CHEMISTRY 1 ST SEMESTER EXAM REVIEW 1 A









![47. Which element has the following electron configuration: [Ne]3 s 1? SODIUM, Na 48. 47. Which element has the following electron configuration: [Ne]3 s 1? SODIUM, Na 48.](https://slidetodoc.com/presentation_image_h/eb10bb981812f6250a047ec4efbc663e/image-12.jpg)





- Slides: 18

CHEMISTRY 1 ST SEMESTER EXAM REVIEW

1. A measure of the quantity of matter is MASS 2. List a process that is a physical change. CUTTING, GRINDING, TEARING 3. List a process that is a chemical change? BURNING 4. A chemical change occurs when MOLECULES ARE REARRANGED TO FORM A NEW COMPOUND 5. A physical change occurs when a MOLECULES ARE MOVED AROUND, BUT ARE STILL MADE OF THE SAME COMPONENTS

6. A state of matter in which a material has no definite shape but has a definite volume is the ____ state. LIQUID 7. Under ordinary conditions of temperature and RANDOMLY SPREAD FAR APART pressure, the particles in a gas are HAVING A DEFINED VOLUME BUT 8. The liquid state of matter can be described as NO DEFINED SHAPE 9. A solid substance is STATE OF MATTER WITH A DEFINED SHAPE AND DEFINED VOLUME

10. If a mixture is uniform in composition, it is said to be HOMOGENEOUS 11. If a mixture is not uniform throughout, it is called HETEROGENEOUS 12. The quantity of matter per unit volume is DENSITY 13. The relationship/equation between the mass m of a material, its volume V, and its density D is D = M/V 14. The density of aluminum is 2. 70 g/cm 3. What is the mass of a solid piece of aluminum with a volume of 1. 50 cm 3? 4. 05 G

15. A sample of gold has a mass of 96. 5 g and a volume of 5. 00 cm 3. The density of gold is 19. 3 G/CM 3 16. The density of pure diamond is 3. 5 g/cm 3. What is the volume of a diamond with a mass of 0. 25 g? . 07 CM 3 17. How many significant figures are in 0. 202 grams? THREE 18. How many significant figures does 30. 00 have? FOUR

19. The speed of light is 300 000 km/s. Rewrite this in scientific notation. 3. 0 X 105 20. The average distance between the Earth and the moon is 386 000 km. Rewrite this in scientific notation. 3. 86 X 105 21. In oxides of nitrogen, such as N 2 O, NO 2, and N 2 O 3, atoms combine in small whole-number ratios. this evidence supports the law of MULTIPLE PROPORTIONS 22. If two or more compounds are composed of the same two elements, the ratio of the masses of one element that combines with a fixed mass of the other element is a simple whole number. This is a statement of the law of MULTIPLE PROPORTIONS

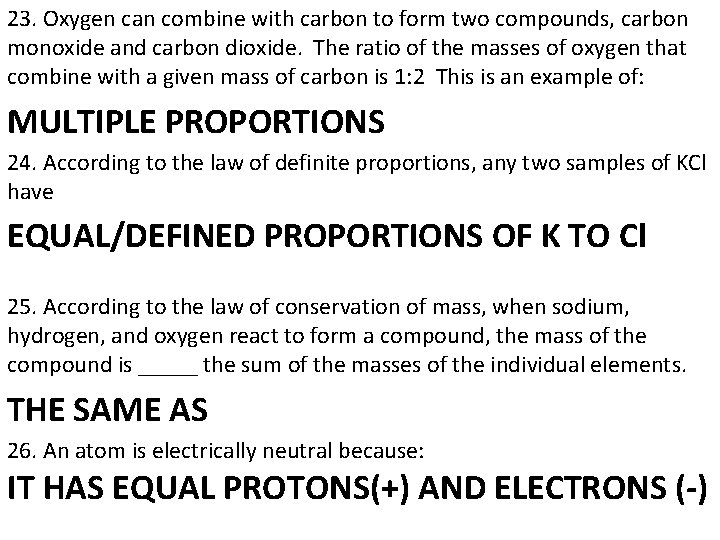
23. Oxygen can combine with carbon to form two compounds, carbon monoxide and carbon dioxide. The ratio of the masses of oxygen that combine with a given mass of carbon is 1: 2 This is an example of: MULTIPLE PROPORTIONS 24. According to the law of definite proportions, any two samples of KCl have EQUAL/DEFINED PROPORTIONS OF K TO Cl 25. According to the law of conservation of mass, when sodium, hydrogen, and oxygen react to form a compound, the mass of the compound is _____ the sum of the masses of the individual elements. THE SAME AS 26. An atom is electrically neutral because: IT HAS EQUAL PROTONS(+) AND ELECTRONS (-)

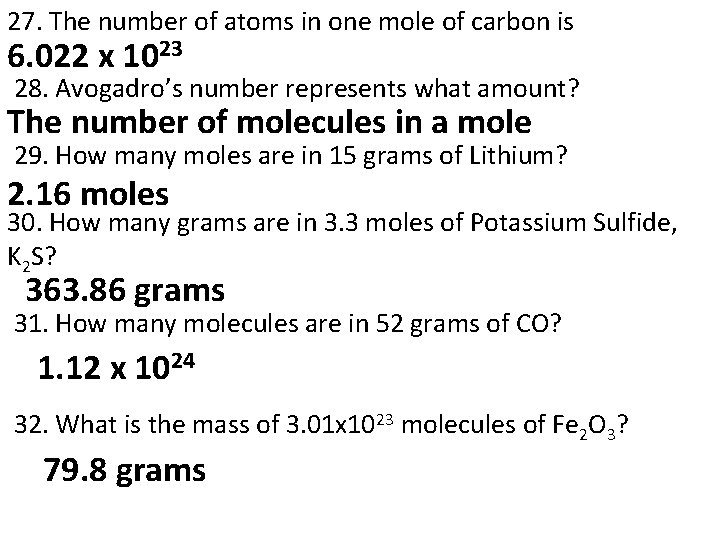
27. The number of atoms in one mole of carbon is 6. 022 x 1023 28. Avogadro’s number represents what amount? The number of molecules in a mole 29. How many moles are in 15 grams of Lithium? 2. 16 moles 30. How many grams are in 3. 3 moles of Potassium Sulfide, K 2 S? 363. 86 grams 31. How many molecules are in 52 grams of CO? 1. 12 x 1024 32. What is the mass of 3. 01 x 1023 molecules of Fe 2 O 3? 79. 8 grams

33. The statement that an electron occupies the lowest available energy orbital is what law/principle? THE AUFBAU PRINCIPLE 34. “Orbitals of equal energy are each occupied by one electron before any is occupied by a second electron, and all electrons in a singly occupied orbital must have the same spin” is a statement of what law/principle? HUND’S RULE 35. The statement that no two electrons in the same atom can have the same four quantum numbers is what law/principle? PAULI’S EXCLUSION PRINCIPLE 36. Which rule requires that each of the P orbitals at a particular energy level receive one electron before any of them can have two electrons? HUND’S RULE

37. The sequence in which energy levels are filled is specified by what law/principle? THE AUFBAU PRINCIPLE 38. The Aufbau Principle states that an electron FILLS UP THE LOWEST ENERGY LEVEL FIRST, THEN BUILDS UP 39. The Pauli Exclusion Principle states that no two electrons in the same atom can HAVE THE SAME SET OF QUANTUM NUMBERS, & CAN’T BE IN THE SAME PLACE AT SAME TIME 40. What is the correct order of atomic orbitals filled according to the Aufbau Principle? 1 S, 2 P, 3 S, 3 P, 4 S, 3 D, 4 P

41. The element with electron configuration 1 s 22 p 63 s 23 p 2 is SILICON, Si 42. The electron configuration for the Carbon atom, C, is 1 s 22 p 2. The atomic number of Carbon is 6 43. What is the electron configuration for Nitrogen, N atomic number 7? 1 S 22 P 3 44. The electron configuration for Aluminum, Al, atomic number 13 is 1 S 22 P 63 S 23 P 1 45. Write the correct electron configuration for Fluorine: 1 s 22 p 5 46. Which element has the following electron configuration: 1 s 22 p 63 s 23 p 64 s 23 d 8? NICKEL, Ni
![47 Which element has the following electron configuration Ne3 s 1 SODIUM Na 48 47. Which element has the following electron configuration: [Ne]3 s 1? SODIUM, Na 48.](https://slidetodoc.com/presentation_image_h/eb10bb981812f6250a047ec4efbc663e/image-12.jpg)
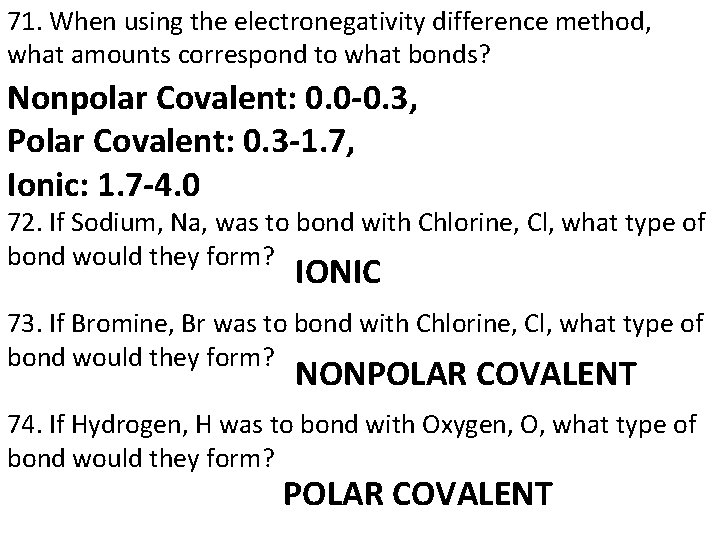
47. Which element has the following electron configuration: [Ne]3 s 1? SODIUM, Na 48. The higher the frequency of a wave, the ____ the energy of that wave. HIGHER 49. The higher the wavelength of a wave, the _____ the energy of the wave. LOWER 50. This individual is responsible for the organization of one of the first periodic tables which was organized based on the atomic mass of the known elements. DIMITRI MENDELEEV 51. This individual is responsible for organization of the present day periodic table which is based on the increasing number of protons in the nucleus. HENRY MOSELEY

52. What is the group of radioactive elements called with the atomic numbers from 90 to 103? ACTINIDES 53. The elements with atomic numbers from 58 to 71 are called? LANTHANIDES 54. Argon, Krypton, and Xenon are all examples of what group of elements? NOBLE GASES 55. The periodic law states that THE PHYSICAL AND CHEMICAL PROPERTIES OF THE ELEMENTS ARE FUNCTIONS OF THEIR ATOMIC NUMBERS 56. Elements in a group or column in the periodic table can be expected to have similar PROPERTIES

57. The elements in Group 1 are also known as the ALKALI METALS 58. The most reactive group of nonmetals are the HALOGENS 59. The soft, silvery, reactive metals, all of which have one electron in its s orbital is known as the ALKALI METALS 60. The most characteristic property of the noble gases is that they are UNREACTIVE

61. A measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound is called ELECTRONEGATIVITY 62. When an electron is acquired by a neutral atom, the energy is called ELECTRON AFFINITY 63. As you move down a group of elements you expect to see the atomic radii INCREASE 64. Across a period in the period table, ionic radii DECREASES 65. Groups 1 -2 and 13 -18 are known as this MAIN GROUP ELEMENTS

66. What is the correct block, period and group for the element with the electron configuration [Kr]5 s 2 ? BLOCK: S PERIOD: 5 GROUP: 2 67. What are the 2 main types of bonds that we have learned about so far? COVALENT AND IONIC 68. During an ionic bond, valence electrons are____ TAKEN OR GIVEN AWAY 69. During a covalent bond, valence electrons are_____ SHARED 70. What periodic trend can be used to determine the type of bond that elements will form? ELECTRONEGATIVITY

71. When using the electronegativity difference method, what amounts correspond to what bonds? Nonpolar Covalent: 0. 0 -0. 3, Polar Covalent: 0. 3 -1. 7, Ionic: 1. 7 -4. 0 72. If Sodium, Na, was to bond with Chlorine, Cl, what type of bond would they form? IONIC 73. If Bromine, Br was to bond with Chlorine, Cl, what type of bond would they form? NONPOLAR COVALENT 74. If Hydrogen, H was to bond with Oxygen, O, what type of bond would they form? POLAR COVALENT

STUDY HARD, AND AS ALWAYS, MAY THE ODDS BE EVER IN YOUR FAVOR
 Chemistry semester 1 exam review answers
Chemistry semester 1 exam review answers Chemistry fall semester exam review answers
Chemistry fall semester exam review answers Chemistry semester exam review
Chemistry semester exam review If a laboratory fire erupts, immediately
If a laboratory fire erupts, immediately World history semester 1 final exam
World history semester 1 final exam Us history semester 2 final exam review
Us history semester 2 final exam review Zoology semester 1 exam review answers
Zoology semester 1 exam review answers Physics fall semester review answers
Physics fall semester review answers Semester 1 final exam study guide us history
Semester 1 final exam study guide us history English 3 fall semester exam review
English 3 fall semester exam review Dramatic irony def
Dramatic irony def Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry 3rd 9 weeks exam review chemistry
3rd 9 weeks exam review chemistry Ap gov review final exam review
Ap gov review final exam review English 4 semester 2 exam
English 4 semester 2 exam Pointing
Pointing Earth science semester 2 final exam answers
Earth science semester 2 final exam answers Algebra 1 final review packet
Algebra 1 final review packet Spanish final exam review packet answer key
Spanish final exam review packet answer key