Inorganic gaseous pollutants IAQ Introduction Inorganic gaseous pollutants



















- Slides: 31

Inorganic gaseous pollutants (IAQ)

Introduction Ø Inorganic gaseous pollutants are the major contributors to Indoor quality problems Ø Combustion appliances and tobacco smoke are the major sources of gases along with other types of pollutants Ø These Inorganic gaseous pollutants include Carbon monoxide. Sulfur dioxide. Nitrous oxide. Nitrogen dioxide.

Factors effecting concentration. Ø The emission patterns and concentrations of the pollutants depend on Following factors: v Types of Fuels used v Combustion efficiency v Appliance design v Ventilation system v Operating conditions v Maintenance v Frequency of use

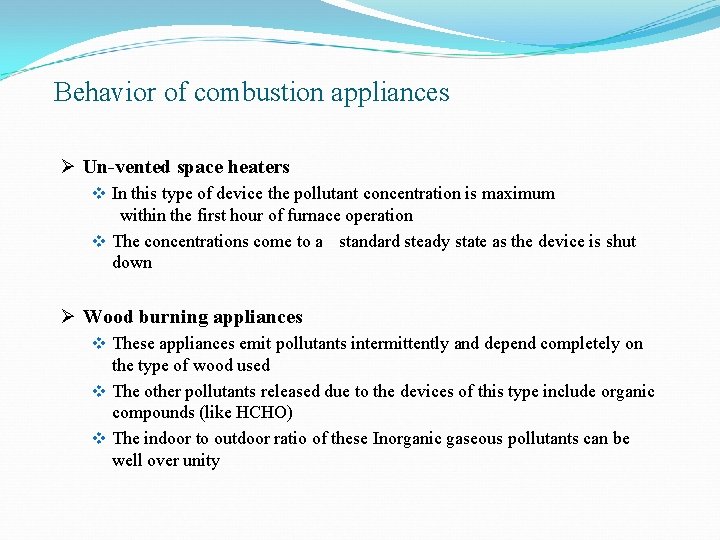
Appliances based on Fuel type and their application Ø Un-vented space heaters Ø Wood burning stoves v Furnaces v Fire places

Behavior of combustion appliances Ø Un-vented space heaters v In this type of device the pollutant concentration is maximum within the first hour of furnace operation v The concentrations come to a standard steady state as the device is shut down Ø Wood burning appliances v These appliances emit pollutants intermittently and depend completely on the type of wood used v The other pollutants released due to the devices of this type include organic compounds (like HCHO) v The indoor to outdoor ratio of these Inorganic gaseous pollutants can be well over unity

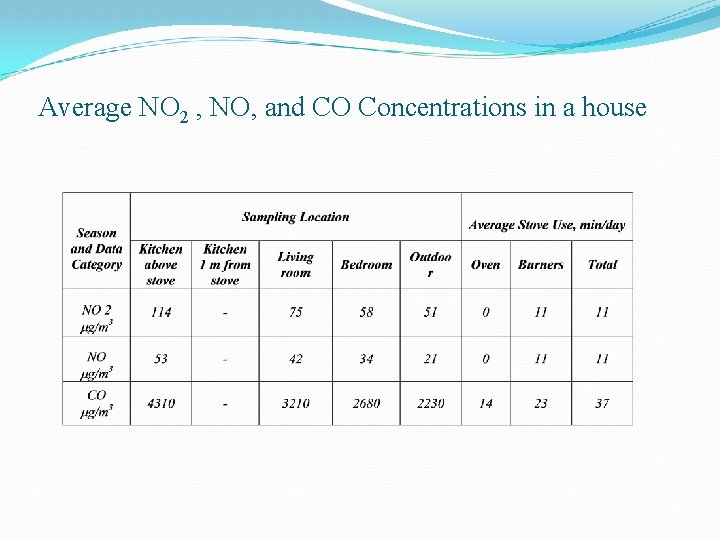
Average NO 2 , NO, and CO Concentrations in a house

Health Effects and standards

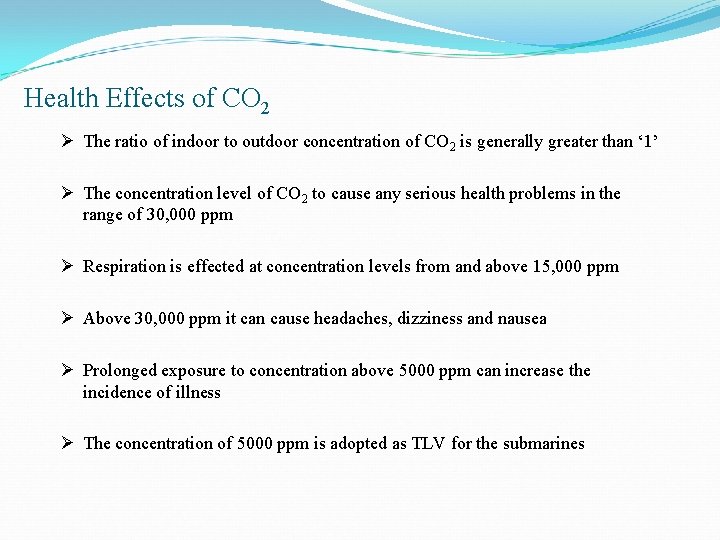
Health Effects of CO 2 Ø The ratio of indoor to outdoor concentration of CO 2 is generally greater than ‘ 1’ Ø The concentration level of CO 2 to cause any serious health problems in the range of 30, 000 ppm Ø Respiration is effected at concentration levels from and above 15, 000 ppm Ø Above 30, 000 ppm it can cause headaches, dizziness and nausea Ø Prolonged exposure to concentration above 5000 ppm can increase the incidence of illness Ø The concentration of 5000 ppm is adopted as TLV for the submarines

Health Effects of CO Ø Health effects due to CO include loss of alertness, impaired perception, learning disorders, sleep deprivation, drowsiness and confusion Ø Health effects due to prolonged exposure to low concentration have been controversial, but acute illness and deaths have been reported Ø CO combines with hemoglobin and myoglobin to stop the supply of oxygen to tissues affecting brain, myocardium and muscle tissues Ø Carboxyhemoglobin cause severe health effects at various percentage levels in blood v Loss of vigilance ability @ 3% - 5% v Loss of hand to eye co-ordination @6% - 10%

Health Effects of NO and NO 2 Ø At high level there can be very serious health effects like coma and eventual death Ø NO and NO 2 are the most reactive species of the nitrogen oxides Ø They combine with other indoor pollutants to form complex toxic substances Ø React with amines like benzo(a)pyrene and pyrelene to form carcinogenic nitrosoamine and mutagens

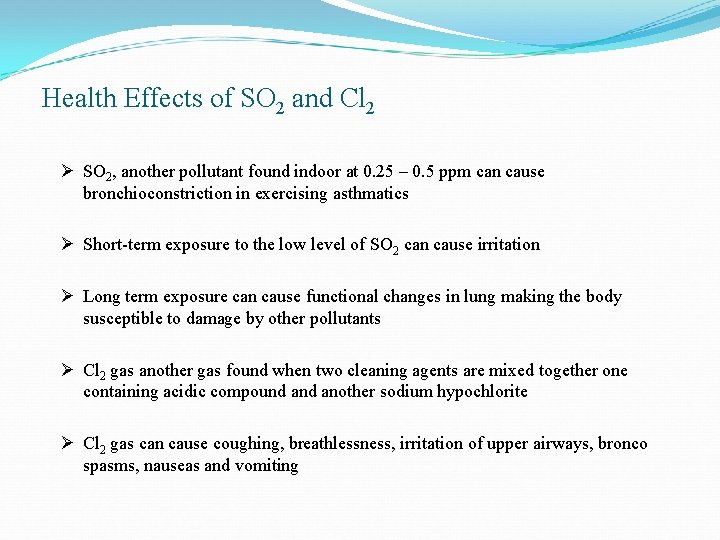
Health Effects of SO 2 and Cl 2 Ø SO 2, another pollutant found indoor at 0. 25 – 0. 5 ppm can cause bronchioconstriction in exercising asthmatics Ø Short-term exposure to the low level of SO 2 can cause irritation Ø Long term exposure can cause functional changes in lung making the body susceptible to damage by other pollutants Ø Cl 2 gas another gas found when two cleaning agents are mixed together one containing acidic compound another sodium hypochlorite Ø Cl 2 gas can cause coughing, breathlessness, irritation of upper airways, bronco spasms, nauseas and vomiting

Sources and Indoor Concentrations

Sources Unvented space heaters Ø The unvented gas heater is one of the major sources of pollutants like Inorganic gaseous pollutants Ø This device can be classified into two categories v Gas space heaters – heaters of this type use gas as fuel v Kerosene space heaters - heaters that use kerosene as fuel

Classification of Kerosene space heaters Ø Convective Ø Radiant Ø Convective / Radiant Ø Two stage Ø Wickless

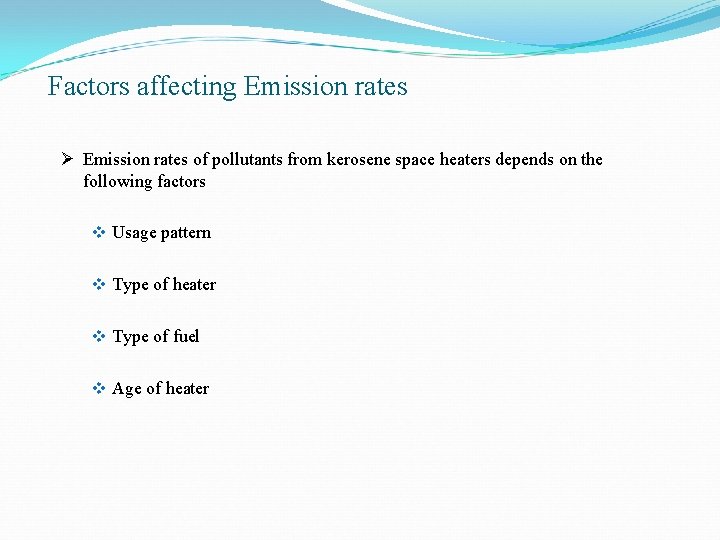
Factors affecting Emission rates Ø Emission rates of pollutants from kerosene space heaters depends on the following factors v Usage pattern v Type of heater v Type of fuel v Age of heater

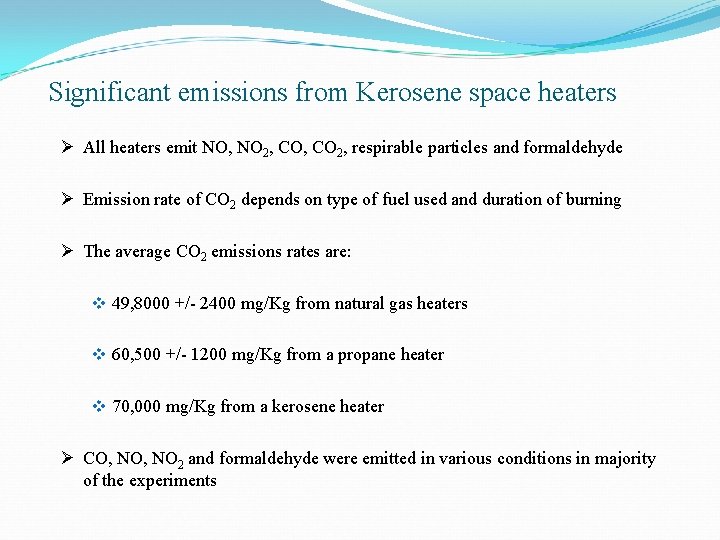
Significant emissions from Kerosene space heaters Ø All heaters emit NO, NO 2, CO 2, respirable particles and formaldehyde Ø Emission rate of CO 2 depends on type of fuel used and duration of burning Ø The average CO 2 emissions rates are: v 49, 8000 +/- 2400 mg/Kg from natural gas heaters v 60, 500 +/- 1200 mg/Kg from a propane heater v 70, 000 mg/Kg from a kerosene heater Ø CO, NO 2 and formaldehyde were emitted in various conditions in majority of the experiments

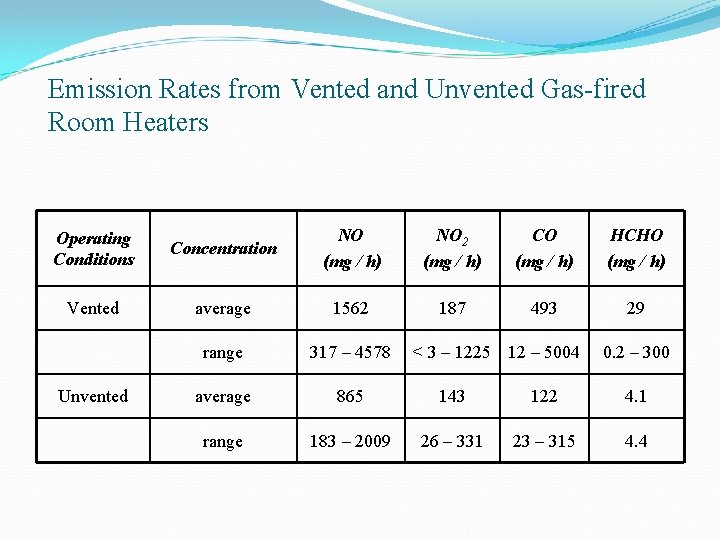
Emission Rates from Vented and Unvented Gas-fired Room Heaters Operating Conditions Concentration NO (mg / h) NO 2 (mg / h) CO (mg / h) HCHO (mg / h) Vented average 1562 187 493 29 range 317 – 4578 average 865 143 122 4. 1 range 183 – 2009 26 – 331 23 – 315 4. 4 Unvented < 3 – 1225 12 – 5004 0. 2 – 300

Wood burning stove’s fireplaces and Furnaces as sources Ø These are potential sources for both indoor and outdoor air Ø They emit NO, NO 2, CO 2, SO 2, respirable suspended particles, benzo(a)pyrene and formaldehyde (which are vented outside) Ø Cracks and leaks in stovepipes, downdrafts, log roll over in fireplaces and negative air pressure cause the indoor pollution Ø The emissions of pollutants per cord of wood are generally: v 0. 5 – 1. 5 lb of sulphur v 0. 7 – 2. 6 lb of NOx v 300 – 1200 lb of CO

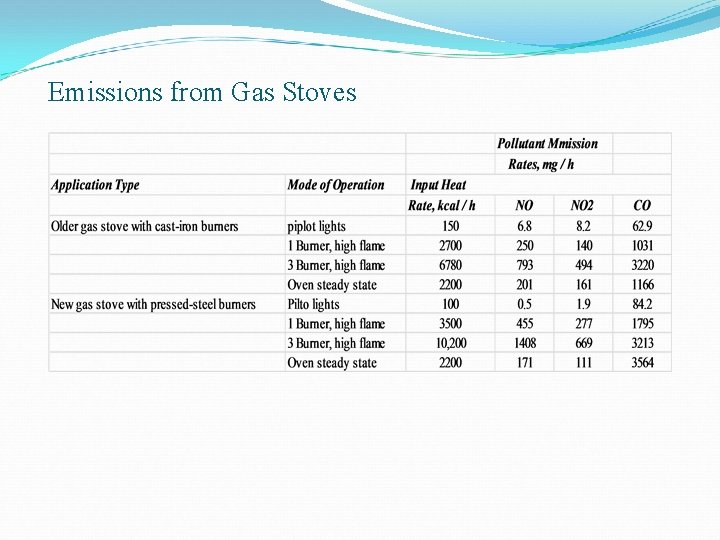
Gas stoves and Ovens Ø Gas stoves produce pollutants like CO, CO 2, NO 2 and aldehyde indoors Ø CO level is increased by 20 – 25 ppm within the first 30 mins of operation, 1. 2 ppm of NO in first 45 mins and 25 ppm of NO 2 Ø CO 2 emissions for ovens in steady state were higher for a new stove compared to an old stove Ø The concentration of indoor pollutant is decided by v Air changes per hour v Number of heaters v Frequency of use v Airspace volume

Emissions from Gas Stoves

Other Combustion sources Ø Other sources of pollutants are water heaters, washers, dryers and attached garages Ø Several hobbies like welding and soldering etc are also sources of indoor air pollution Ø Tobacco smoke is the major contributor of respirable particles Ø Gas water heaters lead to high indoor concentration of Nitrogen oxides

Sampling and Measurement

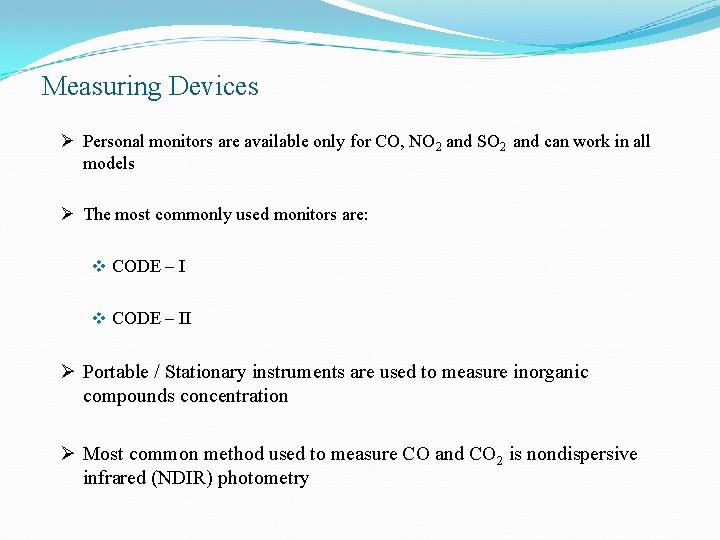
Measuring Devices Ø Personal monitors are available only for CO, NO 2 and SO 2 and can work in all models Ø The most commonly used monitors are: v CODE – II Ø Portable / Stationary instruments are used to measure inorganic compounds concentration Ø Most common method used to measure CO and CO 2 is nondispersive infrared (NDIR) photometry

Other Measuring Devices Ø Individual concentration of gas in a gaseous mixture is measured by Thermal conductivity Detector (TCD) Ø Thermal conductivity Detector is used for quantifying CO, CO 2 and H 2 O Ø Flame photometric method is used for SO 2 and H 2 S

Effective Measuring Methods Ø The Gas filter Correlation method (GFC) is used to measure CO concentration (certified by EPA) Ø Ozone is measured by chemiluminescent method Ø SO 2 concentration is measured by: v Colorimetric method v Flame photometric detection (FPD)

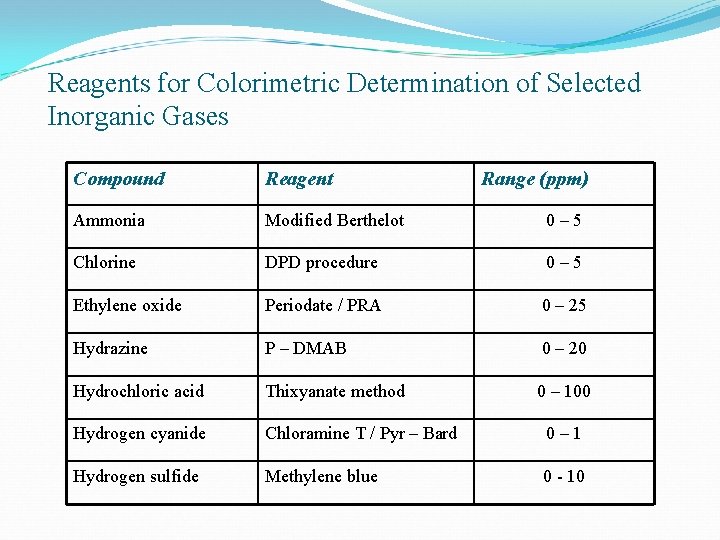
Reagents for Colorimetric Determination of Selected Inorganic Gases Compound Reagent Range (ppm) Ammonia Modified Berthelot 0– 5 Chlorine DPD procedure 0– 5 Ethylene oxide Periodate / PRA 0 – 25 Hydrazine P – DMAB 0 – 20 Hydrochloric acid Thixyanate method 0 – 100 Hydrogen cyanide Chloramine T / Pyr – Bard 0– 1 Hydrogen sulfide Methylene blue 0 - 10

Control Strategies

Source control Ø The major contributors are the wood burning stoves, so removal of them indoors substantially reduces the pollution Ø Replacement of wood burning stoves by oil or gas fired furnaces Ø Modification in the design of the gas and kerosene stoves is another way to subside the indoor pollution Ø Other preventive measures like properation and regular maintenance of the burner could be a possible alternative

Increased ventilation Ø Local and Mechanical ventilation are most effective in removing the pollutants Ø Hood installed over the cooking place is one of the most common local ventilation methods Ø Ductless Cooking ranges with Carbon filters are being used Ø Carbon filters have poor adsorption capacity (which can be improved by careful design) Ø Mechanical ventilation increases the air exchange rate hence decreasing the concentration of the pollutants

Air Cleaning Ø Absorption methods v This method can effectively remove the pollutants indoors v Liquid desiccant based air conditioning systems are being used indoors v Absorber using monoethanolamine is being used in submarines to reduce the concentration of CO 2 v The systems failed when the outdoor concentration was much higher

Air Cleaning Adsorption methods Ø Commonly used adsorbents are: v Silica gel v Activated alumina v Activated carbon v Manganese oxides Ø These adsorbents should be able to remove the moisture from indoor air using solid adsorbents (desiccants)
 Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Inorganic gases
Inorganic gases Pink
Pink Primary pollutants and secondary pollutants
Primary pollutants and secondary pollutants Primary pollutant vs secondary pollutant
Primary pollutant vs secondary pollutant Sustainable iaq units
Sustainable iaq units Sustainable iaq units
Sustainable iaq units What is pharmaceutical inorganic chemistry
What is pharmaceutical inorganic chemistry Introduction to inorganic chemistry
Introduction to inorganic chemistry Solar system inner and outer planets
Solar system inner and outer planets Gaseous exchange in protozoa
Gaseous exchange in protozoa Gaseous exchange in grasshopper
Gaseous exchange in grasshopper Gaseous state chapter
Gaseous state chapter Granular porosity denture
Granular porosity denture Gaseous exchange in animals
Gaseous exchange in animals Gaseous dosage forms
Gaseous dosage forms Gaseous equilibrium
Gaseous equilibrium Gaseous envelope of the sun
Gaseous envelope of the sun Life science grade 11 gaseous exchange practical
Life science grade 11 gaseous exchange practical Pearson
Pearson Gas and liquid solution example
Gas and liquid solution example Phosphate buffer system equation
Phosphate buffer system equation Define doses form
Define doses form At 500 k one mole of gaseous oncl
At 500 k one mole of gaseous oncl Secondary pollutants examples
Secondary pollutants examples Secondary pollutants
Secondary pollutants Primary vs secondary pollutants
Primary vs secondary pollutants Ozone layer depletion
Ozone layer depletion Secondary air pollutants
Secondary air pollutants Primary vs secondary pollutants
Primary vs secondary pollutants Stock pollutants
Stock pollutants Primary and secondary pollutants difference
Primary and secondary pollutants difference