Radioactivity 1 Radioactivity Atomic Physics the study of































- Slides: 67

Radioactivity 1

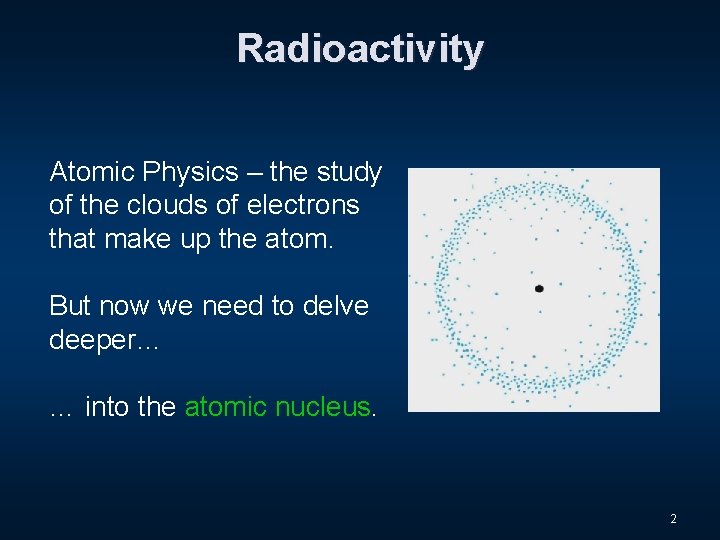
Radioactivity Atomic Physics – the study of the clouds of electrons that make up the atom. But now we need to delve deeper… … into the atomic nucleus. 2

Radioactivity Wilhelm Roentgen 1895 3

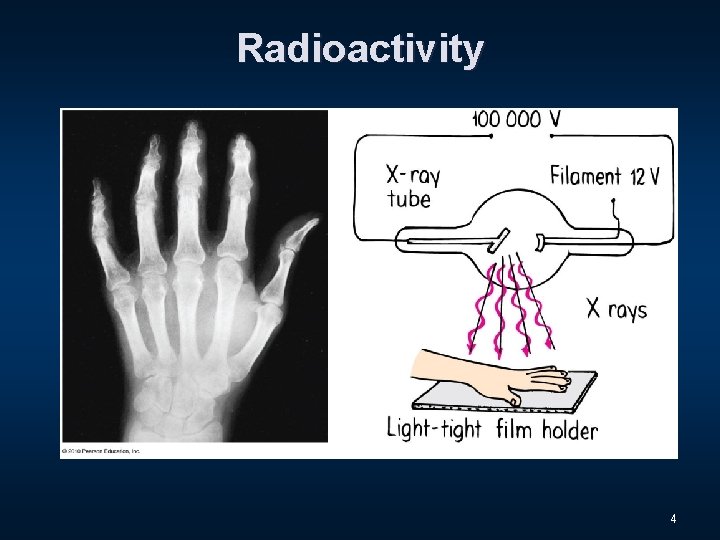
Radioactivity 4

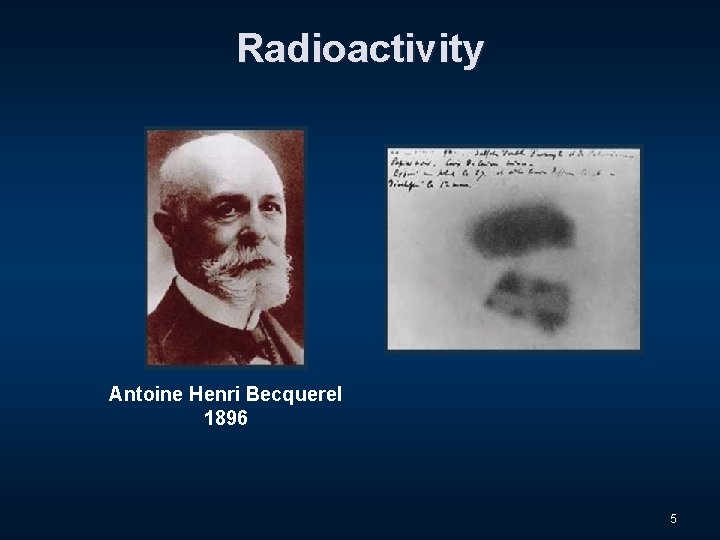
Radioactivity Antoine Henri Becquerel 1896 5

Radioactivity Antoine Henri Becquerel 6

Radioactivity n Radioactivity is the process of the atomic nucleus emitting energetic subatomic particles. n This process is nothing new – it has been around far longer than the human race! 7

Radioactivity 8

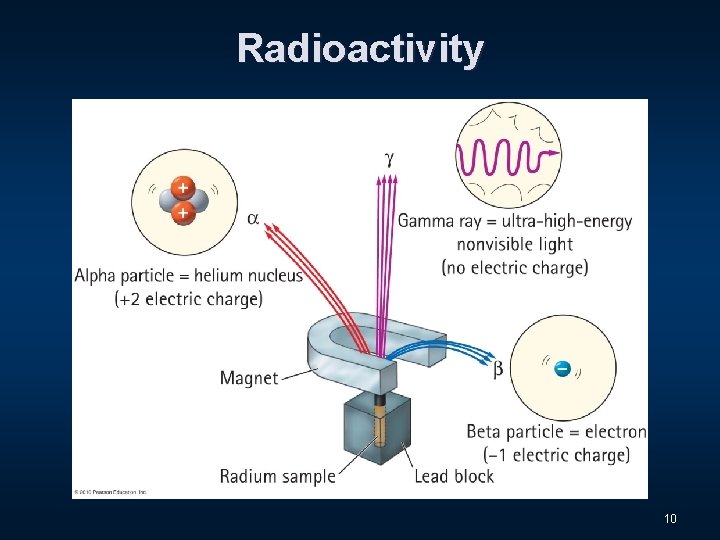
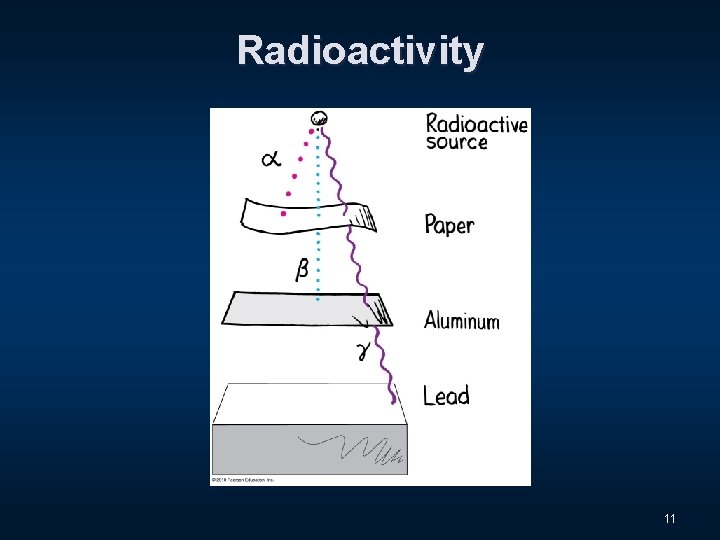
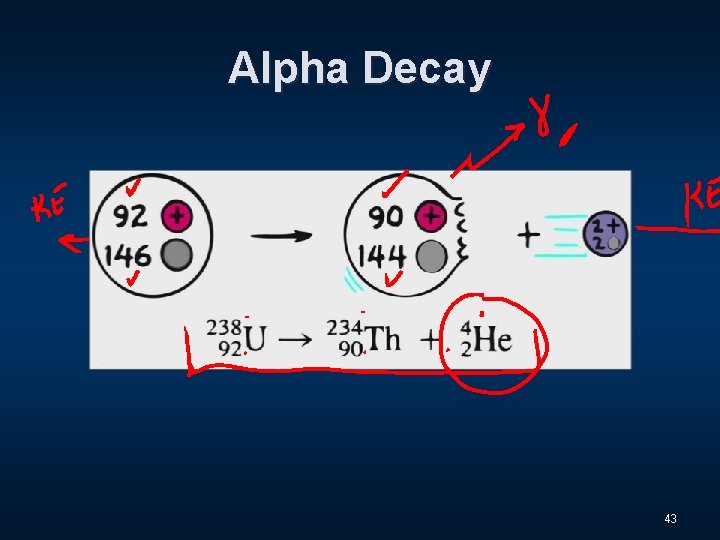
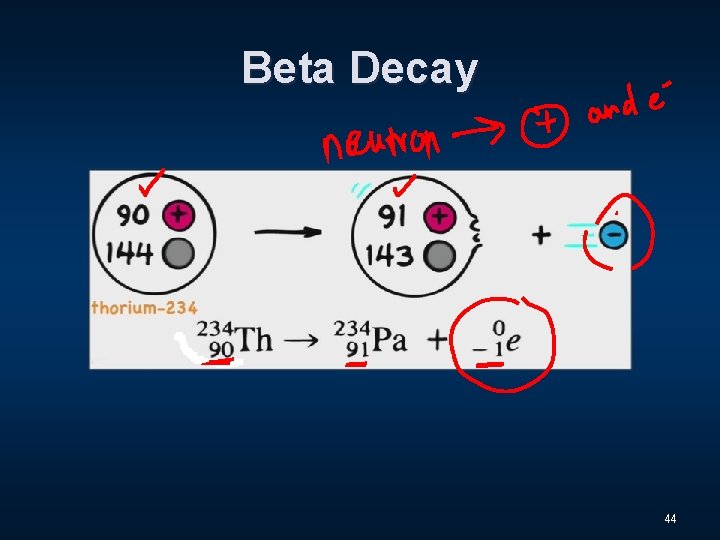
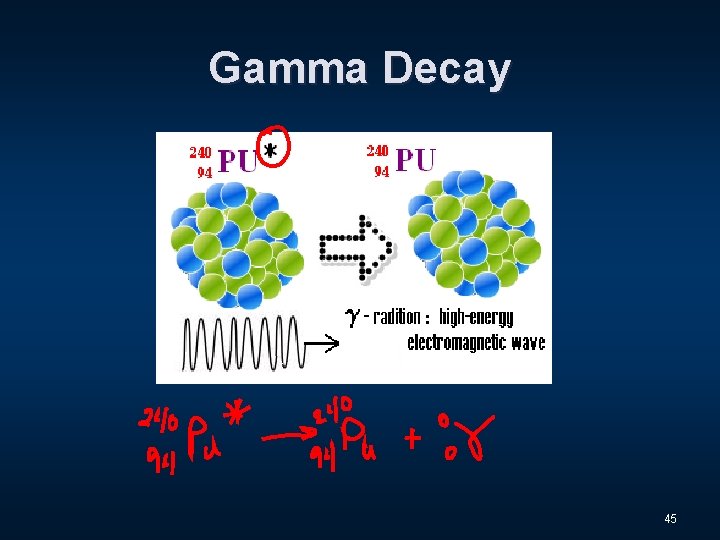
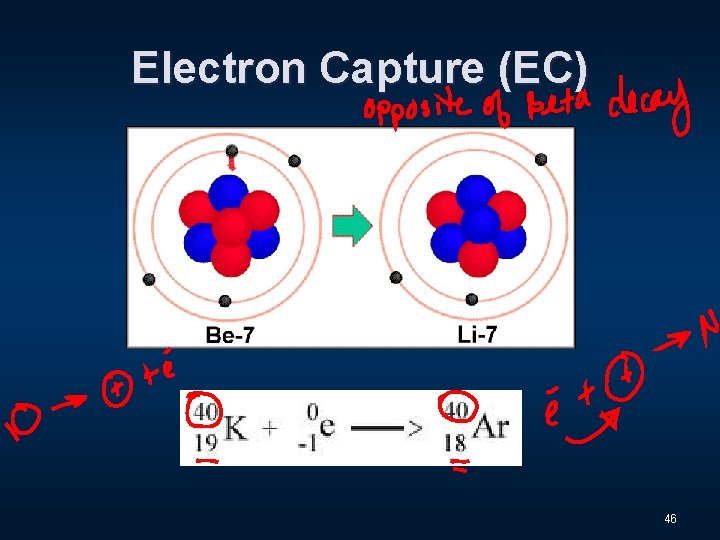
Three Distinct Types of Radiation n Alpha - α n Beta - β n Gamma - Ɣ 9

Radioactivity 10

Radioactivity 11

Radioactivity 12

Radioactivity 13

Radioactivity 14

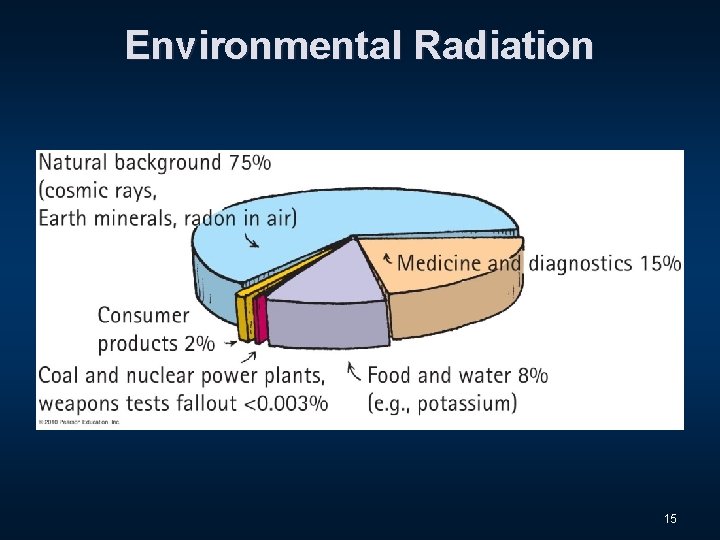
Environmental Radiation 15

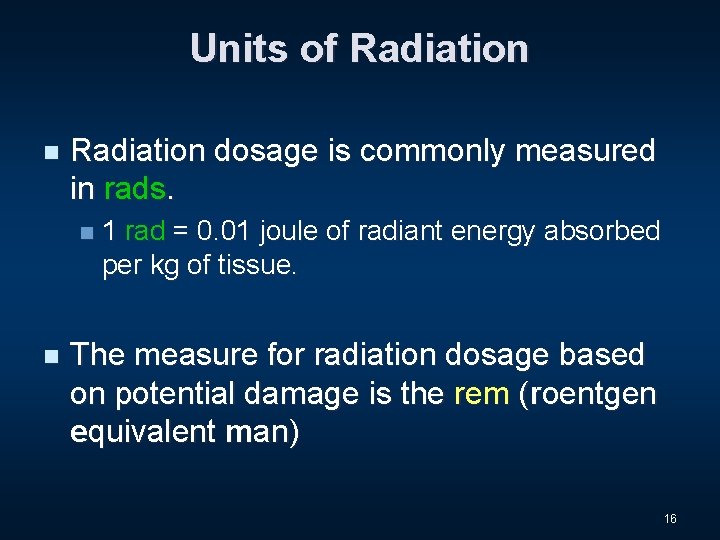
Units of Radiation n Radiation dosage is commonly measured in rads. n n 1 rad = 0. 01 joule of radiant energy absorbed per kg of tissue. The measure for radiation dosage based on potential damage is the rem (roentgen equivalent man) 16

Units of Radiation n A lethal dose of radiation for a human is about 500 rems. You have about a 50% chance of surviving a dose of this magnitude delivered over a short time. n Average person in U. S. is exposed to 360 millirem each year (a millirem is 1/1000 th of a rem) 17

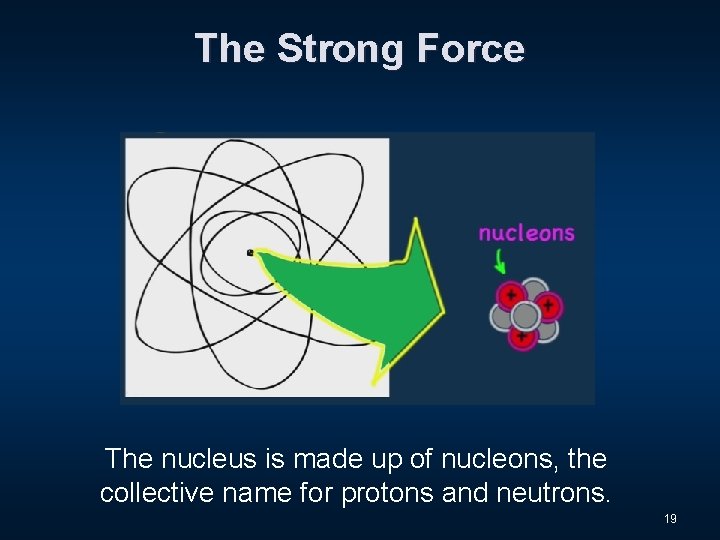
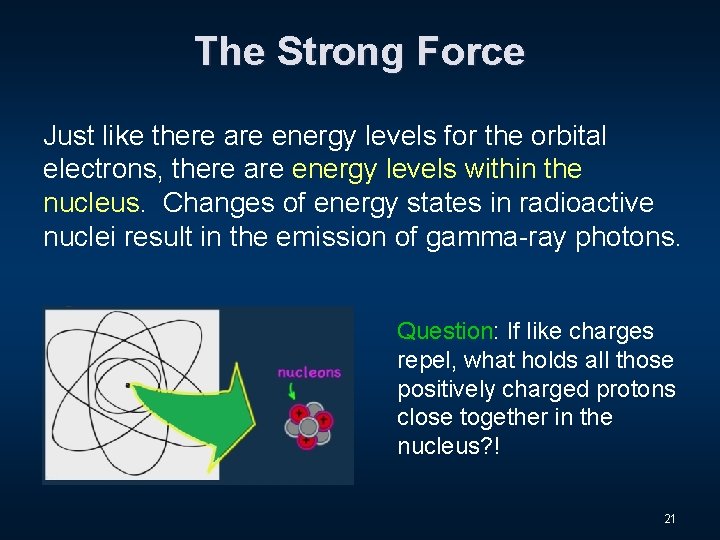
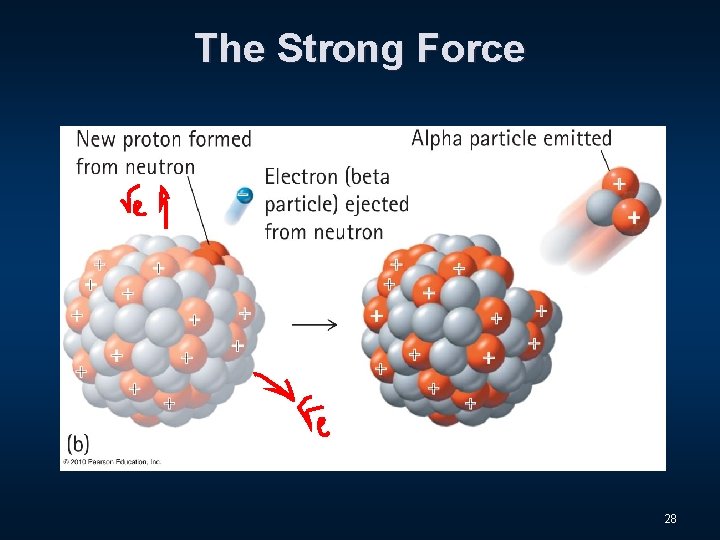
The Strong Force 18

The Strong Force The nucleus is made up of nucleons, the collective name for protons and neutrons. 19

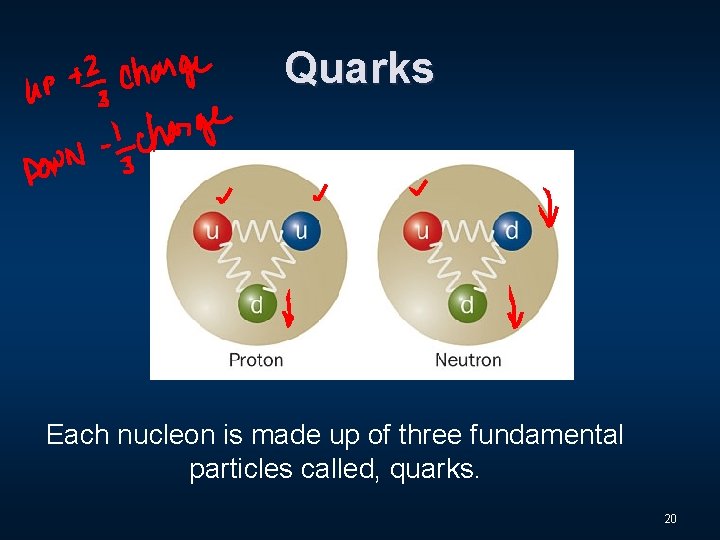
Quarks Each nucleon is made up of three fundamental particles called, quarks. 20

The Strong Force Just like there are energy levels for the orbital electrons, there are energy levels within the nucleus. Changes of energy states in radioactive nuclei result in the emission of gamma-ray photons. Question: If like charges repel, what holds all those positively charged protons close together in the nucleus? ! 21

The Strong Force Comparison of the strengths of the STRONG force and the ELECTRIC force over distance. 22

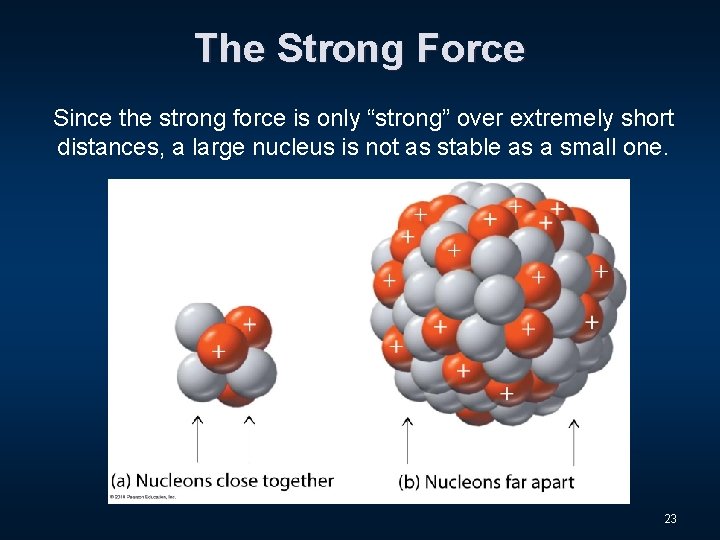
The Strong Force Since the strong force is only “strong” over extremely short distances, a large nucleus is not as stable as a small one. 23

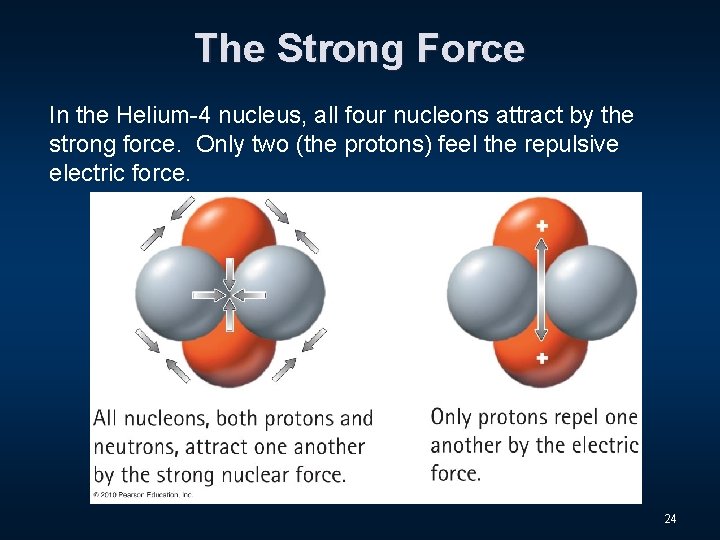
The Strong Force In the Helium-4 nucleus, all four nucleons attract by the strong force. Only two (the protons) feel the repulsive electric force. 24

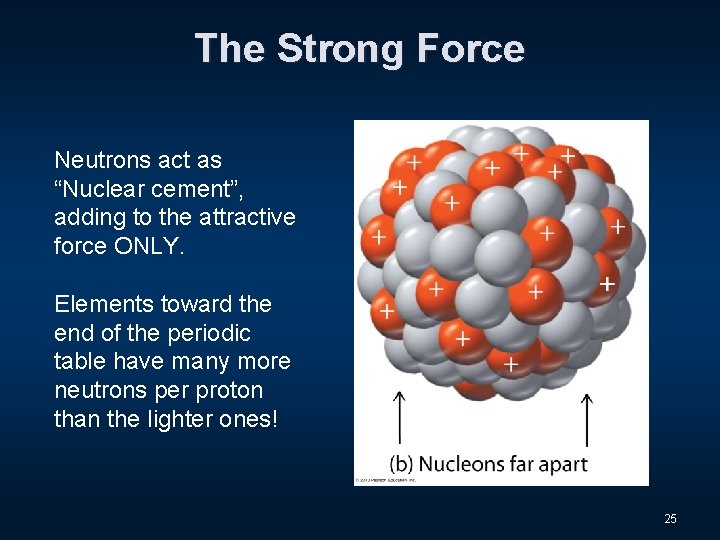
The Strong Force Neutrons act as “Nuclear cement”, adding to the attractive force ONLY. Elements toward the end of the periodic table have many more neutrons per proton than the lighter ones! 25

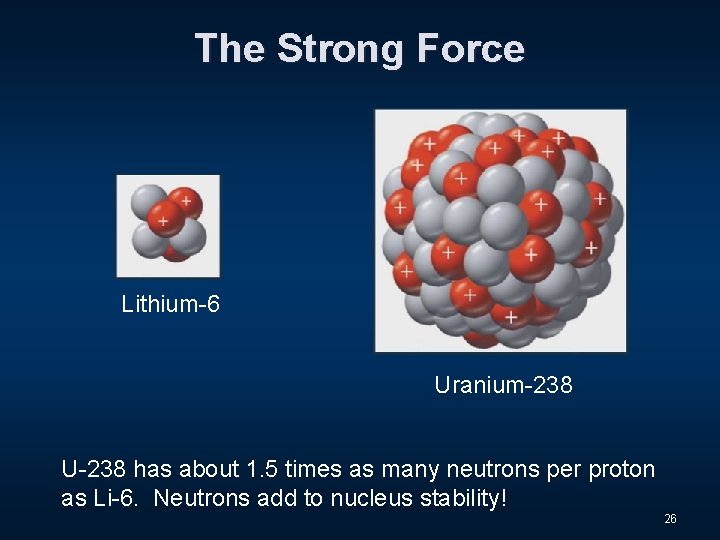
The Strong Force Lithium-6 Uranium-238 U-238 has about 1. 5 times as many neutrons per proton as Li-6. Neutrons add to nucleus stability! 26

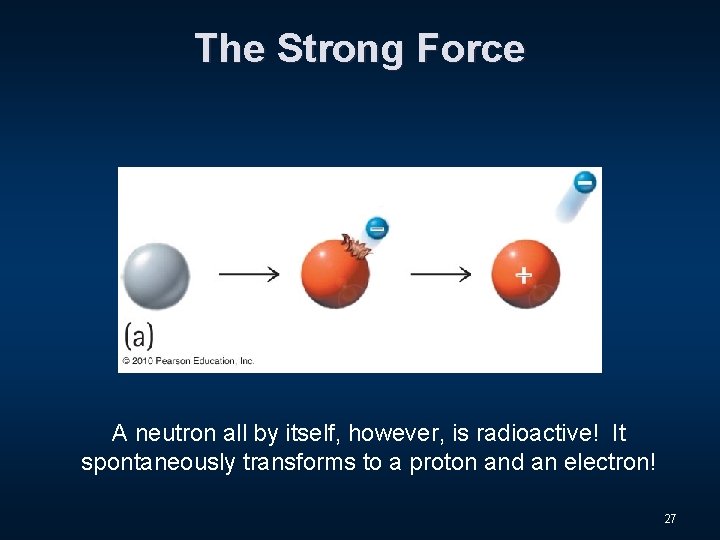
The Strong Force A neutron all by itself, however, is radioactive! It spontaneously transforms to a proton and an electron! 27

The Strong Force 28

Summing Up So Far 1. Which two fundamental forces have visible effects within the nucleus of an atom? 2. Which one acts over greater distance? 3. Which force acts on ALL nucleons? 4. Which force acts only on protons? 29

Summing Up So Far 1. In the nucleus of ordinary hydrogen, does the strong force play a role? 2. How about the electric force? 30

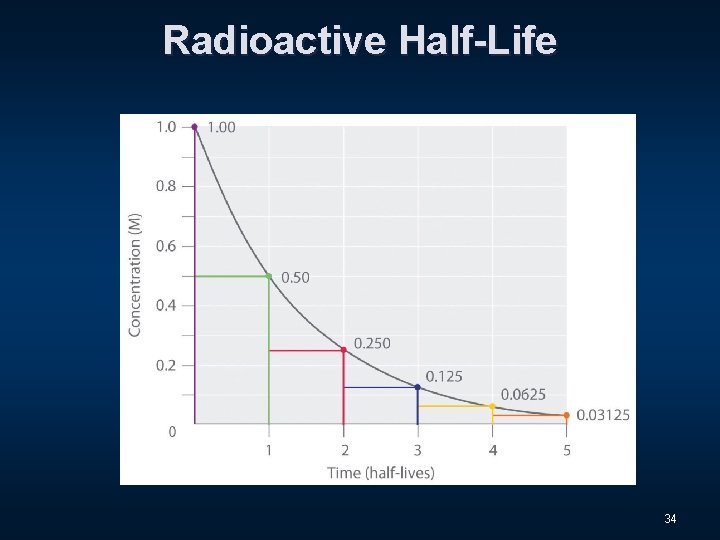
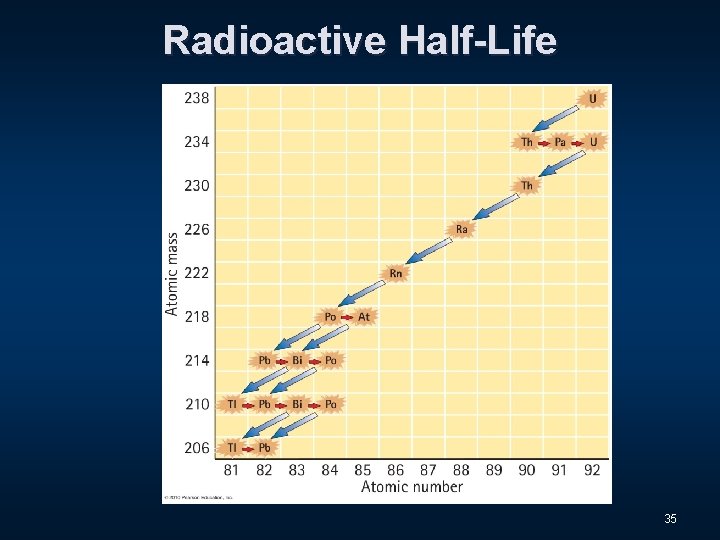
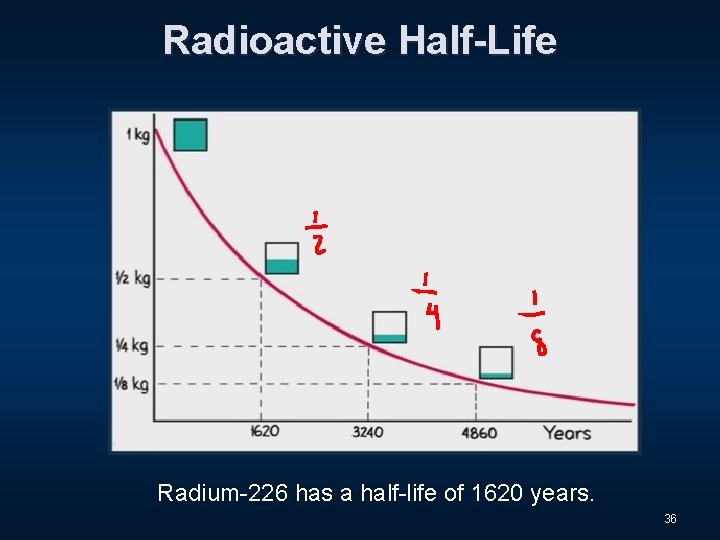
Radioactive Half-Life n The radioactive decay rate of an element is measured in terms of a characteristic time called the half-life. 31

Radioactive Half-Life n The radioactive decay rate of an element is measured in terms of a characteristic time called the half-life. n This is the time it takes for half of an original quantity of a radioactive isotope to decay. 32

Radioactive Half-Life n The radioactive decay rate of an element is measured in terms of a characteristic time called the half-life. n This is the time it takes for half of an original quantity of a radioactive isotope to decay. The half-life of Uranium-238 is 4. 5 billion years! 33

Radioactive Half-Life 34

Radioactive Half-Life 35

Radioactive Half-Life Radium-226 has a half-life of 1620 years. 36

Radioactive Half-Life Check Question: If a sample of a radioactive isotope has a halflife of 1 day, how much of the original sample will be left at the end of the second day? 37

Radiation Detectors A scintillation counter detects incoming radiation by flashes of light produced by particles or gamma rays passing through the counter. A Geiger counter detects incoming radiation by a short pulse of current triggered when radiation ionizes a gas in the tube. 38

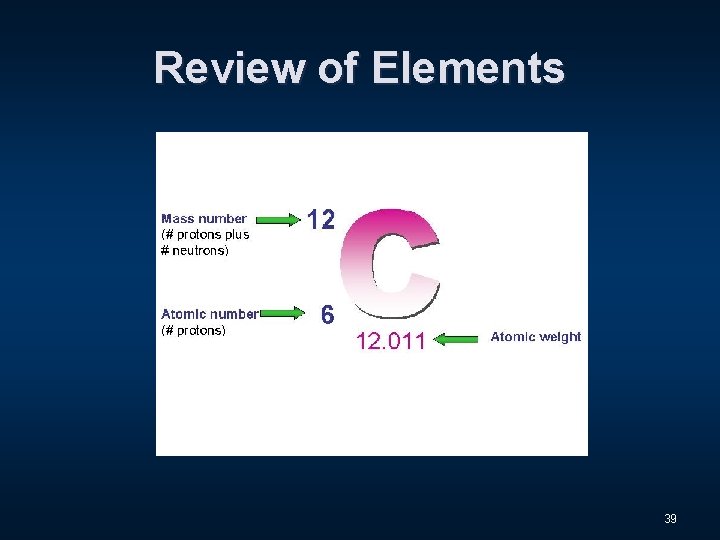
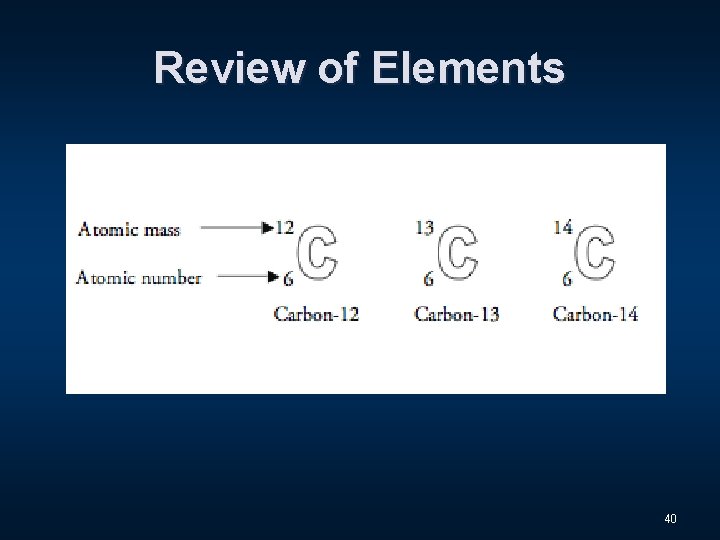
Review of Elements 39

Review of Elements 40

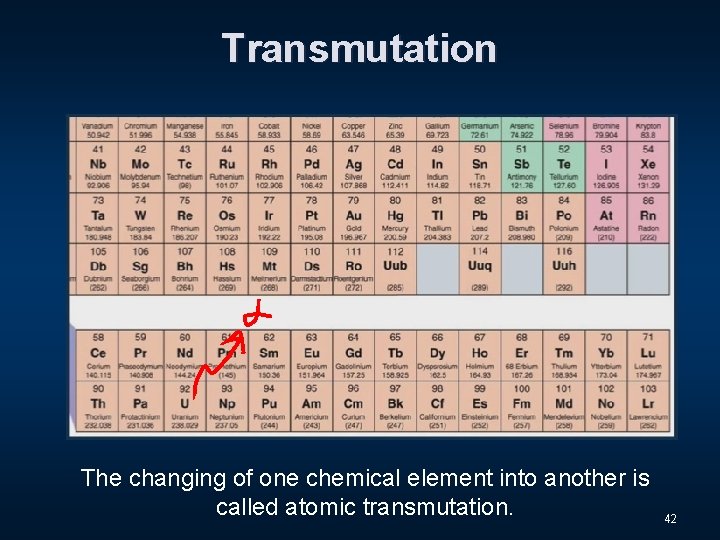
Transmutation 41

Transmutation The changing of one chemical element into another is called atomic transmutation. 42

Alpha Decay 43

Beta Decay 44

Gamma Decay 45

Electron Capture (EC) 46

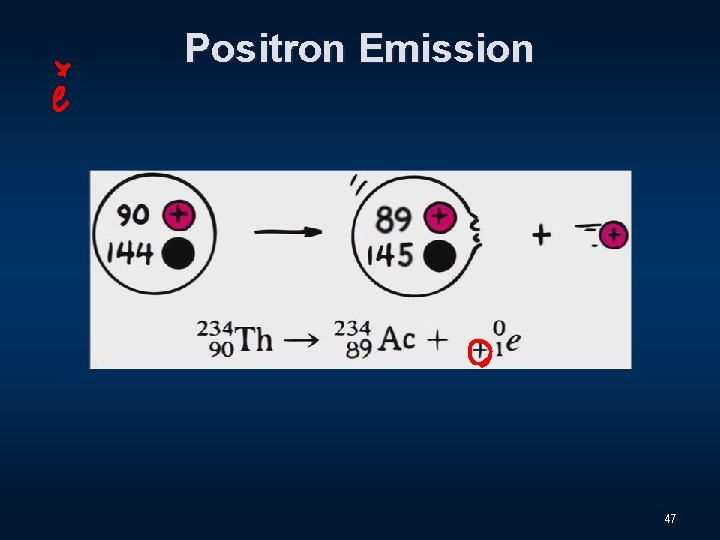
Positron Emission 47

Five types of Decay n Alpha Decay n Beta Decay n Gamma Decay n Electron Capture n Positron Emission 48

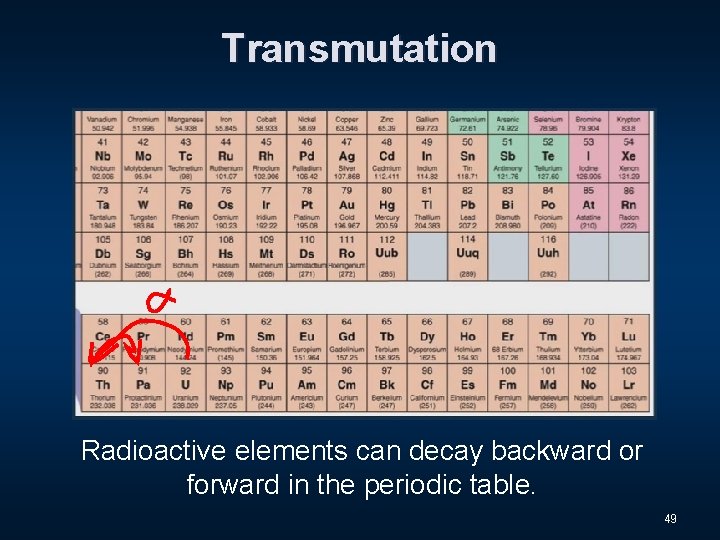
Transmutation Radioactive elements can decay backward or forward in the periodic table. 49

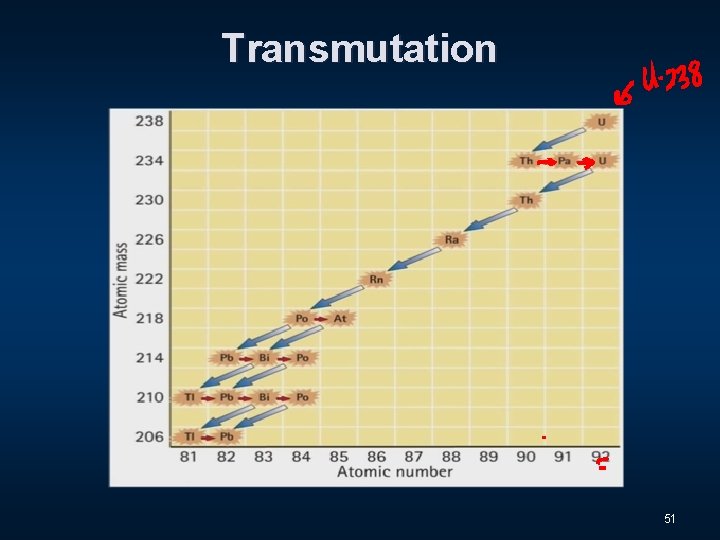
Transmutation 50

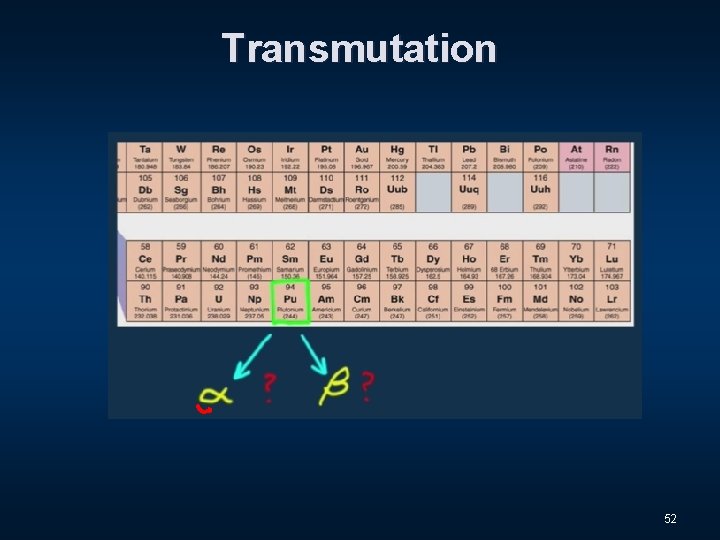
Transmutation 51

Transmutation 52

What nucleus results when Krypton-81 undergoes electron capture? 53

What nucleus results when Silicon-27 undergoes positron emission? 54

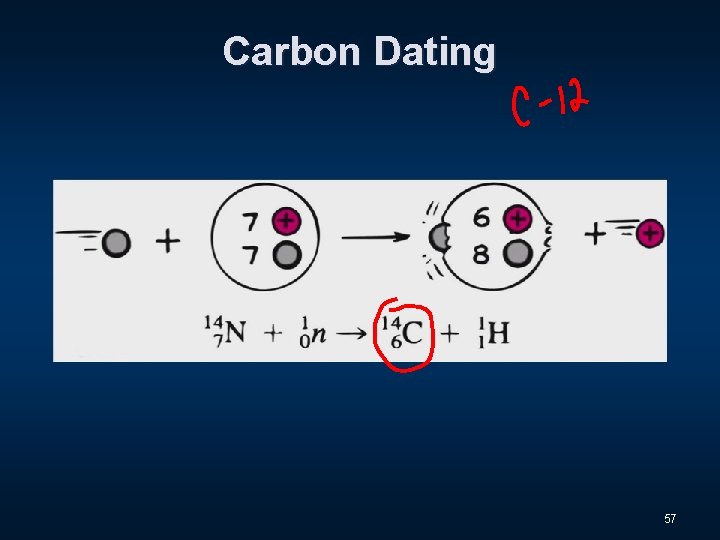
Carbon Dating 55

Carbon Dating 56

Carbon Dating 57

Carbon Dating 58

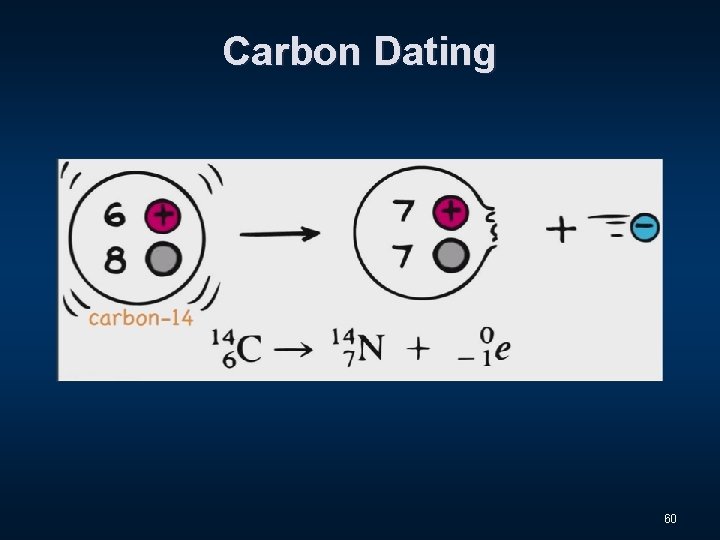
Carbon Dating 59

Carbon Dating 60

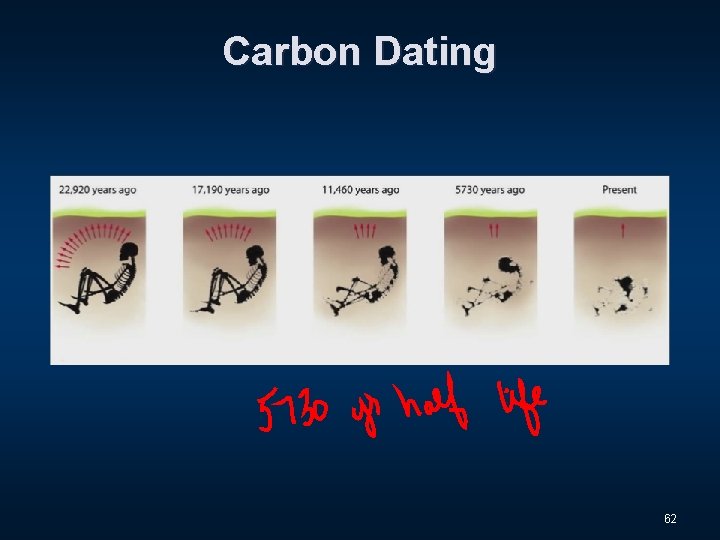
Carbon Dating 61

Carbon Dating 62

Carbon Dating 63

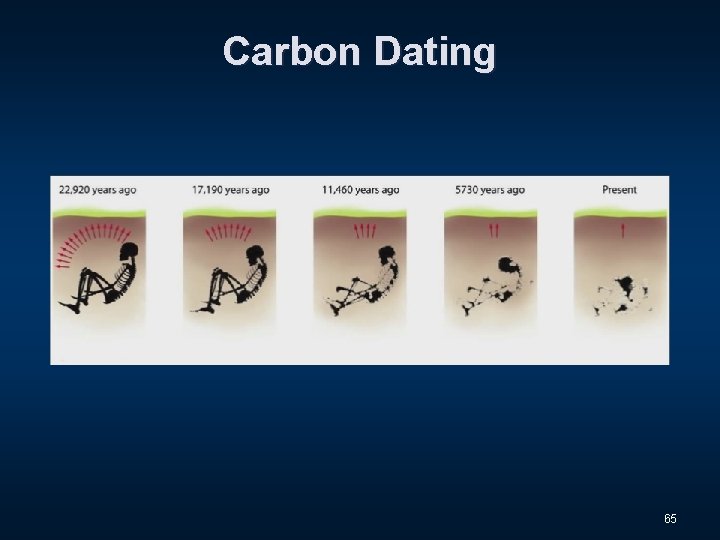
Carbon Dating 64

Carbon Dating 65

Carbon Dating 66

67