Electrolysis Tro Chapter 19 Electrochemistry 19 8 Electrolysis



- Slides: 15

Electrolysis Tro Chapter 19 – Electrochemistry 19. 8 Electrolysis: Driving Nonspontaneous Chemical Reactions with Electricity

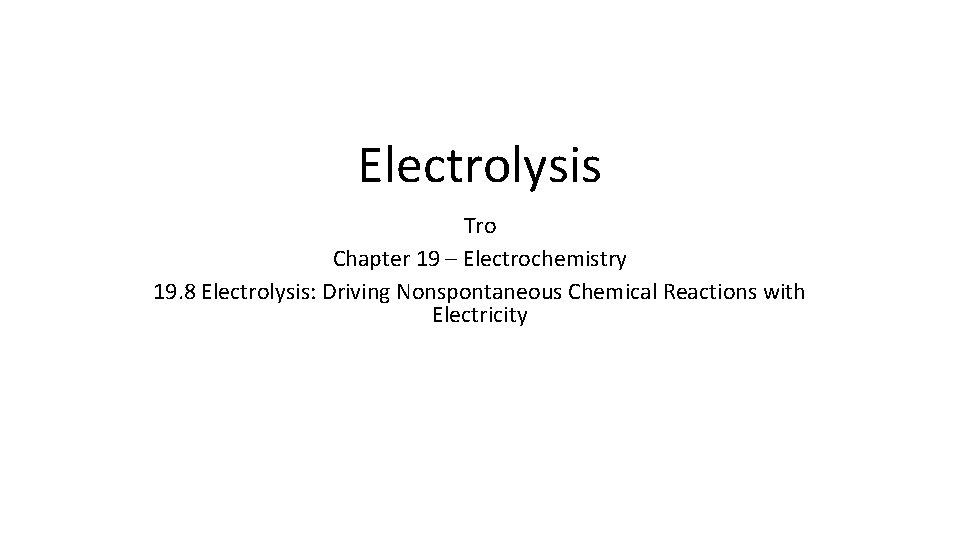
Electrochemical Cells In all cells, whether voltaic or electrolytic, oxidation occurs at the anode, and reduction occurs at the cathode. • Voltaic cells—Spontaneous reaction generates electricity. • Anode is the source of electrons and has a (−) charge. • Cathode draws electrons and has a (+) charge. • Electrolytic cells—nonspontaneous reaction driven by external electrical current. • Electrons are drawn away from the anode, which must be connected to the positive terminal of the external power source (anode +). • Electrons are forced to the cathode, which must be connected to the negative terminal of the power source (cathode -).

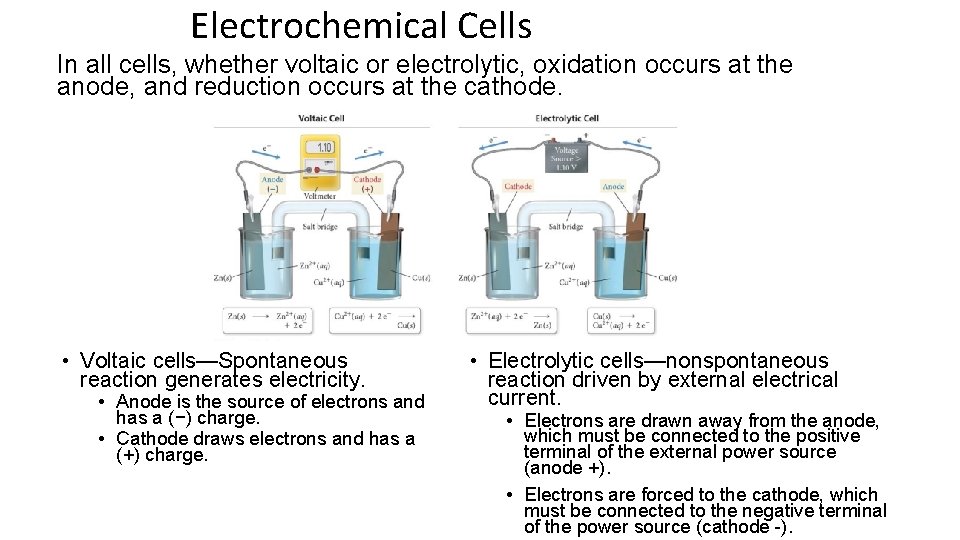
Electrolysis • Electrolysis is the process of using electrical current to drive nonspontaneous reaction. • Electrolysis is carried out in an electrolytic cell. • Electrolytic cells can be used to separate compounds into their elements.

Electrolysis of Aqueous Solutions • Possible cathode reactions • Reduction of cation to metal • Reduction of water to H 2 • 2 H 2 O + 2 e− ® H 2 + 2 OH− • Possible anode reactions E°= − 0. 83 V at stand. cond. E°= − 0. 41 V at p. H 7 • Oxidation of anion to element • Oxidation of H 2 O to O 2 • 2 H 2 O ® O 2 + 4 e− + 4 H+ • Oxidation of electrode E°= − 1. 23 V at stand. cond. E°= − 0. 82 V at p. H 7 • Particularly Cu • Graphite doesn’t oxidize. • Half-reactions that lead to least negative Ecell will occur. • Unless overvoltage changes the conditions

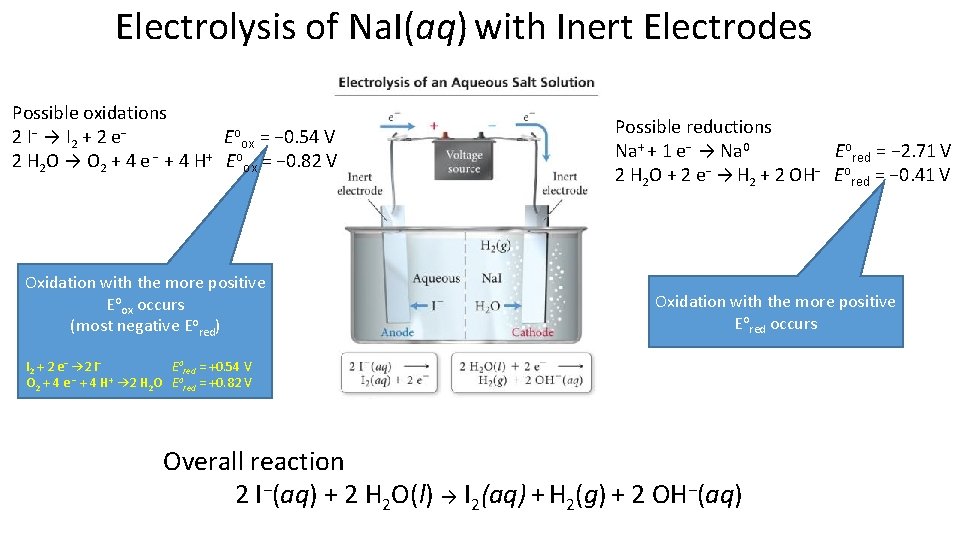
Electrolysis of Na. I(aq) with Inert Electrodes Possible oxidations 2 I − → I 2 + 2 e − Eoox = − 0. 54 V 2 H 2 O → O 2 + 4 e − + 4 H+ Eoox = − 0. 82 V Oxidation with the more positive Eoox occurs (most negative Eored) Possible reductions Na+ + 1 e− → Na 0 Eored = − 2. 71 V 2 H 2 O + 2 e− → H 2 + 2 OH− Eored = − 0. 41 V Oxidation with the more positive Eored occurs I 2 + 2 e− → 2 I− Eored = +0. 54 V O 2 + 4 e − + 4 H+ → 2 H 2 O Eored = +0. 82 V Overall reaction 2 I−(aq) + 2 H 2 O(l) → I 2(aq) + H 2(g) + 2 OH−(aq)

Mixtures of Ions • When more than one cation is present, the cation that is easiest to reduce will be reduced first at the cathode. • Least negative or most positive E°red • When more than one anion is present, the anion that is easiest to oxidize will be oxidized first at the anode. • Least negative or most positive E°ox

If a solution contains Cu 2+, Ag+, and Zn 2+ and the voltage starts out low and is gradually turned up in which order will the metals plate? Given: Ag+ (aq) + e- Ag (s) Eored = 0. 80 V Cu 2+ (aq) + 2 e- Cu (s) Eored = 0. 34 V Zn 2+ (aq) + 2 e- Zn (s) Eored = -0. 76 V

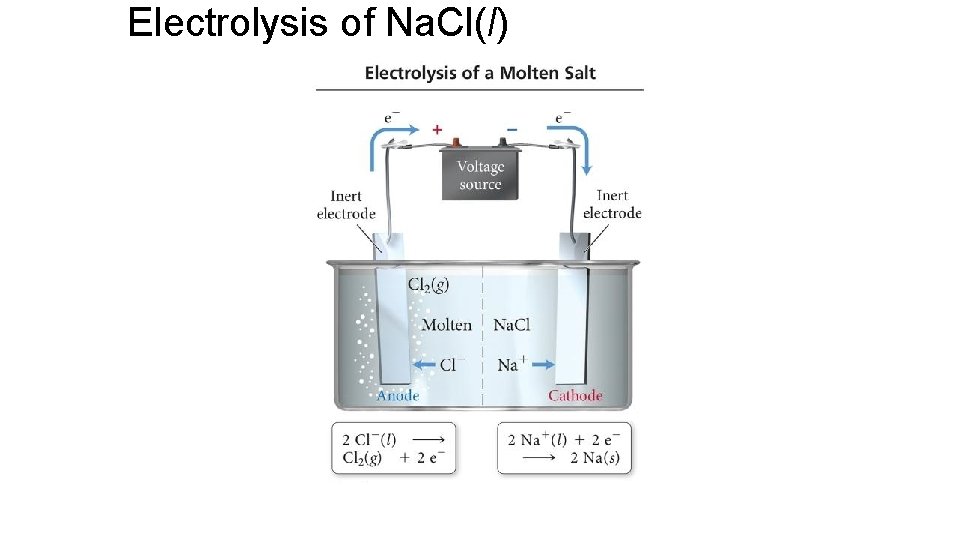
Electrolysis of Na. Cl(l)

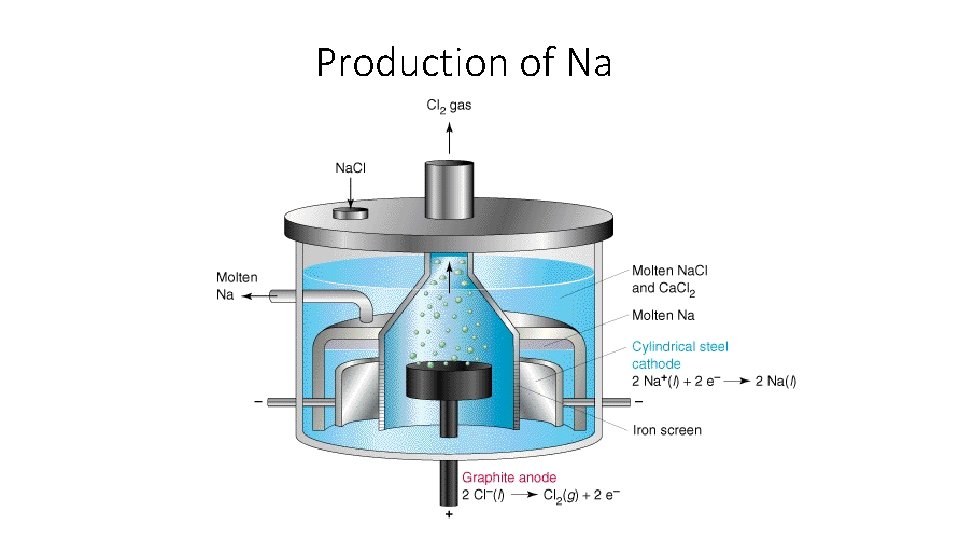
Production of Na

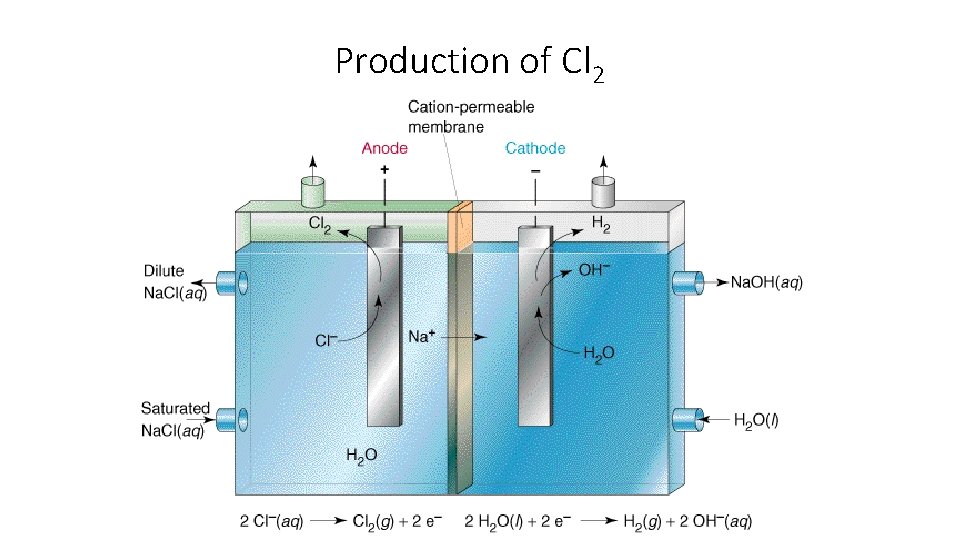
Production of Cl 2

Stoichiometry of Electrolysis • In an electrolytic cell, the amount of product made is related to the number of electrons transferred. • Essentially, the electrons are a reactant. • The number of moles of electrons that flow through the electrolytic cell depends on the current and length of time. • 1 amp = 1 coulomb of charge/second • 1 mole of e− = 96, 485 coulombs of charge • Faraday’s constant

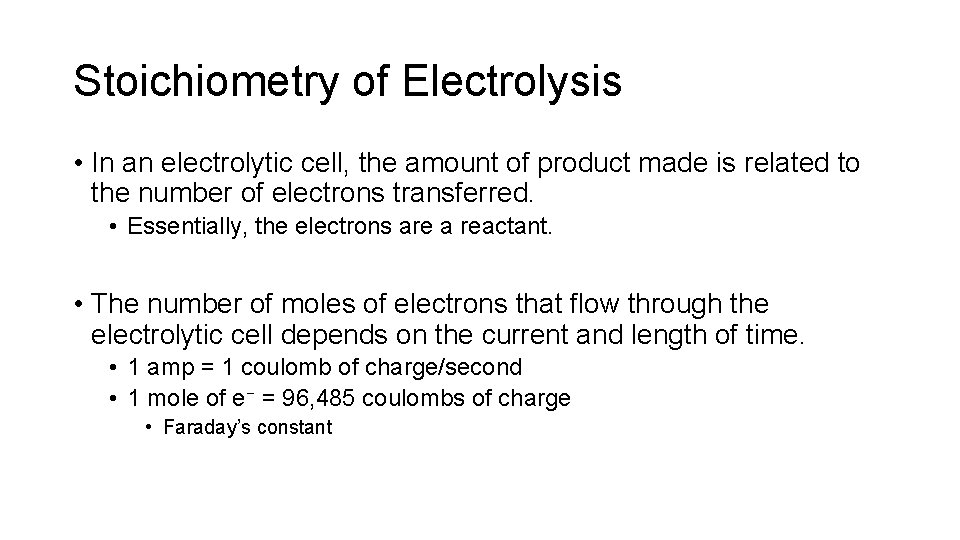
Production of Aluminum How many kg of Al can be produced in 8. 00 hours by passing a constant current of 1. 00× 105 A through a molten mix of aluminum oxide and cryolite?

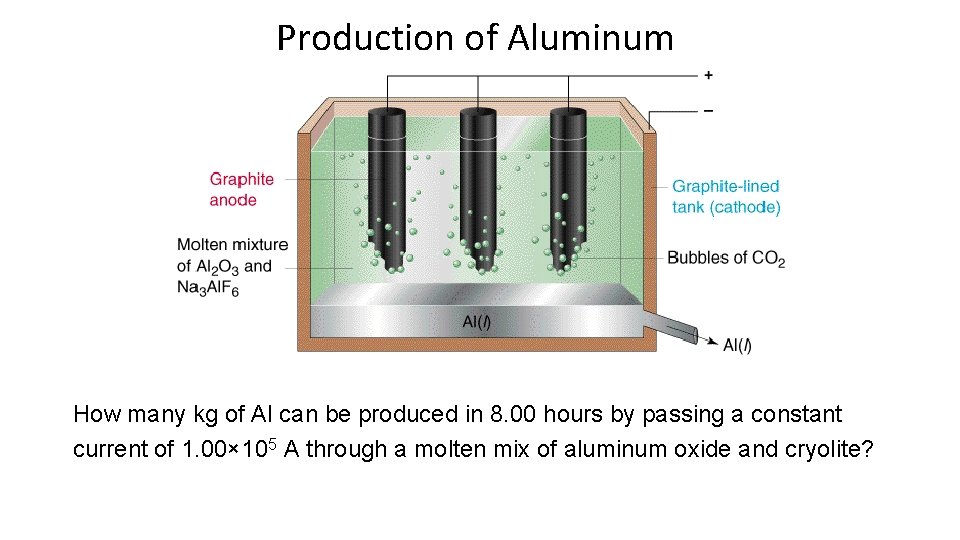
Electroplating How much time is required to deposit 5. 00 g of Ag on a tea pot using 0. 100 A? How much copper can plate out when 10. 0 amps (1 A = 1 C/s) of current is used over a 30. 0 minute period through a solution of 1. 00 M cupric ions (cupric sulfate solution) with a power source greater than 1. 10 V? Cu (s) + Zn 2+ (aq) Zn (s) + Cu 2+ (aq) Tro, Chemistry: A Molecular Approach 13

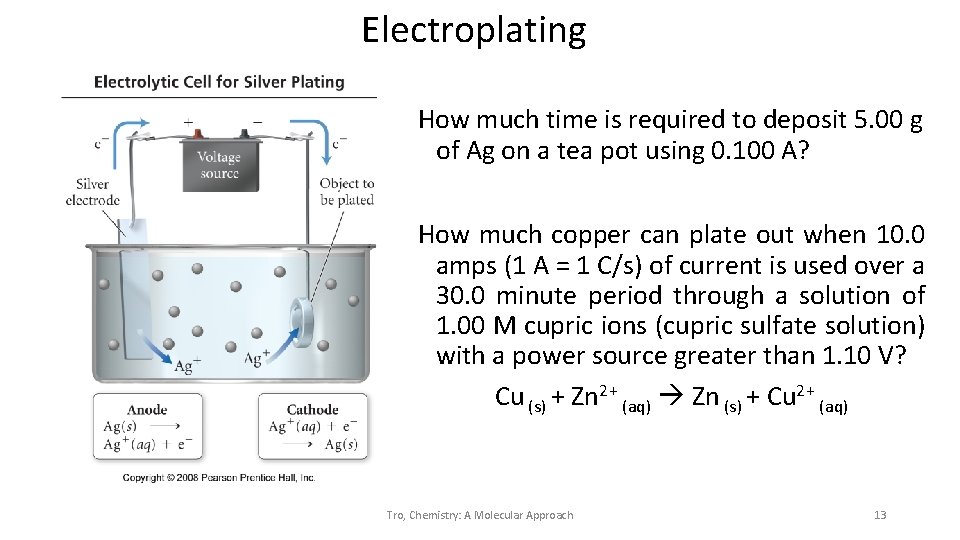
Electrorefining

Challenge Question To electrodeposit all of the Cu and Cd from a solution of Cu. SO 4 and Cd. SO 4 required 1. 20 F of electricity (1 F = 1 mol e-). The mixture of Cu and Cd that was deposited had a mass of 50. 36 g. What mass of Cd. SO 4 was present in the original mixture?