contents Electrolysis of molten compounds Electrolysis of solutions

- Slides: 6

contents • Electrolysis of molten compounds • Electrolysis of solutions • Electroplating • Extraction of metals • Electric cells

electrolysis of molten compounds § an electrolyte is an ionic compound that conducts electricity; molten state or solution in water § molten ionic compounds and solutions of ionic compounds can conduct electricity due to presence of mobile ions that can carry current § electrolysis is the conduction of electricity by an electrolyte, leading to its decomposition § example: electrolysis of molten sodium chloride § inert electrodes (e. g. carbon electrodes) do not react in electrolysis

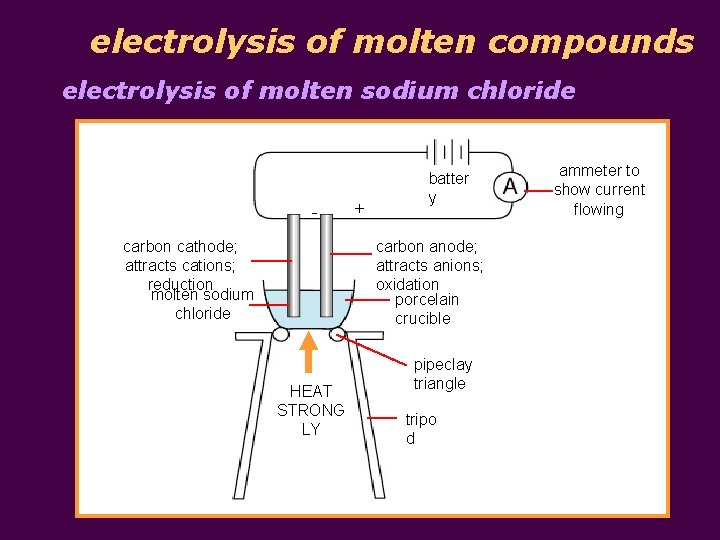
electrolysis of molten compounds electrolysis of molten sodium chloride - + batter y carbon anode; attracts anions; oxidation porcelain crucible carbon cathode; attracts cations; reduction molten sodium chloride HEAT STRONG LY pipeclay triangle tripo d ammeter to show current flowing

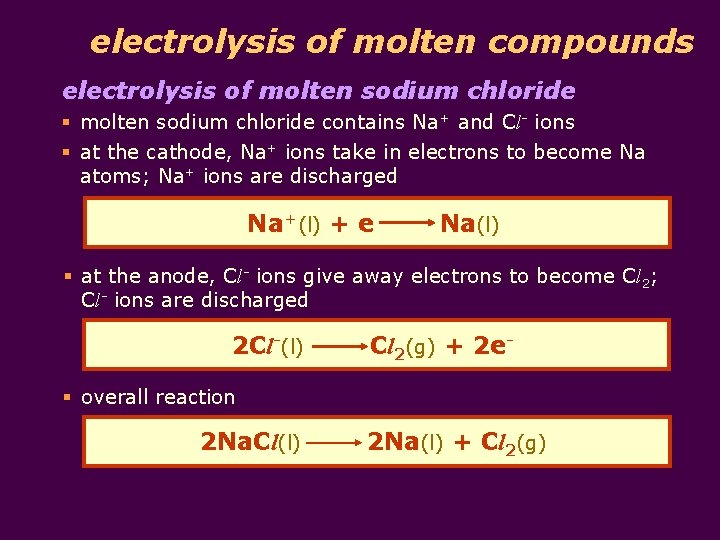
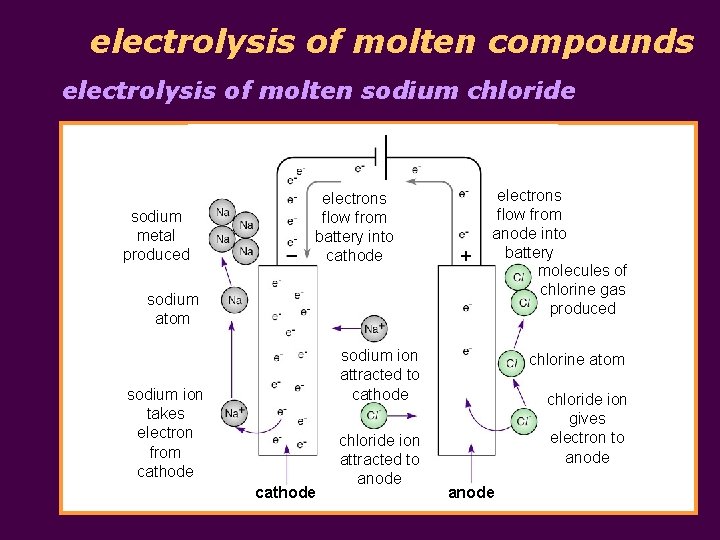
electrolysis of molten compounds electrolysis of molten sodium chloride § molten sodium chloride contains Na+ and Cl- ions § at the cathode, Na+ ions take in electrons to become Na atoms; Na+ ions are discharged Na+(l) + e Na(l) § at the anode, Cl- ions give away electrons to become Cl 2; Cl- ions are discharged 2 Cl-(l) Cl 2(g) + 2 e- § overall reaction 2 Na. Cl(l) 2 Na(l) + Cl 2(g)

electrolysis of molten compounds electrolysis of molten sodium chloride sodium metal produced electrons flow from battery into cathode sodium atom electrons flow from anode into battery molecules of chlorine gas produced sodium ion attracted to cathode sodium ion takes electron from cathode chloride ion attracted to anode chlorine atom chloride ion gives electron to anode

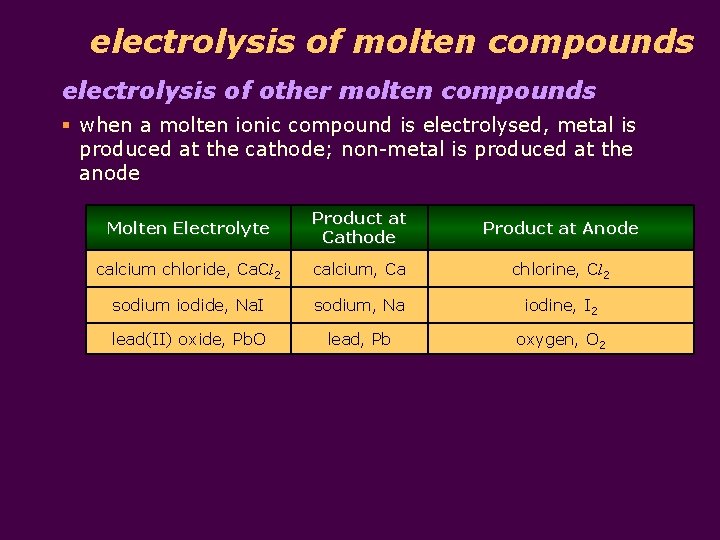
electrolysis of molten compounds electrolysis of other molten compounds § when a molten ionic compound is electrolysed, metal is produced at the cathode; non-metal is produced at the anode Molten Electrolyte Product at Cathode Product at Anode calcium chloride, Ca. Cl 2 calcium, Ca chlorine, Cl 2 sodium iodide, Na. I sodium, Na iodine, I 2 lead(II) oxide, Pb. O lead, Pb oxygen, O 2