Chapter 2 The Chemical Level of Organization Copyright
























- Slides: 62

Chapter 2: The Chemical Level of Organization Copyright 2009, John Wiley & Sons, Inc.

Introduction n Since chemicals compose your body and all body activities are chemical in nature, it is important to become familiar with the language and fundamental concepts of chemistry. Copyright 2009, John Wiley & Sons, Inc.

How Matter is Organized n Chemical Elements q q q All forms of matter are composed of chemical elements which are substances that cannot be split into simpler substances by ordinary chemical means. Elements are given letter abbreviations called chemical symbols. Trace elements are present in tiny amounts Copyright 2009, John Wiley & Sons, Inc.

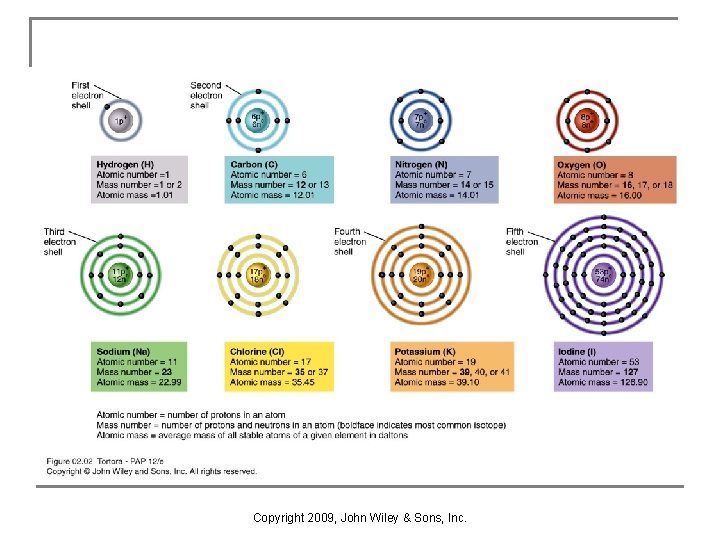
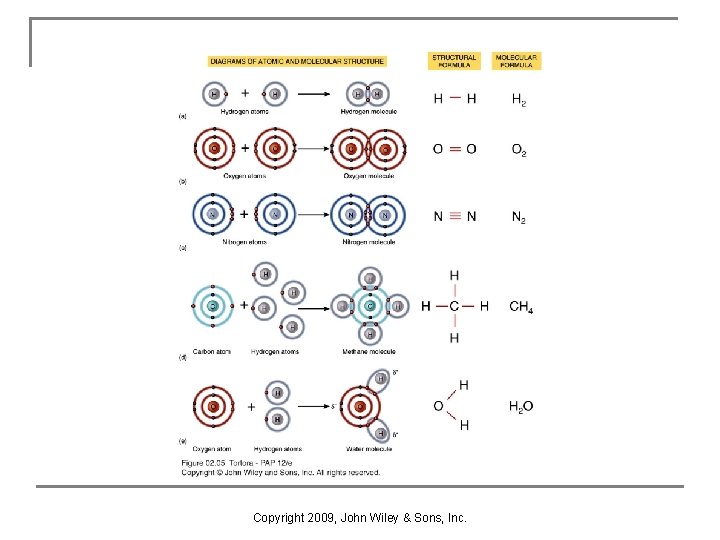
Structure of Atoms Units of matter of all chemical elements are called atoms. An element is a quantity of matter composed of atoms of the same type. Atoms contain: n Nucleus: protons (p+) & neutrons (neutral charge) n Electrons (e-) surround the nucleus as a cloud (electron shells are designated regions of the cloud) n Copyright 2009, John Wiley & Sons, Inc.

2 Representations of the Structure of an Atom Copyright 2009, John Wiley & Sons, Inc.

Atomic Number and Mass Number n n Atomic number is number of protons in the nucleus. Mass number is the sum of its protons and neutrons. Copyright 2009, John Wiley & Sons, Inc.

Copyright 2009, John Wiley & Sons, Inc.

Atomic Mass n n Mass is measured as a dalton (atomic mass unit) Neutron has mass of 1. 008 daltons q q n proton has mass of 1. 007 daltons electron has mass of 0. 0005 dalton Atomic mass (atomic weight) is close to the mass number of its most abundant isotope. Copyright 2009, John Wiley & Sons, Inc.

Atomic Number and Mass Number n Atomic Mass q q The atomic mass, also called the atomic weight, of an element is the average mass of all its naturally occurring isotopes and reflects the relative abundance of isotopes with different mass numbers. The mass of a single atom is slightly less than the sum of the masses of its neutrons, protons, and electrons because some mass (less than 1%) was lost when the atom’s components came together to form an atom. Copyright 2009, John Wiley & Sons, Inc.

Ions, Molecules, & Compounds n Ions q q n an atom that gave up or gained an electron written with its chemical symbol and (+) or (-) Molecule q q atoms share electrons written as molecular formula showing the number of atoms of each element (H 2 O) Copyright 2009, John Wiley & Sons, Inc.

Free Radicals n n A free radical is an electrically charged atom or group of atoms with an unpaired electron in its outermost shell Unstable and highly reactive; can become stable q q q by giving up an electron taking an electron from another molecule Antioxidants are substances that inactivate oxygen-derived free radicals Copyright 2009, John Wiley & Sons, Inc.

Chemical Bonds n n The atoms of a molecule are held together by forces of attraction called chemical bonds. The likelihood that an atom will form a chemical bond with another atom depends on the number of electrons in its outermost shell, also called the valence shell. Copyright 2009, John Wiley & Sons, Inc.

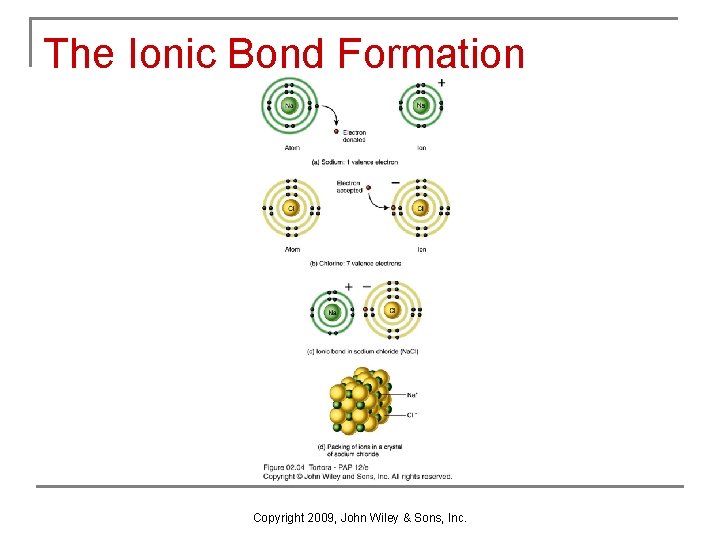
Ionic Bonds n When an atom loses or gains a valence electron, ions are formed (Figure 2. 4 a). q q q Positively and negatively charged ions are attracted to one another. Cations are positively charged ions that have given up one or more electrons (they are electron donors). Anions are negatively charged ions that have picked up one or more electrons that another atom has lost (they are electron acceptors). Copyright 2009, John Wiley & Sons, Inc.

The Ionic Bond Formation Copyright 2009, John Wiley & Sons, Inc.

Covalent Bonds n n Covalent bonds are formed by the atoms of molecules sharing one, two, or three pairs of their valence electrons. q Covalent bonds are common and are the strongest chemical bonds in the body. q Single, double, or triple covalent bonds are formed by sharing one, two, or three pairs of electrons, respectively. Covalent bonds may be nonpolar or polar. q In a nonpolar covalent bond, atoms share the electrons equally; one atom does not attract the shared electrons more strongly than the other atom Copyright 2009, John Wiley & Sons, Inc.

Copyright 2009, John Wiley & Sons, Inc.

Polar Covalent Bonds n Unequal sharing of electrons between atoms. In a water molecule, oxygen attracts the hydrogen electrons more strongly q Oxygen has greater electronegativity as indicated by the negative Greek delta sign. Copyright 2009, John Wiley & Sons, Inc.

Hydrogen Bonds n n n Approximately 5% as strong as covalent bonds Useful in establishing links between molecules or between distant parts of a very large molecule Large 3 -D molecules are often held together by a large number of hydrogen bonds. Copyright 2009, John Wiley & Sons, Inc.

Hydrogen Bonds q q are weak intermolecular bonds; they serve as links between molecules. help determine threedimensional shape give water considerable cohesion which creates a very high surface tension Copyright 2009, John Wiley & Sons, Inc.

Chemical Reactions n n n New bonds form and/or old bonds are broken. Metabolism is “the sum of all the chemical reactions in the body. ” Law of conservation of energy q The total mass of reactants equals the total mass of the products. Copyright 2009, John Wiley & Sons, Inc.

Forms of Energy and Chemical Reactions n Energy is the capacity to do work. n Kinetic energy is the energy associated with matter in motion. n Potential energy is energy stored by matter due to its position. Copyright 2009, John Wiley & Sons, Inc.

Energy Transfer in Chemical Reactions n An exergonic reaction is one in which the bond being broken has more energy than the one formed so that extra energy is released, usually as heat (occurs during catabolism of food molecules). n An endergonic reaction is just the opposite and thus requires that energy be added, usually from a molecule called ATP, to form a bond, as in bonding amino acid molecules together to form proteins. Copyright 2009, John Wiley & Sons, Inc.

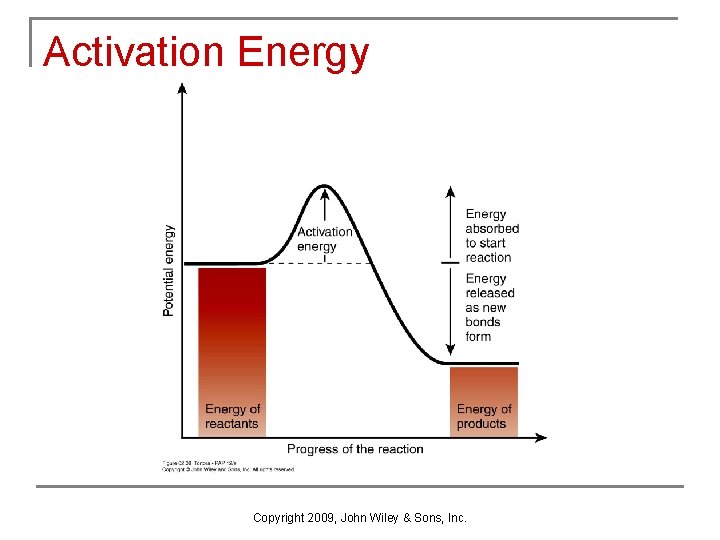
Activation Energy Copyright 2009, John Wiley & Sons, Inc.

Factors that Cause a Collision and Chemical Reaction n n Concentration Temperature Catalysts are chemical compounds that speed up chemical reactions by lowering the activation energy needed for a reaction to occur. A catalyst does not alter the difference in potential energy between the reactants and products. It only lowers the amount of energy needed to get the reaction started. q A catalyst helps to properly orient the colliding particles of matter so that a reaction can occur at a lower collision speed. q The catalyst itself is unchanged at the end of the reaction; it is often re-used many times. Copyright 2009, John Wiley & Sons, Inc.

Catalysts and chemical reactions Copyright 2009, John Wiley & Sons, Inc.

Types of Chemical Reactions n n Synthesis reactions -- Anabolism Decomposition reactions-- Catabolism Exchange reactions Reversible reactions Copyright 2009, John Wiley & Sons, Inc.

Inorganic Compounds and Solutes n Inorganic compounds usually lack carbon and are simple molecules; whereas organic compounds always contain carbon and hydrogen, usually contain oxygen, and always have covalent bonds. Copyright 2009, John Wiley & Sons, Inc.

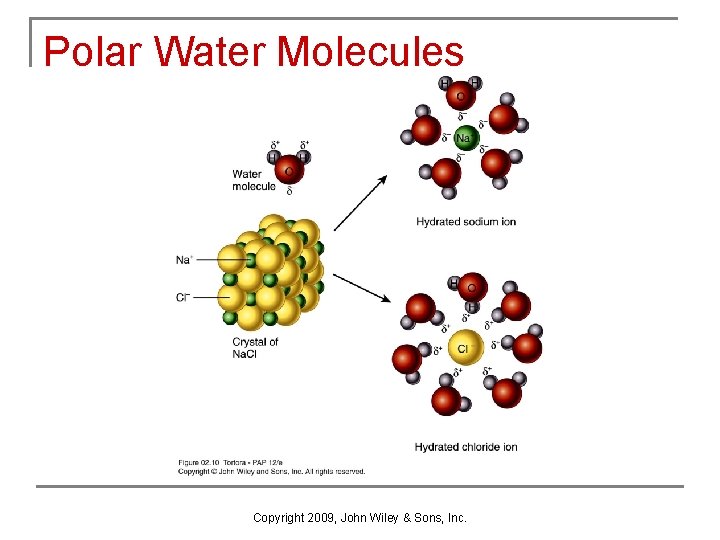
Water q q q Is the most important and abundant inorganic compound in all living systems. Water’s most important property is polarity, the uneven sharing of valence electrons Enables reactants to collide to form products Copyright 2009, John Wiley & Sons, Inc.

Polar Water Molecules Copyright 2009, John Wiley & Sons, Inc.

Water as a Solvent n n In a solution the solvent dissolves the solute. Substances which contain polar covalent bonds and dissolve in water are hydrophilic, while substances which contain non polar covalent bonds are hydrophobic. The polarity of water and its bent shape allow it to interact with several neighboring ions or molecules. Water’s role as a solvent makes it essential for health and survival. Copyright 2009, John Wiley & Sons, Inc.

High Heat Capacity of Water n n Water has a high heat capacity. It can absorb or release a relatively large amount of heat with only a modest change in its own temperature. This property is due to the large number of hydrogen ions in water. Heat of vaporization is also high q amount of heat needed to change from liquid to gas q evaporation of water from the skin removes large amount of heat Copyright 2009, John Wiley & Sons, Inc.

3 Common Mixtures A mixture is a combination of elements or compounds that are physically blended together but are not bound by chemical bonds. n n n Solution: a substance called the solvent dissolves another substance called the solute. Usually there is more solvent than solute in a solution. A colloid differs from a solution mainly on the basis of the size of its particles with the particles in the colloid being large enough to scatter light. Suspension: the suspended material may mix with the liquid or suspending medium for some time, but it will eventually settle out. Copyright 2009, John Wiley & Sons, Inc.

Concentration n The concentration of a molecule is a way of stating the amount of that molecule dissolved in solution. n Percent gives the relative mass of a solute found in a given volume of solution. n A mole is the name for the number of atoms in an atomic weight of that element, or the number of molecules in a molecular weight of that type of molecule, with the molecular weight being the sum of all the atomic weights of the atoms that make up the molecule. Copyright 2009, John Wiley & Sons, Inc.

Dissociation of Acids, Bases, and Salts Copyright 2009, John Wiley & Sons, Inc.

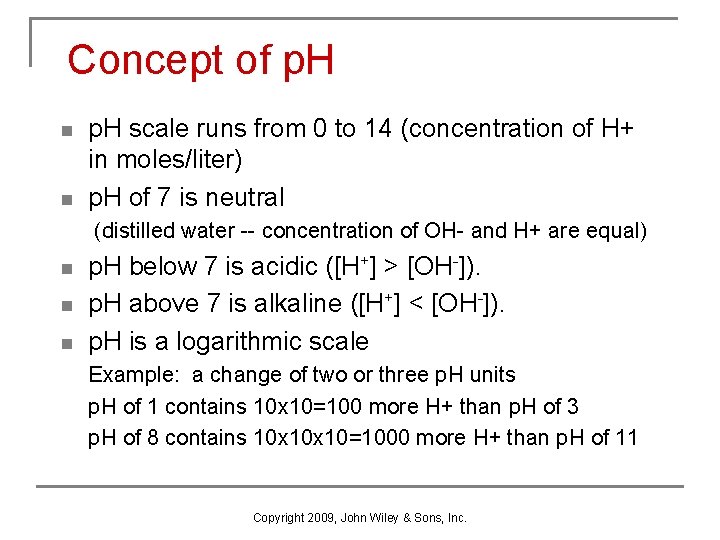
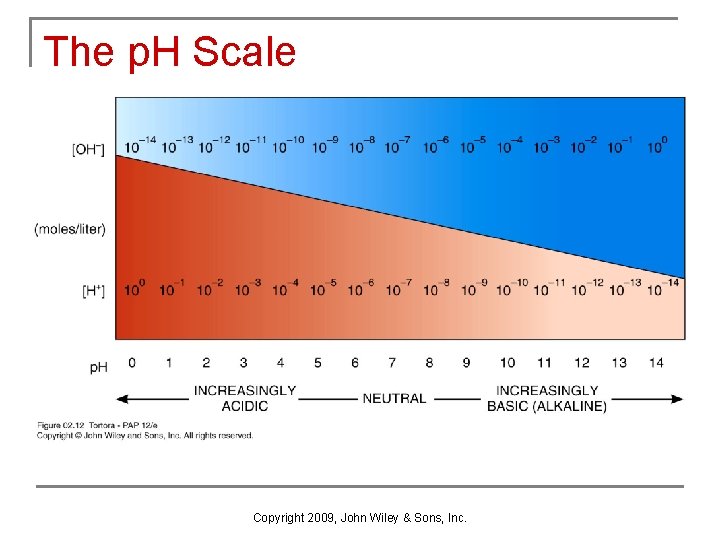
Concept of p. H n n p. H scale runs from 0 to 14 (concentration of H+ in moles/liter) p. H of 7 is neutral (distilled water -- concentration of OH- and H+ are equal) n n n p. H below 7 is acidic ([H+] > [OH-]). p. H above 7 is alkaline ([H+] < [OH-]). p. H is a logarithmic scale Example: a change of two or three p. H units p. H of 1 contains 10 x 10=100 more H+ than p. H of 3 p. H of 8 contains 10 x 10=1000 more H+ than p. H of 11 Copyright 2009, John Wiley & Sons, Inc.

The p. H Scale Copyright 2009, John Wiley & Sons, Inc.

Maintaining p. H: Buffer Systems n The p. H values of different parts of the body are maintained fairly constant by buffer systems, which usually consist of a weak acid and a weak base. q convert strong acids or bases into weak acids or bases. Copyright 2009, John Wiley & Sons, Inc.

Carbon and Its Functional Groups n Many functional groups can attach to carbon skeleton q q n esters, amino, carboxyl, phosphate groups Very large molecules are called macromolecules (or “polymers” if all the monomer subunits are similar) Isomers have the same molecular formulas but different structures (glucose & fructose are both C 6 H 12 O 6) Copyright 2009, John Wiley & Sons, Inc.

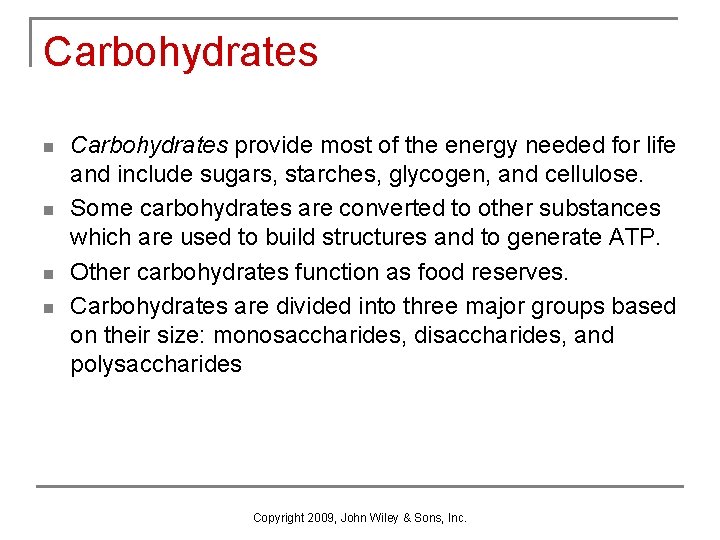
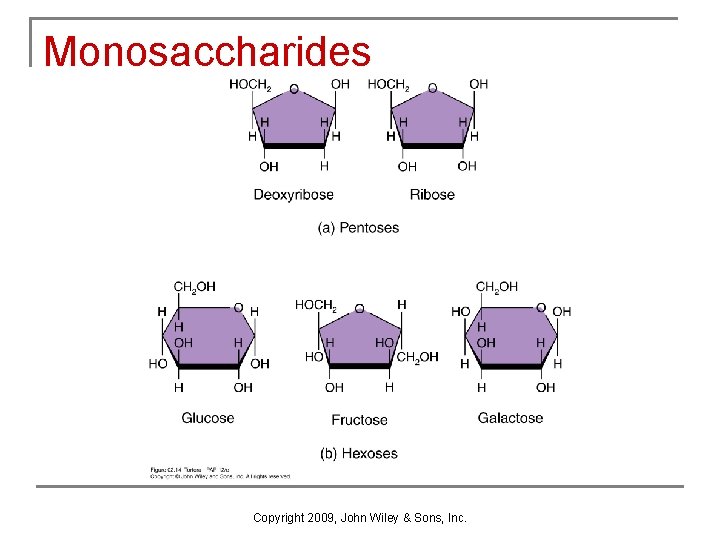
Carbohydrates n n Carbohydrates provide most of the energy needed for life and include sugars, starches, glycogen, and cellulose. Some carbohydrates are converted to other substances which are used to build structures and to generate ATP. Other carbohydrates function as food reserves. Carbohydrates are divided into three major groups based on their size: monosaccharides, disaccharides, and polysaccharides Copyright 2009, John Wiley & Sons, Inc.

Monosaccharides Copyright 2009, John Wiley & Sons, Inc.

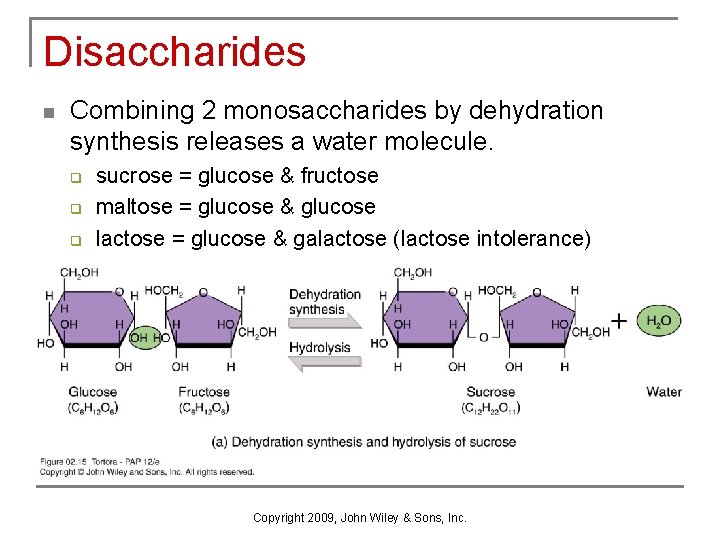
Disaccharides n Combining 2 monosaccharides by dehydration synthesis releases a water molecule. q q q sucrose = glucose & fructose maltose = glucose & glucose lactose = glucose & galactose (lactose intolerance) Copyright 2009, John Wiley & Sons, Inc.

Polysaccharides n n n Polysaccharides are the largest carbohydrates and may contain hundreds of monosaccharides. The principal polysaccharide in the human body is glycogen, which is stored in the liver or skeletal muscles. When blood sugar level drops, the liver hydrolyzes glycogen to yield glucose which is released from the liver into the blood Copyright 2009, John Wiley & Sons, Inc.

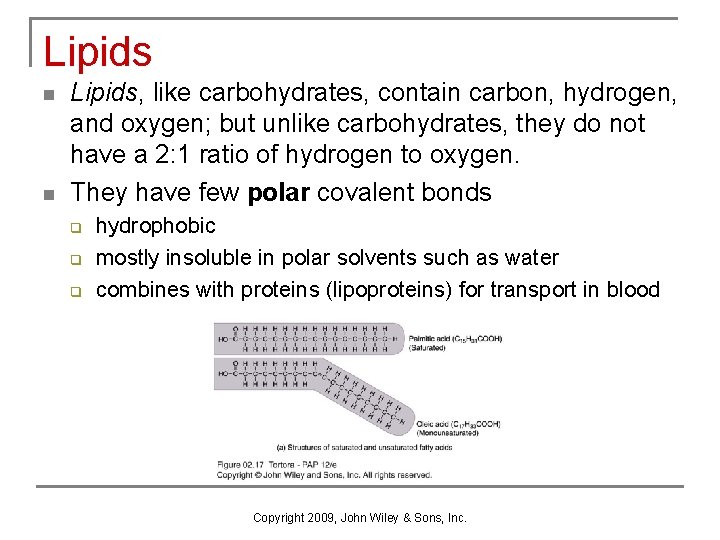
Lipids n n Lipids, like carbohydrates, contain carbon, hydrogen, and oxygen; but unlike carbohydrates, they do not have a 2: 1 ratio of hydrogen to oxygen. They have few polar covalent bonds q q q hydrophobic mostly insoluble in polar solvents such as water combines with proteins (lipoproteins) for transport in blood Copyright 2009, John Wiley & Sons, Inc.

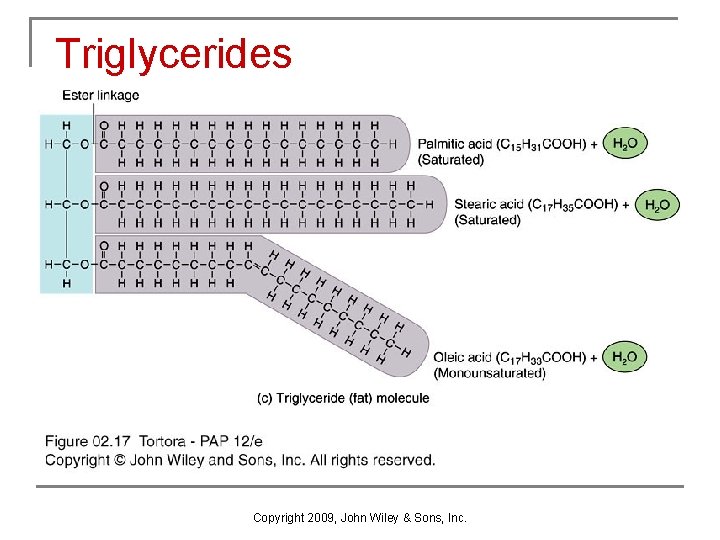
Triglycerides n Triglycerides are the most plentiful lipids in the body and provide protection, insulation, and energy (both immediate and stored). q q At room temperature, triglycerides may be either solid (fats) or liquid (oils). Triglycerides provide more than twice as much energy per gram as either carbohydrates or proteins. Triglyceride storage is virtually unlimited. Excess dietary carbohydrates, proteins, fats, and oils will be deposited in adipose tissue as triglycerides. Copyright 2009, John Wiley & Sons, Inc.

Triglycerides Copyright 2009, John Wiley & Sons, Inc.

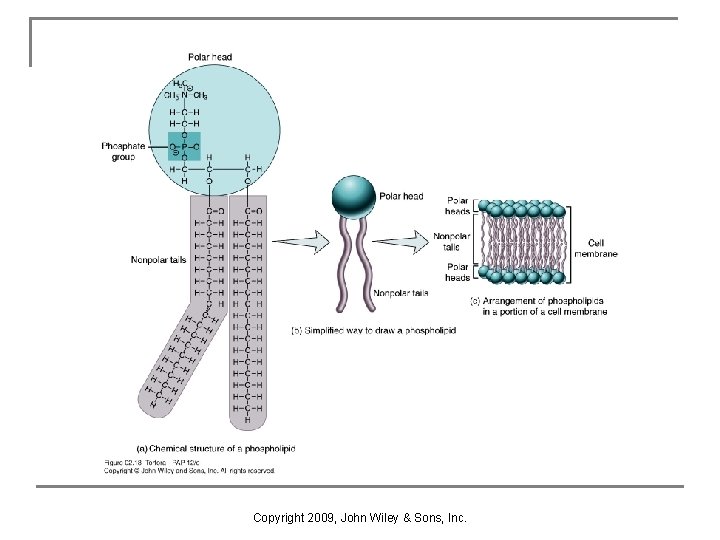
Phospholipids n n Phospholipids are important membrane components. They are amphipathic, with both polar and nonpolar regions (Figure 2. 18). q a polar head n n q a phosphate group (PO 4 -3) & glycerol molecule forms hydrogen bonds with water 2 nonpolar fatty acid tails n interact only with lipids Copyright 2009, John Wiley & Sons, Inc.

Copyright 2009, John Wiley & Sons, Inc.

Steroids n Steroids have four rings of carbon atoms Steroids include q q sex hormone bile salts some vitamins cholesterol, with cholesterol serving as an important component of cell membranes and as starting material for synthesizing other steroids. Copyright 2009, John Wiley & Sons, Inc.

Four Ring Structure of Steroids Copyright 2009, John Wiley & Sons, Inc.

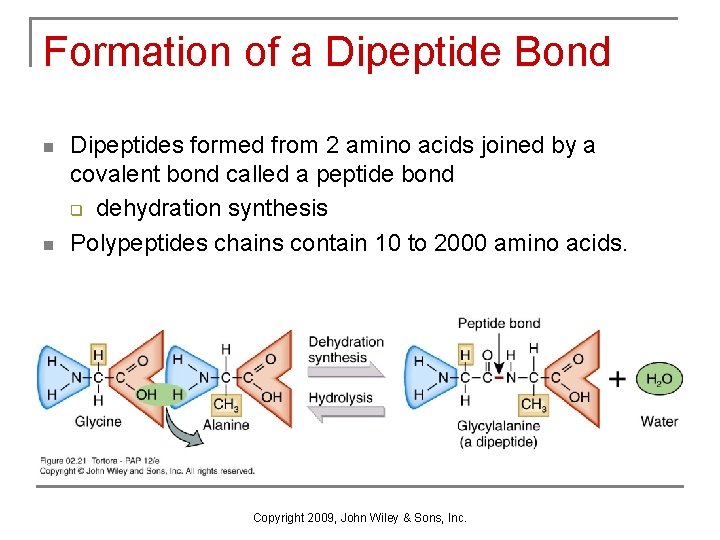
Proteins n Constructed from combinations of 20 amino acids. q dipeptides formed from 2 amino acids joined by a covalent bond called a peptide bond q polypeptides chains formed from 10 to 2000 amino acids. Copyright 2009, John Wiley & Sons, Inc.

Formation of a Dipeptide Bond n n Dipeptides formed from 2 amino acids joined by a covalent bond called a peptide bond q dehydration synthesis Polypeptides chains contain 10 to 2000 amino acids. Copyright 2009, John Wiley & Sons, Inc.

Levels of Structural Organization n Levels of structural organization include q primary q secondary q tertiary q quaternary The resulting shape of the protein greatly influences its ability to recognize and bind to other molecules. Denaturation of a protein by a hostile environment causes loss of its characteristic shape and function. Copyright 2009, John Wiley & Sons, Inc.

Copyright 2009, John Wiley & Sons, Inc.

Enzymes n n Catalysts in living cells are called enzymes. Enzymes are highly specific in terms of the “substrate” with which they react. Enzymes are subject to variety of cellular controls. Enzymes speed up chemical reactions by increasing frequency of collisions, lowering the activation energy and properly orienting the colliding molecules. Copyright 2009, John Wiley & Sons, Inc.

How an Enzyme Works Copyright 2009, John Wiley & Sons, Inc.

H 2 O Substrates Sucrose and Water Enzyme Sucrase Active site of enzyme 1 Enzyme and substrate come together at active site of enzyme, forming an enzyme–substrate complex 3 When reaction is complete, enzyme is unchanged and free to catalyze same reaction again on a new substrate Products Glucose Fructose 2 Enzyme catalyzes reaction and transforms substrate into products Mechanism of enzyme action

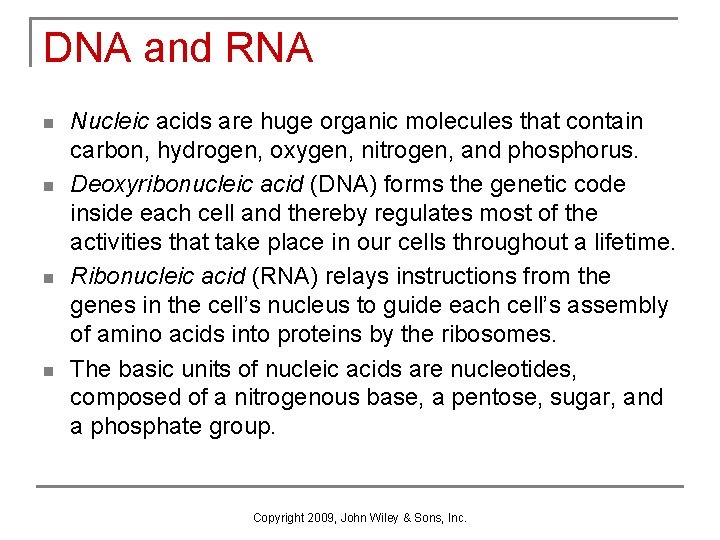
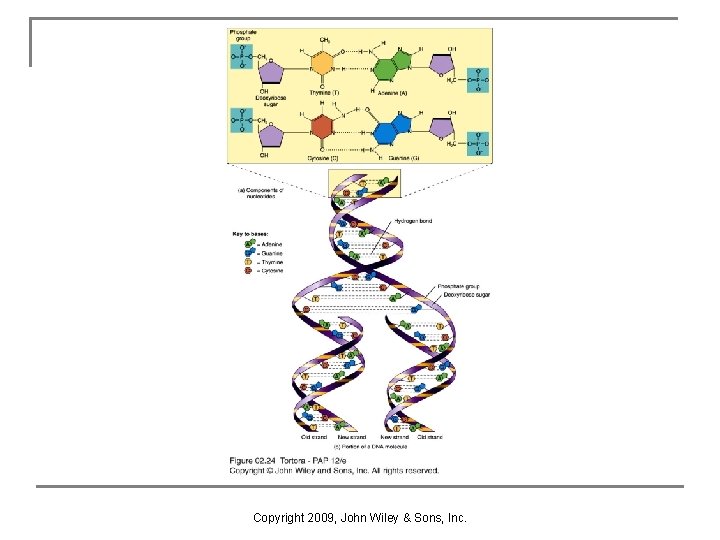
DNA and RNA n n Nucleic acids are huge organic molecules that contain carbon, hydrogen, oxygen, nitrogen, and phosphorus. Deoxyribonucleic acid (DNA) forms the genetic code inside each cell and thereby regulates most of the activities that take place in our cells throughout a lifetime. Ribonucleic acid (RNA) relays instructions from the genes in the cell’s nucleus to guide each cell’s assembly of amino acids into proteins by the ribosomes. The basic units of nucleic acids are nucleotides, composed of a nitrogenous base, a pentose, sugar, and a phosphate group. Copyright 2009, John Wiley & Sons, Inc.

Copyright 2009, John Wiley & Sons, Inc.

RNA Structure n Differs from DNA q q q n single stranded ribose sugar not deoxyribose sugar uracil nitrogenous base replaces thymine Types of RNA within the cell, each with a specific function q q q messenger RNA ribosomal RNA transfer RNA Copyright 2009, John Wiley & Sons, Inc.

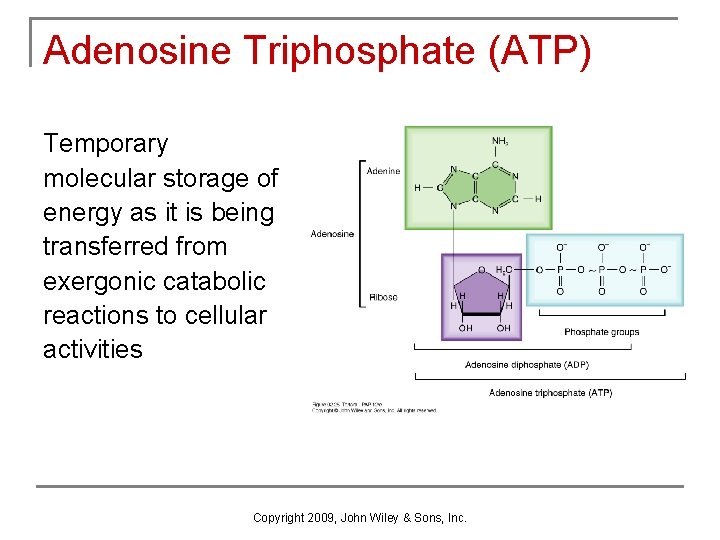
Adenosine Triphosphate (ATP) Temporary molecular storage of energy as it is being transferred from exergonic catabolic reactions to cellular activities Copyright 2009, John Wiley & Sons, Inc.

Formation & Usage of ATP n Hydrolysis of ATP (removal of terminal phosphate group by enzyme -- ATPase) q q n releases energy leaves ADP (adenosine diphosphate) Synthesis of ATP q q enzyme ATP synthase catalyzes the addition of the terminal phosphate group to ADP energy from 1 glucose molecule is used during both anaerobic and aerobic respiration to create 36 to 38 molecules of ATP Copyright 2009, John Wiley & Sons, Inc.

End of Chapter 2 Copyright 2009 John Wiley & Sons, Inc. All rights reserved. Reproduction or translation of this work beyond that permitted in section 117 of the 1976 United States Copyright Act without express permission of the copyright owner is unlawful. Request for further information should be addressed to the Permission Department, John Wiley & Sons, Inc. The purchaser may make back-up copies for his/her own use only and not for distribution or resale. The Publishers assumes no responsibility for errors, omissions, or damages caused by the use of theses programs or from the use of the information herein. Copyright 2009, John Wiley & Sons, Inc.
 The chemical level of organization chapter 2
The chemical level of organization chapter 2 The chemical level of organization chapter 2
The chemical level of organization chapter 2 Empirical formula pogil
Empirical formula pogil Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chapter 4 the tissue level of organization
Chapter 4 the tissue level of organization Chapter 4 the tissue level of organization
Chapter 4 the tissue level of organization Chapter 4 the tissue level of organization
Chapter 4 the tissue level of organization Chapter 3 the cellular level of organization
Chapter 3 the cellular level of organization Process organization in computer organization
Process organization in computer organization Point-by-point arrangement
Point-by-point arrangement Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 1 chemical changes
Section 1 chemical changes Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton Chúa sống lại
Chúa sống lại Kể tên các môn thể thao
Kể tên các môn thể thao Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V. c c
V. c c Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Số nguyên tố là
Số nguyên tố là Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Chapter 10 chemical reactions
Chapter 10 chemical reactions Chapter 9 chapter assessment chemical reactions
Chapter 9 chapter assessment chemical reactions Chapter 9 chemical names and formulas chapter quiz answers
Chapter 9 chemical names and formulas chapter quiz answers Organization level
Organization level Organization-level diagnostic model
Organization-level diagnostic model Middle level management examples
Middle level management examples What is the least complex level of organization
What is the least complex level of organization