Chapter 2 The Chemical Level of Organization KEY



















- Slides: 40

Chapter 2: The Chemical Level of Organization

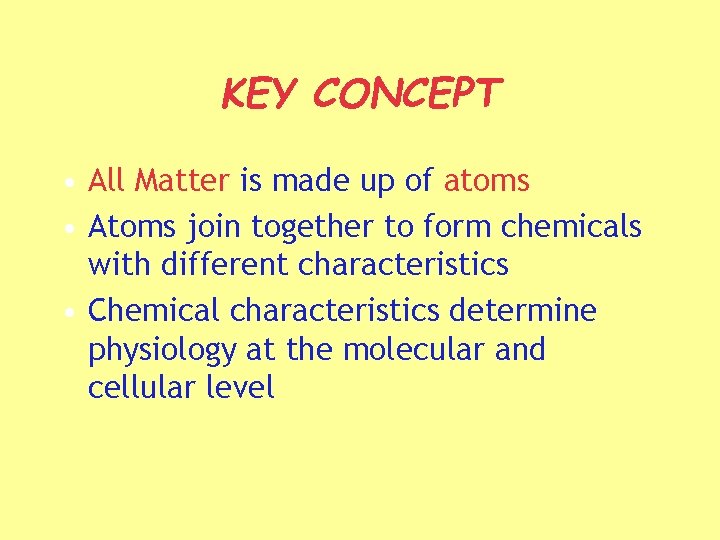
KEY CONCEPT • All Matter is made up of atoms • Atoms join together to form chemicals with different characteristics • Chemical characteristics determine physiology at the molecular and cellular level

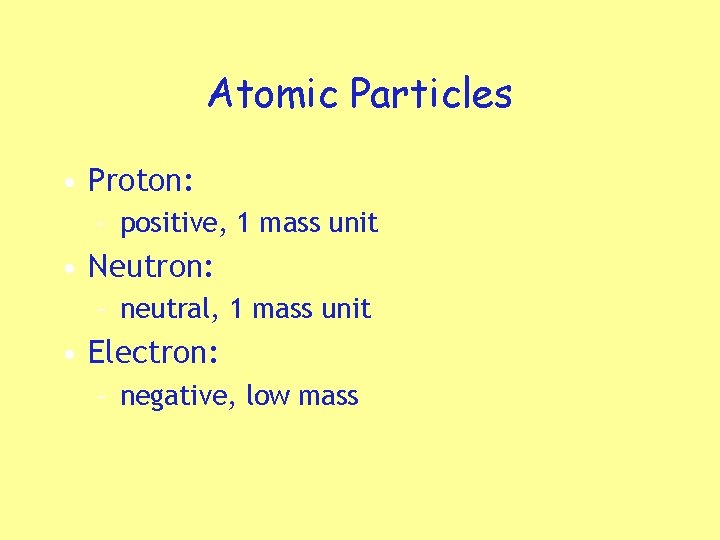
Atomic Particles • Proton: – positive, 1 mass unit • Neutron: – neutral, 1 mass unit • Electron: – negative, low mass

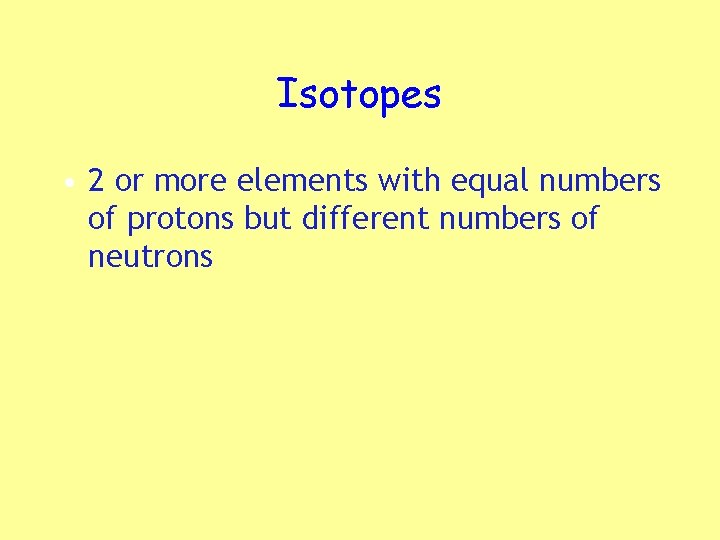
Isotopes • 2 or more elements with equal numbers of protons but different numbers of neutrons

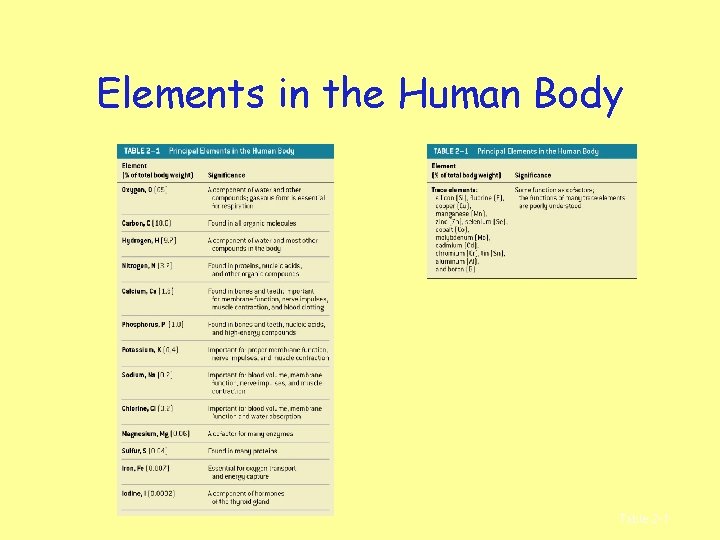
Elements in the Human Body Table 2– 1

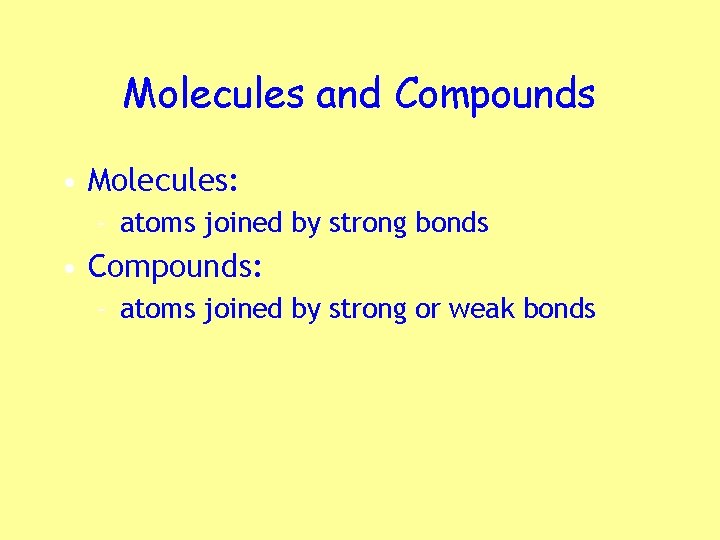
Molecules and Compounds • Molecules: – atoms joined by strong bonds • Compounds: – atoms joined by strong or weak bonds

States of Matter • Solid: – constant volume and shape • Liquid: – constant volume but change shape • Gas: – change volume and shape

Chemical reactions & Physiology? • Energy: – the power to do work • Work: – a change in mass or distance

Forms of Energy • Kinetic energy - energy of motion • Potential energy - stored energy • Chemical energy - potential energy stored in chemical bonds

KEY CONCEPT • When energy is exchanged, heat is produced, but cells cannot capture it or use it for work

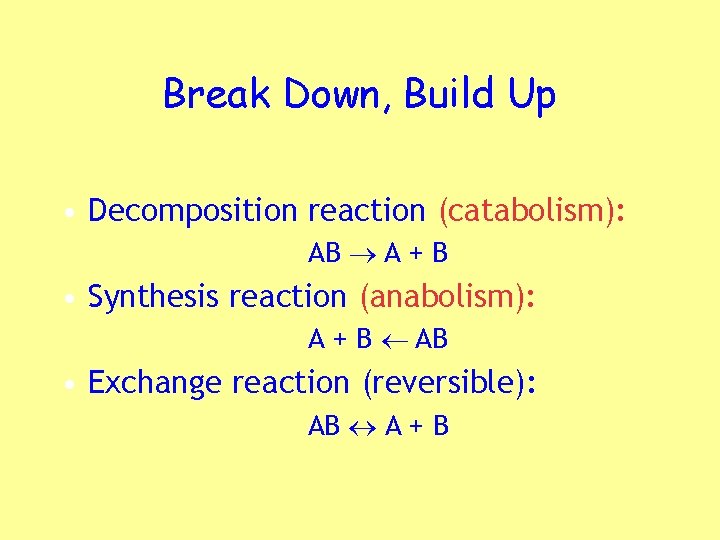
Break Down, Build Up • Decomposition reaction (catabolism): AB A + B • Synthesis reaction (anabolism): A + B AB • Exchange reaction (reversible): AB A + B

KEY CONCEPT • Reversible reactions seek equilibrium, balancing opposing reaction rates • Add or remove reactants: – reaction rates adjust to reach a new equilibrium

How do enzymes control metabolism?

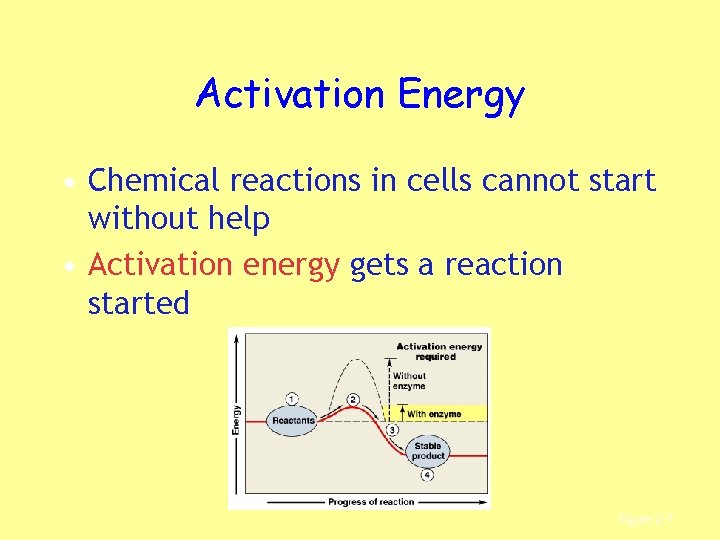
Activation Energy • Chemical reactions in cells cannot start without help • Activation energy gets a reaction started Figure 2– 7

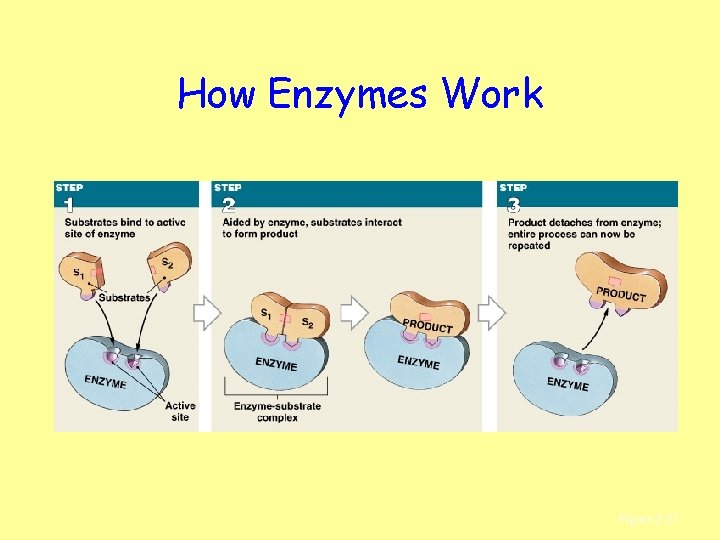
How Enzymes Work Figure 2– 21

KEY CONCEPT • Most chemical reactions that sustain life cannot occur unless the right enzymes are present

Organic and Inorganic Molecules • Organic: – molecules based on carbon and hydrogen • Inorganic: – molecules not based on carbon and hydrogen

Why is water so important to life?

Properties of Water (1 of 2) • Solubility: – water’s ability to dissolve a solute to make a solution • Reactivity: – most body chemistry uses or occurs in water

Properties of Water (2 of 2) • High heat capacity: – water’s ability to absorb and retain heat • Lubrication: – to moisten and reduce friction

KEY CONCEPT • Most of our body weight is water • Water is the key structural and functional component of cells and their control mechanisms, the nucleic acids

Electrolytes • Inorganic ions conduct electricity in solution • Electrolyte imbalance seriously disturbs vital body functions – Fluid balance – Blood pressure – Muscular contractions

p. H • p. H: – the concentration of hydrogen ions (H+) in a solution • Neutral p. H: – a balance of H+ and OH— – pure water = 7. 0

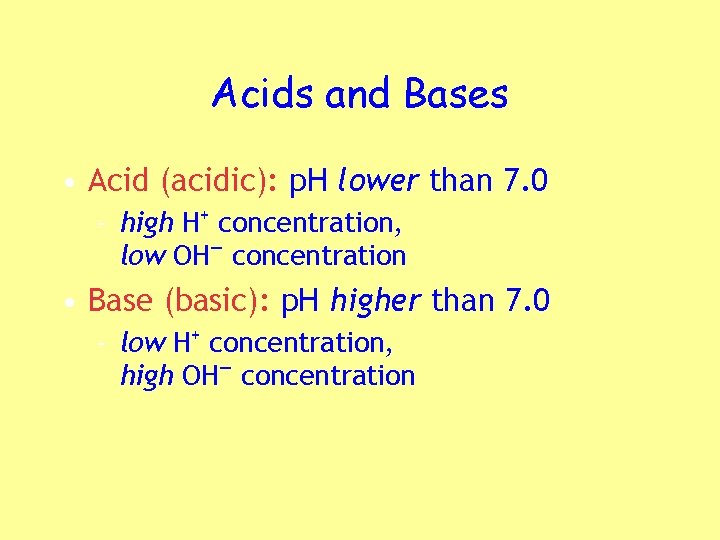
Acids and Bases • Acid (acidic): p. H lower than 7. 0 – high H+ concentration, low OH— concentration • Base (basic): p. H higher than 7. 0 – low H+ concentration, high OH— concentration

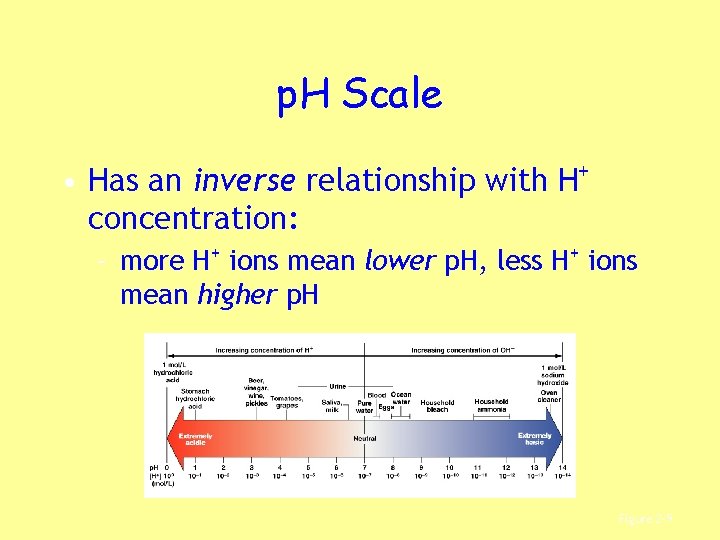
p. H Scale • Has an inverse relationship with H+ concentration: – more H+ ions mean lower p. H, less H+ ions mean higher p. H Figure 2– 9

KEY CONCEPT • p. H of body fluids measures free H+ ions in solution • Excess H+ ions (low p. H): – damages cells and tissues, alters proteins – interferes with normal functions • Excess OH— ions (high p. H) also problem • Normal blood p. H – 7. 35 to 7. 45 • Incompatibile with life – 6. 8 to 7. 8

Acid and Alkaline • Acidosis: – excess H+ in body fluid (low p. H) – Loss of bicarbonate – Blood level < 7. 2 • Alkalosis: – excess OH— in body fluid (high p. H) – Blood level > 7. 5

Nucleic Acids • Large organic molecules, found in the nucleus, which store and process information at the molecular level • DNA and RNA

Deoxyribonucleic Acid (DNA) • Deoxyribonucleic Acid (DNA) – – Determines inherited characteristics Directs protein synthesis Controls enzyme production Controls metabolism • Ribonucleic Acid (RNA) – Codes intermediate steps in protein synthesis

KEY CONCEPT • DNA in the cell nucleus contains the information needed to construct all of the proteins in the body

Nucleotides • Building blocks of DNA • Have 3 molecular parts: – sugar (deoxyribose) – phosphate group – nitrogenous base (A, G, T, C)

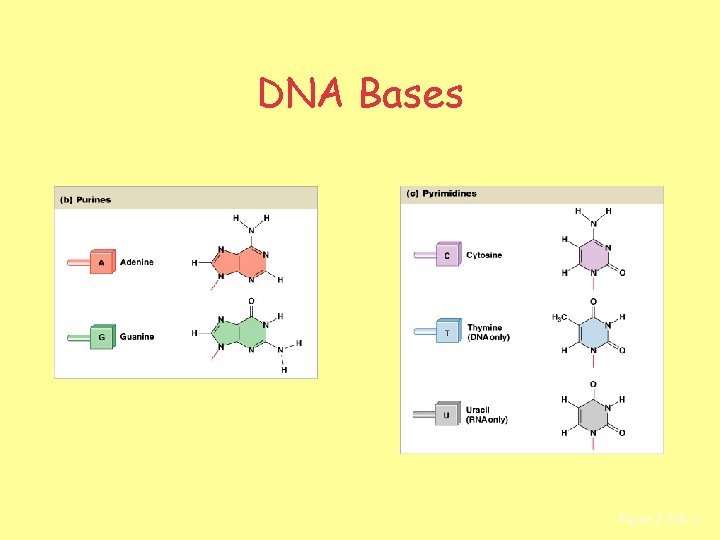
DNA Bases Figure 2– 22 b, c

Complementary Bases • Complementary base pairs: – purines pair with pyrimidines: • DNA: – adenine (A) and thymine (T) – cytosine (C) and guanine (G) • RNA: – uracil (U) replaces thymine (T)

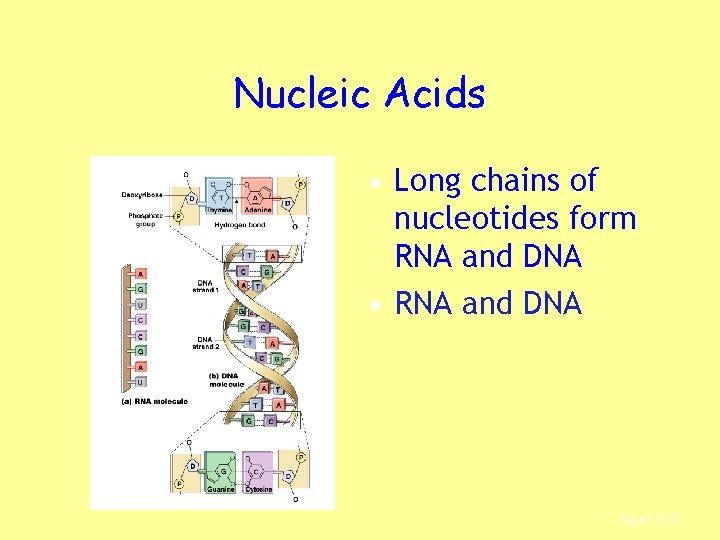
Nucleic Acids • Long chains of nucleotides form RNA and DNA • RNA and DNA Figure 2– 23

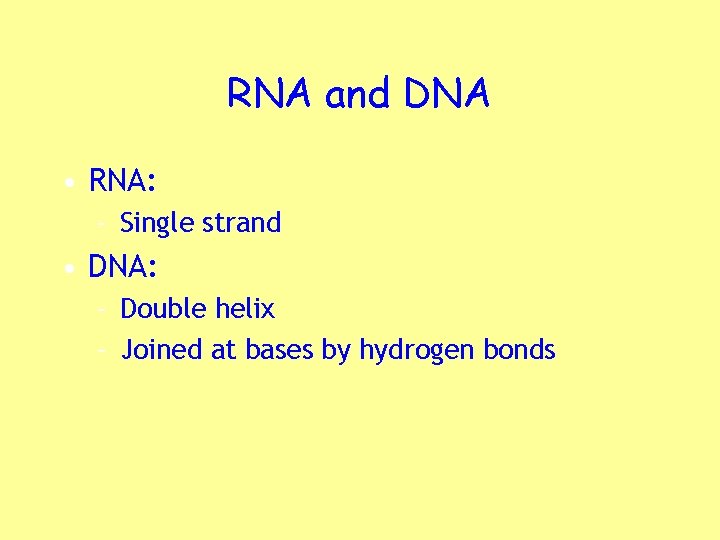
RNA and DNA • RNA: – Single strand • DNA: – Double helix – Joined at bases by hydrogen bonds

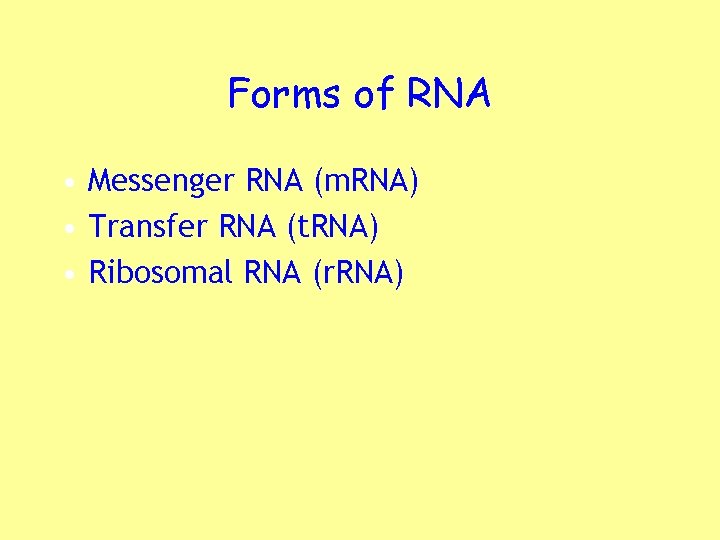
Forms of RNA • Messenger RNA (m. RNA) • Transfer RNA (t. RNA) • Ribosomal RNA (r. RNA)

ADP and ATP • Adenosine diphosphate (ADP): – 2 phosphate groups • di = 2 • Adenosine triphosphate (ATP): – 3 phosphate groups • tri = 3 – Energy for muscular contractions

KEY CONCEPT • Body recycles/renews all chemical components at intervals ranging from minutes to years • Metabolic turnover lets your body: – Grow – Change – Adapt

SUMMARY (1 of 2) • Atoms, molecules, and chemical bonds control cellular physiology • Metabolism and energy work within the cell • Importance of organic and inorganic nutrients and metabolites

SUMMARY (2 of 2) • Role of water and solubility in metabolism and cell structure • Chemistry of acids and bases, p. H and buffers • Structure and function of carbohydrates, lipids, proteins, and nucleic acids
 The chemical level of organization
The chemical level of organization The chemical level of organization chapter 2
The chemical level of organization chapter 2 Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Formula of love
Formula of love Are kc and kp equal
Are kc and kp equal Chapter 4 the tissue level of organization
Chapter 4 the tissue level of organization Chapter 4 the tissue level of organization
Chapter 4 the tissue level of organization Chapter 4 the tissue level of organization
Chapter 4 the tissue level of organization Chapter 3 the cellular level of organization
Chapter 3 the cellular level of organization Business model canvas example
Business model canvas example Contoh bisnis model canvas makanan pdf
Contoh bisnis model canvas makanan pdf Chapter 18 review chemical equilibrium
Chapter 18 review chemical equilibrium Reaction rates and equilibrium worksheet answers chapter 19
Reaction rates and equilibrium worksheet answers chapter 19 Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas Label an atom
Label an atom Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Chapter 7 chemical quantities answer key
Chapter 7 chemical quantities answer key Chapter 10 chemical reactions
Chapter 10 chemical reactions Writing formulas criss-cross method worksheet
Writing formulas criss-cross method worksheet Chapter 10 chemical quantities practice problems answer key
Chapter 10 chemical quantities practice problems answer key Chapter 18 chemical equilibrium answer key
Chapter 18 chemical equilibrium answer key Synthesis reaction
Synthesis reaction Process organization in computer organization
Process organization in computer organization Point by point organization essay
Point by point organization essay Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 1 chemical changes
Section 1 chemical changes Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton
Tư thế worm breton Chúa sống lại
Chúa sống lại Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Làm thế nào để 102-1=99
Làm thế nào để 102-1=99