Inorganic Chemistry Professor Pickett Inorganic Reaction Mechanisms Introduction




![kobs Hexane Methanol [Y] = NHMe 2 • The methanol can attack the metal kobs Hexane Methanol [Y] = NHMe 2 • The methanol can attack the metal](https://slidetodoc.com/presentation_image_h2/7b6fed57123016f29898ba8aba705cd1/image-12.jpg)

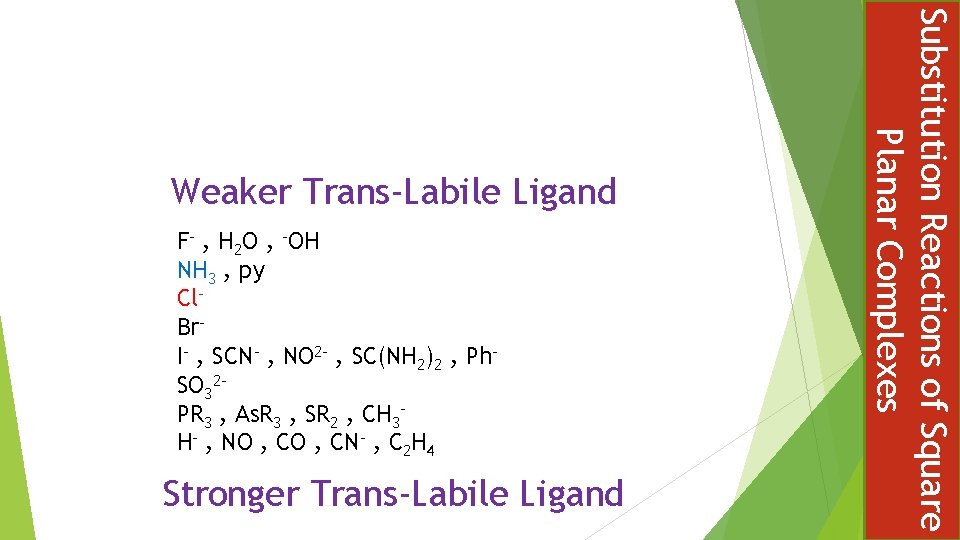





![Briefly discuss the following. [15%] (i) The influence of the leaving group in substitution Briefly discuss the following. [15%] (i) The influence of the leaving group in substitution](https://slidetodoc.com/presentation_image_h2/7b6fed57123016f29898ba8aba705cd1/image-34.jpg)

![Discuss the mechanism of substitution of [Co(NH 3)5 Cl] + by hydroxide. What happens Discuss the mechanism of substitution of [Co(NH 3)5 Cl] + by hydroxide. What happens](https://slidetodoc.com/presentation_image_h2/7b6fed57123016f29898ba8aba705cd1/image-36.jpg)
![Substitution one dinitrogen ligand of trans-[Mo(N 2)2(dppe)2)] by Me. CN to give trans-[Mo(N 2)Me. Substitution one dinitrogen ligand of trans-[Mo(N 2)2(dppe)2)] by Me. CN to give trans-[Mo(N 2)Me.](https://slidetodoc.com/presentation_image_h2/7b6fed57123016f29898ba8aba705cd1/image-38.jpg)





![Extremely slow due to change in multiplicity of the system [Co(NH 3)6]2+ + Extremely slow due to change in multiplicity of the system [Co(NH 3)6]2+ +](https://slidetodoc.com/presentation_image_h2/7b6fed57123016f29898ba8aba705cd1/image-63.jpg)

![Cross reactions and the Marcus Equation k 1, 1 [MLn]y+ + [MLn]y+ K 1, Cross reactions and the Marcus Equation k 1, 1 [MLn]y+ + [MLn]y+ K 1,](https://slidetodoc.com/presentation_image_h2/7b6fed57123016f29898ba8aba705cd1/image-66.jpg)
![Past Exam Question What is the trans-effect and how does it operate? [25%] Past Past Exam Question What is the trans-effect and how does it operate? [25%] Past](https://slidetodoc.com/presentation_image_h2/7b6fed57123016f29898ba8aba705cd1/image-71.jpg)
![Past Exam Question What is the trans-effect and how does it operate? [25%] Past Past Exam Question What is the trans-effect and how does it operate? [25%] Past](https://slidetodoc.com/presentation_image_h2/7b6fed57123016f29898ba8aba705cd1/image-72.jpg)


- Slides: 76

Inorganic Chemistry Professor Pickett

Inorganic Reaction Mechanisms Introduction Substitution Reactions of Octahedral Complexes Insertion Reactions Case Study of an Inorganic Reaction Mechanism Substitution Reactions of Tetrahedral Complexes Electron Transfer Reactions Introduction Substitution Reactions of Square Planar Complexes

EVERYONE SAY THIS OUT ALOUD TOGETHER THE EFFECT OF A LIGAND, UPON THE RATE OF LIGAND REPLACEMENT, OF THE GROUP TRANS TO ITSELF Introduction THE TRANS EFFECT

The Kinetic Model Introduction

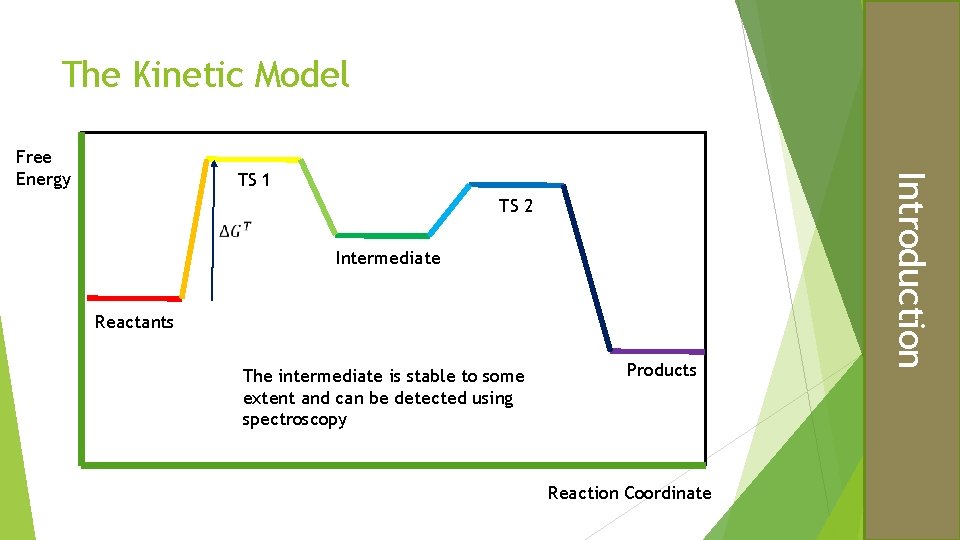
The Kinetic Model TS 1 TS 2 Intermediate Reactants The intermediate is stable to some extent and can be detected using spectroscopy Products Reaction Coordinate Introduction Free Energy

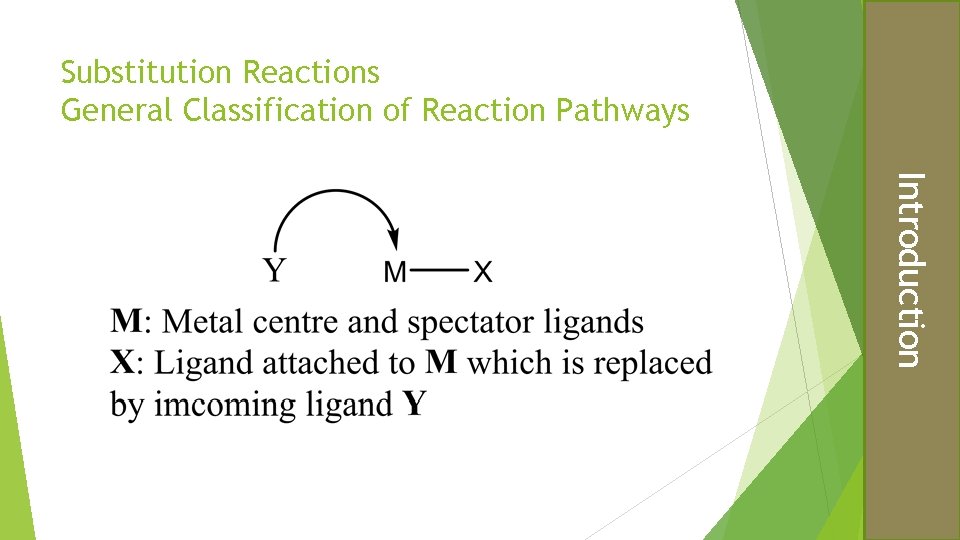
Substitution Reactions General Classification of Reaction Pathways Introduction

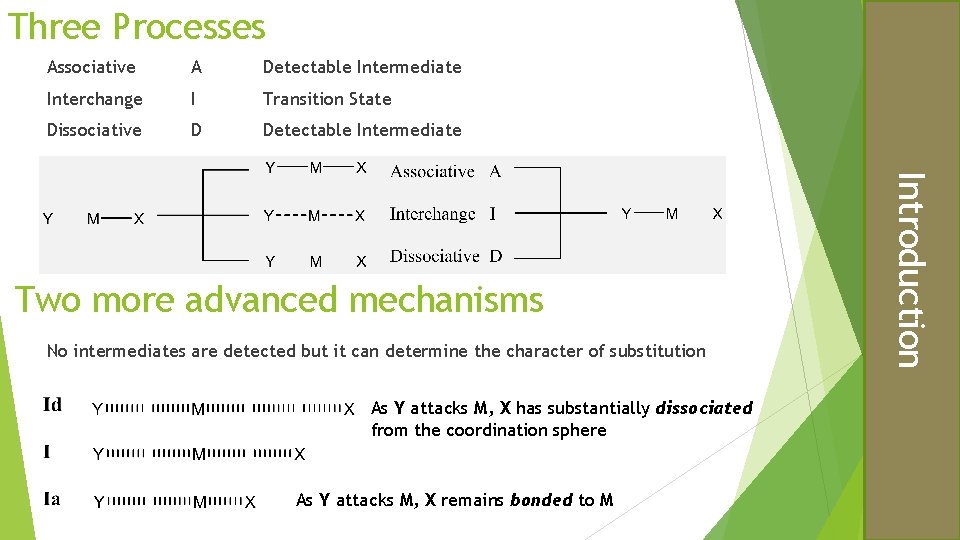
Three Processes Associative A Detectable Intermediate Interchange I Transition State Dissociative D Detectable Intermediate No intermediates are detected but it can determine the character of substitution As Y attacks M, X has substantially dissociated from the coordination sphere As Y attacks M, X remains bonded to M Introduction Two more advanced mechanisms

Introduction Substitution Reactions of Square Planar Complexes Substitution Reactions of Octahedral Complexes Insertion Reactions Case Study of an Inorganic Reaction Mechanism Substitution Reactions of Tetrahedral Complexes Electron Transfer Reactions Substitution Reactions of Square Planar Complexes Inorganic Reaction Mechanisms

THE TRANS EFFECT THE EFFECT OF A LIGAND, UPON THE RATE OF LIGAND REPLACEMENT, OF THE GROUP TRANS TO ITSELF Substitution Reactions of Square Planar Complexes EVERYONE SAY THIS OUT ALOUD TOGETHER

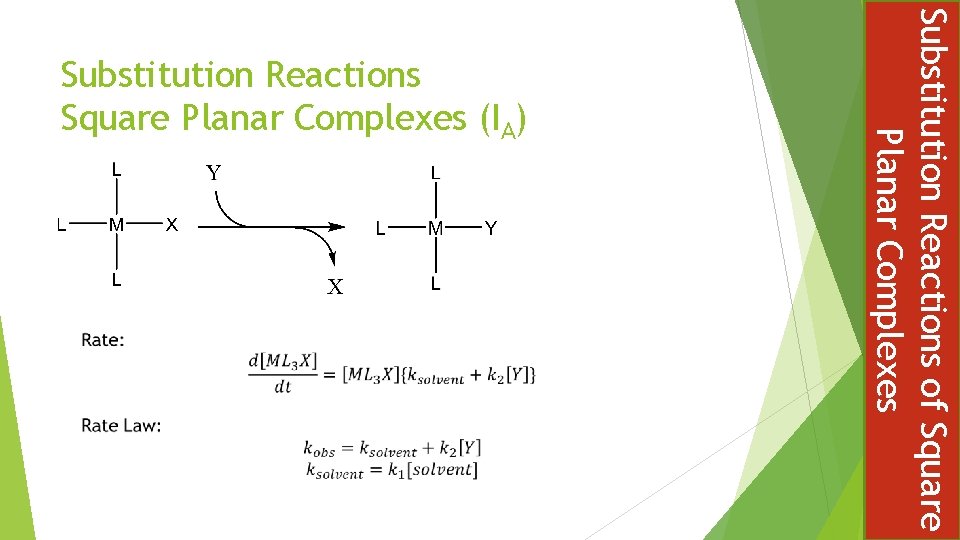
Substitution Reactions of Square Planar Complexes Substitution Reactions Square Planar Complexes (IA)

STEREOCHEMISTRY IS CONSERVED IN BOTH PATHWAYS Substitution Reactions of Square Planar Complexes Due to the associative mechanism
![kobs Hexane Methanol Y NHMe 2 The methanol can attack the metal kobs Hexane Methanol [Y] = NHMe 2 • The methanol can attack the metal](https://slidetodoc.com/presentation_image_h2/7b6fed57123016f29898ba8aba705cd1/image-12.jpg)
kobs Hexane Methanol [Y] = NHMe 2 • The methanol can attack the metal centre due to the lone pair on oxygen • The concentration of methanol does not effect the observed rate • Hexane can not coordinate Substitution Reactions of Square Planar Complexes Role of solvent

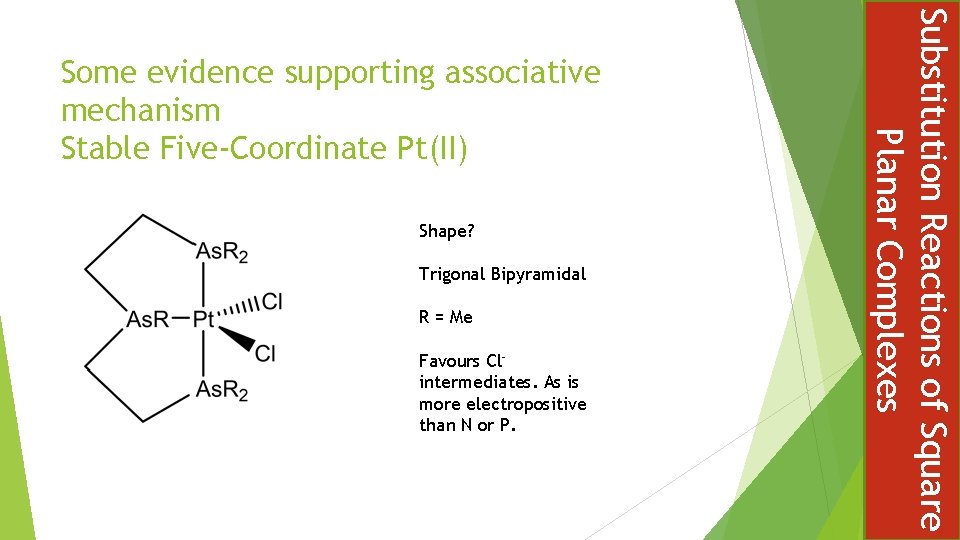
Shape? Trigonal Bipyramidal R = Me Favours Clintermediates. As is more electropositive than N or P. Substitution Reactions of Square Planar Complexes Some evidence supporting associative mechanism Stable Five-Coordinate Pt(II)

Test to whether the solvent has a role in the substitution In water, trap the intermediate using a base. Hydroxyl ligand is formed Substitution Reactions of Square Planar Complexes Evidence for solvent role

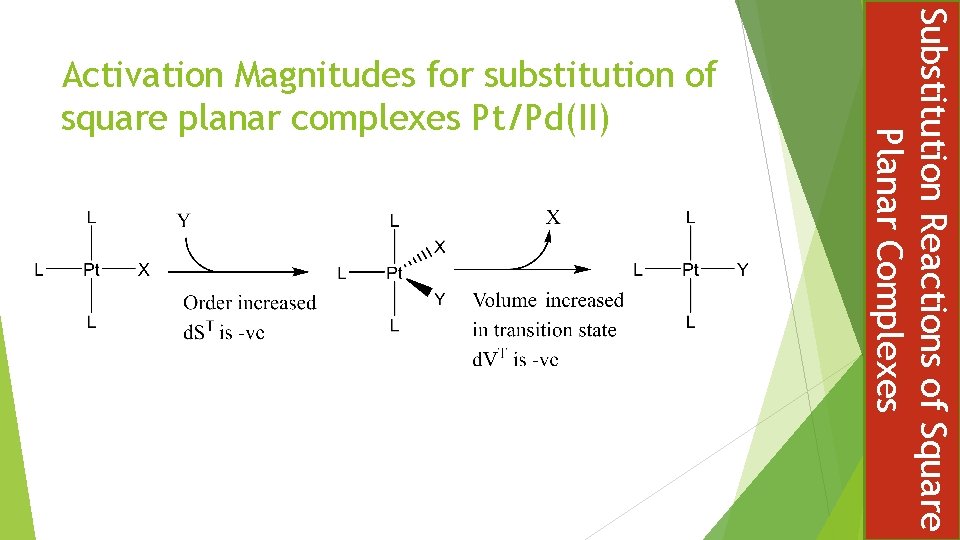
Substitution Reactions of Square Planar Complexes Activation Magnitudes for substitution of square planar complexes Pt/Pd(II)

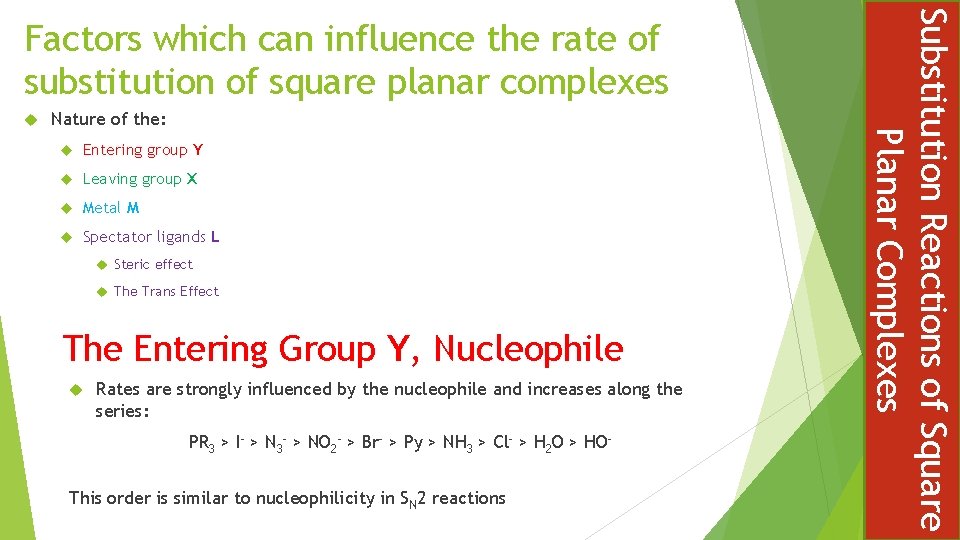
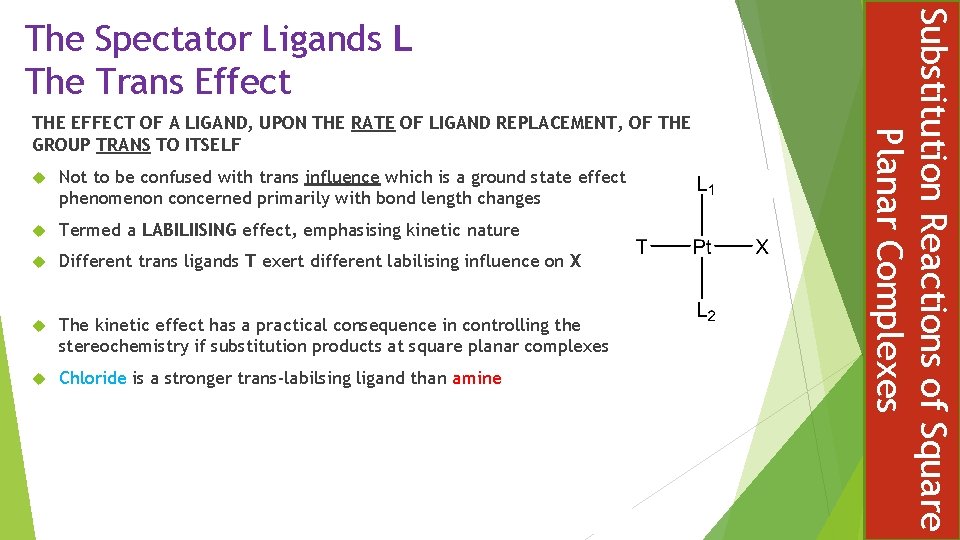
Nature of the: Entering group Y Leaving group X Metal M Spectator ligands L Steric effect The Trans Effect The Entering Group Y, Nucleophile Rates are strongly influenced by the nucleophile and increases along the series: PR 3 > I- > N 3 - > NO 2 - > Br- > Py > NH 3 > Cl- > H 2 O > HO- This order is similar to nucleophilicity in SN 2 reactions Substitution Reactions of Square Planar Complexes Factors which can influence the rate of substitution of square planar complexes

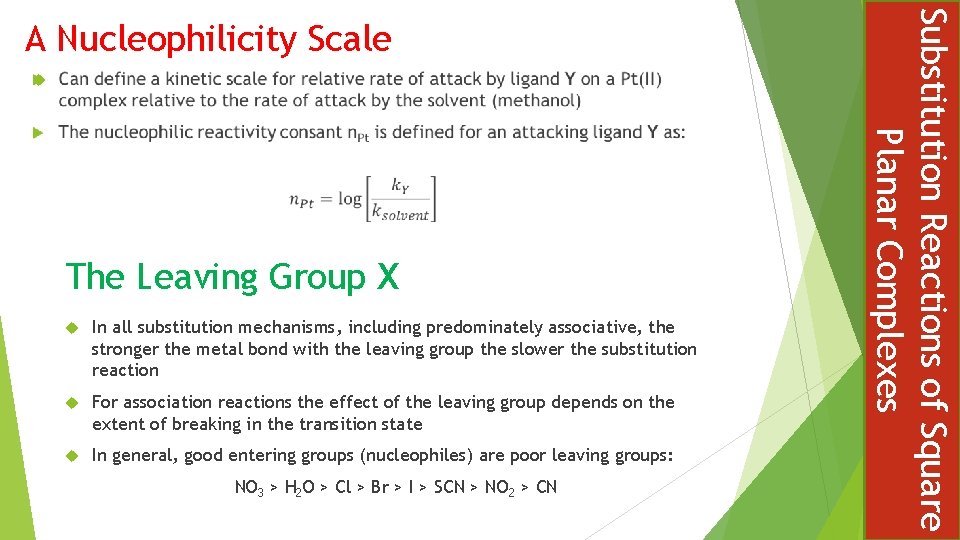
The Leaving Group X In all substitution mechanisms, including predominately associative, the stronger the metal bond with the leaving group the slower the substitution reaction For association reactions the effect of the leaving group depends on the extent of breaking in the transition state In general, good entering groups (nucleophiles) are poor leaving groups: NO 3 > H 2 O > Cl > Br > I > SCN > NO 2 > CN Substitution Reactions of Square Planar Complexes A Nucleophilicity Scale

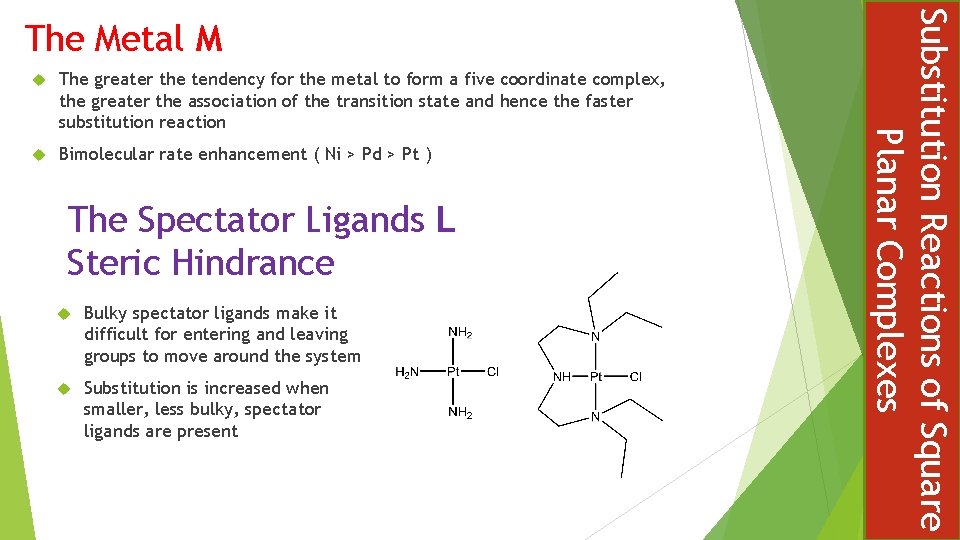
The greater the tendency for the metal to form a five coordinate complex, the greater the association of the transition state and hence the faster substitution reaction Bimolecular rate enhancement ( Ni > Pd > Pt ) The Spectator Ligands L Steric Hindrance Bulky spectator ligands make it difficult for entering and leaving groups to move around the system Substitution is increased when smaller, less bulky, spectator ligands are present Substitution Reactions of Square Planar Complexes The Metal M

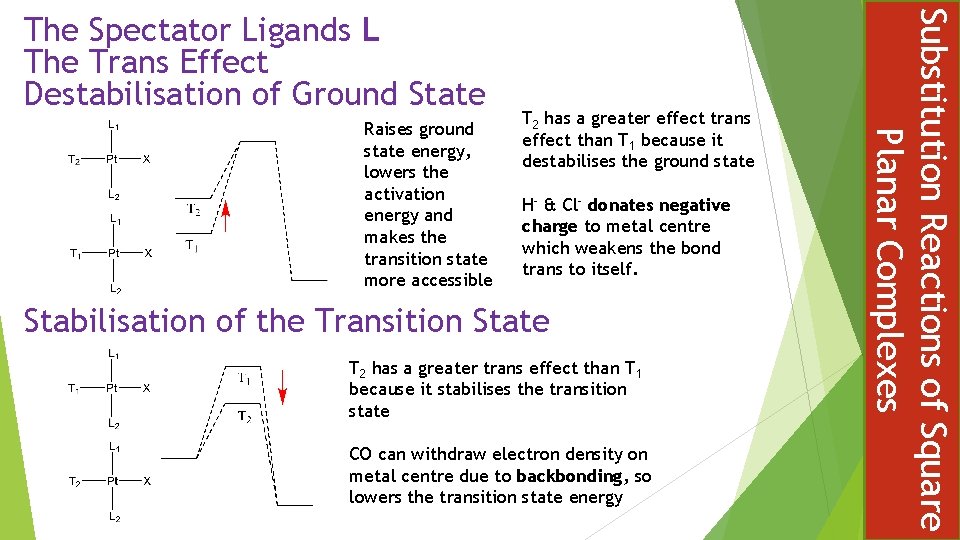
THE EFFECT OF A LIGAND, UPON THE RATE OF LIGAND REPLACEMENT, OF THE GROUP TRANS TO ITSELF Not to be confused with trans influence which is a ground state effect phenomenon concerned primarily with bond length changes Termed a LABILIISING effect, emphasising kinetic nature Different trans ligands T exert different labilising influence on X The kinetic effect has a practical consequence in controlling the stereochemistry if substitution products at square planar complexes Chloride is a stronger trans-labilsing ligand than amine Substitution Reactions of Square Planar Complexes The Spectator Ligands L The Trans Effect
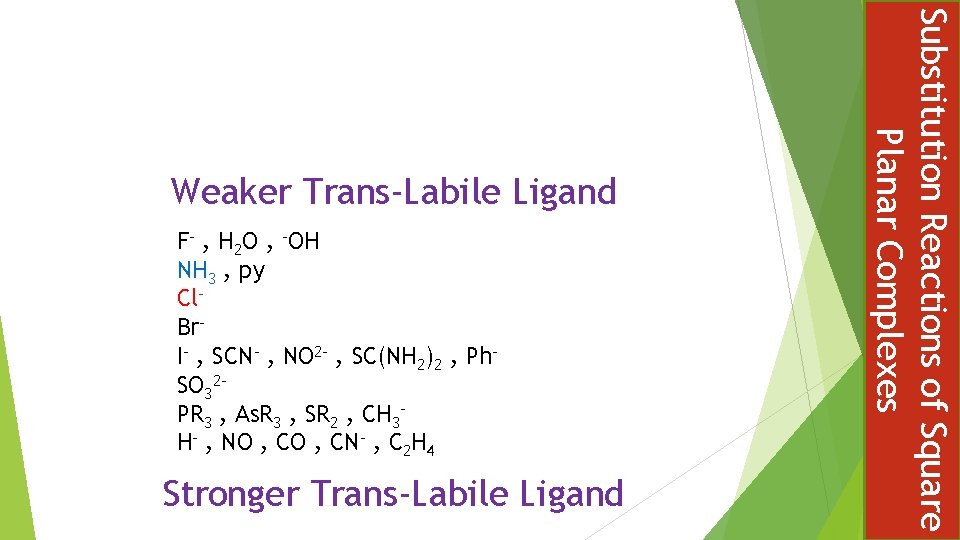
F- , H 2 O , -OH NH 3 , py Cl. Br. I- , SCN- , NO 2 - , SC(NH 2)2 , Ph. SO 32 PR 3 , As. R 3 , SR 2 , CH 3 H- , NO , CN- , C 2 H 4 Stronger Trans-Labile Ligand Substitution Reactions of Square Planar Complexes Weaker Trans-Labile Ligand

Raises ground state energy, lowers the activation energy and makes the transition state more accessible T 2 has a greater effect trans effect than T 1 because it destabilises the ground state H- & Cl- donates negative charge to metal centre which weakens the bond trans to itself. Stabilisation of the Transition State T 2 has a greater trans effect than T 1 because it stabilises the transition state CO can withdraw electron density on metal centre due to backbonding, so lowers the transition state energy Substitution Reactions of Square Planar Complexes The Spectator Ligands L The Trans Effect Destabilisation of Ground State

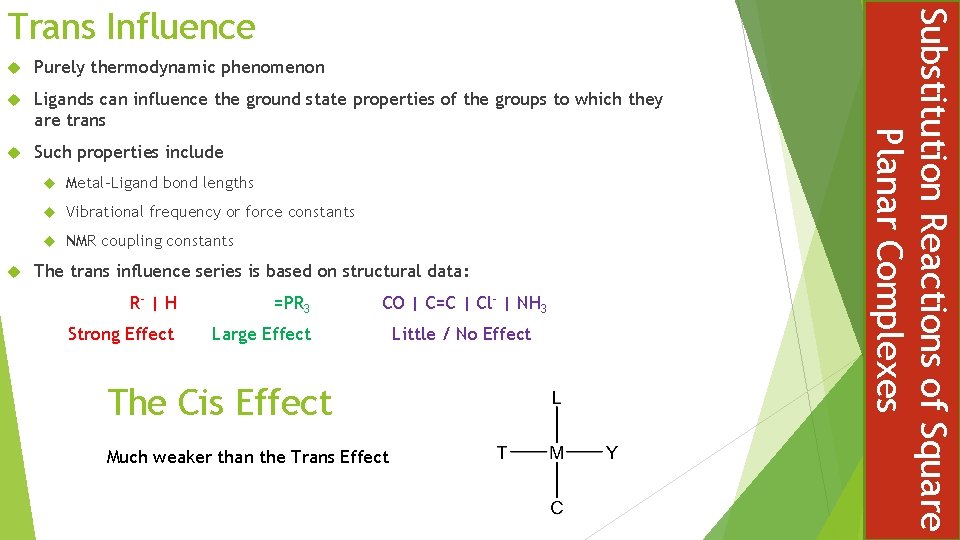
Purely thermodynamic phenomenon Ligands can influence the ground state properties of the groups to which they are trans Such properties include Metal-Ligand bond lengths Vibrational frequency or force constants NMR coupling constants The trans influence series is based on structural data: R- | H =PR 3 Strong Effect Large Effect CO | C=C | Cl- | NH 3 The Cis Effect Much weaker than the Trans Effect Little / No Effect Substitution Reactions of Square Planar Complexes Trans Influence

Define the trans effect. 10% Definition ‘The effect of a ligand upon the rate of ligand replacement of the group trans to itself, primarily refers to substitution at group VIII M(II) square planar complexes ’ A simple diagram would aid definition. Substitution Reactions of Square Planar Complexes Test Question

Hydride and carbon monoxide are both strong trans effect ligands, discuss how their trans effect operates. 10% Hydride lowers the activation energy for subsitution at eg Pt(II) by destabilising the ground state, it places more electron density onto the metal centre, weakening the bond trans to it moreso than does eg Cl -. Carbon monoxide lowers the activation energy by stabilising the TS. It relieves the increased electron density brought in by the lone pair/negative charge on the incoming ligand by accepting this into pi * anti bonding orbitals. Substitution Reactions of Square Planar Complexes Test Question

Briefly discuss how the trans effect can provide a means of kinetically controlling the nature of the isomer formation in Pt(II) substitution chemistry. [10%] The selective formation of cis or trans isomers of [Pt. Cl 2(NH 3)2] can controlled by either starting with [Pt (NH 3)4]2+ and reacting with Cl- which gives exclusively the trans-product or by starting with [Pt Cl 4]2 - whch gives the cisproduct. This is a consequence of Cl- being a greater trans effect than has NH 3. Scheme as in notes would aid clarity. Substitution Reactions of Square Planar Complexes Test Question

Introduction Substitution Reactions of Square Planar Complexes Substitution Reactions of Octahedral Complexes Insertion Reactions Case Study of an Inorganic Reaction Mechanism Substitution Reactions of Tetrahedral Complexes Electron Transfer Reactions Substitution Reactions of Octahedral Complexes Inorganic Reaction Mechanisms

THE TRANS EFFECT THE EFFECT OF A LIGAND, UPON THE RATE OF LIGAND REPLACEMENT, OF THE GROUP TRANS TO ITSELF Substitution Reactions of Octahedral Complexes EVERYONE SAY THIS OUT ALOUD TOGETHER

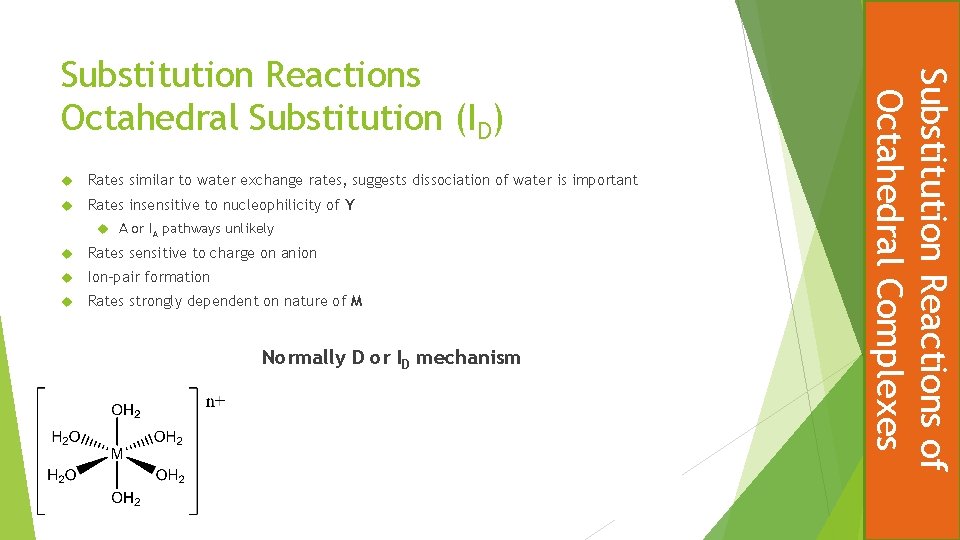
Rates similar to water exchange rates, suggests dissociation of water is important Rates insensitive to nucleophilicity of Y A or IA pathways unlikely Rates sensitive to charge on anion Ion-pair formation Rates strongly dependent on nature of M Normally D or ID mechanism Substitution Reactions of Octahedral Complexes Substitution Reactions Octahedral Substitution (ID)

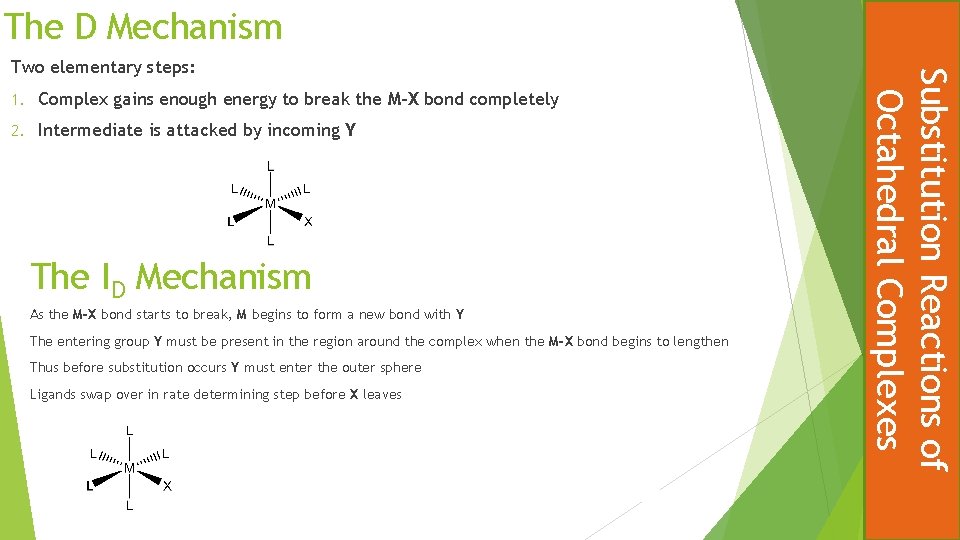
The D Mechanism 1. Complex gains enough energy to break the M-X bond completely 2. Intermediate is attacked by incoming Y The ID Mechanism As the M-X bond starts to break, M begins to form a new bond with Y The entering group Y must be present in the region around the complex when the M-X bond begins to lengthen Thus before substitution occurs Y must enter the outer sphere Ligands swap over in rate determining step before X leaves Substitution Reactions of Octahedral Complexes Two elementary steps:

Nature of the: Entering group Y Metal M Leaving group X Spectator ligands L Electronic Effects Jahn-Teller Effect The Entering Group Y Expect that rate of a dissociative substitution would be insensitive to the entering group Y By varying the nature of the entering group the effect is much smaller The Metal M The relatively small 3 d transition metals do not sterically easily accommodate 7 coordination, D or ID mechanisms dominate their substitution chemistry Substitution Reactions of Octahedral Complexes Factors which can influence the rate of substitution of octahedral complexes

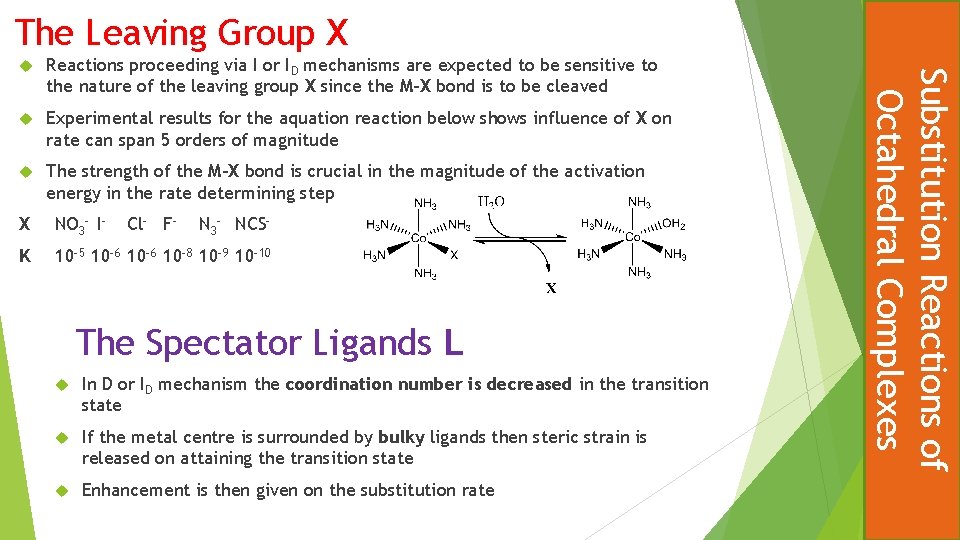
The Leaving Group X Reactions proceeding via I or ID mechanisms are expected to be sensitive to the nature of the leaving group X since the M-X bond is to be cleaved Experimental results for the aquation reaction below shows influence of X on rate can span 5 orders of magnitude The strength of the M-X bond is crucial in the magnitude of the activation energy in the rate determining step X NO 3 - I- Cl- F- N 3 - NCS- K 10 -5 10 -6 10 -8 10 -9 10 -10 The Spectator Ligands L In D or ID mechanism the coordination number is decreased in the transition state If the metal centre is surrounded by bulky ligands then steric strain is released on attaining the transition state Enhancement is then given on the substitution rate Substitution Reactions of Octahedral Complexes

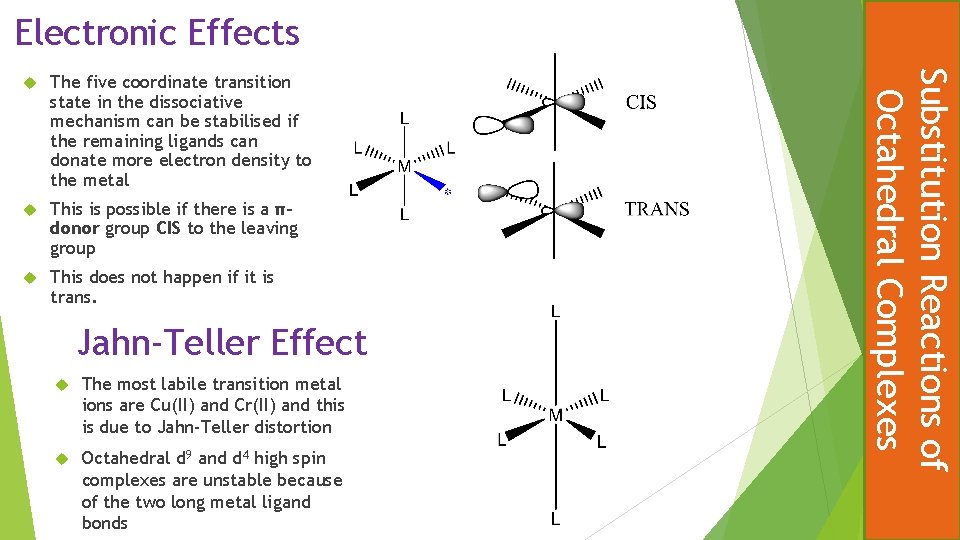
Electronic Effects The five coordinate transition state in the dissociative mechanism can be stabilised if the remaining ligands can donate more electron density to the metal This is possible if there is a πdonor group CIS to the leaving group This does not happen if it is trans. Jahn-Teller Effect The most labile transition metal ions are Cu(II) and Cr(II) and this is due to Jahn-Teller distortion Octahedral d 9 and d 4 high spin complexes are unstable because of the two long metal ligand bonds Substitution Reactions of Octahedral Complexes

Base Hydrolysis Replacement of ligand by HO- Often faster than acid hydrolysis Sensitive to nature of entering group Only observed when deprotonatable group present First step is removal of proton from acidic ligand Dissociative Conjugate Base Mechanism (DCB) Garrick Mechanism Addition of charge makes it easier to remove the chloride Substitution Reactions of Octahedral Complexes
![Briefly discuss the following 15 i The influence of the leaving group in substitution Briefly discuss the following. [15%] (i) The influence of the leaving group in substitution](https://slidetodoc.com/presentation_image_h2/7b6fed57123016f29898ba8aba705cd1/image-34.jpg)
Briefly discuss the following. [15%] (i) The influence of the leaving group in substitution reactions of (a) square planar complexes (b) first transition series octahedral complexes A metal ligand bond begins breaking in the TBP TS therefore the stronger this bond the slower is the substitution reaction. For example NO 3 - is weakly bound, it is a labile ligand its complexes Pt(II) are rapidly substituted whereas SCN- is tightly bound and its corresponding substitution reactions are very slow. Again bond breaking takes place in the TS the stronger the M-X bond the slower is the substitution reaction. For example, [Co(NH) 5 X]2+ aquates 5 orders of magnitude faster for X = NO 3 - than for SCN- Substitution Reactions of Octahedral Complexes Test Question

Briefly discuss how the steric properties of spectator ligands can influence the rate of substitution of (a) Octahedral and (b) Square planar complexes [10%] As TS becomes less crowded by departure of leaving group then steric strain relieved in the GS is relieved. This leads to a lower activation energy and faster substitution kinetics. An example would aid discussion. The converse to above, higher steric strain in TS on going from 4 to 5 coordinate can raise activation energy decreasing substitution rate. Substitution Reactions of Octahedral Complexes Test Question
![Discuss the mechanism of substitution of CoNH 35 Cl by hydroxide What happens Discuss the mechanism of substitution of [Co(NH 3)5 Cl] + by hydroxide. What happens](https://slidetodoc.com/presentation_image_h2/7b6fed57123016f29898ba8aba705cd1/image-36.jpg)
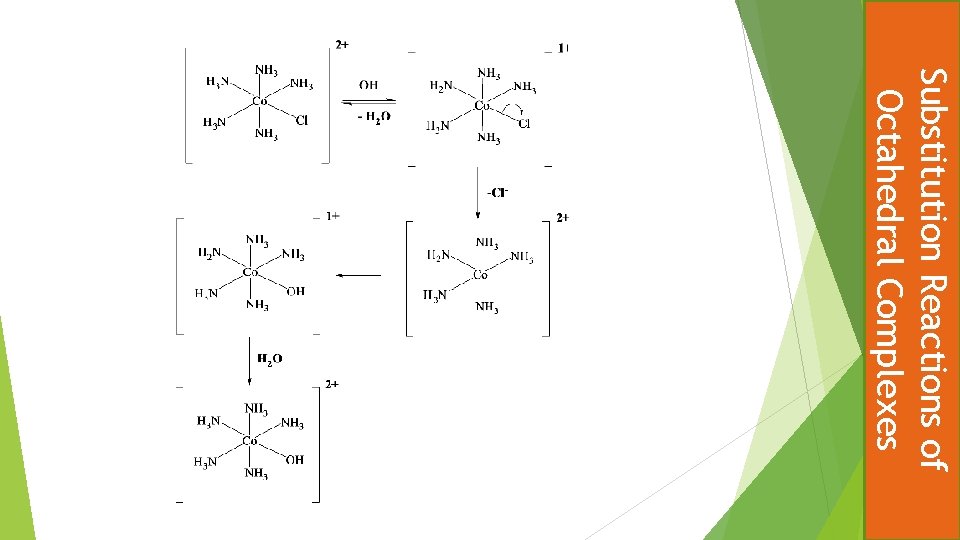
Discuss the mechanism of substitution of [Co(NH 3)5 Cl] + by hydroxide. What happens if another ligand such as SCN - is present when hydroxide is added to the aqueous solution of the complex? Garrick Mechanism, Dissociative, conjugate base mechanism D-CB. A key point is that substitution shows first order dependence on complex and first-order on [OH]-concentrations, ie overall 2 nd order kinetics. This is explained by hydroxide removing a proton from an ‘acidic’ NH 3 ligand. This decreases the net charge on the complex making the electrostatic separation of Cl - from an 1+ charged complex easier (faster) than from the 2+ charged parent complex. Following Cl- dissociation hydroxide attacks the five coordinated intermediate. In the presence of SCN- competive quenching of this intermediate can occur giving both OH- and SCN- substitution products after the re-protonation step. A scheme as below would aid discussion. Substitution Reactions of Octahedral Complexes Test Question

Substitution Reactions of Octahedral Complexes
![Substitution one dinitrogen ligand of transMoN 22dppe2 by Me CN to give transMoN 2Me Substitution one dinitrogen ligand of trans-[Mo(N 2)2(dppe)2)] by Me. CN to give trans-[Mo(N 2)Me.](https://slidetodoc.com/presentation_image_h2/7b6fed57123016f29898ba8aba705cd1/image-38.jpg)
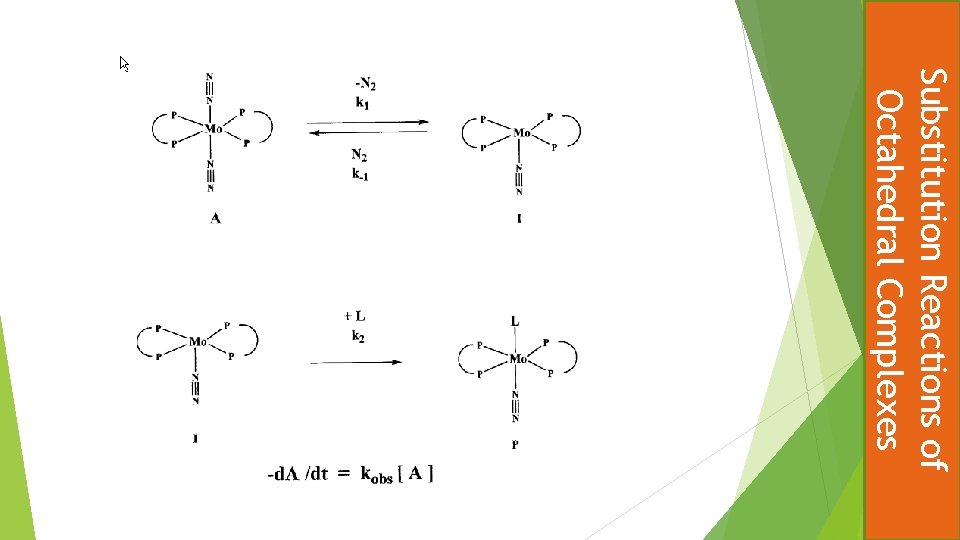
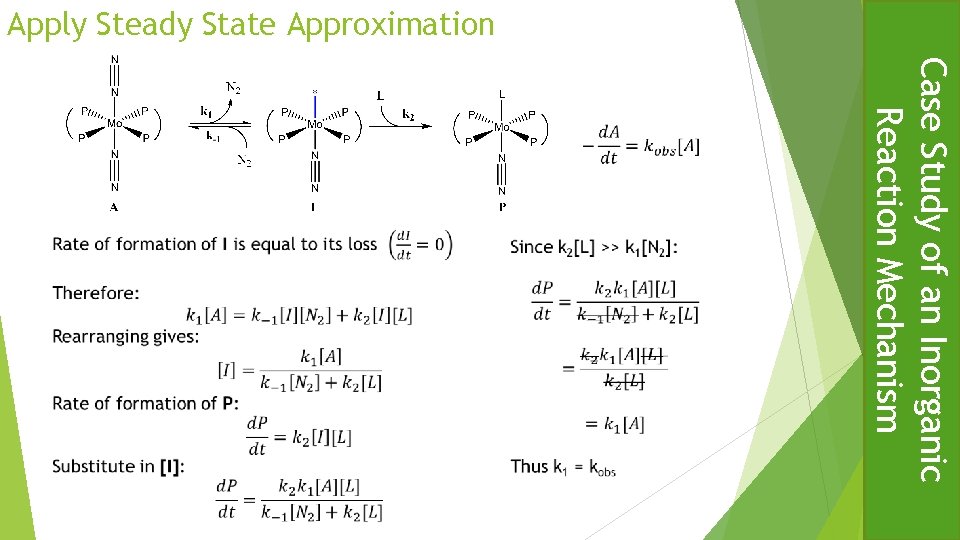
Substitution one dinitrogen ligand of trans-[Mo(N 2)2(dppe)2)] by Me. CN to give trans-[Mo(N 2)Me. CN)(dppe)2)] shows a first order dependence on the concentration of the complex and a zero order dependence on concentration of the incoming ligand under pseudo-first order conditions. Use the steady state approximation to derive an expression for the rate equation consistent with these observations and a mechanism involving loss of dinitrogen as the rds. Briefly explain why the rate of alkylation of dinitrogen by Me. Br in the same complex to give a diazenide shows identical kinetics. 25% Substitution Reactions of Octahedral Complexes Test Question

Substitution Reactions of Octahedral Complexes

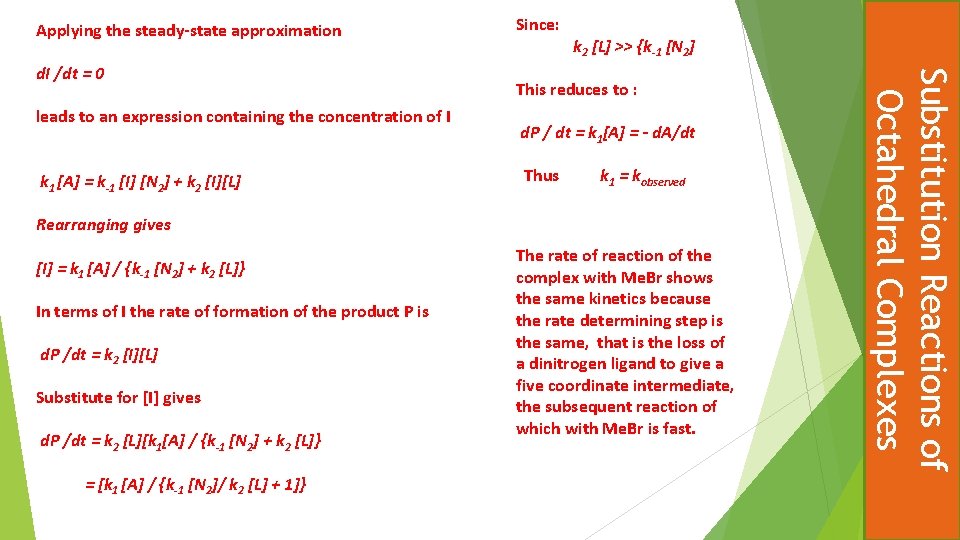
Applying the steady-state approximation leads to an expression containing the concentration of I k 1 [A] = k-1 [I] [N 2] + k 2 [I][L] k 2 [L] >> {k-1 [N 2] This reduces to : d. P / dt = k 1[A] = - d. A/dt Thus k 1 = kobserved Rearranging gives [I] = k 1 [A] / {k-1 [N 2] + k 2 [L]} In terms of I the rate of formation of the product P is d. P /dt = k 2 [I][L] Substitute for [I] gives d. P /dt = k 2 [L][k 1[A] / {k-1 [N 2] + k 2 [L]} = [k 1 [A] / {k-1 [N 2]/ k 2 [L] + 1]} The rate of reaction of the complex with Me. Br shows the same kinetics because the rate determining step is the same, that is the loss of a dinitrogen ligand to give a five coordinate intermediate, the subsequent reaction of which with Me. Br is fast. Substitution Reactions of Octahedral Complexes d. I /dt = 0 Since:

Introduction Substitution Reactions of Square Planar Complexes Substitution Reactions of Octahedral Complexes Insertion Reactions Case Study of an Inorganic Reaction Mechanism Substitution Reactions of Tetrahedral Complexes Electron Transfer Reactions Insertion Reactions Inorganic Reaction Mechanisms

THE TRANS EFFECT THE EFFECT OF A LIGAND, UPON THE RATE OF LIGAND REPLACEMENT, OF THE GROUP TRANS TO ITSELF Insertion Reactions EVERYONE SAY THIS OUT ALOUD TOGETHER

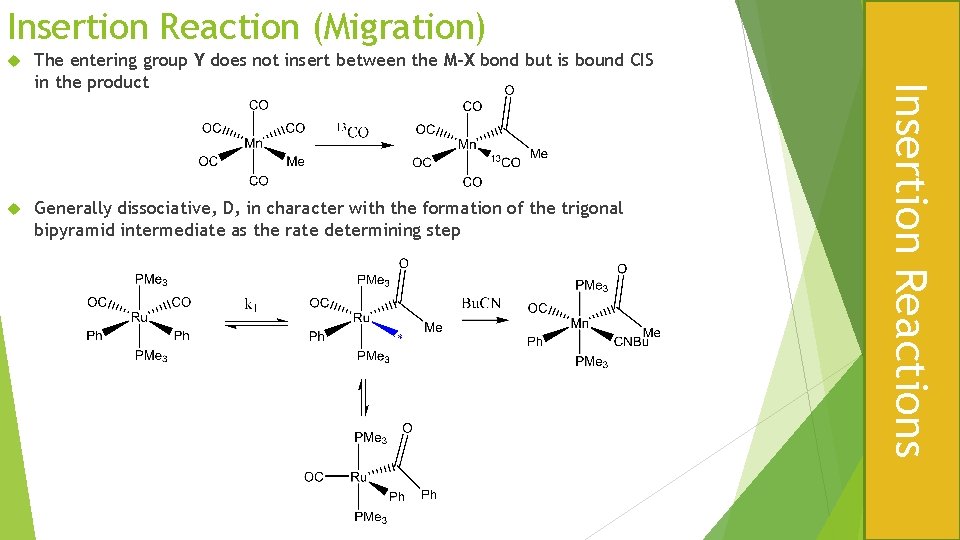
Insertion Reaction (Migration) The entering group Y does not insert between the M-X bond but is bound CIS in the product Generally dissociative, D, in character with the formation of the trigonal bipyramid intermediate as the rate determining step Insertion Reactions

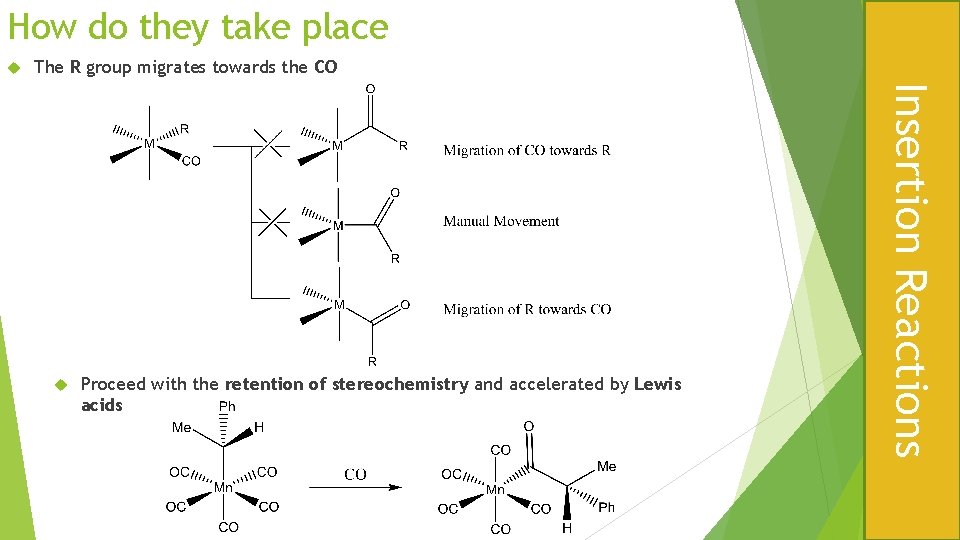
How do they take place The R group migrates towards the CO Proceed with the retention of stereochemistry and accelerated by Lewis acids Insertion Reactions

Introduction Substitution Reactions of Square Planar Complexes Substitution Reactions of Octahedral Complexes Insertion Reactions Case Study of an Inorganic Reaction Mechanism Substitution Reactions of Tetrahedral Complexes Electron Transfer Reactions Case Study of an Inorganic Reaction Mechanisms

THE TRANS EFFECT THE EFFECT OF A LIGAND, UPON THE RATE OF LIGAND REPLACEMENT, OF THE GROUP TRANS TO ITSELF Case Study of an Inorganic Reaction Mechanism EVERYONE SAY THIS OUT ALOUD TOGETHER

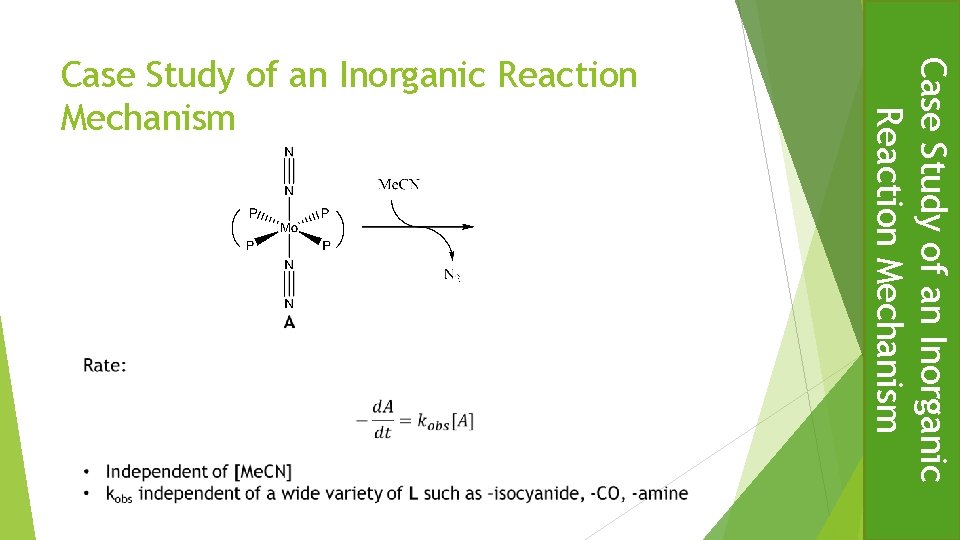
Case Study of an Inorganic Reaction Mechanism

Apply Steady State Approximation Case Study of an Inorganic Reaction Mechanism

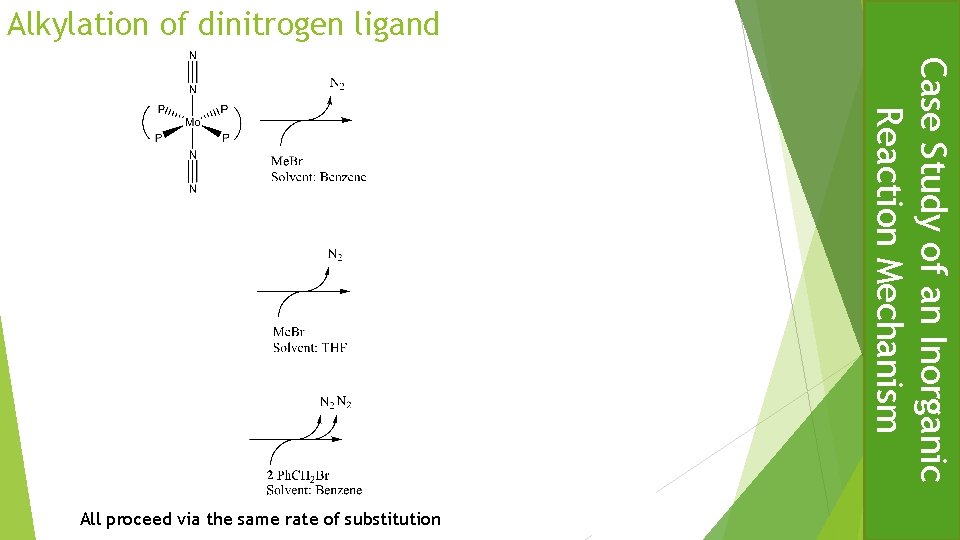
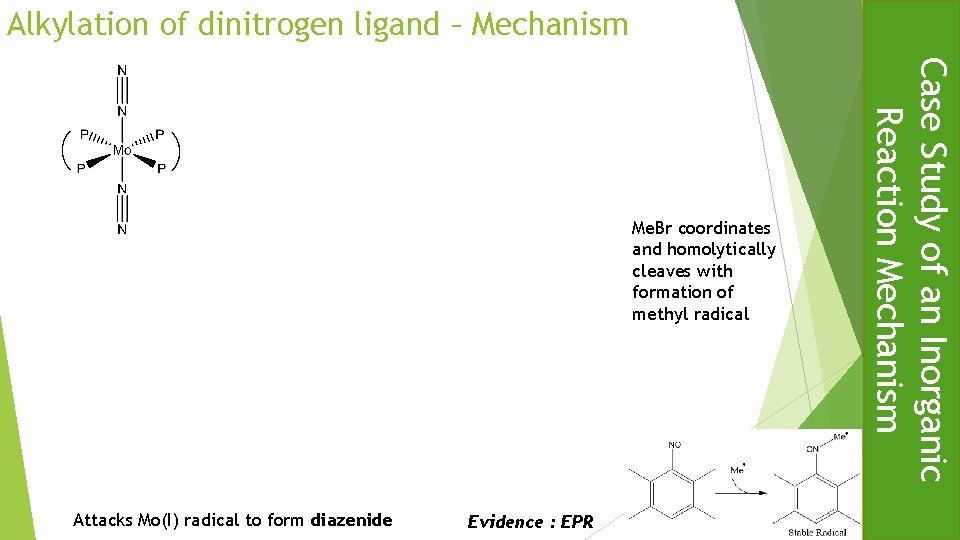
Alkylation of dinitrogen ligand Case Study of an Inorganic Reaction Mechanism All proceed via the same rate of substitution

Alkylation of dinitrogen ligand – Mechanism Attacks Mo(I) radical to form diazenide Evidence : EPR Case Study of an Inorganic Reaction Mechanism Me. Br coordinates and homolytically cleaves with formation of methyl radical

Introduction Substitution Reactions of Square Planar Complexes Substitution Reactions of Octahedral Complexes Insertion Reactions Case Study of an Inorganic Reaction Mechanism Substitution Reactions of Tetrahedral Complexes Electron Transfer Reactions Substitution Reactions of Tetrahedral Complexes Inorganic Reaction Mechanisms

THE TRANS EFFECT THE EFFECT OF A LIGAND, UPON THE RATE OF LIGAND REPLACEMENT, OF THE GROUP TRANS TO ITSELF Substitution Reactions of Tetrahedral Complexes EVERYONE SAY THIS OUT ALOUD TOGETHER

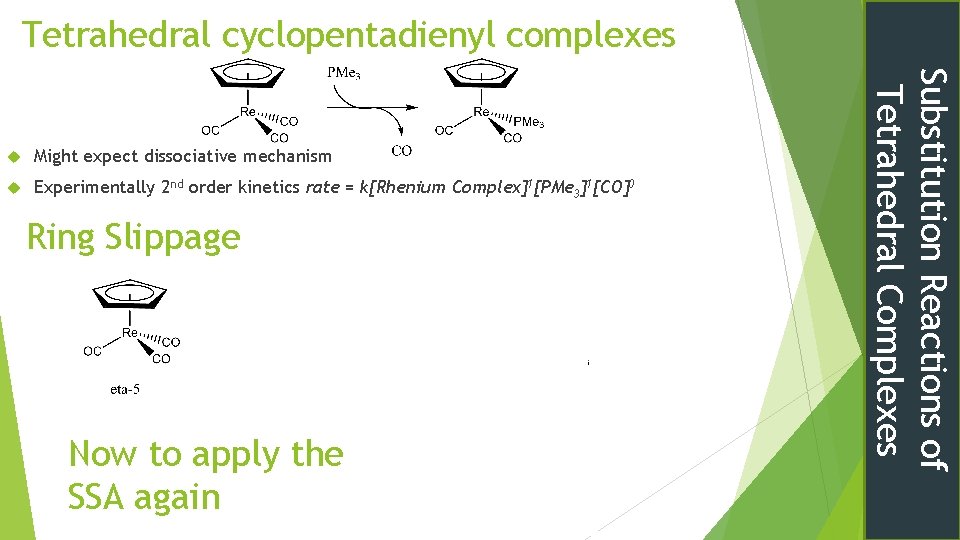
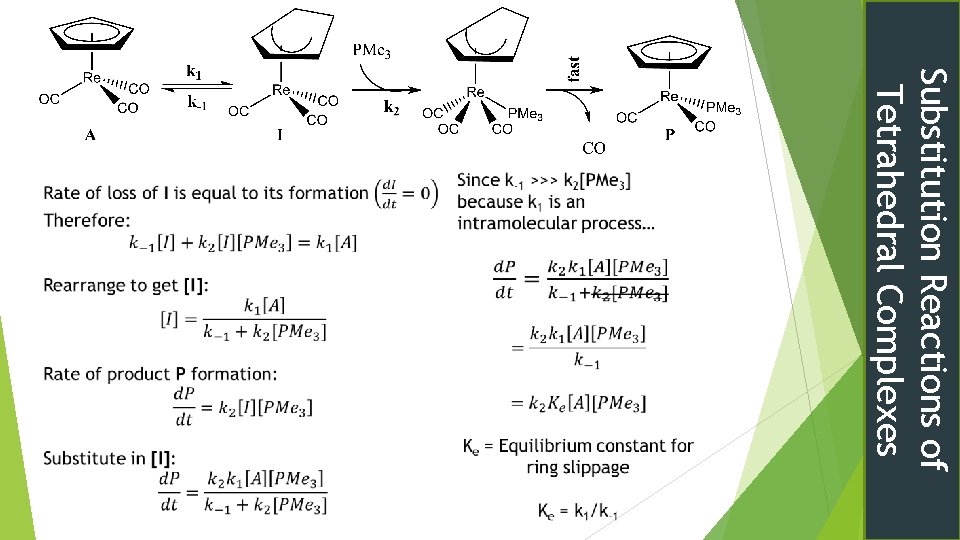
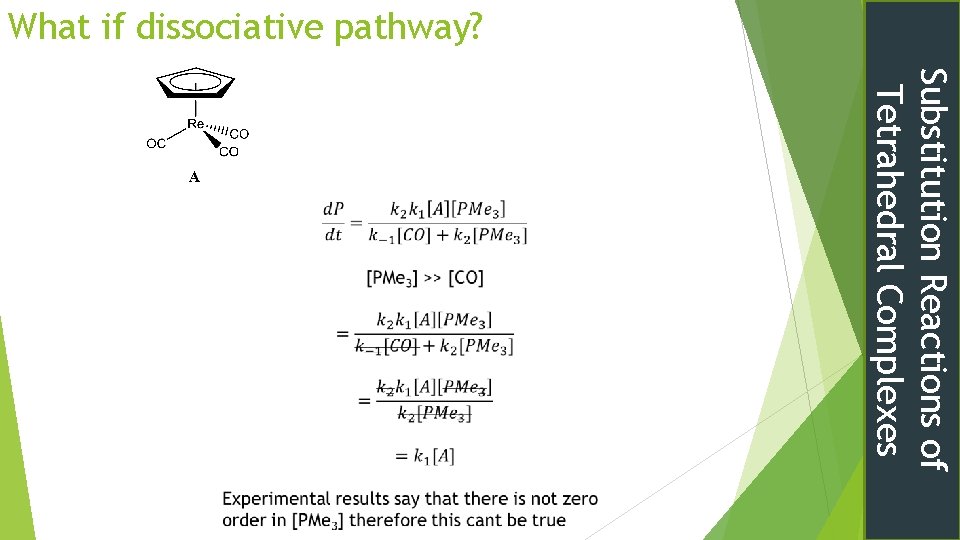
Tetrahedral cyclopentadienyl complexes Might expect dissociative mechanism Experimentally 2 nd order kinetics rate = k[Rhenium Complex]1[PMe 3]1[CO]0 Ring Slippage Now to apply the SSA again Substitution Reactions of Tetrahedral Complexes

Substitution Reactions of Tetrahedral Complexes

What if dissociative pathway? Substitution Reactions of Tetrahedral Complexes

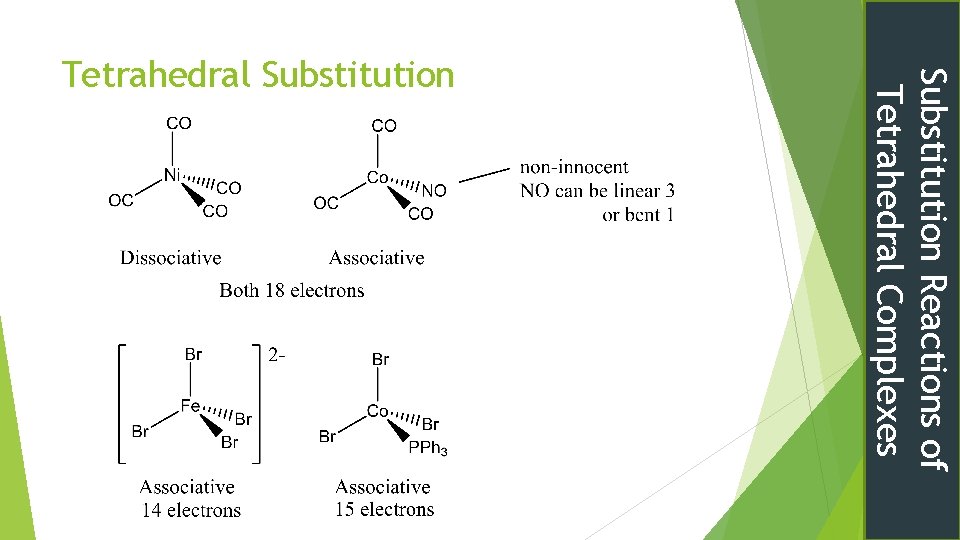
Substitution Reactions of Tetrahedral Complexes Tetrahedral Substitution

Introduction Substitution Reactions of Square Planar Complexes Substitution Reactions of Octahedral Complexes Insertion Reactions Case Study of an Inorganic Reaction Mechanism Substitution Reactions of Tetrahedral Complexes Electron Transfer Reactions Inorganic Reaction Mechanisms

THE TRANS EFFECT THE EFFECT OF A LIGAND, UPON THE RATE OF LIGAND REPLACEMENT, OF THE GROUP TRANS TO ITSELF Electron Transfer Reactions EVERYONE SAY THIS OUT ALOUD TOGETHER

May occur by either or both of two mechanisms Outer Sphere Electron Transfer Inner Sphere Electron Transfer Outer Sphere Electron Transfer In principle all outer sphere mechanisms involve electron transfer from reductant to oxidant with the coordination shells or spheres staying intact One reactant becomes involved in the outer or second coordination sphere of the other reactant and an electron flows from the reductant to oxidant Such a mechanism is established when rapid electron transfer occurs between two substitution inert complexes Electron Transfer Reactions

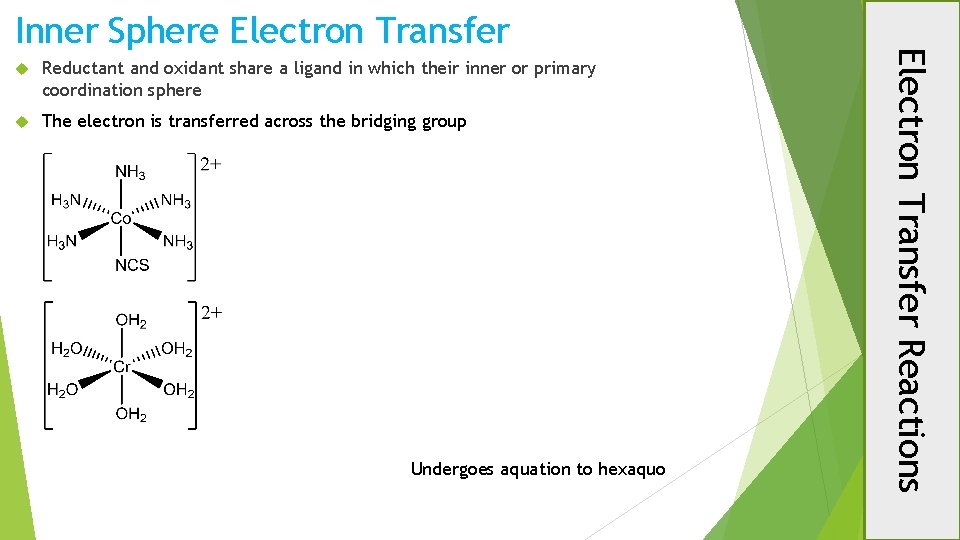
Reductant and oxidant share a ligand in which their inner or primary coordination sphere The electron is transferred across the bridging group Undergoes aquation to hexaquo Electron Transfer Reactions Inner Sphere Electron Transfer

1. Formation of precursor complex in a shared solvent cage A + B {A---B} KAB 2. Activation of precursor, electron transfer, and relaxation of successor complex {A---B} {A+---B-} k. ET 3. Products separate from solvent cage {A+---B-} A+ + BImportant Factors Solvent reorganisation Electronic structure Metal-Ligand reorganisation is small fast Electron Transfer Reactions Outer Sphere Electron Transfer Elementary Steps

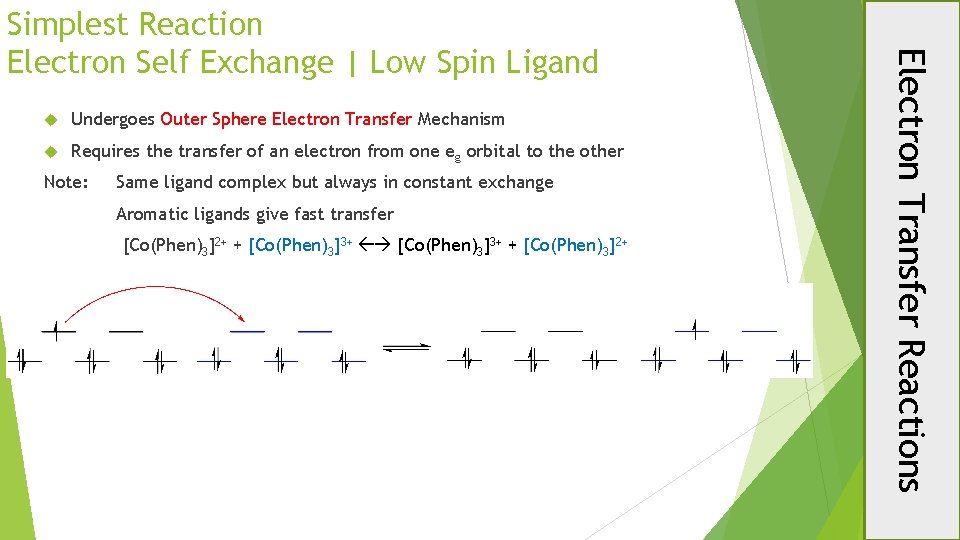
Undergoes Outer Sphere Electron Transfer Mechanism Requires the transfer of an electron from one eg orbital to the other Note: Same ligand complex but always in constant exchange Aromatic ligands give fast transfer [Co(Phen)3]2+ + [Co(Phen)3]3+ + [Co(Phen)3]2+ Electron Transfer Reactions Simplest Reaction Electron Self Exchange | Low Spin Ligand
![Extremely slow due to change in multiplicity of the system CoNH 362 Extremely slow due to change in multiplicity of the system [Co(NH 3)6]2+ +](https://slidetodoc.com/presentation_image_h2/7b6fed57123016f29898ba8aba705cd1/image-63.jpg)
Extremely slow due to change in multiplicity of the system [Co(NH 3)6]2+ + [Co(NH 3)6]3+ + [Co(NH 3)6]2+ Electron Transfer Reactions Electron Self Exchange | High Spin Ligand

Electron Transfer Reactions Electron Transfer Bond Length changes

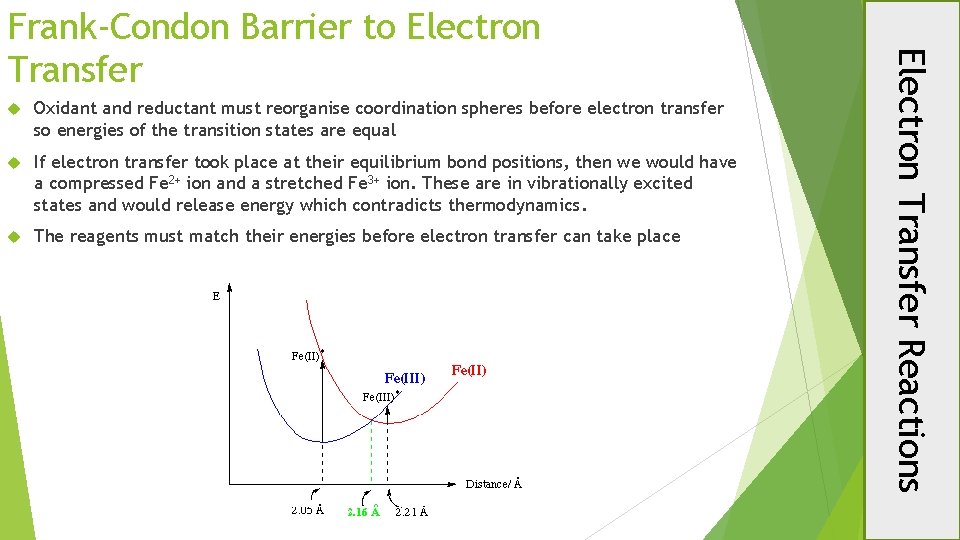
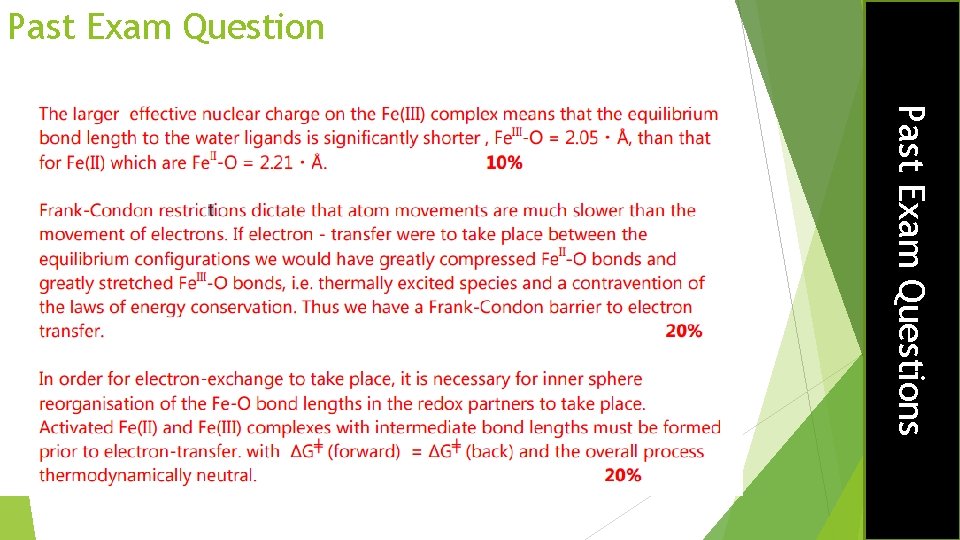
Oxidant and reductant must reorganise coordination spheres before electron transfer so energies of the transition states are equal If electron transfer took place at their equilibrium bond positions, then we would have a compressed Fe 2+ ion and a stretched Fe 3+ ion. These are in vibrationally excited states and would release energy which contradicts thermodynamics. The reagents must match their energies before electron transfer can take place Electron Transfer Reactions Frank-Condon Barrier to Electron Transfer
![Cross reactions and the Marcus Equation k 1 1 MLny MLny K 1 Cross reactions and the Marcus Equation k 1, 1 [MLn]y+ + [MLn]y+ K 1,](https://slidetodoc.com/presentation_image_h2/7b6fed57123016f29898ba8aba705cd1/image-66.jpg)
Cross reactions and the Marcus Equation k 1, 1 [MLn]y+ + [MLn]y+ K 1, 2 [MLn]y+ + [MLn]x+ k 2, 2 [MLn]x+ f 12 = z 21, 2 / z 1, 1. z 2, 2 k 12 = (k 11 k 22 K 12 f 12)1/2 Equilibrium constant, can consider as driving force. WITH APPROXIMATION f = 1 k 12 = (k 1 k 2 K 12)1/2 Can be calculated from redox potentials of reactants Rate constant Electron-self exchange rate constants Electron Transfer Reactions [MLn]x+ +

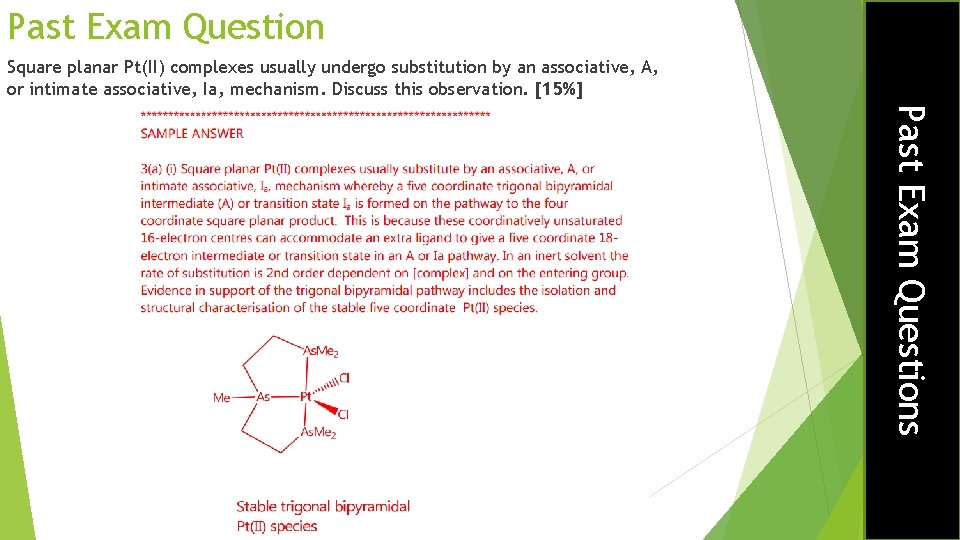
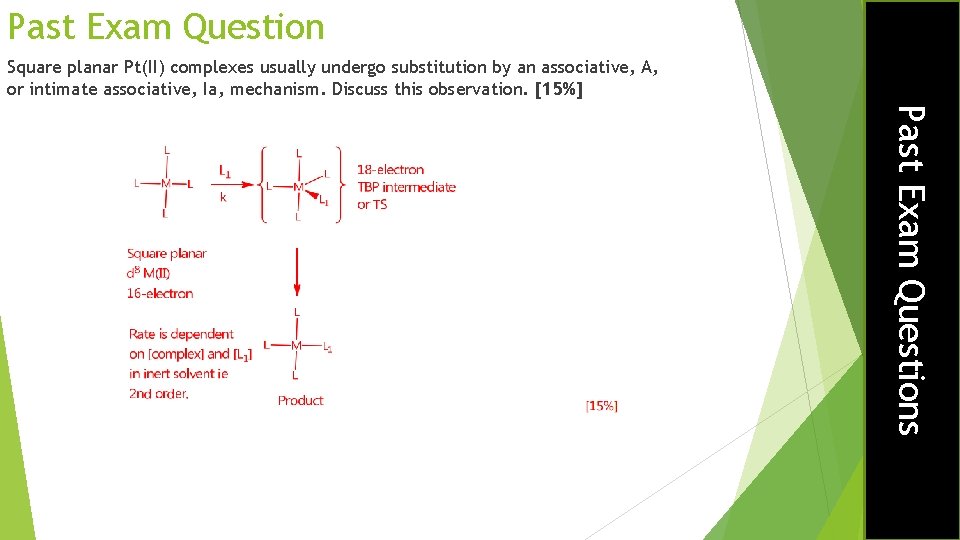
Past Exam Question Square planar Pt(II) complexes usually undergo substitution by an associative, A, or intimate associative, Ia, mechanism. Discuss this observation. [15%] Past Exam Questions

Past Exam Question Square planar Pt(II) complexes usually undergo substitution by an associative, A, or intimate associative, Ia, mechanism. Discuss this observation. [15%] Past Exam Questions

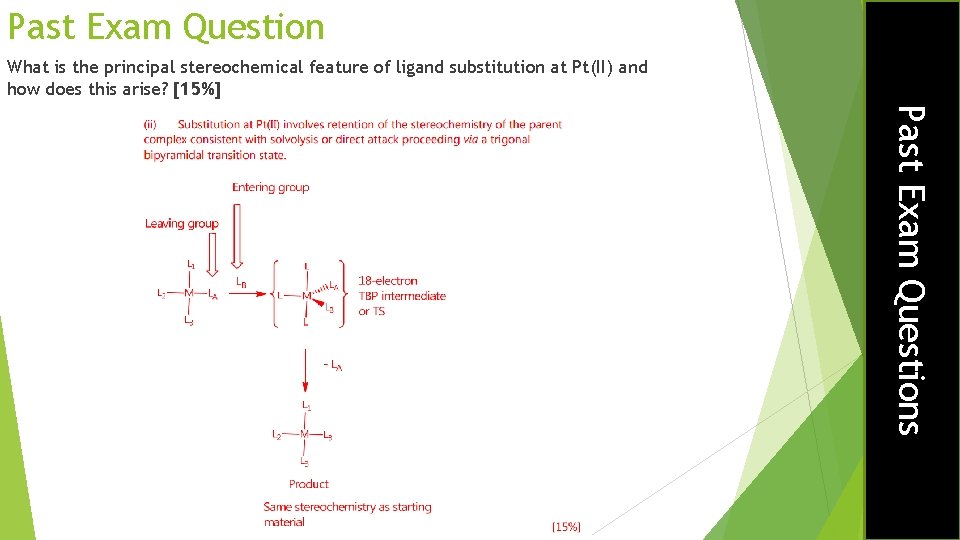
Past Exam Question What is the principal stereochemical feature of ligand substitution at Pt(II) and how does this arise? [15%] Past Exam Questions

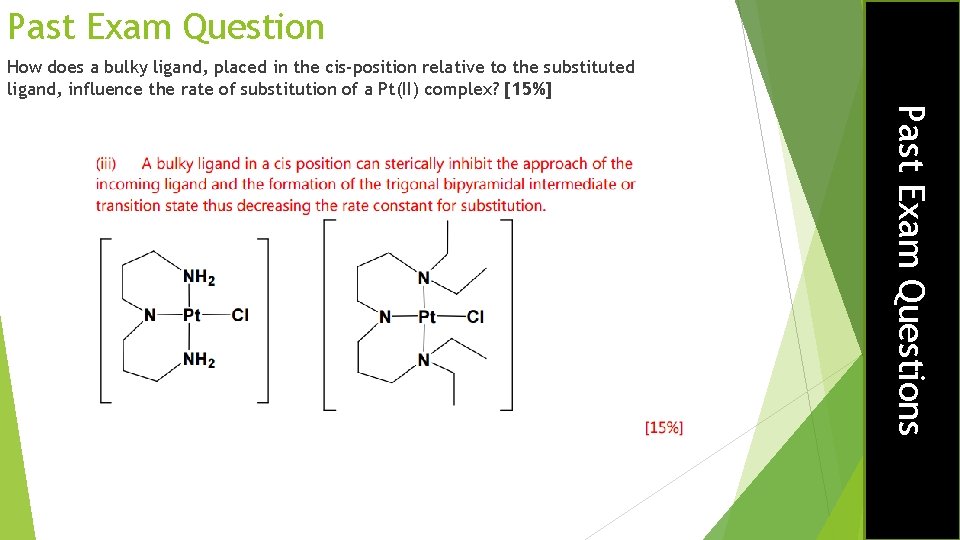
Past Exam Question How does a bulky ligand, placed in the cis-position relative to the substituted ligand, influence the rate of substitution of a Pt(II) complex? [15%] Past Exam Questions
![Past Exam Question What is the transeffect and how does it operate 25 Past Past Exam Question What is the trans-effect and how does it operate? [25%] Past](https://slidetodoc.com/presentation_image_h2/7b6fed57123016f29898ba8aba705cd1/image-71.jpg)
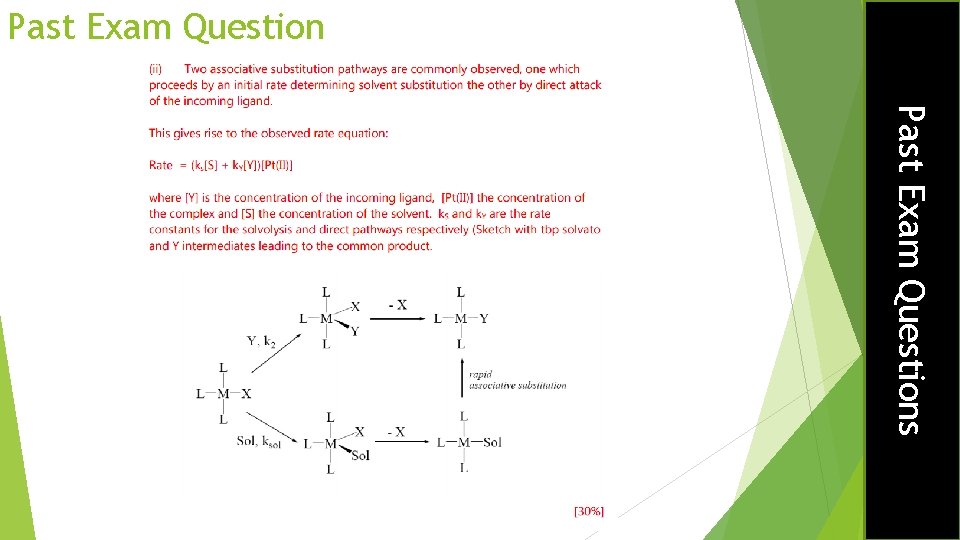
Past Exam Question What is the trans-effect and how does it operate? [25%] Past Exam Questions
![Past Exam Question What is the transeffect and how does it operate 25 Past Past Exam Question What is the trans-effect and how does it operate? [25%] Past](https://slidetodoc.com/presentation_image_h2/7b6fed57123016f29898ba8aba705cd1/image-72.jpg)
Past Exam Question What is the trans-effect and how does it operate? [25%] Past Exam Questions

Past Exam Questions

Past Exam Questions

Past Exam Questions

Past Exam Questions