is needed to remove the hardness unsightly scum is formed during washing Advantages calcium strengthens teeth and bones. better for preventing heart diseases

WATER POLLUTION

WATER POLLUTION Origin nitrates and phosphates in farm fertilizers animal waste products industrial chemical waste lead ions (Pb 2+) from old pipes

WATER POLLUTION Origin nitrates and phosphates in farm fertilizers animal waste products industrial chemical waste lead ions (Pb 2+) from old pipes Effects Nitrates from fertilizers encourage plant and algae growth. When algae die they are decomposed by aerobic bacteria which need oxygen. The oxygen is thus removed from the water making the river “dead” and incapable of supporting fish populations. Nitrates can also be converted to nitrites (which affect haemoglobin in blood) and into carcinogenic nitrosamines. Industrial waste can be in many forms including cyanide, detergents and heavy metals and radioactive products. A build up of lead can lead to brain damage.

WATER POLLUTION Origin nitrates and phosphates in farm fertilizers animal waste products industrial chemical waste lead ions (Pb 2+) from old pipes Effects Nitrates from fertilizers encourage plant and algae growth. When algae die they are decomposed by aerobic bacteria which need oxygen. The oxygen is thus removed from the water making the river “dead” and incapable of supporting fish populations. Nitrates can also be converted to nitrites (which affect haemoglobin in blood) and into carcinogenic nitrosamines. Industrial waste can be in many forms including cyanide, detergents, heavy metals and radioactive products. A build up of lead can lead to brain damage.

WATER PURIFICATION

WATER PURIFICATION Water of the correct quality is essential for life. For humans, drinking water should have sufficiently low levels of dissolved salts and microbes.

WATER PURIFICATION Water of the correct quality is essential for life. For humans, drinking water should have sufficiently low levels of dissolved salts and microbes. Filtration removes insoluble organic matter; the sludge is digested to produce methane. Aeration removes organic waste using oxygen-requiring bacteria. Chlorination kills bacteria to allow water to be used for drinking purposes.

WATER PURIFICATION Water of the correct quality is essential for life. For humans, drinking water should have sufficiently low levels of dissolved salts and microbes. Filtration removes insoluble organic matter; the sludge is digested to produce methane. Aeration removes organic waste using oxygen-requiring bacteria. Chlorination kills bacteria to allow water to be used for drinking purposes. IT CAN THEN BE SENT TO HOUSES THROUGH THE MAINS

WATER PURIFICATION Water of the correct quality is essential for life. For humans, drinking water should have sufficiently low levels of dissolved salts and microbes. Filtration removes insoluble organic matter; the sludge is digested to produce methane. Aeration removes organic waste using oxygen-requiring bacteria. Chlorination kills bacteria to allow water to be used for drinking purposes. OTHER THINGS THAT CAN BE DONE Fluoridation Fluoride compounds are added to water in some areas to aid the prevention of tooth decay. The fluoride is only helpful to children and can be given in tablet form. Many people object to the addition of fluoride to drinking water.

WATER PURIFICATION Water of the correct quality is essential for life. For humans, drinking water should have sufficiently low levels of dissolved salts and microbes. Filtration removes insoluble organic matter; the sludge is digested to produce methane. Aeration removes organic waste using oxygen-requiring bacteria. Chlorination kills bacteria to allow water to be used for drinking purposes. OTHER THINGS THAT CAN BE DONE Fluoridation Fluoride compounds are added to water in some areas to aid the prevention of tooth decay. The fluoride is only helpful to children and can be given in tablet form. Many people object to the addition of fluoride to drinking water. Water filters These contain carbon, silver and ion exchange resins and can remove some dissolved substances from tap water to improve the taste and quality.

WATER PURIFICATION Water of the correct quality is essential for life. For humans, drinking water should have sufficiently low levels of dissolved salts and microbes. Filtration removes insoluble organic matter; the sludge is digested to produce methane. Aeration removes organic waste using oxygen-requiring bacteria. Chlorination kills bacteria to allow water to be used for drinking purposes. OTHER THINGS THAT CAN BE DONE Fluoridation Fluoride compounds are added to water in some areas to aid the prevention of tooth decay. The fluoride is only helpful to children and can be given in tablet form. Many people object to the addition of fluoride to drinking water. Water filters These contain carbon, silver and ion exchange resins and can remove some dissolved substances from tap water to improve the taste and quality. Distillation Needs a lot of energy so is expensive but makes the purest water.

WATER THE END © 2011 JONATHAN HOPTON & KNOCKHARDY PUBLISHING
 Knockhardy publishing
Knockhardy publishing Knockhardy gcse
Knockhardy gcse Knockhardy
Knockhardy Knockhardy gcse
Knockhardy gcse Water and water and water water
Water and water and water water Eduardo de lete
Eduardo de lete Vtac guide 2010 online
Vtac guide 2010 online Ocr gcse modern world history revision guide
Ocr gcse modern world history revision guide Aqa maths gcse revision guide
Aqa maths gcse revision guide Knockhardy science
Knockhardy science Knockhardy chemistry
Knockhardy chemistry Knockhardy publishing
Knockhardy publishing First row transition metals
First row transition metals Knockhardy a level chemistry
Knockhardy a level chemistry Knockhardy
Knockhardy Br2 color
Br2 color Knockhardy chemistry
Knockhardy chemistry Knockhardy
Knockhardy Atom economy
Atom economy Knockhardy publishing
Knockhardy publishing Enthalpy change calculations a level
Enthalpy change calculations a level Acid and carbonate reaction
Acid and carbonate reaction Knockhardy notes
Knockhardy notes Halogenoalkane to alcohol
Halogenoalkane to alcohol Knockhardy chemistry
Knockhardy chemistry Knockhardy ppt
Knockhardy ppt Knockhardy publishing
Knockhardy publishing Water potential gcse
Water potential gcse Fspos vägledning för kontinuitetshantering
Fspos vägledning för kontinuitetshantering Typiska drag för en novell
Typiska drag för en novell Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Ekologiskt fotavtryck
Ekologiskt fotavtryck Varför kallas perioden 1918-1939 för mellankrigstiden?
Varför kallas perioden 1918-1939 för mellankrigstiden? En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Kassaregister ideell förening
Kassaregister ideell förening Tidbok för yrkesförare
Tidbok för yrkesförare A gastrica
A gastrica Förklara densitet för barn
Förklara densitet för barn Datorkunskap för nybörjare
Datorkunskap för nybörjare Boverket ka
Boverket ka Mall för debattartikel
Mall för debattartikel Magnetsjukhus
Magnetsjukhus Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Kraft per area
Kraft per area Offentlig förvaltning
Offentlig förvaltning Lyckans minut erik lindorm analys
Lyckans minut erik lindorm analys Presentera för publik crossboss
Presentera för publik crossboss Vad är ett minoritetsspråk
Vad är ett minoritetsspråk Plats för toran ark
Plats för toran ark Klassificeringsstruktur för kommunala verksamheter
Klassificeringsstruktur för kommunala verksamheter Epiteltyper
Epiteltyper Claes martinsson
Claes martinsson Cks
Cks Programskede byggprocessen
Programskede byggprocessen Bra mat för unga idrottare
Bra mat för unga idrottare Verktyg för automatisering av utbetalningar
Verktyg för automatisering av utbetalningar Rutin för avvikelsehantering
Rutin för avvikelsehantering Smärtskolan kunskap för livet
Smärtskolan kunskap för livet Ministerstyre för och nackdelar
Ministerstyre för och nackdelar Tack för att ni har lyssnat
Tack för att ni har lyssnat Referat mall
Referat mall Redogör för vad psykologi är
Redogör för vad psykologi är Matematisk modellering eksempel
Matematisk modellering eksempel Tack för att ni har lyssnat
Tack för att ni har lyssnat Borra hål för knoppar
Borra hål för knoppar Vilken grundregel finns det för tronföljden i sverige?
Vilken grundregel finns det för tronföljden i sverige? Varians
Varians