The Chemical Level of Organization Unit I Organization










- Slides: 39

The Chemical Level of Organization Unit I. Organization of the Human Body

Essential Concepts • Matter is composed of atoms held together by chemical bonds • During a chemical reaction, bonds are formed, rearranged, or broken • Water is the most important and abundant inorganic compound in the body • Carbohydrates, lipids, proteins, nucleic acids, and ATP are the most important organic compounds in the body

Chemical Elements HOW MATTER IS ORGANIZED

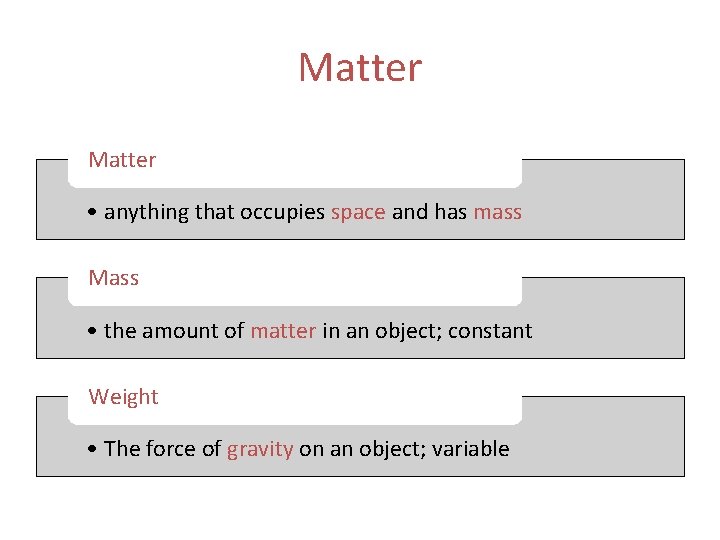
Matter • anything that occupies space and has mass Mass • the amount of matter in an object; constant Weight • The force of gravity on an object; variable

Chemical Elements • Matter exists in three states: – Solids – Liquids – Gases • All matter is composed of chemical elements • Elements - the building blocks of matter

Major Elements Carbon Hydrogen Oxygen Nitrogen

Minor Elements Calcium Phosphorus Potassium Sulfur Sodium Chlorine Magnesium Iodine Iron

Trace Elements • Examples include copper and zinc – Copper works with iron to form RBCs. Keeps vessels, nerves, bones, immune system healthy. – Zinc is necessary for immunity. Helps with mitosis, interphase and healing. Needed for smell and taste.

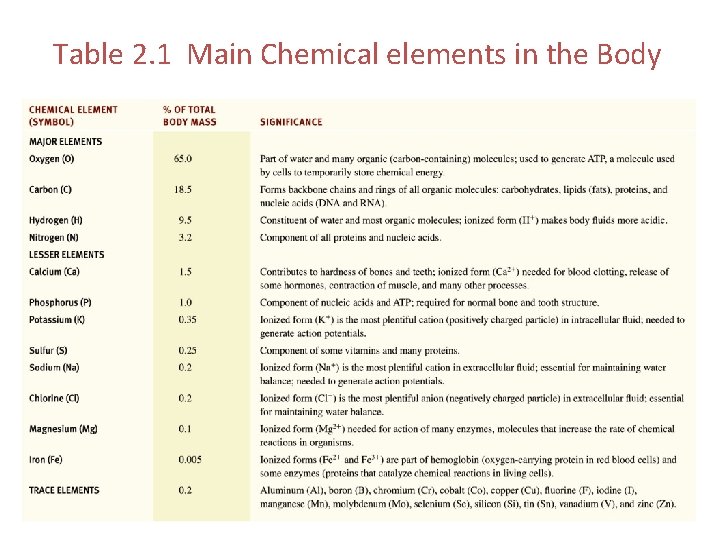
Table 2. 1 Main Chemical elements in the Body

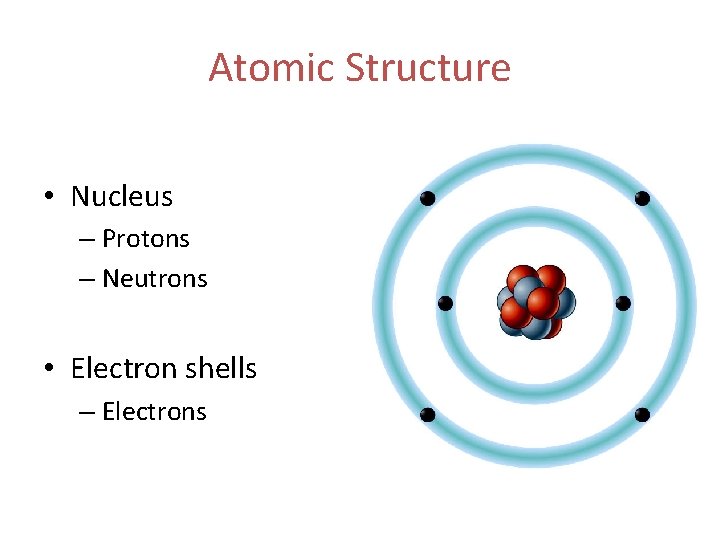
Atomic Structure HOW MATTER IS ORGANIZED

Atoms • Atom – the smallest unit of matter • An element contains the same kind of atoms • Example: a pure sample of the element carbon contains only carbon atoms

Atomic Structure • Nucleus – Protons – Neutrons • Electron shells – Electrons

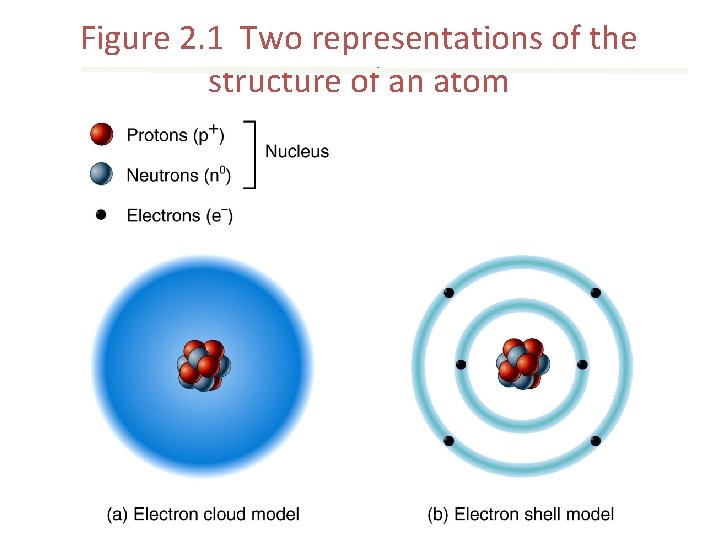
Figure 2. 1 Two representations of the structure of an atom

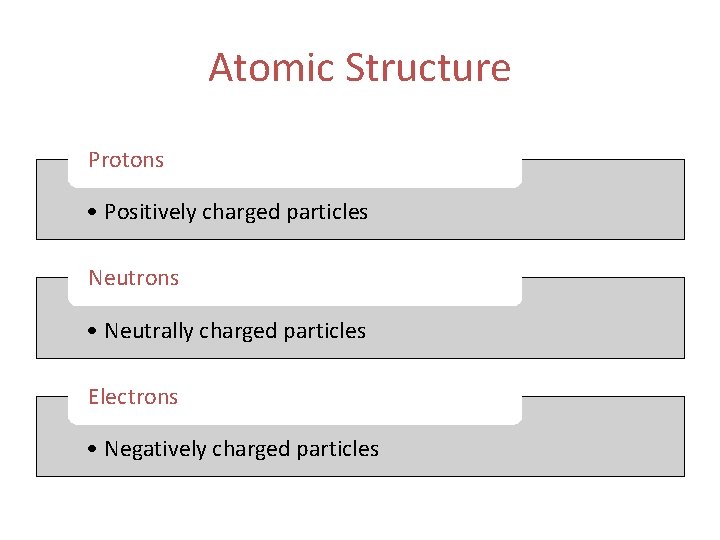
Atomic Structure Protons • Positively charged particles Neutrons • Neutrally charged particles Electrons • Negatively charged particles

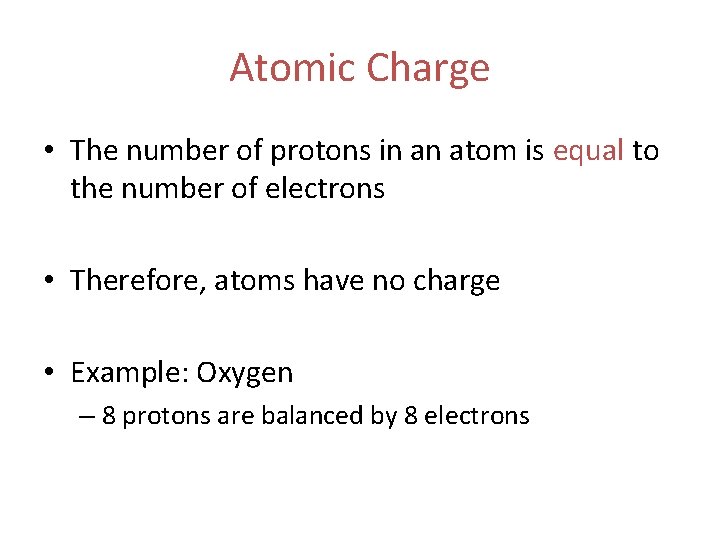
Atomic Charge • The number of protons in an atom is equal to the number of electrons • Therefore, atoms have no charge • Example: Oxygen – 8 protons are balanced by 8 electrons

Atomic Number and Mass Number HOW MATTER IS ORGANIZED

Atomic Number and Atomic Mass Atomic number • the number of protons in an atom’s nucleus Mass number • the sum of the number of protons and neutrons in an atom’s nucleus

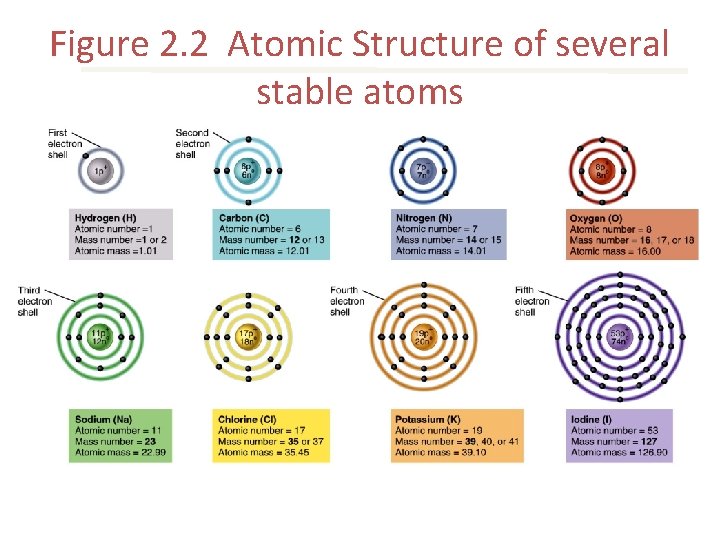
Figure 2. 2 Atomic Structure of several stable atoms

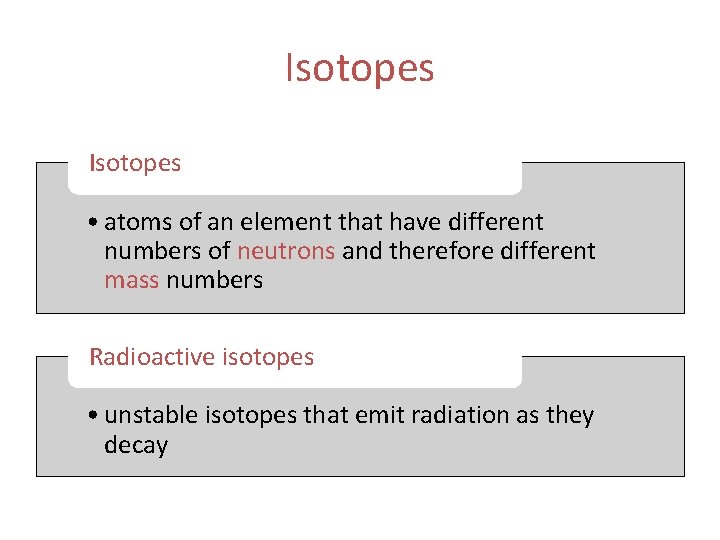
Isotopes • atoms of an element that have different numbers of neutrons and therefore different mass numbers Radioactive isotopes • unstable isotopes that emit radiation as they decay

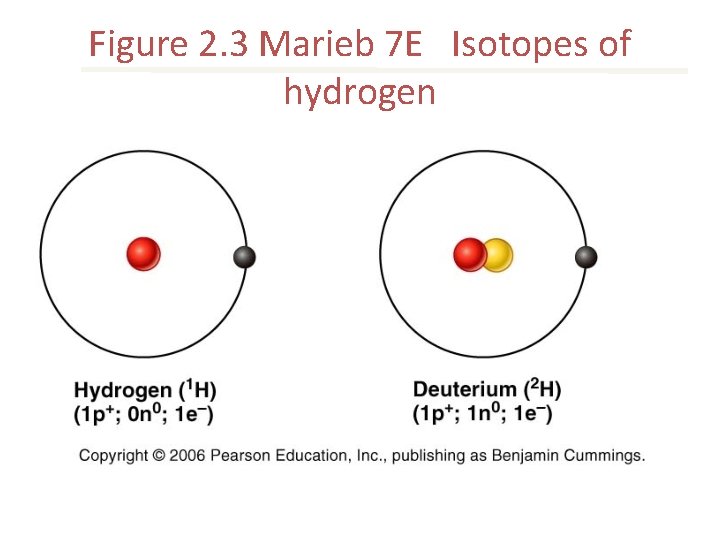
Figure 2. 3 Marieb 7 E Isotopes of hydrogen

Ions, Molecules, and Compounds HOW MATTER IS ORGANIZED

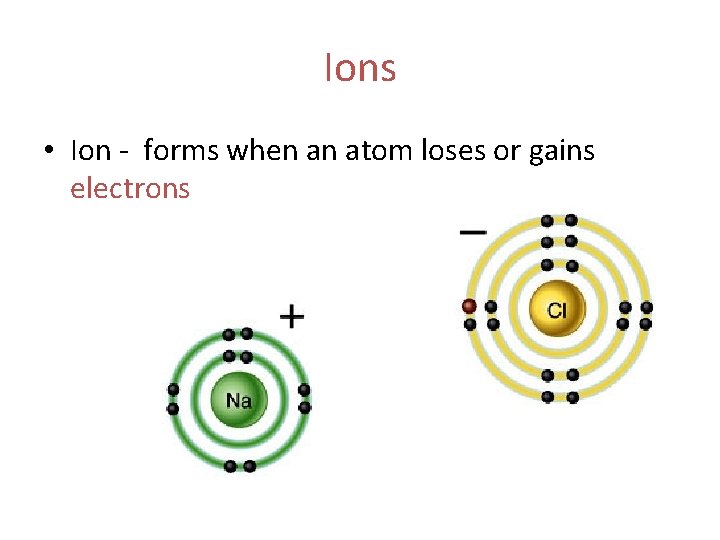
Ions • Ion - forms when an atom loses or gains electrons

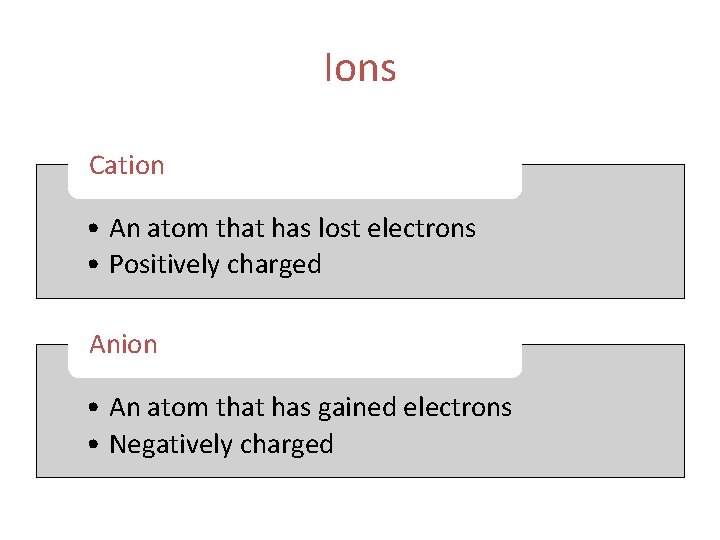
Ions Cation • An atom that has lost electrons • Positively charged Anion • An atom that has gained electrons • Negatively charged

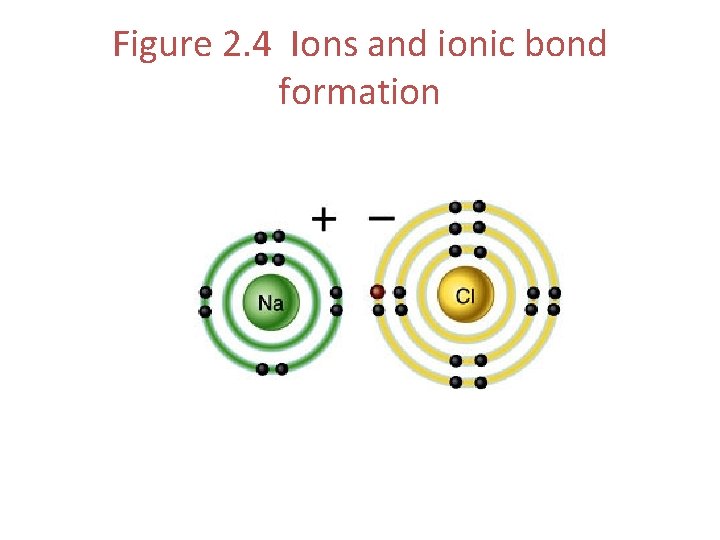
Figure 2. 4 Ions and ionic bond formation

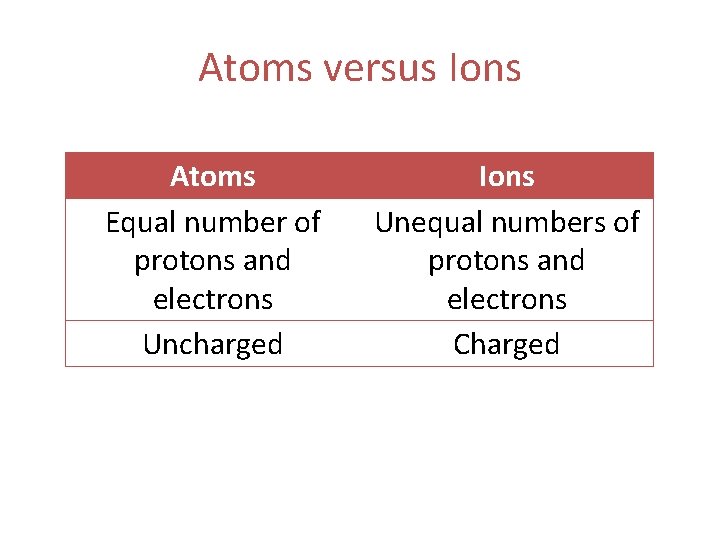
Atoms versus Ions Atoms Equal number of protons and electrons Uncharged Ions Unequal numbers of protons and electrons Charged

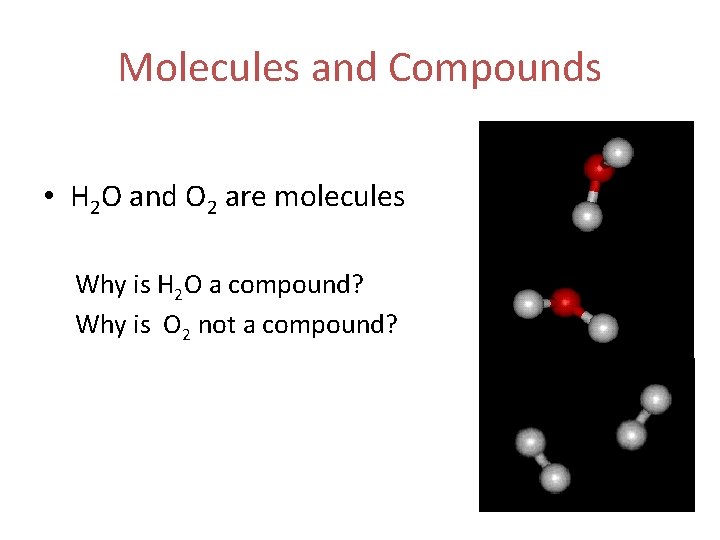
Molecules and Compounds Molecule • Forms when two or more atoms share electrons Compound • A type of molecule composed of two or more different atoms

Molecules and Compounds • H 2 O and O 2 are molecules Why is H 2 O a compound? Why is O 2 not a compound?

Free Radicals • Free radical – an ion or electrically charged molecule with an unpaired electron in its outermost shell • Are extremely unstable and highly reactive • Become stable by donating or accepting electrons, which may destroy nearby molecules • Like robbers deficient in energy • Snatch energy from stable molecules to satisfy themselves • Antioxidants help inactivate free radicals

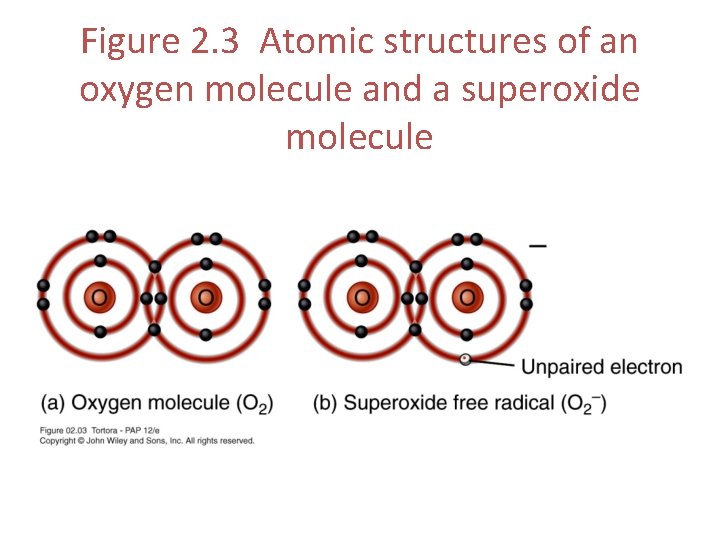
Figure 2. 3 Atomic structures of an oxygen molecule and a superoxide molecule

CHEMICAL BONDS

Chemical Bonds • The forces that hold together a molecule’s atoms • Chemical bonds occur between reacting atoms’ electrons

The Role of Electrons • Electrons are found in shells • Each shell has space for a specific number of electrons – First shell has room for two electrons – Second shell has room for eight electrons • Only the outermost valence shell is important in bonding

The Octet Rule • Two atoms will bond with each other if doing so leaves both with eight valence electrons • Can “get to eight” by giving up, accepting, or sharing electrons • Hydrogen has to “get to two”

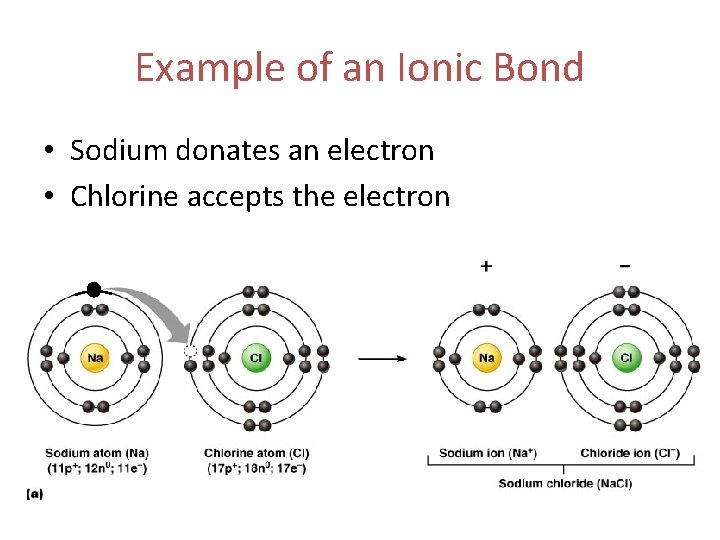
Ionic Bonds CHEMICAL BONDS

Ionic Bonds • Ionic Bond – the force of attraction that holds a cation and anion together • Formed when one atom donates an electron and another atom accepts it

Example of an Ionic Bond • Sodium donates an electron • Chlorine accepts the electron

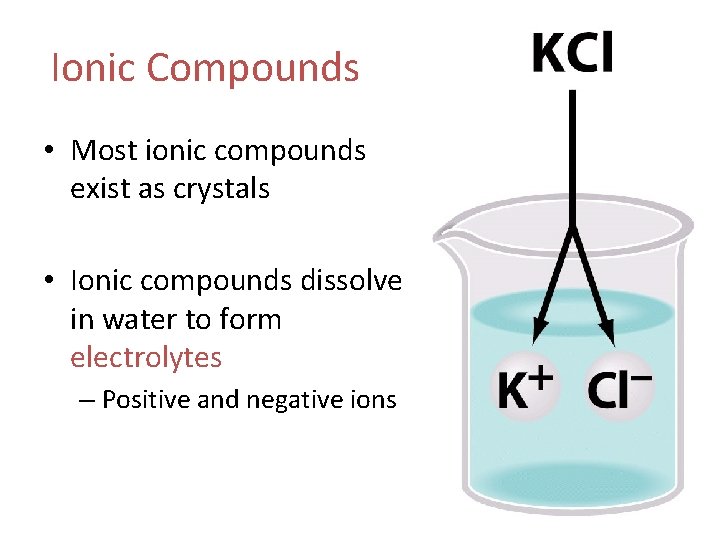
Ionic Compounds • Most ionic compounds exist as crystals • Ionic compounds dissolve in water to form electrolytes – Positive and negative ions

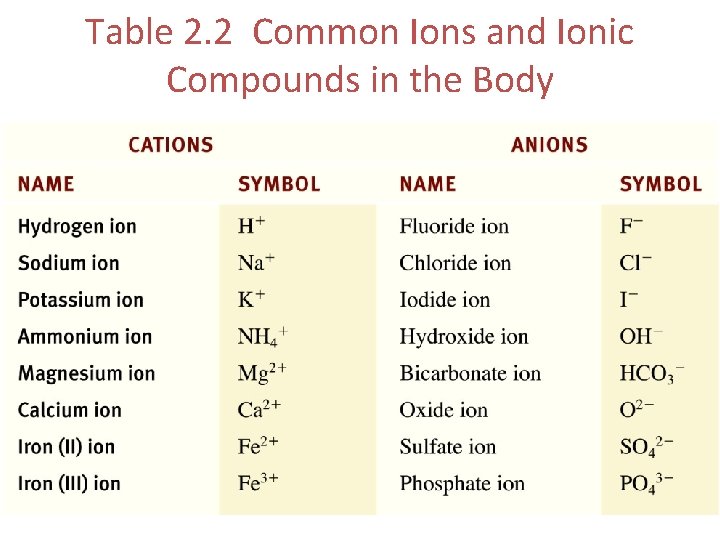
Table 2. 2 Common Ions and Ionic Compounds in the Body

Covalent Bonds CHEMICAL BONDS
 The chemical level of organization
The chemical level of organization The chemical level of organization chapter 2
The chemical level of organization chapter 2 Unit 6 review questions
Unit 6 review questions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Modern chemistry chapter 7
Modern chemistry chapter 7 Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên?
Thế nào là giọng cùng tên? Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là