Electrochemistry Part IV Spontaneity Nernst Equation Electrolysis Jespersen






- Slides: 17

Electrochemistry Part IV: Spontaneity, Nernst Equation & Electrolysis Jespersen Chap. 20 Sec 4 to 7 Skipping Sec 6 & 8 Dr. C. Yau Spring 2013 1

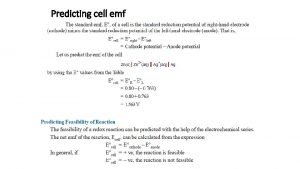
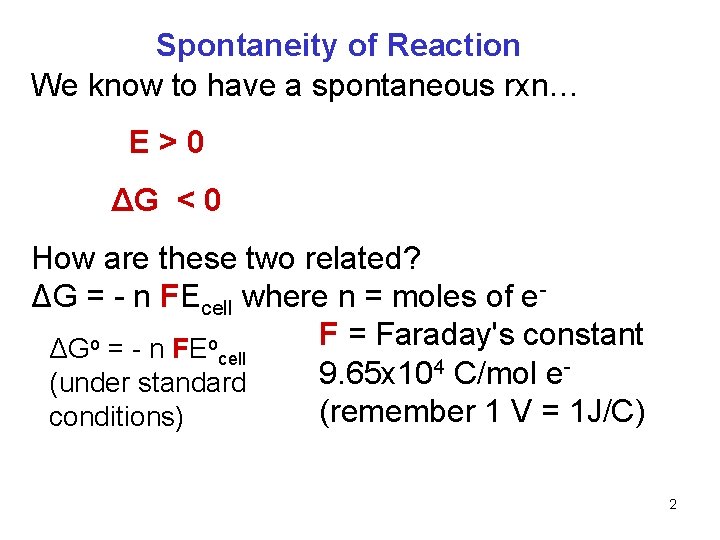
Spontaneity of Reaction We know to have a spontaneous rxn… E>0 ΔG < 0 How are these two related? ΔG = - n FEcell where n = moles of e. F = Faraday's constant ΔGo = - n FEocell 4 C/mol e 9. 65 x 10 (under standard (remember 1 V = 1 J/C) conditions) 2

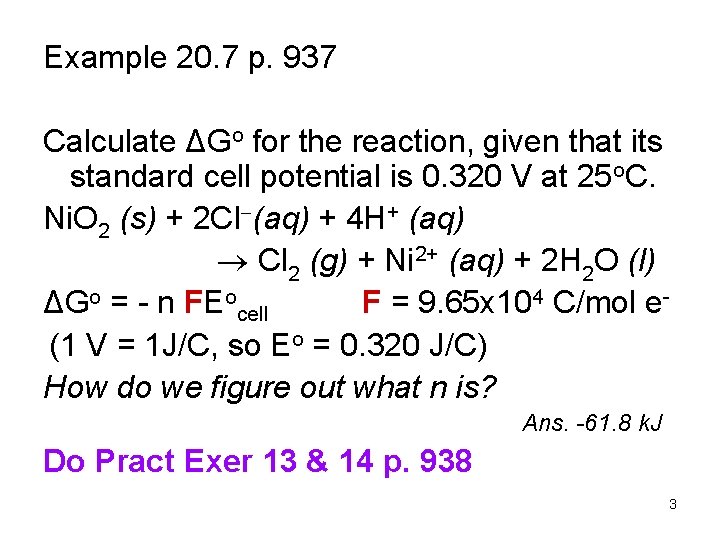
Example 20. 7 p. 937 Calculate ΔGo for the reaction, given that its standard cell potential is 0. 320 V at 25 o. C. Ni. O 2 (s) + 2 Cl (aq) + 4 H+ (aq) Cl 2 (g) + Ni 2+ (aq) + 2 H 2 O (l) ΔGo = - n FEocell F = 9. 65 x 104 C/mol e(1 V = 1 J/C, so Eo = 0. 320 J/C) How do we figure out what n is? Ans. -61. 8 k. J Do Pract Exer 13 & 14 p. 938 3

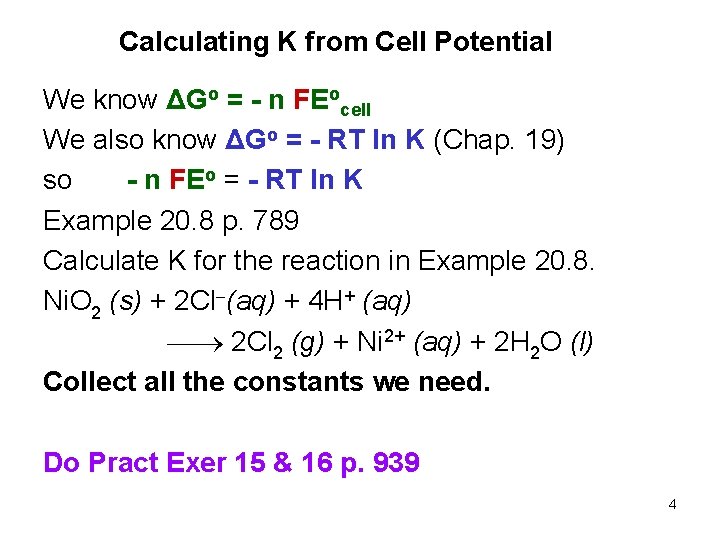
Calculating K from Cell Potential We know ΔGo = - n FEocell We also know ΔGo = - RT ln K (Chap. 19) so - n FEo = - RT ln K Example 20. 8 p. 789 Calculate K for the reaction in Example 20. 8. Ni. O 2 (s) + 2 Cl (aq) + 4 H+ (aq) 2 Cl 2 (g) + Ni 2+ (aq) + 2 H 2 O (l) Collect all the constants we need. Do Pract Exer 15 & 16 p. 939 4

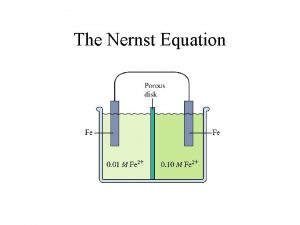
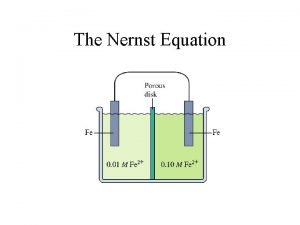
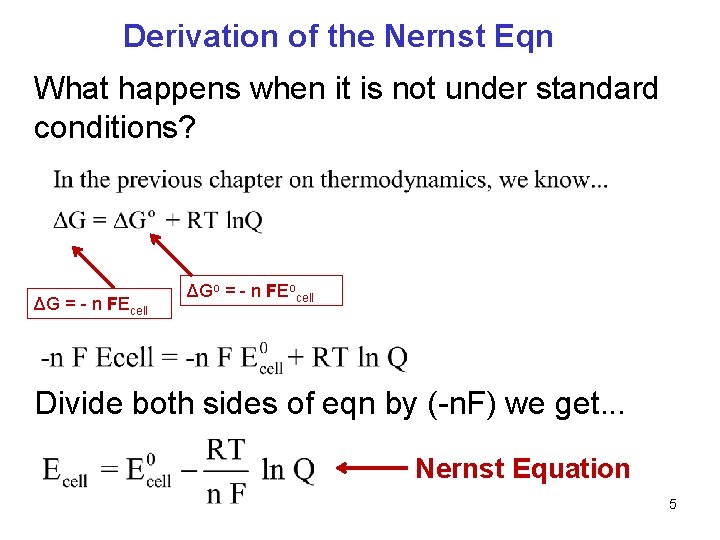
Derivation of the Nernst Eqn What happens when it is not under standard conditions? ΔG = - n FEcell ΔGo = - n FEocell Divide both sides of eqn by (-n. F) we get. . . Nernst Equation 5

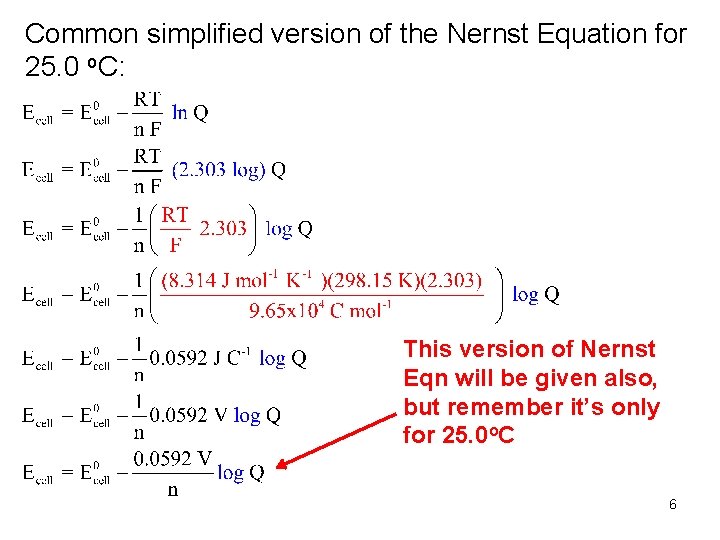
Common simplified version of the Nernst Equation for 25. 0 o. C: This version of Nernst Eqn will be given also, but remember it’s only for 25. 0 o. C 6

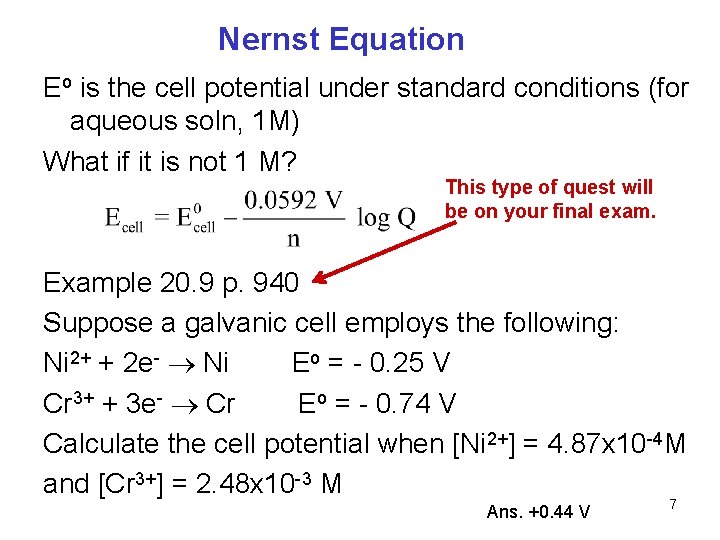
Nernst Equation Eo is the cell potential under standard conditions (for aqueous soln, 1 M) What if it is not 1 M? This type of quest will be on your final exam. Example 20. 9 p. 940 Suppose a galvanic cell employs the following: Ni 2+ + 2 e- Ni Eo = - 0. 25 V Cr 3+ + 3 e- Cr Eo = - 0. 74 V Calculate the cell potential when [Ni 2+] = 4. 87 x 10 -4 M and [Cr 3+] = 2. 48 x 10 -3 M Ans. +0. 44 V 7

Example 20. 10 p. 941 The rxn of tin metal with acid can be written as Sn (s) + 2 H+ (aq) Sn 2+ (aq) + H 2 (g) Calculate the cell potential (a) when the system is at standard state. Ans. +0. 02 V (b) when the p. H = 2. 00 Ans. -0. 16 V (c) when the p. H is 5. 00. Assume that [Sn 2+] = 1. 00 M and the partial pressure of H 2 is also 1. 00 atm. Do Pract Exer 17, 18, 20 p. 942 8

What we are skipping in Chap. 20: pp. 943 -951 Concentration from E Measurements Sec 20. 6 Electricity Batteries: Lead Storage Batteries Zinc-Manganese Dioxide Cells (Le. Clanche cell) Nickel-Cadmium Rechargeable Batteries Nickel-Metal Hydride Batteries Lithium Ion Cells Fuel Cells Photovoltaic Cells 9

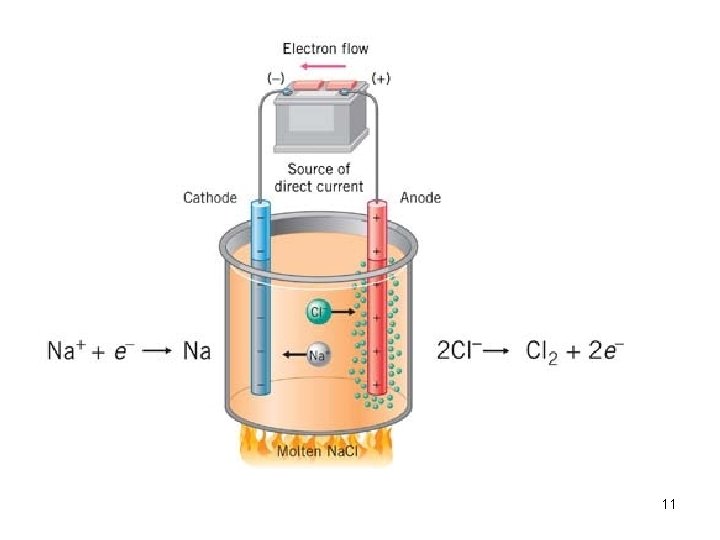
Electrolytic Cell (Sec. 20. 7) We had been considering 2 half-reactions, one of which is the "drive" for the forward reaction. In the electrolytic cell (or electrolysis cell), the driving force is the electric current. "Lysis" means to break apart. "Electrolysis" means to break with an electric current. We know 2 Na + Cl 2 2 Na. Cl is spontaneous. We can do the reverse reaction by applying an electric current. 2 Na. Cl 2 Na + Cl 2 What are the physical states? Usually the electrolytic cell has only one chamber. 10

11

Electrolysis in Aqueous Solutions Can we "break apart" potassium sulfate? It is not easy to melt an ionic compound. What if we did this in an aqueous solution? Water can be oxidized as well as reduced. Why? How? H 2 O H 2 reduction at the … H 2 O O 2 oxidation at the… Overall: 2 H 2 O (l) 2 H 2 (g) + O 2 (g) This cannot take place without an electrolyte present (acting as a salt bridge). Do Pract Exer 23, 24 p. 958 12

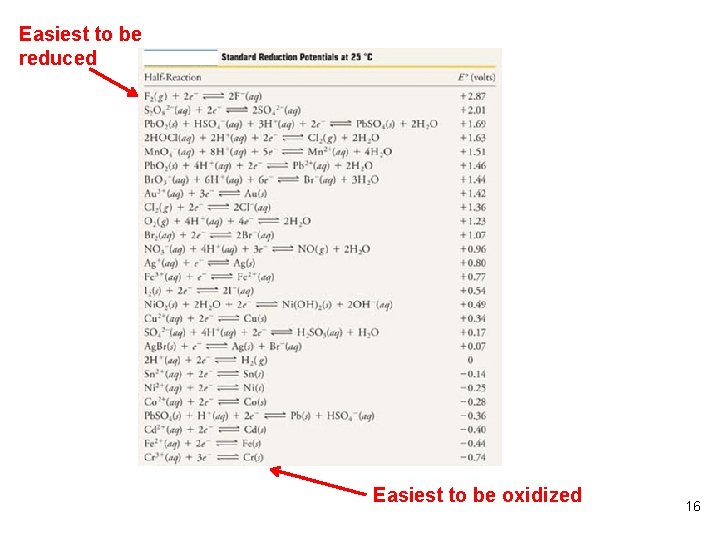
Example 20. 12 p. 957 Electrolysis is planned for an aq soln that contains a mixture of 0. 50 M Zn. SO 4 and 0. 50 M Ni. SO 4. On the basis of standard reduction potentials, what products are expected to be observed at the electrodes? What is the expected net cell reaction? First determine which species are present. Next determine which might be reduced, which might be oxidized. (Remember those to be reduced are on the left side of Table 19. 1, those to be oxidized are on the right side. Which is reduced the easiest on the table? Which is oxidized the easiest? ) 13

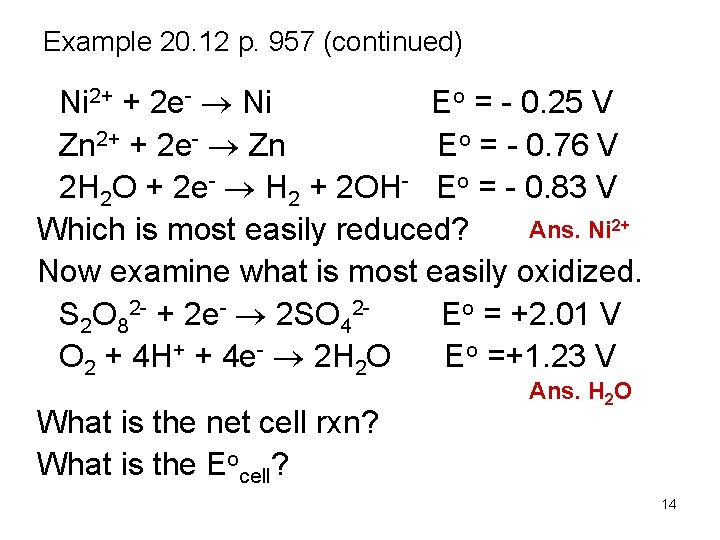
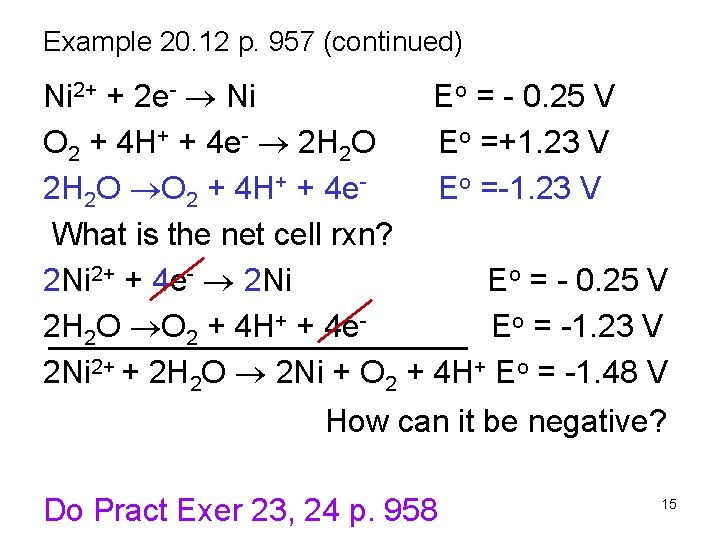
Example 20. 12 p. 957 (continued) Ni 2+ + 2 e- Ni Eo = - 0. 25 V Zn 2+ + 2 e- Zn Eo = - 0. 76 V 2 H 2 O + 2 e- H 2 + 2 OH- Eo = - 0. 83 V Ans. Ni 2+ Which is most easily reduced? Now examine what is most easily oxidized. S 2 O 82 - + 2 e- 2 SO 42 Eo = +2. 01 V O 2 + 4 H+ + 4 e- 2 H 2 O Eo =+1. 23 V What is the net cell rxn? What is the Eocell? Ans. H 2 O 14

Example 20. 12 p. 957 (continued) Ni 2+ + 2 e- Ni Eo = - 0. 25 V O 2 + 4 H+ + 4 e- 2 H 2 O Eo =+1. 23 V 2 H 2 O O 2 + 4 H+ + 4 e. Eo =-1. 23 V What is the net cell rxn? 2 Ni 2+ + 4 e- 2 Ni Eo = - 0. 25 V 2 H 2 O O 2 + 4 H+ + 4 e. Eo = -1. 23 V 2 Ni 2+ + 2 H 2 O 2 Ni + O 2 + 4 H+ Eo = -1. 48 V How can it be negative? Do Pract Exer 23, 24 p. 958 15

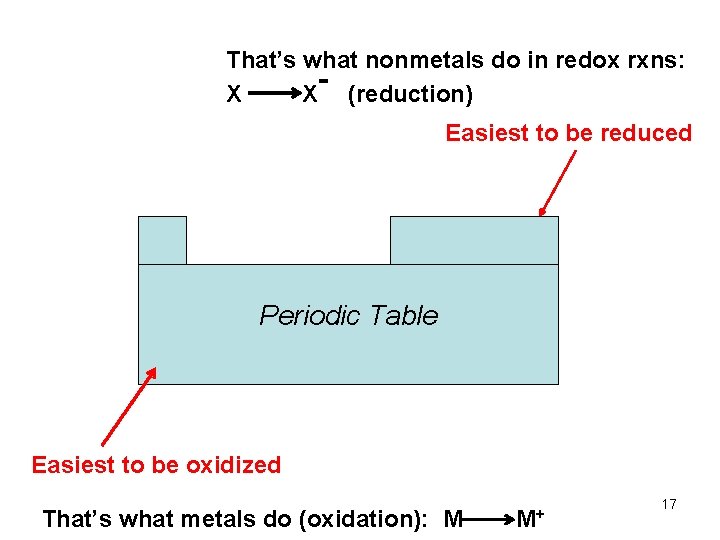
Easiest to be reduced Easiest to be oxidized 16

That’s what nonmetals do in redox rxns: X X (reduction) Easiest to be reduced Periodic Table Easiest to be oxidized That’s what metals do (oxidation): M M+ 17
 Nernst equation derivation
Nernst equation derivation Spontaneity of redox reaction
Spontaneity of redox reaction Pamela fishman gender theory
Pamela fishman gender theory Jespersen tryk
Jespersen tryk Integrated rate equation
Integrated rate equation Rate law for first order reaction
Rate law for first order reaction Laws jespersen
Laws jespersen Brugertyper
Brugertyper Nernst einstein equation
Nernst einstein equation Nernst equation calculator
Nernst equation calculator What is e in nernst equation
What is e in nernst equation Goldman equation
Goldman equation Redox reaction
Redox reaction Fintonal
Fintonal Nernst equation derivation
Nernst equation derivation Nernst equation simplified
Nernst equation simplified Nernst equation derivation
Nernst equation derivation Khan academy electrolysis
Khan academy electrolysis