Introduction Molten electrolyte Aqueous electrolyte Summary We have













- Slides: 48


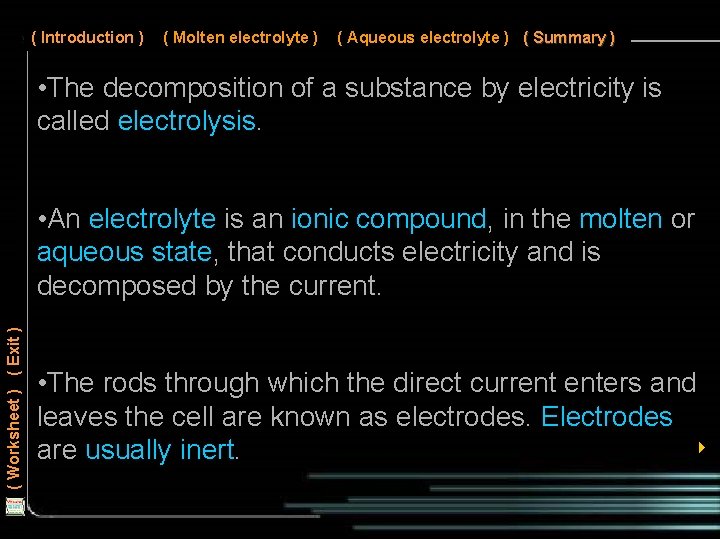
( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) We have learnt that ionic compounds conduct electricity when molten or aqueous. When an electric current passes through such compounds, the compounds are decomposed in a chemical reaction. This is known as electrolysis. ( Worksheet ) ( Exit ) The ionic compound is called an electrolyte. In this lesson, we will learn about the electrolysis of • Molten ionic compounds • Aqueous ionic compounds 4

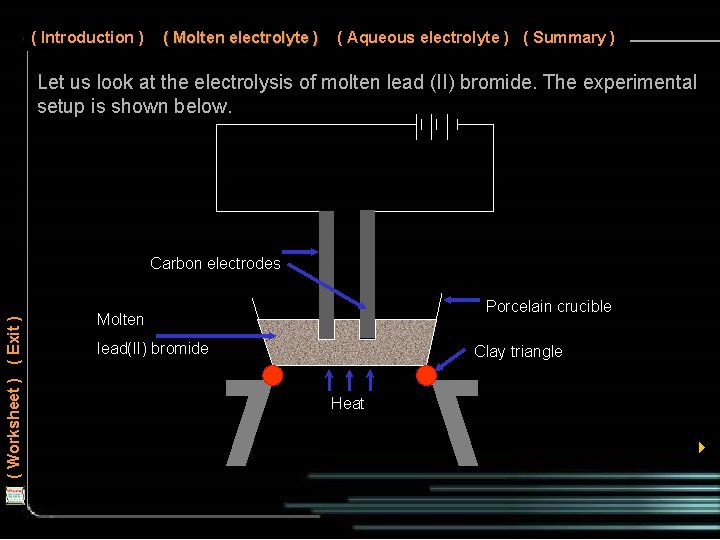
( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) Let us look at the electrolysis of molten lead (II) bromide. The experimental setup is shown below. ( Worksheet ) ( Exit ) Carbon electrodes Porcelain crucible Molten lead(II) bromide Clay triangle Heat 4

( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) ELECTRODES ( Worksheet ) ( Exit ) Anode Cathode • The electrode attached to the positive terminal of the cell. • The electrode attached to the negative terminal of the cell. • Anions are attracted to it. • Cations are attracted to it. Electrodes are usually made of carbon or platinum, as they are unreactive or inert. They do not react with the compounds in electrolysis. 4

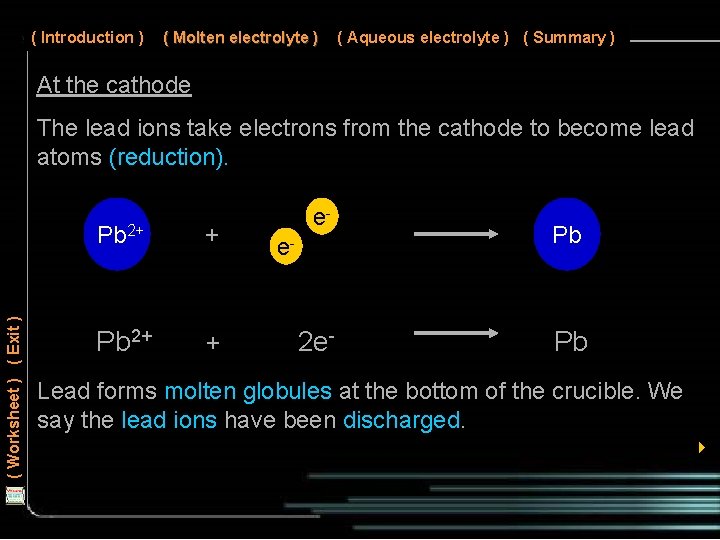
( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) At the cathode ( Worksheet ) ( Exit ) The lead ions take electrons from the cathode to become lead atoms (reduction). Pb 2+ + ee- 2 e- Pb Pb Lead forms molten globules at the bottom of the crucible. We say the lead ions have been discharged. 4

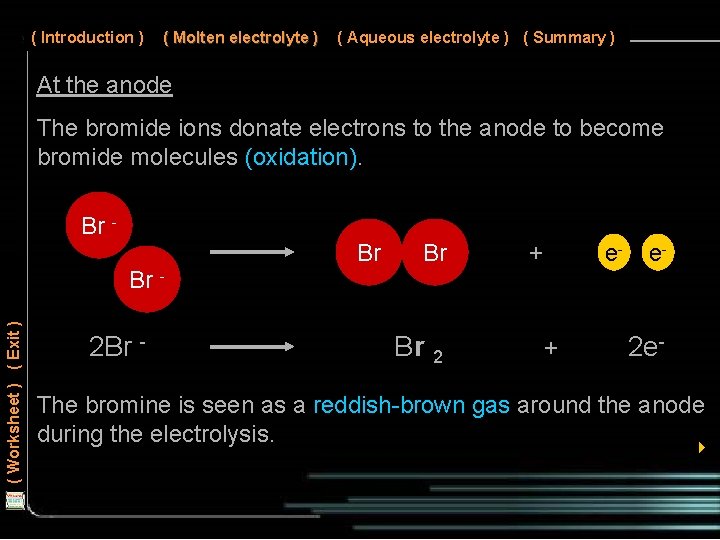
( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) At the anode The bromide ions donate electrons to the anode to become bromide molecules (oxidation). Br Br Br + e- e- ( Worksheet ) ( Exit ) Br - 2 Br - Br 2 + 2 e- The bromine is seen as a reddish-brown gas around the anode during the electrolysis. 4

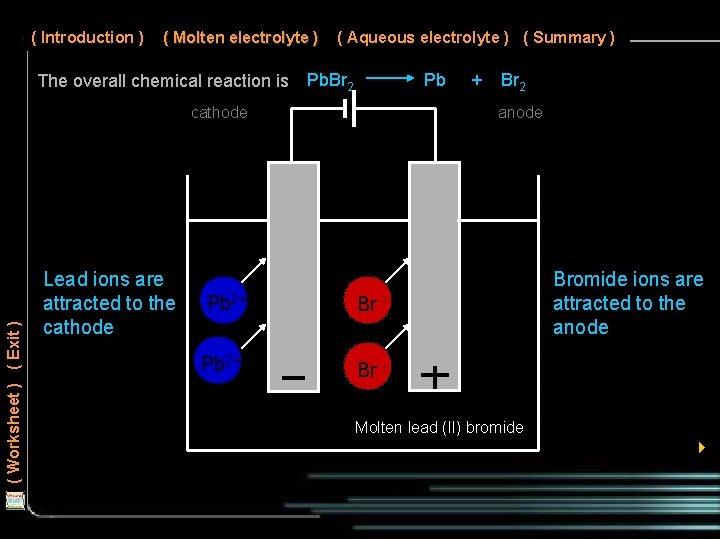
( Introduction ) ( Molten electrolyte ) The overall chemical reaction is ( Aqueous electrolyte ) ( Summary ) Pb. Br 2 Pb ( Worksheet ) ( Exit ) cathode Lead ions are attracted to the cathode Pb 2+ + Br 2 anode Br - Bromide ions are attracted to the anode Br Molten lead (II) bromide 4

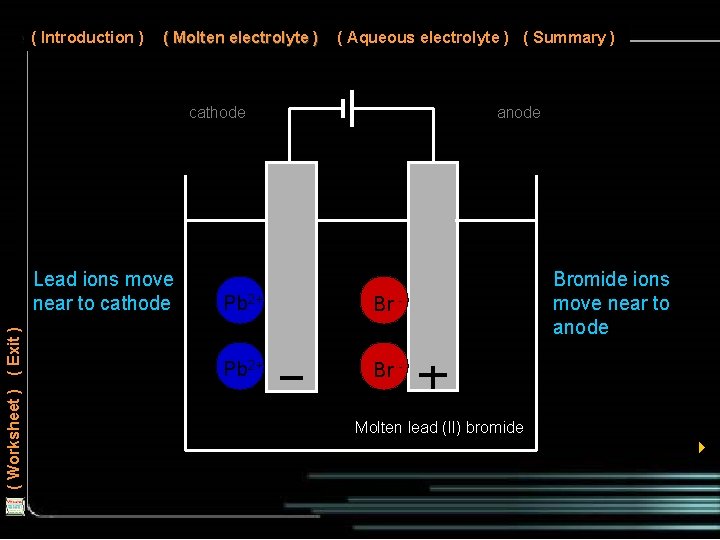
( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) cathode ( Worksheet ) ( Exit ) Lead ions move near to cathode anode Pb 2+ Br - Bromide ions move near to anode Molten lead (II) bromide 4

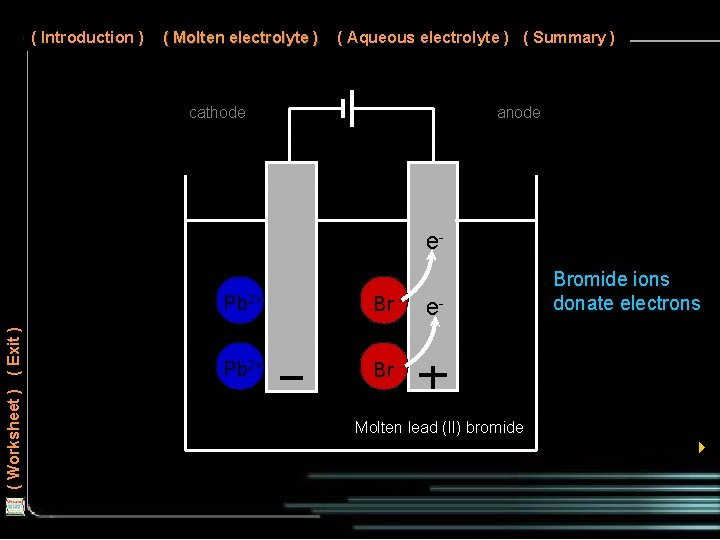
( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) cathode anode ( Worksheet ) ( Exit ) e. Pb 2+ Br - e- Bromide ions donate electrons Molten lead (II) bromide 4

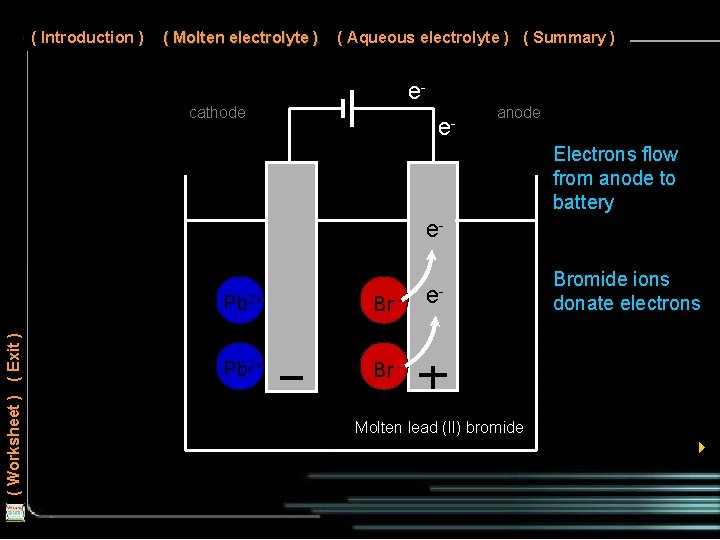
( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) e- cathode e- anode Electrons flow from anode to battery ( Worksheet ) ( Exit ) e. Pb 2+ Br - e- Bromide ions donate electrons Molten lead (II) bromide 4

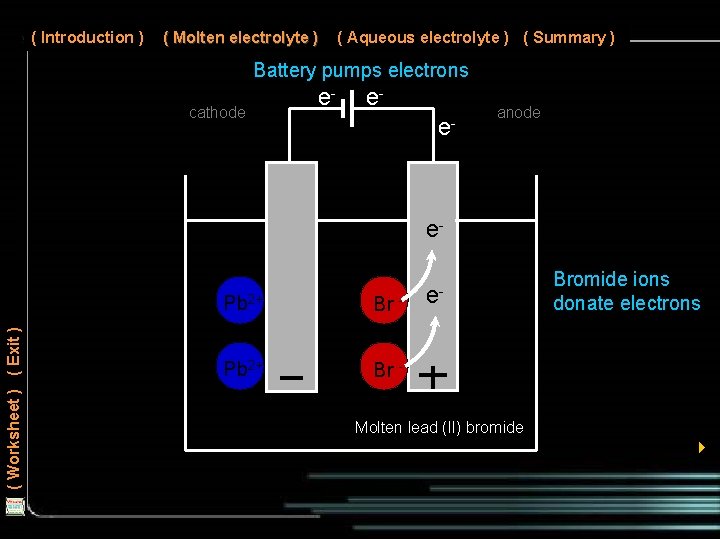
( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) Battery pumps electrons cathode e- ee- anode ( Worksheet ) ( Exit ) e. Pb 2+ Br - e- Bromide ions donate electrons Molten lead (II) bromide 4

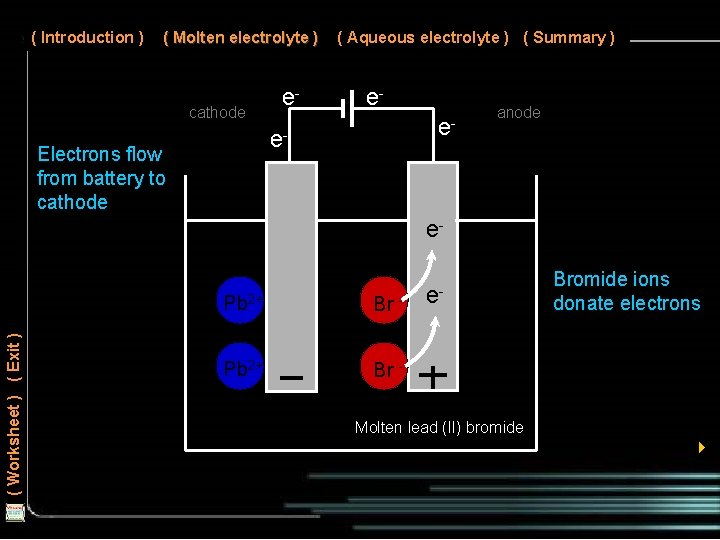
( Introduction ) ( Molten electrolyte ) cathode e- ( Aqueous electrolyte ) ( Summary ) ee- e- Electrons flow from battery to cathode anode ( Worksheet ) ( Exit ) e. Pb 2+ Br - e- Bromide ions donate electrons Molten lead (II) bromide 4

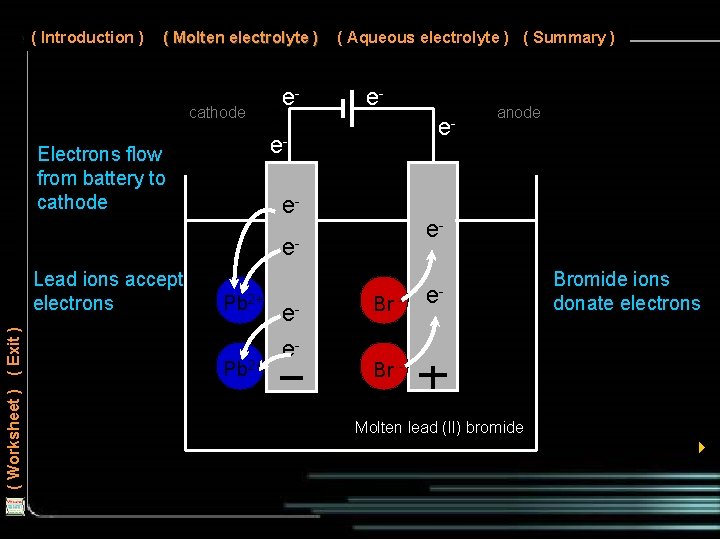
( Introduction ) ( Molten electrolyte ) cathode Electrons flow from battery to cathode e- ( Aqueous electrolyte ) ( Summary ) ee- e- ( Worksheet ) ( Exit ) Lead ions accept electrons Pb 2+ ee- anode Br - e- Bromide ions donate electrons Br Molten lead (II) bromide 4

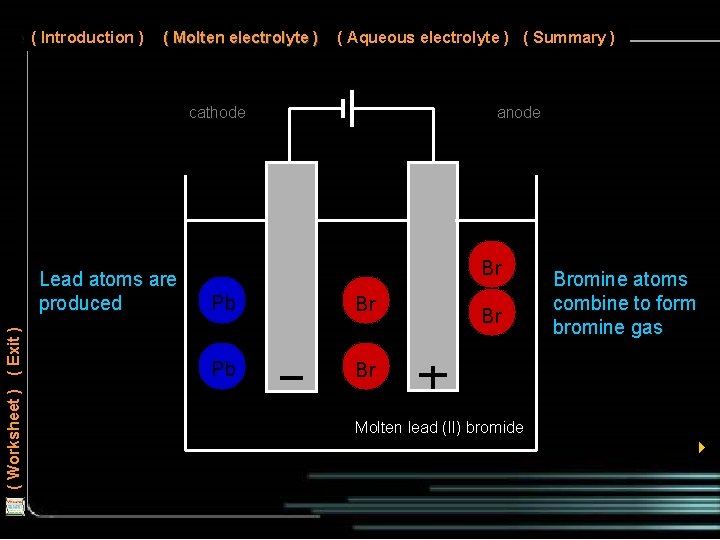
( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) cathode ( Worksheet ) ( Exit ) Lead atoms are produced anode Br Mg Pb Br Cl Mg Pb Cl Br Br Bromine atoms combine to form bromine gas Molten lead (II) bromide 4

( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) QUIZ 1 ( Worksheet ) ( Exit ) 1. The electrolysis of molten iron (III) chloride yields iron metal and a gas. What is the colour of this gas produced? A. Reddish-brown. B. Colourless. C. Yellowish-green. Click on the correct answer Next question 4

( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) QUIZ 1 ( Worksheet ) ( Exit ) 2. With reference to the previous question, at which electrode is the gas formed? A. Anode. B. Cathode. Click on the correct answer 4

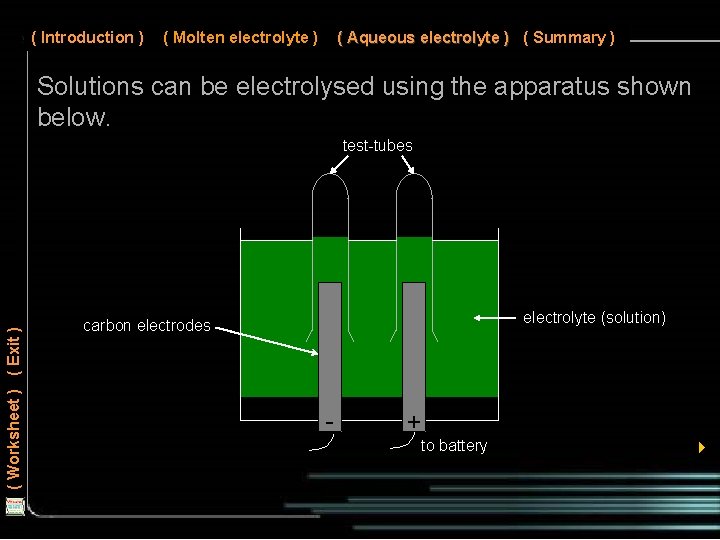
( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) Solutions can be electrolysed using the apparatus shown below. ( Worksheet ) ( Exit ) test-tubes electrolyte (solution) carbon electrodes - + to battery 4

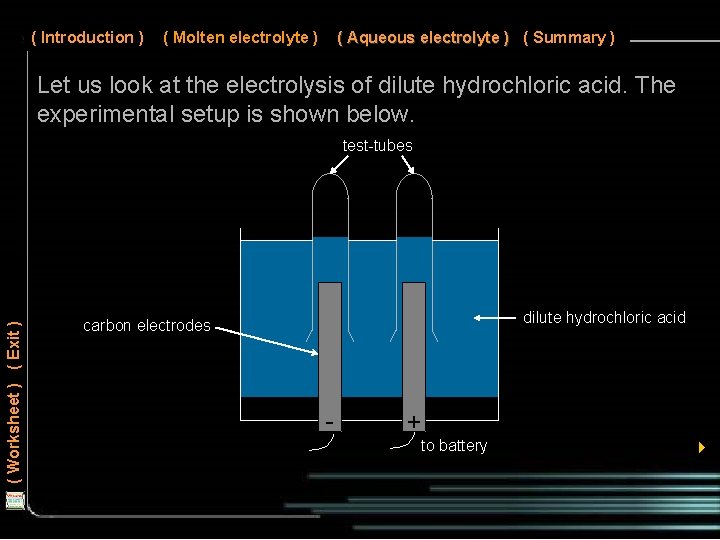
( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) Let us look at the electrolysis of dilute hydrochloric acid. The experimental setup is shown below. ( Worksheet ) ( Exit ) test-tubes dilute hydrochloric acid carbon electrodes - + to battery 4

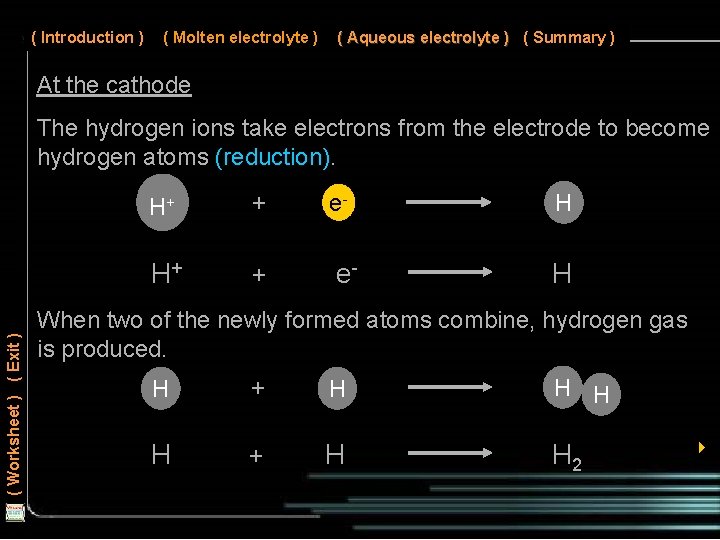
( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) At the cathode ( Worksheet ) ( Exit ) The hydrogen ions take electrons from the electrode to become hydrogen atoms (reduction). H+ + e- H When two of the newly formed atoms combine, hydrogen gas is produced. H + H H 2 4

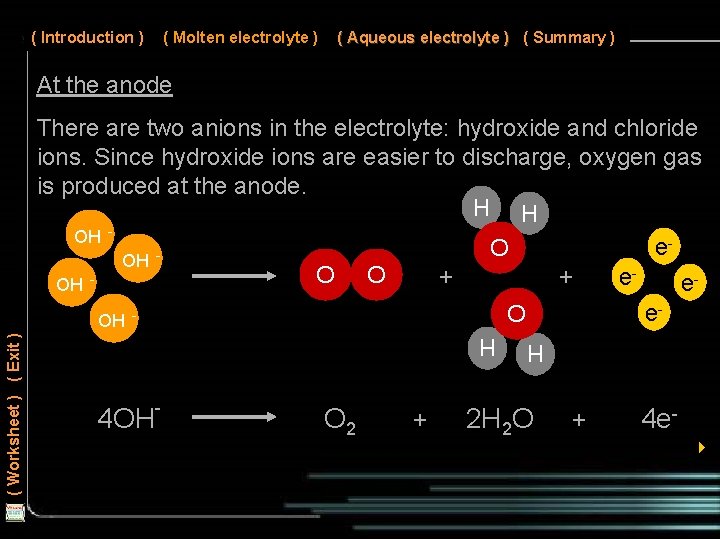
( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) At the anode There are two anions in the electrolyte: hydroxide and chloride ions. Since hydroxide ions are easier to discharge, oxygen gas is produced at the anode. H H OH e O OH O O + + e. OH ee. OH H O 2 + H ( Worksheet ) ( Exit ) O 4 OH- 2 H 2 O + 4 e 4

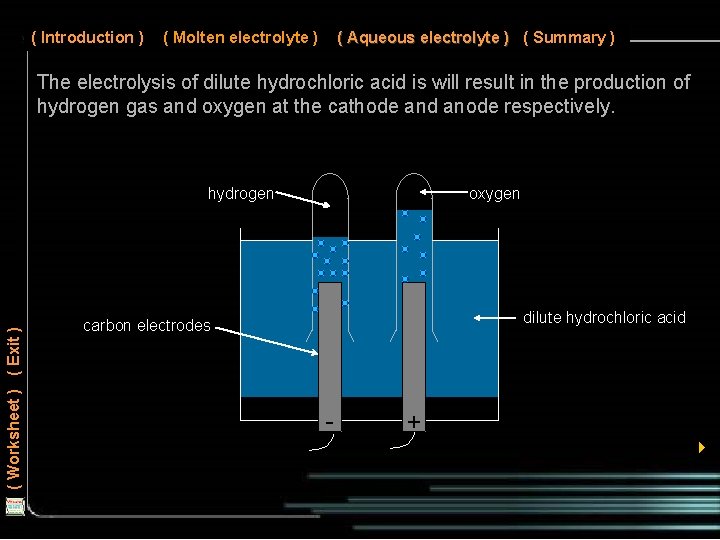
( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) The electrolysis of dilute hydrochloric acid is will result in the production of hydrogen gas and oxygen at the cathode and anode respectively. ( Worksheet ) ( Exit ) hydrogen oxygen dilute hydrochloric acid carbon electrodes - + 4

( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) What gas do you think will be found at the anode when aqueous copper(II) sulphate is electrolysed? Sulphur dioxide? No, not quite. Oxygen gas is evolved at the anode instead and copper metal is deposited at the cathode. ( Worksheet ) ( Exit ) How do you explain this phenomenon? The products can come from the electrolyte or from the water present. The product that is discharged depends on the nature of the ions. 4

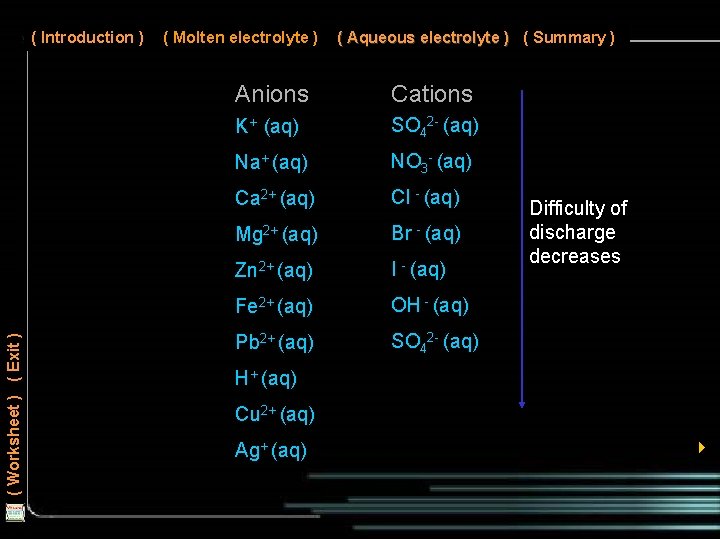
( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) Negative ions from the electrolyte are discharged if they are chloride, bromide or iodide ions. For sulphates and nitrates, oxygen from water is discharged. ( Worksheet ) ( Exit ) Positive ions from the electrolyte that are below Ni 2+(aq) in the electrochemical (or reactivity) series are discharged at the negative cathode. If the positive ions are those of reactive metals above Ni 2+(aq) (e. g. . Na+, K+ and Ca 2+), hydrogen gas from water is discharged. 4

( Worksheet ) ( Exit ) ( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) Anions Cations K+ (aq) SO 42 - (aq) Na+ (aq) NO 3 - (aq) Ca 2+ (aq) Cl - (aq) Mg 2+ (aq) Br - (aq) Zn 2+ (aq) I - (aq) Fe 2+ (aq) OH - (aq) Pb 2+ (aq) SO 42 - (aq) Difficulty of discharge decreases H+ (aq) Cu 2+ (aq) Ag+ (aq) 4

( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) QUIZ 2 ( Worksheet ) ( Exit ) 1. When we electrolyse a solution, what takes place at the cathode? A. Oxidation. B. Reduction. Click on the correct answer Next question 4

( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) QUIZ 2 ( Worksheet ) ( Exit ) 2. During the electrolysis of sulphuric acid, hydrogen gas and another gas are produced. What is the other gas? A. Oxygen. B. Sulphur dioxide. Click on the correct answer 4

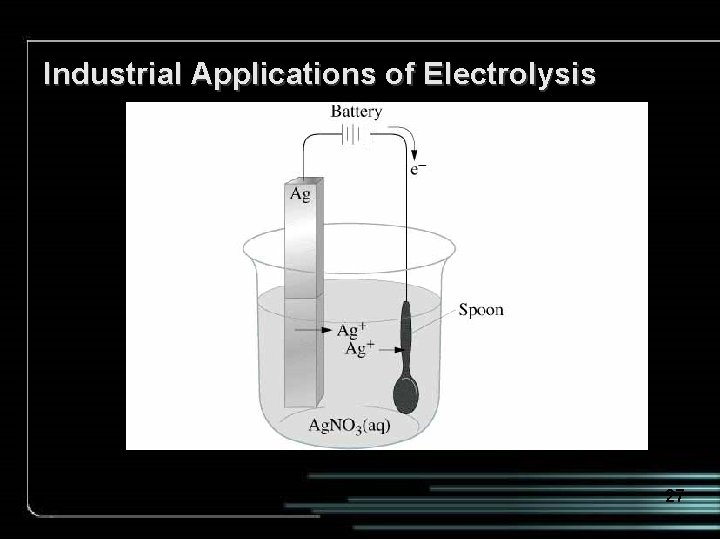
Industrial Applications of Electrolysis 27

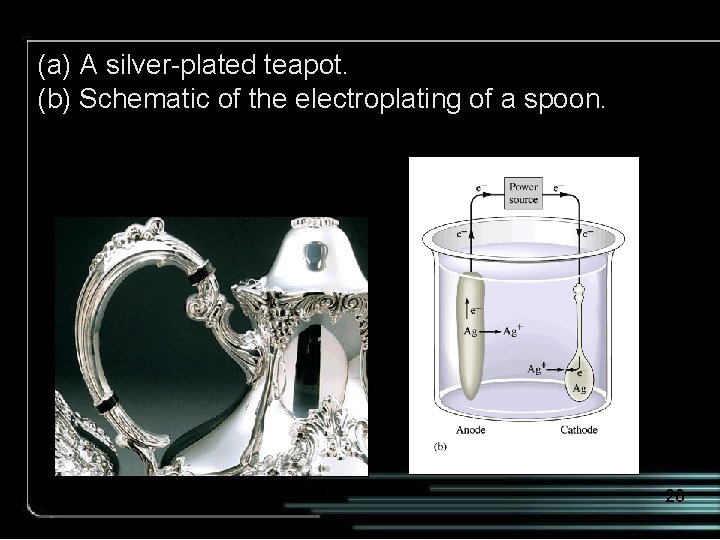
(a) A silver-plated teapot. (b) Schematic of the electroplating of a spoon. 28

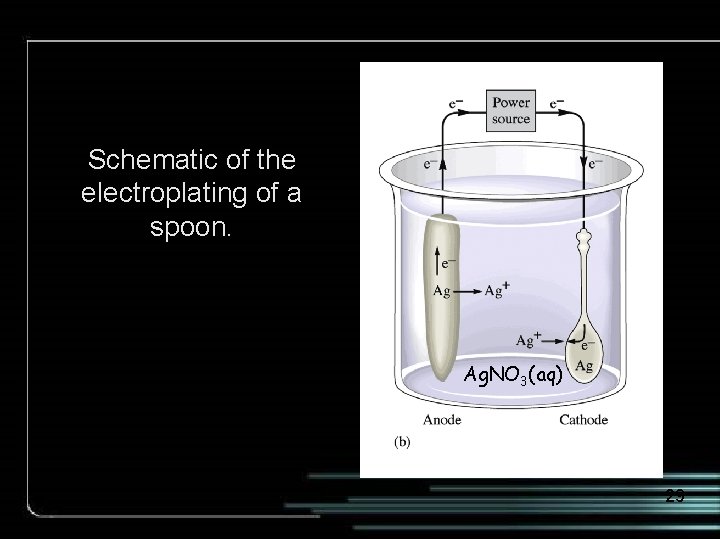
Schematic of the electroplating of a spoon. Ag. NO 3(aq) 29

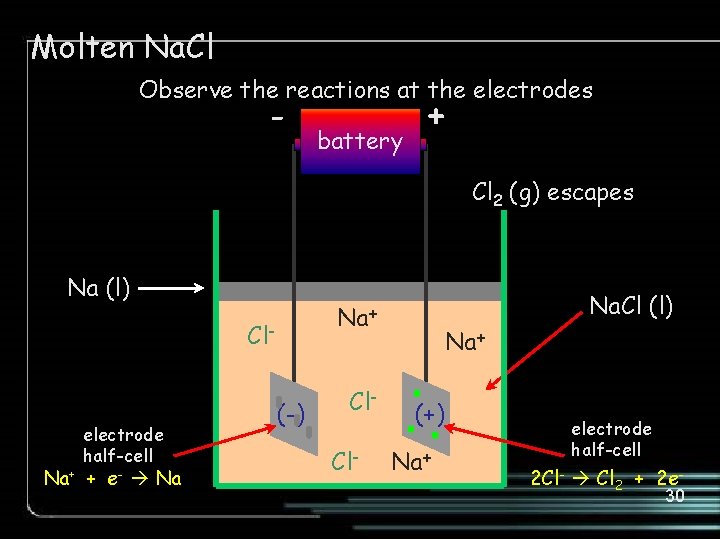
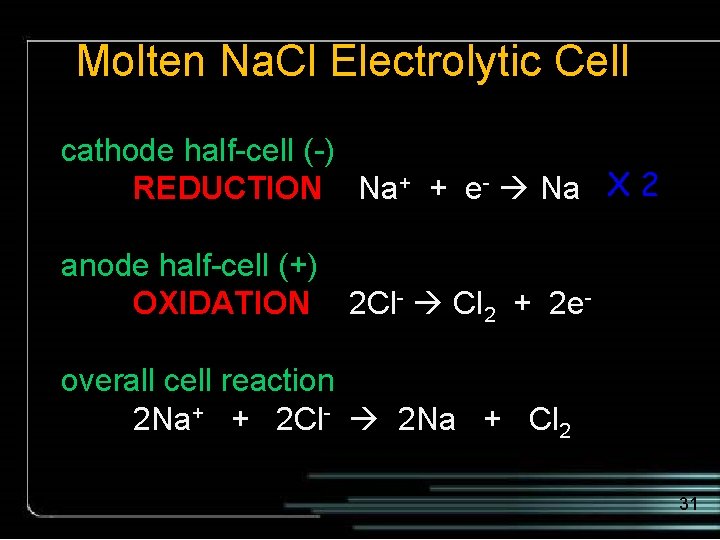
Molten Na. Cl Observe the reactions at the electrodes - battery + Cl 2 (g) escapes Na (l) Cl- electrode half-cell Na+ + e- Na Na. Cl (l) Na+ (-) Cl. Cl- Na+ (+) Na+ electrode half-cell 2 Cl- Cl 2 + 2 e 30

Molten Na. Cl Electrolytic Cell cathode half-cell (-) REDUCTION Na+ + e- Na X 2 anode half-cell (+) OXIDATION 2 Cl- Cl 2 + 2 eoverall cell reaction 2 Na+ + 2 Cl- 2 Na + Cl 2 31

32

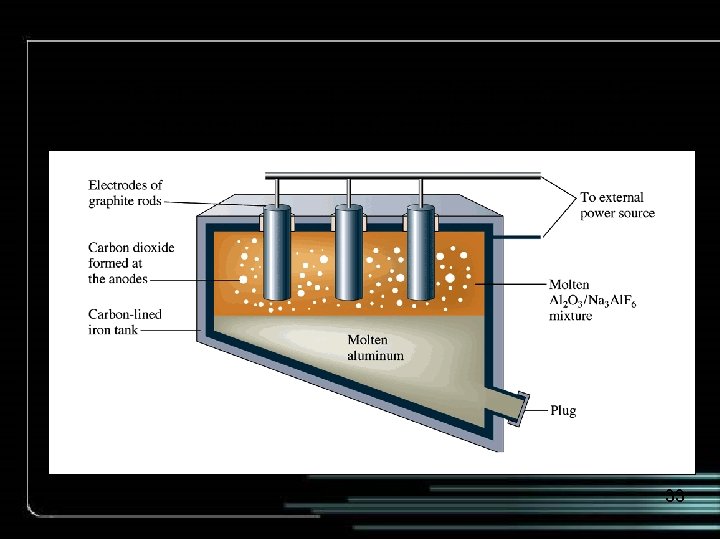
A schematic diagram of an electrolytic cell for producing aluminum by the Hall-Heroult process. 33

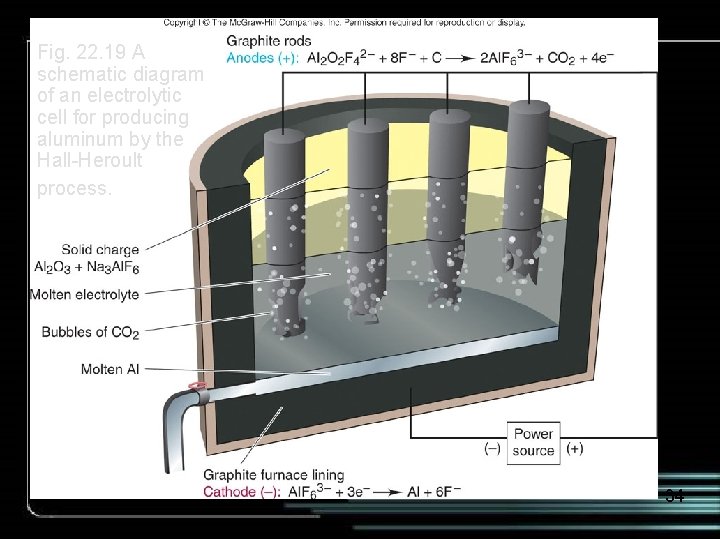
Fig. 22. 19 A schematic diagram of an electrolytic cell for producing aluminum by the Hall-Heroult process. 34

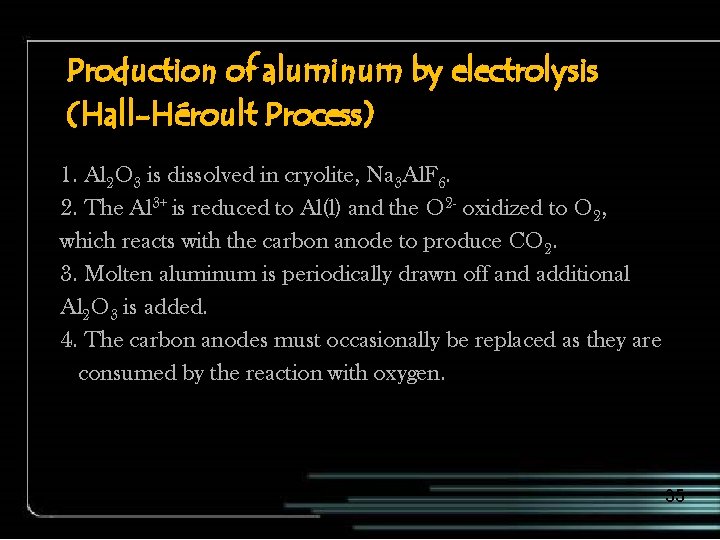
Production of aluminum by electrolysis (Hall-Héroult Process) 1. Al 2 O 3 is dissolved in cryolite, Na 3 Al. F 6. 2. The Al 3+ is reduced to Al(l) and the O 2 - oxidized to O 2, which reacts with the carbon anode to produce CO 2. 3. Molten aluminum is periodically drawn off and additional Al 2 O 3 is added. 4. The carbon anodes must occasionally be replaced as they are consumed by the reaction with oxygen. 35

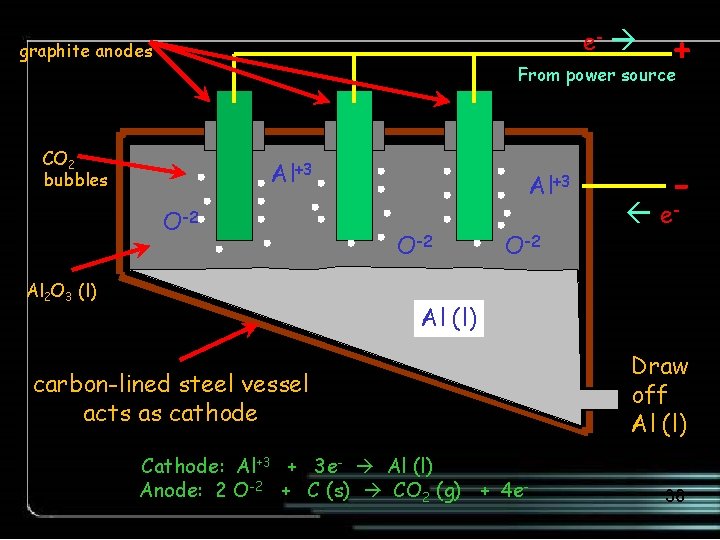
e- graphite anodes + From power source CO 2 bubbles Al+3 O-2 Al 2 O 3 (l) Al+3 O-2 - e- Al (l) carbon-lined steel vessel acts as cathode Cathode: Al+3 + 3 e- Al (l) Anode: 2 O-2 + C (s) CO 2 (g) + 4 e- Draw off Al (l) 36

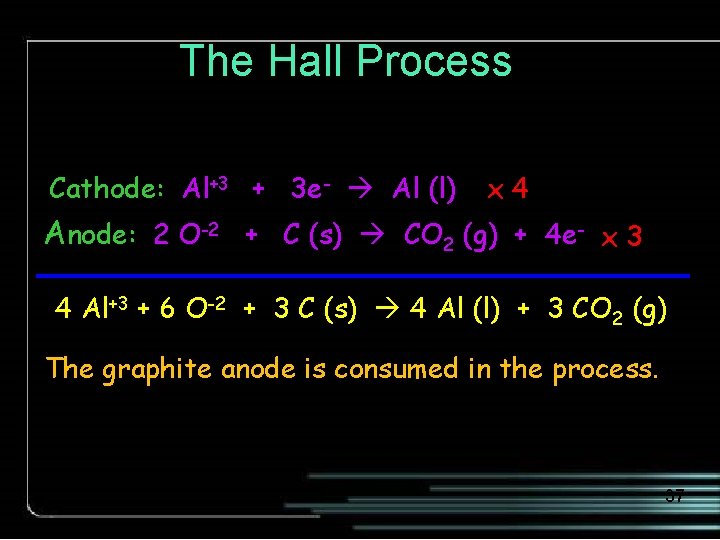
The Hall Process Cathode: Al+3 + 3 e- Al (l) x 4 Anode: 2 O-2 + C (s) CO 2 (g) + 4 e- x 3 4 Al+3 + 6 O-2 + 3 C (s) 4 Al (l) + 3 CO 2 (g) The graphite anode is consumed in the process. 37

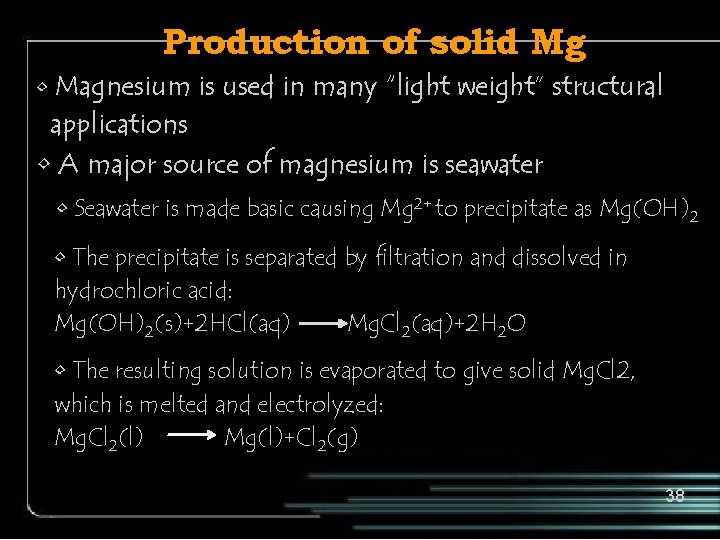
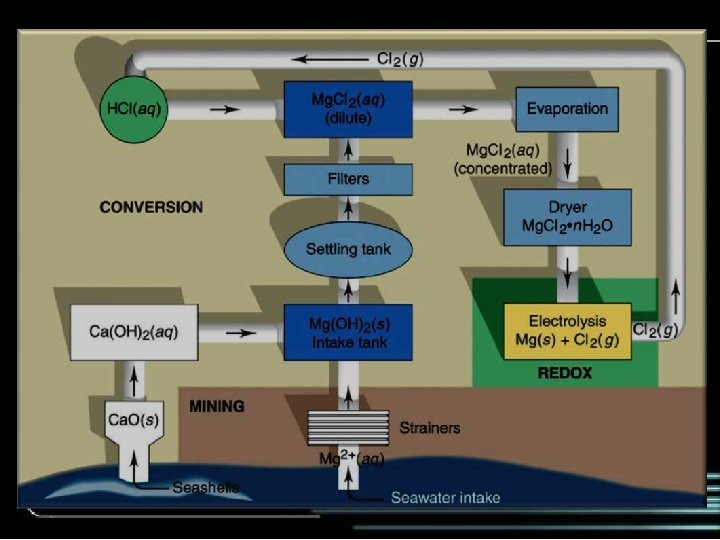
Production of solid Mg • Magnesium is used in many “light weight” structural applications • A major source of magnesium is seawater • Seawater is made basic causing Mg 2+ to precipitate as Mg(OH)2 • The precipitate is separated by filtration and dissolved in hydrochloric acid: Mg(OH)2(s)+2 HCl(aq) Mg. Cl 2(aq)+2 H 2 O • The resulting solution is evaporated to give solid Mg. Cl 2, which is melted and electrolyzed: Mg. Cl 2(l) Mg(l)+Cl 2(g) 38

39

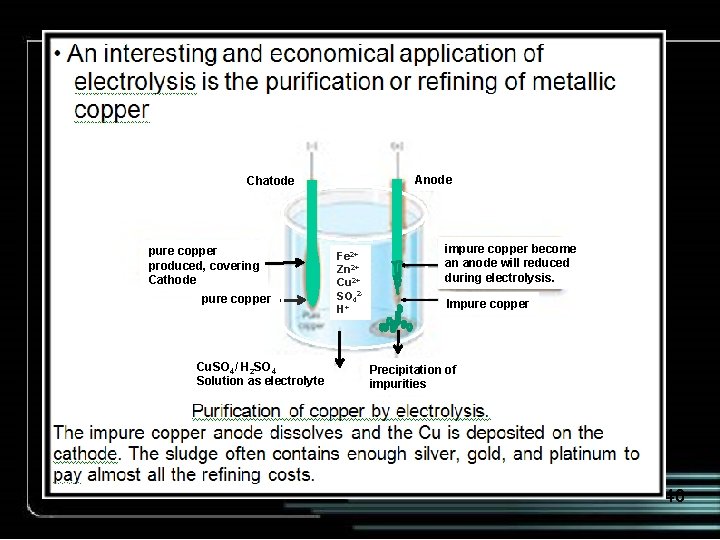
Anode Chatode pure copper produced, covering Cathode pure copper Cu. SO 4/ H 2 SO 4 Solution as electrolyte Fe 2+ Zn 2+ Cu 2+ SO 42 H+ impure copper become an anode will reduced during electrolysis. Impure copper Precipitation of impurities 40

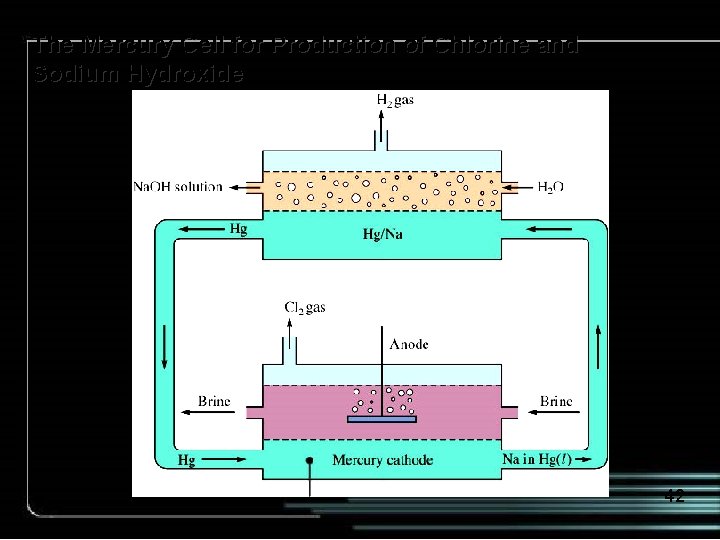
Electrolysis of brine using a mercury cell. • At the anode, chloride ions are oxidized to chlorine. • At the cathode, sodium ions are reduced to sodium atoms, which dissolve in the mercury. • The mercury is pumped into a separate compartment and exposed to water. • The dissolved sodium reacts with the water to form H 2 and Na. OH. 41

The Mercury Cell for Production of Chlorine and Sodium Hydroxide 42

( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) • The decomposition of a substance by electricity is called electrolysis. ( Worksheet ) ( Exit ) • An electrolyte is an ionic compound, in the molten or aqueous state, that conducts electricity and is decomposed by the current. • The rods through which the direct current enters and leaves the cell are known as electrodes. Electrodes 4 are usually inert.

( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) ( Worksheet ) ( Exit ) • The anode is the electrode which is connected to the positive terminal of a cell. Anions are attracted to it. Oxidation occurs at the anode. • The cathode is the electrode connected to the negative terminal of the cell. Cations are attracted to the cathode. Reduction occurs at this electrode. 4

( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) When a molten electrolyte is electrolysed, • a metal (from the positive ions) is discharged at the cathode. ( Worksheet ) ( Exit ) • a non-metal (from the negative ions) is discharged at the anode. 4

( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) When a aqueous electrolyte is electrolysed, • the products come from either the electrolyte or water present. ( Worksheet ) ( Exit ) • The product at the cathode is a metal or hydrogen gas. • The product at the anode is a non-metal. 4

( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) • Reactive metals are not discharged at the cathode. Instead, hydrogen from water is evolved. ( Worksheet ) ( Exit ) • Sulphate and nitrate ions are not discharged at the anode. Instead, oxygen from water is produced. 4

( Introduction ) ( Molten electrolyte ) ( Aqueous electrolyte ) ( Summary ) return to 4 ( Worksheet ) ( Exit ) Credits micro lessons