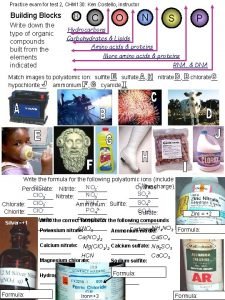
Chem I Semester 1 Review All of the







- Slides: 71

Chem I Semester 1 Review

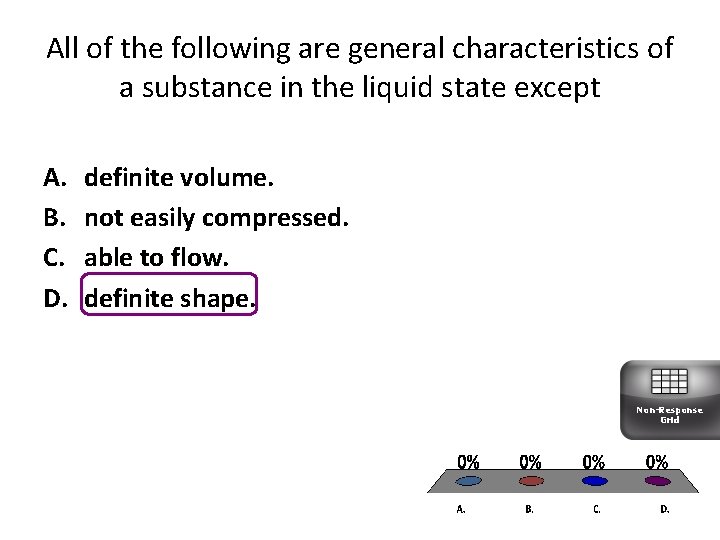
All of the following are general characteristics of a substance in the liquid state except A. B. C. D. definite volume. not easily compressed. able to flow. definite shape. Non-Response Grid

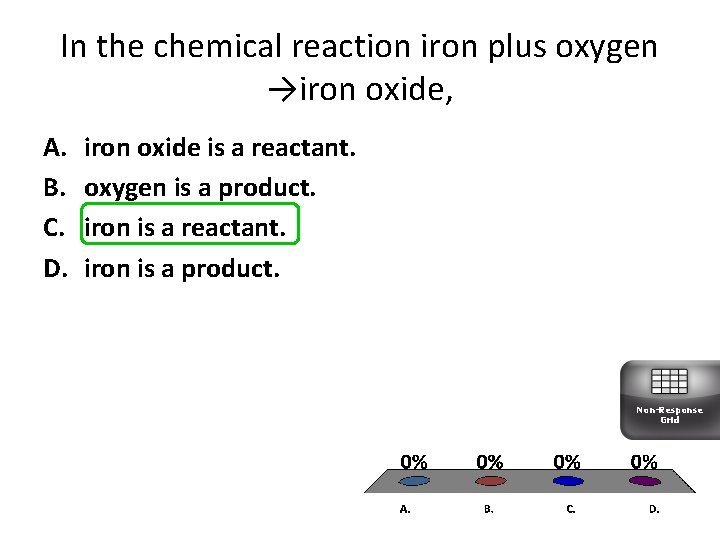
In the chemical reaction iron plus oxygen →iron oxide, A. B. C. D. iron oxide is a reactant. oxygen is a product. iron is a reactant. iron is a product. Non-Response Grid

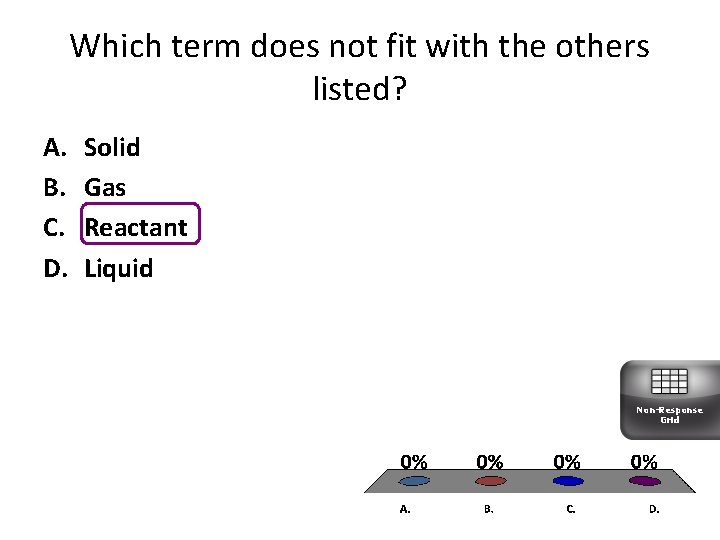
Which term does not fit with the others listed? A. B. C. D. Solid Gas Reactant Liquid Non-Response Grid

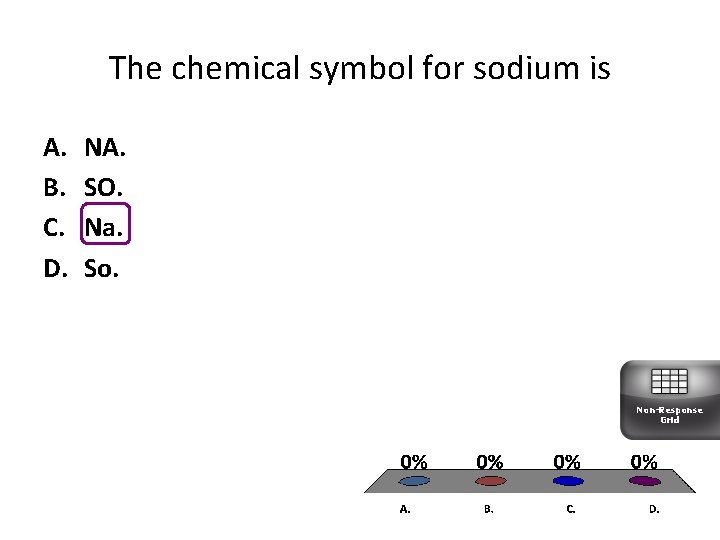
The chemical symbol for sodium is A. B. C. D. NA. SO. Na. So. Non-Response Grid

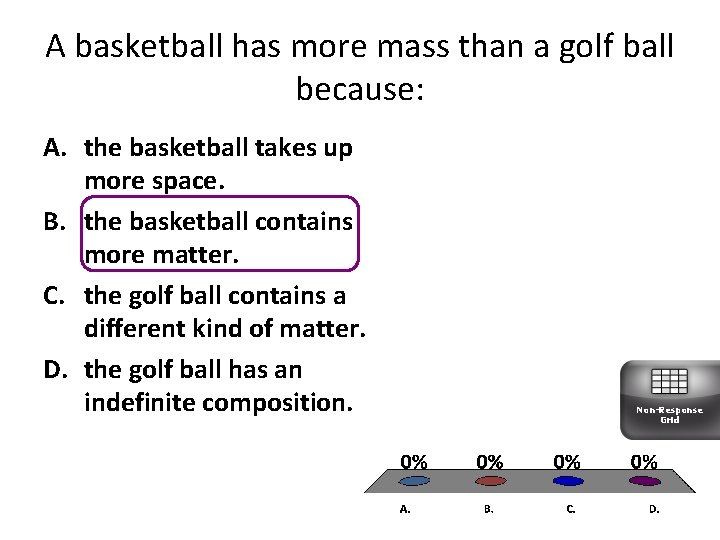
A basketball has more mass than a golf ball because: A. the basketball takes up more space. B. the basketball contains more matter. C. the golf ball contains a different kind of matter. D. the golf ball has an indefinite composition. Non-Response Grid

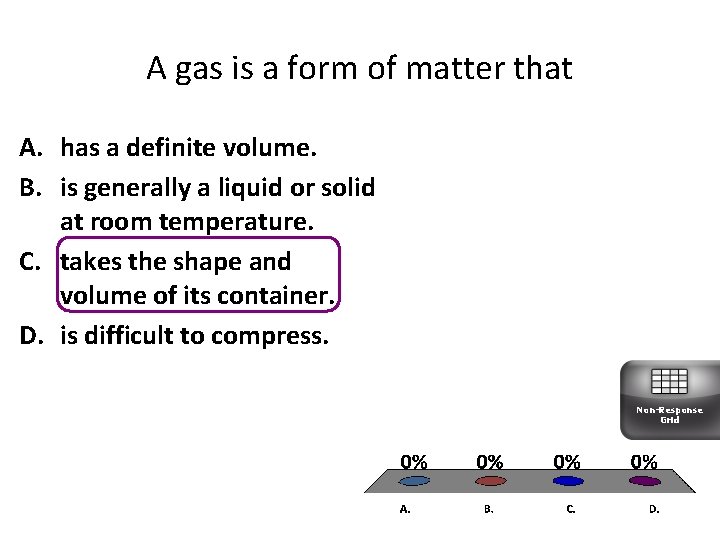
A gas is a form of matter that A. has a definite volume. B. is generally a liquid or solid at room temperature. C. takes the shape and volume of its container. D. is difficult to compress. Non-Response Grid

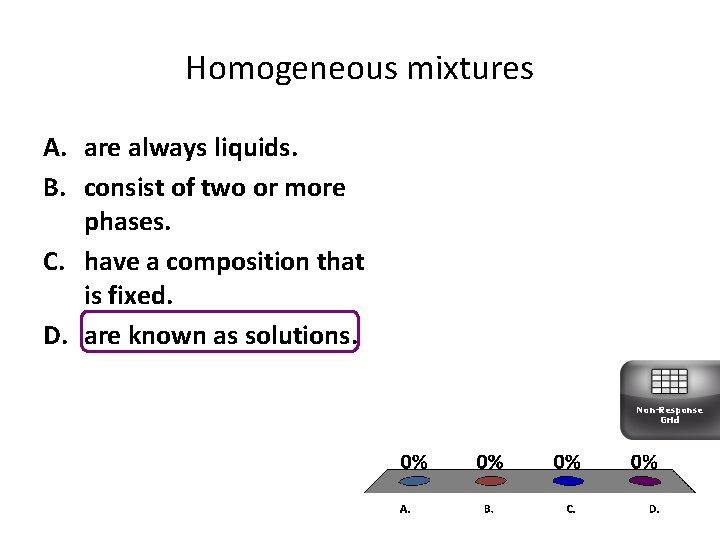
Homogeneous mixtures A. are always liquids. B. consist of two or more phases. C. have a composition that is fixed. D. are known as solutions. Non-Response Grid

A compound A. is a pure substance. B. has a composition that varies. C. can be physically separated into its elements. D. has properties similar to those of its elements. Non-Response Grid

Physical properties of a substance include A. B. C. D. color and odor. malleability. melting & boiling points. all of the above. Non-Response Grid

When iron and oxygen combine to form iron oxide, A. B. C. D. a physical change occurs. a change of state occurs. a change in mass occurs. a chemical change occurs. Non-Response Grid

How many significant figures are in the measurement 2103. 2 g? A. B. C. D. 2 4 5 3 Non-Response Grid

Which of these equalities is not correct? A. B. C. D. 10 kg= 1 g 100 cg= 1 g 1 cm 3= 1 m. L 1000 mm= 1 m Non-Response Grid

How many of the zeros in the measurement 0. 000 040 200 m are significant? A. B. C. D. 2 7 3 8 Non-Response Grid

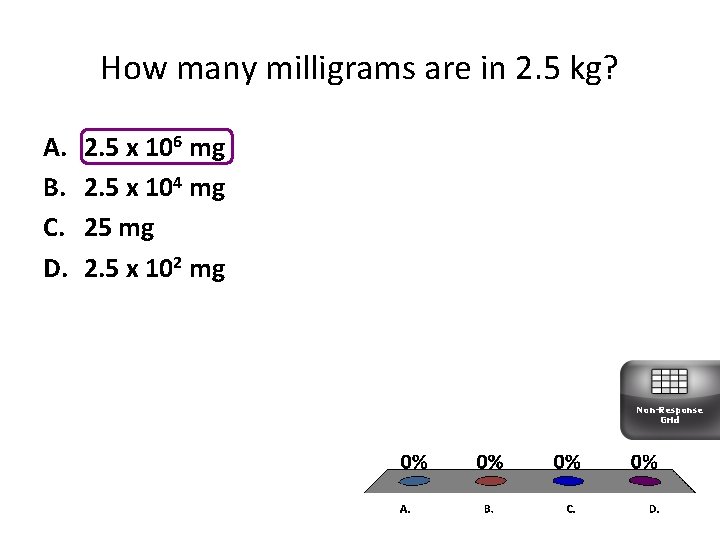
How many milligrams are in 2. 5 kg? A. B. C. D. 2. 5 x 106 mg 2. 5 x 104 mg 25 mg 2. 5 x 102 mg Non-Response Grid

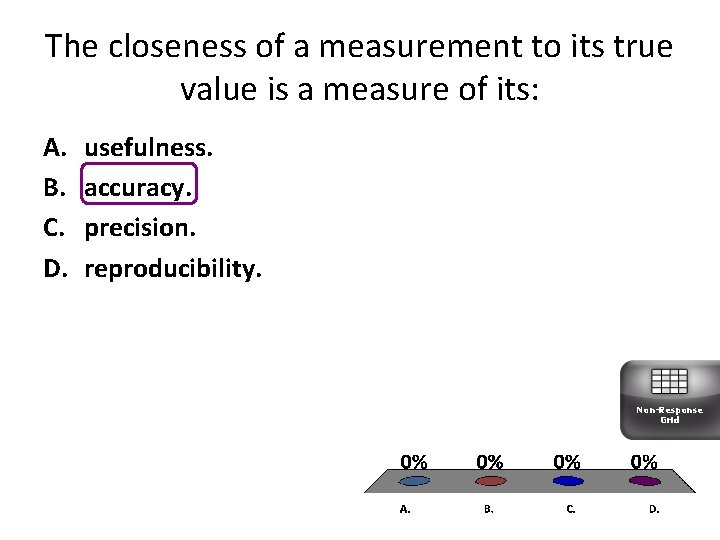
The closeness of a measurement to its true value is a measure of its: A. B. C. D. usefulness. accuracy. precision. reproducibility. Non-Response Grid

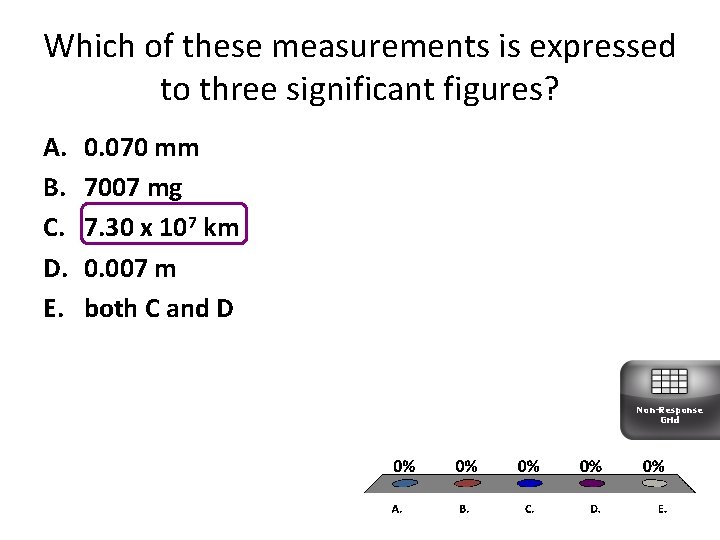
Which of these measurements is expressed to three significant figures? A. B. C. D. E. 0. 070 mm 7007 mg 7. 30 x 107 km 0. 007 m both C and D Non-Response Grid

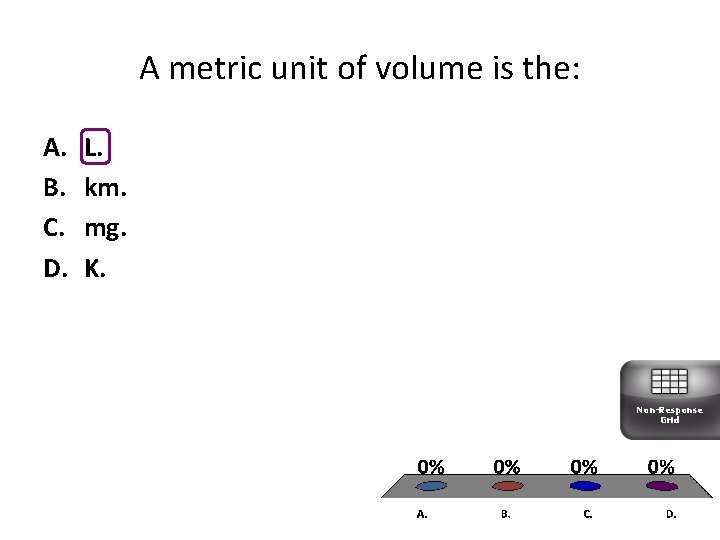
A metric unit of volume is the: A. B. C. D. L. km. mg. K. Non-Response Grid

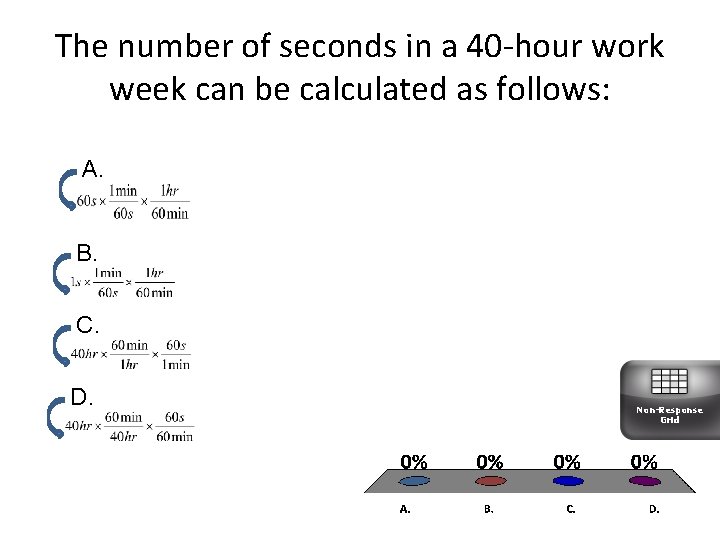
The number of seconds in a 40 -hour work week can be calculated as follows: A. B. C. D. Non-Response Grid

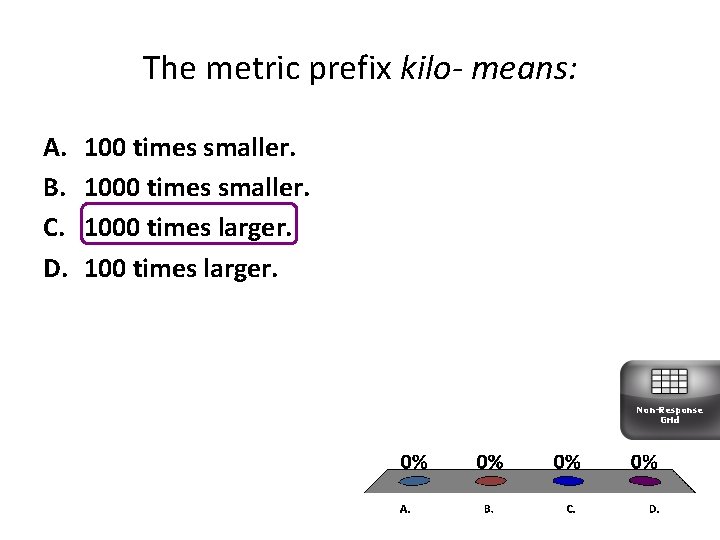
The metric prefix kilo- means: A. B. C. D. 100 times smaller. 1000 times larger. 100 times larger. Non-Response Grid

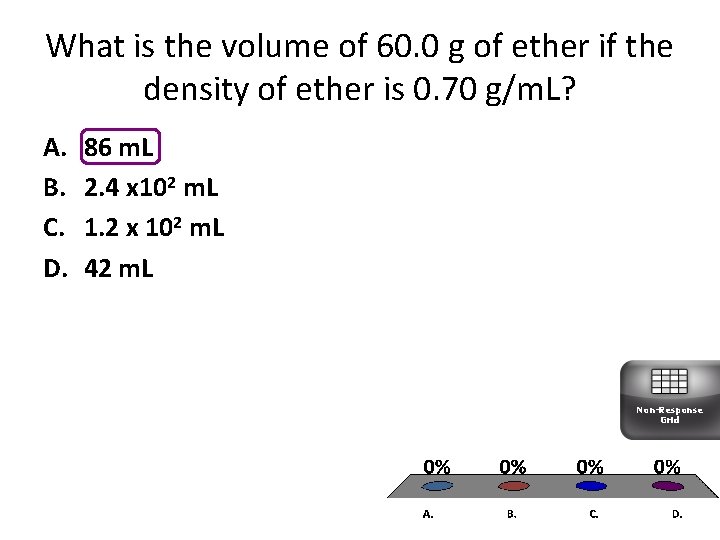
What is the volume of 60. 0 g of ether if the density of ether is 0. 70 g/m. L? A. B. C. D. 86 m. L 2. 4 x 102 m. L 1. 2 x 102 m. L 42 m. L Non-Response Grid

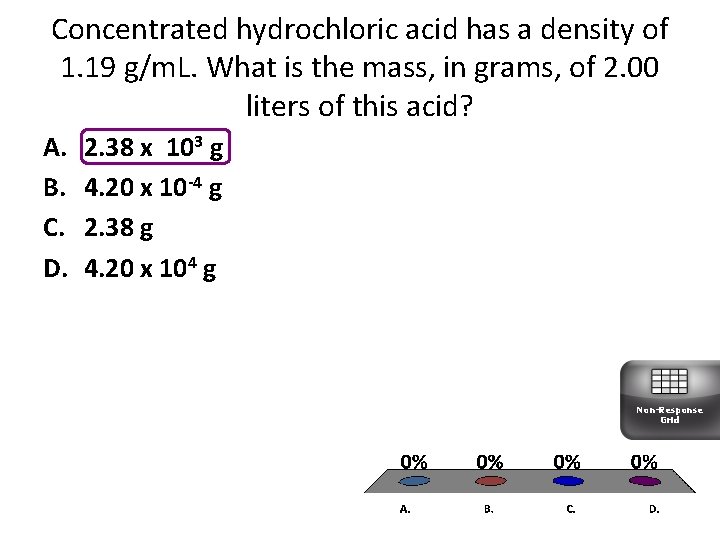
Concentrated hydrochloric acid has a density of 1. 19 g/m. L. What is the mass, in grams, of 2. 00 liters of this acid? A. B. C. D. 2. 38 x 103 g 4. 20 x 10 -4 g 2. 38 g 4. 20 x 104 g Non-Response Grid

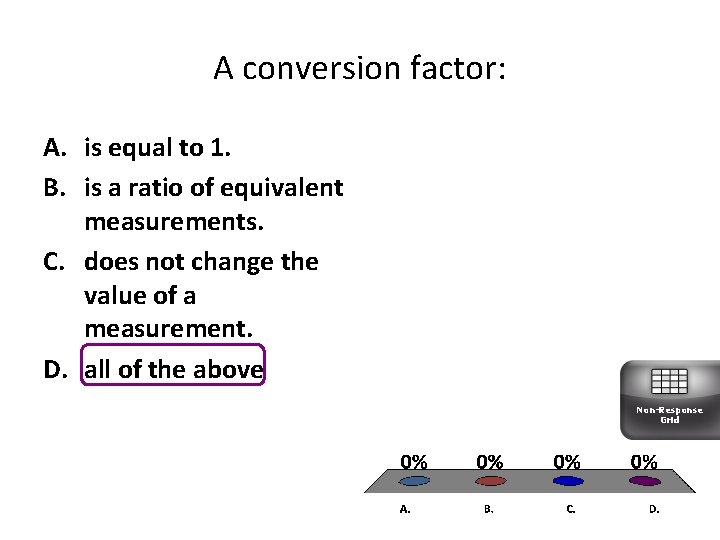
A conversion factor: A. is equal to 1. B. is a ratio of equivalent measurements. C. does not change the value of a measurement. D. all of the above Non-Response Grid

Which of the following is not a part of Dalton’s atomic theory? A. All elements are composed of atoms. B. Atoms of the same element are alike. C. Atoms are always in motion. D. Atoms that combine do so in simple wholenumber ratios. Non-Response Grid

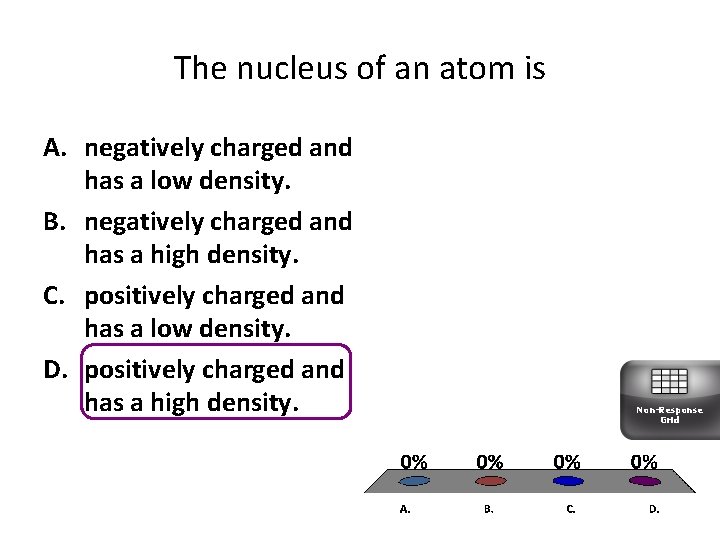
The nucleus of an atom is A. negatively charged and has a low density. B. negatively charged and has a high density. C. positively charged and has a low density. D. positively charged and has a high density. Non-Response Grid

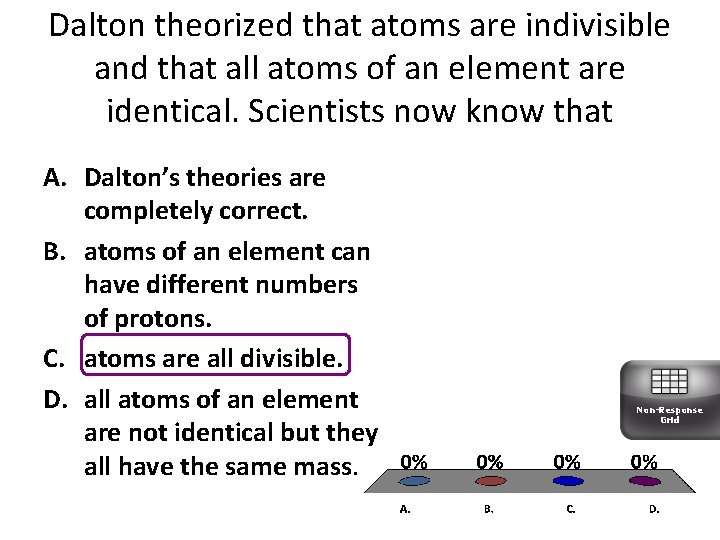
Dalton theorized that atoms are indivisible and that all atoms of an element are identical. Scientists now know that A. Dalton’s theories are completely correct. B. atoms of an element can have different numbers of protons. C. atoms are all divisible. D. all atoms of an element are not identical but they all have the same mass. Non-Response Grid

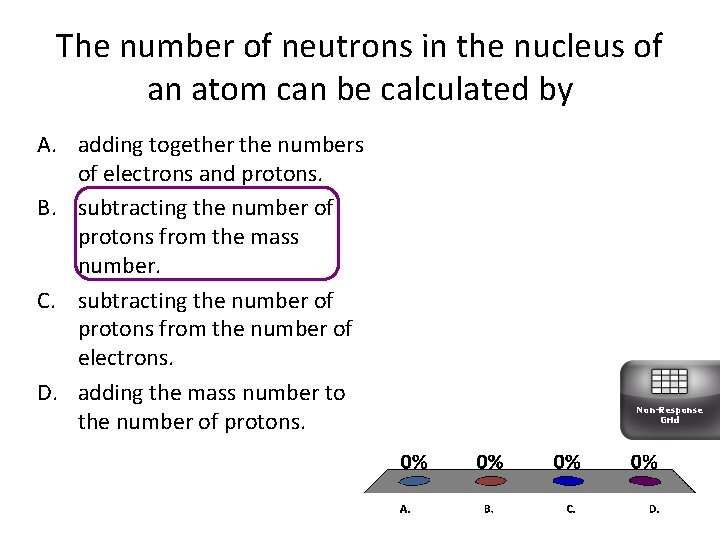
The number of neutrons in the nucleus of an atom can be calculated by A. adding together the numbers of electrons and protons. B. subtracting the number of protons from the mass number. C. subtracting the number of protons from the number of electrons. D. adding the mass number to the number of protons. Non-Response Grid

The sum of the protons and neutrons in an atom equals the A. B. C. D. atomic number. atomic mass. number of electrons. mass number. Non-Response Grid

All atoms of the same element have the same: A. B. C. D. number of protons. mass number of neutrons. mass. Non-Response Grid

Which of these statements is false? A. Electrons have a negative charge. B. Electrons have a mass of 1 amu. C. The nucleus of an atom is positively charged. D. The neutron is found in the nucleus of an atom. Non-Response Grid

An atom of an element with atomic number 48 and mass number 120 contains A. 48 protons, 48 electrons, and 72 neutrons. B. 72 protons, 48 electrons, and 48 neutrons. C. 120 protons, 48 electrons, and 72 neutrons. D. 72 protons, 72 electrons, and 48 neutrons. Non-Response Grid

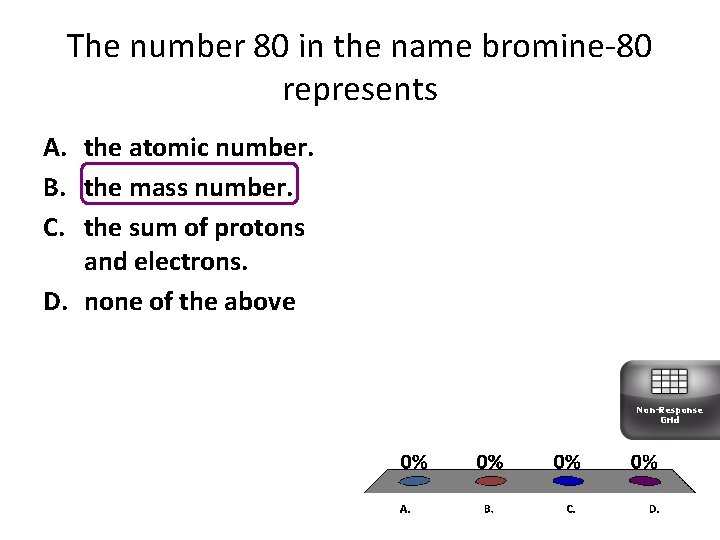
The number 80 in the name bromine-80 represents A. the atomic number. B. the mass number. C. the sum of protons and electrons. D. none of the above Non-Response Grid

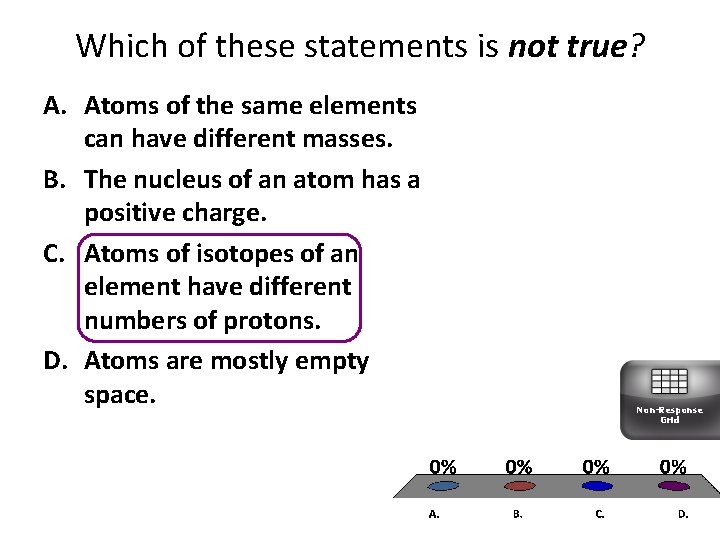
Which of these statements is not true? A. Atoms of the same elements can have different masses. B. The nucleus of an atom has a positive charge. C. Atoms of isotopes of an element have different numbers of protons. D. Atoms are mostly empty space. Non-Response Grid

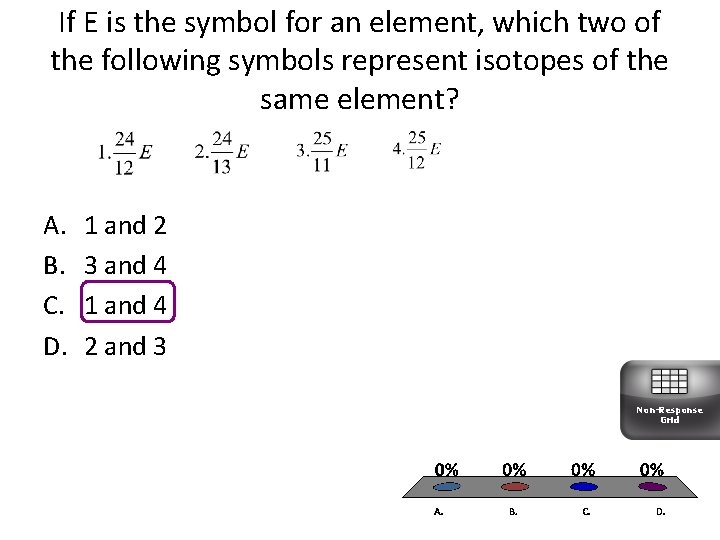
If E is the symbol for an element, which two of the following symbols represent isotopes of the same element? A. B. C. D. 1 and 2 3 and 4 1 and 4 2 and 3 Non-Response Grid

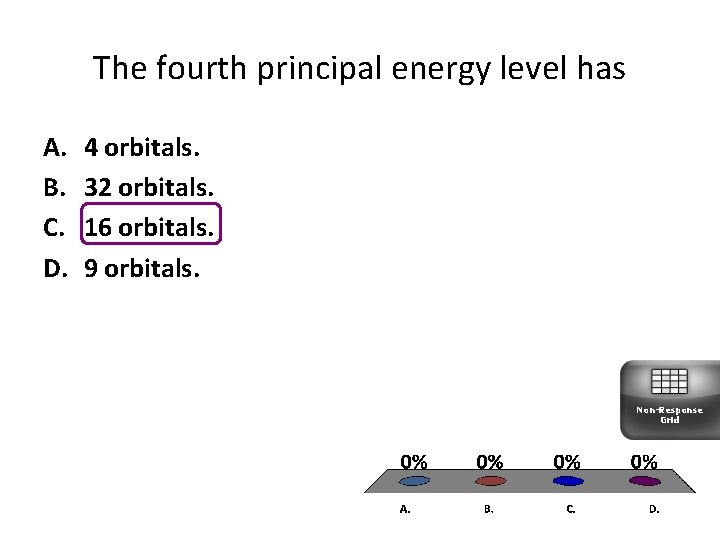
The fourth principal energy level has A. B. C. D. 4 orbitals. 32 orbitals. 16 orbitals. 9 orbitals. Non-Response Grid

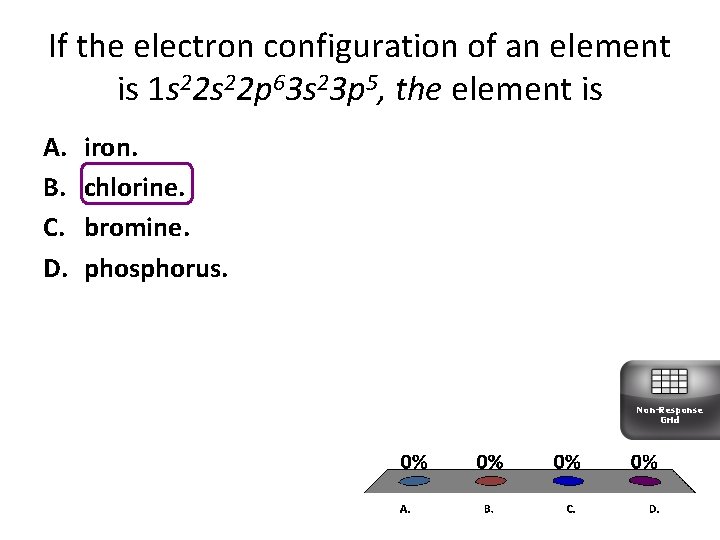
If the electron configuration of an element is 1 s 22 p 63 s 23 p 5, the element is A. B. C. D. iron. chlorine. bromine. phosphorus. Non-Response Grid

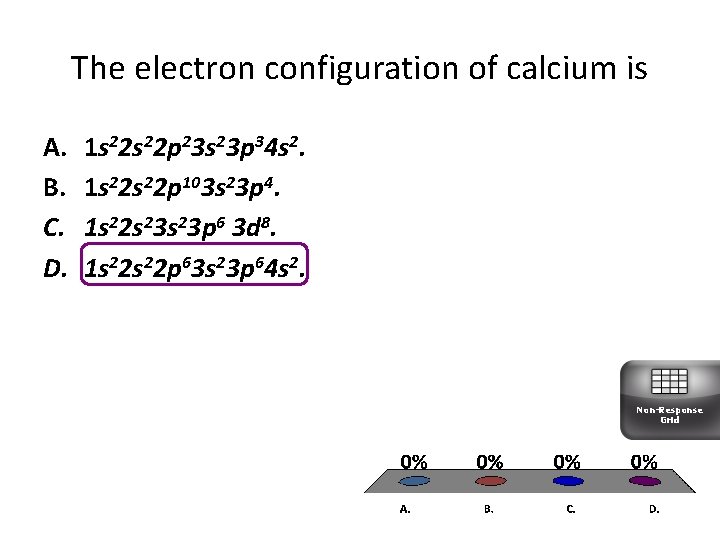
The electron configuration of calcium is A. B. C. D. 1 s 22 p 23 s 23 p 34 s 2. 1 s 22 p 103 s 23 p 4. 1 s 22 s 23 p 6 3 d 8. 1 s 22 p 63 s 23 p 64 s 2. Non-Response Grid

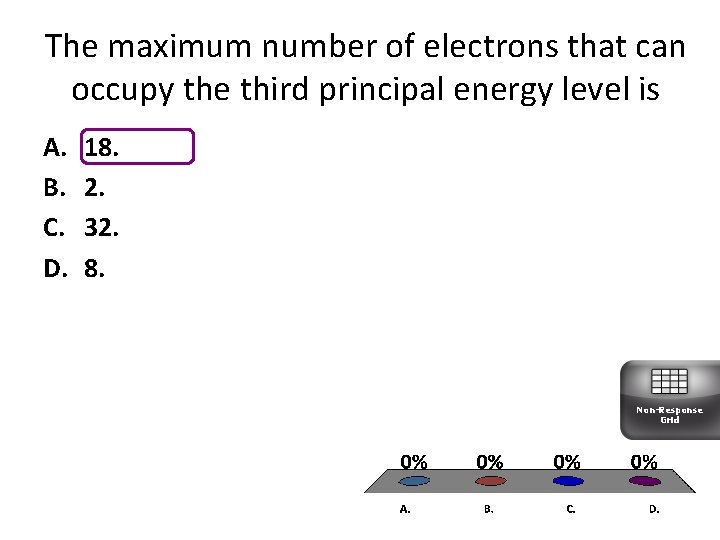
The maximum number of electrons that can occupy the third principal energy level is A. B. C. D. 18. 2. 32. 8. Non-Response Grid

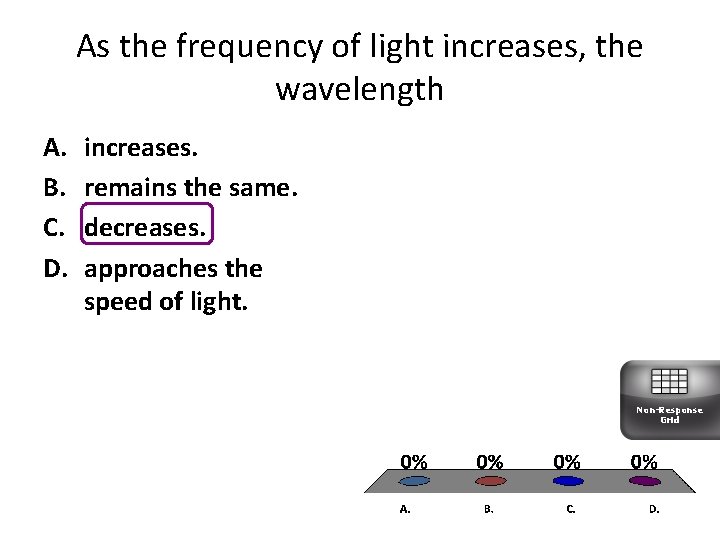
As the frequency of light increases, the wavelength A. B. C. D. increases. remains the same. decreases. approaches the speed of light. Non-Response Grid

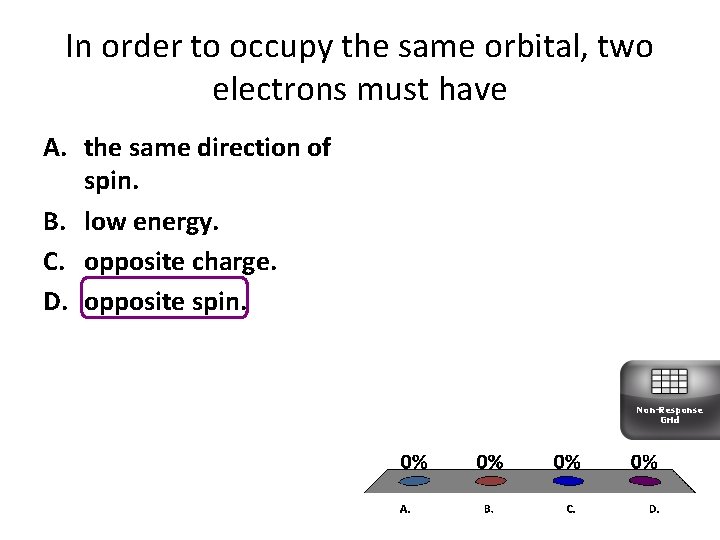
In order to occupy the same orbital, two electrons must have A. the same direction of spin. B. low energy. C. opposite charge. D. opposite spin. Non-Response Grid

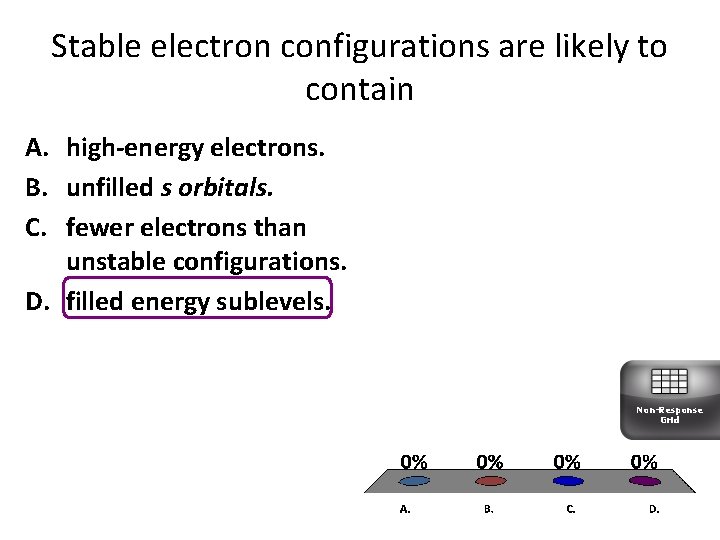
Stable electron configurations are likely to contain A. high-energy electrons. B. unfilled s orbitals. C. fewer electrons than unstable configurations. D. filled energy sublevels. Non-Response Grid

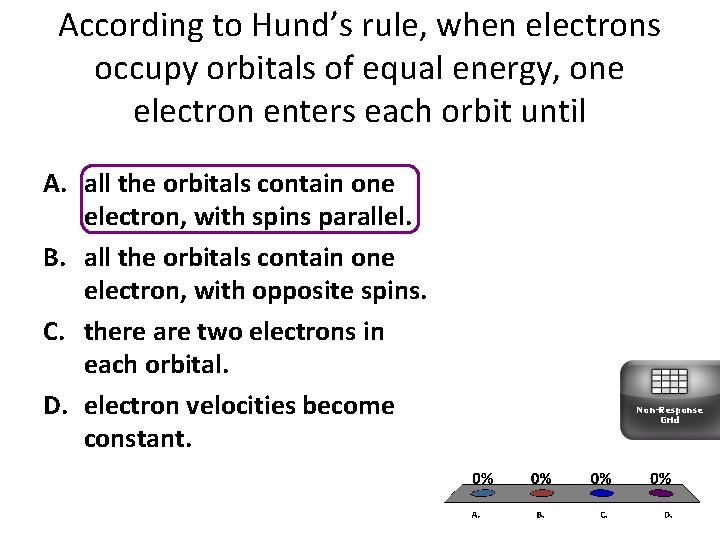
According to Hund’s rule, when electrons occupy orbitals of equal energy, one electron enters each orbit until A. all the orbitals contain one electron, with spins parallel. B. all the orbitals contain one electron, with opposite spins. C. there are two electrons in each orbital. D. electron velocities become constant. Non-Response Grid

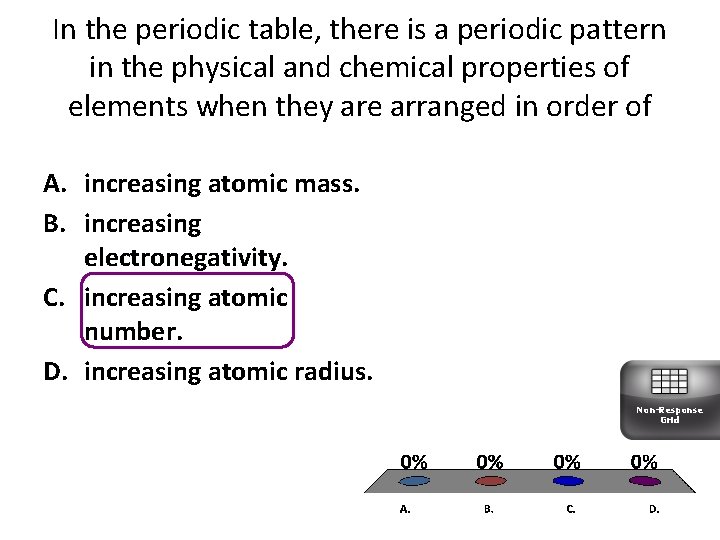
In the periodic table, there is a periodic pattern in the physical and chemical properties of elements when they are arranged in order of A. increasing atomic mass. B. increasing electronegativity. C. increasing atomic number. D. increasing atomic radius. Non-Response Grid

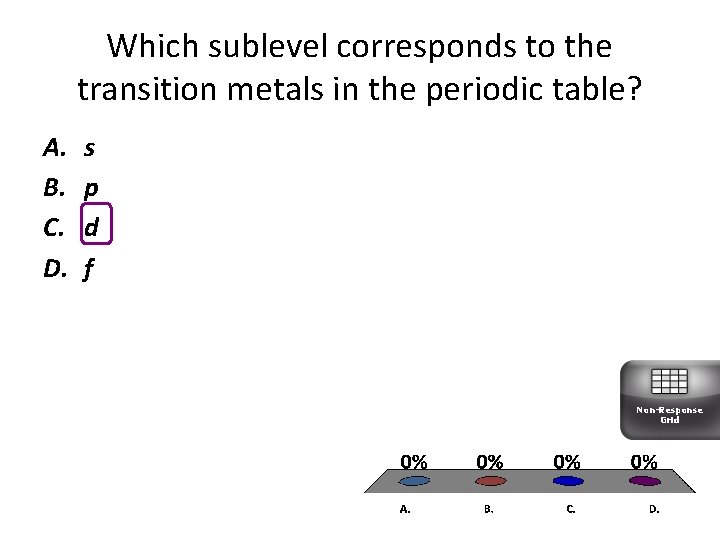
Which sublevel corresponds to the transition metals in the periodic table? A. B. C. D. s p d f Non-Response Grid

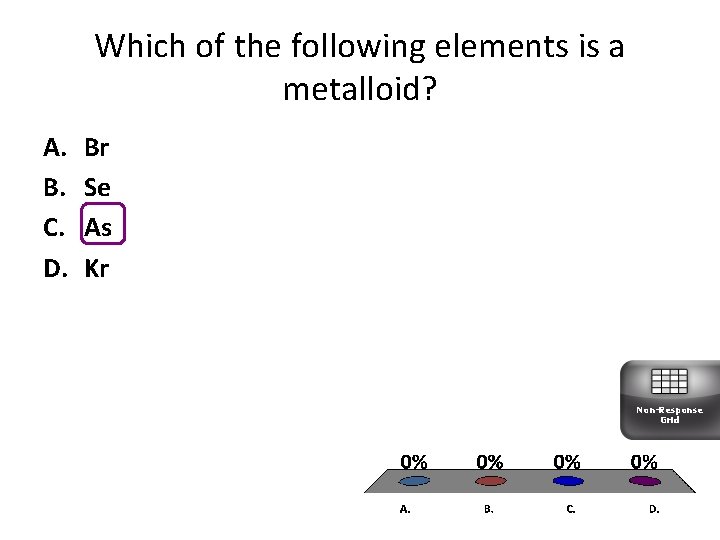
Which of the following elements is a metalloid? A. B. C. D. Br Se As Kr Non-Response Grid

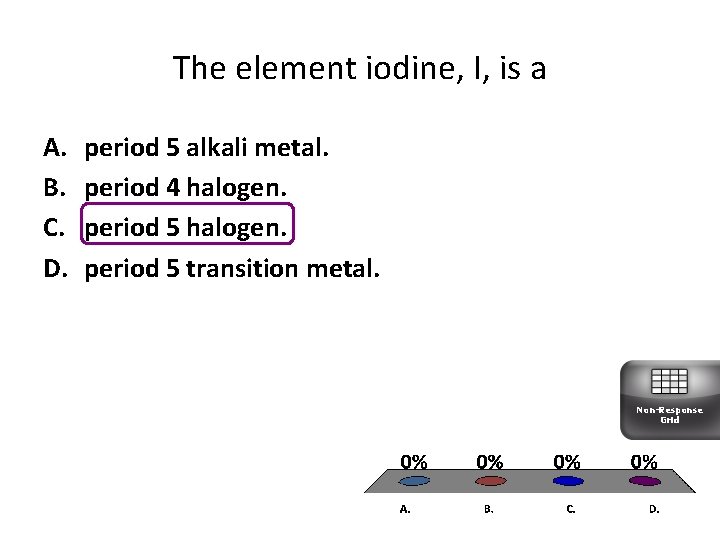
The element iodine, I, is a A. B. C. D. period 5 alkali metal. period 4 halogen. period 5 transition metal. Non-Response Grid

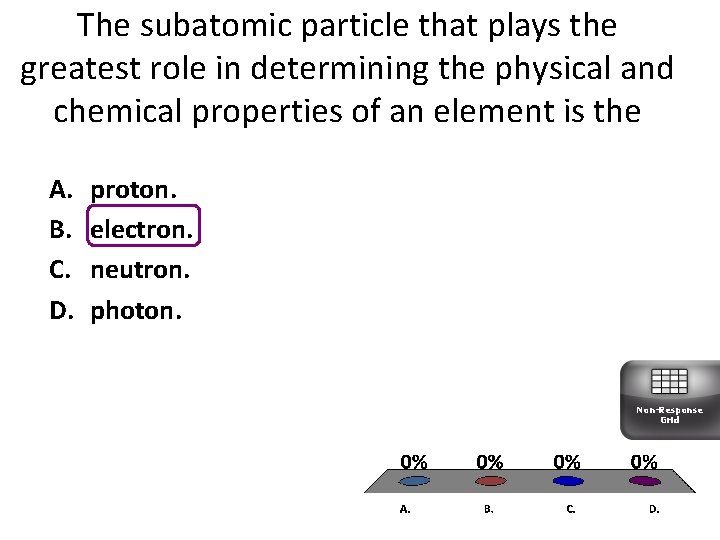
The subatomic particle that plays the greatest role in determining the physical and chemical properties of an element is the A. B. C. D. proton. electron. neutron. photon. Non-Response Grid

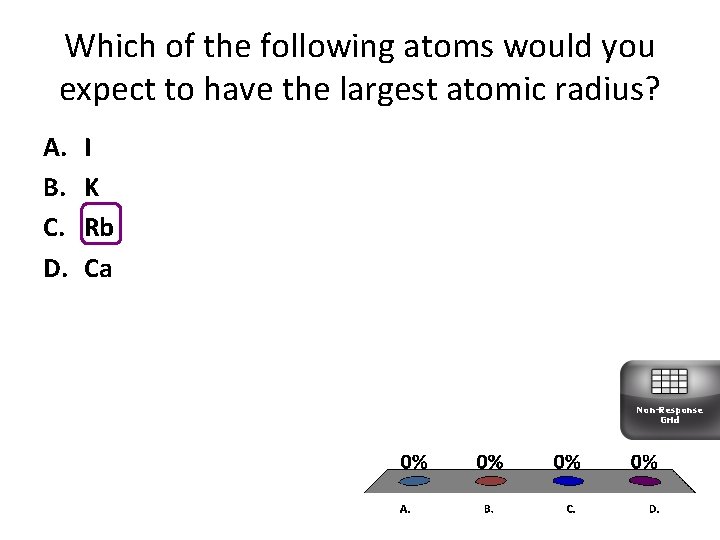
Which of the following atoms would you expect to have the largest atomic radius? A. B. C. D. I K Rb Ca Non-Response Grid

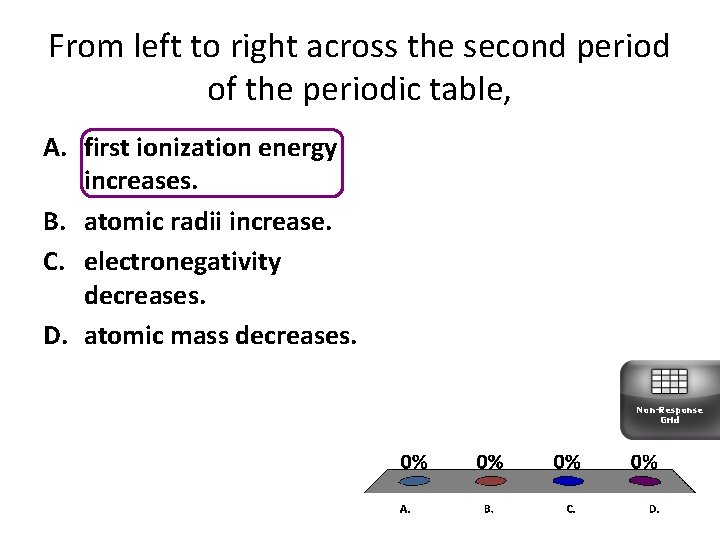
From left to right across the second period of the periodic table, A. first ionization energy increases. B. atomic radii increase. C. electronegativity decreases. D. atomic mass decreases. Non-Response Grid

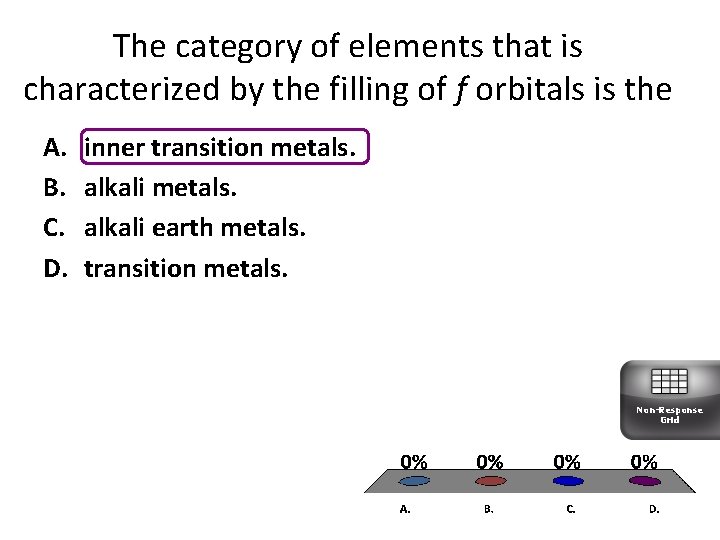
The category of elements that is characterized by the filling of f orbitals is the A. B. C. D. inner transition metals. alkali earth metals. transition metals. Non-Response Grid

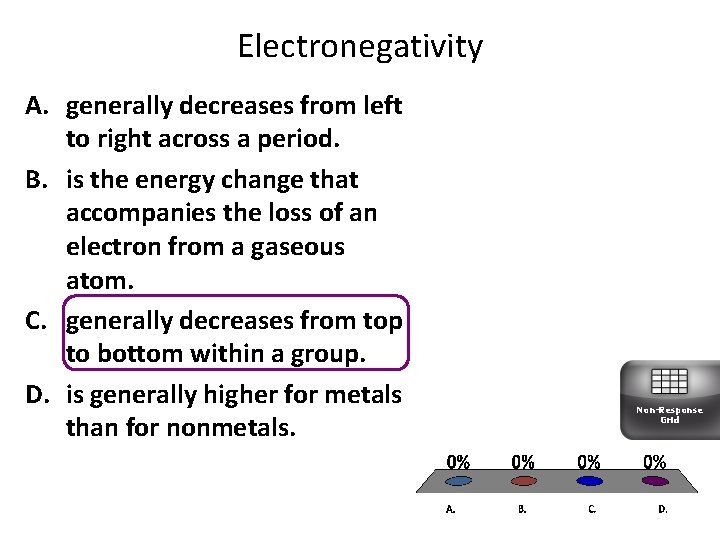
Electronegativity A. generally decreases from left to right across a period. B. is the energy change that accompanies the loss of an electron from a gaseous atom. C. generally decreases from top to bottom within a group. D. is generally higher for metals than for nonmetals. Non-Response Grid

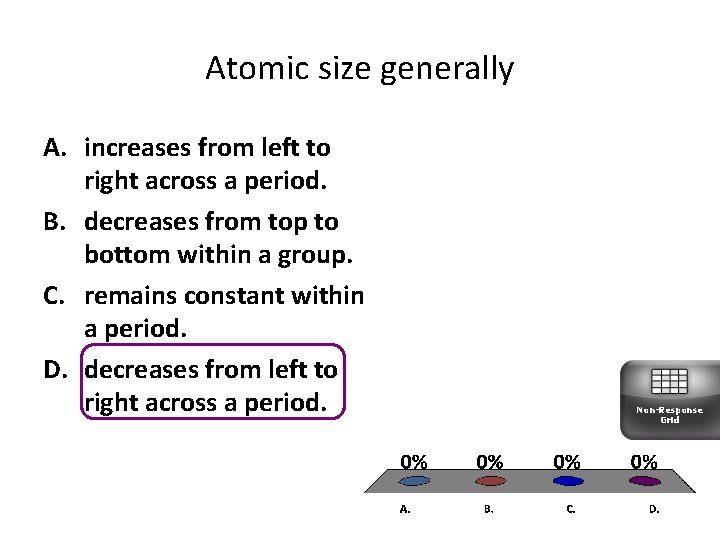
Atomic size generally A. increases from left to right across a period. B. decreases from top to bottom within a group. C. remains constant within a period. D. decreases from left to right across a period. Non-Response Grid

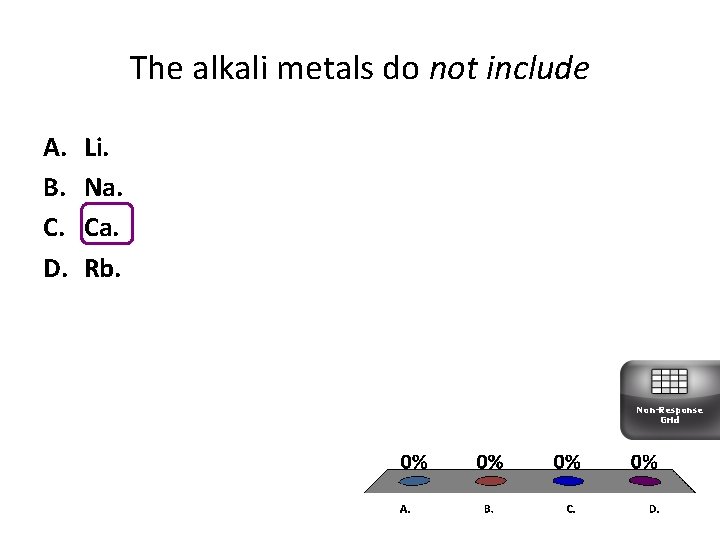
The alkali metals do not include A. B. C. D. Li. Na. Ca. Rb. Non-Response Grid

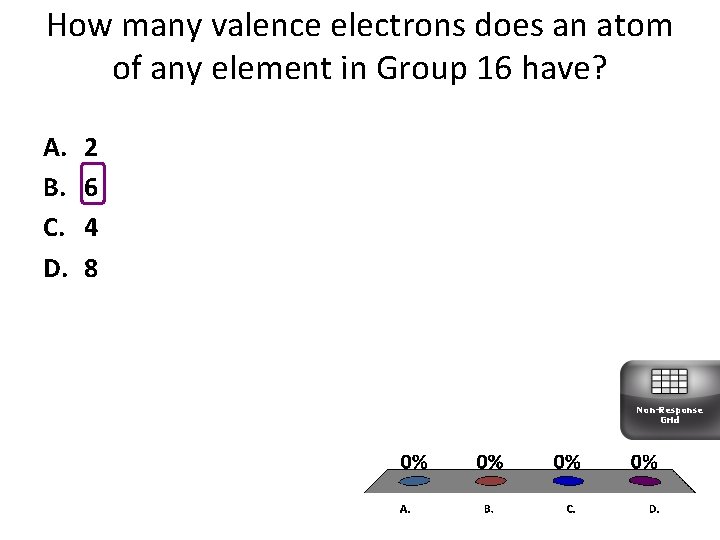
How many valence electrons does an atom of any element in Group 16 have? A. B. C. D. 2 6 4 8 Non-Response Grid

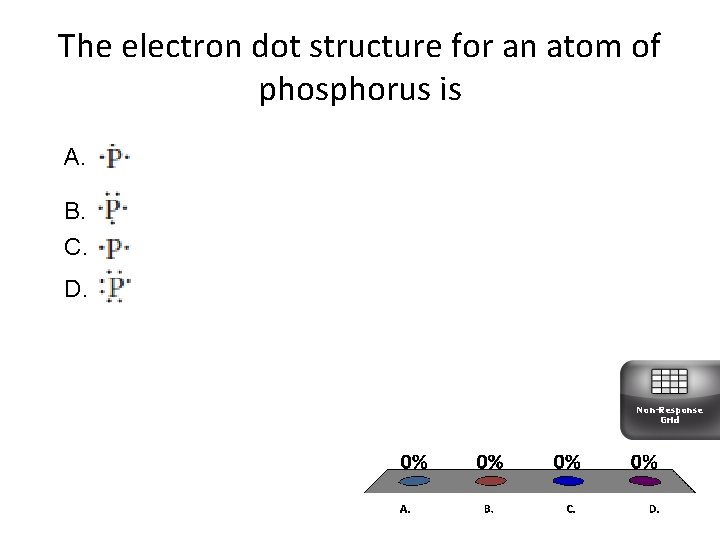
The electron dot structure for an atom of phosphorus is A. B. C. D. Non-Response Grid

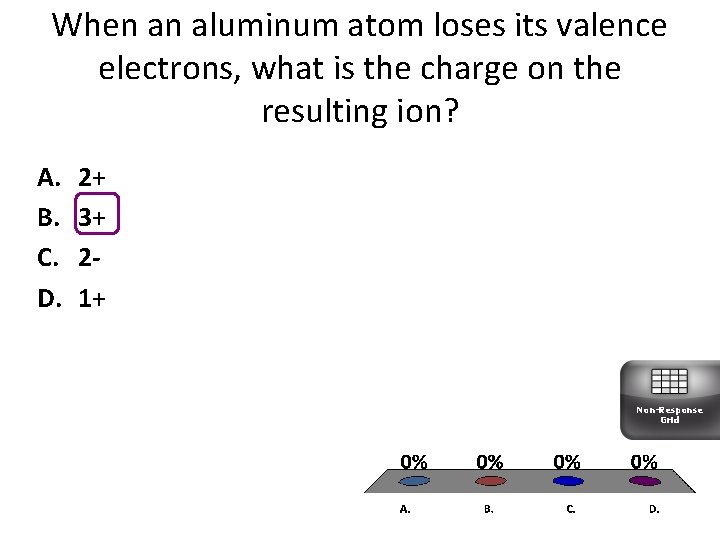
When an aluminum atom loses its valence electrons, what is the charge on the resulting ion? A. B. C. D. 2+ 3+ 21+ Non-Response Grid

15 • The high surface tension of water is due to the: • a. small size of water molecules. • b. low mass of water molecules. • c. hydrogen bonding between water molecules. • d. covalent bonds in water molecules. c.

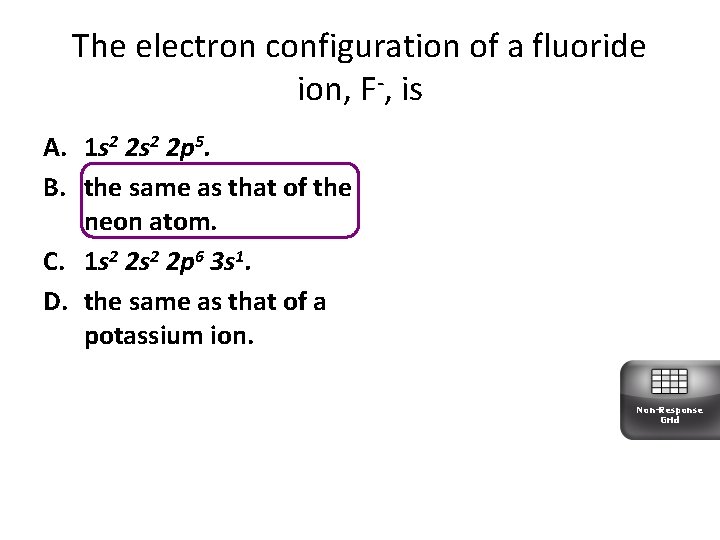
The electron configuration of a fluoride ion, F-, is A. 1 s 2 2 p 5. B. the same as that of the neon atom. C. 1 s 2 2 p 6 3 s 1. D. the same as that of a potassium ion. Non-Response Grid

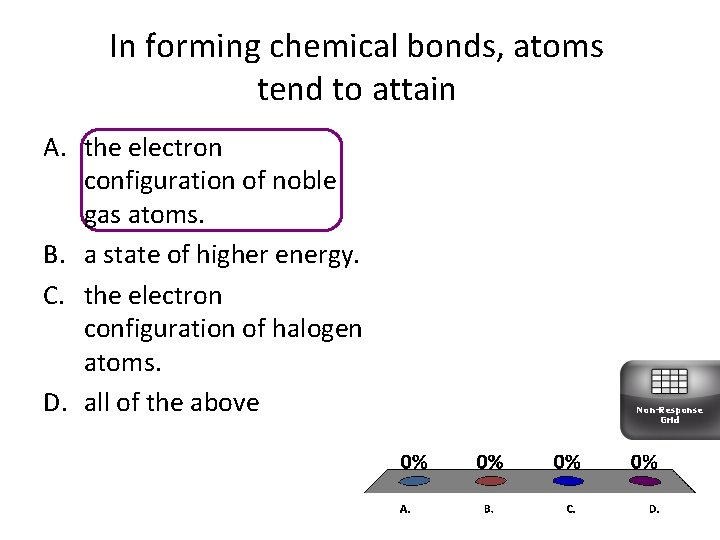
In forming chemical bonds, atoms tend to attain A. the electron configuration of noble gas atoms. B. a state of higher energy. C. the electron configuration of halogen atoms. D. all of the above Non-Response Grid

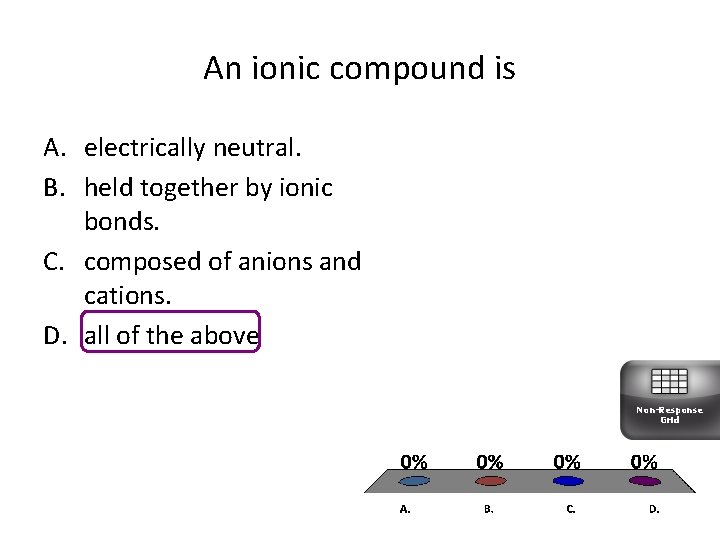
An ionic compound is A. electrically neutral. B. held together by ionic bonds. C. composed of anions and cations. D. all of the above Non-Response Grid

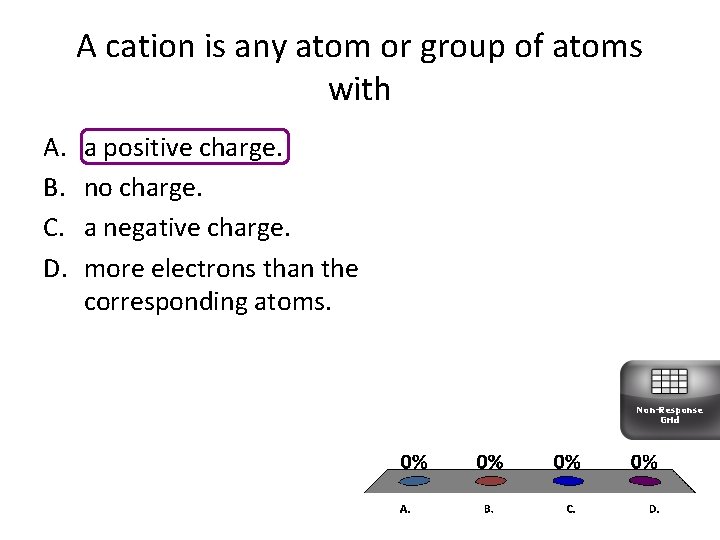
A cation is any atom or group of atoms with A. B. C. D. a positive charge. no charge. a negative charge. more electrons than the corresponding atoms. Non-Response Grid

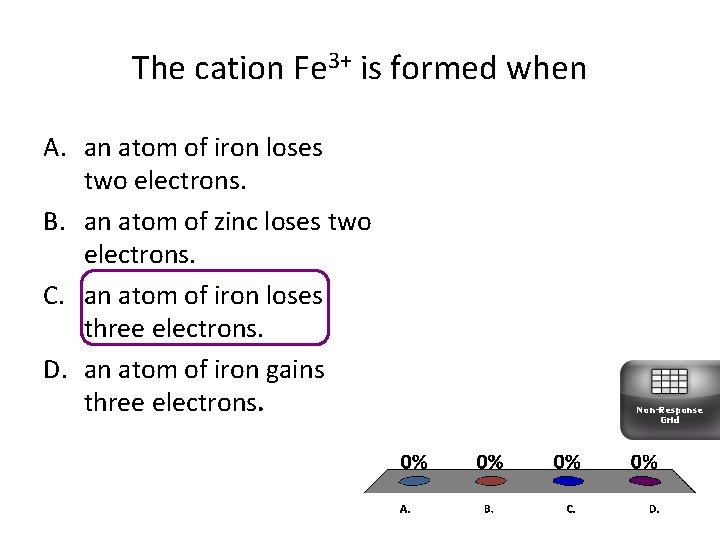
The cation Fe 3+ is formed when A. an atom of iron loses two electrons. B. an atom of zinc loses two electrons. C. an atom of iron loses three electrons. D. an atom of iron gains three electrons. Non-Response Grid

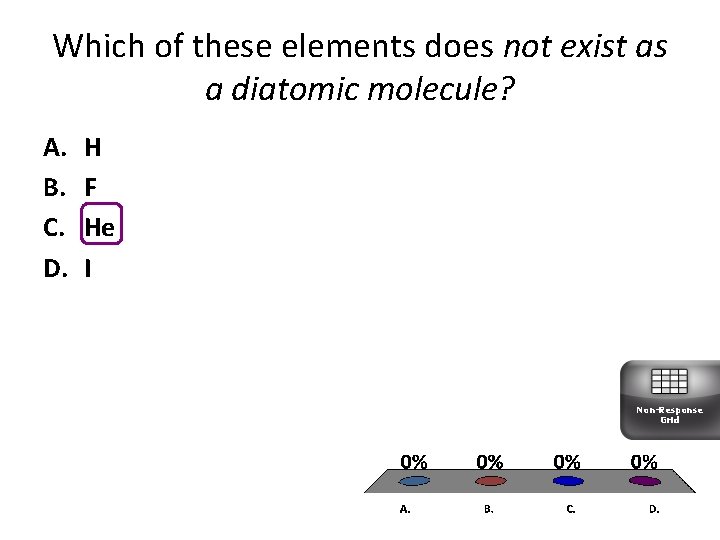
Which of these elements does not exist as a diatomic molecule? A. B. C. D. H F He I Non-Response Grid

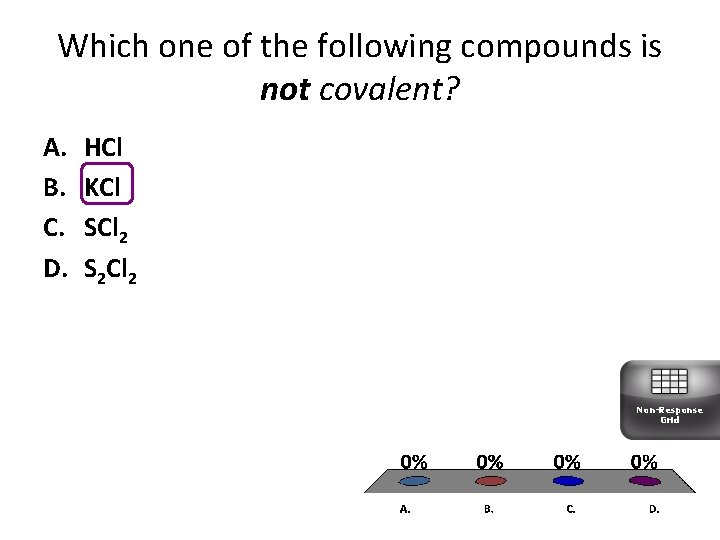
Which one of the following compounds is not covalent? A. B. C. D. HCl KCl SCl 2 S 2 Cl 2 Non-Response Grid

How many valence electrons does an atom of any halogen have? A. B. C. D. 4 2 1 7 Non-Response Grid

If a bonding pair of electrons is unequally shared between two atoms, the bond is A. B. C. D. ionic. coordinate covalent. nonpolar covalent. Non-Response Grid

A covalent bond forms A. when an element becomes a noble gas. B. when atoms share electrons. C. between metals and nonmetals. D. when electrons are transferred from one atom to another. Non-Response Grid

The correct name for the N 3 - ion is the: A. B. C. D. nitrate ion. nitride ion. nitric ion. nitrite ion. Non-Response Grid

A molecular formula: A. gives information about molecular geometry. B. can be written for ionic compounds. C. shows the number and kinds of atoms in a molecule of a compound. D. uses superscripts to show the number of atoms of each kind. Non-Response Grid

The metals in Groups 1 A, 2 A, and 3 A: A. gain electrons when they form ions. B. form ions with a charge found by subtracting 8 from the group number. C. all form ions with a 1 charge. D. lose electrons when they form ions. Non-Response Grid

Which of the following is not an empirical formula? A. B. C. D. Na 2 SO 4 N 2 H 4 C 6 H 5 Cl Sn 3(PO 4)4 Non-Response Grid
 Gen chem review for ochem
Gen chem review for ochem Ap chemistry equilibrium review
Ap chemistry equilibrium review Ap chem electrochemistry review
Ap chem electrochemistry review Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Physics semester 1 review
Physics semester 1 review World history semester 2 final review packet
World history semester 2 final review packet World history semester 2 review
World history semester 2 review Us history semester 2 final exam review
Us history semester 2 final exam review Algebra 1 final review packet
Algebra 1 final review packet Zoology semester 1 exam review answers
Zoology semester 1 exam review answers Geometry unit 1 review
Geometry unit 1 review Apes semester 1 final review
Apes semester 1 final review Physics fall semester exam review
Physics fall semester exam review Us history final exam
Us history final exam English 3 fall semester exam review
English 3 fall semester exam review Verbal irony def
Verbal irony def Chemistry semester 1 exam study guide answers
Chemistry semester 1 exam study guide answers World history semester 1 final exam study guide answers
World history semester 1 final exam study guide answers Biology semester 1 review 2018
Biology semester 1 review 2018 Chemistry semester exam review
Chemistry semester exam review World history semester 1 final exam study guide answers
World history semester 1 final exam study guide answers Name all the rays
Name all the rays Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton là gì
Tư thế worm breton là gì Hát lên người ơi alleluia
Hát lên người ơi alleluia Kể tên các môn thể thao
Kể tên các môn thể thao Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên?
Thế nào là giọng cùng tên? Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tia chieu sa te
Tia chieu sa te Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot So nguyen to
So nguyen to Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Iannonechem
Iannonechem Ap chem unit 7
Ap chem unit 7 Imf chem
Imf chem Chm 130 chapter 12 practice problems answer key
Chm 130 chapter 12 practice problems answer key Alkanes formula
Alkanes formula Principles of kmt
Principles of kmt June 2018 chemistry regents
June 2018 chemistry regents Beer's law ap chemistry free response
Beer's law ap chemistry free response