Chapter 2 The Chemical Level of Organization Matter




























- Slides: 37

Chapter 2 The Chemical Level of Organization • Matter – Anything that has mass and takes up space • Atom- The smallest particle into which a substance can be broken by ordinary chemical means. • Element – Groups of the same type of atom • Compound – Two or more elements chemically combined ex. H 2 O

MOLECULE- GROUPS OF ATOMS BONDED TOGETHER & ACTING AS A GROUP ORGANELLES- BASIC STRUCTURES WITHIN CELLS – has a specific function CELL- BASIC UNIT OF LIVING ORGANISMS

• TISSUE- TISSUES ARE GROUPS OF CELLS WITH A COMMON FUNCTION. • ORGAN- OFTEN LARGE AND COMPOSED OF SEVERAL DIFFERENT TISSUES • ORGAN SYSTEM- A GROUP OF ORGANS CARRYING OUT A MAJOR BODY PROCESS • ORGANISM- (A GROUP OF ORGAN SYSTEMS IN AN INDIVIDUAL) – any living thing

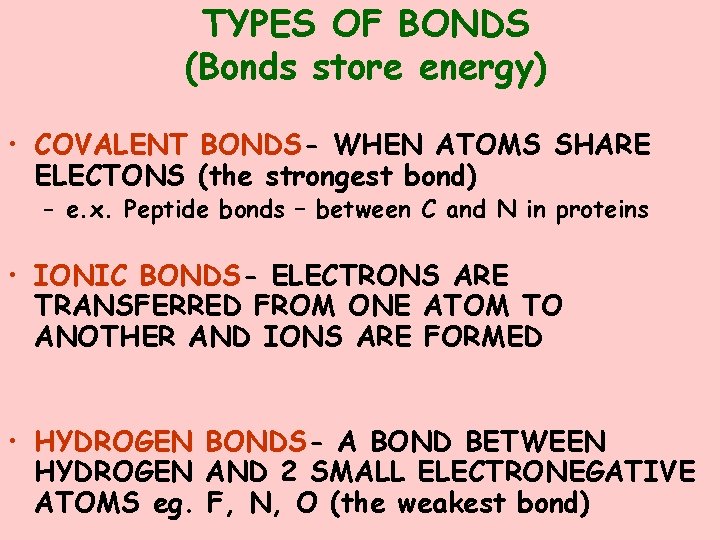
TYPES OF BONDS (Bonds store energy) • COVALENT BONDS- WHEN ATOMS SHARE ELECTONS (the strongest bond) – e. x. Peptide bonds – between C and N in proteins • IONIC BONDS- ELECTRONS ARE TRANSFERRED FROM ONE ATOM TO ANOTHER AND IONS ARE FORMED • HYDROGEN BONDS- A BOND BETWEEN HYDROGEN AND 2 SMALL ELECTRONEGATIVE ATOMS eg. F, N, O (the weakest bond)

Reactions • Chemical Reactions – the process of breaking down chemical bonds and/or forming new ones (a chemical equations shows this) – To do this an activation energy is needed ( the energy that is needed to get the reaction going) • Exothermic – there is a net release of energy (it feels warm) • Endothermic – there is a net absorption of energy (feels cool)

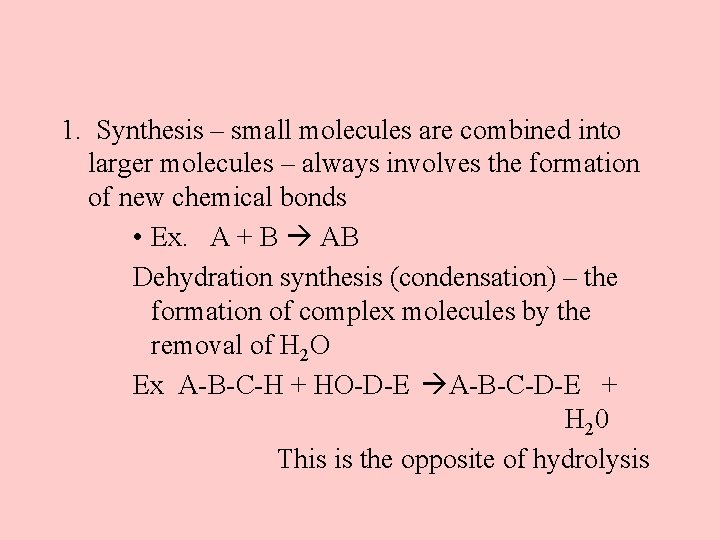
1. Synthesis – small molecules are combined into larger molecules – always involves the formation of new chemical bonds • Ex. A + B AB Dehydration synthesis (condensation) – the formation of complex molecules by the removal of H 2 O Ex A-B-C-H + HO-D-E A-B-C-D-E + H 20 This is the opposite of hydrolysis

• Anabolism – the synthesis of complex organic compounds from simpler compounds (within the body) * requires energy (anabolic steroids)

2. Decomposition – breaks large molecules into smaller ones • AB A + B – Ex. Food broken down – If water is used to breakdown the bonds then it is called hydrolysis (opposite of dehydration synthesis) Ex A-B-C-D-E + H 20 A-B-C-H + HO-D-E

• Catabolism – the breakdown of complex organic molecules into simpler components, it releases E that can do work • Ex. Growth, movement, reproduction

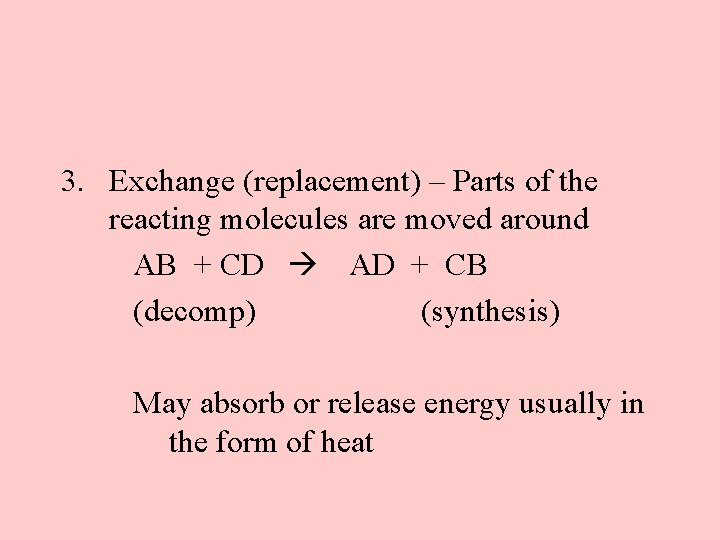
3. Exchange (replacement) – Parts of the reacting molecules are moved around AB + CD AD + CB (decomp) (synthesis) May absorb or release energy usually in the form of heat

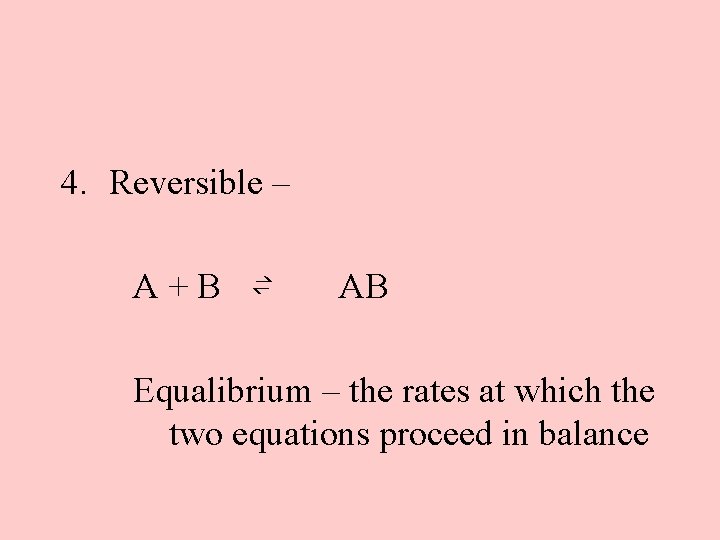
4. Reversible – A+B ⇌ AB Equalibrium – the rates at which the two equations proceed in balance

Enzymes and Chemical Reactions Activation Energy – the amount of E necessary to start a reaction. Enzymes – protein in nature that catalyzes a specific biochemical reaction. (catalyst – accelerates chemical reactions – they enter into a reaction but do not become part of it). They do this by lowering the activation E …… this makes it “safe” for the cell

• Remember enzymes are made up of proteins enzyme A + B AB

Inorganic Compounds • Generally do not contain C – Include H 2 O, Na. Cl, CO 2, NO, and CO – H 2 O – the universal solvent • 67% of body is water • Dehydration synthesis and Hydrolysis • Lubrication (reduces friction) • High heat capacity (Carries heat away) • Requires a lot of E to change temp. – So thermoregulation

– Hydrophilic – reacts with water (hydro – water philos – loving) – Hydrophobic – doesn’t react w/water (phobic – fear) Electrolytes – soluble inorganic compounds whose ions conduct electrical current in solution ex Na. Cl Na+ Cl-

Mixtures • Mixtures – two or more substances not chemically combined – therefore they retain their own properties – Solutions – evenly combined • Solvent – does the dissolving ex. Water • Solute – the substance being dissolved ex. Sugar – Suspensions –The particles will spread out and eventually settle – large particles

• Example sand in water (whole blood) -Colloid – a mixture that has medium particles – held in solution by their association with water ex. Liquid jello…. . Mayonnaise

p. H • p. H IS A WAY TO MEASURE THE ACIDITY OR ALKALINITY (BASICITY) OF A SUBSTANCE • ACIDS HAVE A HIGH CONCENTRATION OF (H+) H 3 O + • BASES HAVE A HIGH CONCENTRATION OF OH-

• Acids and bases are measured on a scale called the p. H scale (power of Hydrogen). This scale measures how many hydronium ions (H 3 O+) are present in a solution (now called just H+). The more hydronium (hydrogen) ions the more acidic (lower) the p. H, also the fewer the hydroxide ions (OH-). The more hydroxide ions the higher the p. H (more alkaline – meaning a base) and the fewer the hydronium ions

• THE p. H SCALE RANGES FROM 1 -14 • ON THE p. H SCALE, 7 IS NEUTRAL • A p. H BELOW 7 IS ACIDIC • A p. H ABOVE 7 IS (BASIC) Alkaline • (see drawings on board)

• THE FARTHER A p. H IS FROM 7, THE STRONGER THE SUBSTANCE IS • BUFFERS ARE SUBSTANCES THAT HELP STABILIZE p. H IN THE BODY • BICARBONATE IS THE MOST Important buffer (how CO 2 mostly appears in the blood – keeps the blood from becoming to acidic or alkaline)

ORGANIC MOLELCULES • ALL ORGANIC MOLECULES CONTAIN CARBON AND ARE FOUND IN LIVING ORGANISMS • THERE ARE 4 MAIN GROUPS – CARBOHYDRATES – PROTEINS – LIPIDS – NUCLEIC ACIDS

CARBOHYDRATES • ARE SUGARS, STARCHES AND RELATED COMPOUNDS • THEIR MOST IMPORTANT FUNCTION IS TO SERVE AS A FUEL SOURCE FOR CELLS • THEY ARE ALSO COMPONENTS OF CELL MEMBRANES AND NUCLEIC ACIDS

• CARBOHYDRATES ARE CHAINS OF CARBON ATOMS THAT ARE BONDED TO HYDROXYL (OH) GROUPS AND HYDROGEN ATOMS (H) • CARBOHYDRATES ARE MADE OF C, H & O • C, H & O ARE IN A 1: 2: 1 RATIO • THERE ARE 3 MAIN GROUPS OF CARBOHYDRATES

3 GROUPS OF CARBOHYDRATES • MONOSACCHARIDES- SIMPLE SUGARS eg. GLUCOSE, FRUCTOSE, GALACTOSE • DISACCHARIDES- 2 SIMPLE SUGARS eg, LACTOSE, SUCROSE, MALTOSE • POLYSACCHARIDES- MANY SIMPLE SUGARS eg. STARCH, CELLULOSE, GLYCOGEN • SUGARS END IN - OSE

MONOSACCHARIDES • GLUCOSE, FRUCTOSE & GALACTOSE ARE ISOMERS. • ALL HAVE THE CHEMICAL FORMULA C 6 H 12 O 6 • ISOMERS- ARE MOLECULES WITH THE SAME CHEMICAL FORMULA, BUT A DIFFERENT ARRANGEMENT OF ATOMS.

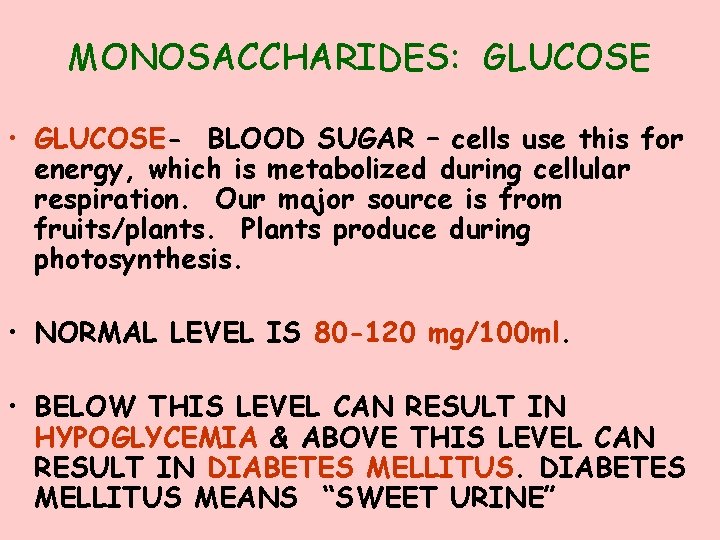
MONOSACCHARIDES: GLUCOSE • GLUCOSE- BLOOD SUGAR – cells use this for energy, which is metabolized during cellular respiration. Our major source is from fruits/plants. Plants produce during photosynthesis. • NORMAL LEVEL IS 80 -120 mg/100 ml. • BELOW THIS LEVEL CAN RESULT IN HYPOGLYCEMIA & ABOVE THIS LEVEL CAN RESULT IN DIABETES MELLITUS MEANS “SWEET URINE”

FRUCTOSE FRUIT SUGAR THE SWEETEST OF ALL THE SUGARS

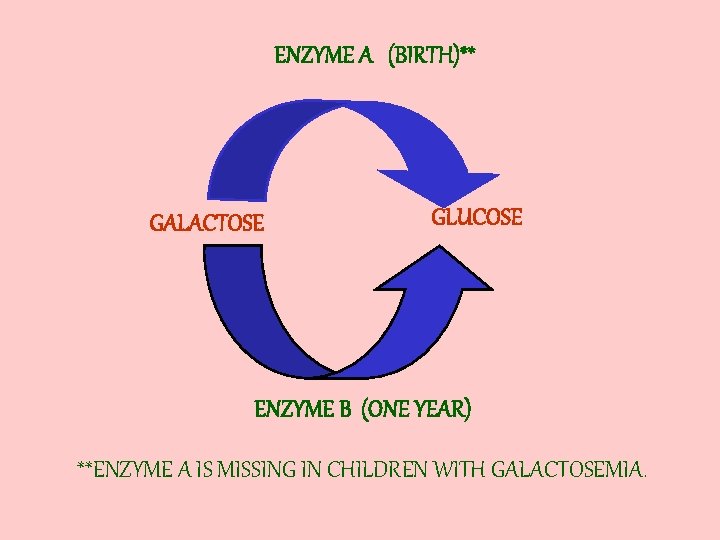
GALACTOSE • NOT FOUND FREE IN NATURE, ONLY IN MILK • WE CANNOT USE GALACTOSE BUT MUST CHANGE IT TO GLUCOSE

ENZYME A (BIRTH)** GALACTOSE GLUCOSE ENZYME B (ONE YEAR) **ENZYME A IS MISSING IN CHILDREN WITH GALACTOSEMIA.

THIS DISORDER IS CALLED GALACTOSEMIA WHICH MEANS GALACTOSE IN THE BLOOD.

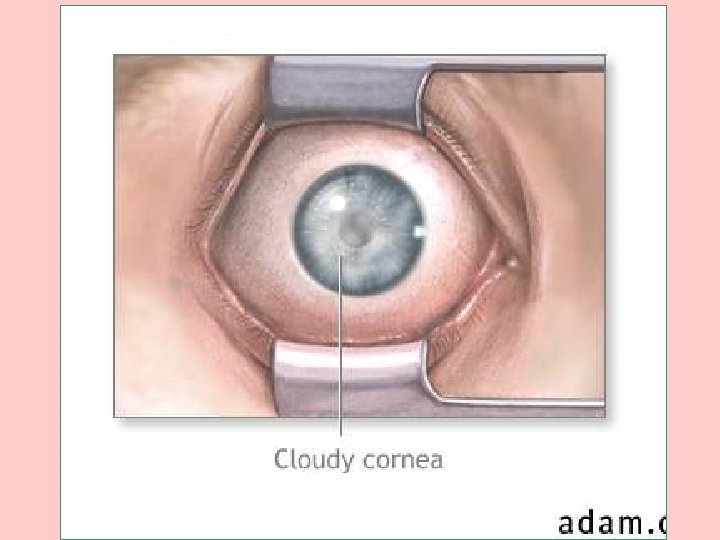
Sx OF GALACTOSEMIA • CLOUDING OF THE CORNEA AT ABOUT 4 WEEKS • ENLARGED LIVER AND SPLEEN AT ABOUT 4 MONTHS • CATARACTS AT ABOUT 6 MONTHS • MENTAL RETARDATION AT 12 MONTHS • ALL ARE REVERSIBLE, EXCEPT MENTAL RETARDATION



HOW WOULD YOU TREAT GALACTOSEMIA? FEED THE BABY A GALACTOSE FREE FORMULA, NO COW’S OR MOTHER’S MILK. BOTH CONTAIN THIS SUGAR.

DISACCHARIDES – when two monosaccharides bond in a dehydration synthesis reaction H+ and OH- are removed and form water LACTOSE: MILK SUGAR GLUCOSE + GALACTOSE SUCROSE: TABLE SUGAR GLUCOSE + FRUCTOSE MALTOSE: MALT SUGAR BREWING INDUSTRY GLUCOSE + GLUCOSE

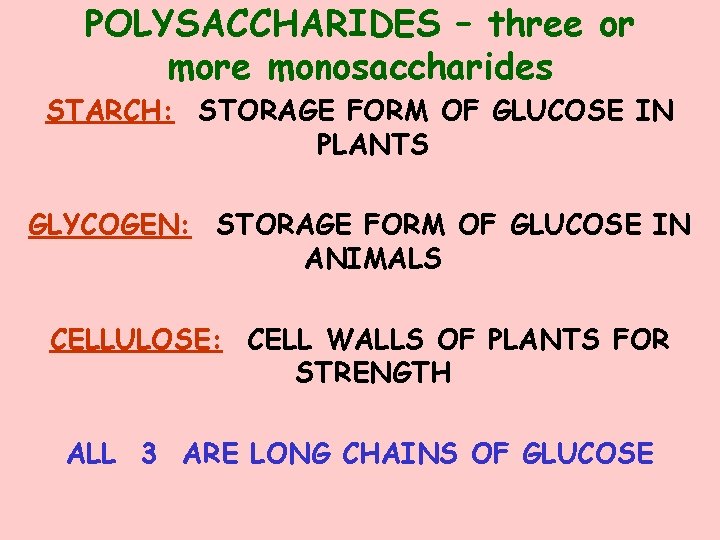
POLYSACCHARIDES – three or more monosaccharides STARCH: STORAGE FORM OF GLUCOSE IN PLANTS GLYCOGEN: STORAGE FORM OF GLUCOSE IN ANIMALS CELLULOSE: CELL WALLS OF PLANTS FOR STRENGTH ALL 3 ARE LONG CHAINS OF GLUCOSE
 The chemical level of organization chapter 2
The chemical level of organization chapter 2 The chemical level of organization chapter 2
The chemical level of organization chapter 2 Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Empirical formula pogil
Empirical formula pogil Modern chemistry chapter 7 test
Modern chemistry chapter 7 test Are kc and kp equal
Are kc and kp equal Chapter 4 the tissue level of organization
Chapter 4 the tissue level of organization Chapter 4 the tissue level of organization
Chapter 4 the tissue level of organization Chapter 4 the tissue level of organization
Chapter 4 the tissue level of organization Chapter 3 the cellular level of organization
Chapter 3 the cellular level of organization Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter White matter
White matter Section 1 composition of matter
Section 1 composition of matter Label the cranial dura septa and associated sinuses.
Label the cranial dura septa and associated sinuses. Section 1 composition of matter chapter 15 answer key
Section 1 composition of matter chapter 15 answer key Gray matter and white matter
Gray matter and white matter What is grey matter
What is grey matter Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter Chemical property definition
Chemical property definition Measurable properties examples
Measurable properties examples Chemical properties of matter
Chemical properties of matter True or false: chemical and physical changes alter matter.
True or false: chemical and physical changes alter matter. Chemical equation states of matter
Chemical equation states of matter Process organization in computer organization
Process organization in computer organization Point by point arrangement
Point by point arrangement Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Thang điểm glasgow
Thang điểm glasgow Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng tiếng nhảy
Các môn thể thao bắt đầu bằng tiếng nhảy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới