Topic 7 AtomicNuclear Physics Structure properties of the


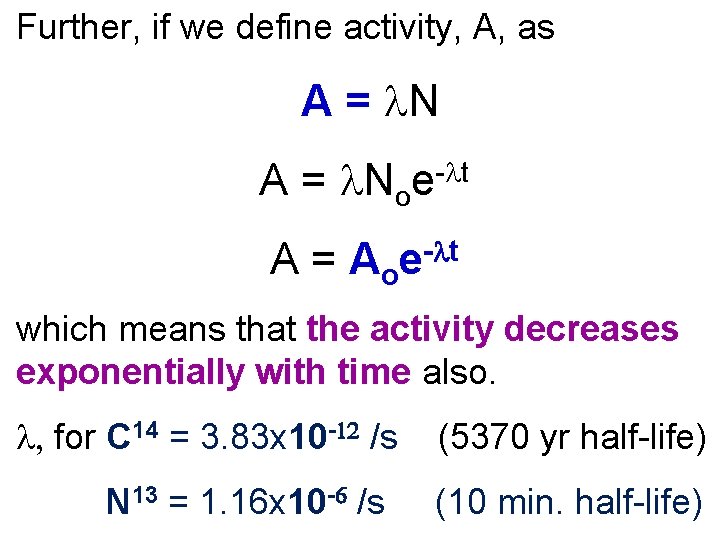
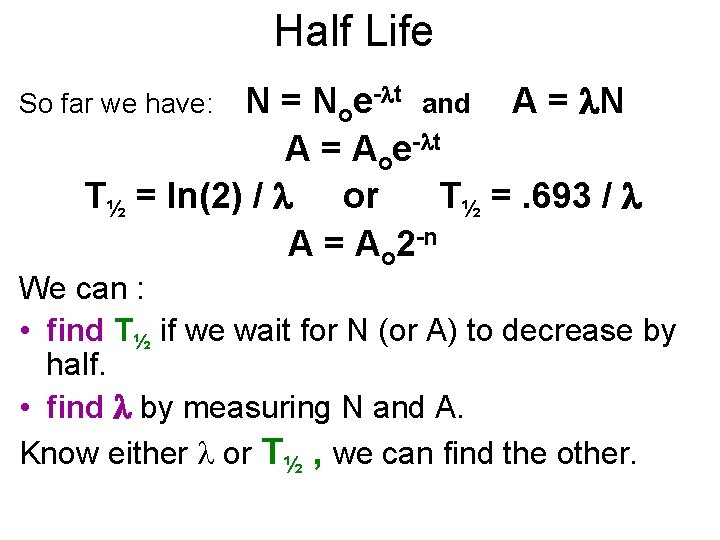














- Slides: 60

Topic 7: Atomic/Nuclear Physics. • Structure & properties of the nucleus • Binding energy & nuclear forces • Radioactivity & Decay – Alpha – Beta – Gamma • Half-life • Radiocarbon dating • Applications

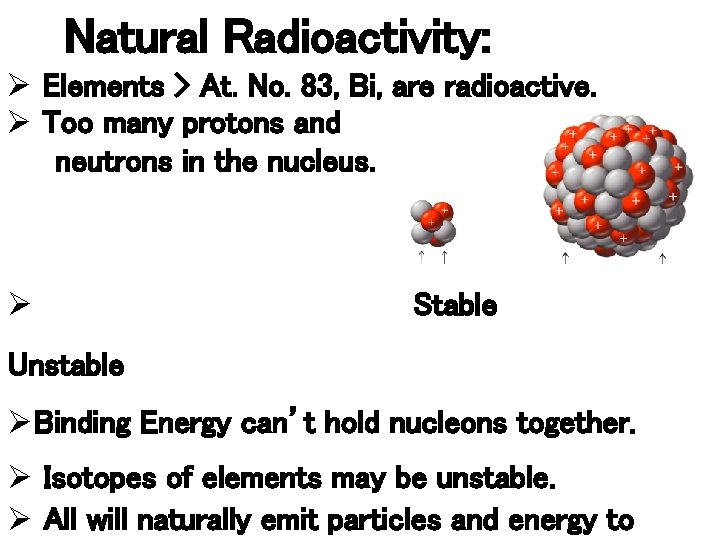
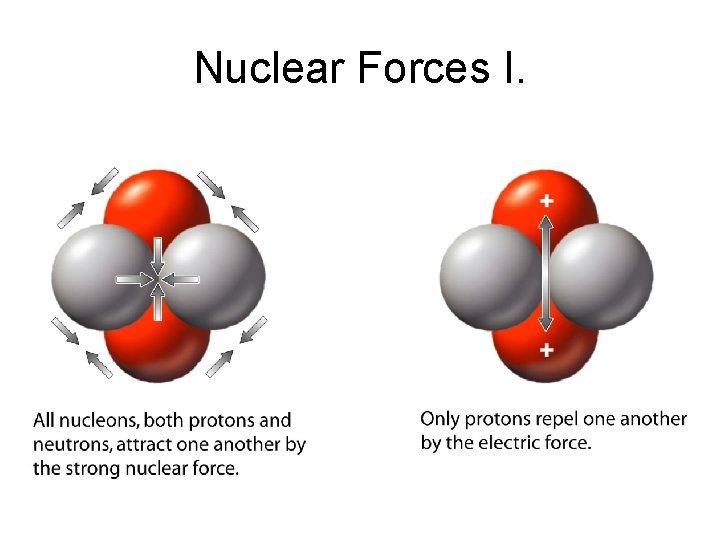
Natural Radioactivity: Ø Elements > At. No. 83, Bi, are radioactive. Ø Too many protons and neutrons in the nucleus. Ø Stable Unstable ØBinding Energy can’t hold nucleons together. Ø Isotopes of elements may be unstable. Ø All will naturally emit particles and energy to

Radioactive Decay

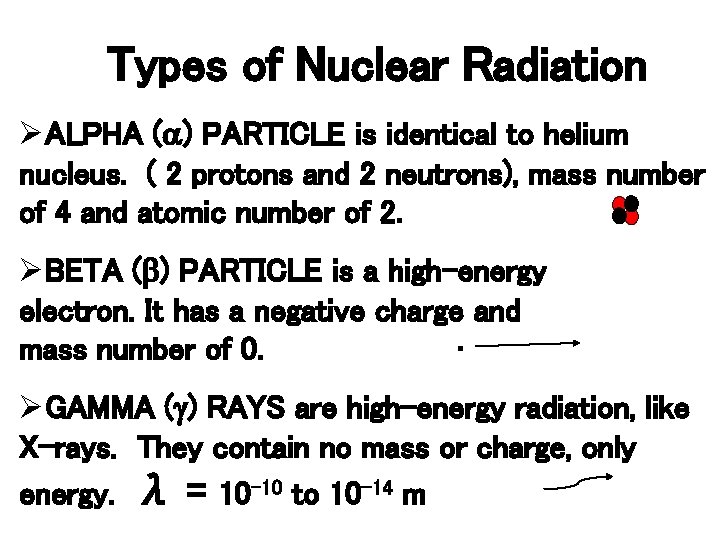
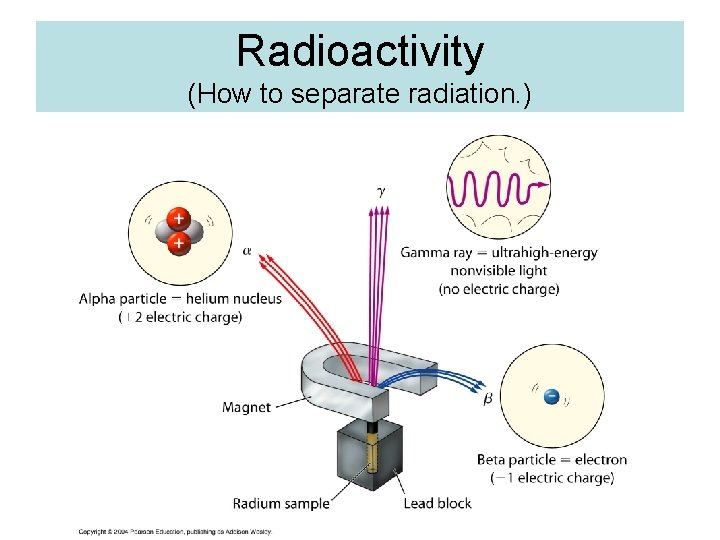
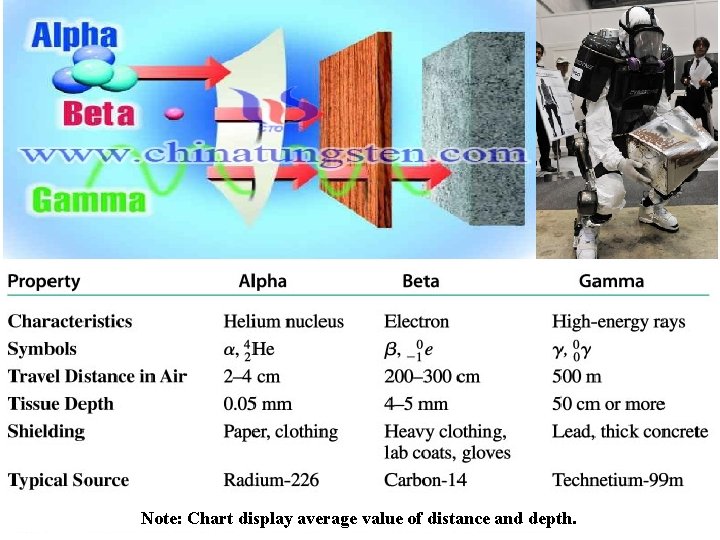
Types of Nuclear Radiation ØALPHA (a) PARTICLE is identical to helium nucleus. ( 2 protons and 2 neutrons), mass number of 4 and atomic number of 2. ØBETA (b) PARTICLE is a high-energy electron. It has a negative charge and mass number of 0. ∙ ØGAMMA (g) RAYS are high-energy radiation, like X-rays. They contain no mass or charge, only energy. λ = 10 -10 to 10 -14 m

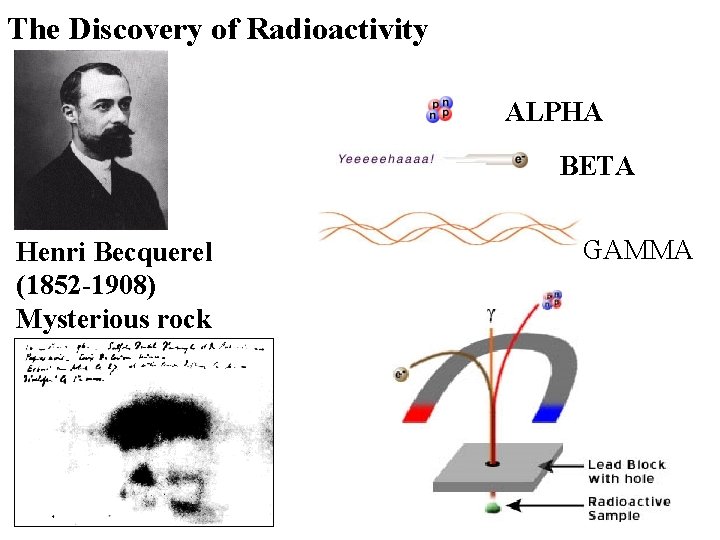
The Discovery of Radioactivity ALPHA BETA Henri Becquerel (1852 -1908) Mysterious rock GAMMA

Radioactivity: Historical Overview 1896: Becquerel accidentally discovered that a mysterious rock emitted invisible radiation onto a photographic plate. 1898: Marie and Pierre Curie discovered polonium (Z=84) and radium (Z = 88), two new radioactive elements. The Nobel Prize in Physics 1903 "in recognition of the extraordinary services they have rendered by their joint researches on the radiation phenomena discovered by Professor Henri Becquerel" Pierre Curie France Marie Curie France "in recognition of the extraordinary services he has rendered by his discovery of spontaneous radioactivity" Antoine Henri Becquerel France

Radioactivity (How to separate radiation. )

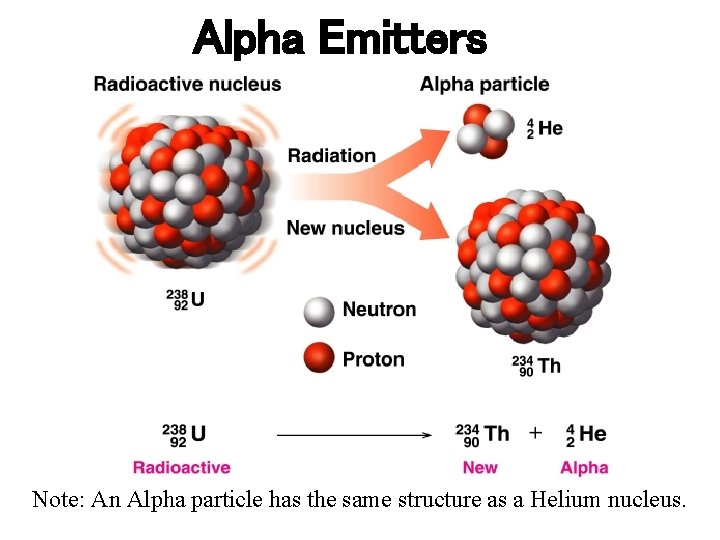
Alpha Emitters Note: An Alpha particle has the same structure as a Helium nucleus.

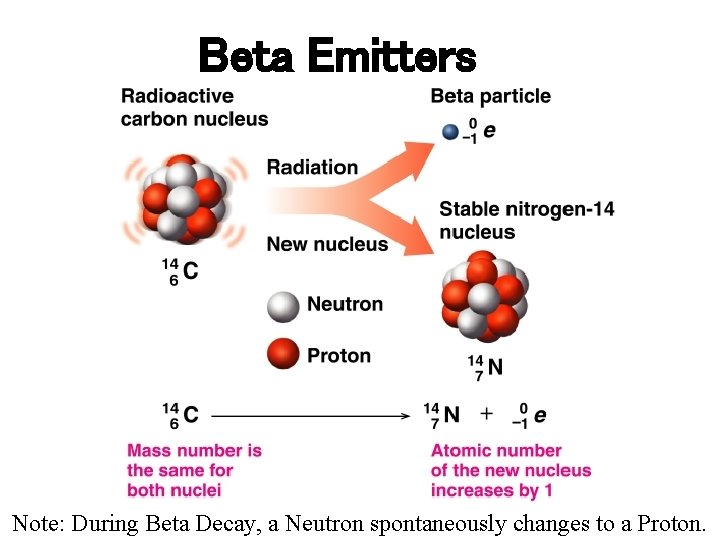
Beta Emitters Note: During Beta Decay, a Neutron spontaneously changes to a Proton.

Radioactivity

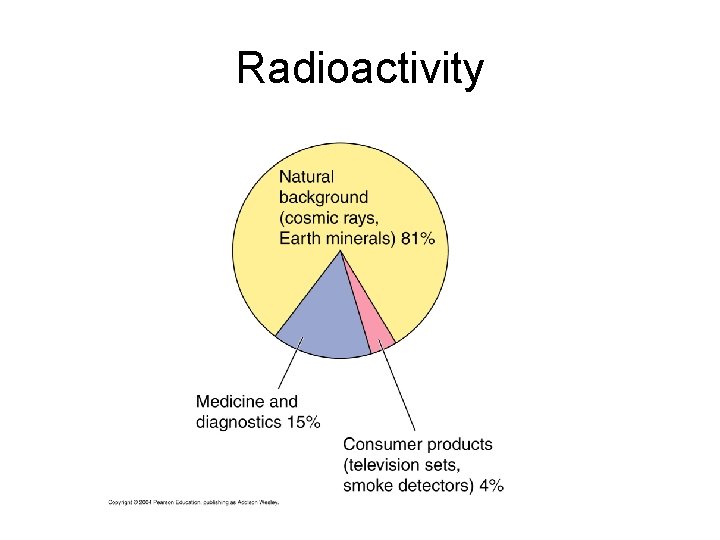
Radiation Exposure: ØBACKGROUND RADIATION is the radiation that is in the environment. ØBackground radiation can come from food, building materials, cosmic rays, etc. ØThe air molecules in the Earth’s atmosphere block out some cosmic rays.

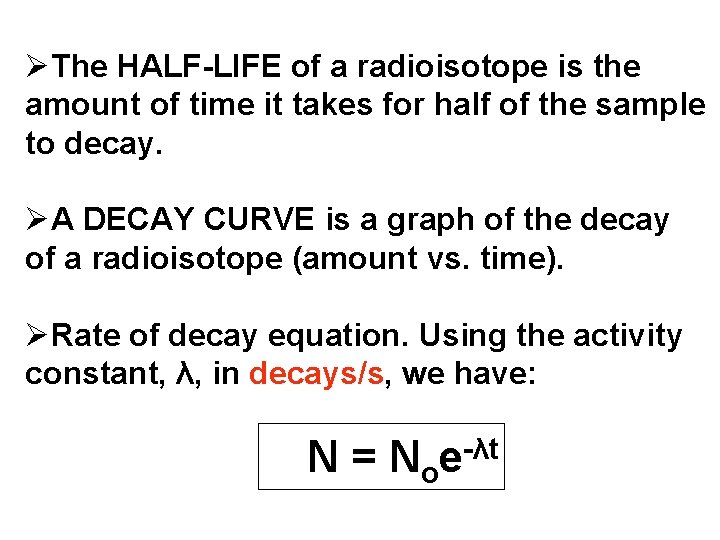
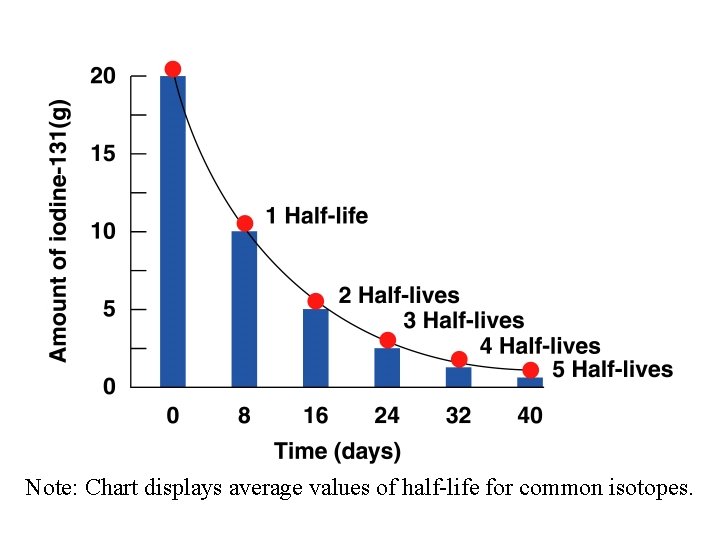
ØThe HALF-LIFE of a radioisotope is the amount of time it takes for half of the sample to decay. ØA DECAY CURVE is a graph of the decay of a radioisotope (amount vs. time). ØRate of decay equation. Using the activity constant, λ, in decays/s, we have: N = No -λt e

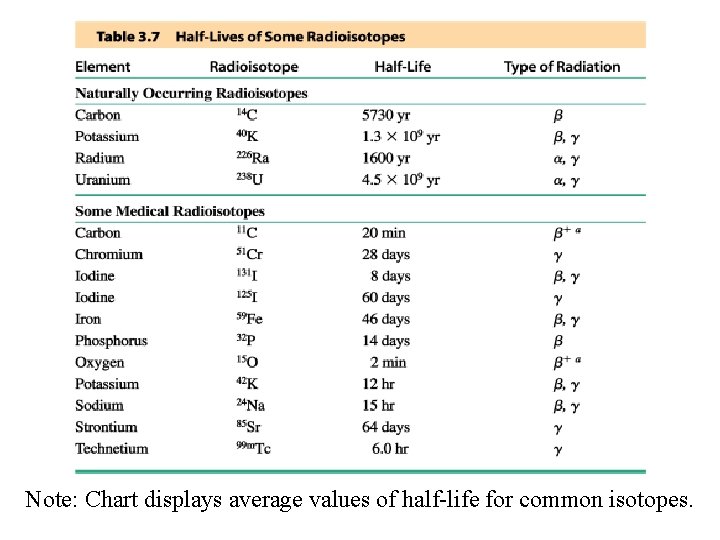
Note: Chart displays average values of half-life for common isotopes.
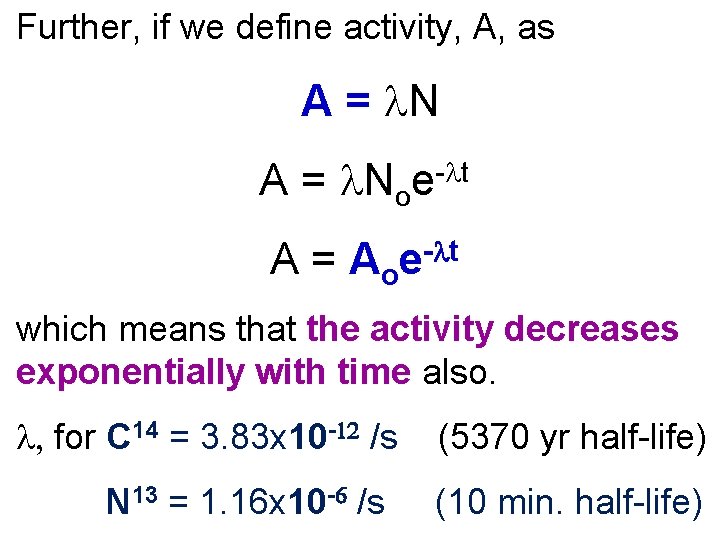
Further, if we define activity, A, as A = Noe- t A = Aoe- t which means that the activity decreases exponentially with time also. , for C 14 = 3. 83 x 10 -12 /s (5370 yr half-life) N 13 = 1. 16 x 10 -6 /s (10 min. half-life)
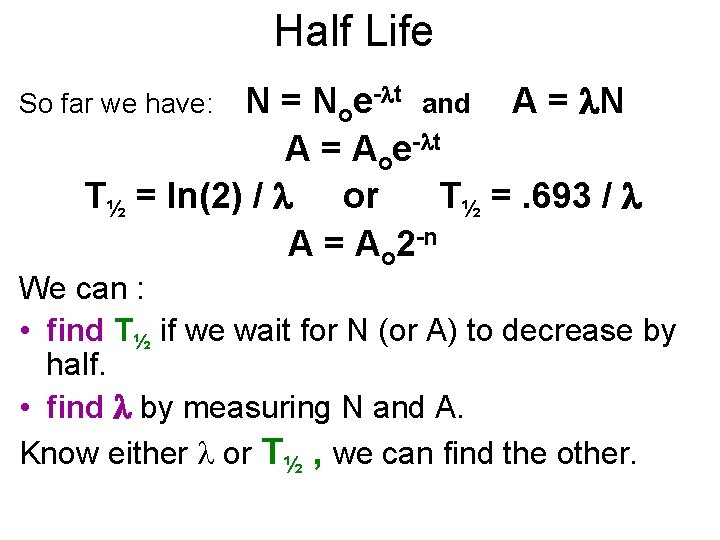
Half Life N = Noe- t and A = N A = Aoe- t T½ = ln(2) / or T½ =. 693 / A = Ao 2 -n So far we have: We can : • find T½ if we wait for N (or A) to decrease by half. • find by measuring N and A. Know either or T½ , we can find the other.

Note: Chart displays average values of half-life for common isotopes.

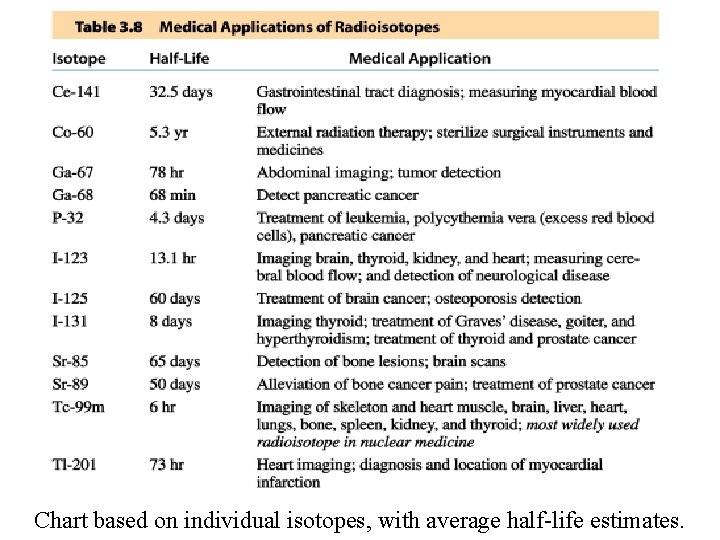
Chart based on individual isotopes, with average half-life estimates.

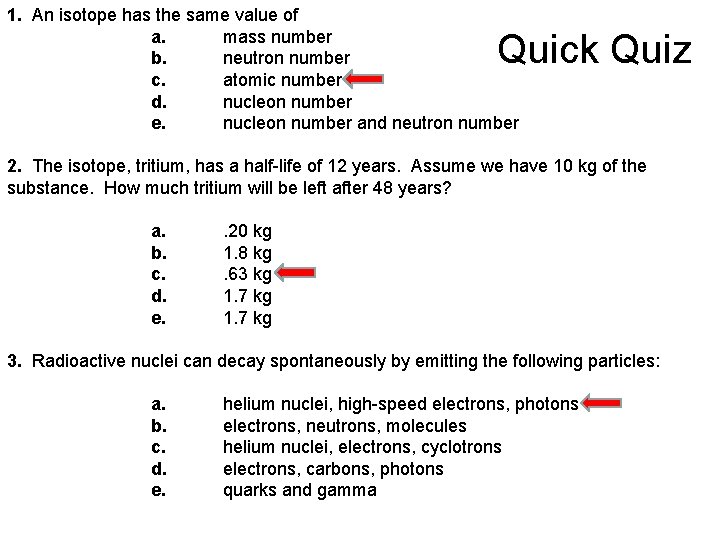
1. An isotope has the same value of a. mass number b. neutron number c. atomic number d. nucleon number e. nucleon number and neutron number Quick Quiz 2. The isotope, tritium, has a half-life of 12 years. Assume we have 10 kg of the substance. How much tritium will be left after 48 years? a. . 20 kg b. 1. 8 kg c. . 63 kg d. 1. 7 kg e. 1. 7 kg 3. Radioactive nuclei can decay spontaneously by emitting the following particles: a. helium nuclei, high-speed electrons, photons b. electrons, neutrons, molecules c. helium nuclei, electrons, cyclotrons d. electrons, carbons, photons e. quarks and gamma

Nuclear Forces I.

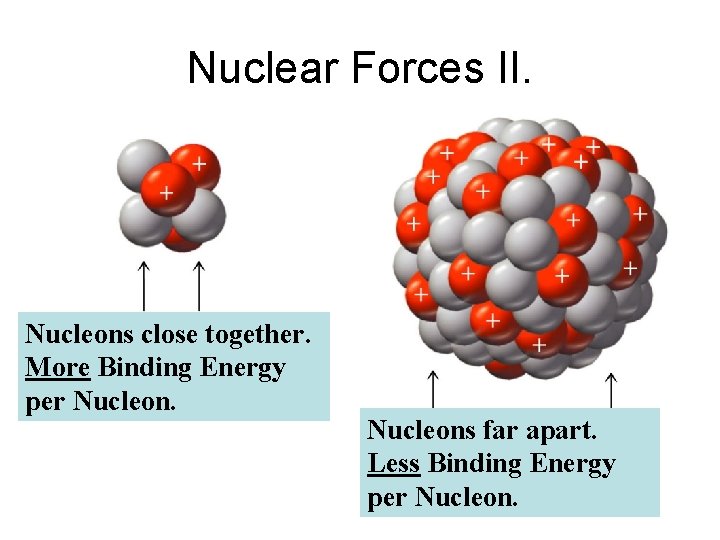
Nuclear Forces II. Nucleons close together. More Binding Energy per Nucleons far apart. Less Binding Energy per Nucleon.

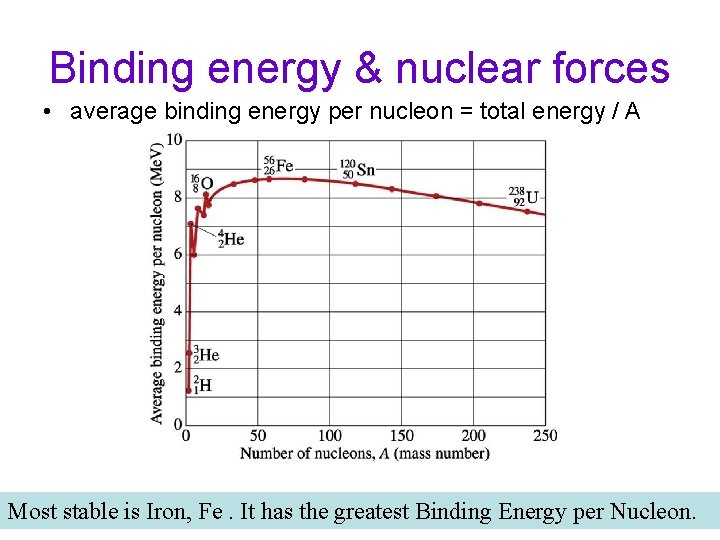
Binding energy & nuclear forces • average binding energy per nucleon = total energy / A Most stable is Iron, Fe. It has the greatest Binding Energy per Nucleon.

4. The smallest particle of any chemical element that can exist by itself and yet retain the qualities that distinguish it as that element is: A. an electron B. a proton C. a neutron D. an atom E. a molecule Quick Quiz 5. The mass of an electron: A. is almost the same as that of a neutron B. is negative C. equals that of a proton D. is zero if the electron is at rest E. is much less than that of a proton 6. The mass of a neutron in amu’s: A. exactly equals that of an electron B. exactly equals that of a proton C. is a little more than that of a proton D. is exactly that of a proton plus an electron E. is as yet unmeasured

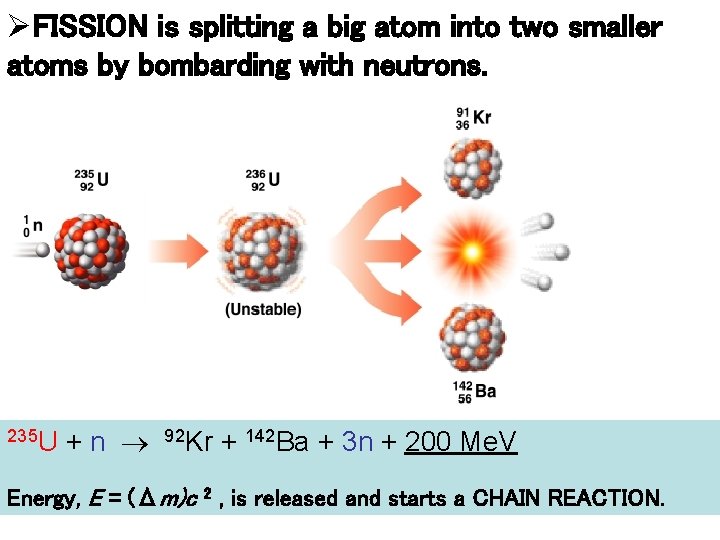
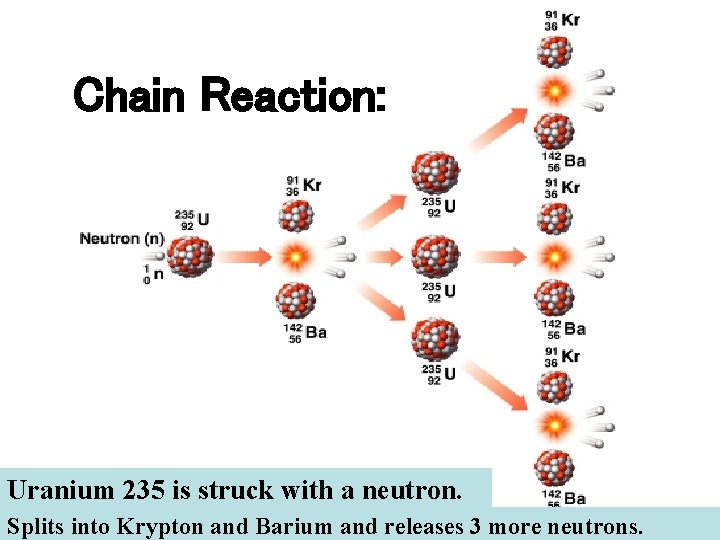
ØFISSION is splitting a big atom into two smaller atoms by bombarding with neutrons. 235 U + n 92 Kr + 142 Ba + 3 n + 200 Me. V Energy, E = (Δm)c 2 , is released and starts a CHAIN REACTION.

Chain Reaction: Uranium 235 is struck with a neutron. Splits into Krypton and Barium and releases 3 more neutrons.

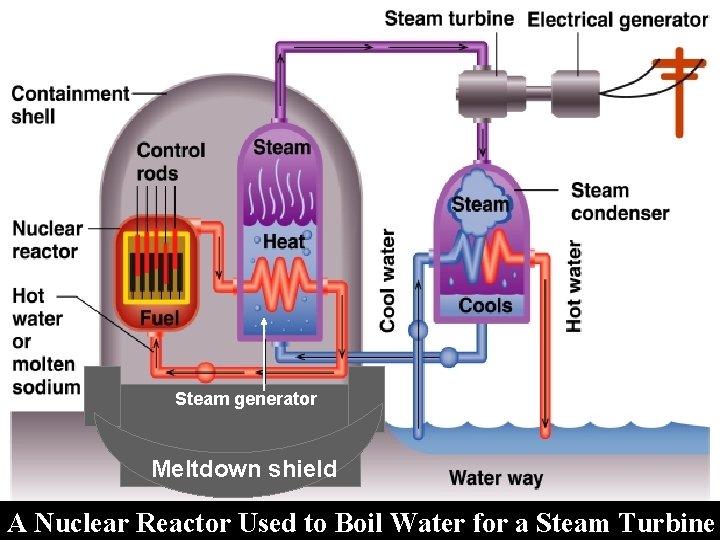
Nuclear Power: a Fission Reaction. ØMass of uranium is kept small and CONTROL RODS absorb neutrons to prevent chain reaction. ØProblems are: • Public perception • Security • Hazardous radioactive waste with a half-life of thousands of years • Breeder Reactors can produce Plutonium or

In a flash, what a nuclear explosion can do to a building. .

Quick Quiz 7. The atomic number of an element is: A. the whole number nearest to its mass B. the number of protons in its nucleus C. the total number of protons and neutrons in its nucleus D. the number of neutrons in its nucleus E. its order of discovery on the Periodic Table 8. Iron has atomic number 26. Naturally mined iron contains isotopes of mass numbers 54, 56, 57, and 58. Which of the following statements is FALSE? A. Every atom of iron has 26 protons B. Some iron atoms have 30 neutrons C. Some iron atoms have 54 neutrons D. Iron is most stable 9. A femtometer is: A. larger than 10− 9 m B. 10− 9 m C. 10− 12 m D. 10− 15 m E. 10− 18 m

Steam generator Meltdown shield A Nuclear Reactor Used to Boil Water for a Steam Turbine (

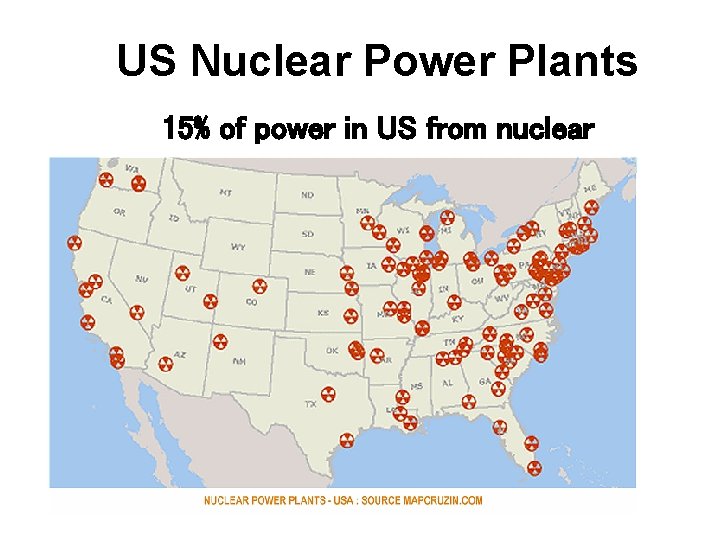
US Nuclear Power Plants 15% of power in US from nuclear

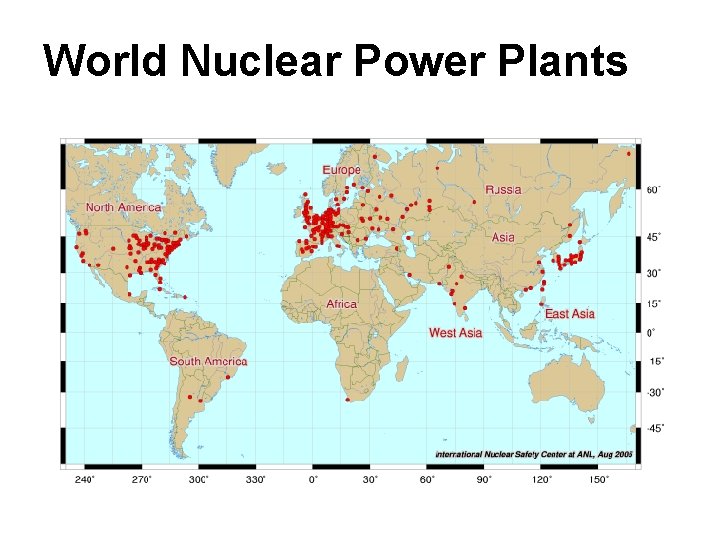
World Nuclear Power Plants

10. The half-life of a radioactive substance is: A. half the time it takes for the entire substance to decay B. usually about 50 years C. the time for radium to change into lead D. calculated from E = mc 2 E. the time for half the substance to decay Quick Quiz 11. The half-life of radium is about 1600 years. If a rock initially contains 1000 g of radium, the amount left after 6400 years will be about: A. 938 g B. 63 g C. 31 g D. 16 g E. less than 16 g 12. Radioactive 90 Sr has a half-life of 30 years. What percent of a sample of 90 Sr will remain after 60 years? A. 0% B. 25% C. 50% D. 75% E. 14%

What Happened at Chernobl. 1. Steam Explosion. 2. Partial Meltdown. 3. Containment held.

• FISSION: a Neutron collides with a 235 U nucleus to form an excited state that decays into two smaller nuclei (plus neutrons) plus ENERGY! • Equation is: 235 U + n 92 Kr + 142 Ba + 3 n + 200 Me. V 235 U will not fission without being “kicked” by neutron. Process also works with 252 Cf.

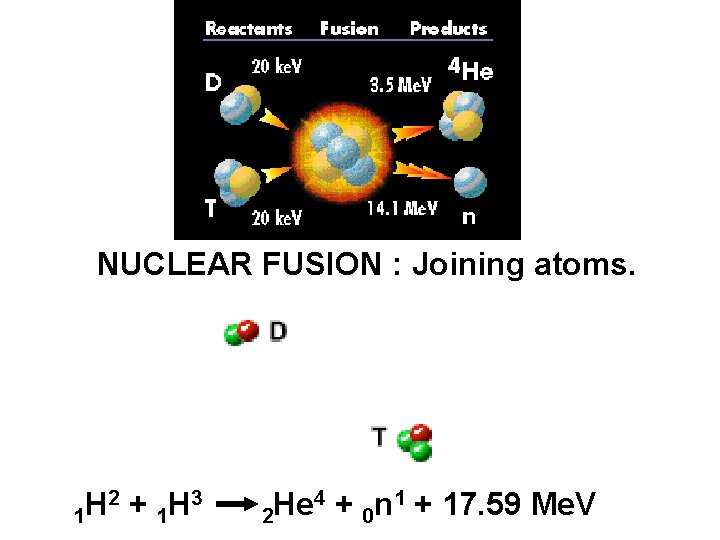
FUSION is the combining of two small atoms into one bigger atom with release of energy. ØMore energy is released than fission. ØOccurs continuously in the sun and stars ØRequires temperature of 100, 000 C ØProblem to reach and maintain this temp ØGood source of future energy – lots of H in ocean ØWaste products decay much faster than fission

NUCLEAR FUSION : Joining atoms. 2 + H 3 H 1 1 4 + n 1 + 17. 59 Me. V He 2 0

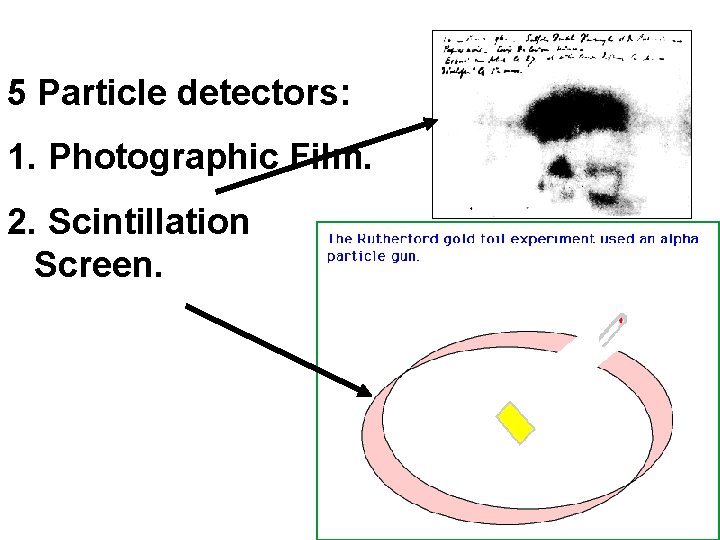
5 Particle detectors: 1. Photographic Film. 2. Scintillation Screen.

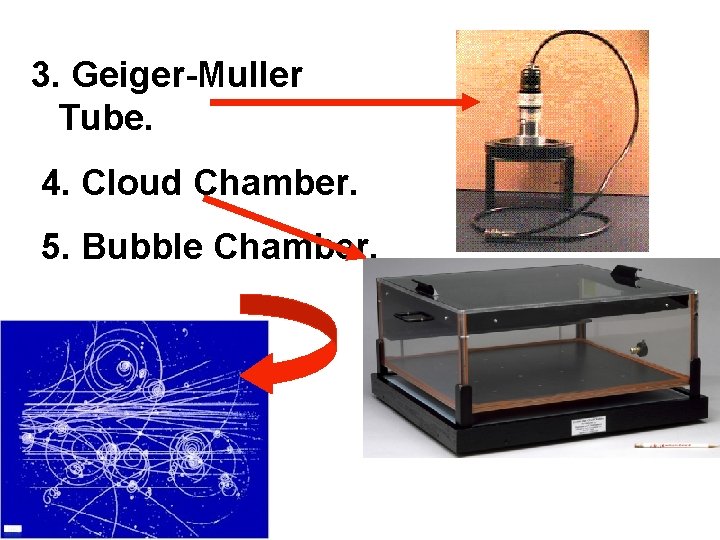
3. Geiger-Muller Tube. 4. Cloud Chamber. 5. Bubble Chamber.

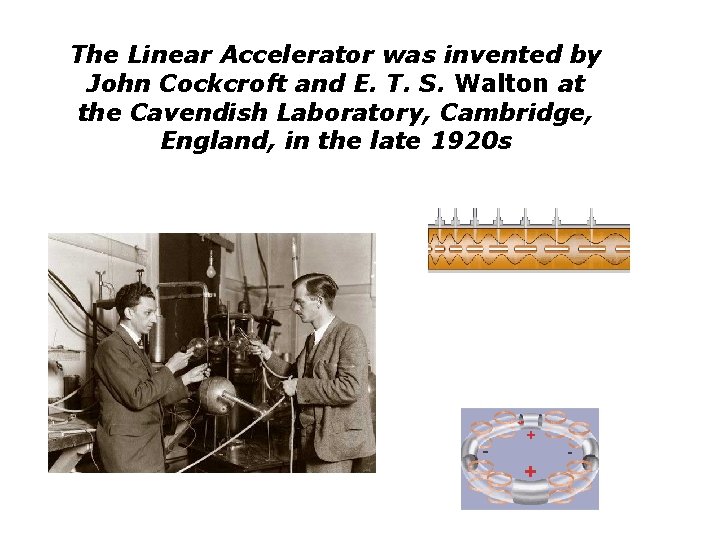
The Linear Accelerator was invented by John Cockcroft and E. T. S. Walton at the Cavendish Laboratory, Cambridge, England, in the late 1920 s

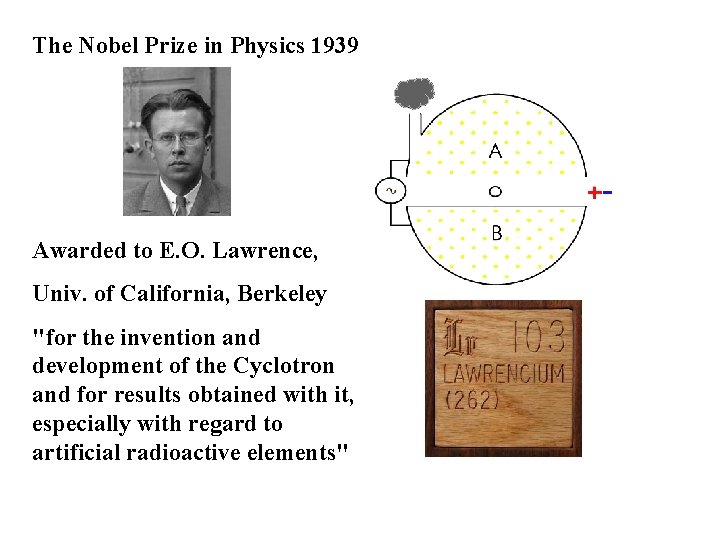
The Nobel Prize in Physics 1939 Awarded to E. O. Lawrence, Univ. of California, Berkeley "for the invention and development of the Cyclotron and for results obtained with it, especially with regard to artificial radioactive elements"

The “Marie Curie’s” of Today Women In Physics • Her work involved slowing down light to 17 m/s in an ultra-cold gas. • Did 7 months at CERN, the European Laboratory for Particle Physics near Geneva. • From Netherlands, now teaches at Harvard. Lene Vestergaard • “After I discovered quantum physics, I've been hooked ever since. I would rather do physics than go to the movies. ”

Women In Physics Sau Lan Wu • Born in Hong Kong • “Reading the biography of Marie Curie inspired me so much that I decided to devote my life to physics. ” • Graduated from Vassar summa cum laude and Phi Beta Kappa, and then earned her Ph. D. from Harvard. • Working at MIT, she was a member of the team that discovered a new particle known as J/psi, or the charm quark. • She played a key role in the discovery of the gluon, the “particle that holds quarks together to form protons and neutrons. ”

Women In Physics Lisa Randall • She was the 1 st tenured woman in physics at Princeton; the 1 st tenured woman theorist in Physics at Harvard & MIT. • She's the most cited theoretical physicist in the world in the last five years. • Research in theoretical high energy physics. • “I really like that my work is getting more people interested in Physics. ”

Finding the Age of Rocks • You need a radioactive decay that has a longer half-life than 14 C (5700 yrs. ). • Potassium-Argon – 40 K decays to 40 Ar with a 1200 Myr half-life. • Uranium-Lead – 235 U to 207 Pb with 700 Myr half-life. • But, only works with volcanic layers. • So, the ages of fossils are interpolated from ages of volcanic layers above and below them.

Note: Chart display average value of distance and depth.

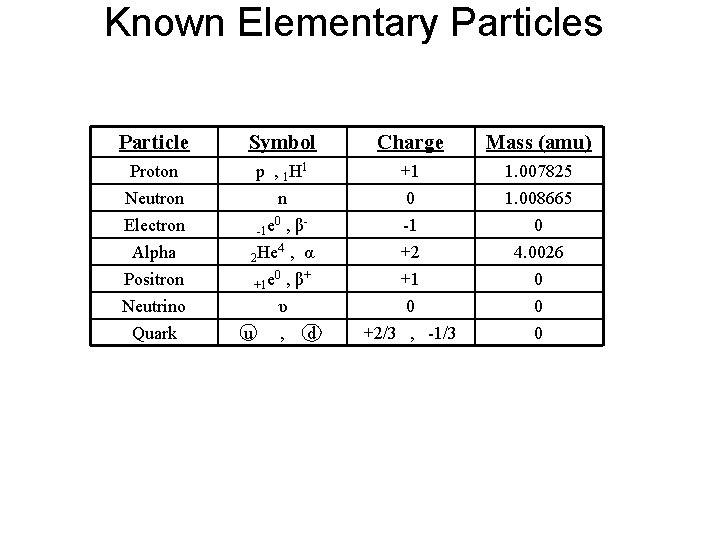
Known Elementary Particles Particle Symbol Charge Mass (amu) Proton Neutron Electron p , 1 H 1 n 0 -1 e , β +1 0 -1 1. 007825 1. 008665 0 Alpha Positron Neutrino Quark 4 2 He , α 0 + +1 e , β +2 +1 0 +2/3 , -1/3 4. 0026 0 0 0 u υ , d

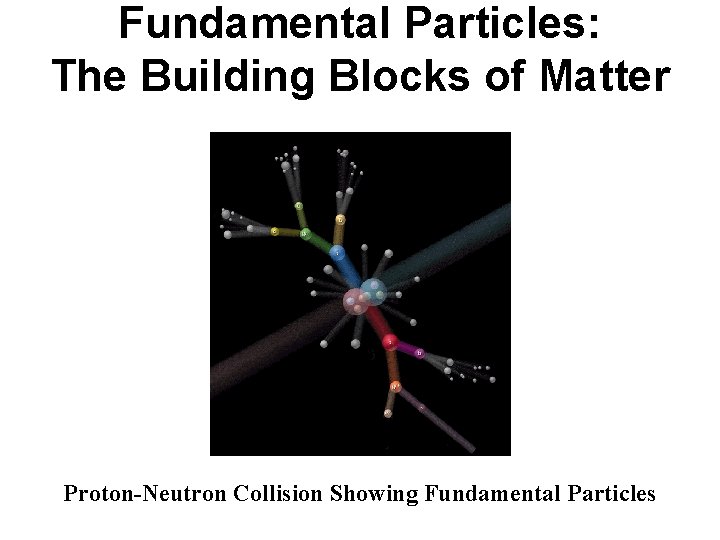
Fundamental Particles: The Building Blocks of Matter Proton-Neutron Collision Showing Fundamental Particles

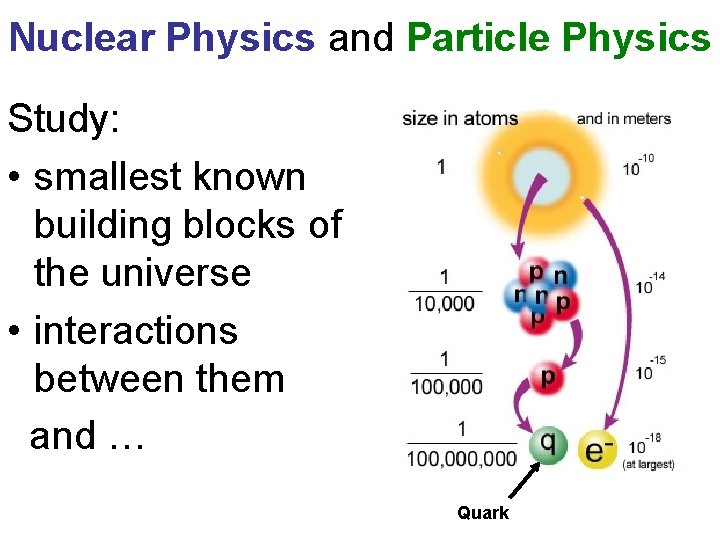
Nuclear Physics and Particle Physics Study: • smallest known building blocks of the universe • interactions between them and … Quark

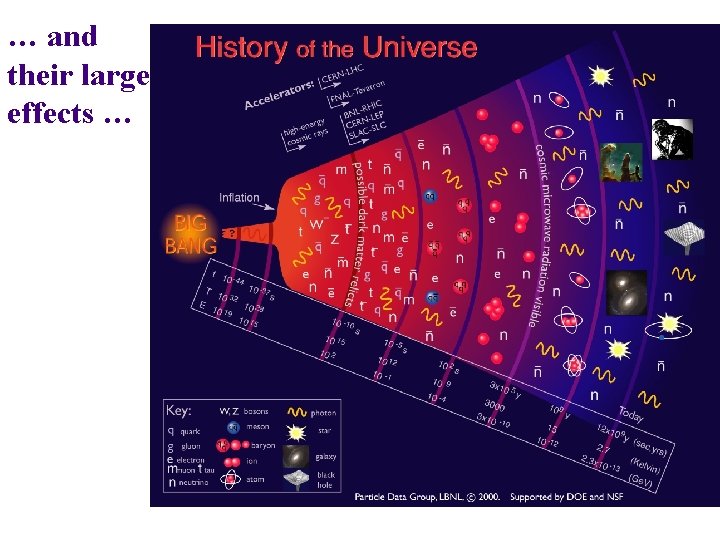
… and their large effects …

and affect us all. – History: alchemy, atomic weapons – Astronomy: sunlight, “metals”, cosmology – Medicine: PET, MRI, chemotherapy – Household: smoke detectors, radon – Nanotechnology: the study of the very small – Archaeology & Earth Sciences: dating

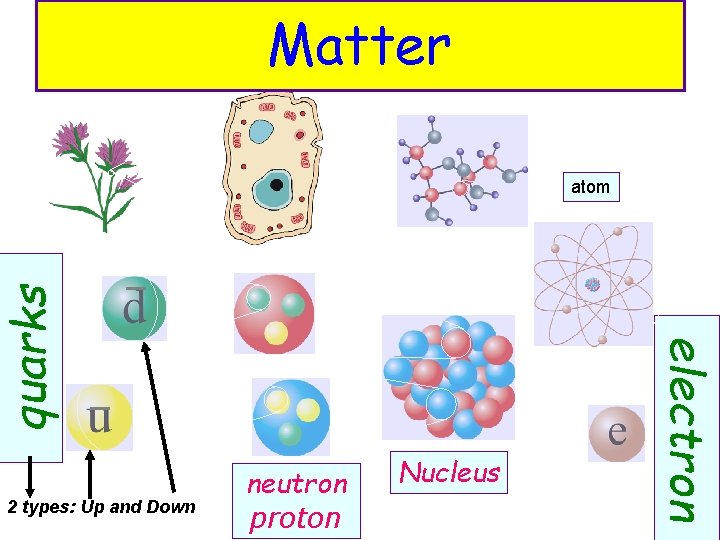
Matter 2 types: Up and Down neutron proton Nucleus electron quarks atom

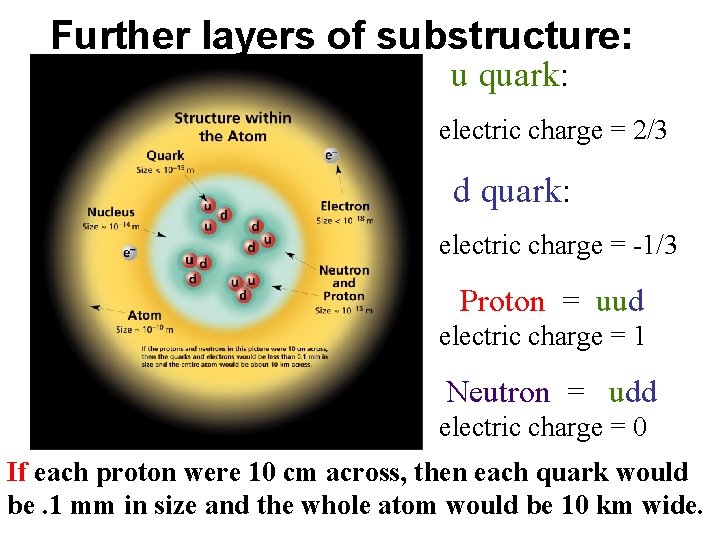
Further layers of substructure: u quark: electric charge = 2/3 d quark: electric charge = -1/3 Proton = uud electric charge = 1 Neutron = udd electric charge = 0 If each proton were 10 cm across, then each quark would be. 1 mm in size and the whole atom would be 10 km wide.

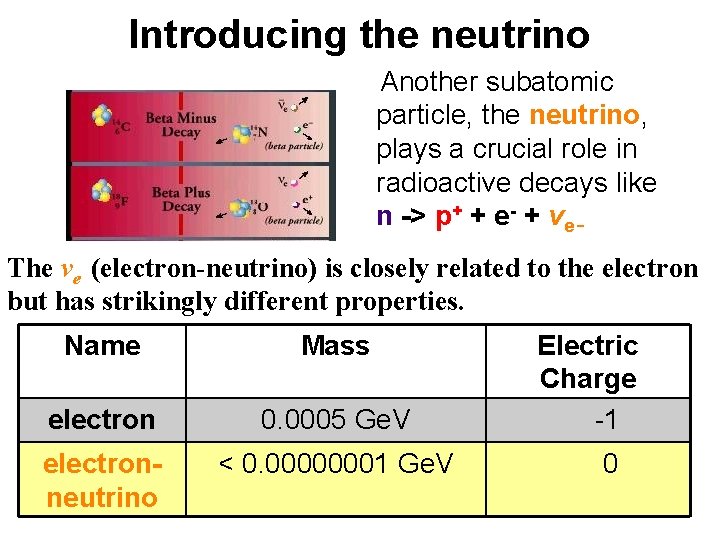
Introducing the neutrino Another subatomic particle, the neutrino, plays a crucial role in radioactive decays like n -> p+ + e- + ve The ve (electron-neutrino) is closely related to the electron but has strikingly different properties. Name Mass electron 0. 0005 Ge. V Electric Charge -1 electronneutrino < 0. 00000001 Ge. V 0

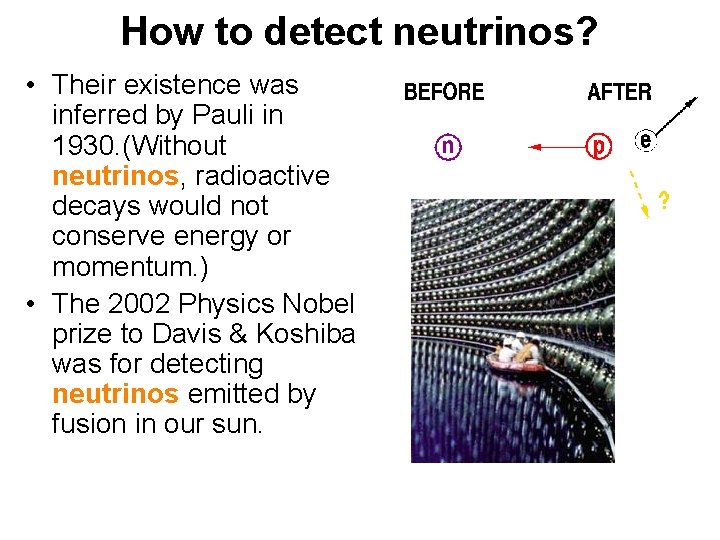
How to detect neutrinos? • Their existence was inferred by Pauli in 1930. (Without neutrinos, radioactive decays would not conserve energy or momentum. ) • The 2002 Physics Nobel prize to Davis & Koshiba was for detecting neutrinos emitted by fusion in our sun.

Producing Elementary Particles Causing particle collisions powerful enough to produce smaller ones requires an enormous and powerful particle accelerator: the Large Hadron Collider (LHC).

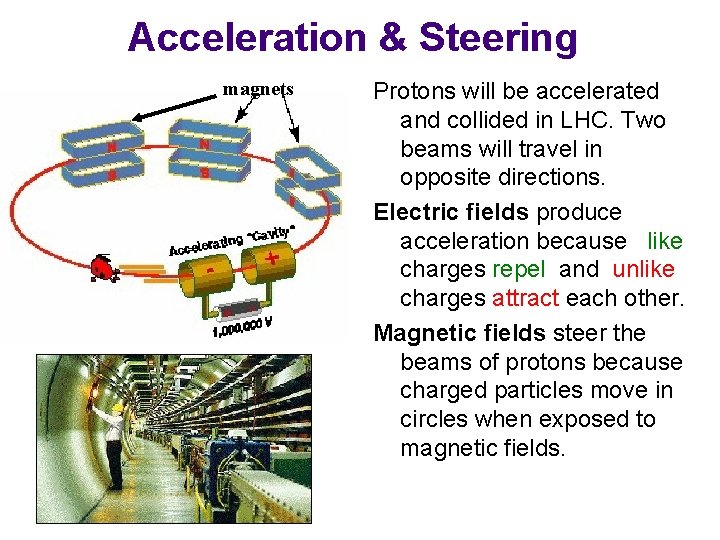
Acceleration & Steering magnets Protons will be accelerated and collided in LHC. Two beams will travel in opposite directions. Electric fields produce acceleration because like charges repel and unlike charges attract each other. Magnetic fields steer the beams of protons because charged particles move in circles when exposed to magnetic fields.

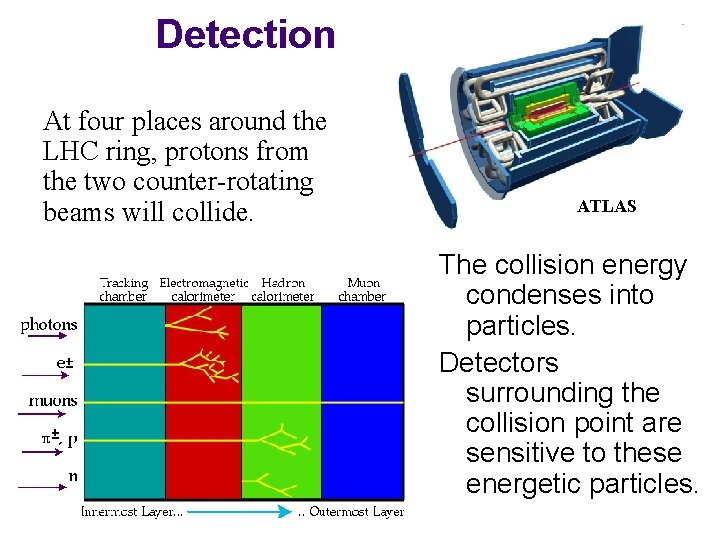
Detection At four places around the LHC ring, protons from the two counter-rotating beams will collide. ATLAS The collision energy condenses into particles. Detectors surrounding the collision point are sensitive to these energetic particles.

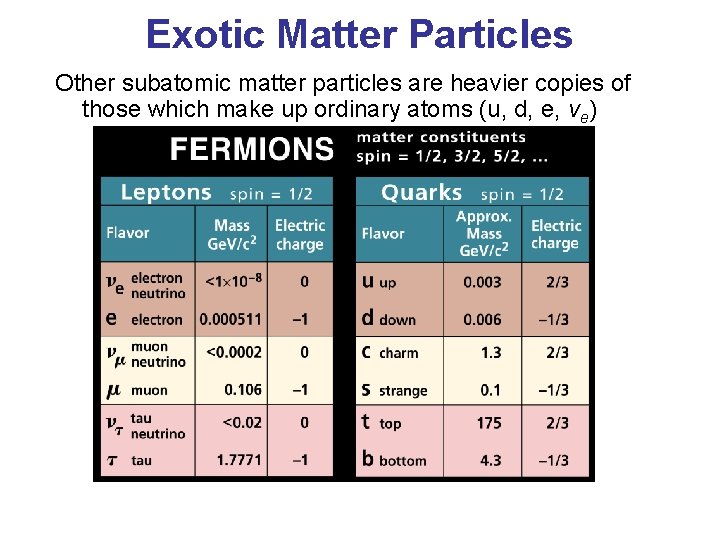
Exotic Matter Particles Other subatomic matter particles are heavier copies of those which make up ordinary atoms (u, d, e, ve)

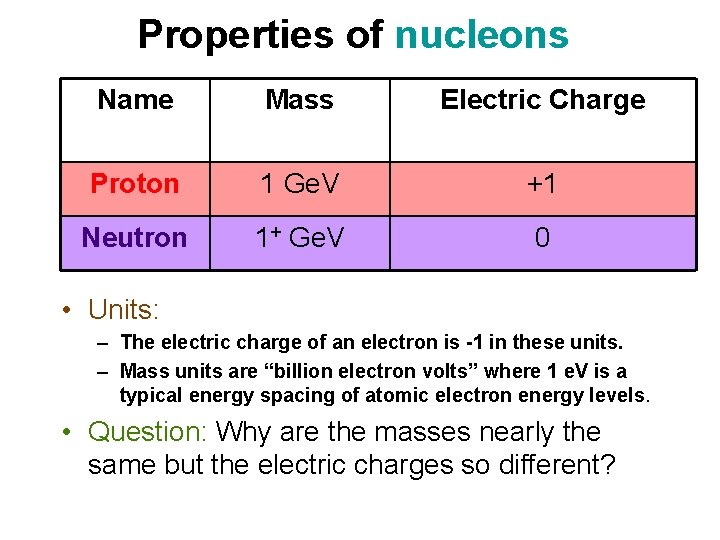
Properties of nucleons Name Mass Electric Charge Proton 1 Ge. V +1 Neutron 1+ Ge. V 0 • Units: – The electric charge of an electron is -1 in these units. – Mass units are “billion electron volts” where 1 e. V is a typical energy spacing of atomic electron energy levels. • Question: Why are the masses nearly the same but the electric charges so different?

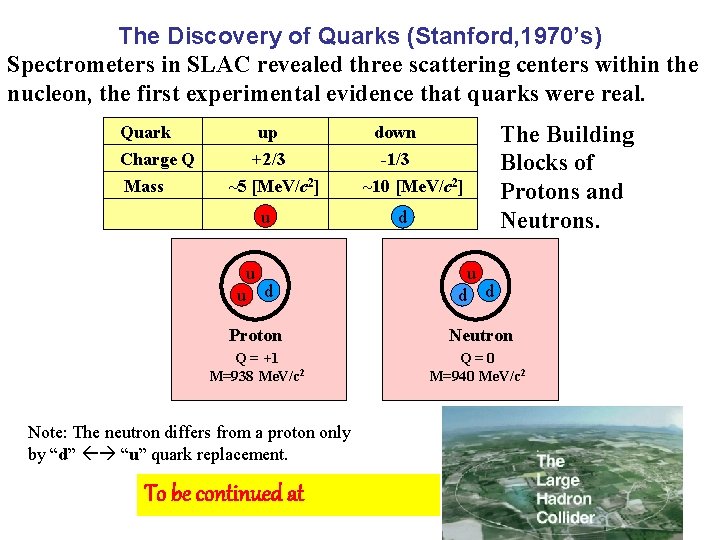
The Discovery of Quarks (Stanford, 1970’s) Spectrometers in SLAC revealed three scattering centers within the nucleon, the first experimental evidence that quarks were real. Quark Charge Q Mass up +2/3 ~5 [Me. V/c 2] u u u d The Building Blocks of Protons and Neutrons. down -1/3 ~10 [Me. V/c 2] d u d d Proton Neutron Q = +1 M=938 Me. V/c 2 Q = 0 M=940 Me. V/c 2 Note: The neutron differs from a proton only by “d” “u” quark replacement. To be continued at

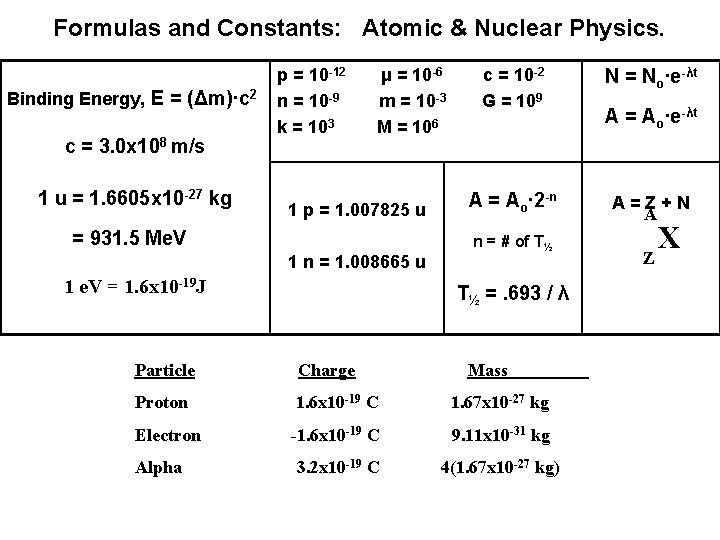
Formulas and Constants: Atomic & Nuclear Physics. Binding Energy, E = (Δm)·c 2 c = 3. 0 x 108 m/s 1 u = 1. 6605 x 10 -27 kg p = 10 -12 n = 10 -9 k = 103 μ = 10 -6 m = 10 -3 M = 106 1 p = 1. 007825 u = 931. 5 Me. V 1 n = 1. 008665 u 1 e. V = 1. 6 x 10 -19 J c = 10 -2 G = 109 N = No∙e-λt A = Ao∙ 2 -n A=Z+N A n = # of T½ T½ =. 693 / λ Particle Charge Mass Proton 1. 6 x 10 -19 C 1. 67 x 10 -27 kg Electron -1. 6 x 10 -19 C 9. 11 x 10 -31 kg Alpha 3. 2 x 10 -19 C 4(1. 67 x 10 -27 kg) A = Ao∙e-λt Z X