Chapter 2 The Chemical Level of Organization Lecture





- Slides: 72

Chapter 2 The Chemical Level of Organization Lecture Presentation by Lee Ann Frederick University of Texas at Arlington

An Introduction to the Chemical Level of Organization • Learning Outcomes • 2 -1 Describe an atom and how atomic structure affects interactions between atoms. • 2 -2 Compare the ways in which atoms combine to form molecules and compounds. • 2 -3 Distinguish among the major types of chemical reactions that are important for studying physiology. • 2 -4 Describe the crucial role of enzymes in metabolism. © 2015 Pearson Education, Inc.

An Introduction to the Chemical Level of Organization • Learning Outcomes • 2 -5 Distinguish between organic and inorganic compounds. • 2 -6 Explain how the chemical properties of water make life possible. • 2 -7 Discuss the importance of p. H and the role of buffers in body fluids. • 2 -8 Describe the physiological roles of inorganic compounds. • 2 -9 Discuss the structures and functions of carbohydrates. © 2015 Pearson Education, Inc.

An Introduction to the Chemical Level of Organization • Learning Outcomes • 2 -10 Discuss the structures and functions of lipids. • 2 -11 Discuss the structures and functions of proteins. • 2 -12 Discuss the structures and functions of nucleic acids. • 2 -13 Discuss the structures and functions of high-energy compounds. • 2 -14 Explain the relationship between chemicals and cells. © 2015 Pearson Education, Inc.

An Introduction to the Chemical Level of Organization • Chemistry • Is the science of change • Topics of this chapter include: • The structure of atoms • The basic chemical building blocks • How atoms combine to form increasingly complex structures © 2015 Pearson Education, Inc.

2 -1 Atoms and Atomic Structure • Matter • Is made up of atoms • Atoms join together to form chemicals with different characteristics • Chemical characteristics determine physiology at the molecular and cellular levels © 2015 Pearson Education, Inc.

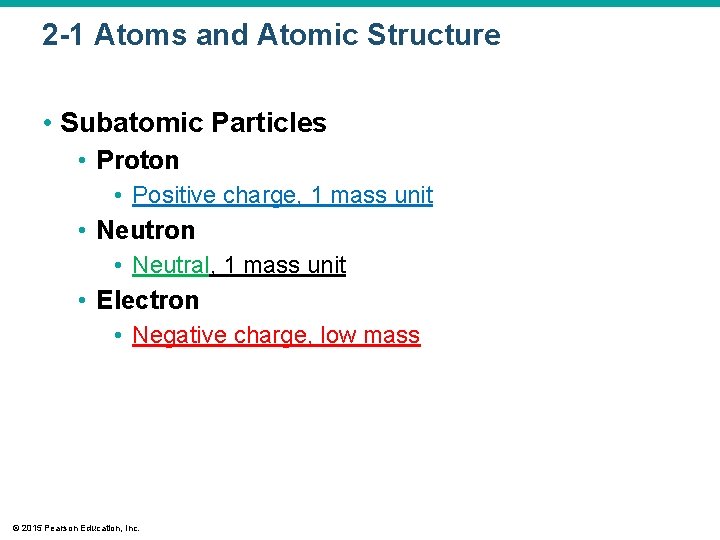
2 -1 Atoms and Atomic Structure • Subatomic Particles • Proton • Positive charge, 1 mass unit • Neutron • Neutral, 1 mass unit • Electron • Negative charge, low mass © 2015 Pearson Education, Inc.

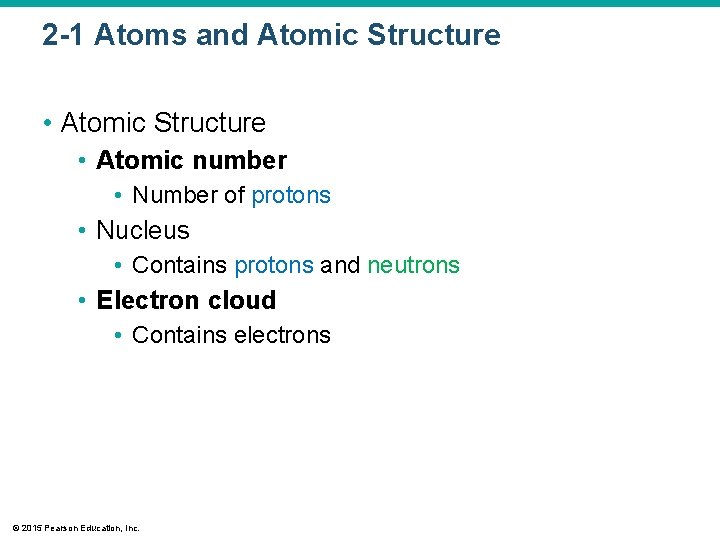
2 -1 Atoms and Atomic Structure • Atomic number • Number of protons • Nucleus • Contains protons and neutrons • Electron cloud • Contains electrons © 2015 Pearson Education, Inc.

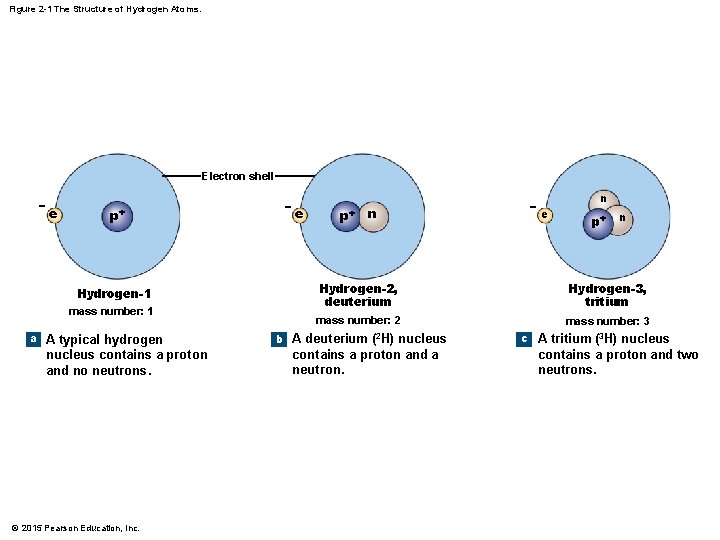
Figure 2 -1 The Structure of Hydrogen Atoms. Electron shell − e p+ Hydrogen-1 mass number: 1 a A typical hydrogen nucleus contains a proton and no neutrons. © 2015 Pearson Education, Inc. − e p+ n − n e p+ n Hydrogen-2, deuterium Hydrogen-3, tritium mass number: 2 mass number: 3 b A deuterium (2 H) nucleus contains a proton and a neutron. c A tritium (3 H) nucleus contains a proton and two neutrons.

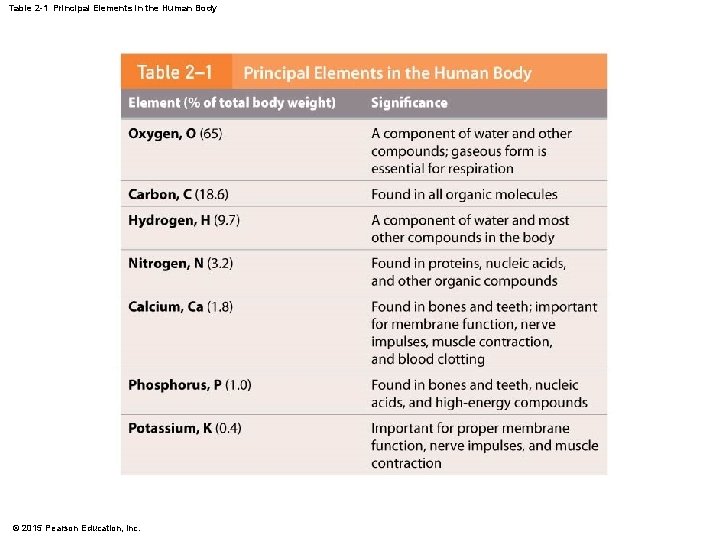
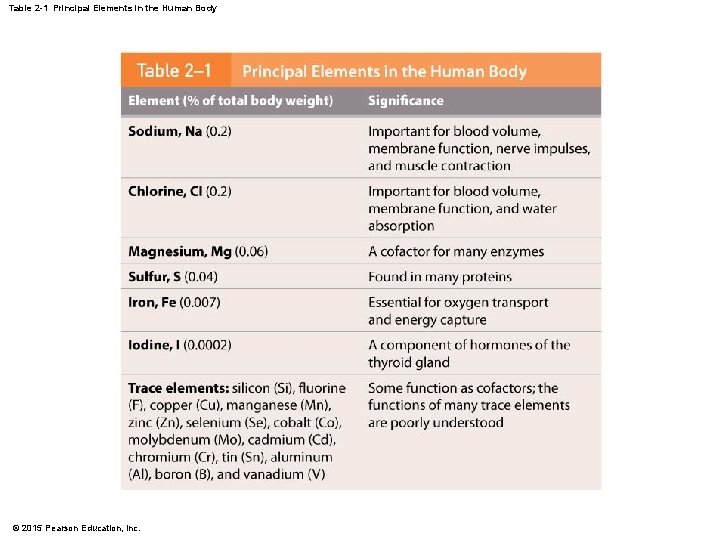
Table 2 -1 Principal Elements in the Human Body © 2015 Pearson Education, Inc.

Table 2 -1 Principal Elements in the Human Body © 2015 Pearson Education, Inc.

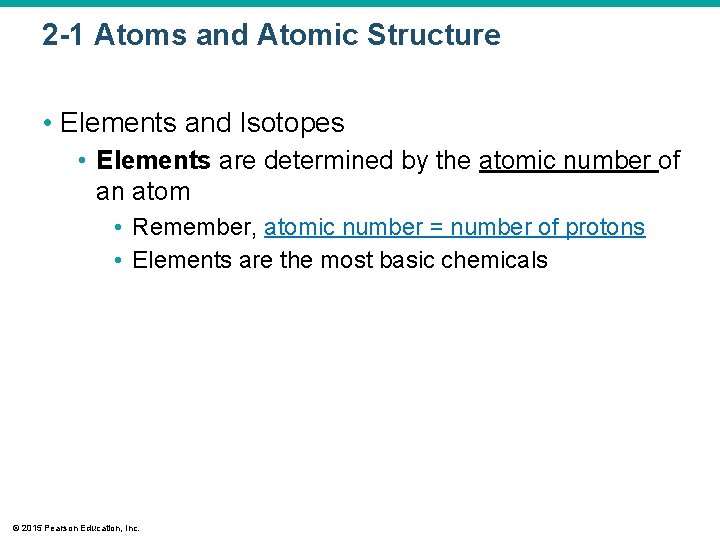
2 -1 Atoms and Atomic Structure • Elements and Isotopes • Elements are determined by the atomic number of an atom • Remember, atomic number = number of protons • Elements are the most basic chemicals © 2015 Pearson Education, Inc.

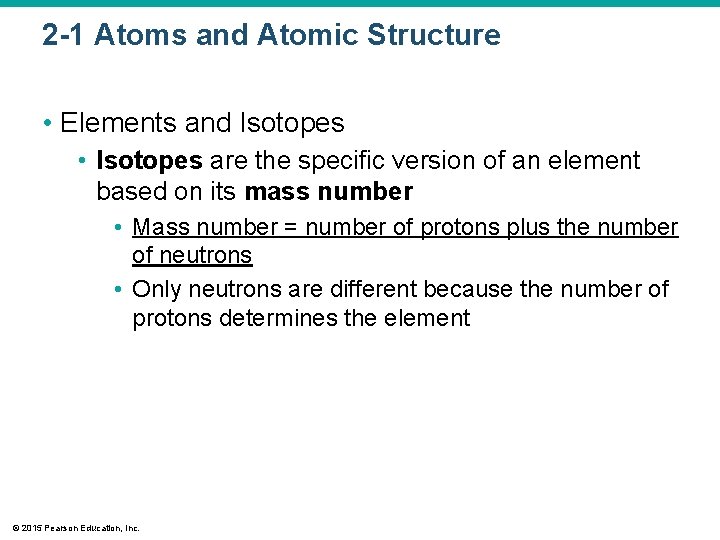
2 -1 Atoms and Atomic Structure • Elements and Isotopes • Isotopes are the specific version of an element based on its mass number • Mass number = number of protons plus the number of neutrons • Only neutrons are different because the number of protons determines the element © 2015 Pearson Education, Inc.

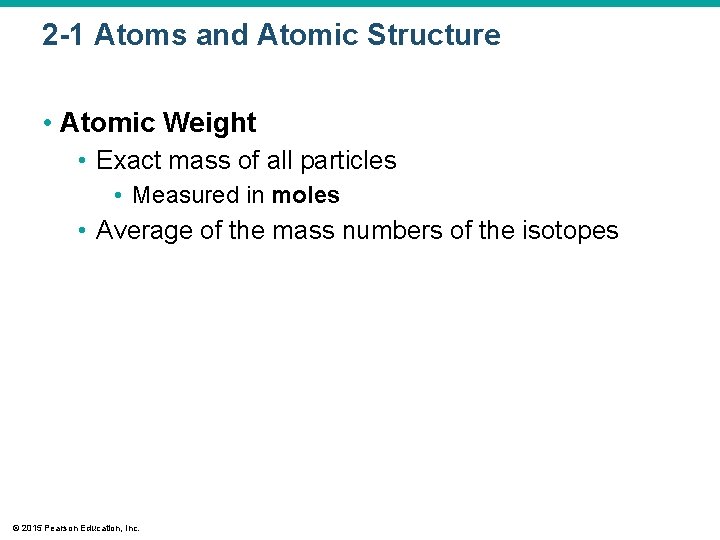
2 -1 Atoms and Atomic Structure • Atomic Weight • Exact mass of all particles • Measured in moles • Average of the mass numbers of the isotopes © 2015 Pearson Education, Inc.

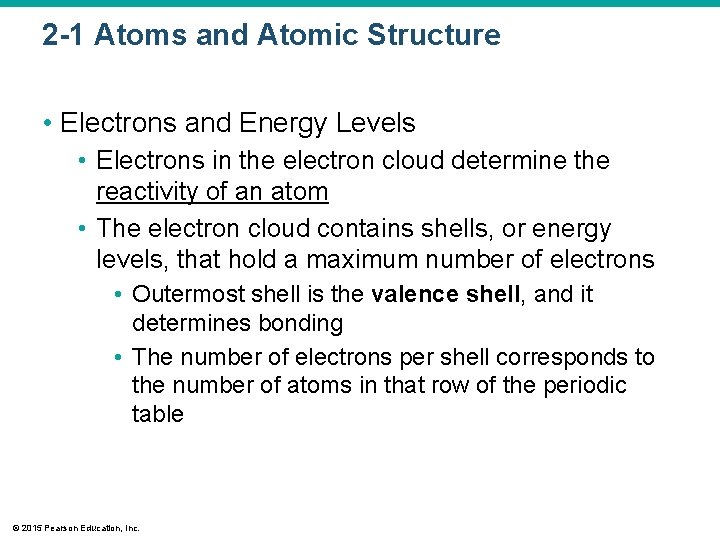
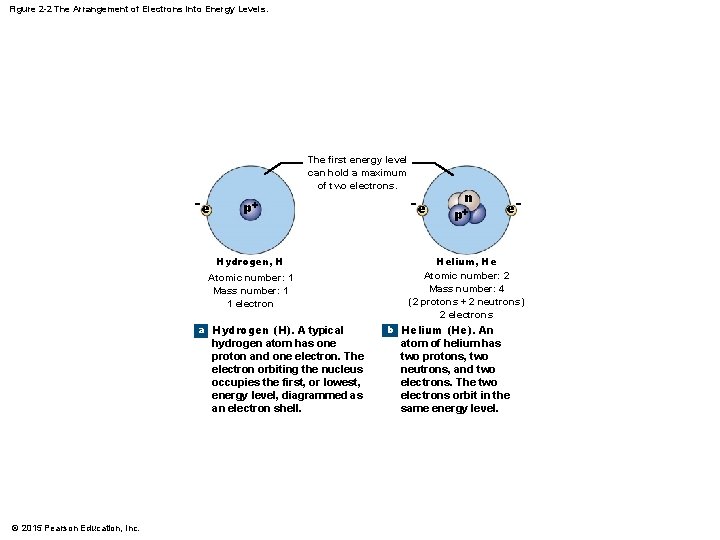
2 -1 Atoms and Atomic Structure • Electrons and Energy Levels • Electrons in the electron cloud determine the reactivity of an atom • The electron cloud contains shells, or energy levels, that hold a maximum number of electrons • Outermost shell is the valence shell, and it determines bonding • The number of electrons per shell corresponds to the number of atoms in that row of the periodic table © 2015 Pearson Education, Inc.

Figure 2 -2 The Arrangement of Electrons into Energy Levels. The first energy level can hold a maximum of two electrons. −e p+ Hydrogen, H Atomic number: 1 Mass number: 1 1 electron a Hydrogen (H). A typical hydrogen atom has one proton and one electron. The electron orbiting the nucleus occupies the first, or lowest, energy level, diagrammed as an electron shell. © 2015 Pearson Education, Inc. −e n p+ e− Helium, He Atomic number: 2 Mass number: 4 (2 protons + 2 neutrons) 2 electrons b Helium (He). An atom of helium has two protons, two neutrons, and two electrons. The two electrons orbit in the same energy level.

Figure 2 -2 The Arrangement of Electrons into Energy Levels. The second and third energy levels can each contain up to 8 electrons. −e −e n p+ e− −e −e c Lithium (Li). A lithium atom has three protons, three neutrons, and three electrons. The first energy level can hold only two electrons, so the third electron occupies a second energy level. © 2015 Pearson Education, Inc. e− − e e− n −e p+ −e Lithium, Li Atomic number: 3 Mass number: 6 (3 protons + 3 neutrons) 3 electrons e− −e e− Neon, Ne Atomic number: 10 Mass number: 20 (10 protons + 10 neutrons) 10 electrons d Neon (Ne). A neon atom has 10 protons, 10 neutrons, and 10 electrons. The second level can hold up to eight electrons; thus, both the first and second energy levels are filled.

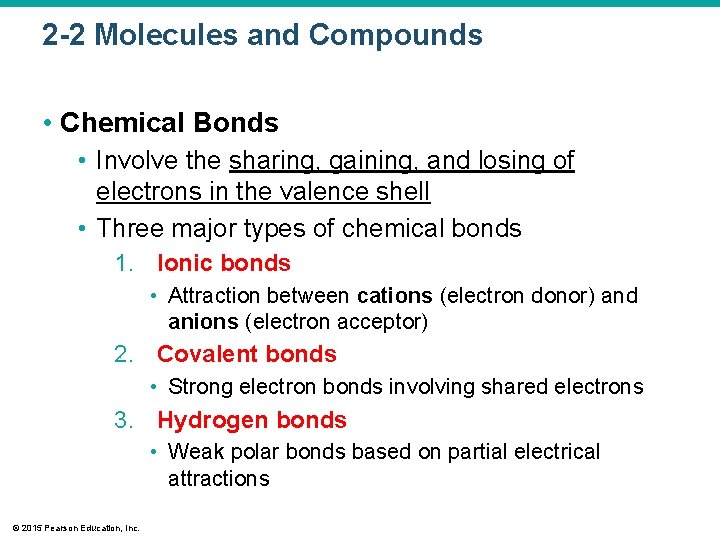
2 -2 Molecules and Compounds • Chemical Bonds • Involve the sharing, gaining, and losing of electrons in the valence shell • Three major types of chemical bonds 1. Ionic bonds • Attraction between cations (electron donor) and anions (electron acceptor) 2. Covalent bonds • Strong electron bonds involving shared electrons 3. Hydrogen bonds • Weak polar bonds based on partial electrical attractions © 2015 Pearson Education, Inc.

2 -2 Molecules and Compounds • Chemical Bonds • Form molecules and/or compounds • Molecules • Two or more atoms joined by strong bonds • Compounds are all molecules, but not all molecules are compounds • H 2 = molecule only • H 2 O = molecule and compound © 2015 Pearson Education, Inc.

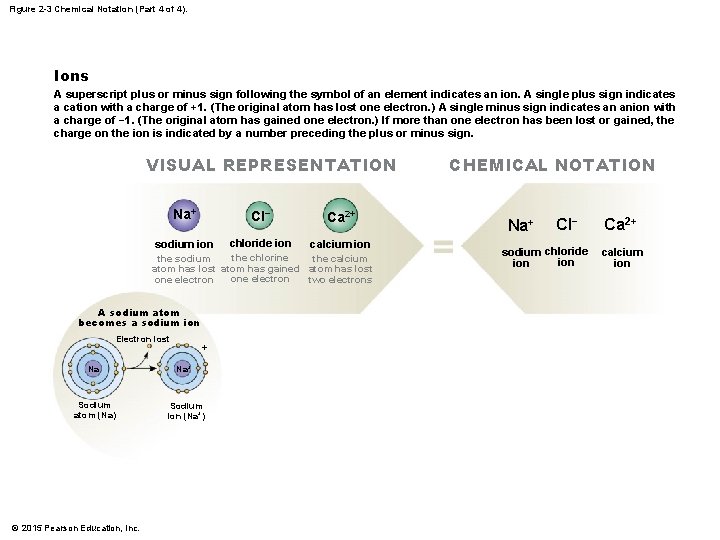
Figure 2 -3 Chemical Notation (Part 4 of 4). Ions A superscript plus or minus sign following the symbol of an element indicates an ion. A single plus sign indicates a cation with a charge of +1. (The original atom has lost one electron. ) A single minus sign indicates an anion with a charge of − 1. (The original atom has gained one electron. ) If more than one electron has been lost or gained, the charge on the ion is indicated by a number preceding the plus or minus sign. VISUAL REPRESENTATION Na+ Cl− Ca 2+ sodium ion chloride ion calcium ion the chlorine the sodium the calcium atom has lost atom has gained atom has lost one electron two electrons A sodium atom becomes a sodium ion Electron lost Na Sodium atom (Na) © 2015 Pearson Education, Inc. + Na+ Sodium ion (Na+) CHEMICAL NOTATION Na+ Cl− sodium chloride ion Ca 2+ calcium ion

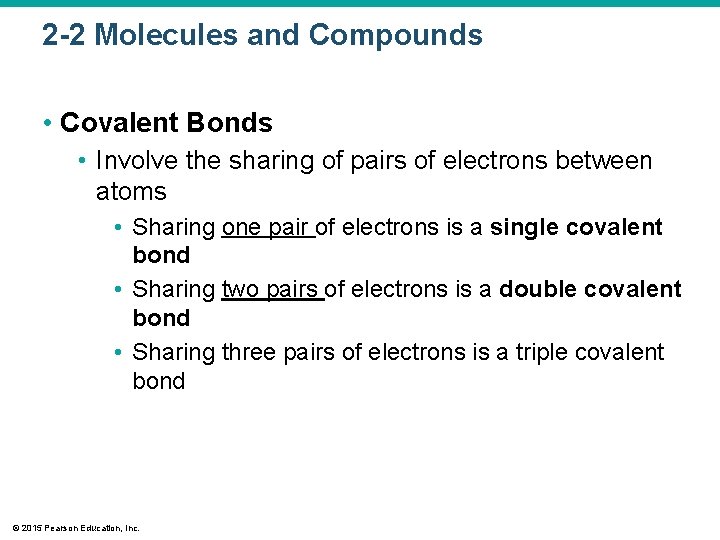
2 -2 Molecules and Compounds • Covalent Bonds • Involve the sharing of pairs of electrons between atoms • Sharing one pair of electrons is a single covalent bond • Sharing two pairs of electrons is a double covalent bond • Sharing three pairs of electrons is a triple covalent bond © 2015 Pearson Education, Inc.

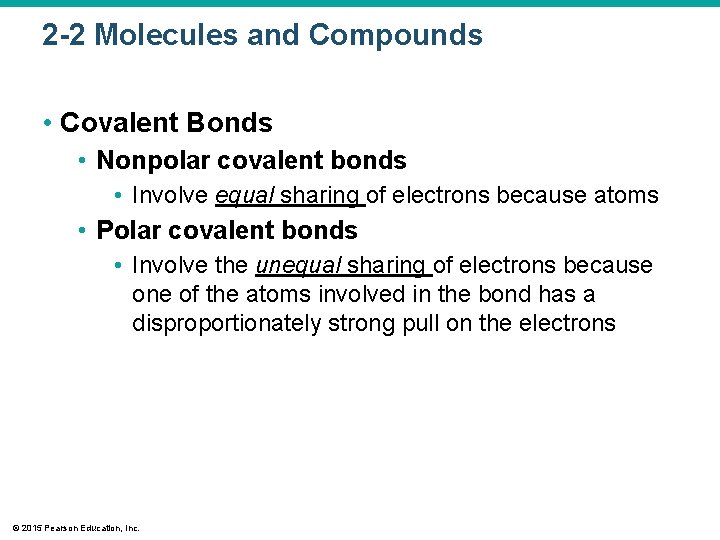
2 -2 Molecules and Compounds • Covalent Bonds • Nonpolar covalent bonds • Involve equal sharing of electrons because atoms • Polar covalent bonds • Involve the unequal sharing of electrons because one of the atoms involved in the bond has a disproportionately strong pull on the electrons © 2015 Pearson Education, Inc.

2 -2 Molecules and Compounds • Hydrogen Bonds • Bonds between adjacent molecules, not atoms • Hydrogen bonds between H 2 O molecules cause surface tension © 2015 Pearson Education, Inc.

2 -2 Molecules and Compounds • States of Matter • Solid • Constant volume and shape • Liquid • Constant volume but changes shape • Gas • Changes volume and shape © 2015 Pearson Education, Inc.

2 -3 Chemical Reactions • In a Chemical Reaction • Either new bonds are formed or existing bonds are broken • Reactants • Materials going into a reaction • Products • Materials coming out of a reaction • Metabolism • All of the reactions that are occurring at one time © 2015 Pearson Education, Inc.

2 -3 Chemical Reactions • Basic Energy Concepts • Energy • The power to do work • Work • A change in mass or distance • Kinetic energy • Energy of motion • Potential energy • Stored energy • Chemical energy • Potential energy stored in chemical bonds © 2015 Pearson Education, Inc.

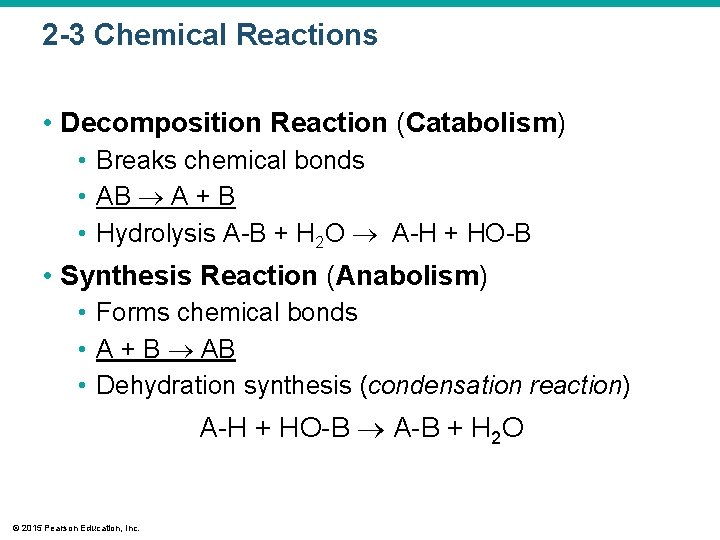
2 -3 Chemical Reactions • Decomposition Reaction (Catabolism) • Breaks chemical bonds • AB A + B • Hydrolysis A-B + H 2 O A-H + HO-B • Synthesis Reaction (Anabolism) • Forms chemical bonds • A + B AB • Dehydration synthesis (condensation reaction) A-H + HO-B A-B + H 2 O © 2015 Pearson Education, Inc.

2 -3 Chemical Reactions • Exchange Reaction • Involves decomposition first, then synthesis • AB + CD AD + CB © 2015 Pearson Education, Inc.

2 -3 Chemical Reactions • Reversible Reaction • A + B ↔ AB • At equilibrium the amounts of chemicals do not change even though the reactions are still occurring © 2015 Pearson Education, Inc.

2 -4 Enzymes • Chemical Reactions • In cells, cannot start without help • Activation energy is the amount of energy needed to get a reaction started • Enzymes are protein catalysts that lower the activation energy of reactions © 2015 Pearson Education, Inc.

2 -4 Enzymes • Exergonic (Exothermic) Reactions • Produce more energy than they use • Endergonic (Endothermic) Reactions • Use more energy than they produce © 2015 Pearson Education, Inc.

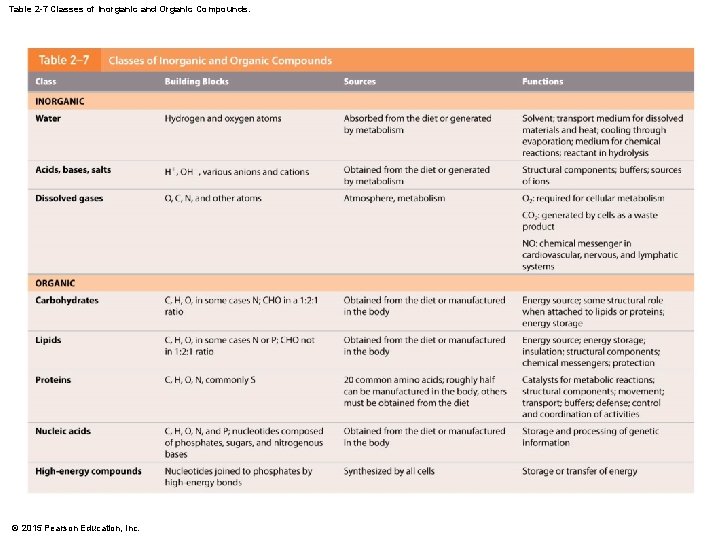
2 -5 Inorganic and Organic Compounds • Nutrients • Essential molecules obtained from food • Metabolites • Molecules made or broken down in the body • Inorganic Compounds • Molecules not based on carbon and hydrogen • Carbon dioxide, oxygen, water, and inorganic acids, bases, and salts • Organic Compounds • Molecules based on carbon and hydrogen • Carbohydrates, proteins, lipids, and nucleic acids © 2015 Pearson Education, Inc.

2 -6 Properties of Water • The Properties of Aqueous Solutions • Electrolytes are inorganic ions that conduct electricity in solution • Electrolyte imbalance seriously disturbs vital body functions • Water accounts for up to two-thirds of your total body weight © 2015 Pearson Education, Inc.

2 -6 Properties of Water • The Properties of Aqueous Solutions • Hydrophilic and hydrophobic compounds • Hydrophilic • hydro- = water, philos = loving • Interacts with water • Includes ions and polar molecules • Hydrophobic • phobos = fear • Does NOT interact with water • Includes nonpolar molecules, fats, and oils © 2015 Pearson Education, Inc.

2 -6 Properties of Water • Colloids and Suspensions • Colloid • A solution of very large organic molecules • For example, blood plasma • Suspension • A solution in which particles settle (sediment) • For example, whole blood • Concentration • The amount of solute in a solvent (mol/L, mg/m. L) © 2015 Pearson Education, Inc.

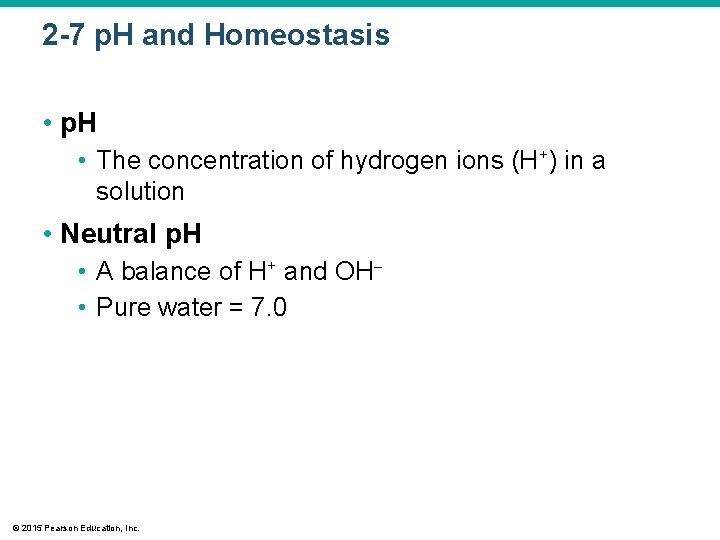
2 -7 p. H and Homeostasis • p. H • The concentration of hydrogen ions (H+) in a solution • Neutral p. H • A balance of H+ and OH • Pure water = 7. 0 © 2015 Pearson Education, Inc.

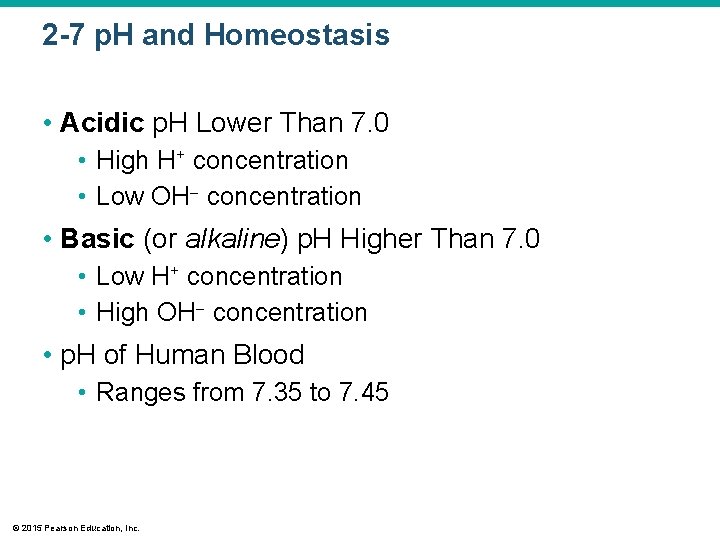
2 -7 p. H and Homeostasis • Acidic p. H Lower Than 7. 0 • High H+ concentration • Low OH concentration • Basic (or alkaline) p. H Higher Than 7. 0 • Low H+ concentration • High OH concentration • p. H of Human Blood • Ranges from 7. 35 to 7. 45 © 2015 Pearson Education, Inc.

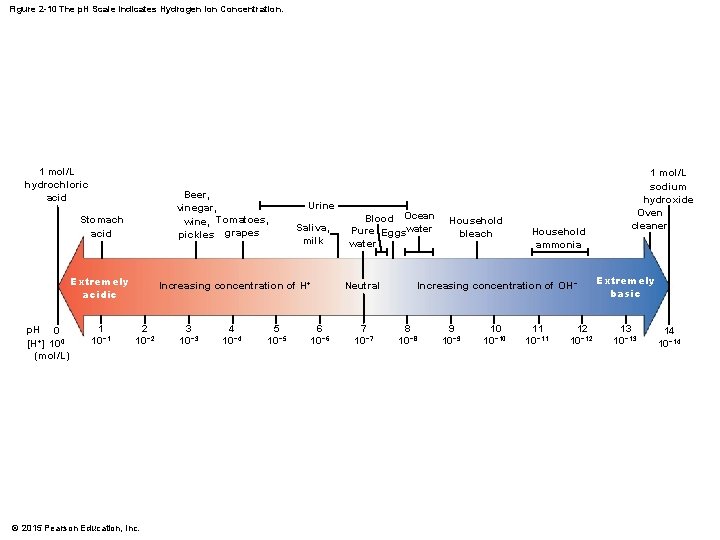
2 -7 p. H and Homeostasis • p. H Scale • Has an inverse relationship with H+ concentration • More H+ ions means lower p. H, fewer H+ ions means higher p. H © 2015 Pearson Education, Inc.

Figure 2 -10 The p. H Scale Indicates Hydrogen Ion Concentration. 1 mol/L hydrochloric acid Beer, vinegar, wine, Tomatoes, pickles grapes Stomach acid Extremely acidic p. H 0 [H+] 100 (mol/L) 1 10− 1 Urine Saliva, milk Increasing concentration of H+ 2 10− 2 © 2015 Pearson Education, Inc. 3 10− 3 4 10− 4 5 10− 5 6 10− 6 Blood Ocean Pure Eggswater Neutral 7 10− 7 Household bleach Household ammonia Increasing concentration of OH− 8 10− 8 9 10− 9 10 10− 10 11 10− 11 12 10− 12 1 mol/L sodium hydroxide Oven cleaner Extremely basic 13 10− 13 14 10− 14

2 -8 Inorganic Compounds • Buffers • Weak acid/salt compounds • Neutralize either strong acid or strong base • Antacids • Basic compounds that neutralize acid and form a salt • Alka-Seltzer, Tums, Rolaids, etc. • Salts • Solutes that dissociate into cations and anions other than hydrogen ions and hydroxide ions © 2015 Pearson Education, Inc.

2 -9 Carbohydrates • Organic Molecules • Contain H, C, and usually O • Are covalently bonded • • Carbohydrates Lipids Proteins (or amino acids) Nucleic acids © 2015 Pearson Education, Inc.

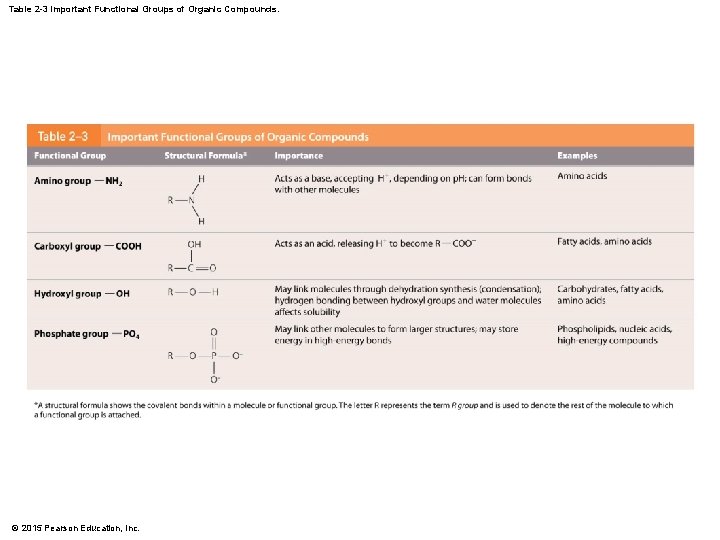
Table 2 -3 Important Functional Groups of Organic Compounds. © 2015 Pearson Education, Inc.

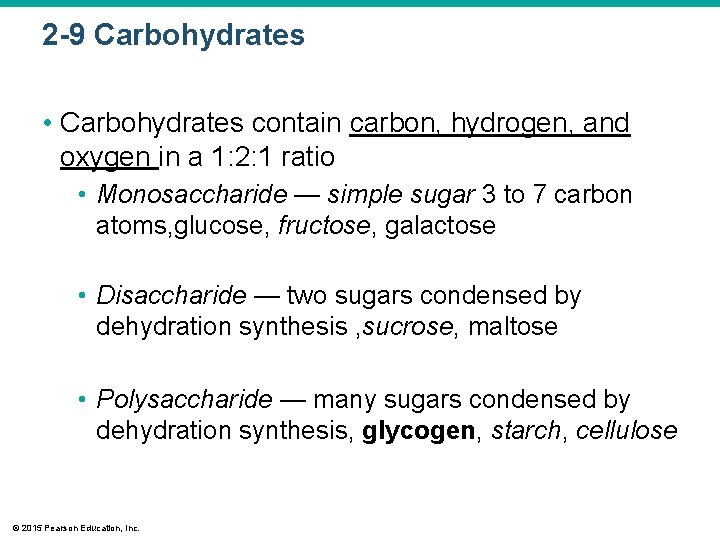
2 -9 Carbohydrates • Carbohydrates contain carbon, hydrogen, and oxygen in a 1: 2: 1 ratio • Monosaccharide — simple sugar 3 to 7 carbon atoms, glucose, fructose, galactose • Disaccharide — two sugars condensed by dehydration synthesis , sucrose, maltose • Polysaccharide — many sugars condensed by dehydration synthesis, glycogen, starch, cellulose © 2015 Pearson Education, Inc.

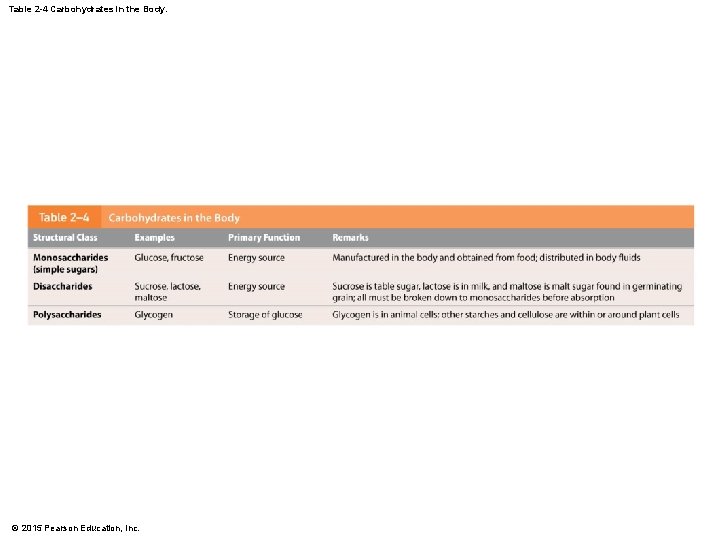
Table 2 -4 Carbohydrates in the Body. © 2015 Pearson Education, Inc.

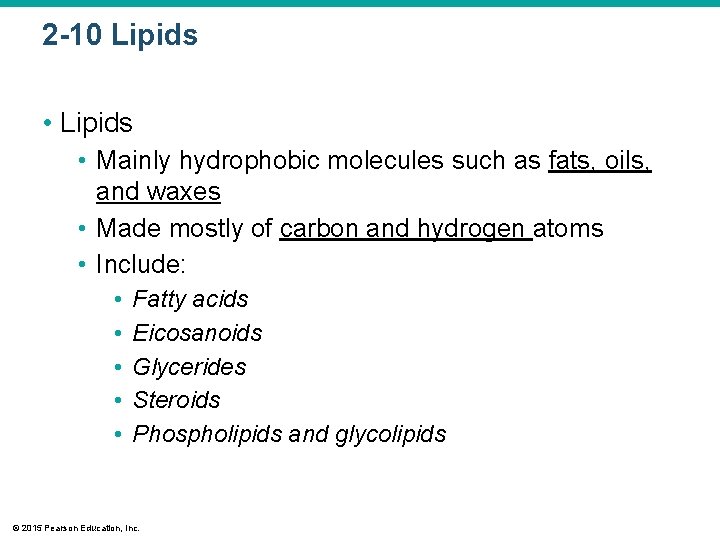
2 -10 Lipids • Mainly hydrophobic molecules such as fats, oils, and waxes • Made mostly of carbon and hydrogen atoms • Include: • • • Fatty acids Eicosanoids Glycerides Steroids Phospholipids and glycolipids © 2015 Pearson Education, Inc.

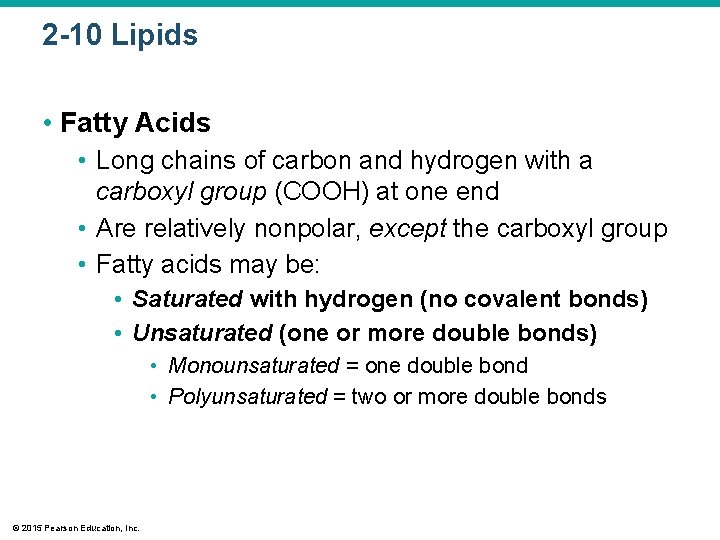
2 -10 Lipids • Fatty Acids • Long chains of carbon and hydrogen with a carboxyl group (COOH) at one end • Are relatively nonpolar, except the carboxyl group • Fatty acids may be: • Saturated with hydrogen (no covalent bonds) • Unsaturated (one or more double bonds) • Monounsaturated = one double bond • Polyunsaturated = two or more double bonds © 2015 Pearson Education, Inc.

Figure 2 -14 b Fatty Acids. Saturated Unsaturated b © 2015 Pearson Education, Inc. A fatty acid is either saturated (has single covalent bonds only) or unsaturated (has one or more double covalent bonds). The presence of a double bond causes a sharp bend in the molecule.

2 -10 Lipids • Glycerides • Fatty acids attached to a glycerol molecule • Triglycerides are three fatty-acid tails • Also called triacylglycerols or neutral fats • Have three important functions 1. Energy source 2. Insulation 3. Protection © 2015 Pearson Education, Inc.

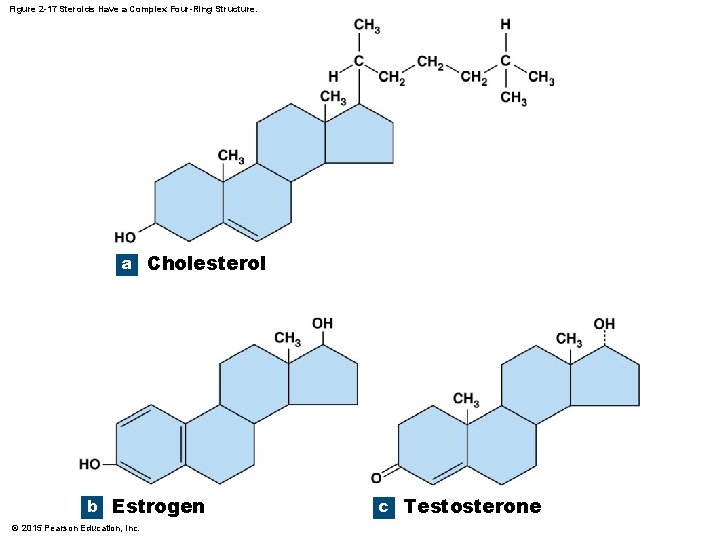
2 -10 Lipids • Steroids • Four rings of carbon and hydrogen with an assortment of functional groups • Types of steroids • Cholesterol • Component of plasma (cell) membranes • Estrogens and testosterone • Sex hormones • Corticosteroids and calcitriol • Metabolic regulation • Bile salts • Derived from steroids © 2015 Pearson Education, Inc.

Figure 2 -17 Steroids Have a Complex Four-Ring Structure. a Cholesterol b Estrogen © 2015 Pearson Education, Inc. c Testosterone

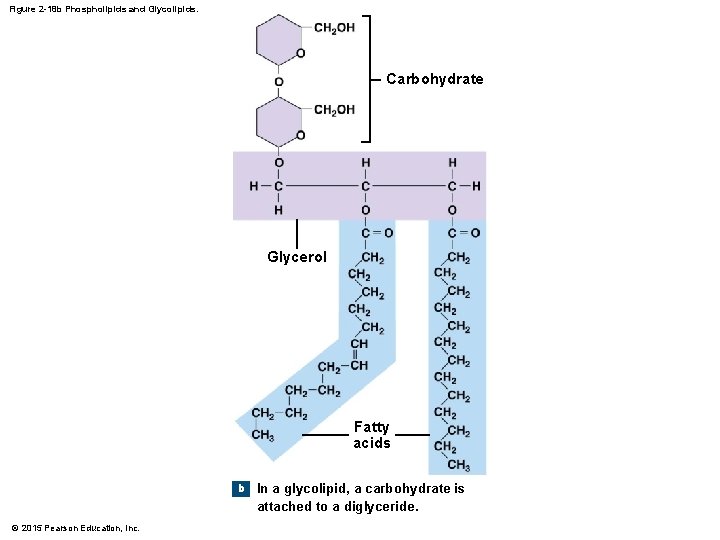
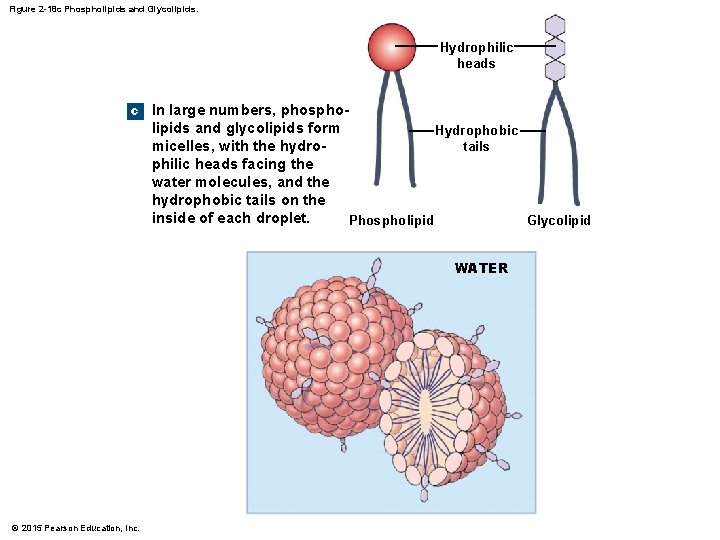
2 -10 Lipids • Phospholipids and Glycolipids • Diglycerides attached to either a phosphate group (phospholipid) or a sugar (glycolipid) • Generally, both have hydrophilic heads and hydrophobic tails and are structural lipids, components of plasma (cell) membranes © 2015 Pearson Education, Inc.

Figure 2 -18 b Phospholipids and Glycolipids. Carbohydrate Glycerol Fatty acids b © 2015 Pearson Education, Inc. In a glycolipid, a carbohydrate is attached to a diglyceride.

Figure 2 -18 c Phospholipids and Glycolipids. Hydrophilic heads c In large numbers, phospholipids and glycolipids form Hydrophobic tails micelles, with the hydrophilic heads facing the water molecules, and the hydrophobic tails on the inside of each droplet. Glycolipid Phospholipid WATER © 2015 Pearson Education, Inc.

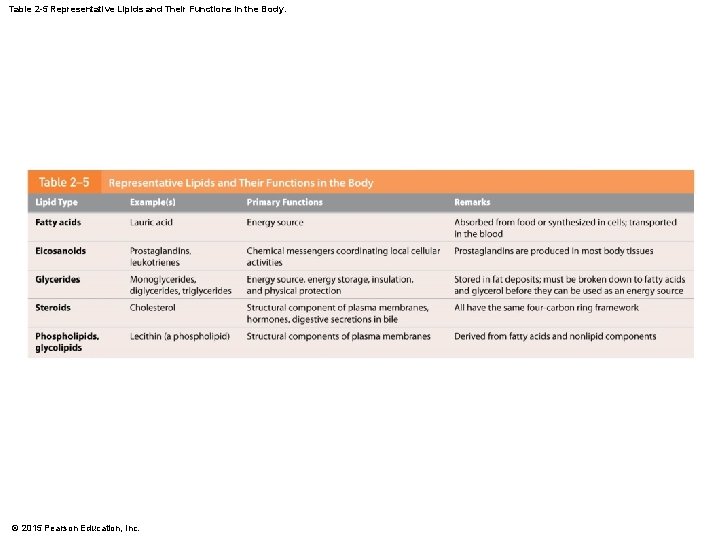
Table 2 -5 Representative Lipids and Their Functions in the Body. © 2015 Pearson Education, Inc.

2 -11 Proteins • Are the most abundant and important organic molecules • Contain basic elements • Carbon (C), hydrogen (H), oxygen (O), and nitrogen (N) • Basic building blocks • 20 amino acids © 2015 Pearson Education, Inc.

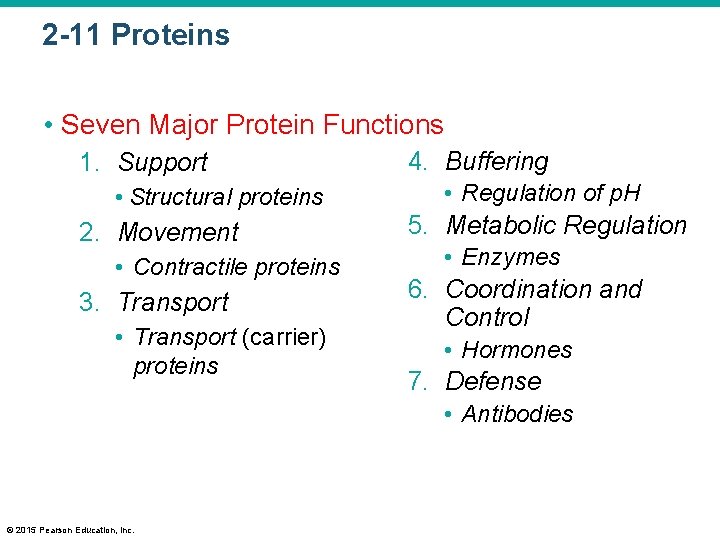
2 -11 Proteins • Seven Major Protein Functions 1. Support • Structural proteins 2. Movement • Contractile proteins 3. Transport • Transport (carrier) proteins 4. Buffering • Regulation of p. H 5. Metabolic Regulation • Enzymes 6. Coordination and Control • Hormones 7. Defense • Antibodies © 2015 Pearson Education, Inc.

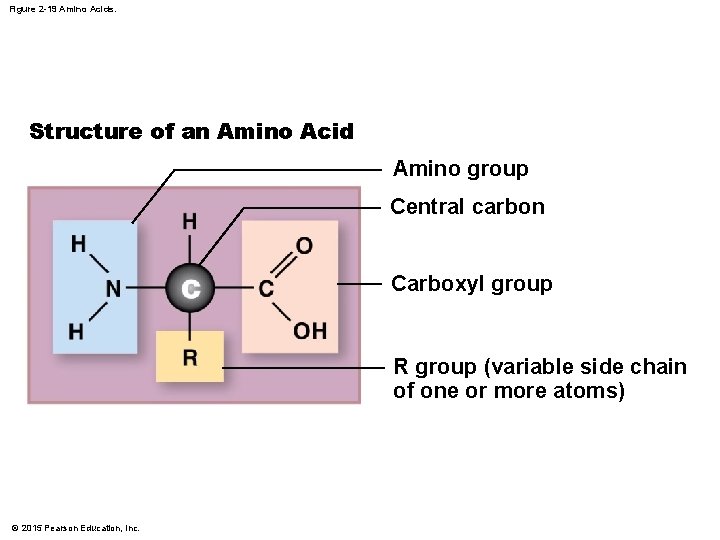
Figure 2 -19 Amino Acids. Structure of an Amino Acid Amino group Central carbon Carboxyl group R group (variable side chain of one or more atoms) © 2015 Pearson Education, Inc.

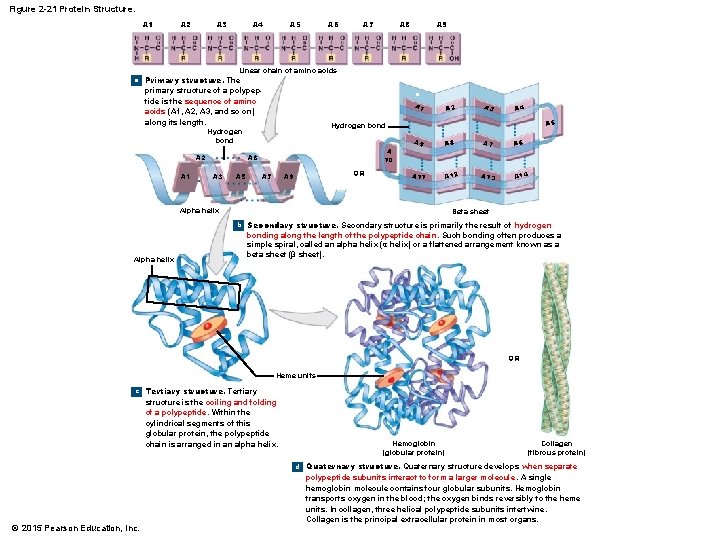
2 -11 Proteins • Protein Shape • Primary structure • The sequence of amino acids along a polypeptide • Secondary structure • Hydrogen bonds form spirals or pleats • Tertiary structure • Secondary structure folds into a unique shape • Quaternary structure • Final protein shape — several tertiary structures together © 2015 Pearson Education, Inc.

Figure 2 -21 Protein Structure. A 1 A 2 A 3 A 4 A 5 A 6 A 7 A 8 A 9 Linear chain of amino acids a Primary structure. The primary structure of a polypeptide is the sequence of amino acids (A 1, A 2, A 3, and so on) along its length. a A 1 A 10 A 6 A 3 A 5 A 3 A 4 A 5 Hydrogen bond A 2 A 7 OR A 9 A 8 A 7 A 6 A 11 A 12 A 13 A 14 Alpha helix Beta sheet b Secondary structure is primarily the result of hydrogen Alpha helix bonding along the length of the polypeptide chain. Such bonding often produces a simple spiral, called an alpha helix (α helix) or a flattened arrangement known as a beta sheet (β sheet). OR Heme units c Tertiary structure is the coiling and folding of a polypeptide. Within the cylindrical segments of this globular protein, the polypeptide chain is arranged in an alpha helix. Hemoglobin (globular protein) Collagen (fibrous protein) d Quaternary structure develops when separate © 2015 Pearson Education, Inc. polypeptide subunits interact to form a larger molecule. A single hemoglobin molecule contains four globular subunits. Hemoglobin transports oxygen in the blood; the oxygen binds reversibly to the heme units. In collagen, three helical polypeptide subunits intertwine. Collagen is the principal extracellular protein in most organs.

2 -11 Proteins • Fibrous Proteins • Structural sheets or strands • Globular Proteins • Soluble spheres with active functions • Protein function is based on shape • Shape is based on sequence of amino acids © 2015 Pearson Education, Inc.

2 -11 Proteins • Enzyme Function • Enzymes are catalysts • Proteins that lower the activation energy of a chemical reaction • Cofactor • An ion or molecule that binds to an enzyme before substrates can bind • Coenzyme • Nonprotein organic cofactors (vitamins) • Isozymes • Two enzymes that can catalyze the same reaction © 2015 Pearson Education, Inc.

2 -12 Nucleic Acids • Are large organic molecules, found in the nucleus, which store and process information at the molecular level • Deoxyribonucleic acid (DNA) • • Determines inherited characteristics Directs protein synthesis Controls enzyme production Controls metabolism • Ribonucleic acid (RNA) • Controls intermediate steps in protein synthesis © 2015 Pearson Education, Inc.

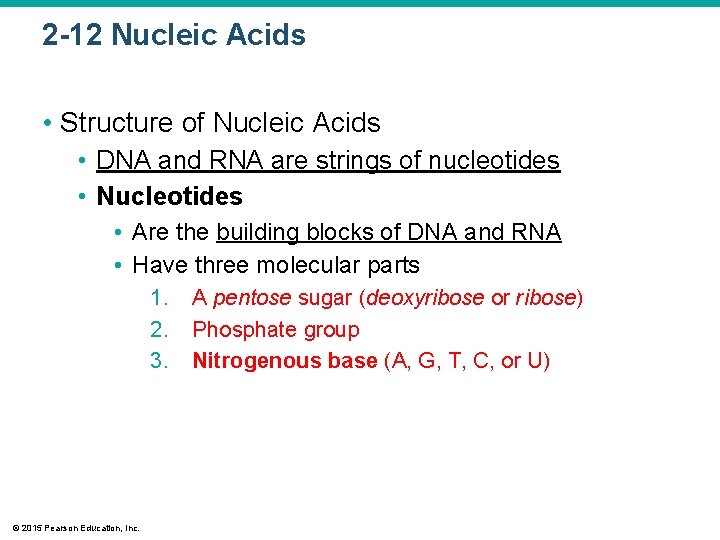
2 -12 Nucleic Acids • Structure of Nucleic Acids • DNA and RNA are strings of nucleotides • Nucleotides • Are the building blocks of DNA and RNA • Have three molecular parts 1. 2. 3. © 2015 Pearson Education, Inc. A pentose sugar (deoxyribose or ribose) Phosphate group Nitrogenous base (A, G, T, C, or U)

Figure 2 -23 a Nucleotides and Nitrogenous Bases. a Nucleotide structure The nitrogenous base may be a purine or a pyrimidine. Phosphate group Sugar Nitrogenous base © 2015 Pearson Education, Inc.

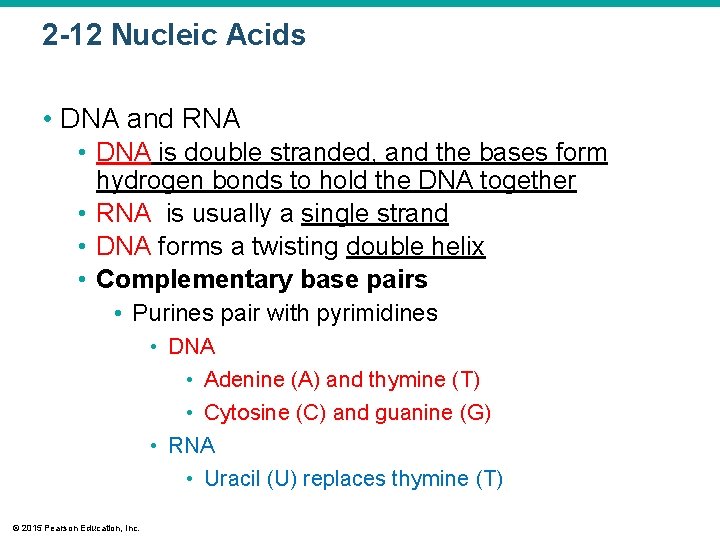
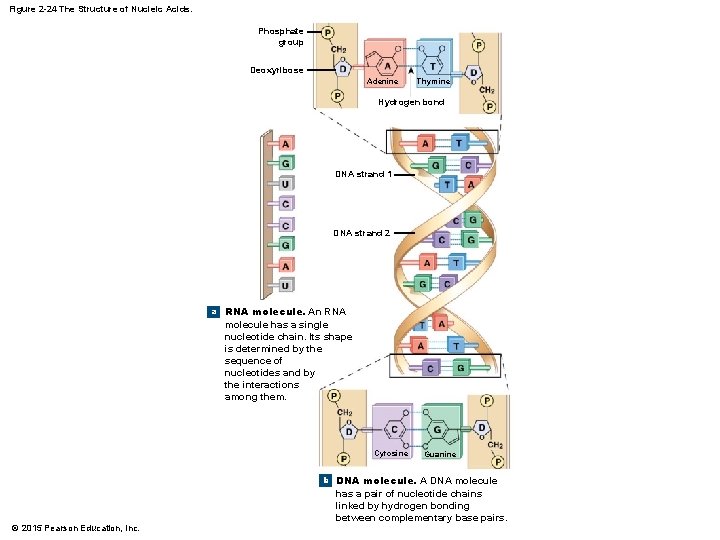
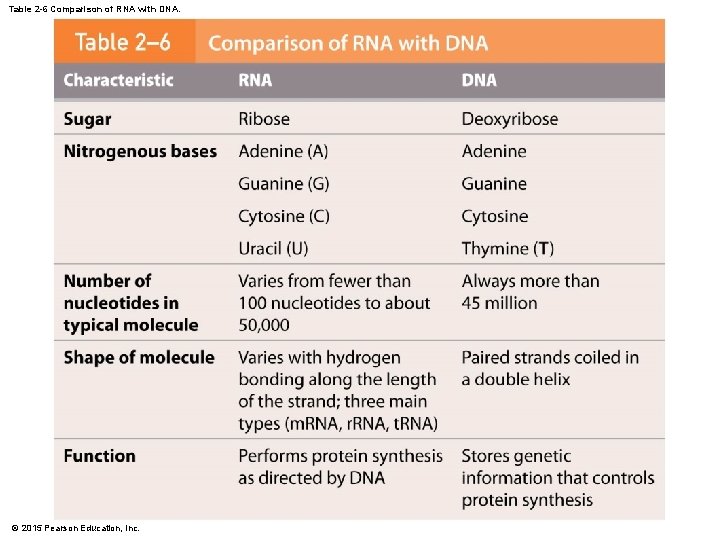
2 -12 Nucleic Acids • DNA and RNA • DNA is double stranded, and the bases form hydrogen bonds to hold the DNA together • RNA is usually a single strand • DNA forms a twisting double helix • Complementary base pairs • Purines pair with pyrimidines • DNA • Adenine (A) and thymine (T) • Cytosine (C) and guanine (G) • RNA • Uracil (U) replaces thymine (T) © 2015 Pearson Education, Inc.

Figure 2 -24 The Structure of Nucleic Acids. Phosphate group Deoxyribose Adenine Thymine Hydrogen bond DNA strand 1 DNA strand 2 a RNA molecule. An RNA molecule has a single nucleotide chain. Its shape is determined by the sequence of nucleotides and by the interactions among them. Cytosine Guanine b DNA molecule. A DNA molecule © 2015 Pearson Education, Inc. has a pair of nucleotide chains linked by hydrogen bonding between complementary base pairs.

2 -12 Nucleic Acids • Types of RNA • Messenger RNA (m. RNA) • Transfer RNA (t. RNA) • Ribosomal RNA (r. RNA) © 2015 Pearson Education, Inc.

Table 2 -6 Comparison of RNA with DNA. © 2015 Pearson Education, Inc.

2 -13 High-Energy Compounds • Nucleotides Can Be Used to Store Energy • Adenosine diphosphate (ADP) • Two phosphate groups; di- = 2 • Adenosine triphosphate (ATP) • Three phosphate groups; tri- = 3 © 2015 Pearson Education, Inc.

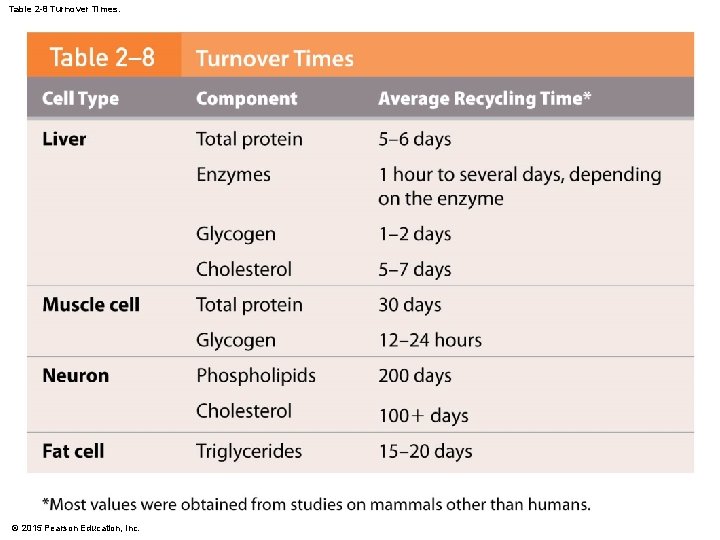
2 -14 Chemicals and Cells • Biochemical building blocks form functional units called cells • Metabolic turnover lets your body grow, change, and adapt to new conditions and activities • Your body recycles and renews all of its chemical components at intervals ranging from minutes to years © 2015 Pearson Education, Inc.

Table 2 -7 Classes of Inorganic and Organic Compounds. © 2015 Pearson Education, Inc.

Table 2 -8 Turnover Times. © 2015 Pearson Education, Inc.
 The chemical level of organization
The chemical level of organization The chemical level of organization chapter 2
The chemical level of organization chapter 2 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Trinitrogen monosulfide formula
Trinitrogen monosulfide formula Are kc and kp equal
Are kc and kp equal Chapter 4 the tissue level of organization
Chapter 4 the tissue level of organization Chapter 4 the tissue level of organization
Chapter 4 the tissue level of organization Chapter 4 the tissue level of organization
Chapter 4 the tissue level of organization Chapter 3 the cellular level of organization
Chapter 3 the cellular level of organization Process organization in computer organization
Process organization in computer organization Block organization essay
Block organization essay Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worm breton
Tư thế worm breton Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Các môn thể thao bắt đầu bằng từ đua
Các môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V. c c
V. c c Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tia chieu sa te
Tia chieu sa te Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Bảng số nguyên tố
Bảng số nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Chapter 10 chemical reactions
Chapter 10 chemical reactions Chapter 9 chapter assessment chemical reactions
Chapter 9 chapter assessment chemical reactions Representative metal
Representative metal Organization level
Organization level Organization-level diagnostic model
Organization-level diagnostic model Organization level
Organization level What is the least complex level of organization
What is the least complex level of organization Level of organization in ecology
Level of organization in ecology Level of organization organ system
Level of organization organ system Levels of data management
Levels of data management Smallest to largest level of organization
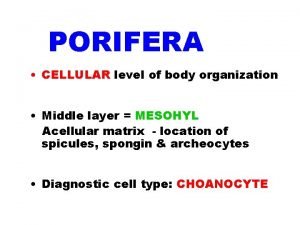
Smallest to largest level of organization Cellular level of organization porifera
Cellular level of organization porifera Example for management information system
Example for management information system Three level cache organization
Three level cache organization What level of organization
What level of organization Cnidarians level of organization
Cnidarians level of organization