AL CHEM REVIEW AL CHEM Written Practical Inorganic

![AL CHEM Written Practical [Inorganic Chemistry] ~ Preparation of Gases ~ dry Cl 2 AL CHEM Written Practical [Inorganic Chemistry] ~ Preparation of Gases ~ dry Cl 2](https://slidetodoc.com/presentation_image_h2/f7441f7397134c6056f427a90ff4d1ae/image-2.jpg)





- Slides: 18

AL CHEM REVIEW
![AL CHEM Written Practical Inorganic Chemistry Preparation of Gases dry Cl 2 AL CHEM Written Practical [Inorganic Chemistry] ~ Preparation of Gases ~ dry Cl 2](https://slidetodoc.com/presentation_image_h2/f7441f7397134c6056f427a90ff4d1ae/image-2.jpg)
AL CHEM Written Practical [Inorganic Chemistry] ~ Preparation of Gases ~ dry Cl 2 dry HCl dry NH 3 dry SO 2 drying agent used ? ? How to collect? ? (upward/downward delivery? ) p. 1

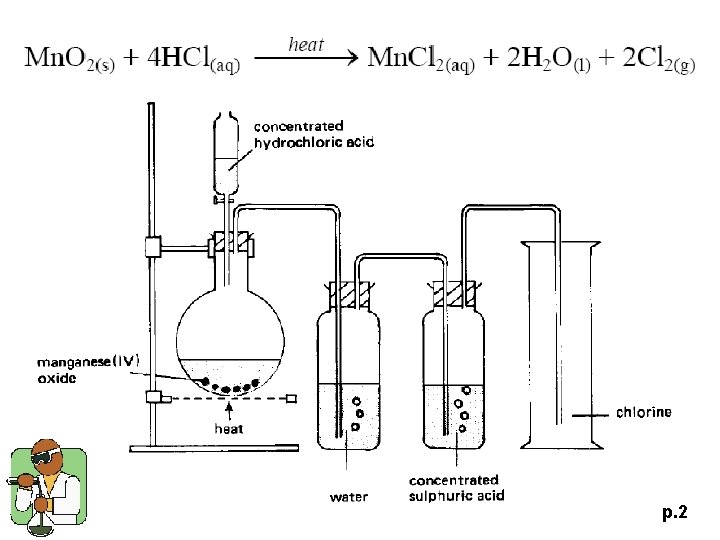
p. 2

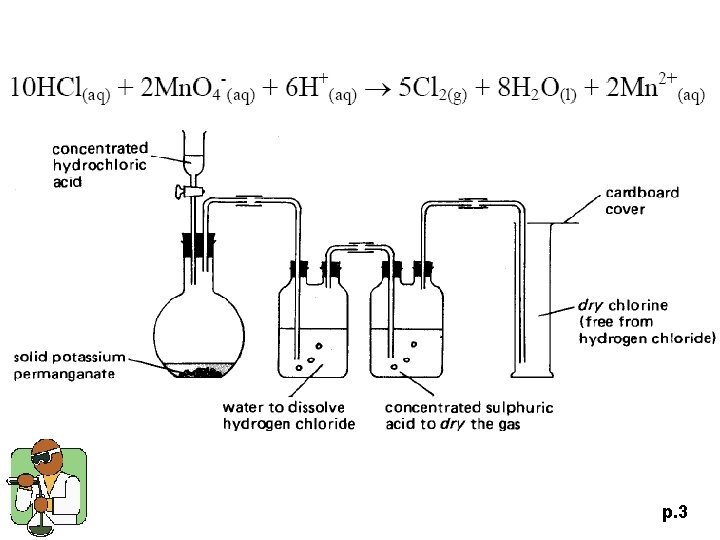
p. 3

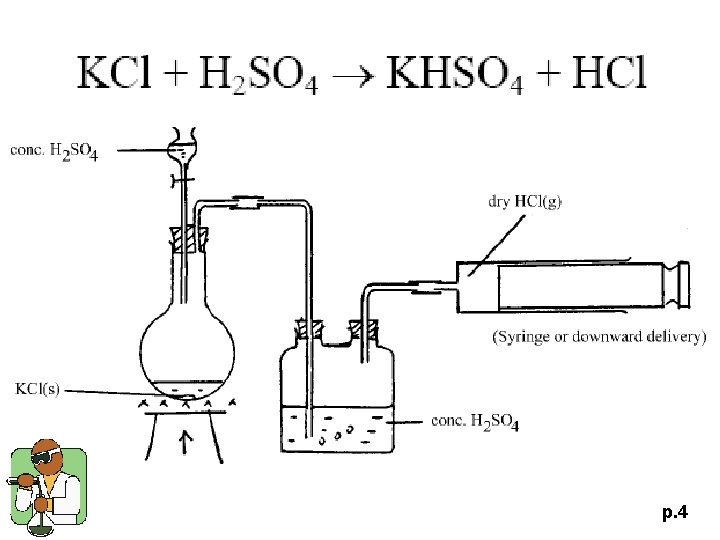
p. 4

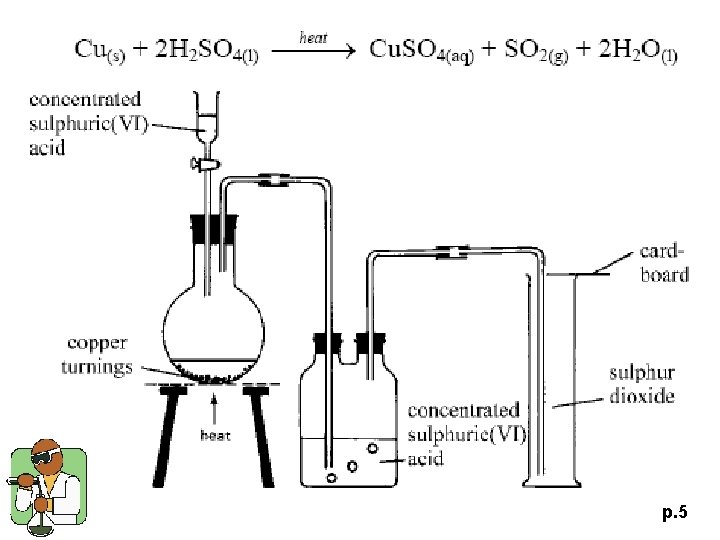
p. 5

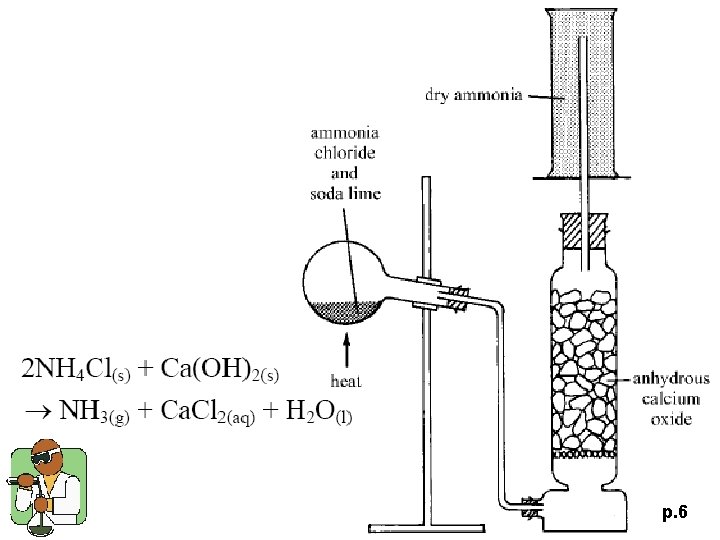
p. 6

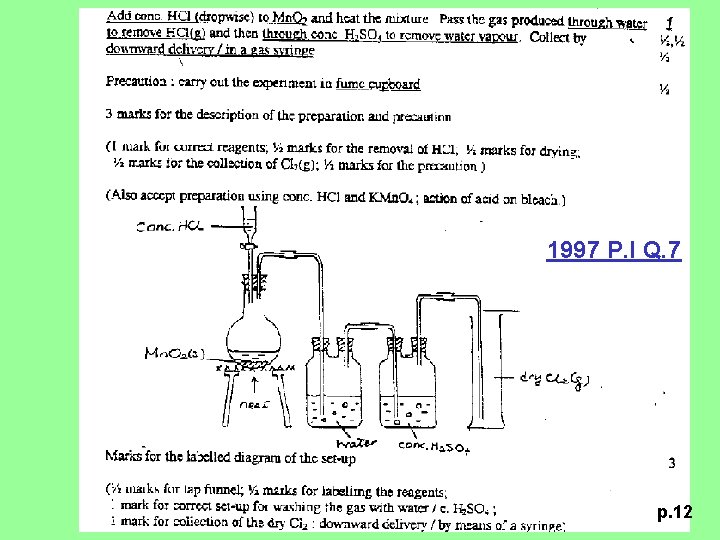
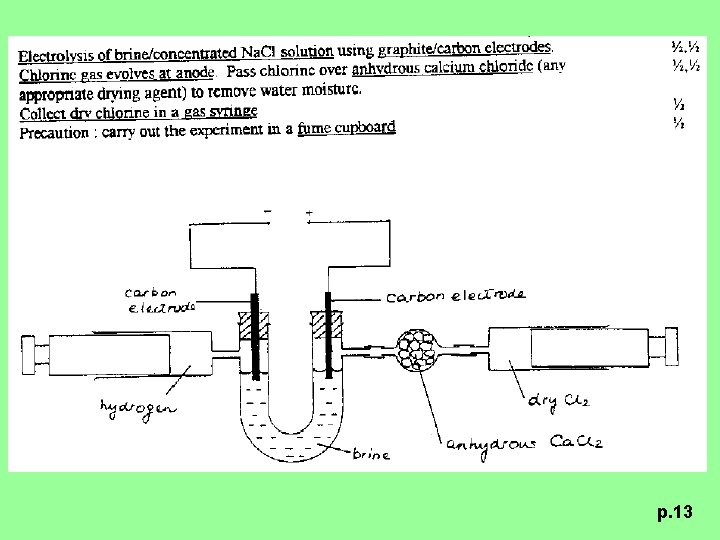
1994 P. II Q. 4 (a) Describe how a sample of dry chlorine is prepared in the laboratory. State the safety precaution(s) that is / are required. (5 M) p. 7

p. 8

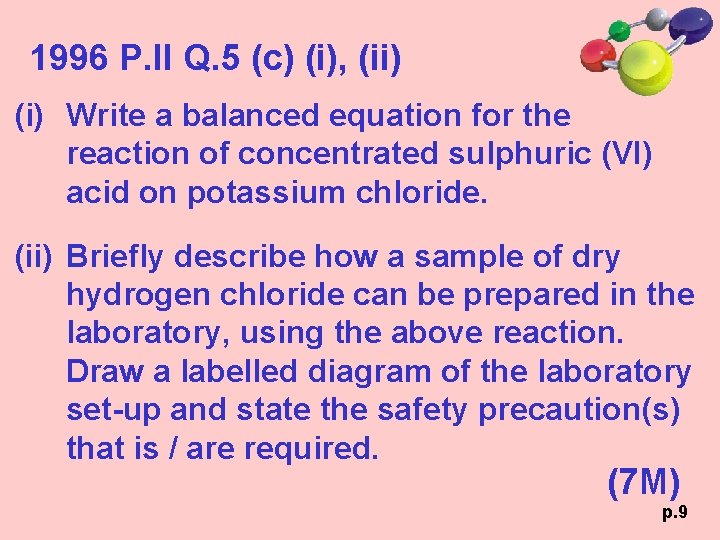
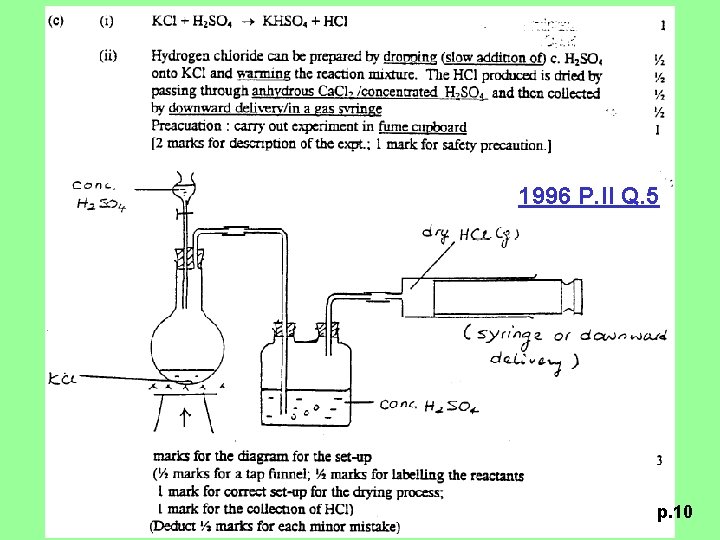
1996 P. II Q. 5 (c) (i), (ii) (i) Write a balanced equation for the reaction of concentrated sulphuric (VI) acid on potassium chloride. (ii) Briefly describe how a sample of dry hydrogen chloride can be prepared in the laboratory, using the above reaction. Draw a labelled diagram of the laboratory set-up and state the safety precaution(s) that is / are required. (7 M) p. 9

1996 P. II Q. 5 p. 10

1997 P. I Q. 7 (a) Briefly describe how a sample of dry chlorine gas can be prepared in the laboratory. Draw a labelled diagram of the laboratory set-up and state the safety precaution(s) that is / are required. (6 M) p. 11

1997 P. I Q. 7 p. 12

p. 13

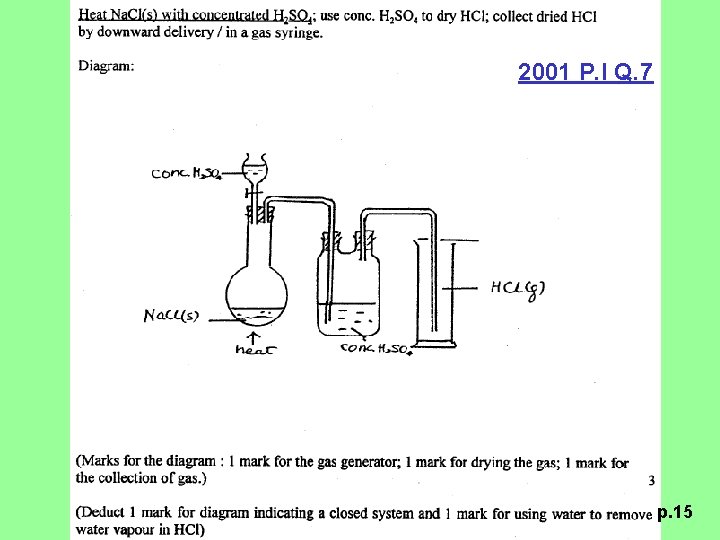
2001 P. I Q. 7 (a) Suggest how you would prepare a sample of dry hydrogen chloride gas in a school laboratory. Draw a labelled diagram of the set-up of apparatus used in the preparation. (4 M) p. 14

2001 P. I Q. 7 p. 15

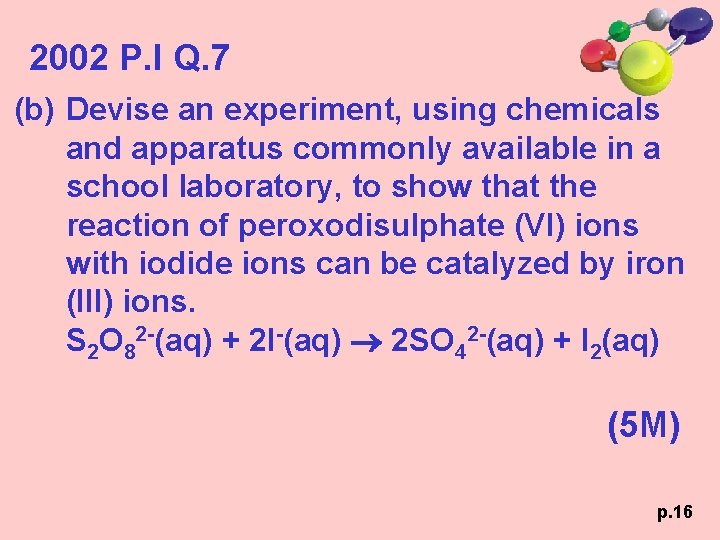
2002 P. I Q. 7 (b) Devise an experiment, using chemicals and apparatus commonly available in a school laboratory, to show that the reaction of peroxodisulphate (VI) ions with iodide ions can be catalyzed by iron (III) ions. S 2 O 82 -(aq) + 2 I-(aq) 2 SO 42 -(aq) + I 2(aq) (5 M) p. 16

2002 P. I Q. 7 p. 17
 Gen chem review for ochem
Gen chem review for ochem Ap chem equilibrium review
Ap chem equilibrium review Chapter 20 review electrochemistry
Chapter 20 review electrochemistry Regents earth science lab practical
Regents earth science lab practical Smear layer in dentistry
Smear layer in dentistry Organic and inorganic compounds experiment
Organic and inorganic compounds experiment Inorganic plant
Inorganic plant Pharmaceutical inorganic chemistry introduction
Pharmaceutical inorganic chemistry introduction Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Organic vs inorganic molecules
Organic vs inorganic molecules Which compound is inorganic
Which compound is inorganic Introduction to organic chemistry
Introduction to organic chemistry Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules What is inorganic matter
What is inorganic matter Inorganic mineral definition
Inorganic mineral definition Organic and inorganic cofactors
Organic and inorganic cofactors Organic vs inorganic compounds
Organic vs inorganic compounds Prosthetic group example
Prosthetic group example Bottle gum method
Bottle gum method