Chapter 7 Chemical Quantities Section 7 1 The












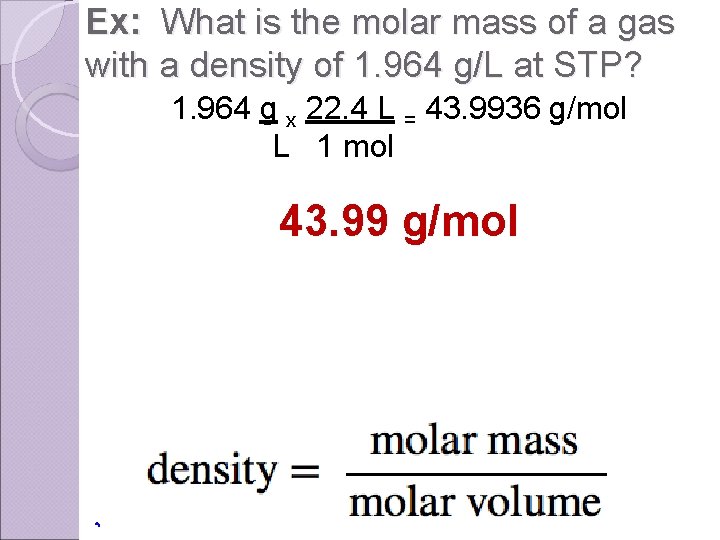



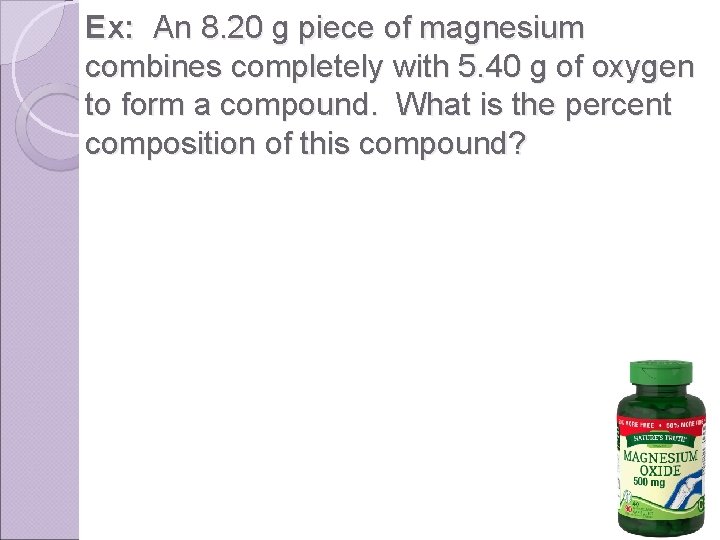


- Slides: 45

Chapter 7 – Chemical Quantities


Section 7. 1 The Mole: A Measurement of Matter Objectives: ◦ Describe how Avogadro’s number is related to a mole of any substance ◦ Calculate the mass of a mole of any substance

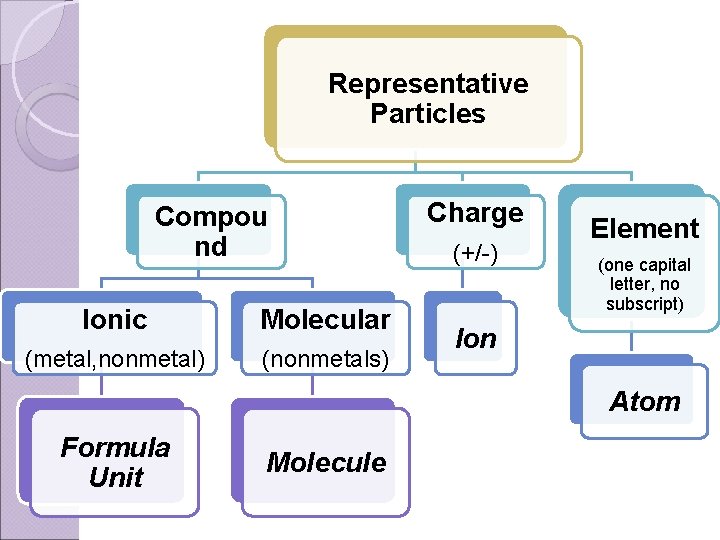
Representative Particles Types of representative particles: ◦ Atoms (Single element) ◦ Molecules (Covalent/Molecular Compound) ◦ Formula Units (Ionic Compound) ◦ Ions (+/- charge)

Representative Particles Compou nd Ionic Molecular (metal, nonmetal) (nonmetals) Charge (+/-) Element (one capital letter, no subscript) Ion Atom Formula Unit Molecule

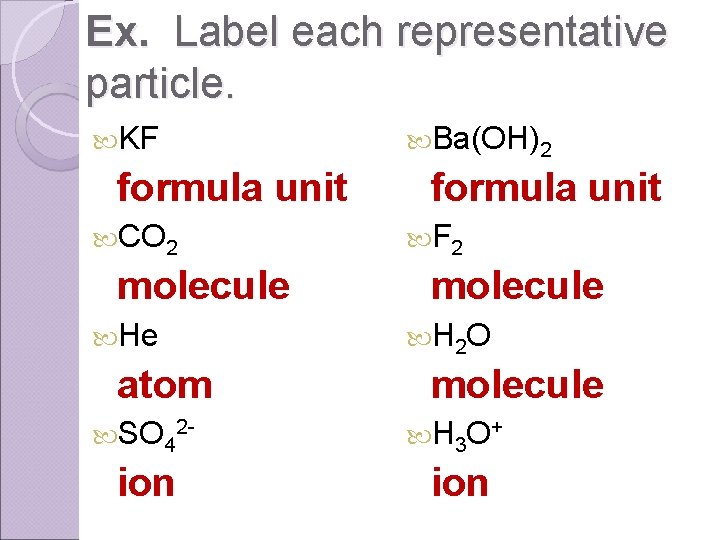
Ex. Label each representative particle. KF formula unit CO 2 molecule He atom Ba(OH)2 formula unit F 2 molecule H 2 O molecule SO 42 - H 3 O + ion

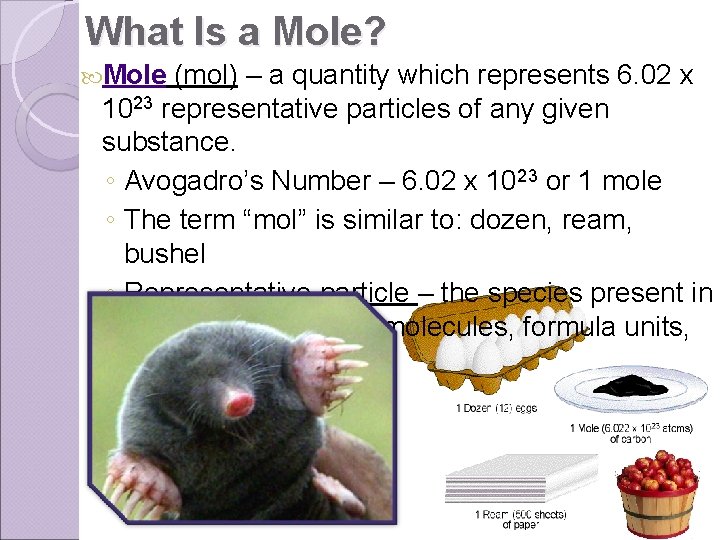
What Is a Mole? Mole (mol) – a quantity which represents 6. 02 x 1023 representative particles of any given substance. ◦ Avogadro’s Number – 6. 02 x 1023 or 1 mole ◦ The term “mol” is similar to: dozen, ream, bushel ◦ Representative particle – the species present in a substance: atoms, molecules, formula units, ions.

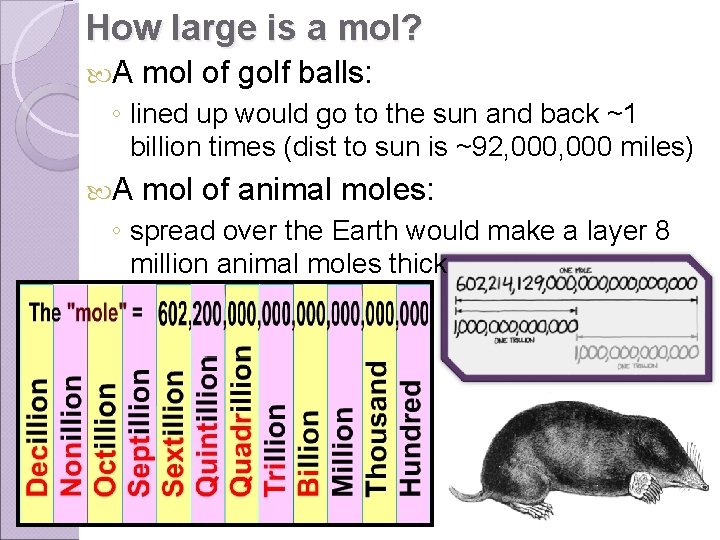
How large is a mol? A mol of golf balls: ◦ lined up would go to the sun and back ~1 billion times (dist to sun is ~92, 000 miles) A mol of animal moles: ◦ spread over the Earth would make a layer 8 million animal moles thick

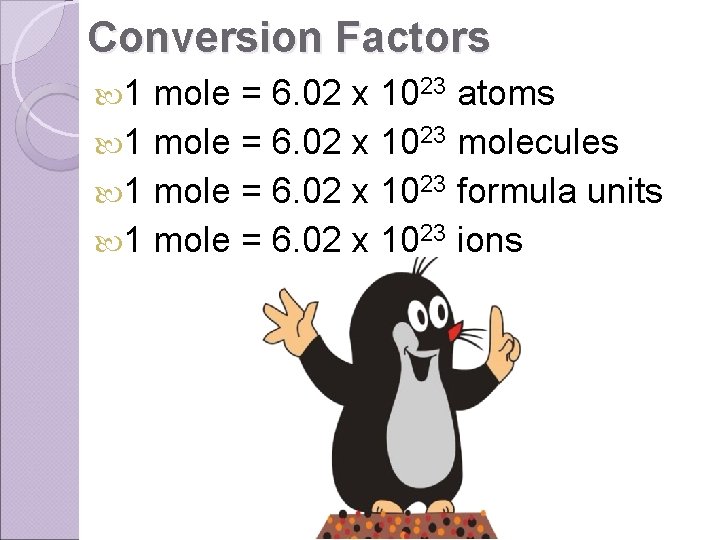
Conversion Factors 1 mole = 6. 02 x 1023 atoms 1 mole = 6. 02 x 1023 molecules 1 mole = 6. 02 x 1023 formula units 1 mole = 6. 02 x 1023 ions

Ex: How many atoms are there in 1. 14 mol Ag? 1. 14 mole Ag x 6. 02 x 1023 atoms = 6. 8628 x 1023 atoms Ag 1 mole Ag 6. 86 x 1023 atoms Ag

Ex: How many moles of magnesium is 1. 25 x 1023 atoms of magnesium? 23 1. 25 x 10 atom Mg x 1 mole Mg = 0. 207641196 mol Mg 6. 02 x 1023 atoms Mg 0. 208 moles Mg

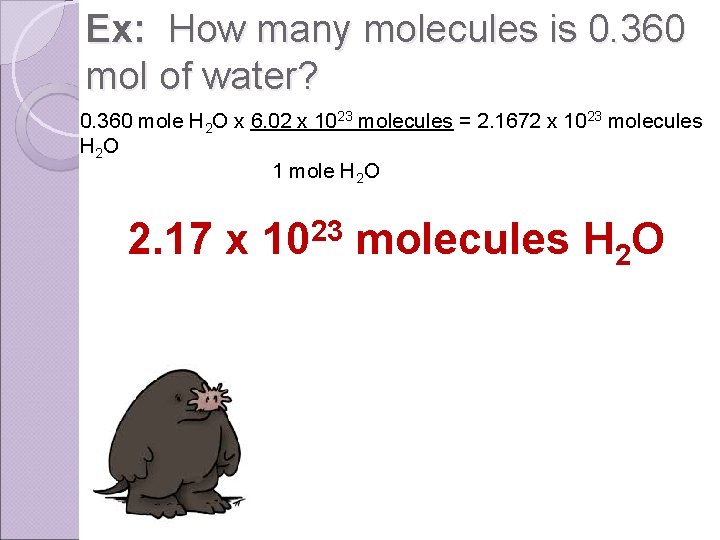
Ex: How many molecules is 0. 360 mol of water? 0. 360 mole H 2 O x 6. 02 x 1023 molecules = 2. 1672 x 1023 molecules H 2 O 1 mole H 2 O 2. 17 x 1023 molecules H 2 O

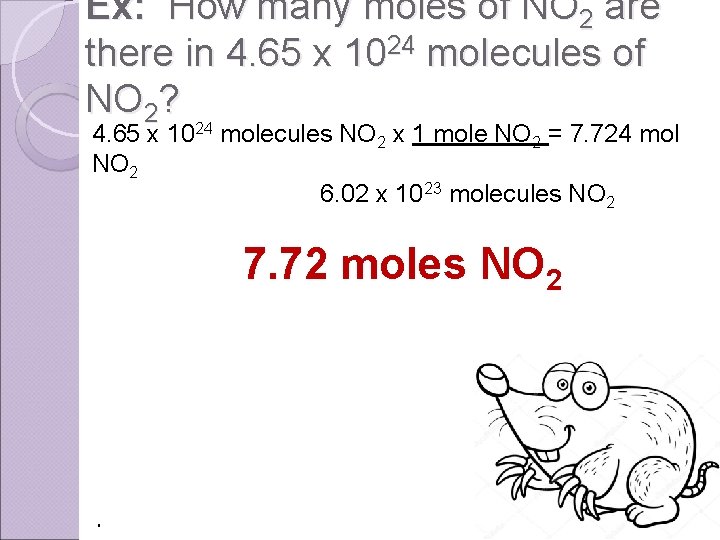
Ex: How many moles of NO 2 are there in 4. 65 x 1024 molecules of NO 2? 4. 65 x 1024 molecules NO 2 x 1 mole NO 2 = 7. 724 mol NO 2 6. 02 x 10 23 molecules NO 2 7. 72 moles NO 2

Caution! Be careful when being asked to convert moles of a compound into atoms! ◦ We will need to multiply the final answer by the number of atoms in the compound. MOLE CROSSING

Ex: How many atoms are there in 1. 14 mol SO 3? 1. 14 mol x 6. 02 x 1023 molecules x 4 atoms = 2. 74512 x 1024 atoms 1 molecule 2. 75 x 1024 atoms

Ex: How many atoms are in 2. 12 mol of propane (C 3 H 8)? 2. 12 mol x 6. 02 x 1023 molecules x 11 atoms = 1. 403864 x 1025 atoms 1 molecule 1. 40 x 1025 atoms

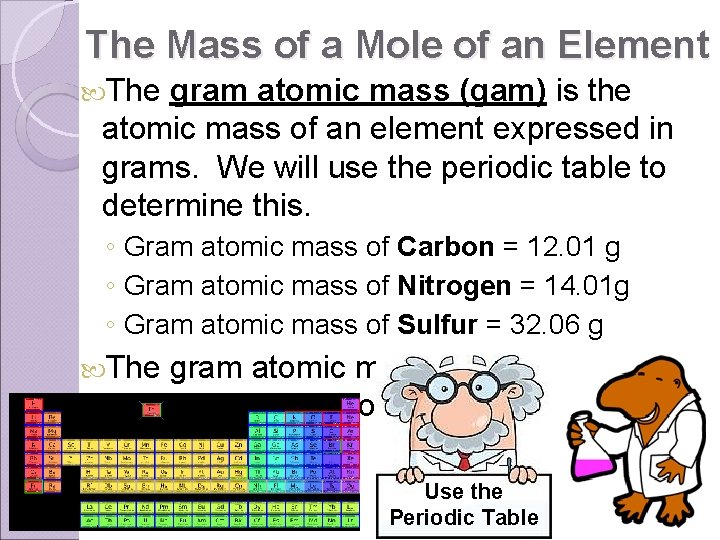
The Mass of a Mole of an Element The gram atomic mass (gam) is the atomic mass of an element expressed in grams. We will use the periodic table to determine this. ◦ Gram atomic mass of Carbon = 12. 01 g ◦ Gram atomic mass of Nitrogen = 14. 01 g ◦ Gram atomic mass of Sulfur = 32. 06 g The gram atomic mass is equivalent to one mole of the atom. Use the Periodic Table

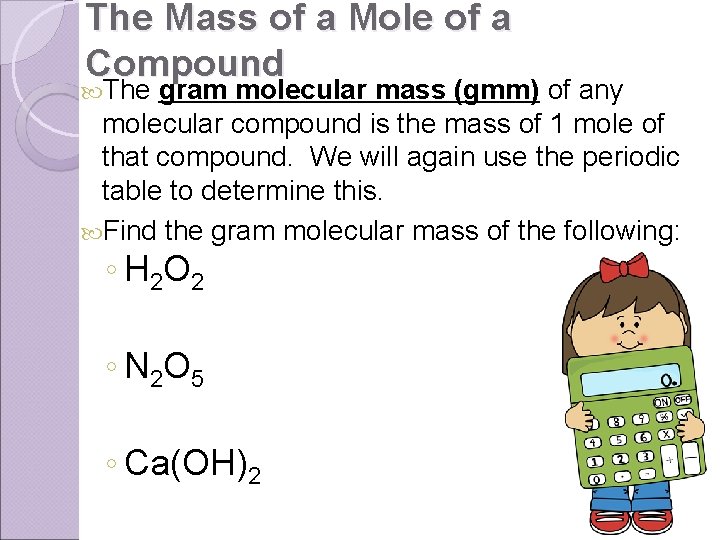
The Mass of a Mole of a Compound The gram molecular mass (gmm) of any molecular compound is the mass of 1 mole of that compound. We will again use the periodic table to determine this. Find the gram molecular mass of the following: ◦ H 2 O 2 ◦ N 2 O 5 ◦ Ca(OH)2

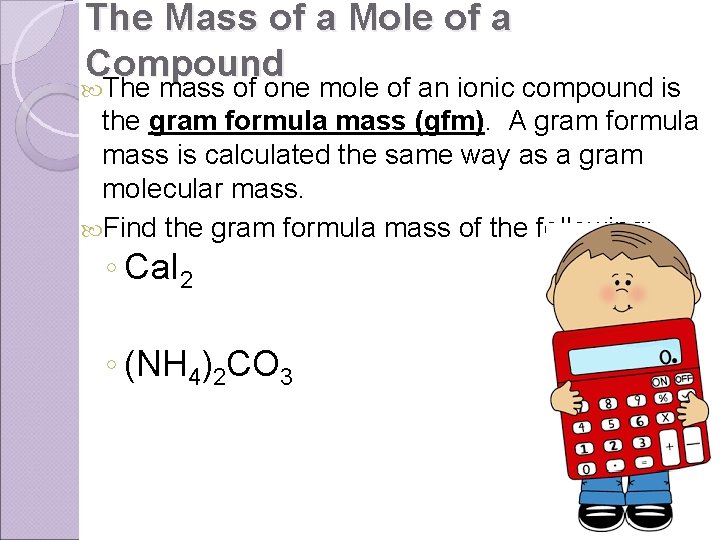
The Mass of a Mole of a Compound The mass of one mole of an ionic compound is the gram formula mass (gfm). A gram formula mass is calculated the same way as a gram molecular mass. Find the gram formula mass of the following: ◦ Ca. I 2 ◦ (NH 4)2 CO 3

Section 7. 1 The Mole: A Measurement of Matter Did We Meet Our Objectives? ◦ Describe how Avogadro’s number is related to a mole of any substance ◦ Calculate the mass of a mole of any substance Charlotte would be proud!

Section 7. 2 Mole-Mass and Mole. Volume Relationships Objectives: ◦ Use the molar mass to convert between mass and moles of a substance ◦ Use the mole to convert among measurements of mass, volume, and number of particles

The Mass of a Mole of an Element Molar mass – mass of 1 mol of any substance. Can be used in calculations involving elements, molecular compounds, and ionic compounds ◦ 1. 0 mol of C has a mass of 12. 01 g/mol ◦ 1. 0 mol of H 2 has a mass of 2. 02 g/mol ◦ 1. 0 mol H 2 O has a mass of 18. 02 g/mol

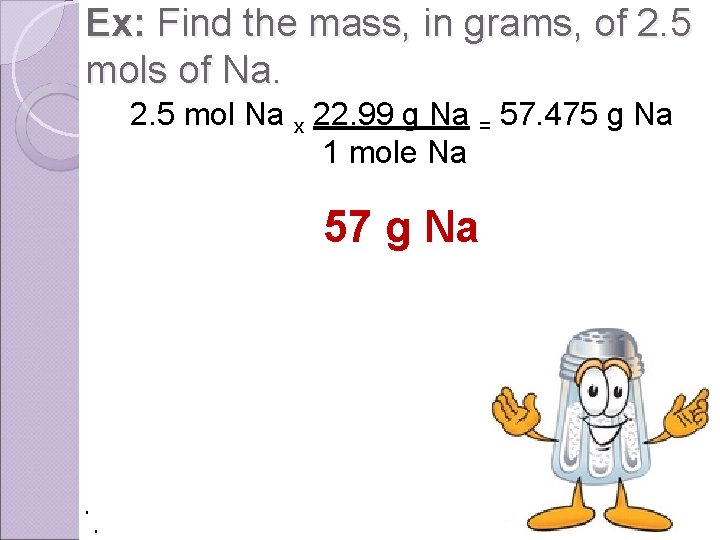
Ex: Find the mass, in grams, of 2. 5 mols of Na. 2. 5 mol Na x 22. 99 g Na = 57. 475 g Na 1 mole Na 57 g Na

Ex: Find the number of mols in 75. 0 g of dinitrogen trioxide (N 2 O 3). 75. 0 g N 2 O 3 x 1 mol N 2 O 3 = 0. 98658 mol N 2 O 3 76. 02 g N 2 O 3 0. 987 mol N 2 O 3

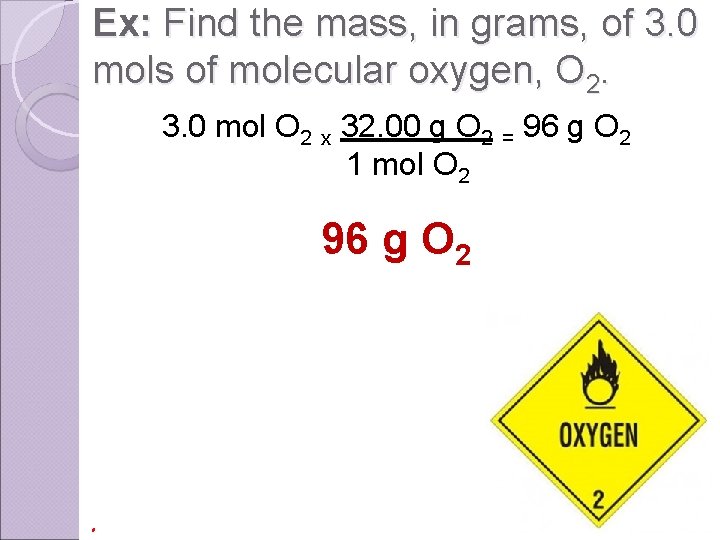
Ex: Find the mass, in grams, of 3. 0 mols of molecular oxygen, O 2. 3. 0 mol O 2 x 32. 00 g O 2 = 96 g O 2 1 mol O 2 96 g O 2

Ex: Find the number of moles in 236. 5 g of Cu. SO 4. 236. 5 g Cu. SO 4 x 1 mol Cu. SO 4 = 1. 4817 mol Cu. SO 4 159. 61 g Cu. SO 4 1. 482 mol Cu. SO 4

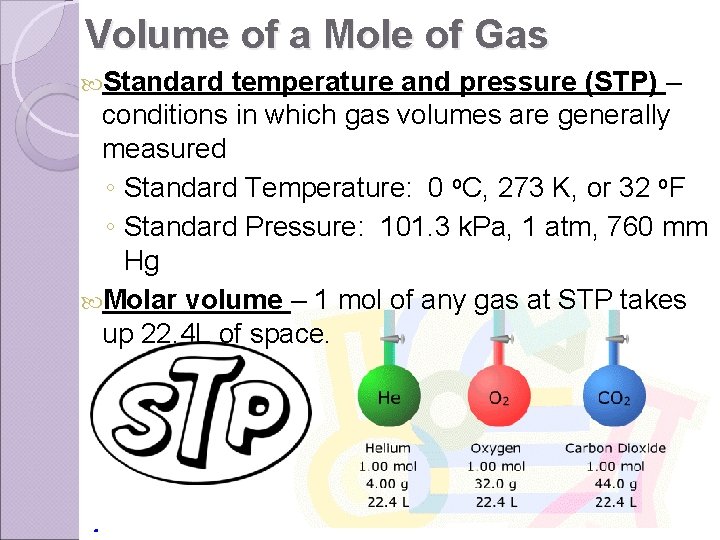
Volume of a Mole of Gas Standard temperature and pressure (STP) – conditions in which gas volumes are generally measured ◦ Standard Temperature: 0 o. C, 273 K, or 32 o. F ◦ Standard Pressure: 101. 3 k. Pa, 1 atm, 760 mm Hg Molar volume – 1 mol of any gas at STP takes up 22. 4 L of space.

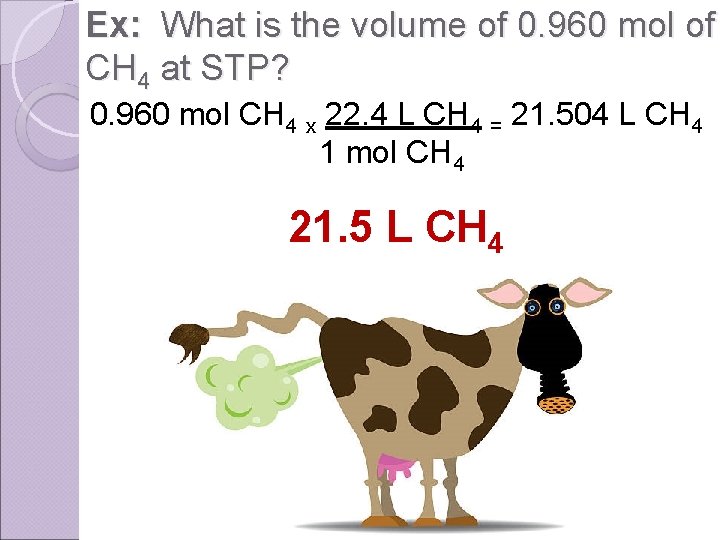
Ex: What is the volume of 0. 960 mol of CH 4 at STP? 0. 960 mol CH 4 x 22. 4 L CH 4 = 21. 504 L CH 4 1 mol CH 4 21. 5 L CH 4

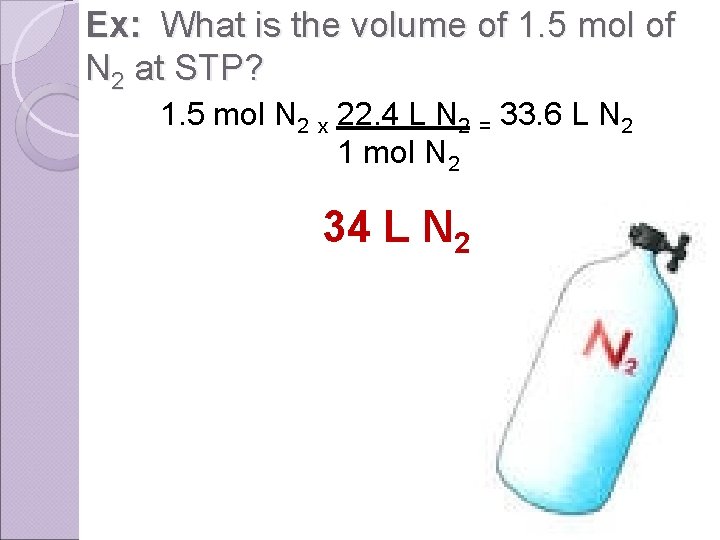
Ex: What is the volume of 1. 5 mol of N 2 at STP? 1. 5 mol N 2 x 22. 4 L N 2 = 33. 6 L N 2 1 mol N 2 34 L N 2

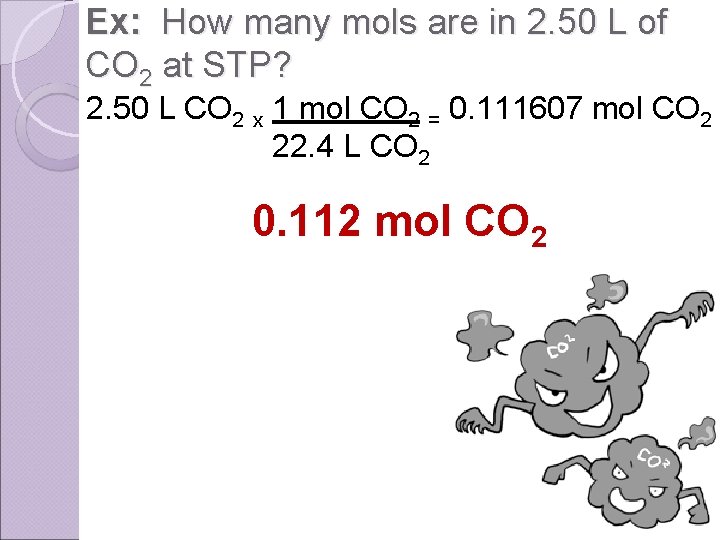
Ex: How many mols are in 2. 50 L of CO 2 at STP? 2. 50 L CO 2 x 1 mol CO 2 = 0. 111607 mol CO 2 22. 4 L CO 2 0. 112 mol CO 2
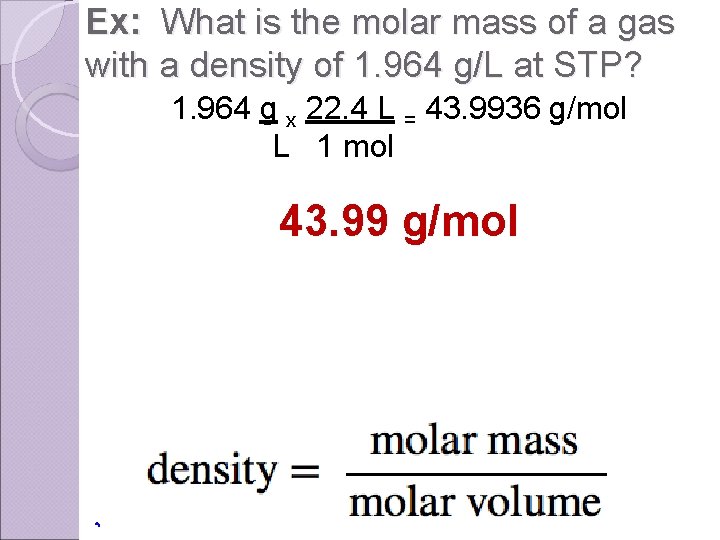
Ex: What is the molar mass of a gas with a density of 1. 964 g/L at STP? 1. 964 g x 22. 4 L = 43. 9936 g/mol L 1 mol 43. 99 g/mol

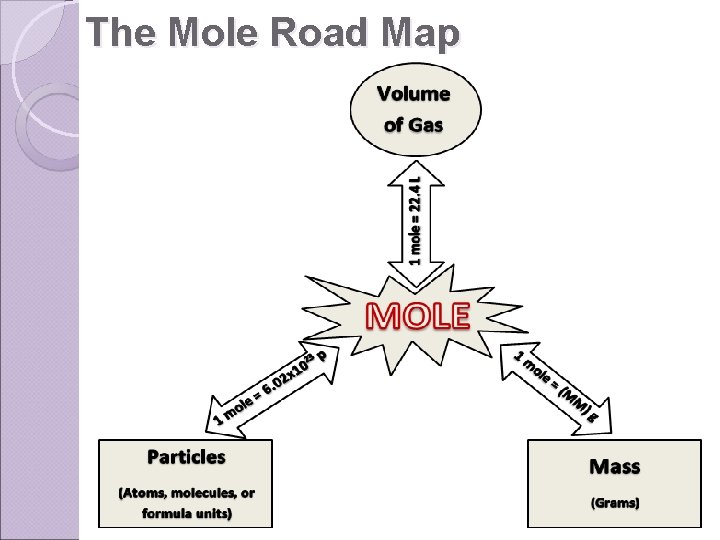
The Mole Road Map

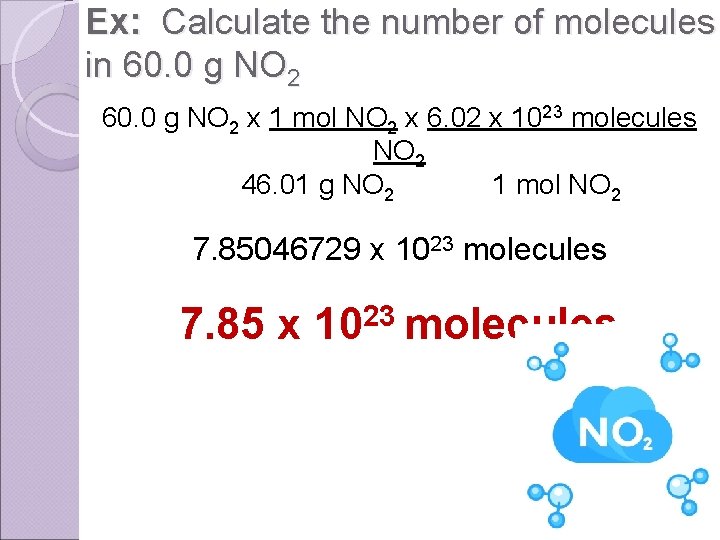
Ex: Calculate the number of molecules in 60. 0 g NO 2 x 1 mol NO 2 x 6. 02 x 1023 molecules NO 2 46. 01 g NO 2 1 mol NO 2 7. 85046729 x 1023 molecules 7. 85 x 1023 molecules

Ex: Calculate the volume, in liters, of 3. 24 x 1022 molecules Cl 2 at STP. 3. 24 x 1022 molecules Cl 2 x 1 mol x 22. 4 L 6. 02 x 10 23 molecules 1 mol 1. 21 L Cl 2 at STP

Section 7. 2 Mole-Mass and Mole. Volume Relationships Did We Meet Our Objectives? ◦ Use the molar mass to convert between mass and moles of a substance ◦ Use the mole to convert among measurements of mass, volume, and number of particles

Section 7. 3 Percent Composition and Chemical Formulas Objectives: ◦ Calculate the percent composition of a substance from its chemical formula or experimental data ◦ Derive the empirical formula and molecular formula of a compound from experimental data

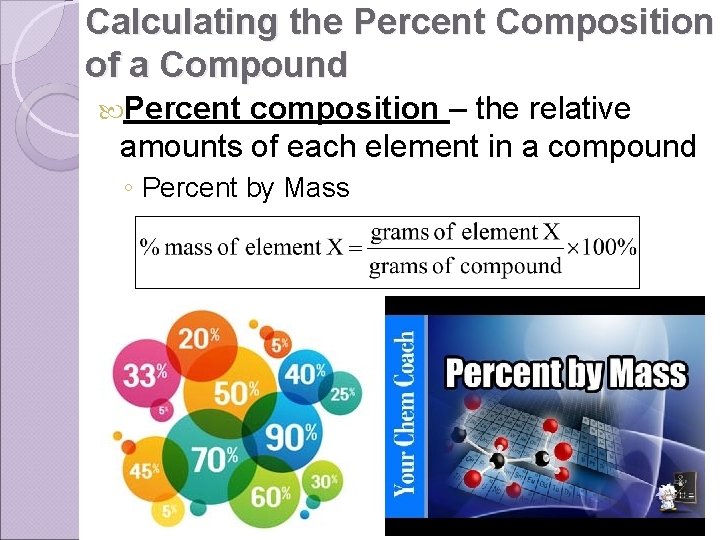
Calculating the Percent Composition of a Compound Percent composition – the relative amounts of each element in a compound ◦ Percent by Mass
Ex: An 8. 20 g piece of magnesium combines completely with 5. 40 g of oxygen to form a compound. What is the percent composition of this compound?

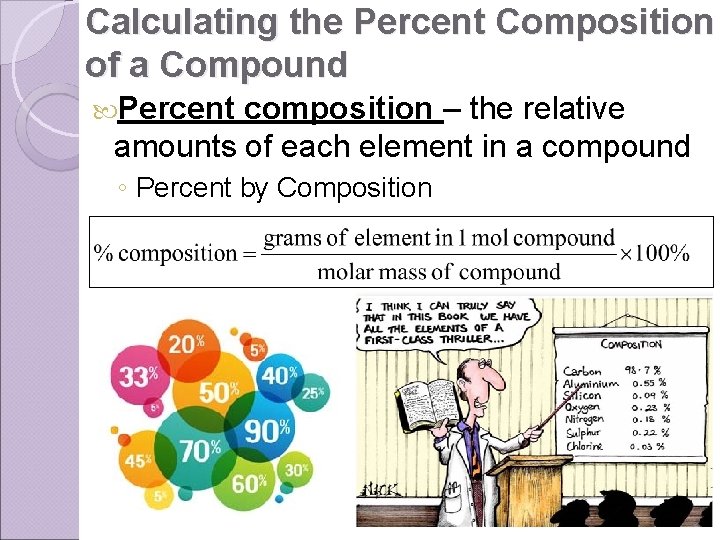
Calculating the Percent Composition of a Compound Percent composition – the relative amounts of each element in a compound ◦ Percent by Composition

Ex: Find the percent composition of propane (C 3 H 8).

Using Percent as a Conversion Factor To do this, you multiply the mass of the compound by a conversion factor that is based on the percent composition. ◦ Ex: Calculate the mass of carbon in 82. 0 g of propane (C 3 H 8). (Remember, carbon is 81. 8%)

Calculating Empirical Formulas Empirical formula – gives the lowest whole number ratio of atoms of the elements in a compound ◦ Empirical formula can sometimes be the molecular formula CO 2 C 6 H 12 O 6 CH 2 O

Calculating Empirical Formulas The polymer used for the nonstick surface of cooking utensils is 24. 0% C and 76. 0% F by mass. ◦ What is the empirical formula of this polymer?

Calculating Molecular Formulas The polymer used for the nonstick surface of cooking utensils is 24. 0% C and 76. 0% F by mass. ◦ What is the empirical formula of this polymer? ◦ If the molecular mass is 100. 02 g, what is the molecular formula?

Section 7. 3 Percent Composition and Chemical Formulas Did We Meet Our Objectives? ◦ Calculate the percent composition of a substance from its chemical formula or experimental data ◦ Derive the empirical formula and molecular formula of a compound from experimental data