Catalyst Put this Catalyst on the same sheet













- Slides: 34

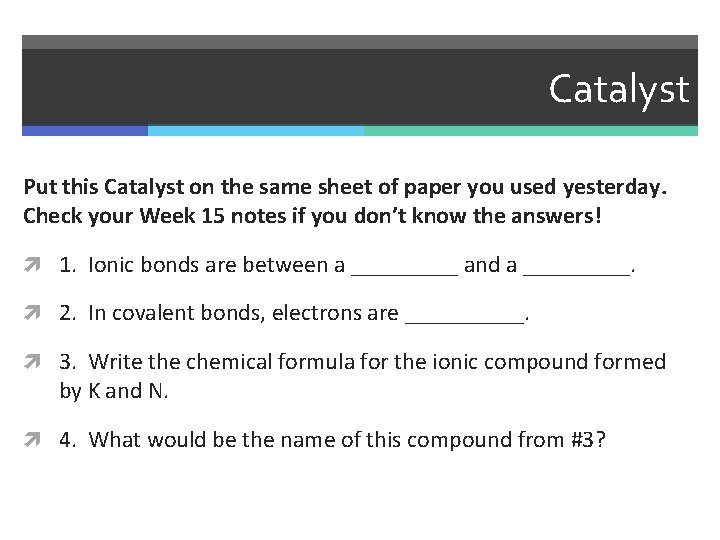
Catalyst Put this Catalyst on the same sheet of paper you used yesterday. Check your Week 15 notes if you don’t know the answers! 1. Ionic bonds are between a _____ and a _____. 2. In covalent bonds, electrons are _____. 3. Write the chemical formula for the ionic compound formed by K and N. 4. What would be the name of this compound from #3?

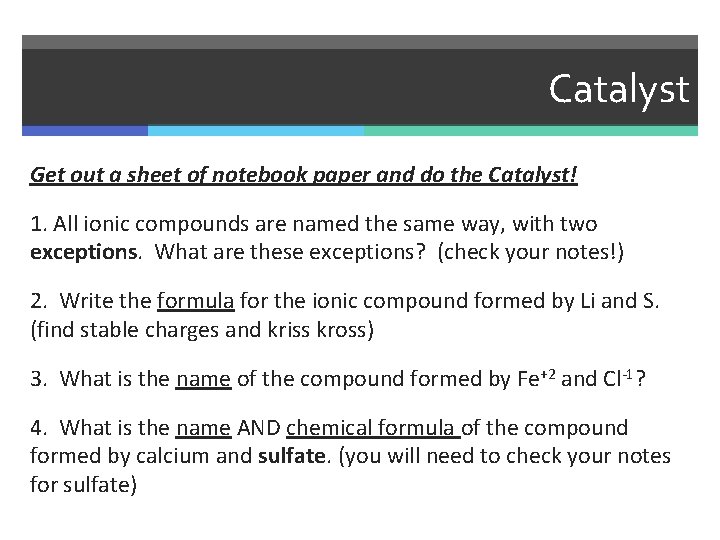
Catalyst Get out a sheet of notebook paper and do the Catalyst! 1. All ionic compounds are named the same way, with two exceptions. What are these exceptions? (check your notes!) 2. Write the formula for the ionic compound formed by Li and S. (find stable charges and kriss kross) 3. What is the name of the compound formed by Fe+2 and Cl-1? 4. What is the name AND chemical formula of the compound formed by calcium and sulfate. (you will need to check your notes for sulfate)

Exit Slip 1. What is the correct formula and compound name for the compound formed by Ga and Cl? a. Ga. Cl, gallium chloride b. Ga. Cl 3, gallium chlorine c. Ga. Cl 3, gallium chloride 2. True or false: K and S would form potassium sulfide

Exit Slip 3. What is the correct name for the compound formed by Fe(II) and Br? 5. What is the correct name for this compound: Al(OH)3? a. b. c. 4. Write the correct name AND formula for the compound formed by Ca and At Aluminum hydroxide Aluminum (III) hydroxide d. Aluminum hydroxide(III)

Today’s Agenda 5 min • Catalyst 10 min • Naming Ionic Compounds 5 min • Chemical Equations for Ionic Compounds 20 min • Stations 5 min • Exit Slip

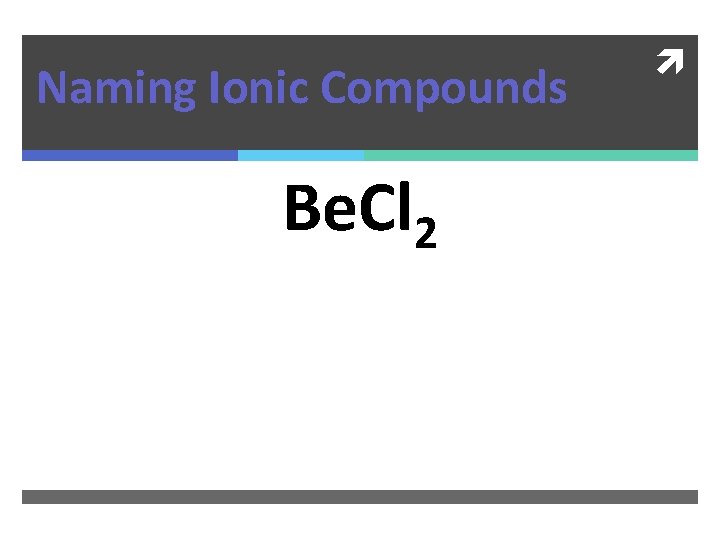
Naming Ionic Compounds Be. Cl 2

Naming Ionic Compounds Be. Cl 2 Barium Chloride

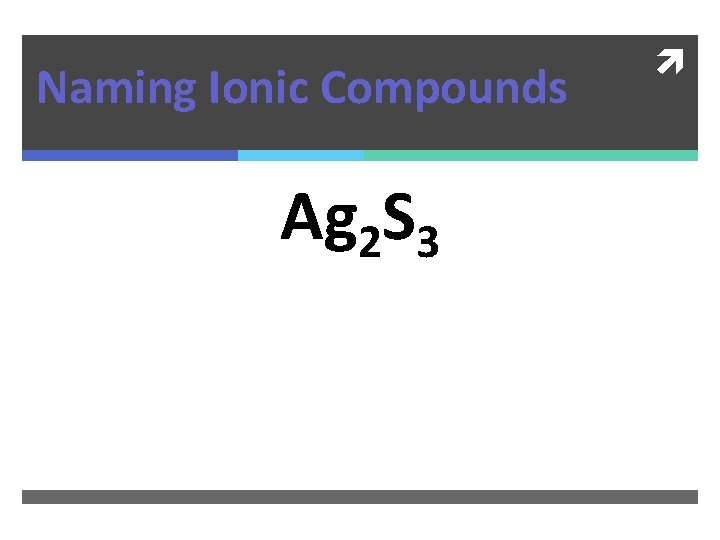
Naming Ionic Compounds Ag 2 S 3

Naming Ionic Compounds Ag 2 S 3 Silver (III) Sulfide

Naming Ionic Compounds Al 2 S 3

Naming Ionic Compounds Al 2 S 3 Aluminum Sulfide

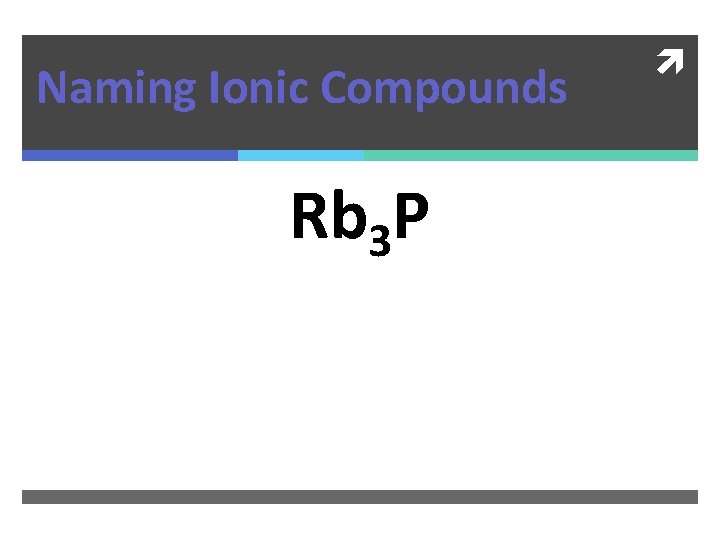
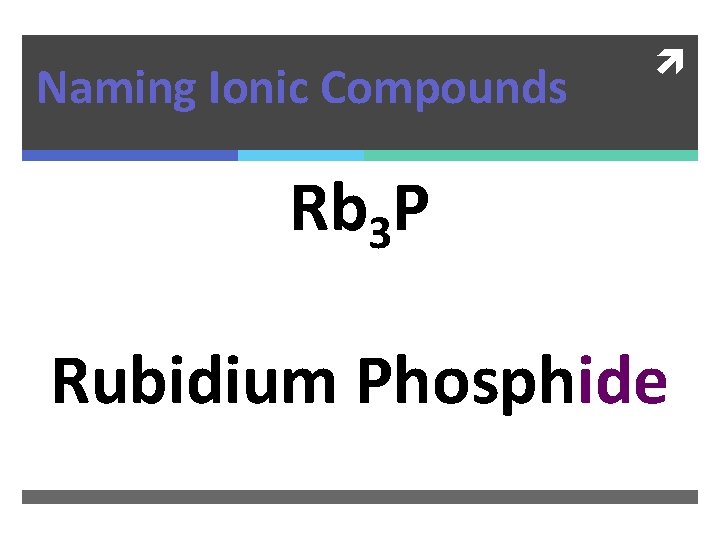
Naming Ionic Compounds Rb 3 P

Naming Ionic Compounds Rb 3 P Rubidium Phosphide

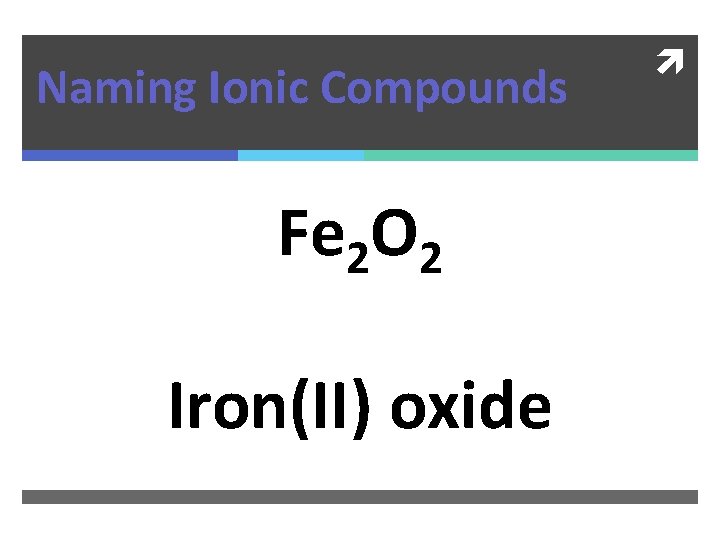
Naming Ionic Compounds Fe 2 O 2

Naming Ionic Compounds Fe 2 O 2 Iron(II) oxide

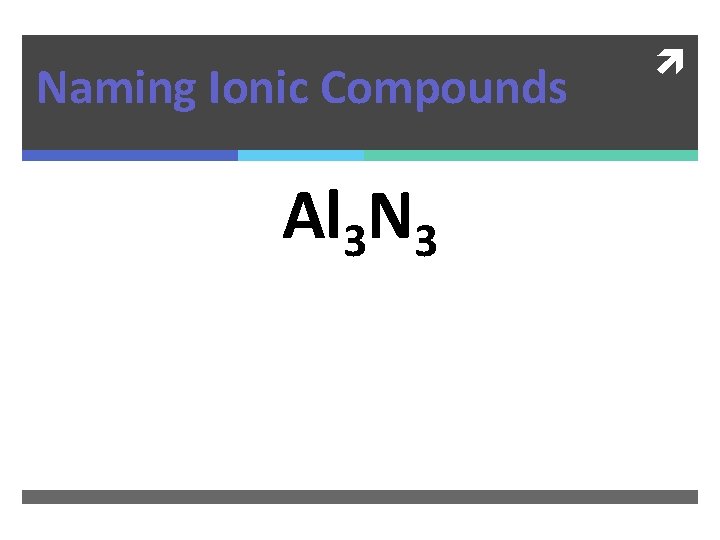
Naming Ionic Compounds Al 3 N 3

Naming Ionic Compounds Al 2 N 3 Aluminum Nitride

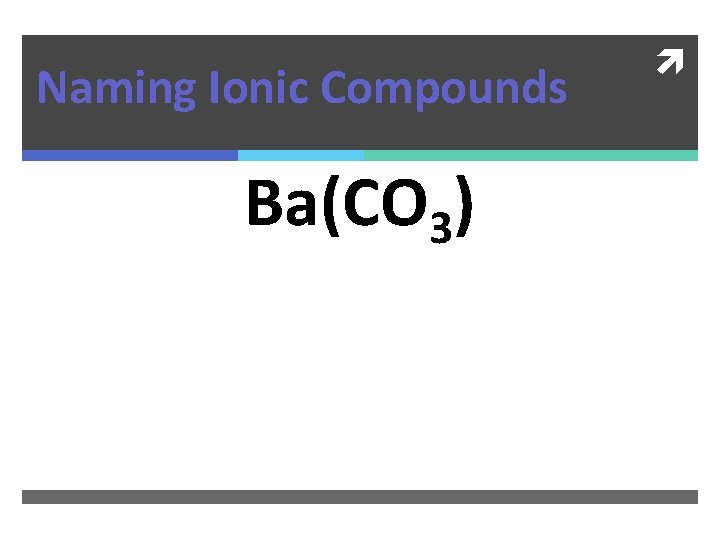
Naming Ionic Compounds Ba(CO 3)

Naming Ionic Compounds Ba(CO 3) Barium Carbonate

Naming Ionic Compounds Al(PO 3)

Naming Ionic Compounds Al(PO 3) Aluminum Phosphite

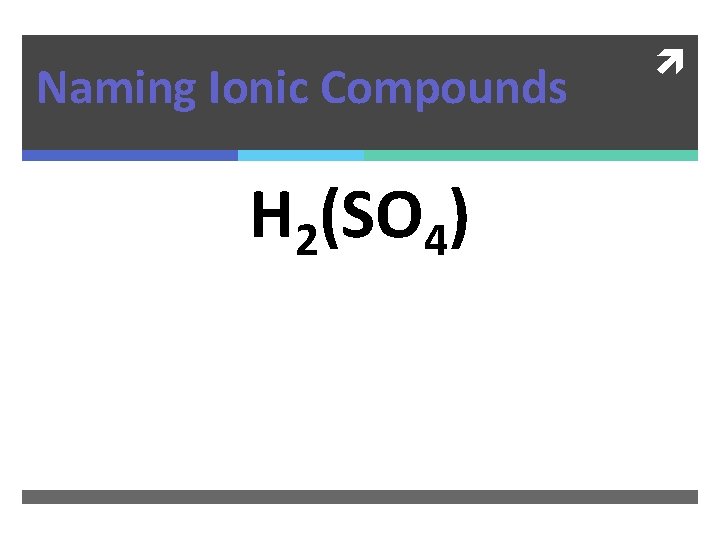
Naming Ionic Compounds H 2(SO 4)

Naming Ionic Compounds H 2(SO 4) Hydrogen Sulfate

Today’s Agenda 5 min • Catalyst 10 min • Naming Ionic Compounds 5 min • Chemical Equations for Ionic Compounds 20 min • Stations 5 min • Exit Slip

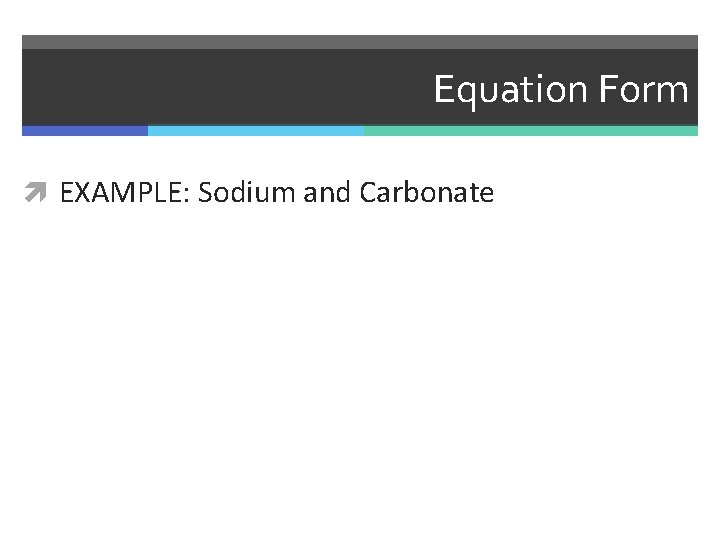
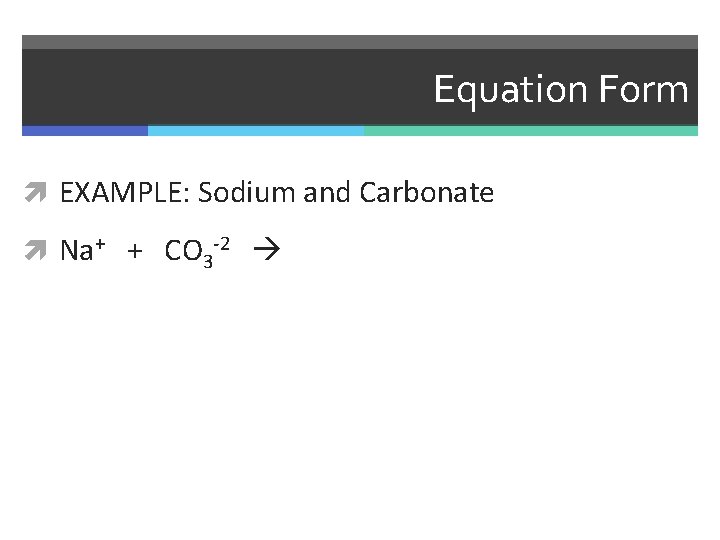
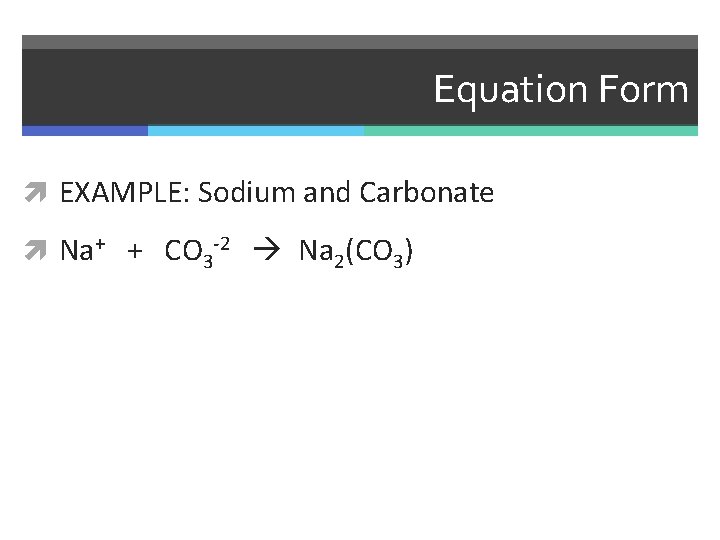
Equation Form EXAMPLE: Sodium and Carbonate

Equation Form EXAMPLE: Sodium and Carbonate Na+ + CO 3 -2

Equation Form EXAMPLE: Sodium and Carbonate Na+ + CO 3 -2 Na 2(CO 3)

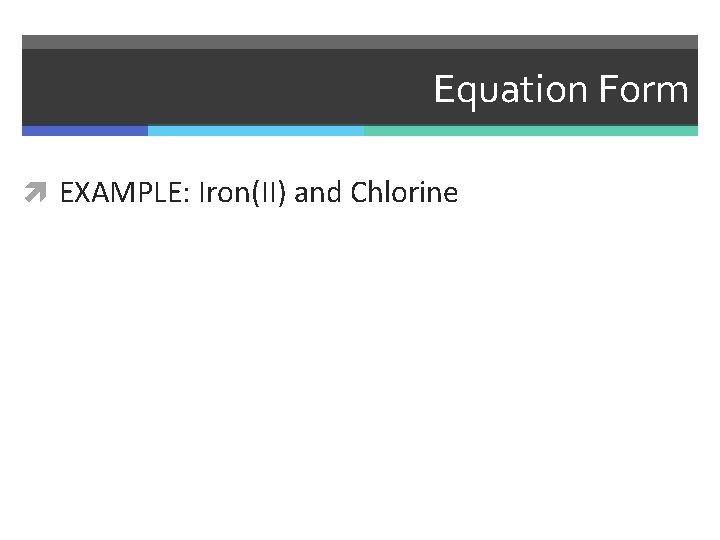
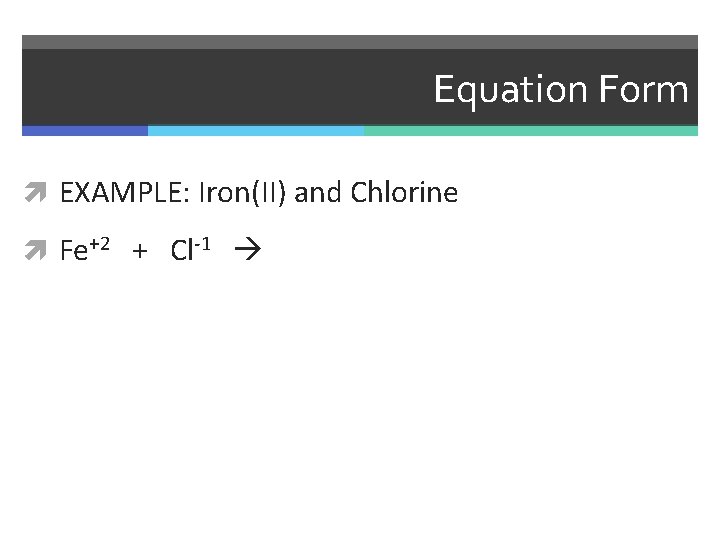
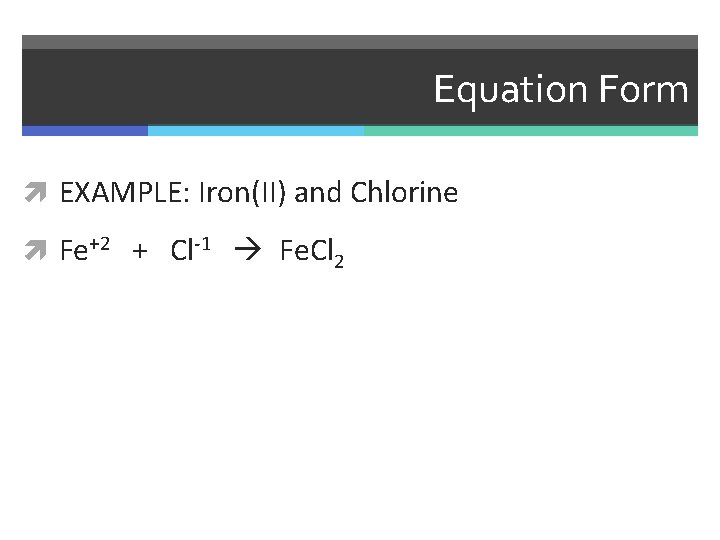
Equation Form EXAMPLE: Iron(II) and Chlorine

Equation Form EXAMPLE: Iron(II) and Chlorine Fe+2 + Cl-1

Equation Form EXAMPLE: Iron(II) and Chlorine Fe+2 + Cl-1 Fe. Cl 2

Today’s Agenda 5 min • Catalyst 10 min • Naming Ionic Compounds 5 min • Chemical Equations for Ionic Compounds 20 min • Stations 5 min • Exit Slip

Today’s Agenda 5 min • Catalyst 10 min • Naming Ionic Compounds 5 min • Chemical Equations for Ionic Compounds 20 min • Stations 5 min • Exit Slip

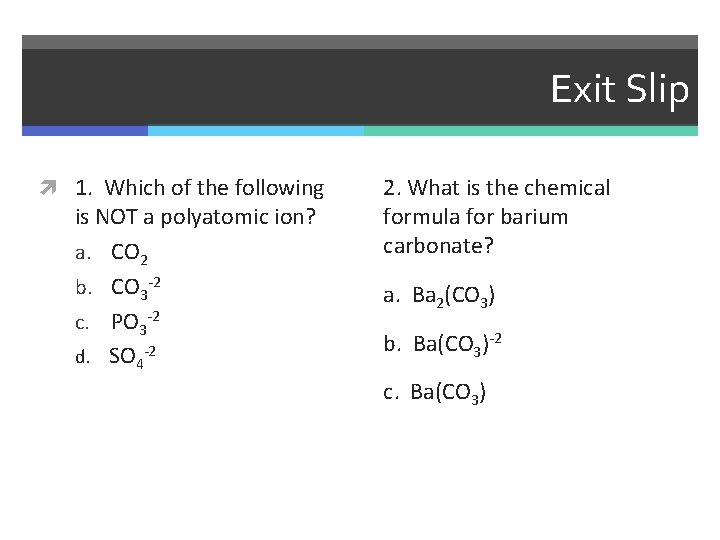
Exit Slip 1. Which of the following is NOT a polyatomic ion? a. CO 2 b. CO 3 -2 c. PO 3 -2 d. SO 4 -2 2. What is the chemical formula for barium carbonate? a. Ba 2(CO 3) b. Ba(CO 3)-2 c. Ba(CO 3)

Exit Slip 3. What is the name of the following compound: H 2(SO 4)? 4. True or false: Aluminum(III) sulfide would be the correct name for Al 2 S 3 a. Hydride sulfate b. Hydrogen sulfate c. Hydrogen sulfite 5. Write the chemical formula for iron(III) sulfide