The Chemical Level of Organization The Chemical Level






![Dissociation p. H - measure or acidity/alkalinity p. H = - log [H+] acidic Dissociation p. H - measure or acidity/alkalinity p. H = - log [H+] acidic](https://slidetodoc.com/presentation_image_h2/ad739cf978f327ccba76ddd4d6b86f23/image-33.jpg)





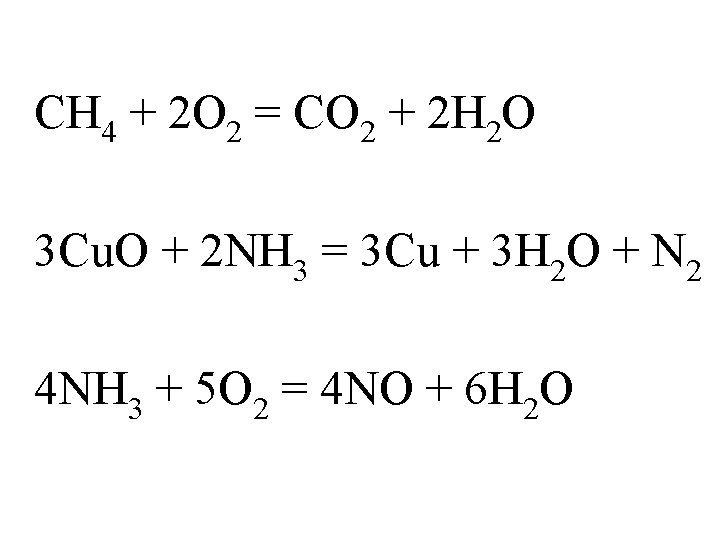







- Slides: 64

The Chemical Level of Organization

The Chemical Level of Organization • Matter – elements – atoms and molecules • • • Chemical bonds Chemical energy Chemical reactions Inorganic compounds Organic compounds

How Matter is Organized • Chemistry is the science of the structure and interactions of matter. – all living things consist of matter. • Matter is anything that occupies space. – mass is the amount of matter in any object. – weight is the force of gravity acting on matter.

Chemical Elements • Elements are substances that can not be split into simpler substances by ordinary means. – 112 elements ( 92 occur naturally ) – 26 of naturally occurring elements are in the body – represented by chemical symbols ( first 1 -2 letters of name ) • 4 elements form 96 % of the body’s mass – hydrogen, oxygen, carbon and nitrogen • Trace elements are present in tiny amounts – such as copper, tin, selenium & zinc

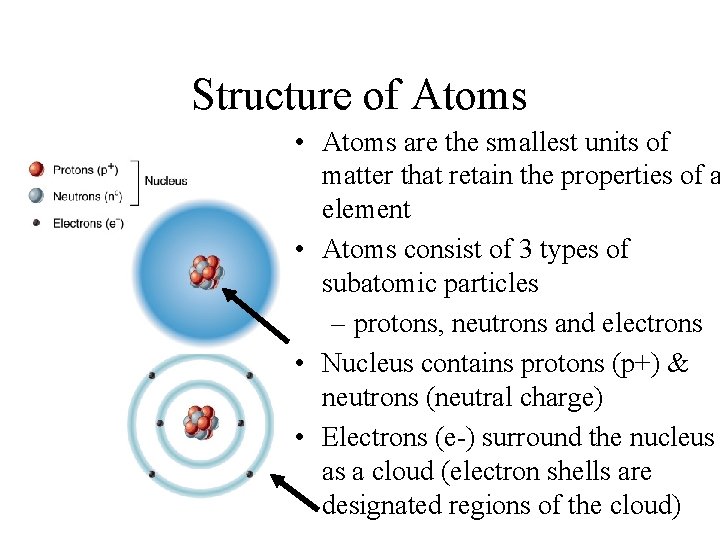
Structure of Atoms • Atoms are the smallest units of matter that retain the properties of a element • Atoms consist of 3 types of subatomic particles – protons, neutrons and electrons • Nucleus contains protons (p+) & neutrons (neutral charge) • Electrons (e-) surround the nucleus as a cloud (electron shells are designated regions of the cloud)

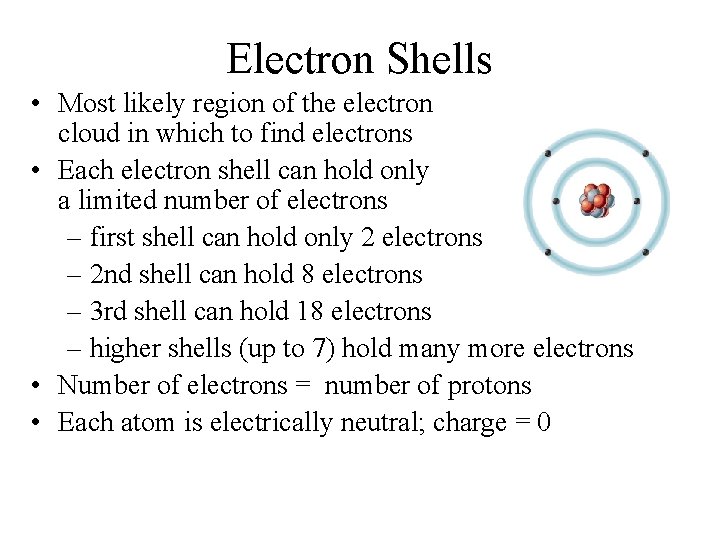
Electron Shells • Most likely region of the electron cloud in which to find electrons • Each electron shell can hold only a limited number of electrons – first shell can hold only 2 electrons – 2 nd shell can hold 8 electrons – 3 rd shell can hold 18 electrons – higher shells (up to 7) hold many more electrons • Number of electrons = number of protons • Each atom is electrically neutral; charge = 0

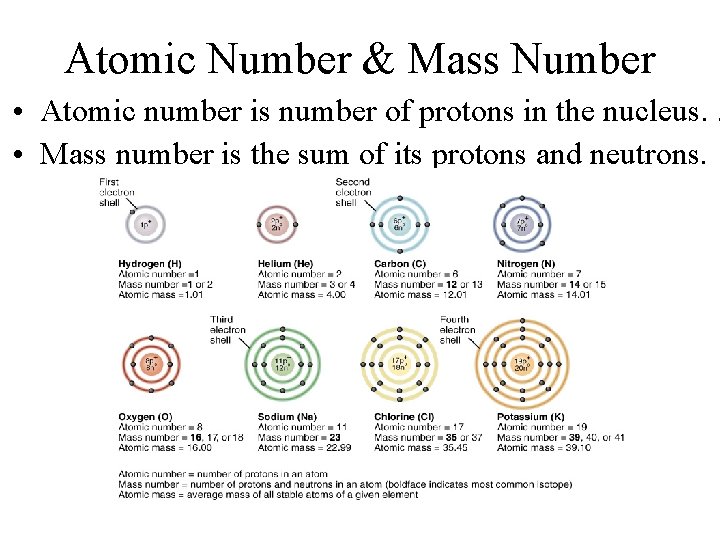
Atomic Number & Mass Number • Atomic number is number of protons in the nucleus. . • Mass number is the sum of its protons and neutrons.

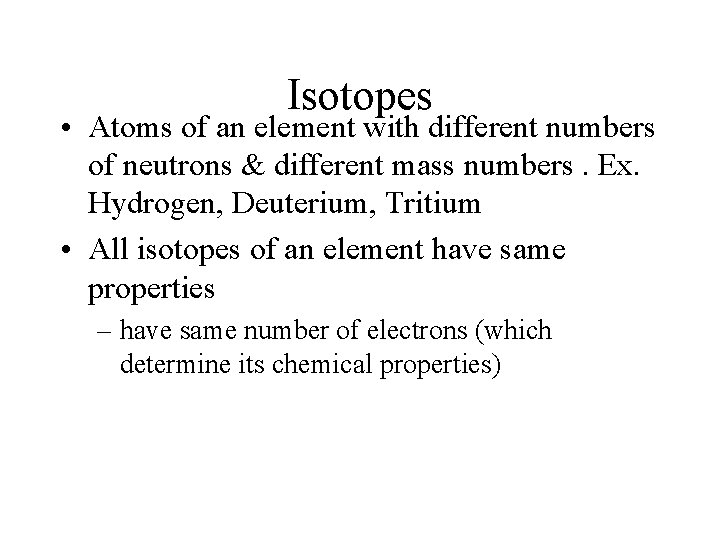
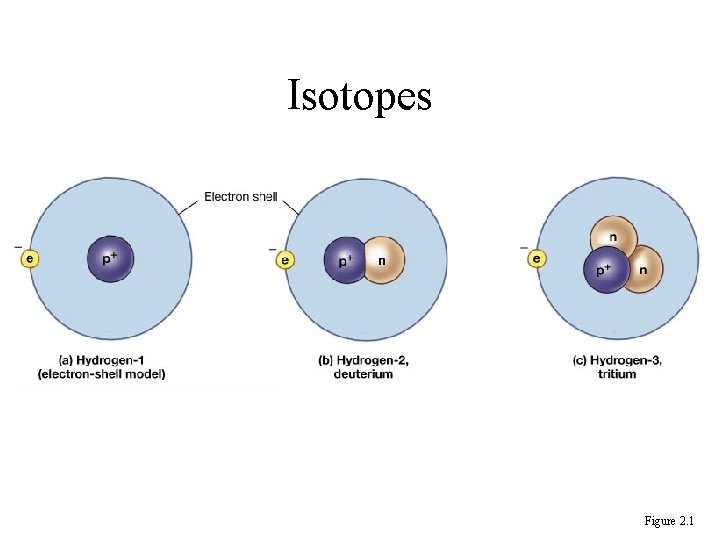
Isotopes • Atoms of an element with different numbers of neutrons & different mass numbers. Ex. Hydrogen, Deuterium, Tritium • All isotopes of an element have same properties – have same number of electrons (which determine its chemical properties)

Isotopes Figure 2. 1

Molecule, Element, compound • Molecule= more than one atom existing in union • Element= Molecule contains identical atoms • Compound: Molecule contains different atoms

IONS: Charged particles When an atom gains or loses electros ions are formed Positively charged= cation Negatively charged = anion

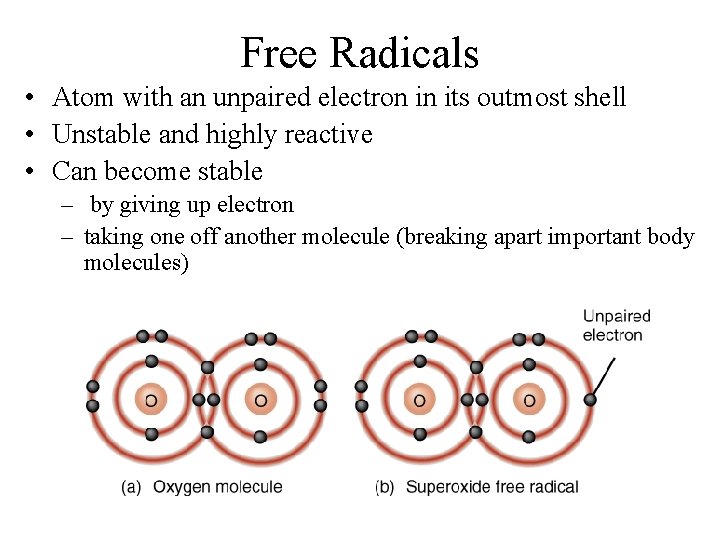
Free Radicals • Atom with an unpaired electron in its outmost shell • Unstable and highly reactive • Can become stable – by giving up electron – taking one off another molecule (breaking apart important body molecules)

Free Radicals & Your Health • Produced in your body by absorption of energy in ultraviolet light in sunlight, x-rays, by breakdown of harmful substances, & during normal metabolic reactions • Linked to many diseases -- cancer, diabetes, Alzheimer, atherosclerosis and arthritis • Damage may be slowed with antioxidants such as vitamins C and E, selenium & beta-carotene (precursor to vitamin A)

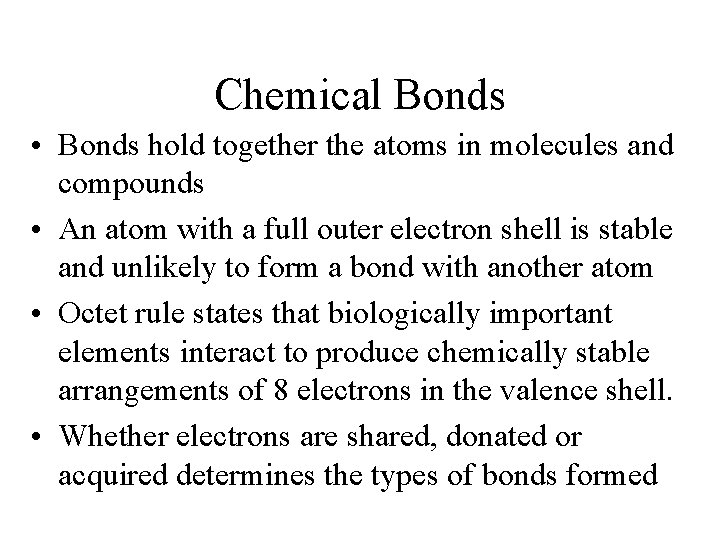
Chemical Bonds • Bonds hold together the atoms in molecules and compounds • An atom with a full outer electron shell is stable and unlikely to form a bond with another atom • Octet rule states that biologically important elements interact to produce chemically stable arrangements of 8 electrons in the valence shell. • Whether electrons are shared, donated or acquired determines the types of bonds formed

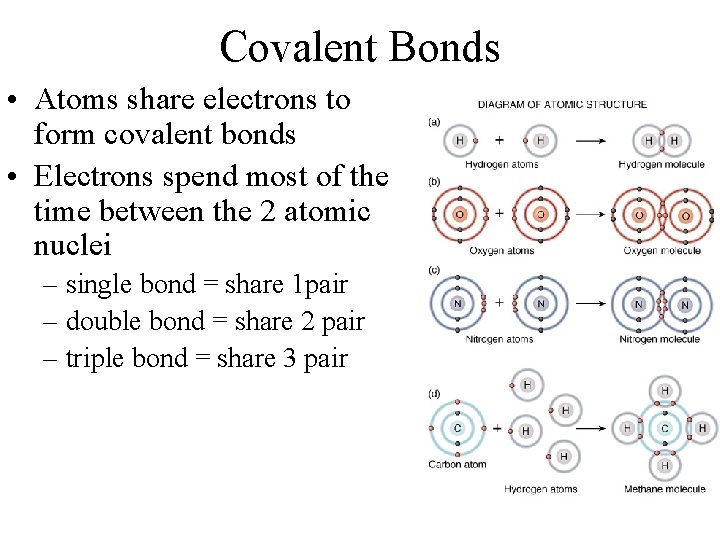
Covalent Bonds • Atoms share electrons to form covalent bonds • Electrons spend most of the time between the 2 atomic nuclei – single bond = share 1 pair – double bond = share 2 pair – triple bond = share 3 pair

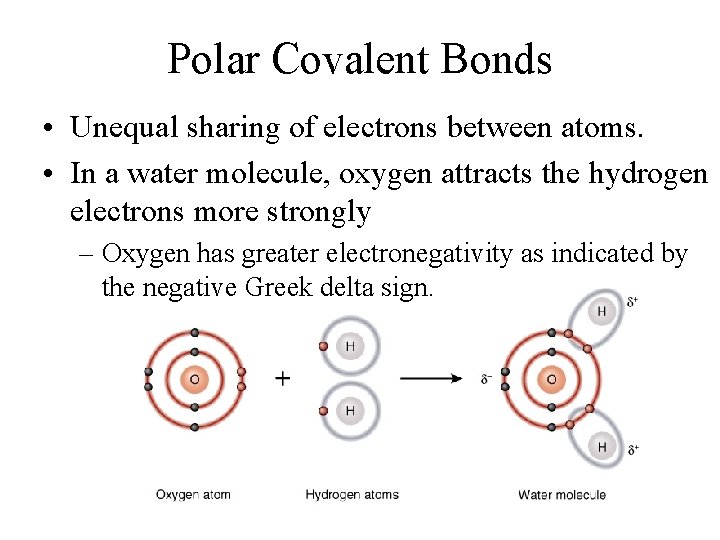
Polar Covalent Bonds • Unequal sharing of electrons between atoms. • In a water molecule, oxygen attracts the hydrogen electrons more strongly – Oxygen has greater electronegativity as indicated by the negative Greek delta sign.

Ionic Bonds • Positively and negatively charged ions attract each other to form an ionic bond • In the body, ionic bonds are found mainly in teeth and bones • An ionic compound that dissociates in water into + and - ions is called an electrolyte

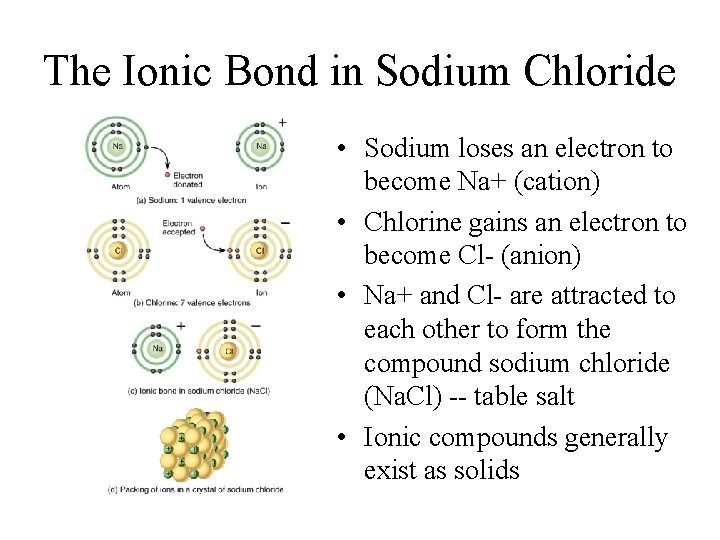
The Ionic Bond in Sodium Chloride • Sodium loses an electron to become Na+ (cation) • Chlorine gains an electron to become Cl- (anion) • Na+ and Cl- are attracted to each other to form the compound sodium chloride (Na. Cl) -- table salt • Ionic compounds generally exist as solids

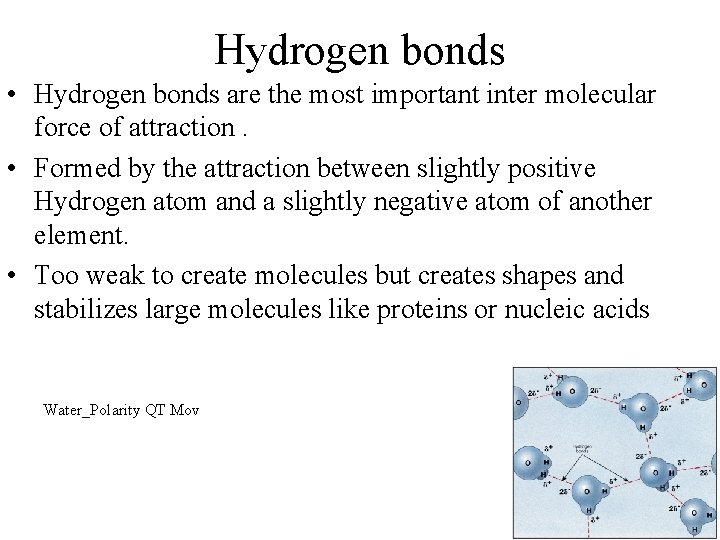
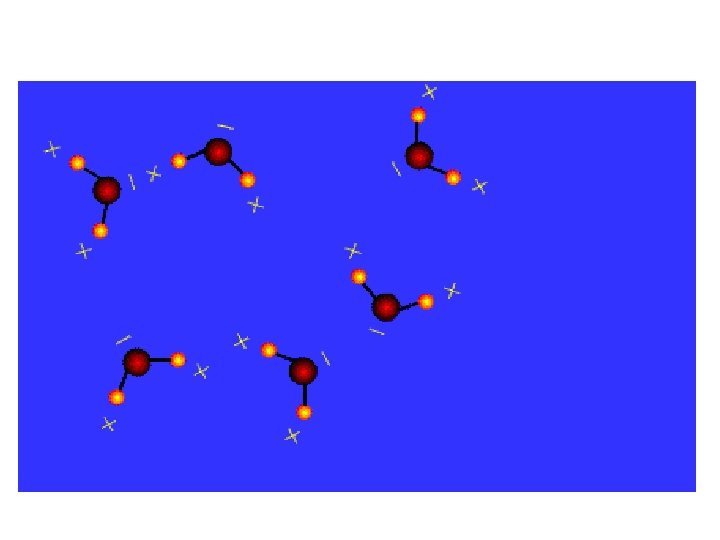
Hydrogen bonds • Hydrogen bonds are the most important inter molecular force of attraction. • Formed by the attraction between slightly positive Hydrogen atom and a slightly negative atom of another element. • Too weak to create molecules but creates shapes and stabilizes large molecules like proteins or nucleic acids Water_Polarity QT Mov


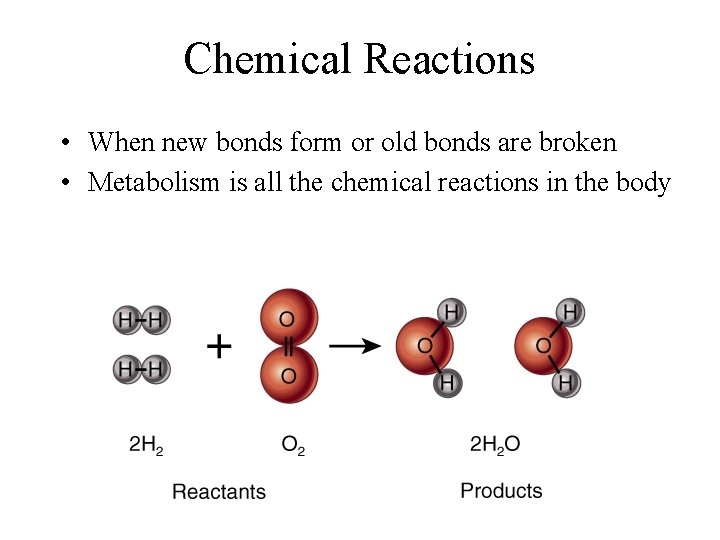
Chemical Reactions • When new bonds form or old bonds are broken • Metabolism is all the chemical reactions in the body

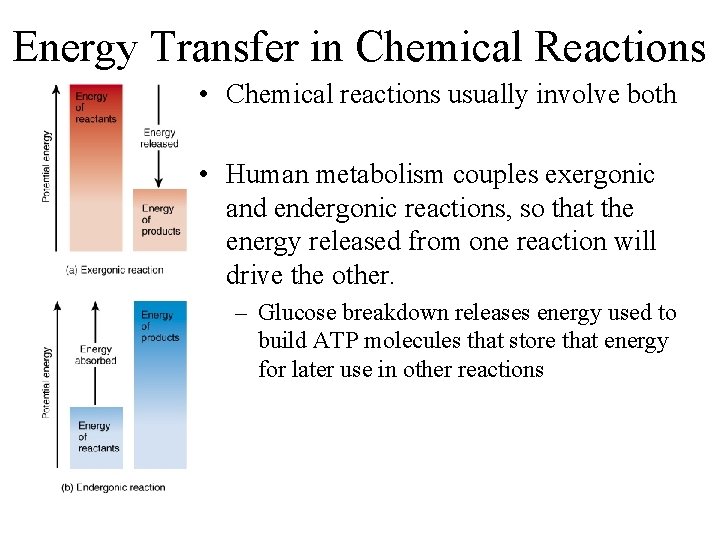
Energy and Chemical Reactions • Chemical reactions involve energy changes • Law of conservation of energy – energy can neither be created nor destroyed--just converted from one form to another • Reactions that yield energy = Exergonic reactions (Larger to smaller mols. ) AB A + B • Reactions that require energy to occur= Endergonic reactions (smaller to larger mols ) A + B AB


Energy Transfer in Chemical Reactions • Chemical reactions usually involve both • Human metabolism couples exergonic and endergonic reactions, so that the energy released from one reaction will drive the other. – Glucose breakdown releases energy used to build ATP molecules that store that energy for later use in other reactions

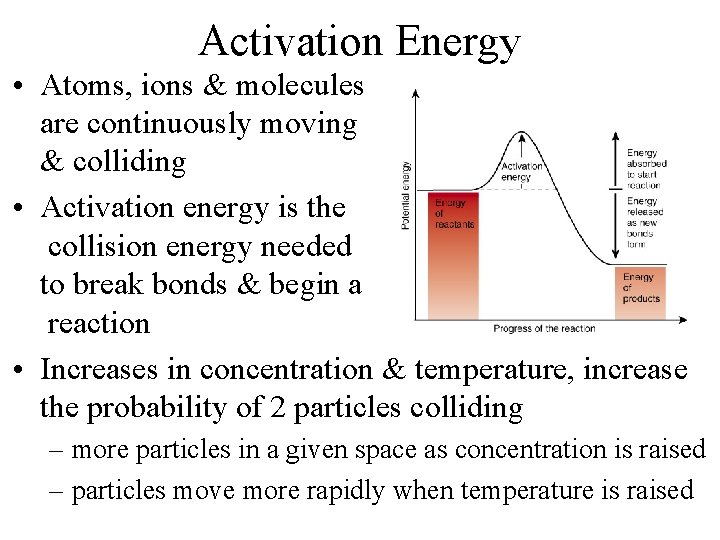
Activation Energy • Atoms, ions & molecules are continuously moving & colliding • Activation energy is the collision energy needed to break bonds & begin a reaction • Increases in concentration & temperature, increase the probability of 2 particles colliding – more particles in a given space as concentration is raised – particles move more rapidly when temperature is raised

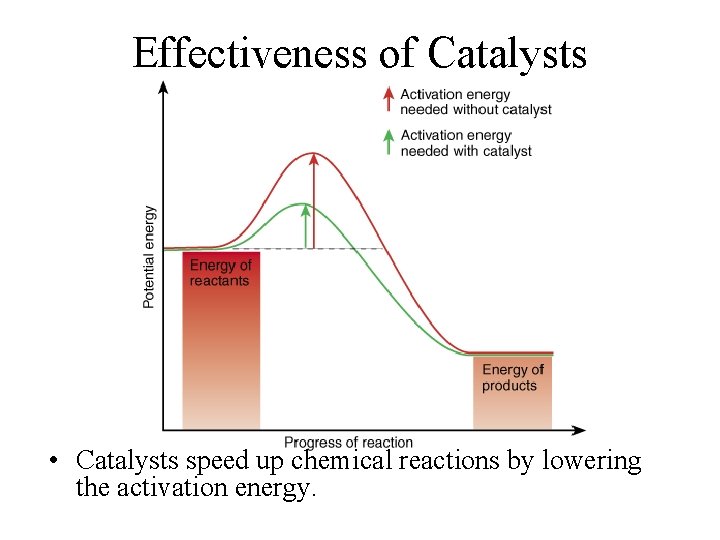
Catalysts or Enzymes • Normal body temperatures and concentrations are too low to cause chemical reactions to occur • Catalysts speed up chemical reactions by lowering the activation energy needed to get it started • Catalysts orient the colliding particles properly so that they touch at the spots that make the reaction happen • Catalyst molecules are unchanged and can be used repeatedly to speed up similar reactions.

Effectiveness of Catalysts • Catalysts speed up chemical reactions by lowering the activation energy.

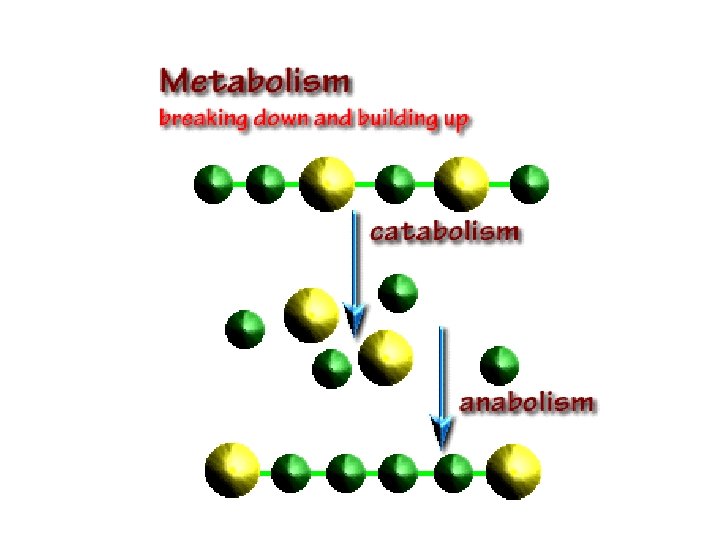
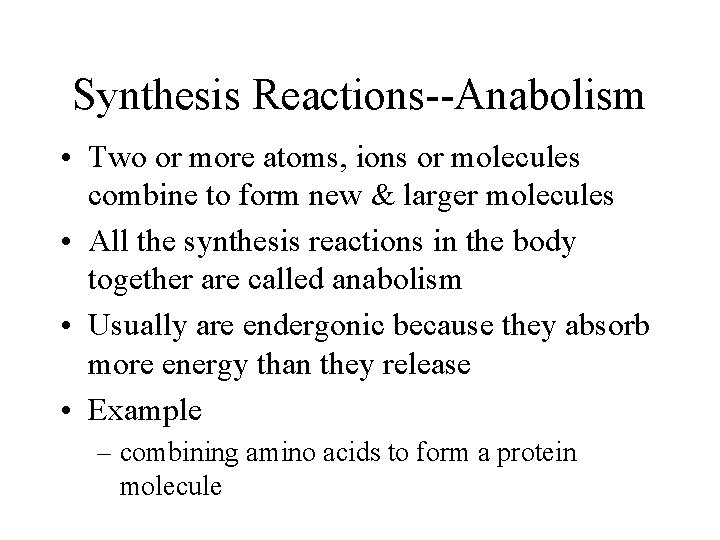
Synthesis Reactions--Anabolism • Two or more atoms, ions or molecules combine to form new & larger molecules • All the synthesis reactions in the body together are called anabolism • Usually are endergonic because they absorb more energy than they release • Example – combining amino acids to form a protein molecule

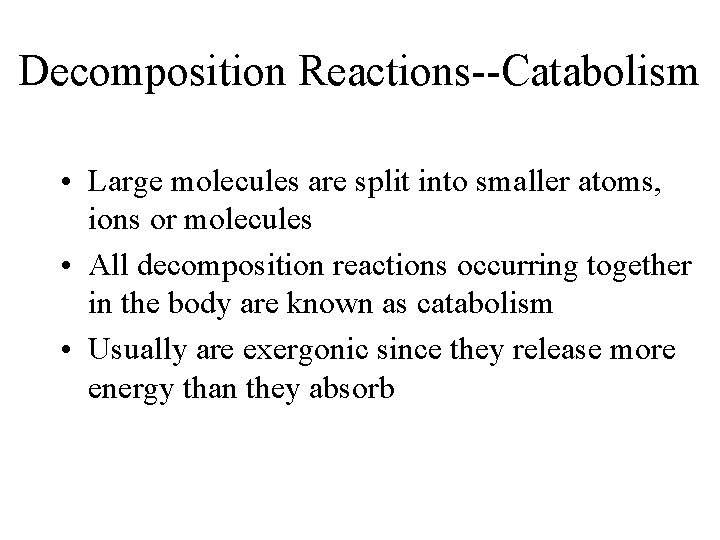
Decomposition Reactions--Catabolism • Large molecules are split into smaller atoms, ions or molecules • All decomposition reactions occurring together in the body are known as catabolism • Usually are exergonic since they release more energy than they absorb

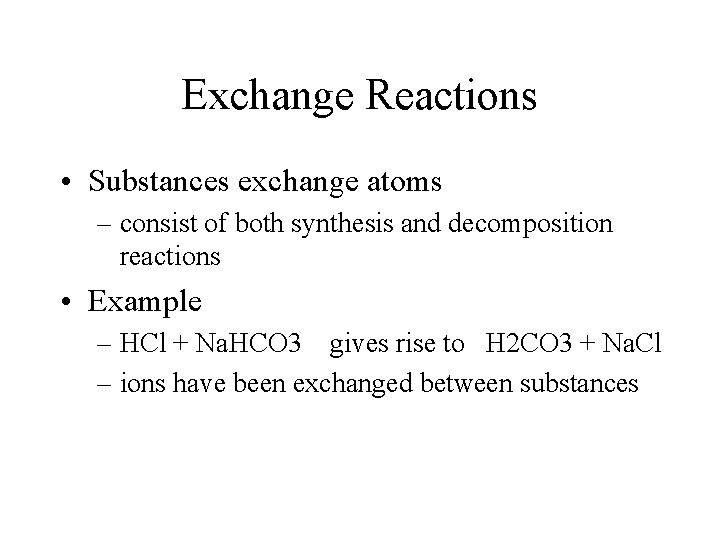
Exchange Reactions • Substances exchange atoms – consist of both synthesis and decomposition reactions • Example – HCl + Na. HCO 3 gives rise to H 2 CO 3 + Na. Cl – ions have been exchanged between substances

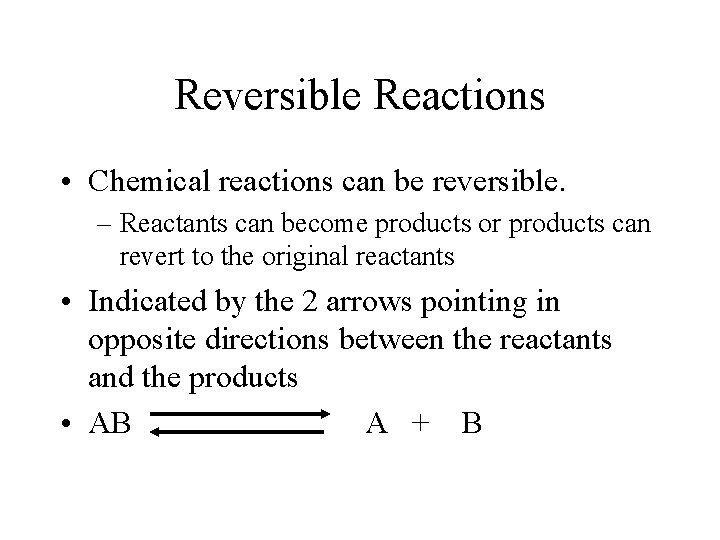
Reversible Reactions • Chemical reactions can be reversible. – Reactants can become products or products can revert to the original reactants • Indicated by the 2 arrows pointing in opposite directions between the reactants and the products • AB A + B

Inorganic Compounds & Solvents • Most of the chemicals in the body are compounds • Inorganic compounds – usually lack carbon & are structurally simple – water, salts, acids and bases • Organic compounds – contain carbon & usually hydrogen – always have covalent bonds
![Dissociation p H measure or acidityalkalinity p H log H acidic Dissociation p. H - measure or acidity/alkalinity p. H = - log [H+] acidic](https://slidetodoc.com/presentation_image_h2/ad739cf978f327ccba76ddd4d6b86f23/image-33.jpg)
Dissociation p. H - measure or acidity/alkalinity p. H = - log [H+] acidic < 7 < basic Acids-raise H+ content Bases-lower H+ content: release OH- or accepts H+ (alkaline) 1 p. H unit = 10 x difference 1000 as many H+ in a p. H of 5 as there are in 8 Buffer-takes up or releases H+ or OH- to prevent changes in p. H. In the bicarbonate system, H 2 CO 3 H+ base acceptor, HCO 3 - acid acceptor

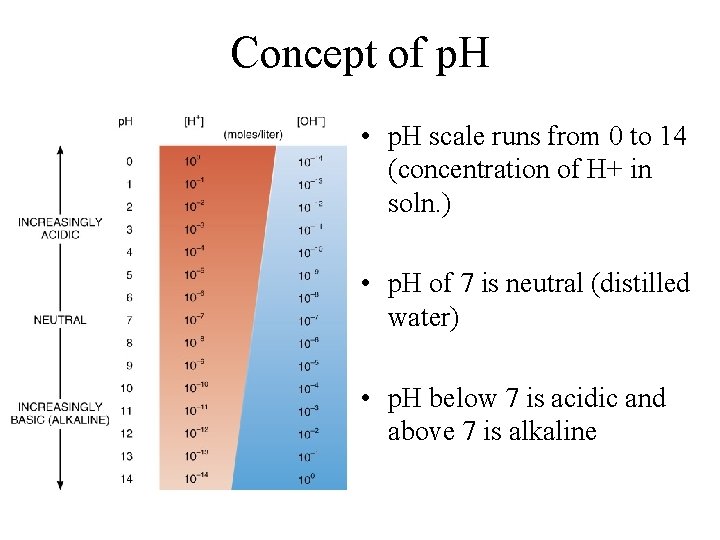
Concept of p. H • p. H scale runs from 0 to 14 (concentration of H+ in soln. ) • p. H of 7 is neutral (distilled water) • p. H below 7 is acidic and above 7 is alkaline

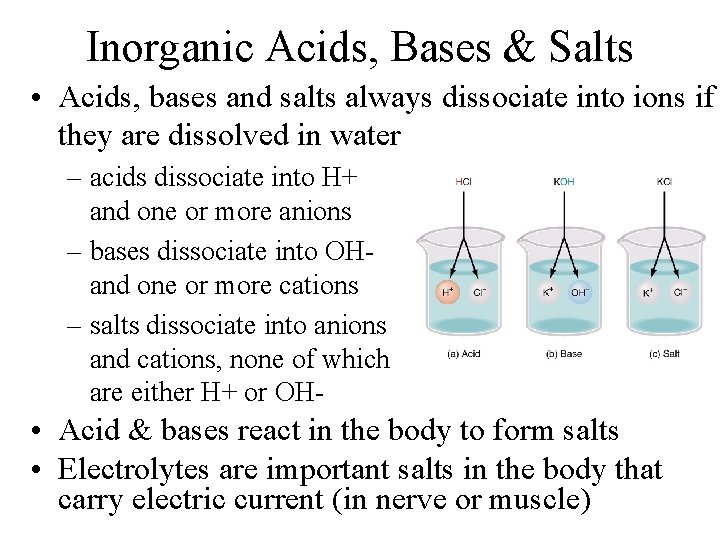
Inorganic Acids, Bases & Salts • Acids, bases and salts always dissociate into ions if they are dissolved in water – acids dissociate into H+ and one or more anions – bases dissociate into OHand one or more cations – salts dissociate into anions and cations, none of which are either H+ or OH- • Acid & bases react in the body to form salts • Electrolytes are important salts in the body that carry electric current (in nerve or muscle)

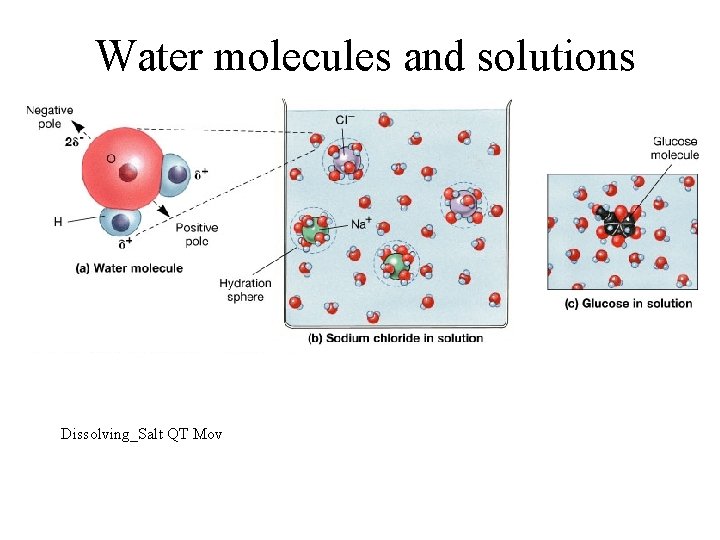
Water & It’s Properties • Most important inorganic compound in living systems • Medium of nearly all chemical reactions • Polarity – uneven sharing of electrons – partial negative charge near oxygen atom and partial positive charge near hydrogen atoms • makes it an excellent solvent for ionic or polar substances • gives water molecules cohesion • allows water to moderate temperature changes

Water has a high surface tension • Water is wet • Water is attracted to itself, and this attraction, due to H bonds is stronger than the attraction to the air above • Adhesion and cohesion allow for capillary action water transport in plants

Water as a Solvent • Most versatile solvent known – polar covalent bonds (hydrophilic versus hydrophobic) – its shape allows each water molecule to interact with neighboring ions/molecules • oxygen attracts sodium • hydrogen attracts chloride • sodium & chloride separate as ionic bonds are broken hydration spheres surround each ion and decrease possibility of bonds being reformed • Water dissolves many substances

Water molecules and solutions Dissolving_Salt QT Mov

Water in Chemical Reactions • Participates as a product or reactant in certain reactions in the body – hydrolysis reactions • water is added to a large molecule to separate it into two smaller molecules • digestion of food – dehydration synthesis reaction • two small molecules are joined to form a larger molecule releasing a water molecule

Heat Capacity of Water • Heat capacity is high – can absorb a large amount of heat with only a small increase in its own temperature • large number of hydrogen bonds in water – bonds are broken as heat is absorbed instead of increasing temperature of water – large amount of water in body helps lessen the impact of environmental changes in temperature

Water is a good evaporative coolant • Heat of vaporization is also high – amount of heat needed to change from liquid to gas – evaporation of water from the skin removes large amount of heat • B/c it takes a lot of energy to change water from a liquid to a gas, it takes energy with it

Water as a Lubricant • Major component of lubricating fluids within the body – mucus in respiratory and digestive systems – synovial fluid in joints – serous fluids in chest and abdominal cavities • organs slide past one another

Ice floats • Water has a high freezing point and lower density as a solid than a liquid

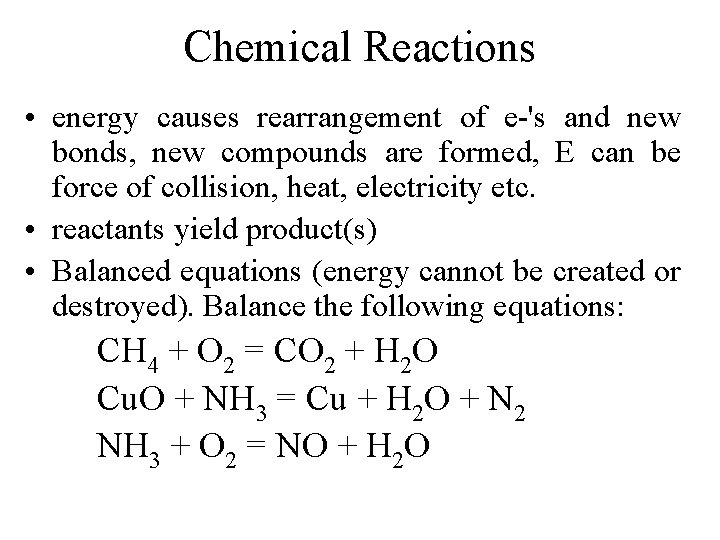
Chemical Reactions • energy causes rearrangement of e-'s and new bonds, new compounds are formed, E can be force of collision, heat, electricity etc. • reactants yield product(s) • Balanced equations (energy cannot be created or destroyed). Balance the following equations: CH 4 + O 2 = CO 2 + H 2 O Cu. O + NH 3 = Cu + H 2 O + N 2 NH 3 + O 2 = NO + H 2 O
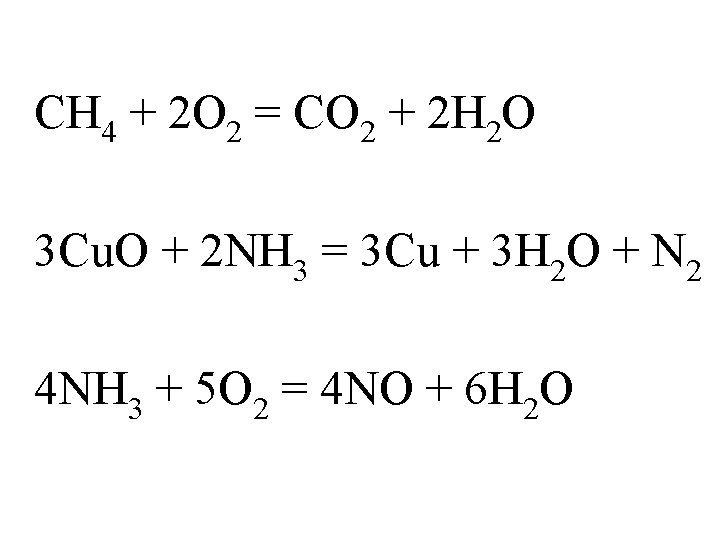
CH 4 + 2 O 2 = CO 2 + 2 H 2 O 3 Cu. O + 2 NH 3 = 3 Cu + 3 H 2 O + N 2 4 NH 3 + 5 O 2 = 4 NO + 6 H 2 O

• Mixtures-combination of substances in which the individual components retain their own properties • solutions-or more substances is distributed evenly in another substance solution = solvent(H 2 O)+solute(dissolved particles) • suspension-- particles of materials are temporarily mixed together • colloid-particles larger than solution, smaller than suspension

Chemistry Tutorial • http: //www. biology. arizona. edu/biochemistr y/tutorials/chemistry/main. html

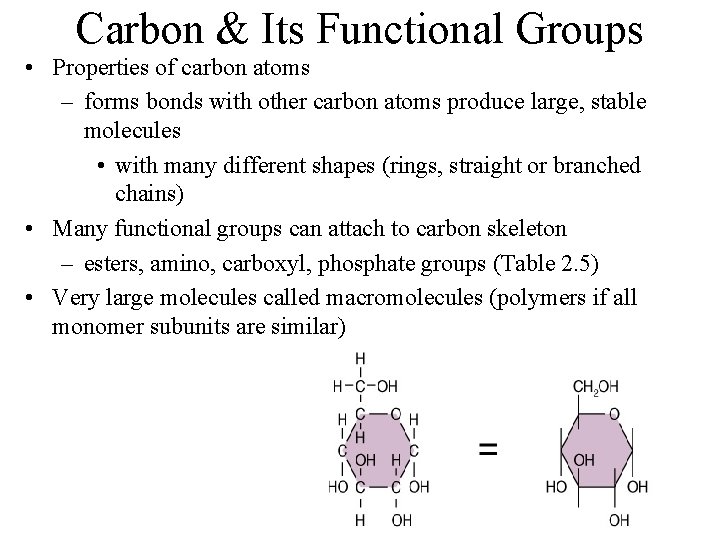
Organic Compounds • Always contain carbon and hydrogen • Usually contain covalent bonds • Usually large, unique molecules with complex functions

Carbon & Its Functional Groups • Properties of carbon atoms – forms bonds with other carbon atoms produce large, stable molecules • with many different shapes (rings, straight or branched chains) • Many functional groups can attach to carbon skeleton – esters, amino, carboxyl, phosphate groups (Table 2. 5) • Very large molecules called macromolecules (polymers if all monomer subunits are similar)

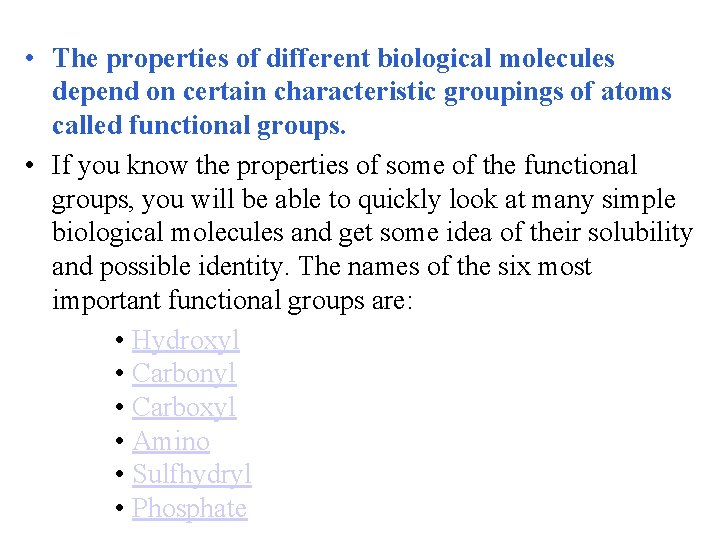
• The properties of different biological molecules depend on certain characteristic groupings of atoms called functional groups. • If you know the properties of some of the functional groups, you will be able to quickly look at many simple biological molecules and get some idea of their solubility and possible identity. The names of the six most important functional groups are: • Hydroxyl • Carbonyl • Carboxyl • Amino • Sulfhydryl • Phosphate

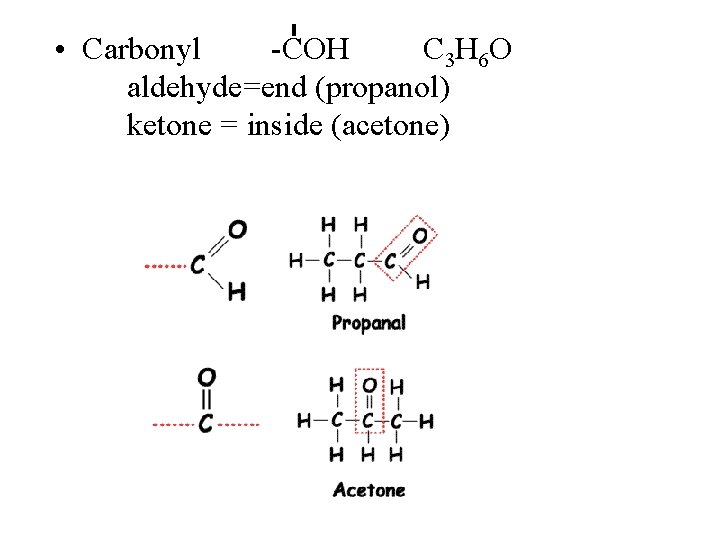
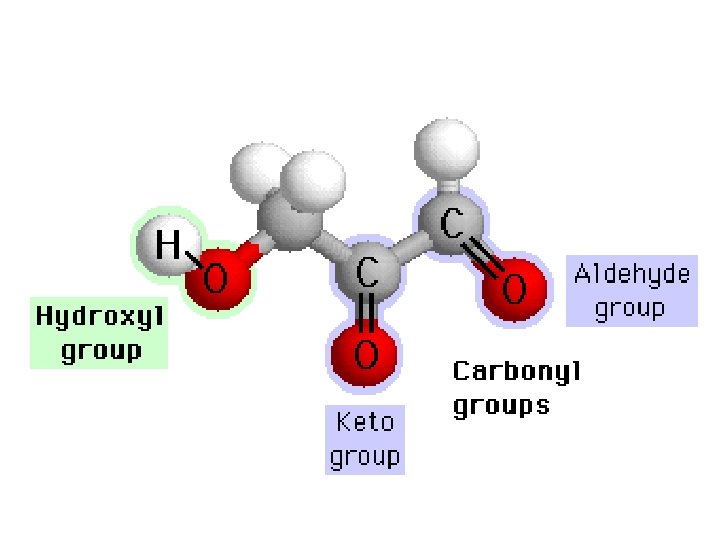
Hydroxyl • Two functional groups containing oxygen, the hydroxyl and carbonyl groups, contribute to water solubility. • Hydroxyl groups have one hydrogen paired with one oxygen atom (symbolized as -OH). Hydroxyl groups are not highly reactive, but they readily form hydrogen bonds and contribute to making molecules soluble in water. Alcohols and sugars are "loaded" with hydroxyl groups.

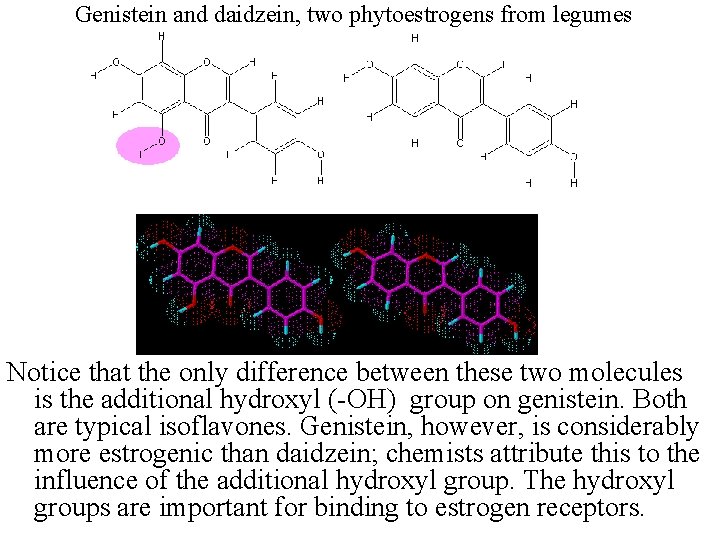
Genistein and daidzein, two phytoestrogens from legumes Notice that the only difference between these two molecules is the additional hydroxyl (-OH) group on genistein. Both are typical isoflavones. Genistein, however, is considerably more estrogenic than daidzein; chemists attribute this to the influence of the additional hydroxyl group. The hydroxyl groups are important for binding to estrogen receptors.

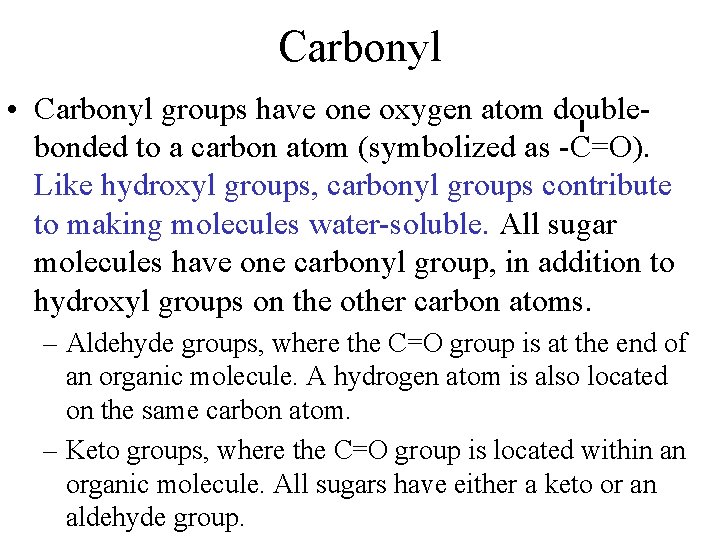
Carbonyl • Carbonyl groups have one oxygen atom doublebonded to a carbon atom (symbolized as -C=O). Like hydroxyl groups, carbonyl groups contribute to making molecules water-soluble. All sugar molecules have one carbonyl group, in addition to hydroxyl groups on the other carbon atoms. – Aldehyde groups, where the C=O group is at the end of an organic molecule. A hydrogen atom is also located on the same carbon atom. – Keto groups, where the C=O group is located within an organic molecule. All sugars have either a keto or an aldehyde group.

• Carbonyl -COH C 3 H 6 O aldehyde=end (propanol) ketone = inside (acetone)


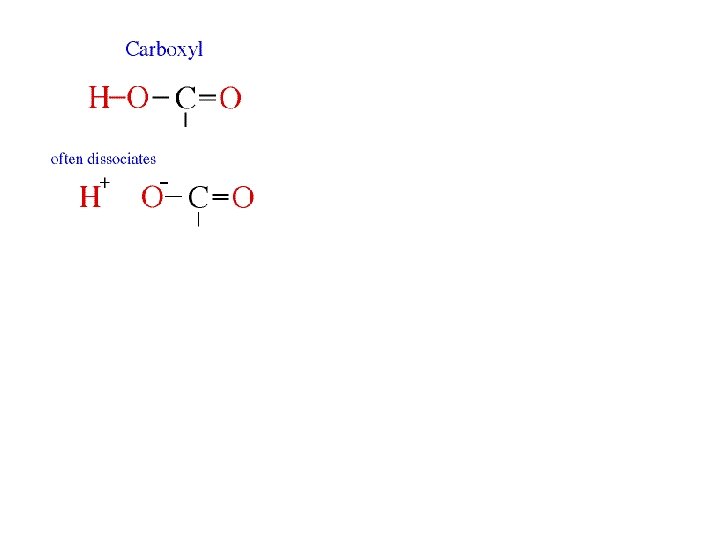
Carboxylic Acids • Carboxyl groups are weak acids, dissociating partially to release hydrogen ions. The carboxyl group (symbolized as COOH) has both a carbonyl and a hydroxyl group attached to the same carbon atom, resulting in new properties. Carboxyl groups frequently ionize, releasing the H from the hydroxyl group as a free proton (H+), with the remaining O carrying a negative charge. Molecules containing carboxyl groups are called carboxylic acids and dissociate partially into H+ and COO–. Carboxyl groups are common in many biological molecules, including amino acids and fatty acids.


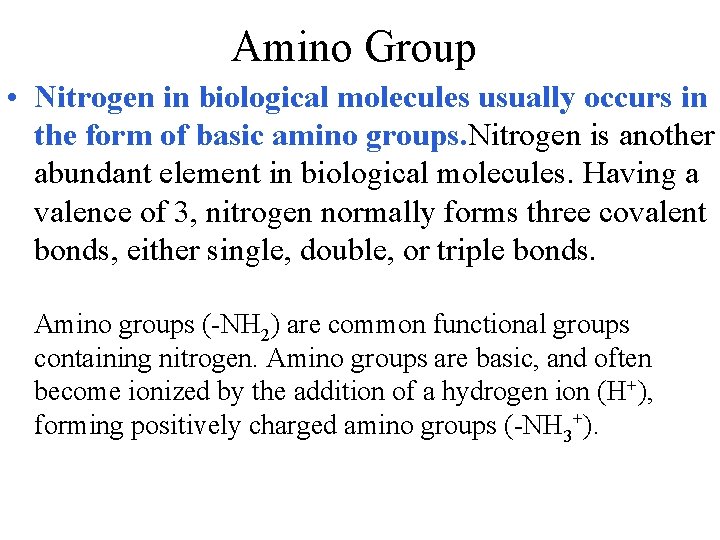
Amino Group • Nitrogen in biological molecules usually occurs in the form of basic amino groups. Nitrogen is another abundant element in biological molecules. Having a valence of 3, nitrogen normally forms three covalent bonds, either single, double, or triple bonds. Amino groups (-NH 2) are common functional groups containing nitrogen. Amino groups are basic, and often become ionized by the addition of a hydrogen ion (H+), forming positively charged amino groups (-NH 3+).

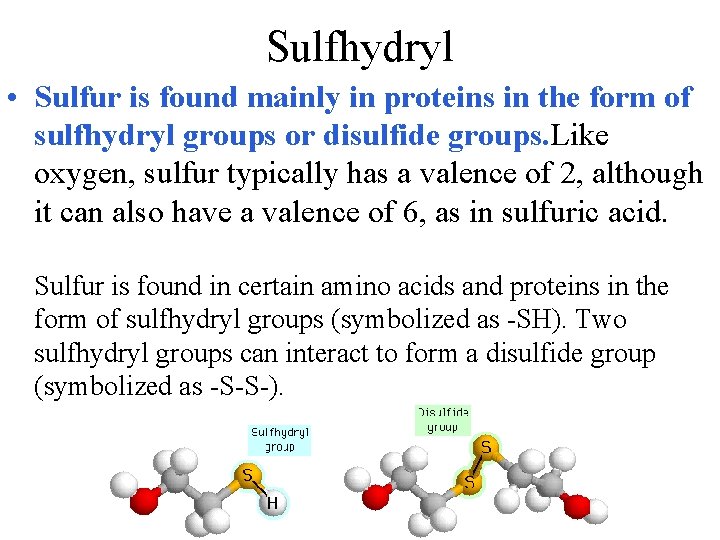
Sulfhydryl • Sulfur is found mainly in proteins in the form of sulfhydryl groups or disulfide groups. Like oxygen, sulfur typically has a valence of 2, although it can also have a valence of 6, as in sulfuric acid. Sulfur is found in certain amino acids and proteins in the form of sulfhydryl groups (symbolized as -SH). Two sulfhydryl groups can interact to form a disulfide group (symbolized as -S-S-).

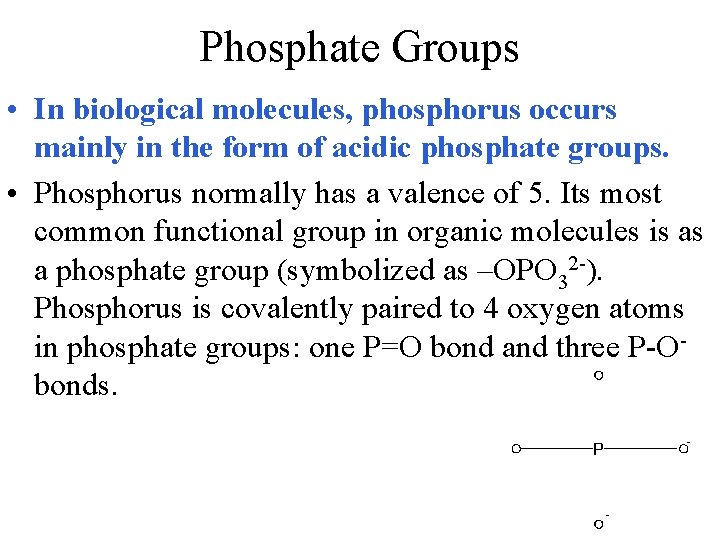
Phosphate Groups • In biological molecules, phosphorus occurs mainly in the form of acidic phosphate groups. • Phosphorus normally has a valence of 5. Its most common functional group in organic molecules is as a phosphate group (symbolized as –OPO 32 -). Phosphorus is covalently paired to 4 oxygen atoms in phosphate groups: one P=O bond and three P-Obonds.

Function Groups Practice

Types of Organic compounds Four major groups of organic compounds, necessary for life are: polymers monomers – Carbohydrates monosacchrides – Lipids fatty acids – Proteins amino acids – Nucleic acids nucleotides

Resources • Bare Bones Chemistry: www. hcs. ohiostate. edu/hcs 300/chem. htm • Bio. Topics Contents: http: //www. biotopics. co. uk/conten. html
 The chemical level of organization
The chemical level of organization The chemical level of organization chapter 2
The chemical level of organization chapter 2 Process organization in computer organization
Process organization in computer organization Block organization vs point by point
Block organization vs point by point Organization level
Organization level Organization-level diagnostic model
Organization-level diagnostic model Middle level management examples
Middle level management examples What is the least complex level of organization
What is the least complex level of organization Levels of organization ecology
Levels of organization ecology Level of organization organ system
Level of organization organ system Mdm maturity model
Mdm maturity model Smallest to largest level of organization
Smallest to largest level of organization Ostia porifera
Ostia porifera Levels of management information system
Levels of management information system Three level cache organization
Three level cache organization Chapter 4 the tissue level of organization
Chapter 4 the tissue level of organization What level of organization
What level of organization Porifera level of organization
Porifera level of organization Level of management
Level of management The levels of ecological organization
The levels of ecological organization Porifera level of organization
Porifera level of organization Chapter 4 the tissue level of organization
Chapter 4 the tissue level of organization Chapter 4 the tissue level of organization
Chapter 4 the tissue level of organization Chapter 3 the cellular level of organization
Chapter 3 the cellular level of organization Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worm breton
Tư thế worm breton Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế
Cái miệng nó xinh thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V cc cc
V cc cc Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tia chieu sa te
Tia chieu sa te Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Các số nguyên tố là gì
Các số nguyên tố là gì Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu