Electrolysis Molten Solutions Aqueous Solutions Applications Cells Electrolysis












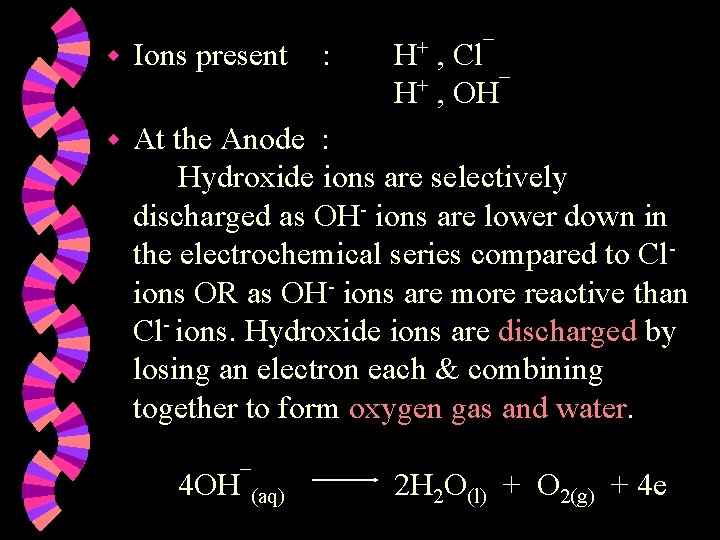





- Slides: 58

Electrolysis Molten Solutions Aqueous Solutions Applications Cells

Electrolysis w Means “splitting with electricity”. w It is the chemical decomposition of a molten liquid or aqueous solution containing mobile ions by electricity. An electric current is passed through the molten liquid or aqueous solution, causing the liquid / solution to decompose.

Terms : Electrolyte : The substance that conducts electricity in the molten / aqueous state. It undergoes w decomposition during electrolysis. w w Electrolytes are usually ionic compounds, but may consist of covalent compounds that dissolve in water to form ions.

w Some ionic compounds are insoluble in water & cannot form aqueous solutions. e. g. Lead (II) chloride Calcium carbonate Zinc Hydroxide These substances cannot act as electrolytes in the aqueous state as they do not exist in this state. They may exist as electrolytes in the molten state if they do not decompose on heating.

w Some covalent compounds dissolve in water and ionize, forming mobile ions in solution. These substances act as electrolytes in the aqueous state. w Examples are : Ammonia gas Hydrogen chloride gas Sulphur dioxide gas Sulphur trioxide gas Chlorine gas

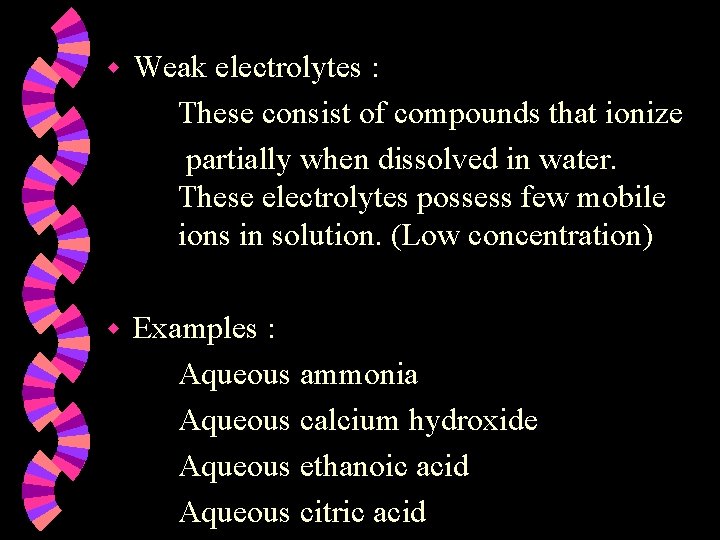
w Weak electrolytes : These consist of compounds that ionize partially when dissolved in water. These electrolytes possess few mobile ions in solution. (Low concentration) w Examples : Aqueous ammonia Aqueous calcium hydroxide Aqueous ethanoic acid Aqueous citric acid

w Strong Electrolytes : These consist of compounds that undergo complete ionisation when dissolved in water. These electrolytes possess a high concentration of ions in solution. w Examples : Dilute sulphuric acid Dilute hydrochloric acid Copper (II) sulphate solution

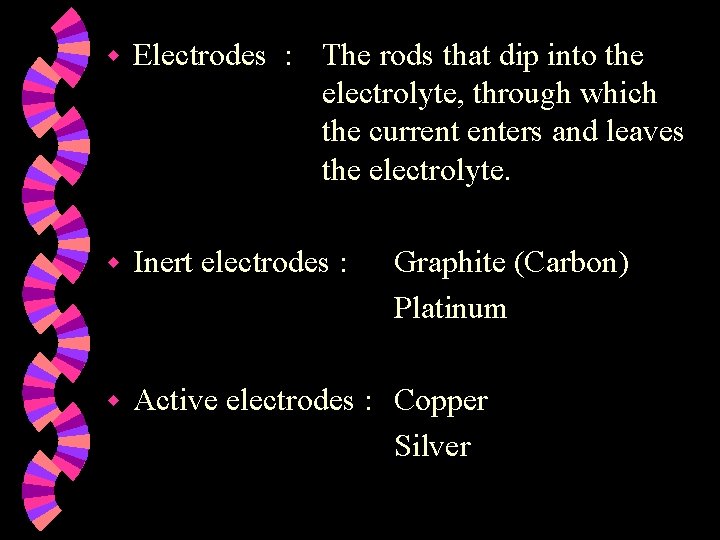
w Electrodes : The rods that dip into the electrolyte, through which the current enters and leaves the electrolyte. w Inert electrodes : w Active electrodes : Copper Silver Graphite (Carbon) Platinum

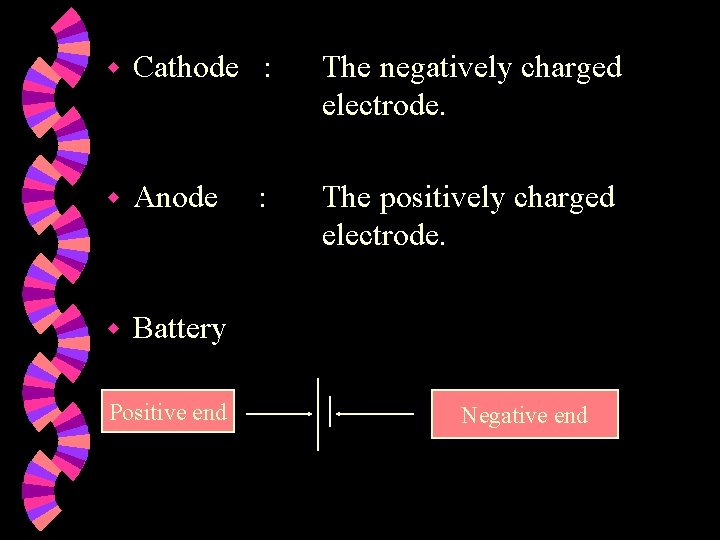
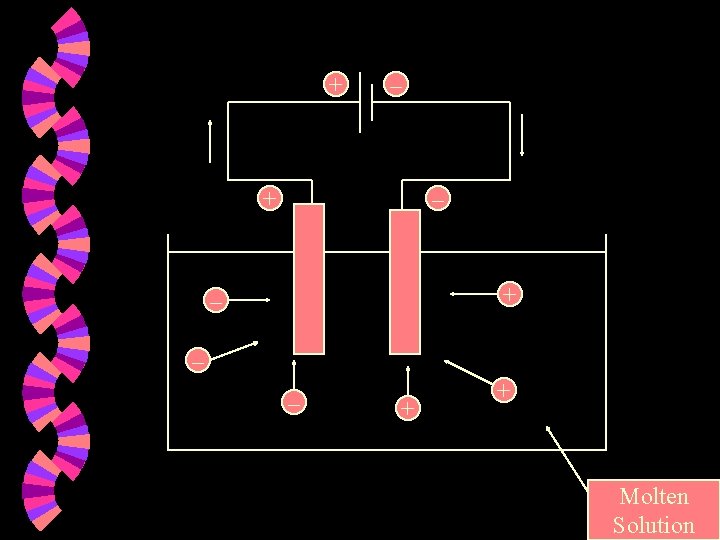
w Cathode : The negatively charged electrode. w Anode The positively charged electrode. w Battery Positive end : Negative end

+ – + – – – + + Molten Solution

Processes in Electrolysis : w Electrons move from the negative end of the battery to the positive end. This forms the external circuit. w Within the electrolyte, the electrodes become charged. w The positive ions (cations) move to the cathode.

w The negative ions (anions) move to the anode. w The flow of ions towards the electrodes constitutes the flow of current through the solution. w At the electrodes, the ions lose their charges to form neutral atoms / molecules. The ions get discharged. Otherwise, the anode may dissolve to form ions in aqueous solutions.

Substances that can conduct electricity : w Metals : Molten / Solid state. w Ionic compounds : Aqueous / Molten state. w Some covalent compounds : Aqueous state, if they dissolve in water to form ions

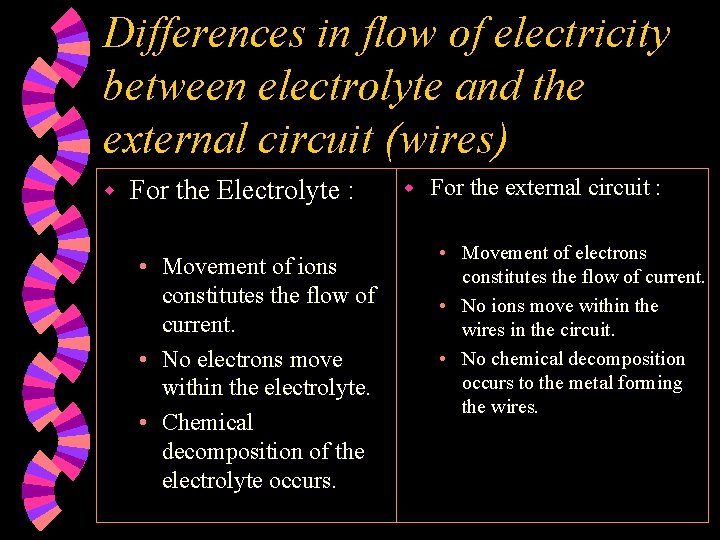
Differences in flow of electricity between electrolyte and the external circuit (wires) w For the Electrolyte : • Movement of ions constitutes the flow of current. • No electrons move within the electrolyte. • Chemical decomposition of the electrolyte occurs. w For the external circuit : • Movement of electrons constitutes the flow of current. • No ions move within the wires in the circuit. • No chemical decomposition occurs to the metal forming the wires.

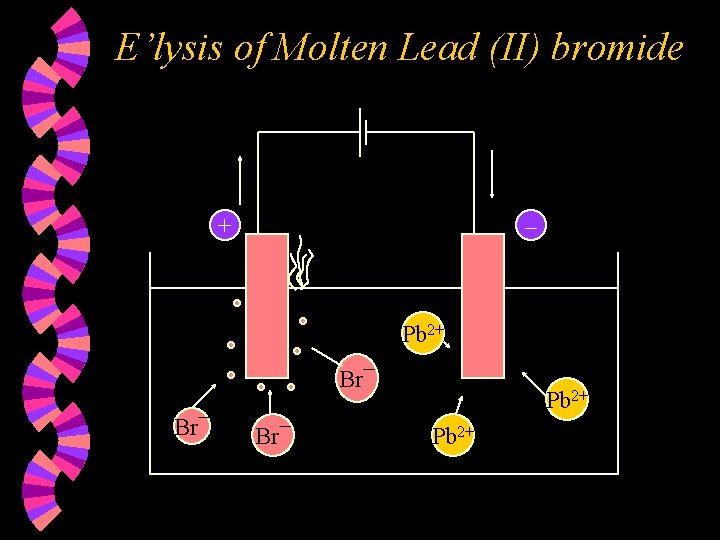
E’lysis of Molten Lead (II) bromide + – Pb 2+ Br¯ Br¯ Pb 2+

w At the Anode : • Br¯ ions are discharged and lose one electron each to form Br atoms. Two Br atoms combine to form Br 2 molecules. • 2 Br¯ (l) – 2 e Br 2(g) • Therefore, bromine vapour bubbles out.

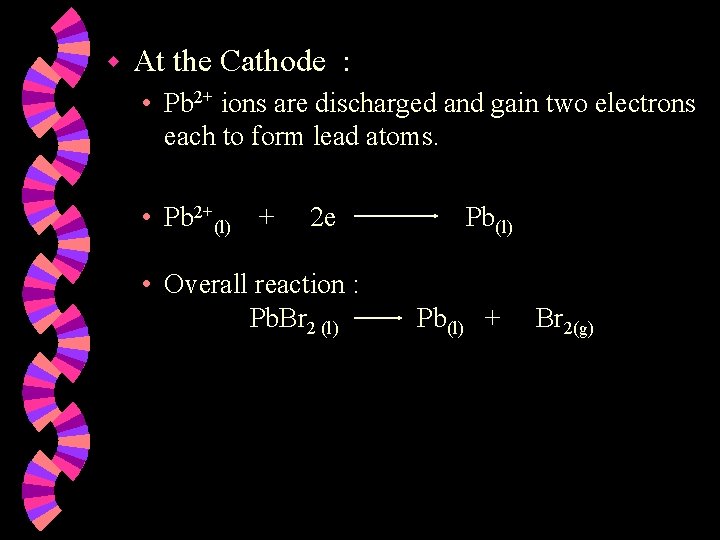
w At the Cathode : • Pb 2+ ions are discharged and gain two electrons each to form lead atoms. • Pb 2+(l) + 2 e • Overall reaction : Pb. Br 2 (l) Pb(l) + Br 2(g)

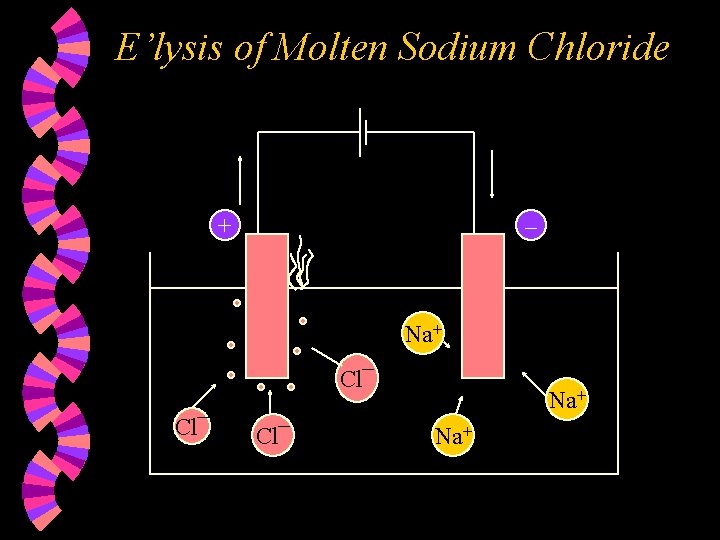
E’lysis of Molten Sodium Chloride + – Na+ Cl¯ Cl¯ Na+

w At the Anode : • Cl¯ ions are discharged and lose one electron each to form Cl atoms. Two Cl atoms combine to form Cl 2 molecules. • 2 Cl¯ (l) – 2 e Cl 2(g) • Therefore, Chlorine vapour bubbles out.

w At the Cathode : • Na+ ions are discharged and gain one electron each to form sodium atoms. • Na+(l) + e • Overall reaction : Na. Cl (l) Na(l) + Cl 2(g)

E’lysis of Aqueous Solutions : w In aqueous solutions of salts, the ions in solution are ions from the salt and H+ and OH¯ ions from water. w With more than one cation / anion in solution, selective discharge of ions will occur i. e. preferred reactions are those that occur easily.

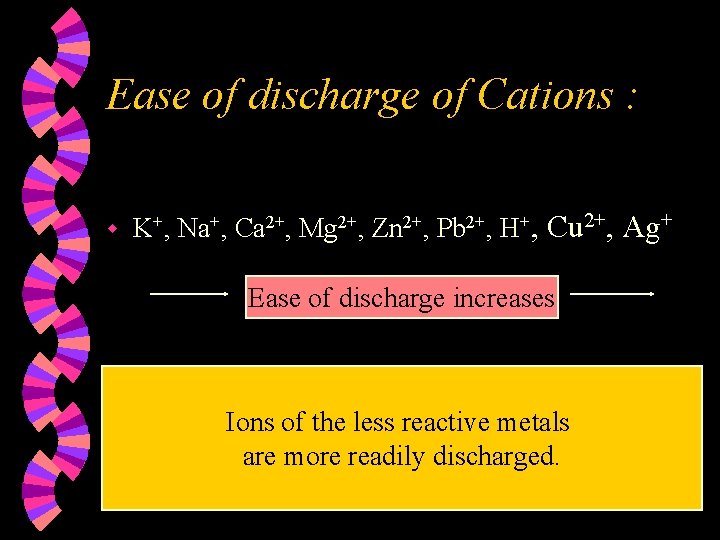
Ease of discharge of Cations : w K+, Na+, Ca 2+, Mg 2+, Zn 2+, Pb 2+, H+, Cu 2+, Ag+ Ease of discharge increases Ions of the less reactive metals are more readily discharged.

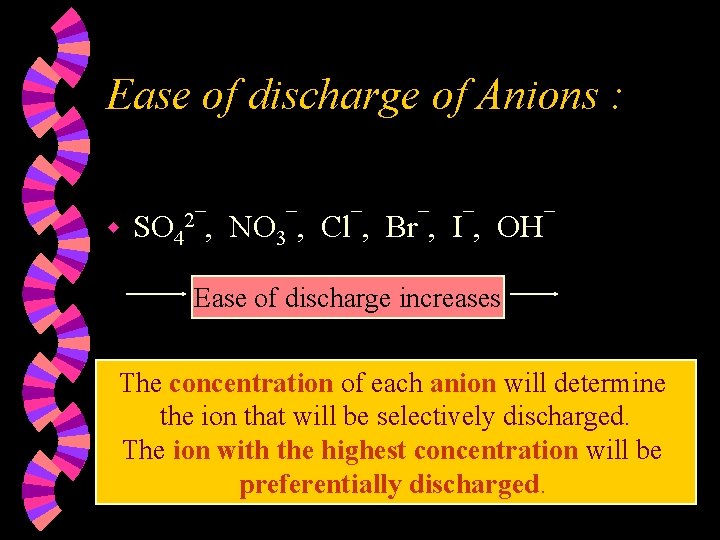
Ease of discharge of Anions : w SO 42¯, NO 3¯, Cl¯, Br¯, I¯, OH¯ Ease of discharge increases The concentration of each anion will determine the ion that will be selectively discharged. The ion with the highest concentration will be preferentially discharged.

Selective discharge of ions depends on : w Position of the ions in the electrochemical series. w Concentration of the anions i. e. ions of higher concentration will be discharged more readily.

w The type of electrode used • If the electrode is inert , like graphite and platinum electrodes , it does not react / dissolve. • If it not inert / active, like copper electrodes, the anode will dissolve to form ions & release electrons.

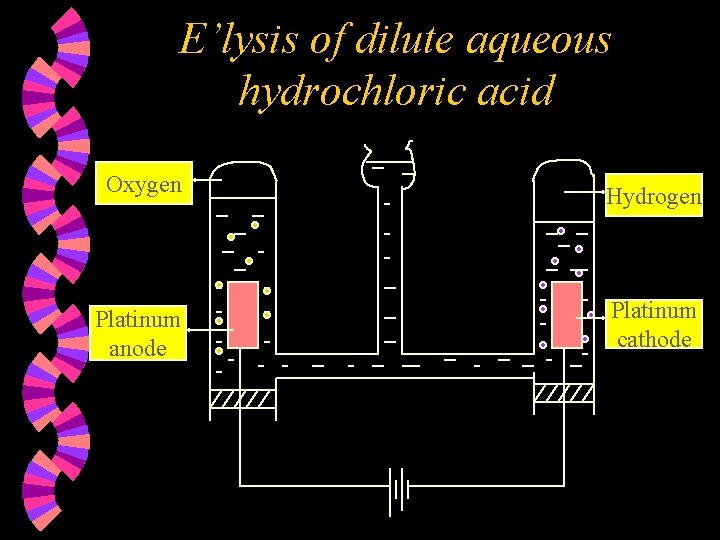
E’lysis of dilute aqueous hydrochloric acid Oxygen Hydrogen Platinum anode Platinum cathode
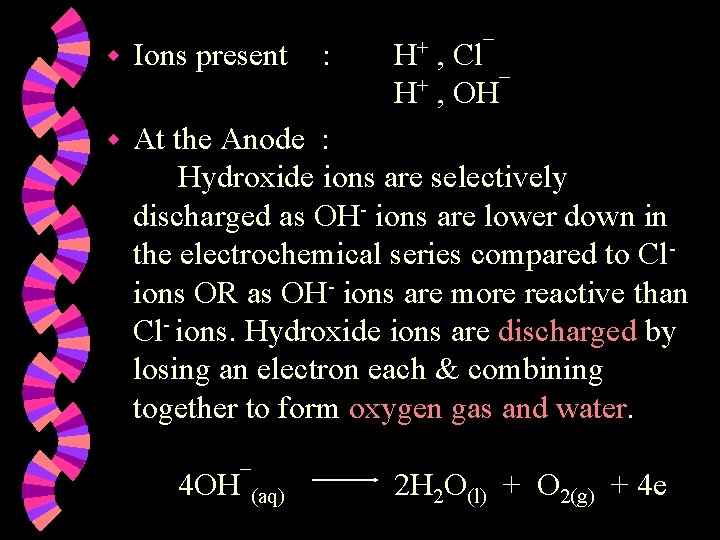
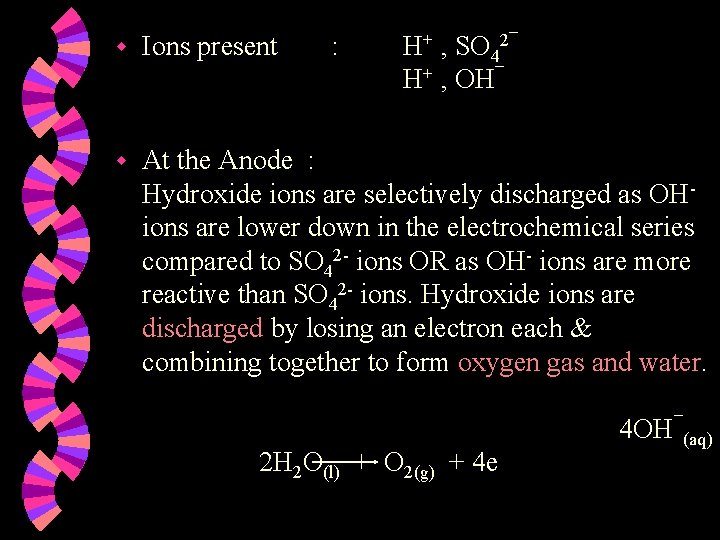
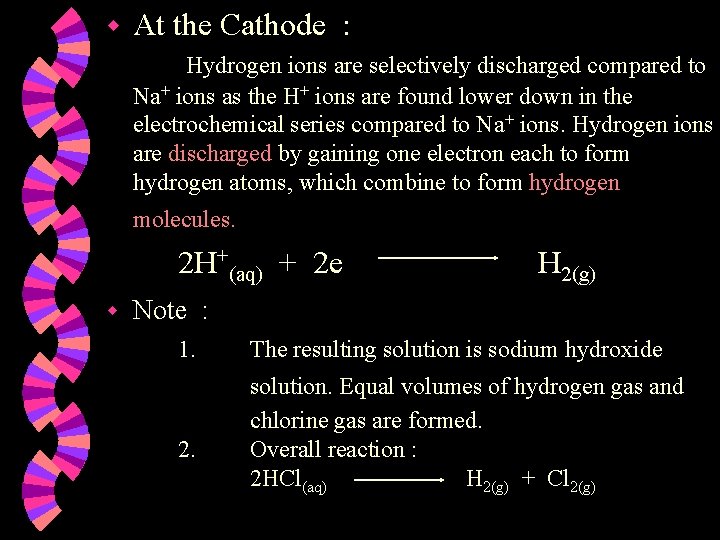
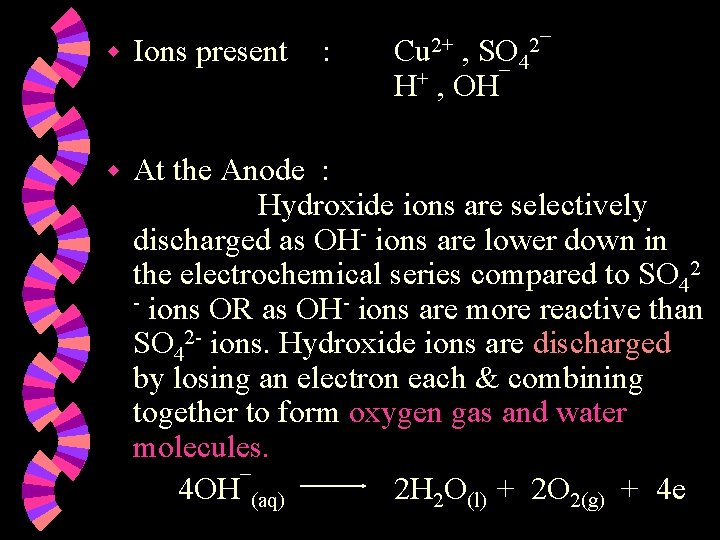
w Ions present w At the Anode : Hydroxide ions are selectively discharged as OH- ions are lower down in the electrochemical series compared to Clions OR as OH- ions are more reactive than Cl- ions. Hydroxide ions are discharged by losing an electron each & combining together to form oxygen gas and water. 4 OH¯(aq) : H+ , Cl¯ H+ , OH¯ 2 H 2 O(l) + O 2(g) + 4 e

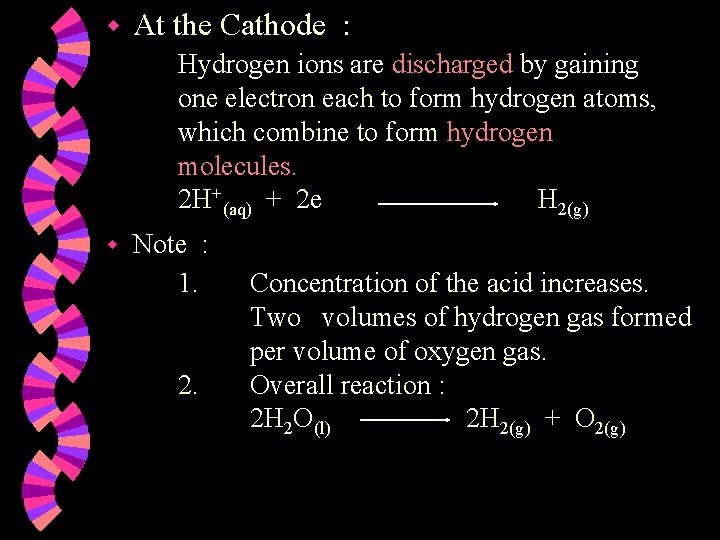
w At the Cathode : Hydrogen ions are discharged by gaining one electron each to form hydrogen atoms, which combine to form hydrogen molecules. 2 H+(aq) + 2 e H 2(g) w Note : 1. 2. Concentration of the acid increases. Two volumes of hydrogen gas formed per volume of oxygen gas. Overall reaction : 2 H 2 O(l) 2 H 2(g) + O 2(g)

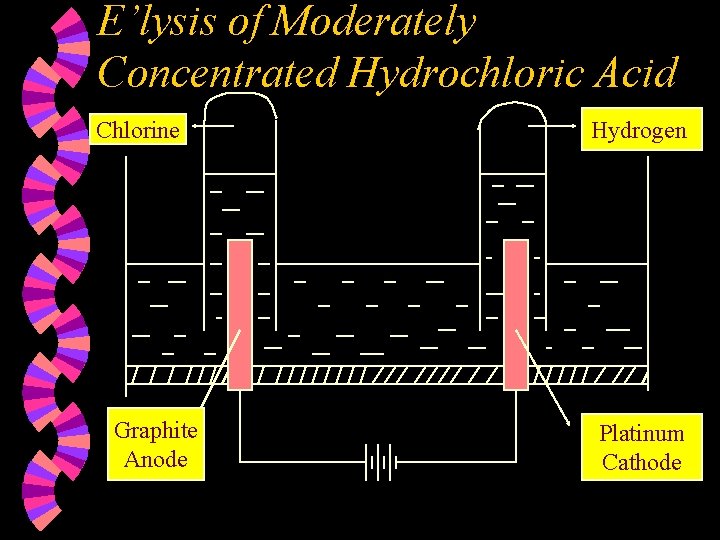
E’lysis of Moderately Concentrated Hydrochloric Acid Chlorine Graphite Anode Hydrogen Platinum Cathode

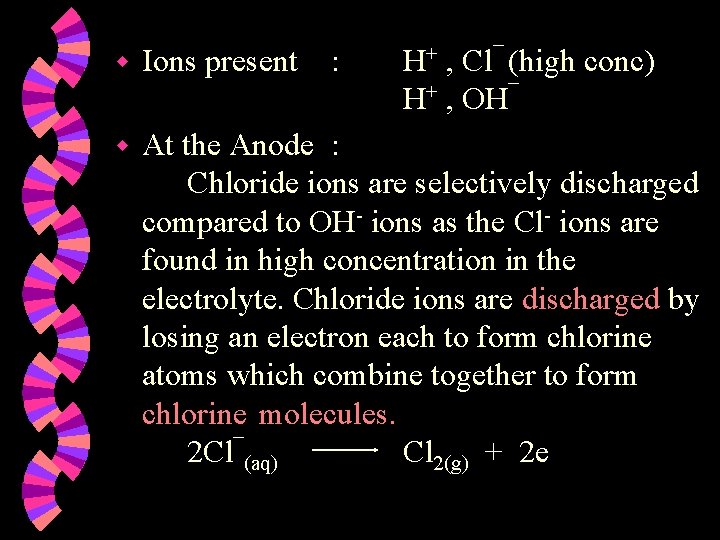
w Ions present : H+ , Cl¯ (high conc) H+ , OH¯ w At the Anode : Chloride ions are selectively discharged compared to OH- ions as the Cl- ions are found in high concentration in the electrolyte. Chloride ions are discharged by losing an electron each to form chlorine atoms which combine together to form chlorine molecules. 2 Cl¯(aq) Cl 2(g) + 2 e

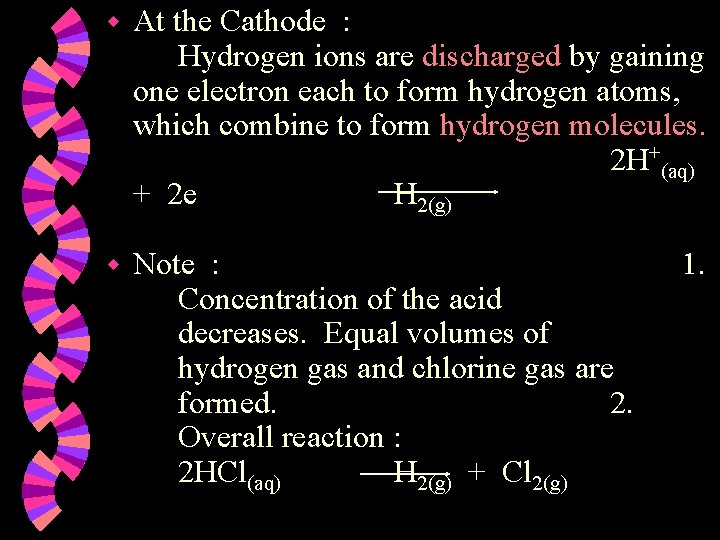
w At the Cathode : Hydrogen ions are discharged by gaining one electron each to form hydrogen atoms, which combine to form hydrogen molecules. 2 H+(aq) + 2 e H 2(g) w Note : Concentration of the acid decreases. Equal volumes of hydrogen gas and chlorine gas are formed. 2. Overall reaction : 2 HCl(aq) H 2(g) + Cl 2(g) 1.

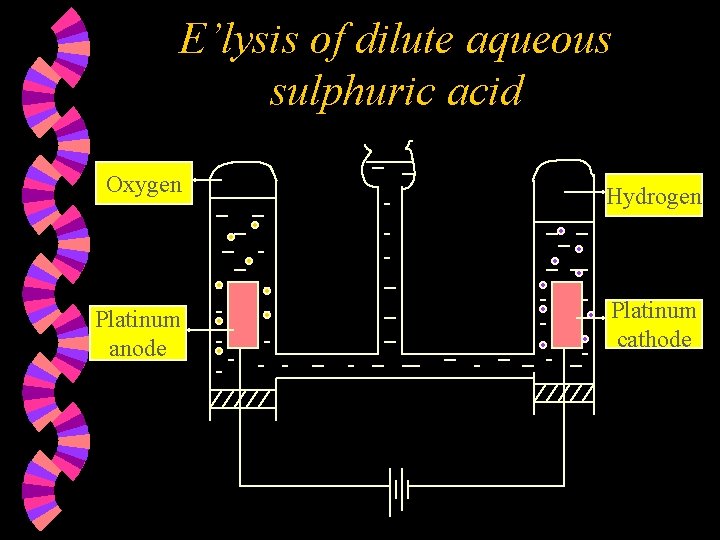
E’lysis of dilute aqueous sulphuric acid Oxygen Hydrogen Platinum anode Platinum cathode

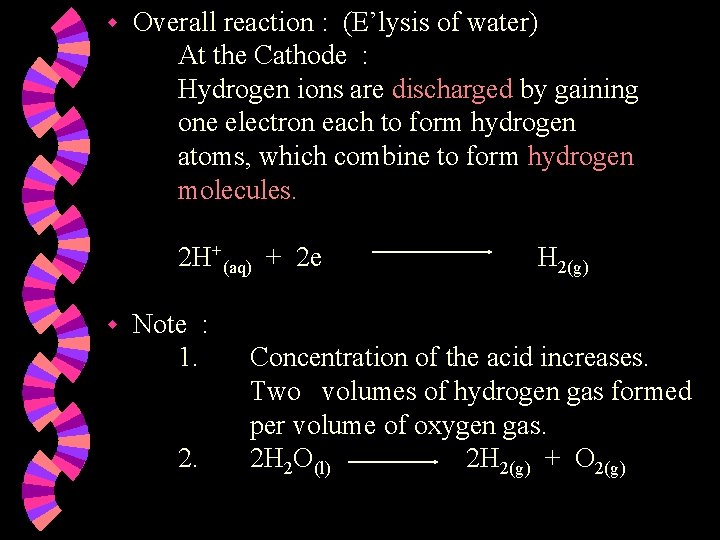
w Ions present : H+ , SO 42¯ H+ , OH¯ w At the Anode : Hydroxide ions are selectively discharged as OHions are lower down in the electrochemical series compared to SO 42 - ions OR as OH- ions are more reactive than SO 42 - ions. Hydroxide ions are discharged by losing an electron each & combining together to form oxygen gas and water. 2 H 2 O(l) + O 2(g) + 4 e 4 OH¯(aq)

w Overall reaction : (E’lysis of water) At the Cathode : Hydrogen ions are discharged by gaining one electron each to form hydrogen atoms, which combine to form hydrogen molecules. 2 H+(aq) + 2 e w Note : 1. 2. H 2(g) Concentration of the acid increases. Two volumes of hydrogen gas formed per volume of oxygen gas. 2 H 2 O(l) 2 H 2(g) + O 2(g)

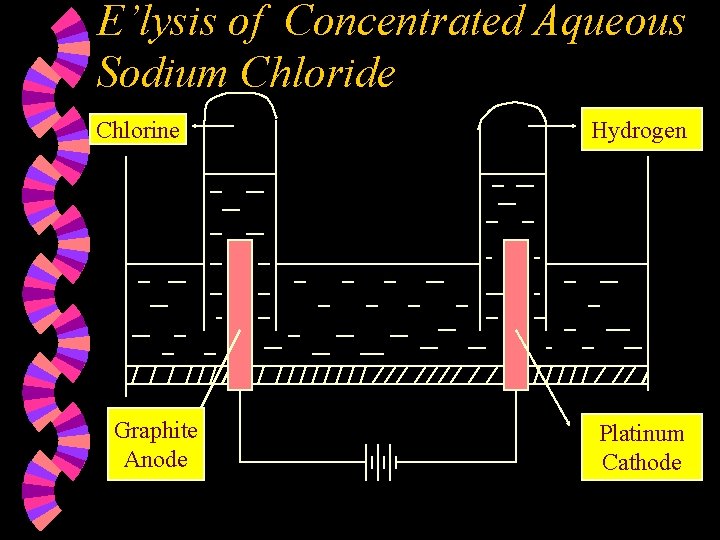
E’lysis of Concentrated Aqueous Sodium Chloride Chlorine Graphite Anode Hydrogen Platinum Cathode

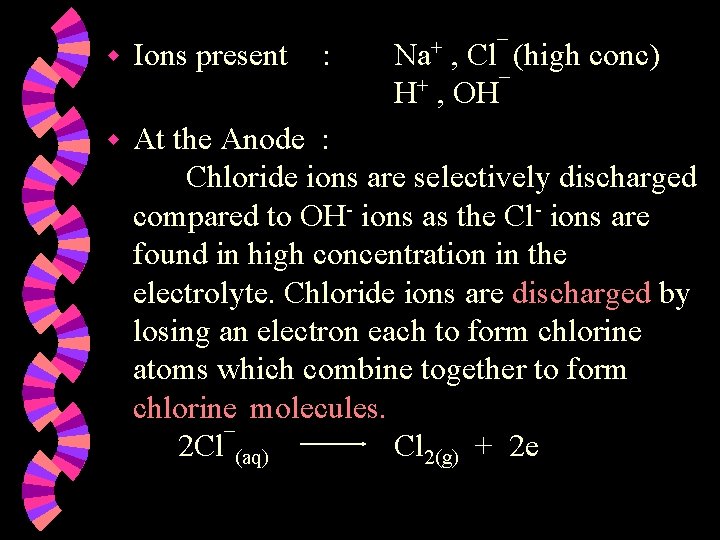
w Ions present : Na+ , Cl¯ (high conc) H+ , OH¯ w At the Anode : Chloride ions are selectively discharged compared to OH- ions as the Cl- ions are found in high concentration in the electrolyte. Chloride ions are discharged by losing an electron each to form chlorine atoms which combine together to form chlorine molecules. 2 Cl¯(aq) Cl 2(g) + 2 e

w At the Cathode : Hydrogen ions are selectively discharged compared to Na+ ions as the H+ ions are found lower down in the electrochemical series compared to Na+ ions. Hydrogen ions are discharged by gaining one electron each to form hydrogen atoms, which combine to form hydrogen molecules. 2 H+(aq) + 2 e w H 2(g) Note : 1. 2. The resulting solution is sodium hydroxide solution. Equal volumes of hydrogen gas and chlorine gas are formed. Overall reaction : 2 HCl(aq) H 2(g) + Cl 2(g)

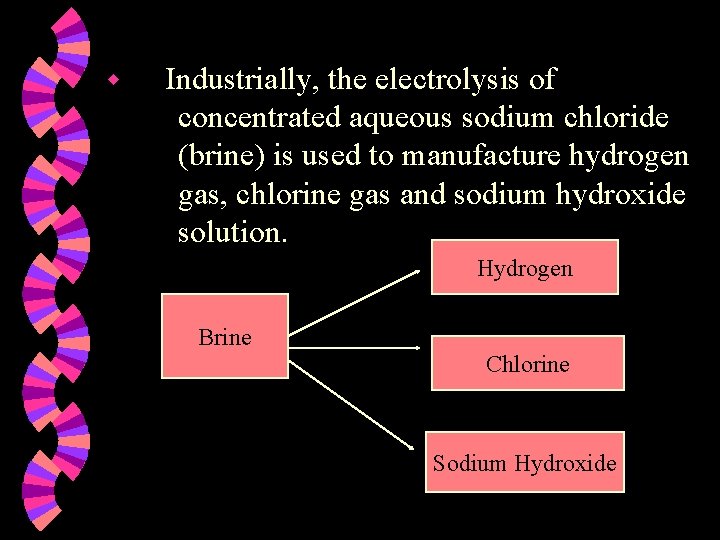
w Industrially, the electrolysis of concentrated aqueous sodium chloride (brine) is used to manufacture hydrogen gas, chlorine gas and sodium hydroxide solution. Hydrogen Brine Chlorine Sodium Hydroxide

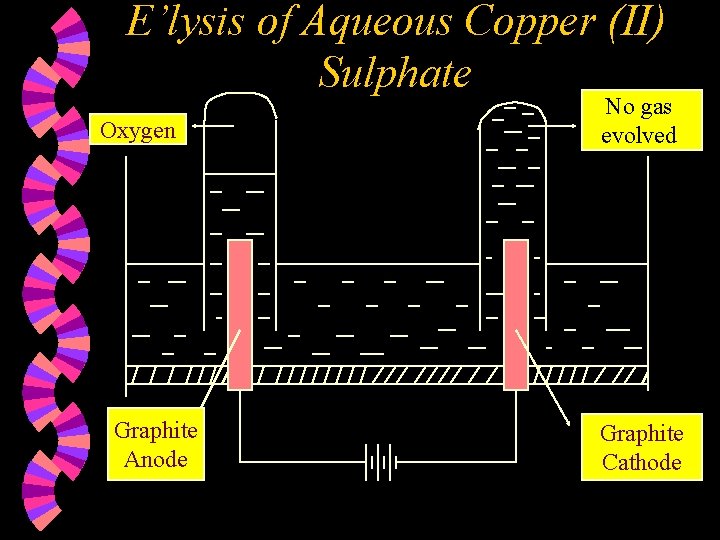
E’lysis of Aqueous Copper (II) Sulphate Oxygen Graphite Anode No gas evolved Graphite Cathode

w Ions present : Cu 2+ , SO 42¯ H+ , OH¯ w At the Anode : Hydroxide ions are selectively discharged as OH- ions are lower down in the electrochemical series compared to SO 42 - ions OR as OH- ions are more reactive than SO 42 - ions. Hydroxide ions are discharged by losing an electron each & combining together to form oxygen gas and water molecules. 4 OH¯(aq) 2 H 2 O(l) + 2 O 2(g) + 4 e

w At the Cathode : Copper (II) ions are selectively discharged compared to H+ ions as the Cu 2+ ions are found lower down in the electrochemical series compared to H+ ions. Copper (II) ions are discharged by gaining two electrons each to form copper atoms. Cu 2+(aq) + 2 e Cu(s) w Note : 1. The cathode is coated with a layer of pinkish-brown solid copper. 2. During electrolysis, the blue colour of the solution will fade. The concentration of Cu 2+ ions will drop.

• 3. The solution left behind in the vessel is sulphuric acid. • 4. Overall reaction : 2 Cu 2+(aq) + 4 OH¯(aq) 2 Cu(s) + 2 H 2 O(l) + O 2(g)

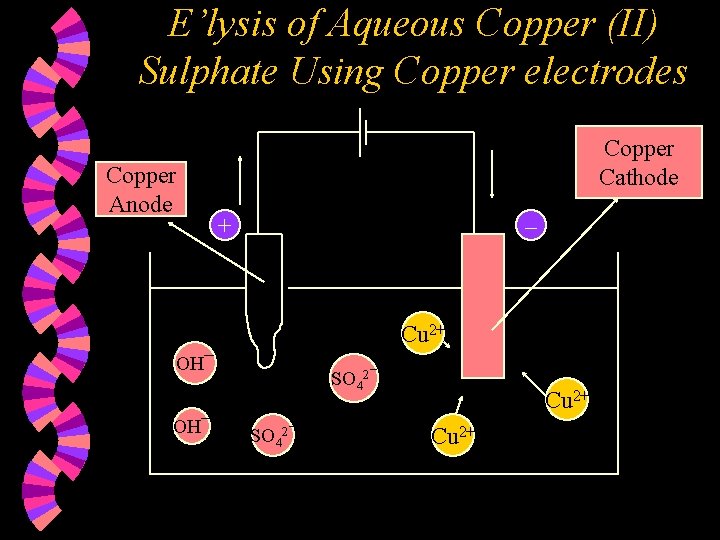
E’lysis of Aqueous Copper (II) Sulphate Using Copper electrodes Copper Anode Copper Cathode + – Cu 2+ OH¯ SO 42¯ Cu 2+

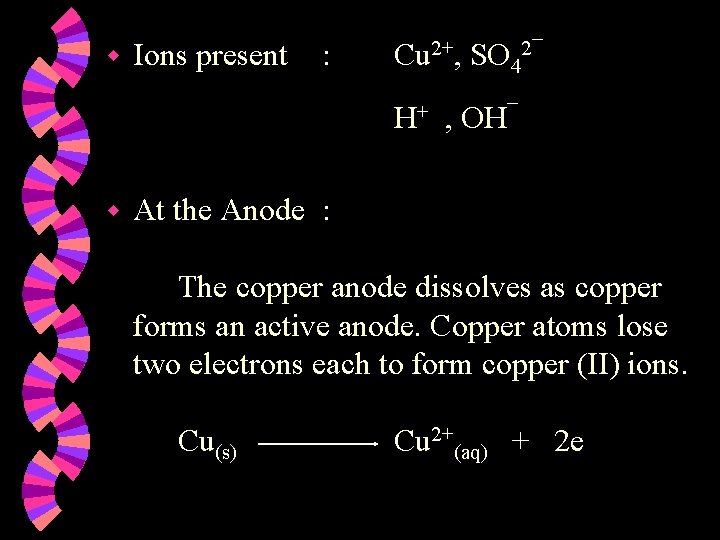
w Ions present : Cu 2+, SO 42¯ H+ , OH¯ w At the Anode : The copper anode dissolves as copper forms an active anode. Copper atoms lose two electrons each to form copper (II) ions. Cu(s) Cu 2+(aq) + 2 e

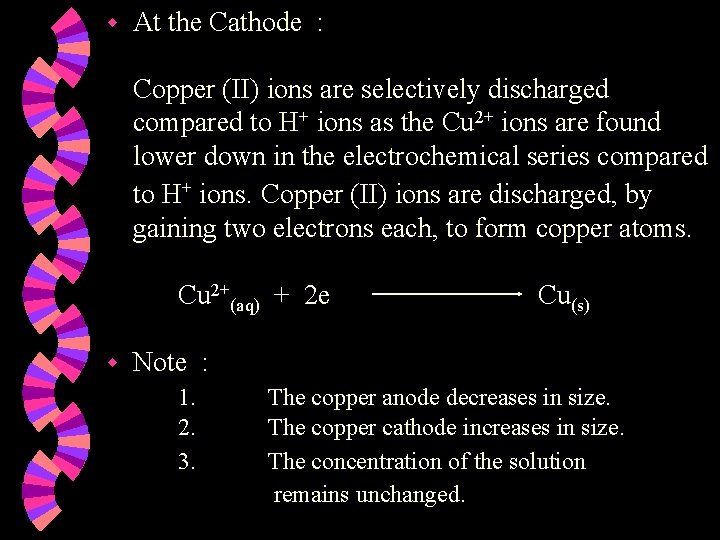
w At the Cathode : Copper (II) ions are selectively discharged compared to H+ ions as the Cu 2+ ions are found lower down in the electrochemical series compared to H+ ions. Copper (II) ions are discharged, by gaining two electrons each, to form copper atoms. Cu 2+(aq) + 2 e w Cu(s) Note : 1. 2. 3. The copper anode decreases in size. The copper cathode increases in size. The concentration of the solution remains unchanged.

Industrial Applications of E’lysis w Extraction of metals from their ores e. g. aluminium from bauxite (Al 2 O 3). w Electroplating w Purification of metals w Manufacture of Chlorine & Sodium Hydroxide

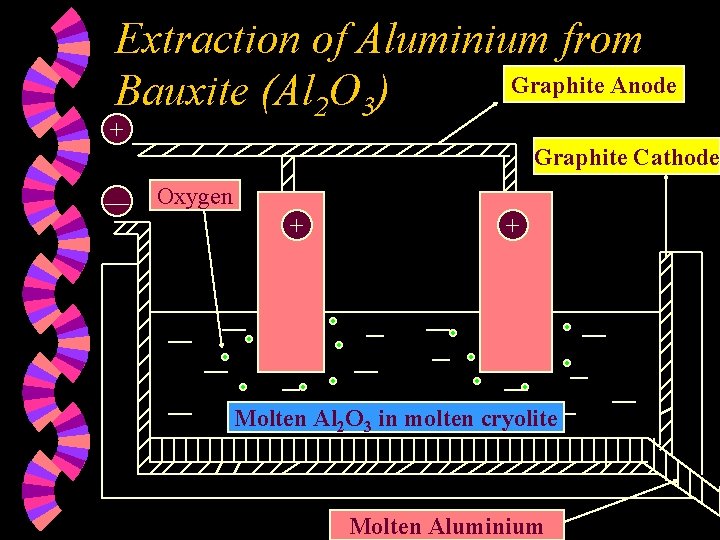
Extraction of Aluminium from Graphite Anode Bauxite (Al 2 O 3) + Graphite Cathode — Oxygen + + Molten Al 2 O 3 in molten cryolite Molten Aluminium

w The ore, bauxite, is first purified to obtain aluminium oxide. w Pure Al 2 O 3 melts at 2045 C. It is dissolved in molten cryolite (Na 3 Al. F 6). The mixture now melts at a lower temperature of 700°C. Molten cryolite lowers the melting point of aluminium oxide. It also behaves as a solvent for Al 2 O 3. w Is molten cryolite ionic or covalent ?

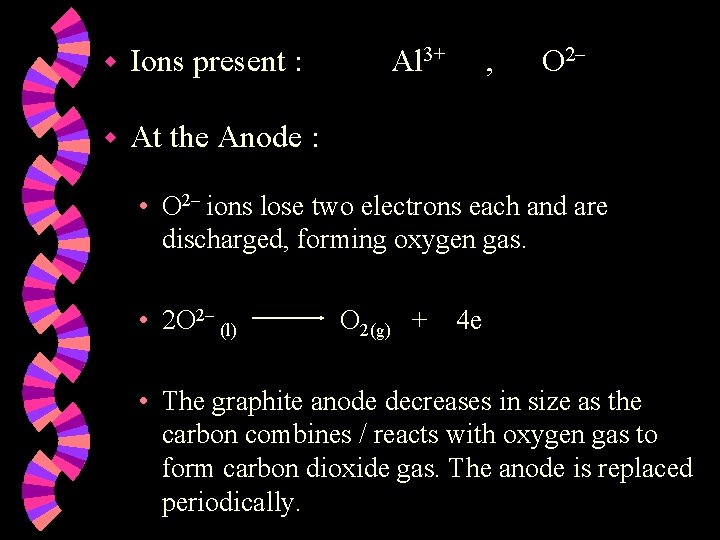
w Ions present : w At the Anode : Al 3+ , O 2 • O 2 ions lose two electrons each and are discharged, forming oxygen gas. • 2 O 2 (l) O 2(g) + 4 e • The graphite anode decreases in size as the carbon combines / reacts with oxygen gas to form carbon dioxide gas. The anode is replaced periodically.

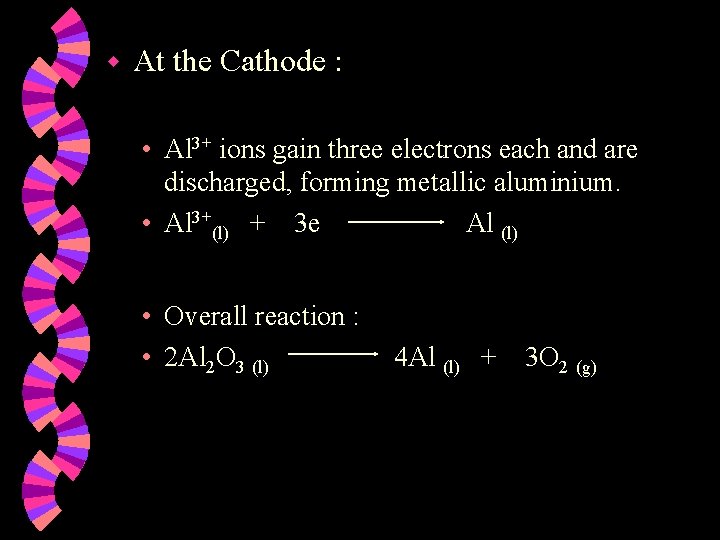
w At the Cathode : • Al 3+ ions gain three electrons each and are discharged, forming metallic aluminium. • Al 3+(l) + 3 e Al (l) • Overall reaction : • 2 Al 2 O 3 (l) 4 Al (l) + 3 O 2 (g)

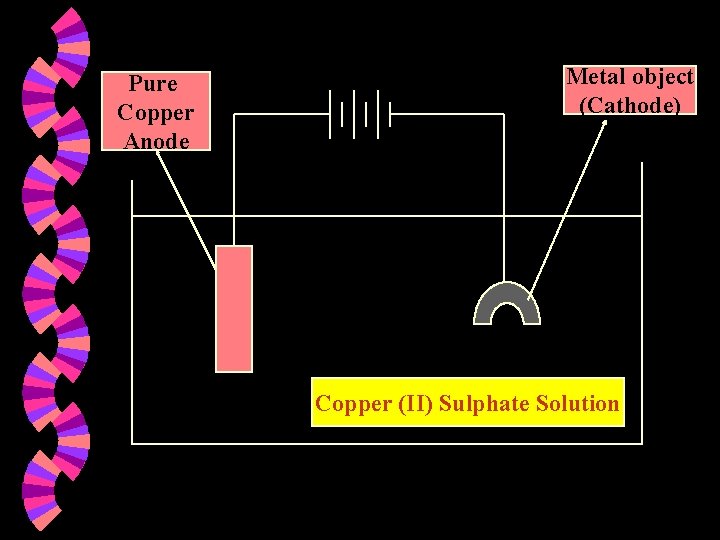
Electroplating w Electrolysis can be used to coat one metal with another. This process is called electroplating. w The object to be electroplated is made the cathode (-). w The anode is a pure metal e. g. copper and is the plating metal.

Pure Copper Anode Metal object (Cathode) Copper (II) Sulphate Solution

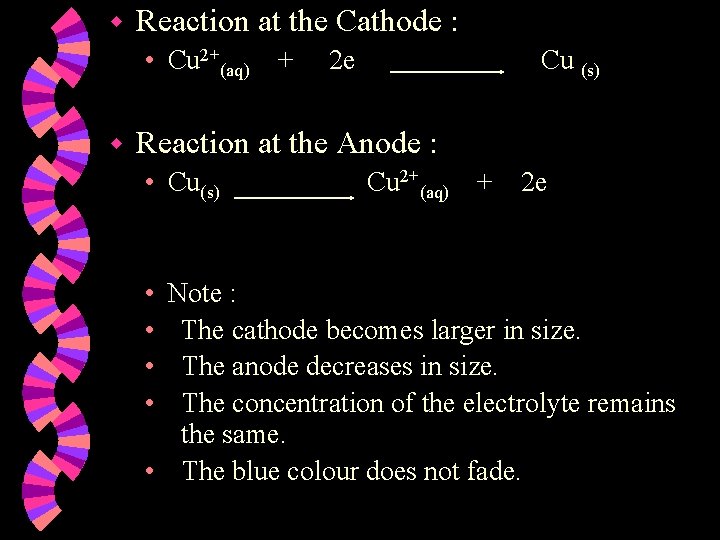
w Reaction at the Cathode : • Cu 2+(aq) + w 2 e Cu (s) Reaction at the Anode : • Cu(s) • • Cu 2+(aq) + 2 e Note : The cathode becomes larger in size. The anode decreases in size. The concentration of the electrolyte remains the same. • The blue colour does not fade.

Uses of Electroplating w Makes a metal look more attractive i. e. car bumpers, door handles & knobs. w Prevents a metal from rusting i. e. tin is electroplated onto iron in making food cans.
w What would the set - up look like if you want to plate an object with silver ?

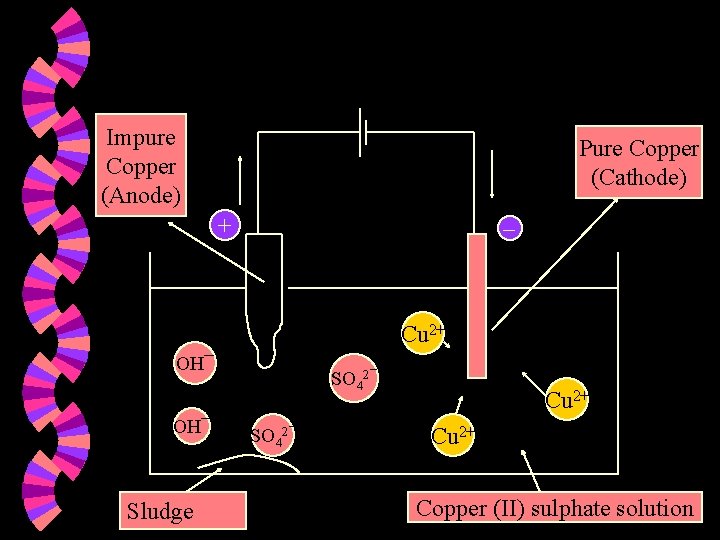
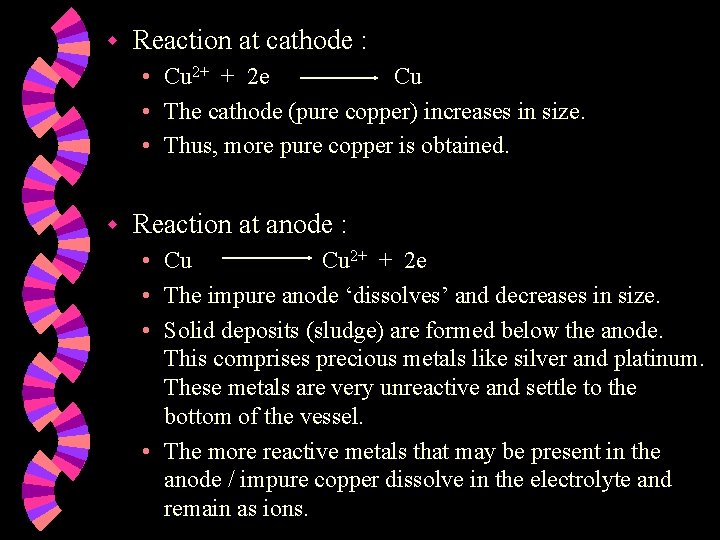
Purification of metals w If you have a piece of impure copper metal, you could purify it using electrolysis. The impure copper metal would be the anode. w A narrow strip of pure copper would be the cathode. w The electrolyte would be a solution containing copper (II) ions. w

Impure Copper (Anode) Pure Copper (Cathode) + Cu 2+ OH¯ Sludge SO 42¯ Cu 2+ Copper (II) sulphate solution

w Reaction at cathode : • Cu 2+ + 2 e Cu • The cathode (pure copper) increases in size. • Thus, more pure copper is obtained. w Reaction at anode : • Cu Cu 2+ + 2 e • The impure anode ‘dissolves’ and decreases in size. • Solid deposits (sludge) are formed below the anode. This comprises precious metals like silver and platinum. These metals are very unreactive and settle to the bottom of the vessel. • The more reactive metals that may be present in the anode / impure copper dissolve in the electrolyte and remain as ions.