Pg 12 Catalyst Answer the Catalyst in this















































- Slides: 73

Pg. 12 Catalyst: Answer the Catalyst in this box! You have 5 minutes! Catalyst: Write 2 observations. Then, turn one of your observations into an inference. Finally, write one testable question about your inference. Topic: EQ: Pg. 13 Copy the Topic and EQ. Topic: Atoms & Matter EQ: What are three most important properties of matter? Summary:

Make sure your Table of Contents looks like this: Date 9/8 Left Hand Topic Left Hand Directions Balloon Reflection 9/9 9/12 The Scientific Method 9/13 Density Lab 9/14 Scientific Method Sort 9/15 Atoms & Matter Page (Even) Right Hand Topic Page (Odd) 2 Right Hand Directions 3 4 Teamwork 5 6 8 10 The Scientific Method 7 9 11 12 Density Lab Observation & Inference Sort Atoms & Matter 13

Consider this picture. What do you see?

What can you observe now? What has changed?

Now have your observations changed?

What do you think this is a picture of?

What do you think it is now?

What is this a picture of?

What has changed about what you see?

All of those pictures were examples of matter! • Matter - is made up of atoms and has a volume (takes up space) 1. What is an example of something that is matter? 2. What is an example of something that is NOT matter?


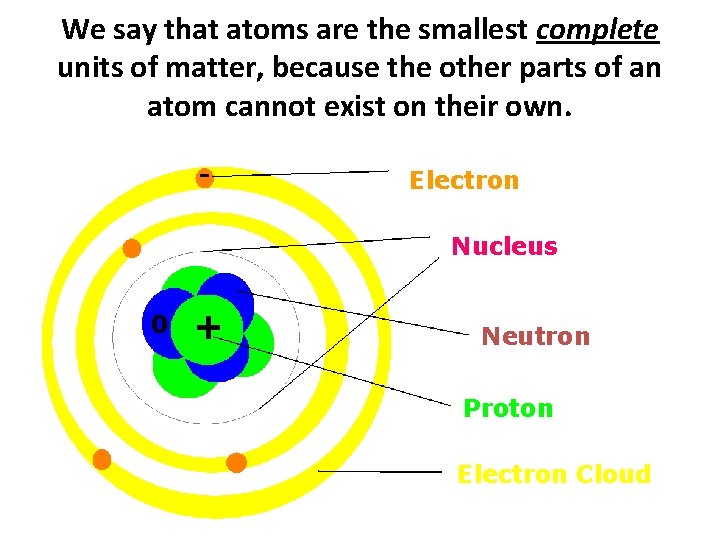
What are Atoms? Atoms - the smallest, complete units of matter Atoms also have a mass and take up space. All matter is made of atoms!


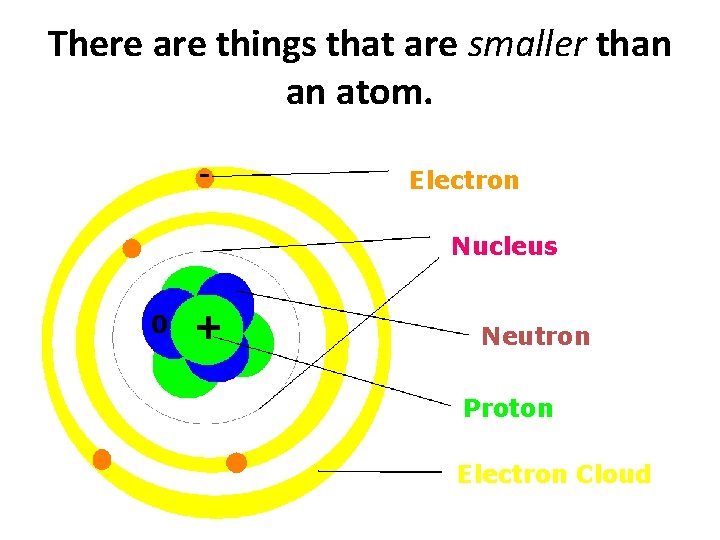
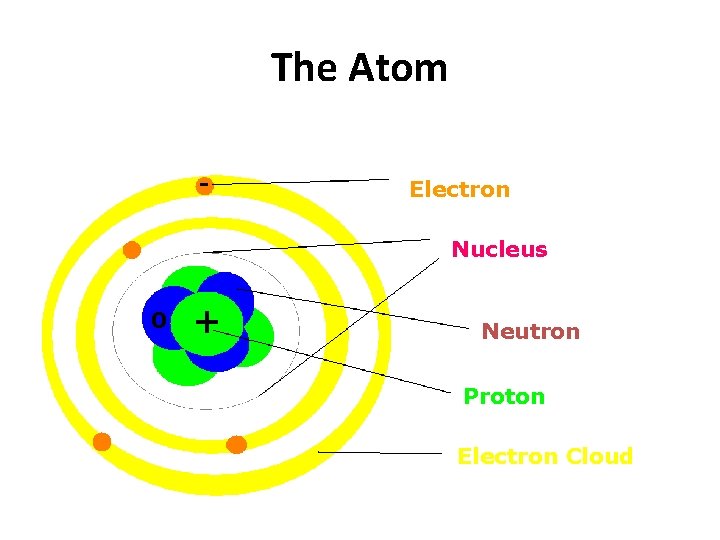
There are things that are smaller than an atom. - Electron Nucleus 0 + Neutron Proton Electron Cloud

We say that atoms are the smallest complete units of matter, because the other parts of an atom cannot exist on their own. - Electron Nucleus 0 + Neutron Proton Electron Cloud

The Atom • “Atom” is from the Greek word “atomos” which means “not able to be divided. ”

The Atom • People can be divided into smaller sections, organs, bones, blood, etc. Those parts can be divided even smaller. For example, organs are made of cells. Cells have cytoplasm, ribosomes and much more. That can be divided even smaller until you get to atoms.

How small is an atom? • A single penny has 2 x 10^22 or – 20, 000, 000, 000 (twenty sextillion) atoms! That’s a lot!

Is air matter? • Discuss the following questions with your table group: – Is air matter? – Does it have a weight? – Does it take up space? – Is it made of atoms? Who can tell me their inference? Phrase it like a hypothesis: I think air is/isn’t matter, because____.

Let’s find out!

Okay, so atoms are small enough that we can’t see them in the air. But just HOW small are they?

Just What Size Is an Atom? Cut 1: This is about the size of a: Hand Pocket 14 cm

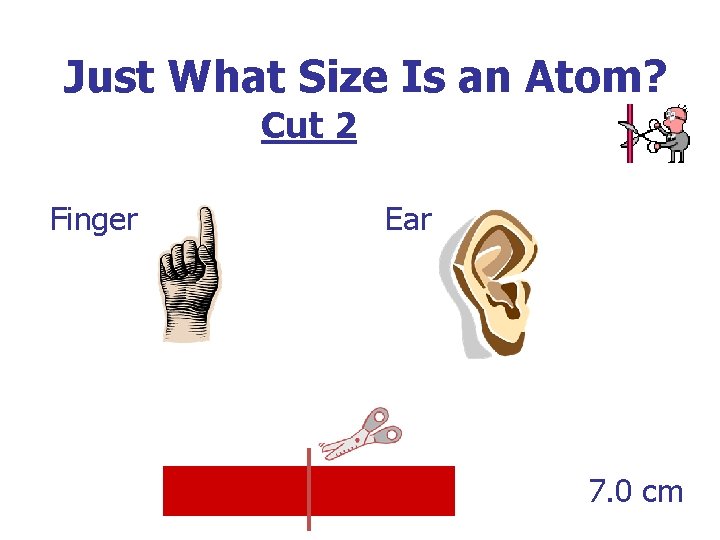
Just What Size Is an Atom? Cut 2 Finger Ear 7. 0 cm

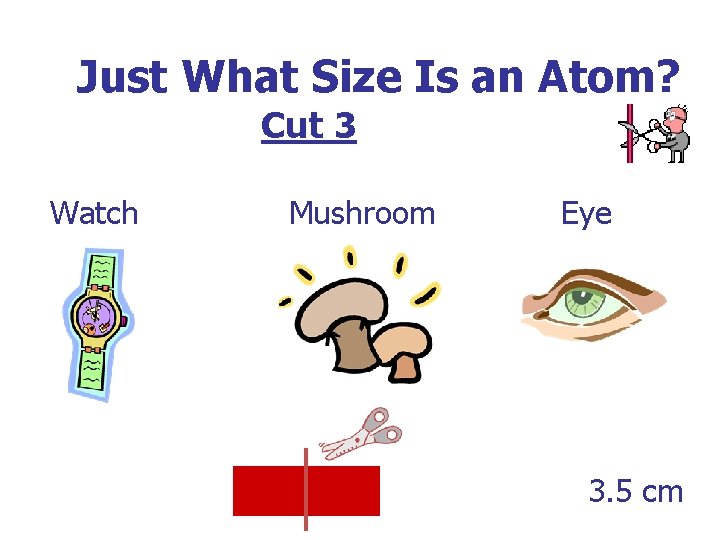
Just What Size Is an Atom? Cut 3 Watch Mushroom Eye 3. 5 cm

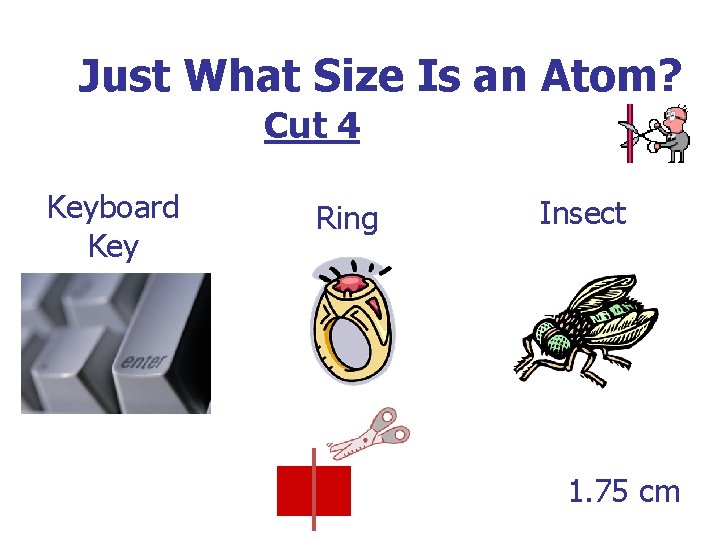
Just What Size Is an Atom? Cut 4 Keyboard Key Ring Insect 1. 75 cm

Just What Size Is an Atom? Cut 5 Keep going … atoms are much smaller! 0. 88 cm

Just What Size Is an Atom? Cut 6 Tiny seeds on top of a Whopper bun 0. 44 cm

Just What Size Is an Atom? Cut 7 Keep going … atoms are still much smaller! 0. 22 cm

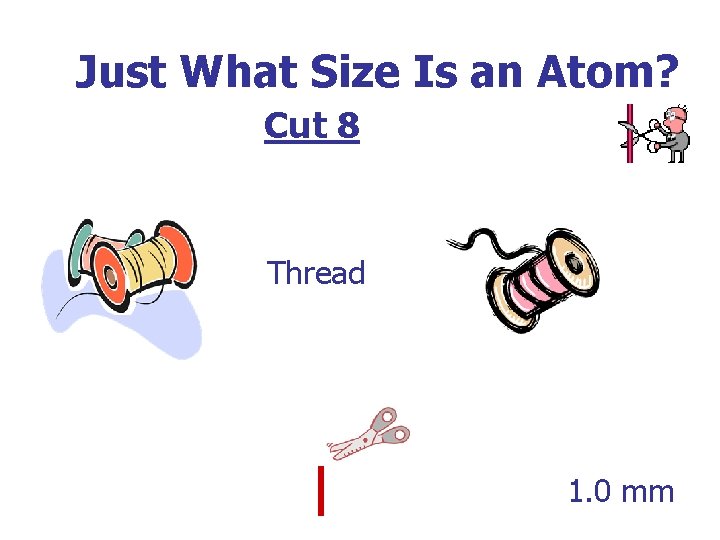
Just What Size Is an Atom? Cut 8 Thread 1. 0 mm

Just What Size Is an Atom? Cut 9 It’s too difficult to keep cutting! Let’s just pretend from now on 0. 5 mm

Just What Size Is an Atom? Cut 10 The size of one tiny light (pixel) on a computer screen 0. 25 mm

Just What Size Is an Atom? Cut 11 Wow, I didn’t think anything was this small! 0. 125 mm

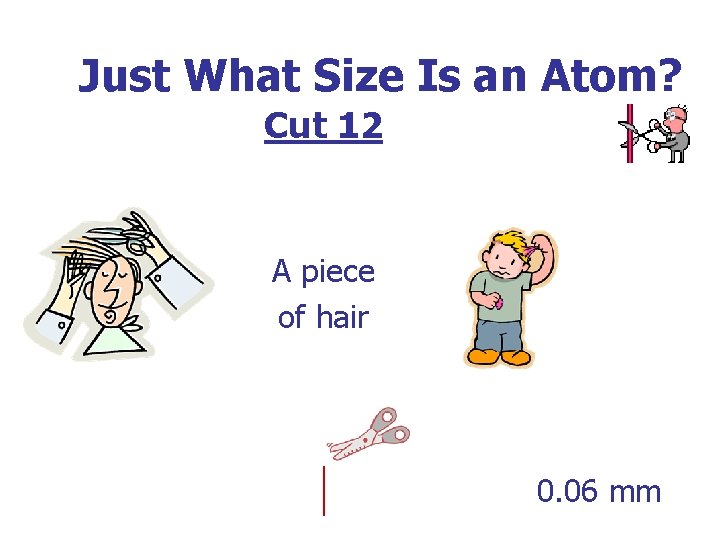
Just What Size Is an Atom? Cut 12 A piece of hair 0. 06 mm

Just What Size Is an Atom? Cut 13 Our meter has disappeared! ? ? ? 0. 03 mm

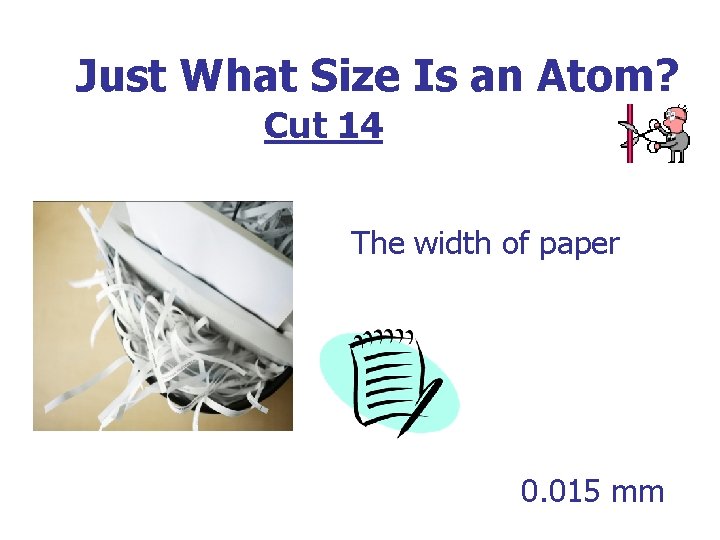
Just What Size Is an Atom? Cut 14 The width of paper 0. 015 mm

Just What Size Is an Atom? Cut 15 We’re not done yet keep cutting! 0. 007 mm

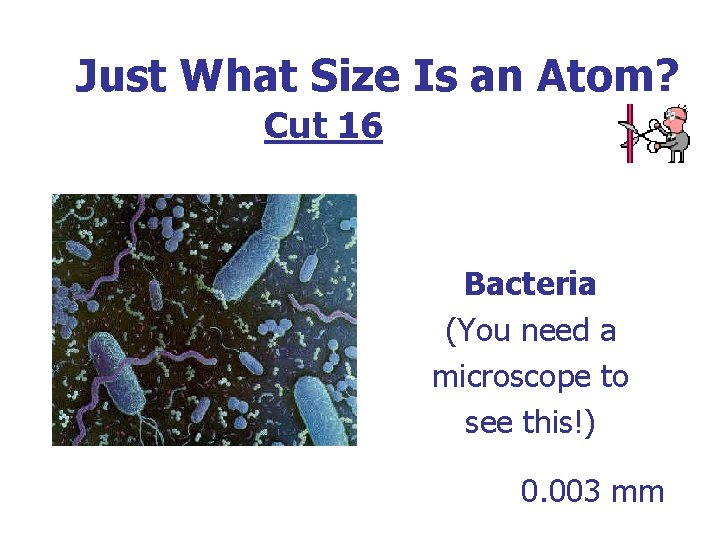
Just What Size Is an Atom? Cut 16 Bacteria (You need a microscope to see this!) 0. 003 mm

Just What Size Is an Atom? Cut 17 We’re not done yet keep cutting! 0. 0015 mm

Just What Size Is an Atom? Cut 18 Water filter 1. 0 micron

Just What Size Is an Atom? Cut 19 We’re not done yet keep cutting! 0. 5 micron

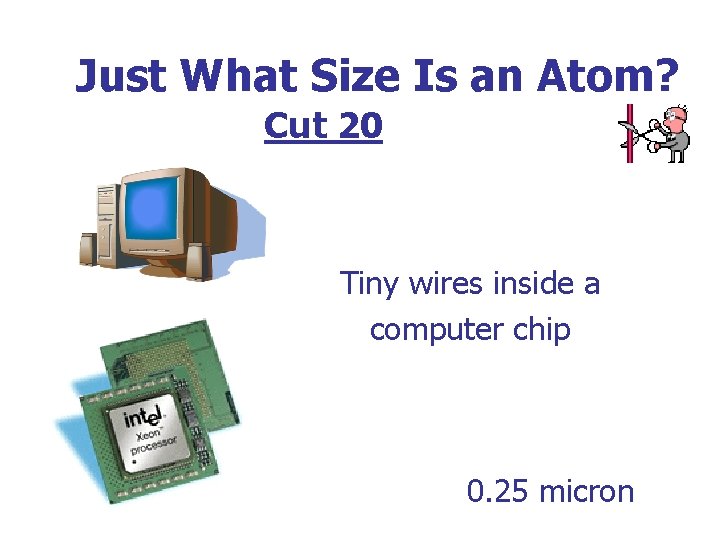
Just What Size Is an Atom? Cut 20 Tiny wires inside a computer chip 0. 25 micron

Just What Size Is an Atom? Cut 21 Keep cutting! 0. 13 micron

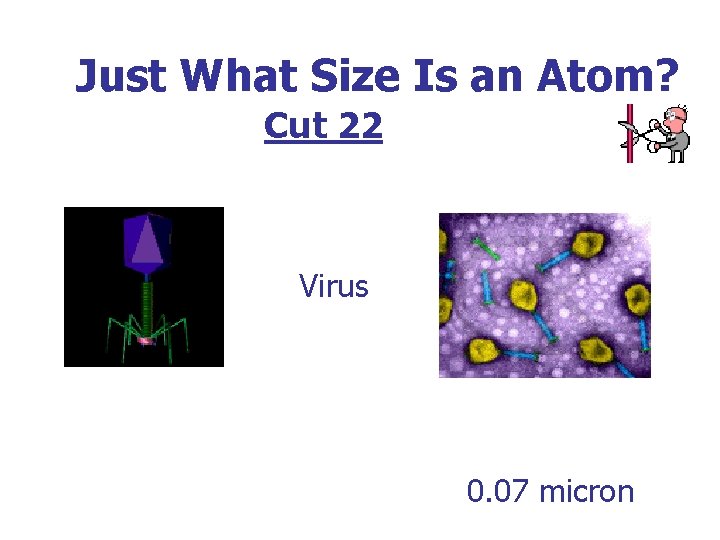
Just What Size Is an Atom? Cut 22 Virus 0. 07 micron

Just What Size Is an Atom? Cut 23 Cut again! 0. 03 micron

Just What Size Is an Atom? Cut 24 And again! 0. 015 micron

Just What Size Is an Atom? Cut 25 We’re not done yet keep cutting! 0. 008 micron

Just What Size Is an Atom? Cut 26 Almost there 5 Cuts left 0. 004 micron

Just What Size Is an Atom? Cut 27 Almost there 4 Cuts left 0. 002 micron

Just What Size Is an Atom? Cut 28 Almost there 3 Cuts left 0. 001 micron

Just What Size Is an Atom? Cut 29 Almost there 2 Cuts left 0. 0005 micron

Just What Size Is an Atom? Cut 30 Almost there 1 Cut left 0. 00025 micron

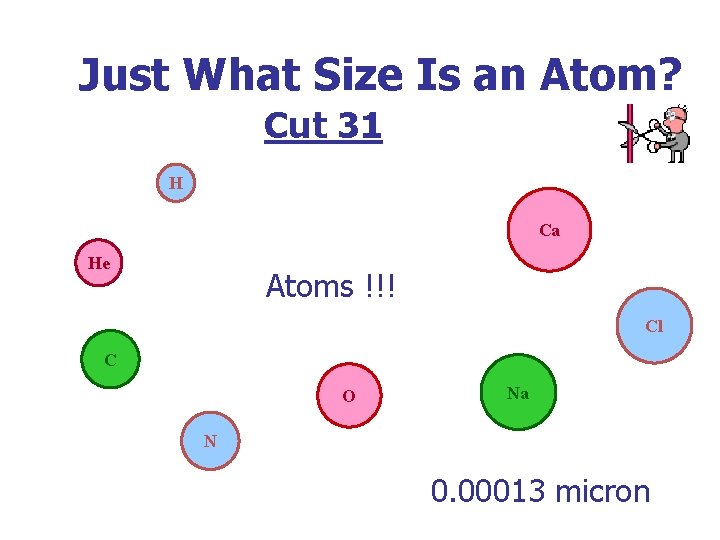
Just What Size Is an Atom? Cut 31 H Ca He Atoms !!! Cl C O Na N 0. 00013 micron

How Many Atoms Are In A Person? Around … 8, 000, 000, 000 that’s… eight octillian atoms Wow!

But don’t take my word for it. Let’s head to Ted Ed for a visual. https: //www. youtube. com/watch? v=y. QP 4 UJh. Nn 0 I

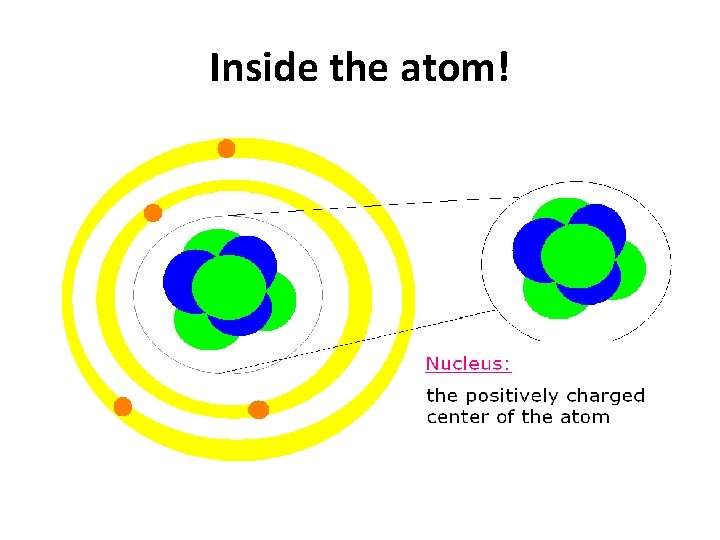
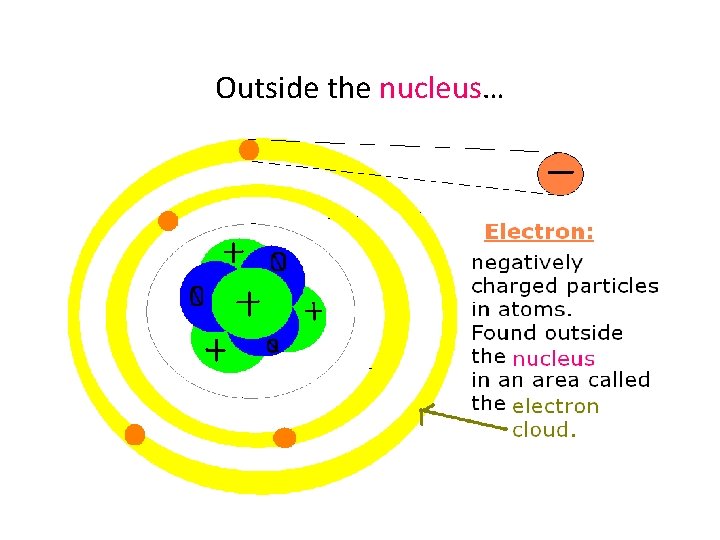
We know that atoms are the smallest complete units of matter, but they have even smaller parts inside!!!

Inside the atom!

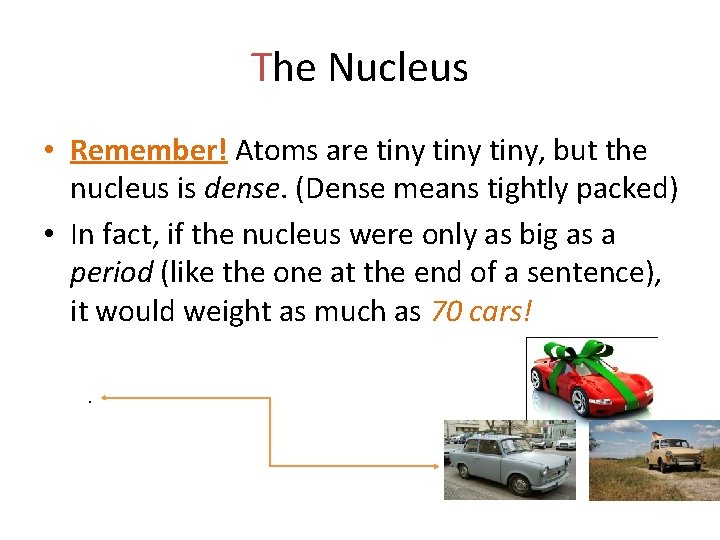
The Nucleus • Remember! Atoms are tiny, but the nucleus is dense. (Dense means tightly packed) • In fact, if the nucleus were only as big as a period (like the one at the end of a sentence), it would weight as much as 70 cars!.

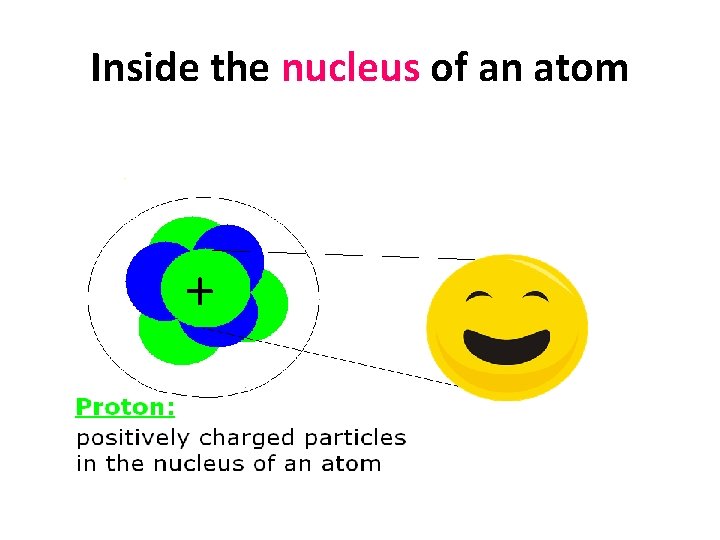
Inside the nucleus of an atom +

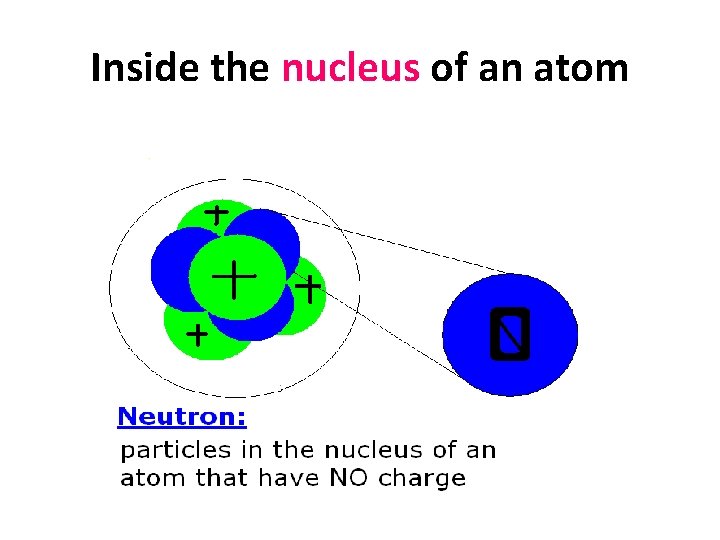
Inside the nucleus of an atom

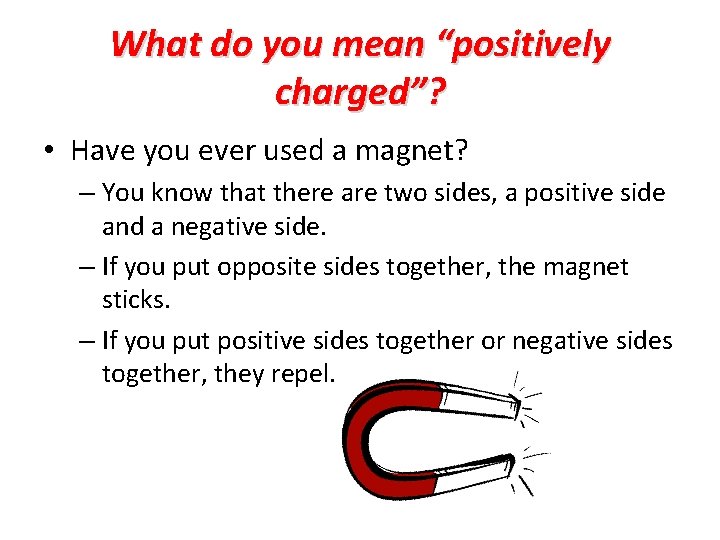
What do you mean “positively charged”? • Have you ever used a magnet? – You know that there are two sides, a positive side and a negative side. – If you put opposite sides together, the magnet sticks. – If you put positive sides together or negative sides together, they repel.

Outside the nucleus…

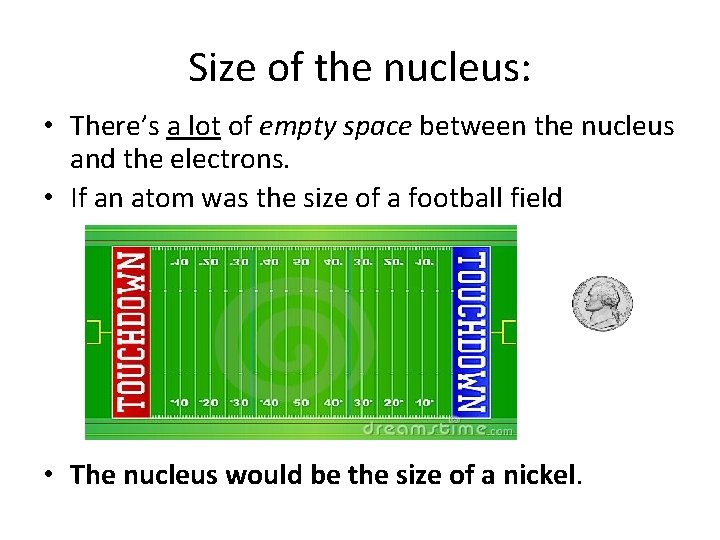
Size of the nucleus: • There’s a lot of empty space between the nucleus and the electrons. • If an atom was the size of a football field • The nucleus would be the size of a nickel.

The Atom - Electron Nucleus 0 + Neutron Proton Electron Cloud

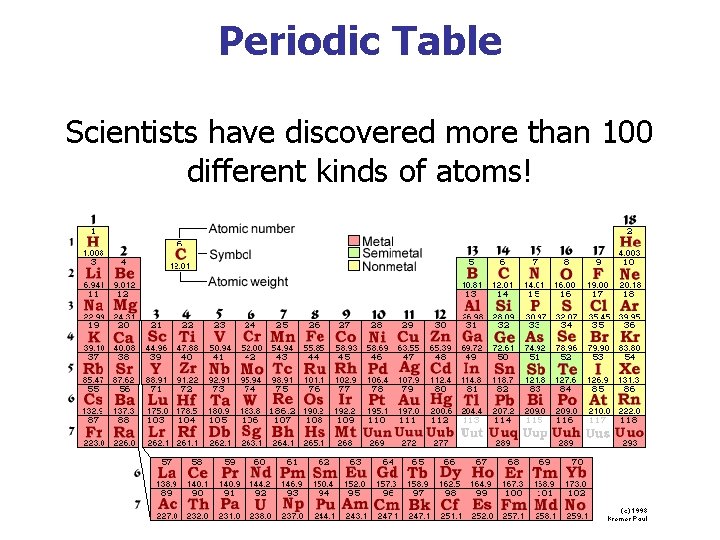
Periodic Table Scientists have discovered more than 100 different kinds of atoms!

Table Group Point! • In order for something to be matter, it has to mass (or be made of atoms) have a ______________ and a volume _______.

What is mass? • Mass – the amount of matter (or atoms) in an object.

How do we measure mass? Mass is usually measured using a balance. Usually, if things weigh more, they have a greater mass. • Weight depends on the amount of gravity, but if you are unsure, it’s a good rule of thumb.

Which do you think has a greater mass? • A cupcake or a paper weight? • A text book or a chapter book? • A car or a bike?

What is volume? • Volume – how much space an object takes up.

How do we measure volume? We can calculate volume all by ourselves! (Sometimes we might need a calculator. ) Volume = length x width x height Since we are measuring 3 things, we write our units in units 3 (in. 3 , cm. 3 , ft. 3)

Let’s Practice! • With your table group, find the volume for each of the following items: 1. A phone is 5 inches tall, 2 inches long, and 1 inch wide. 2. A box is 10 feet tall, 4 feet long, and 2 feet wide.

Which do you think has a greater volume? • • A cupcake or a cookie? A text book or a chapter book? A car or a bike? A deflated balloon or an inflated balloon?
