LIPID MODIFICATION IN THE TREATMENT AND PREVENTION OF













































- Slides: 83

LIPID MODIFICATION IN THE TREATMENT AND PREVENTION OF CARDIOVASCULAR DISEASE: Clinical, Public Health and Ethical Challenges Charles H Hennekens, MD, Dr. PH Sir Richard Doll Research Professor of Medicine Charles E. Schmidt College of Medicine Florida Atlantic University Clinical Professor of Preventive Medicine Nova Southeastern University Voluntary Professor of Family Medicine and Community Health University of Miami Miller School of Medicine

Disclosure • I am funded by the Charles E. Schmidt College of Medicine at Florida Atlantic University (FAU). I have served as Principal Investigator on two investigator initiated research grants funded to FAU by Bayer testing the effects of aspirin dose on platelet and inflammatory biomarkers as well as nitric oxide formation. • I serve as an independent scientist in an advisory role to investigators and sponsors as Chair of Data and Safety Monitoring Boards for Actelion, Amgen, Anthera, Bristol-Myers Squibb, and Sunovion and as a Member of Data and Safety Monitoring Boards for Astra. Zeneca, Bayer , British Heart Foundation, Canadian Institutes of Health Research and Lilly. • I serve as an independent scientist in an advisory role to the U. S. Food and Drug Administration, U. S. National Institutes of Health, Children's Services Council of Palm Beach County and Up. To. Date. • I serve as an independent scientist in an advisory role to legal counsel for Glaxo. Smith. Kline and Stryker. • I serve as speaker for the Association for Research in Vision and Ophthalmology, Baptist Health South Florida, National Association for Continuing Education, Pri. Med, and the International Atherosclerosis Society. • I receive royalties for authorship or editorship of three textbooks. • I receive royalties as co-inventor on patents concerning inflammatory markers and cardiovascular disease which are held by Brigham and Women’s Hospital. • I have an investment management relationship with The West-Bacon Group within Sun. Trust Investment Services who has discretionary investment authority. • I do not own any common or preferred stock in any pharmaceutical or medical device company.

Death is inevitable but premature death is not. Sir Richard Doll


Totality of Evidence • • Basic research (why) Epidemiology (whether) – Descriptive studies • case reports • case series • ecological studies – Analytic studies • observational – case-control – cohort • randomized trials Hennekens CH. Epidemiology in Medicine. Boston, Mass: Little, Brown & Co. ; 1987.

ADVANTAGES AND DISADVANTAGES Basic Research Advantage: Precision Disadvantage: ? Relevance to free living humans Epidemiology Advantage: Relevance to free living humans Disadvantage: Imprecision

QUESTIONABLE RELEVANCE OF BASIC RESEARCH TO FREE LIVING HUMANS Who would have guessed that Homo sapiens would share with the humble guinea pig the unenviable distinction of being unable to synthesize ascorbic acid or with armadillos a susceptibility to the bacterium that causes leprosy or that intestinal cancer usually occurs in the large intestine of humans and the small intestine of sheep? Professor John Cairns

QUESTIONABLE RELEVANCE OF BASIC RESEARCH TO FREE LIVING HUMANS In basic research over 750, 000 chemicals have a potential to cause human cancer but less than 7500 (1%) have any direct relevance to humans Professor Sir Richard Peto

SACCHARIN AND BLADDER CANCER • Canadian rats fed the daily equivalent of 16 gallons of saccharin containing soft drinks developed bladder cancer • • US FDA removed saccharin from diet soft drinks In a case-control study from Harvard School of Public Health published in the New England Journal of Medicine, Massachusetts humans who drank an average of about one to two 12 ounce cans of saccharin containing soft drinks daily had no increased risk of bladder cancer

QUESTIONABLE RELEVANCE OF BASIC RESEARCH TO FREE LIVING HUMANS When the Harvard researcher was asked how to explain the apparent discrepancies he replied that there must be systematic differences between the Canadian rats and the Massachusetts humans. Columbus Georgia Ledger

QUESTIONABLE RELEVANCE OF BASIC RESEARCH TO FREE LIVING HUMANS I guess it takes a researcher from Harvard to put 2 and 2 together. The New Yorker Magazine

THE NEED FOR LARGE SCALE RANDOMIZED EVIDENCE • For hypotheses testing of large effects (i. e. smoking and lung cancer where RR=20, or even smoking and CHD where RR=2. 0) randomized evidence is neither necessary nor desirable • For the most plausible small to moderate effects (i. e. 20%) the amount of uncontrolled and uncontrollable confounding inherent in all case control and cohort studies can be as big as the effect sizes so large scale randomized evidence is crucial. Hennekens CH, De. Mets D: The need for large scale randomized evidence without undue emphasis on small trials, their meta-analyses or subgroup analyses JAMA 2009; 302: 2361 -2362.

CONTRIBUTIONS OF SUBGROUP ANALYSES OF RANDOMIZED TRIALS Subgroup analyses of trials are no longer randomized and so have lower sample sizes; They should be viewed, at best, as hypothesis formulating and, at worst, as rubbish. The biggest danger in interpretation of subgroups is acting as if they provide serious evidence for clinicians and policymakers. Professor Sir Richard Peto


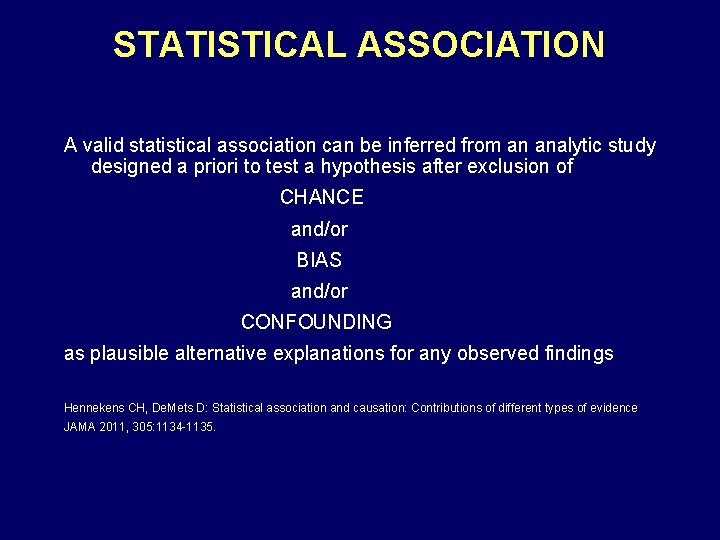
STATISTICAL ASSOCIATION A valid statistical association can be inferred from an analytic study designed a priori to test a hypothesis after exclusion of CHANCE and/or BIAS and/or CONFOUNDING as plausible alternative explanations for any observed findings Hennekens CH, De. Mets D: Statistical association and causation: Contributions of different types of evidence JAMA 2011, 305: 1134 -1135.

STATISTICAL ASSOCIATION AND CAUSE AND EFFECT RELATIONSHIPS • Statistical Association: A matter of fact like death and taxes • Cause and Effect Relationships: A matter of opinion like truth and beauty Hennekens CH, Buring JE: Epidemiology in Medicine, Little, Brown, and Company, Boston, 1987

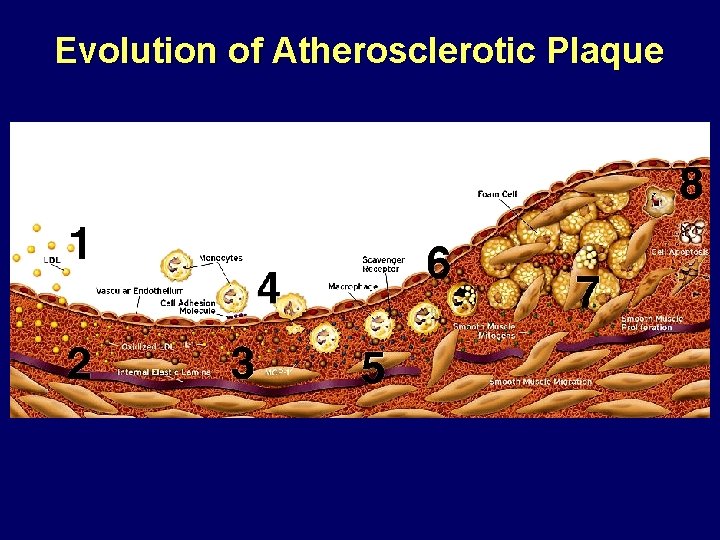
Evolution of Atherosclerotic Plaque Libby P. The Vascular Biology of Atherosclerosis. In: Braunwald, Zipes, Libby, eds. Heart Disease. 6 th edition. 2001.

1985 Nobel Prize in Physiology or Medicine Professors Michael Brown and Joseph Goldstein of Texas Southwestern Medical School discovered the underlying mechanisms of cholesterol metabolism which led to the development of the statin drugs.

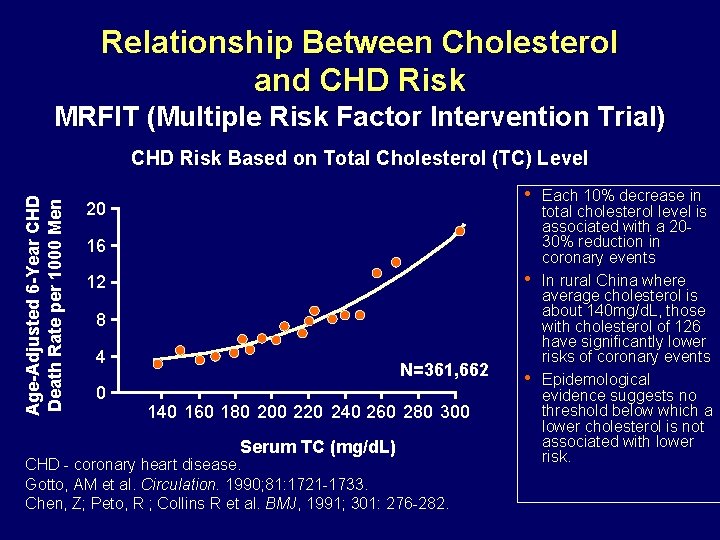
Relationship Between Cholesterol and CHD Risk MRFIT (Multiple Risk Factor Intervention Trial) Age-Adjusted 6 -Year CHD Death Rate per 1000 Men CHD Risk Based on Total Cholesterol (TC) Level • 20 16 • 12 8 4 0 N=361, 662 140 160 180 200 220 240 260 280 300 Serum TC (mg/d. L) CHD - coronary heart disease. Gotto, AM et al. Circulation. 1990; 81: 1721 -1733. Chen, Z; Peto, R ; Collins R et al. BMJ, 1991; 301: 276 -282. • Each 10% decrease in total cholesterol level is associated with a 2030% reduction in coronary events In rural China where average cholesterol is about 140 mg/d. L, those with cholesterol of 126 have significantly lower risks of coronary events Epidemological evidence suggests no threshold below which a lower cholesterol is not associated with lower risk.

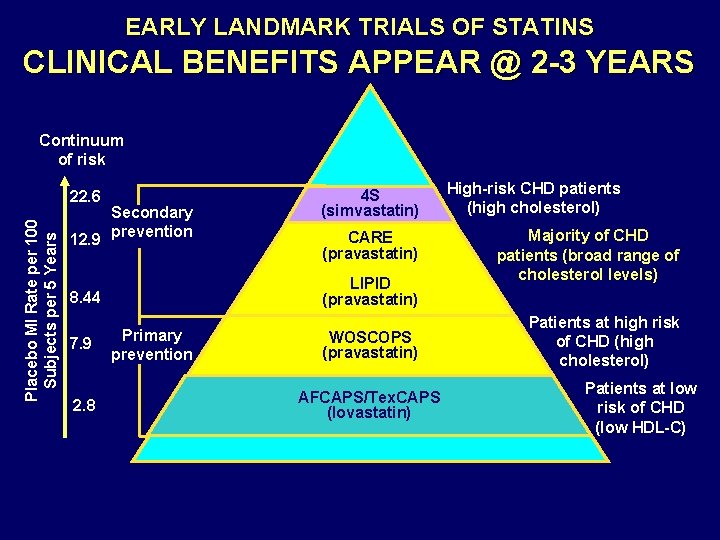
EARLY LANDMARK TRIALS OF STATINS CLINICAL BENEFITS APPEAR @ 2 -3 YEARS Continuum of risk Placebo MI Rate per 100 Subjects per 5 Years 22. 6 12. 9 Secondary prevention 2. 8 CARE (pravastatin) LIPID (pravastatin) 8. 44 7. 9 4 S (simvastatin) Primary prevention WOSCOPS (pravastatin) AFCAPS/Tex. CAPS (lovastatin) High-risk CHD patients (high cholesterol) Majority of CHD patients (broad range of cholesterol levels) Patients at high risk of CHD (high cholesterol) Patients at low risk of CHD (low HDL-C)

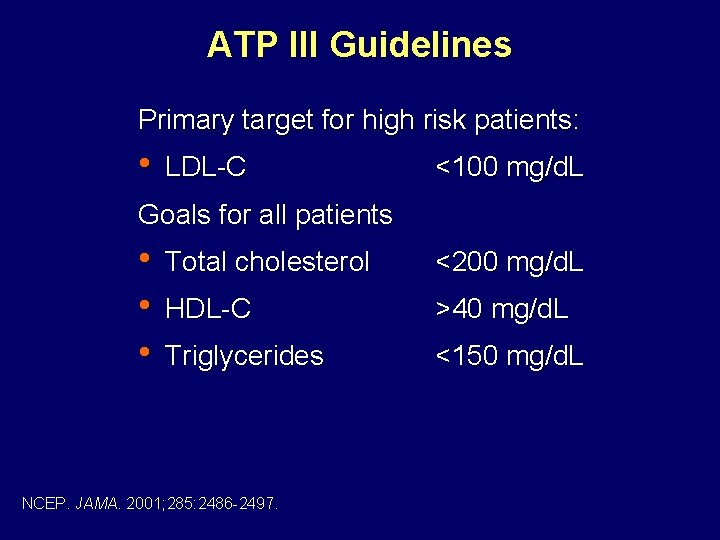
ATP III Guidelines Primary target for high risk patients: • LDL-C <100 mg/d. L Goals for all patients • • • Total cholesterol <200 mg/d. L HDL-C >40 mg/d. L Triglycerides <150 mg/d. L NCEP. JAMA. 2001; 285: 2486 -2497.

ATP III Guidelines: Increased the numbers of US patients eligible for statins from 13 to 36 million • Prior CVD event – – – • CHD Stroke Other clinical forms of atherosclerotic disease (peripheral arterial disease, abdominal aortic aneurysm, and symptomatic carotid artery disease) Risk factors that confer a 10 -year risk for CHD ≥ 20% – Diabetes – ≥ 2 risk factors (metabolic syndrome)

Metabolic Syndrome • Metabolic syndrome is a constellation of obesity, dyslipidemia, hypertension, and insulin resistance. • In the U. S. , based on NCHS data, 20% of adults aged 20 and over as well as 40% of adults aged 40 and older have metabolic syndrome. • Patients with metabolic syndrome have a 10 year risk of a first CHD event of 16 -18%.

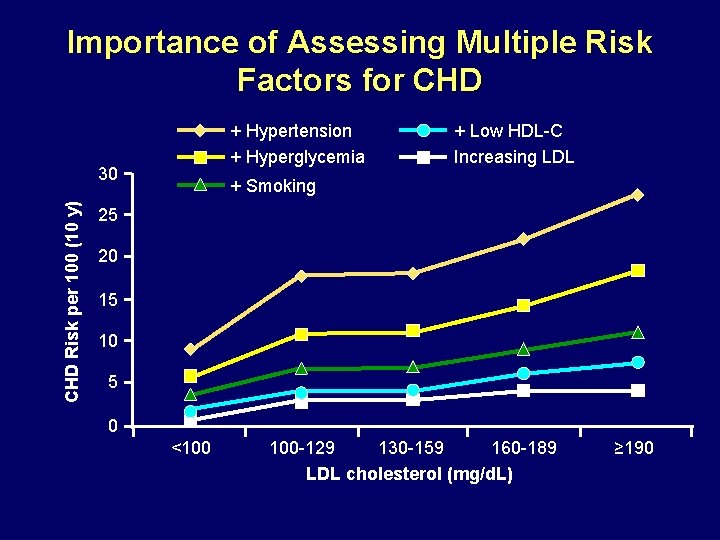
Importance of Assessing Multiple Risk Factors for CHD + Hypertension + Hyperglycemia CHD Risk per 100 (10 y) 30 + Low HDL-C Increasing LDL + Smoking 25 20 15 10 5 0 <100 100 -129 130 -159 160 -189 LDL cholesterol (mg/d. L) ≥ 190


There is absolutely no substitute for good clinical judgment This is because: Randomized trials are necessary to develop guidelines Guidelines are of crucial importance but are not the sole factor in good clinical judgment.

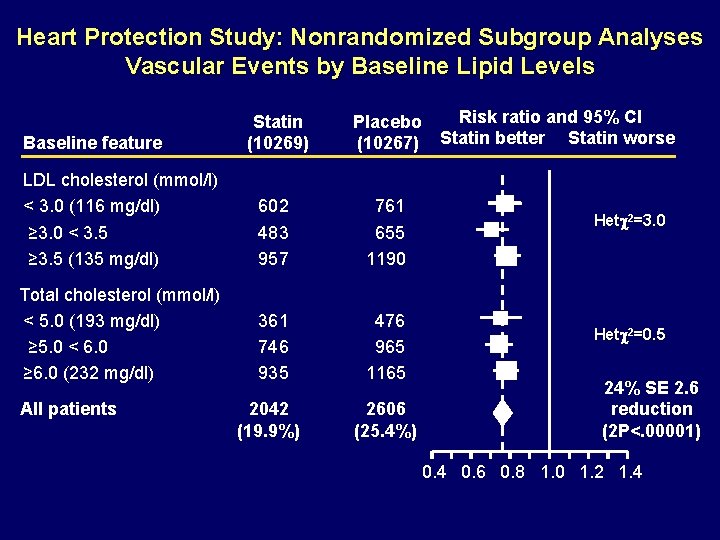
Heart Protection Study: Nonrandomized Subgroup Analyses Vascular Events by Baseline Lipid Levels Baseline feature Statin (10269) Risk ratio and 95% CI Placebo (10267) Statin better Statin worse LDL cholesterol (mmol/l) < 3. 0 (116 mg/dl) ≥ 3. 0 < 3. 5 ≥ 3. 5 (135 mg/dl) 602 483 957 761 655 1190 Total cholesterol (mmol/l) < 5. 0 (193 mg/dl) ≥ 5. 0 < 6. 0 ≥ 6. 0 (232 mg/dl) 361 746 935 476 965 1165 2042 (19. 9%) 2606 (25. 4%) All patients Het 2=3. 0 Het 2=0. 5 24% SE 2. 6 reduction (2 P<. 00001) 0. 4 0. 6 0. 8 1. 0 1. 2 1. 4

PROVE-IT • • • 4162 post acute coronary syndrome (ACS) patients 80 mg atorvastatin vs 40 mg pravastatin Baseline LDL of 106 Achieved LDL: 44(LDL=62) vs 9(LDL=97) 24 -month treatment and follow-up Cannon et al. N Engl J Med 2004; 350: 1495 -1504.

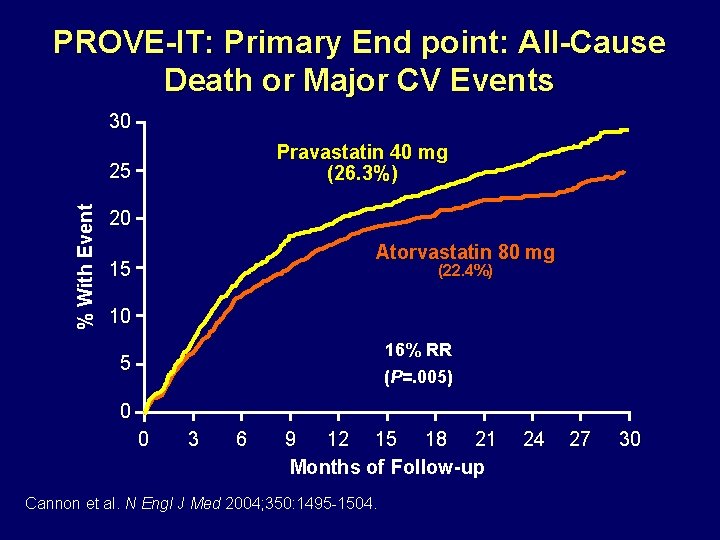
PROVE-IT: Primary End point: All-Cause Death or Major CV Events 30 Pravastatin 40 mg (26. 3%) % With Event 25 20 Atorvastatin 80 mg 15 (22. 4%) 10 16% RR (P=. 005) 5 0 0 3 6 9 12 15 18 21 Months of Follow-up Cannon et al. N Engl J Med 2004; 350: 1495 -1504. 24 27 30

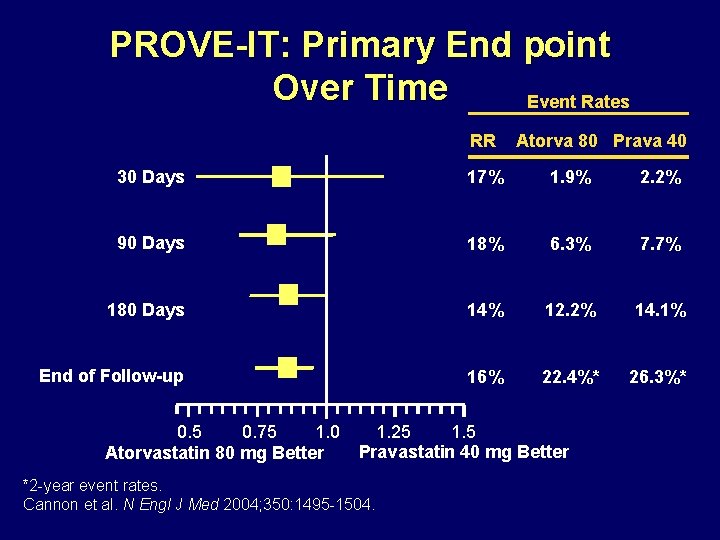
PROVE-IT: Primary End point Over Time Event Rates RR Atorva 80 Prava 40 30 Days 17% 1. 9% 2. 2% 90 Days 18% 6. 3% 7. 7% 180 Days 14% 12. 2% 14. 1% End of Follow-up 16% 22. 4%* 26. 3%* 0. 5 0. 75 1. 0 Atorvastatin 80 mg Better 1. 25 1. 5 Pravastatin 40 mg Better *2 -year event rates. Cannon et al. N Engl J Med 2004; 350: 1495 -1504.

Implications of Recent Clinical Trials for NCEP ATP III Guidelines • • Recent trials of higher versus usual dose statins provide greater rationale for lower target LDL-C levels and more intensive LDL-lowering therapy Key modifications to ATP III treatment algorithm for LDLC: – LDL-C goal <70 mg/d. L is therapeutic option for patients at very high risk – LDL-C goal <100 mg/d. L is therapeutic option for moderately highrisk patients – At least 30% to 40% reduction in LDL-C recommended for high and moderately high risk patients. Grundy et al. Circulation. 2004; 110: 227 -239.

TNT—Treatment-to-New Targets Trial • 10, 002 chronic stable coronary disease patients • • Enrollment in 250 sites from 14 countries • Duration of 5 years or accrual of 750 primary CVD events Randomized, double-blind trial of 80 mg atorvastatin versus 10 mg atorvastatin

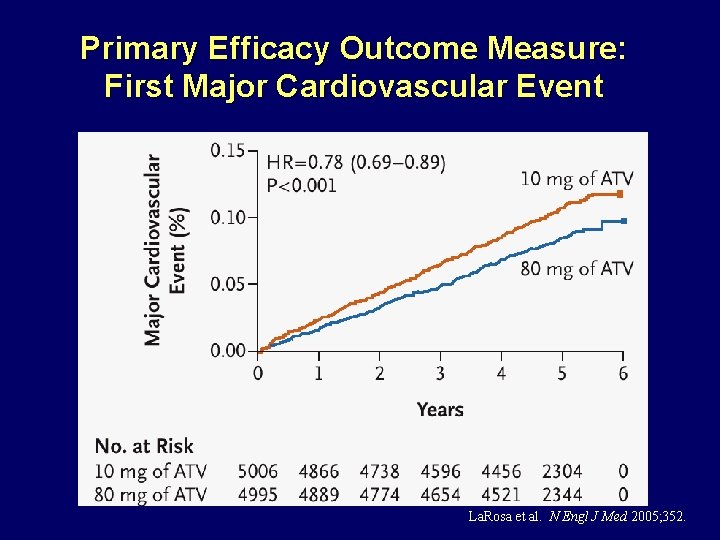
Primary Efficacy Outcome Measure: First Major Cardiovascular Event La. Rosa et al. N Engl J Med 2005; 352.

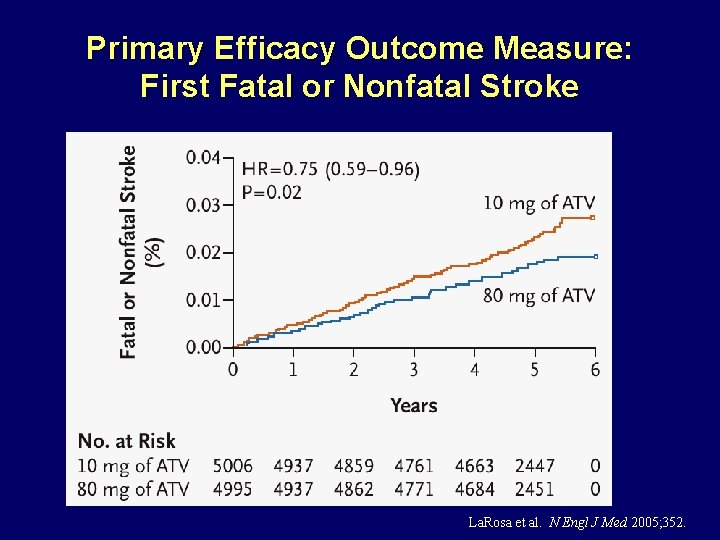
Primary Efficacy Outcome Measure: First Fatal or Nonfatal Stroke La. Rosa et al. N Engl J Med 2005; 352.

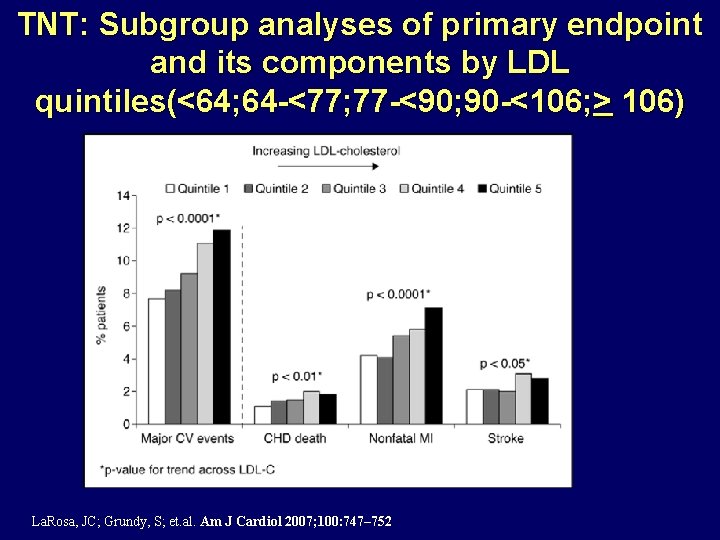
TNT: Subgroup analyses of primary endpoint and its components by LDL quintiles(<64; 64 -<77; 77 -<90; 90 -<106; > 106) La. Rosa, JC; Grundy, S; et. al. Am J Cardiol 2007; 100: 747– 752

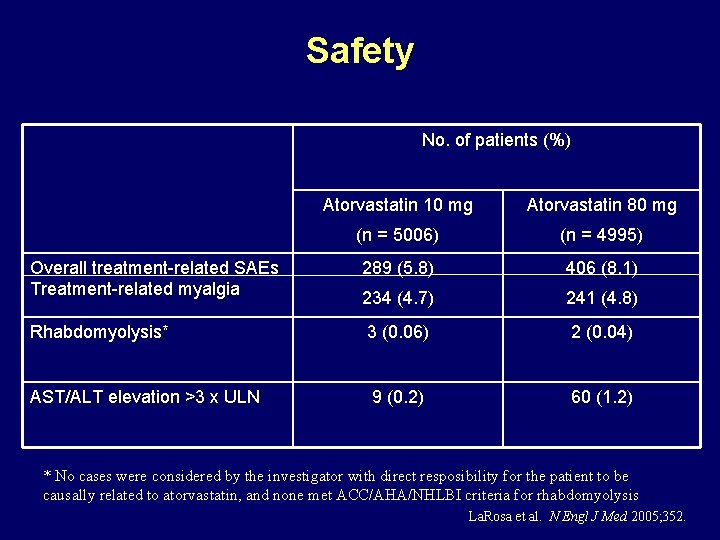
Safety No. of patients (%) Atorvastatin 10 mg Atorvastatin 80 mg (n = 5006) (n = 4995) Overall treatment-related SAEs Treatment-related myalgia 289 (5. 8) 406 (8. 1) 234 (4. 7) 241 (4. 8) Rhabdomyolysis* 3 (0. 06) 2 (0. 04) AST/ALT elevation >3 x ULN 9 (0. 2) 60 (1. 2) * No cases were considered by the investigator with direct resposibility for the patient to be causally related to atorvastatin, and none met ACC/AHA/NHLBI criteria for rhabdomyolysis La. Rosa et al. N Engl J Med 2005; 352.

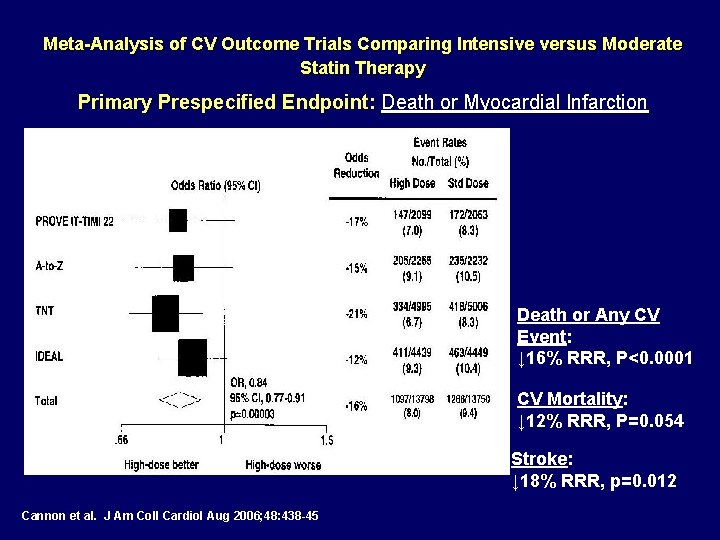
Meta-Analysis of CV Outcome Trials Comparing Intensive versus Moderate Statin Therapy Primary Prespecified Endpoint: Death or Myocardial Infarction Death or Any CV Event: ↓ 16% RRR, P<0. 0001 CV Mortality: ↓ 12% RRR, P=0. 054 Stroke: ↓ 18% RRR, p=0. 012 Cannon et al. J Am Coll Cardiol Aug 2006; 48: 438 -45

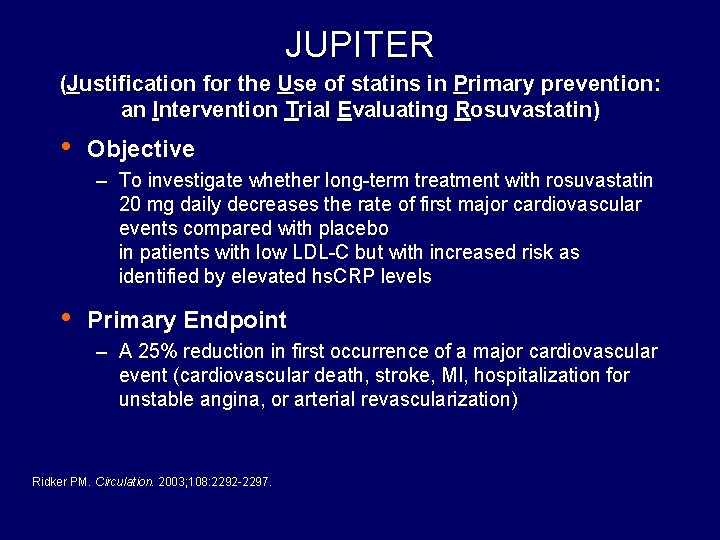
JUPITER (Justification for the Use of statins in Primary prevention: an Intervention Trial Evaluating Rosuvastatin) • Objective – To investigate whether long-term treatment with rosuvastatin 20 mg daily decreases the rate of first major cardiovascular events compared with placebo in patients with low LDL-C but with increased risk as identified by elevated hs. CRP levels • Primary Endpoint – A 25% reduction in first occurrence of a major cardiovascular event (cardiovascular death, stroke, MI, hospitalization for unstable angina, or arterial revascularization) Ridker PM. Circulation. 2003; 108: 2292 -2297.

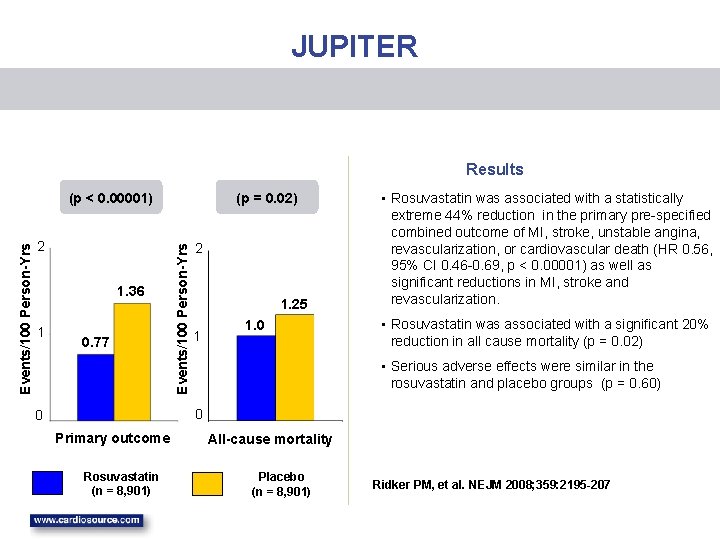
EARLY TERMINATION OF JUPITER • The 10 year risk of a first CHD event in patients randomized into JUPITER was about 16% (about 50% had metabolic syndrome and about 50% had hypertension). • On March 29, 2008 JUPITER was terminated early after a median follow up of 1. 9 years based on the unanimous recommendation of the independent Data and Safety Monitoring Board (DSMB) The DSMB recommendation was based on “unequivocal evidence of a reduction in cardiovascular morbidity and mortality among patients assigned at random to rosuvastatin when compared to placebo. ” JUPITER achieved an average LDL of 55 in the rosuvastatin group and 109 in placebo which resulted in a 44% reduction in the primary prespecified endpoint. • •

JUPITER Results 2 1. 36 1 0. 77 (p = 0. 02) Events/100 Person-Yrs (p < 0. 00001) 2 1. 25 1 1. 0 • Rosuvastatin was associated with a statistically extreme 44% reduction in the primary pre-specified combined outcome of MI, stroke, unstable angina, revascularization, or cardiovascular death (HR 0. 56, 95% CI 0. 46 -0. 69, p < 0. 00001) as well as significant reductions in MI, stroke and revascularization. • Rosuvastatin was associated with a significant 20% reduction in all cause mortality (p = 0. 02) • Serious adverse effects were similar in the rosuvastatin and placebo groups (p = 0. 60) 0 0 Primary outcome Rosuvastatin (n = 8, 901) All-cause mortality Placebo (n = 8, 901) Ridker PM, et al. NEJM 2008; 359: 2195 -207

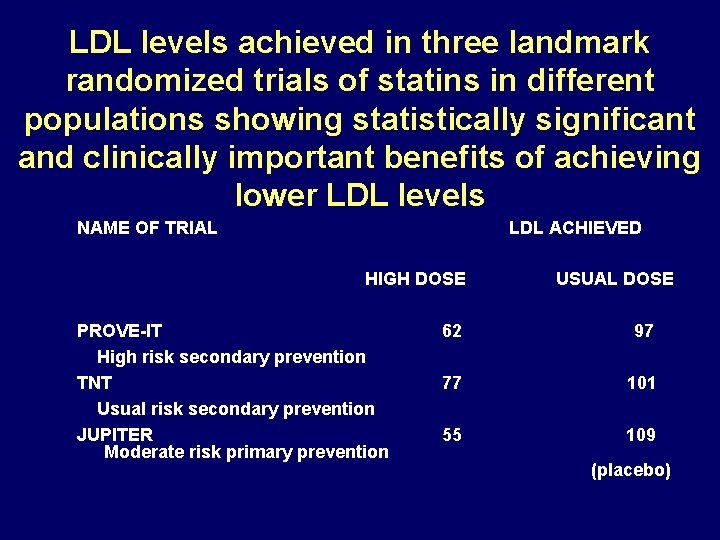
LDL levels achieved in three landmark randomized trials of statins in different populations showing statistically significant and clinically important benefits of achieving lower LDL levels NAME OF TRIAL LDL ACHIEVED HIGH DOSE USUAL DOSE PROVE-IT High risk secondary prevention TNT Usual risk secondary prevention JUPITER Moderate risk primary prevention 62 97 77 101 55 109 (placebo)

Diabetes and Cardiovascular Disease • • • Diabetes is a major risk factor for CVD and can be a component to the metabolic syndrome which markedly increases risks of CVD The CARDS trial of diabetics in primary prevention was terminated early due to a statistically extreme 37% reduction in the primary pre-specified outcome The US National Cholesterol Education Program (NCEP) III has elevated diabetes from a major risk factor to a CHD risk equivalent and recommends that all patients with diabetes should be treated as aggressively as survivors of a CVD event (i. e. , MI or stroke).

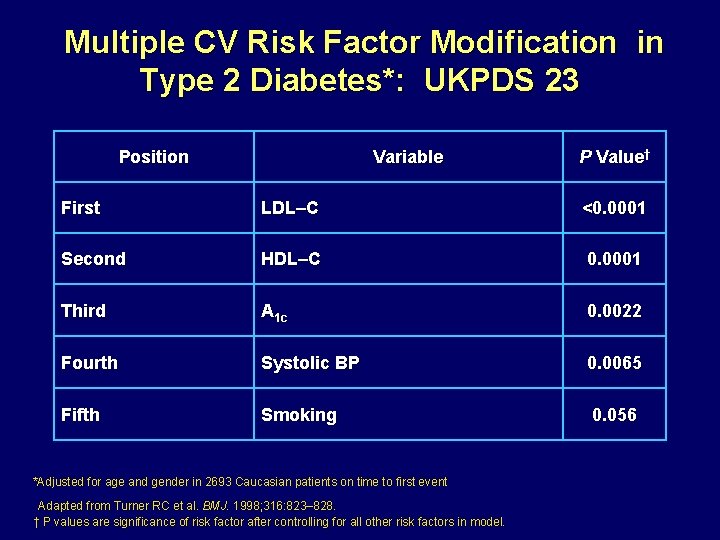
Multiple CV Risk Factor Modification in Type 2 Diabetes*: UKPDS 23 Position Variable P Value† First LDL–C <0. 0001 Second HDL–C 0. 0001 Third A 1 c 0. 0022 Fourth Systolic BP 0. 0065 Fifth Smoking 0. 056 *Adjusted for age and gender in 2693 Caucasian patients on time to first event Adapted from Turner RC et al. BMJ. 1998; 316: 823– 828. † P values are significance of risk factor after controlling for all other risk factors in model.

STATINS AND DIABETES • In a meta-analysis of 13 statin trials with 91, 140 participants 4278 developed diabetes (2226 assigned statins and 2052 assigned control) This increase (OR=1. 09, 95%CI 1. 02 -1. 17) in risk, if real, is low both in absolute terms and when compared with the reductions in cardiovascular events • Clinical practice in patients with moderate or high cardiovascular risk or existing cardiovascular disease should not change. Lancet, 2010; 375: 735 -742

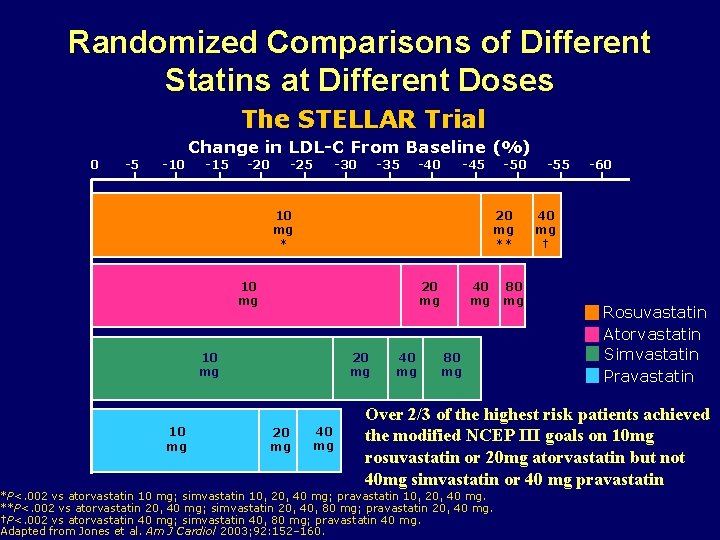
Randomized Comparisons of Different Statins at Different Doses The STELLAR Trial Change in LDL-C From Baseline (%) 0 -5 -10 -15 -20 -25 -30 -35 -40 -45 10 mg * 20 mg ** 10 mg 20 mg -50 40 mg 80 mg -55 -60 40 mg † Rosuvastatin Atorvastatin Simvastatin Pravastatin Over 2/3 of the highest risk patients achieved the modified NCEP III goals on 10 mg rosuvastatin or 20 mg atorvastatin but not 40 mg simvastatin or 40 mg pravastatin *P<. 002 vs atorvastatin 10 mg; simvastatin 10, 20, 40 mg; pravastatin 10, 20, 40 mg. **P<. 002 vs atorvastatin 20, 40 mg; simvastatin 20, 40, 80 mg; pravastatin 20, 40 mg. †P<. 002 vs atorvastatin 40 mg; simvastatin 40, 80 mg; pravastatin 40 mg. Adapted from Jones et al. Am J Cardiol 2003; 92: 152– 160.

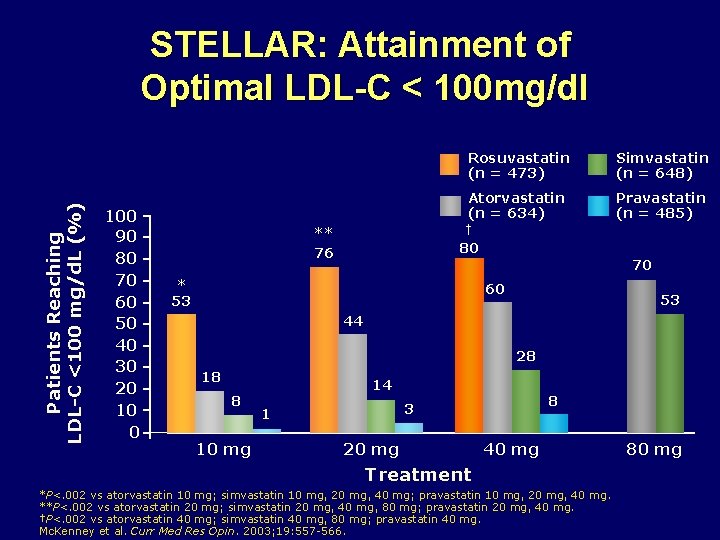
Patients Reaching LDL-C <100 mg/d. L (%) STELLAR: Attainment of Optimal LDL-C < 100 mg/dl 100 90 80 70 60 50 40 30 20 10 0 Rosuvastatin (n = 473) Simvastatin (n = 648) Atorvastatin (n = 634) Pravastatin (n = 485) † ** 76 80 70 * 53 60 53 44 28 18 8 10 mg 14 8 3 1 20 mg 40 mg Treatment *P<. 002 vs atorvastatin 10 mg; simvastatin 10 mg, 20 mg, 40 mg; pravastatin 10 mg, 20 mg, 40 mg. **P<. 002 vs atorvastatin 20 mg; simvastatin 20 mg, 40 mg, 80 mg; pravastatin 20 mg, 40 mg. †P<. 002 vs atorvastatin 40 mg; simvastatin 40 mg, 80 mg; pravastatin 40 mg. Mc. Kenney et al. Curr Med Res Opin. 2003; 19: 557 -566. 80 mg

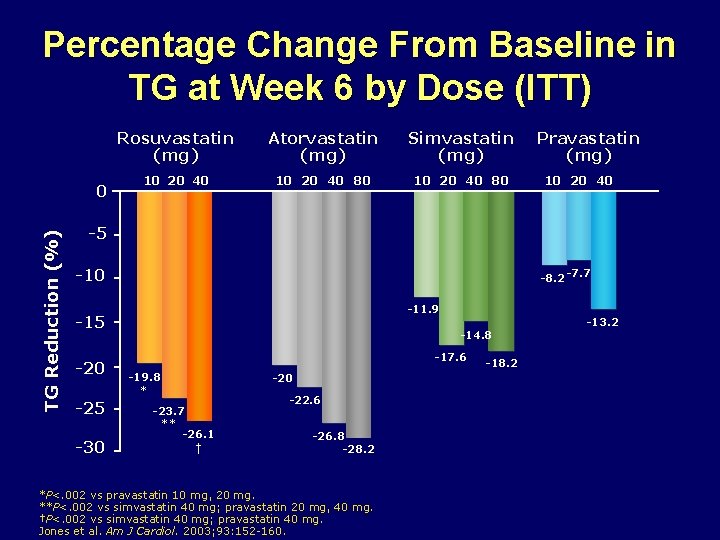
Percentage Change From Baseline in TG at Week 6 by Dose (ITT) TG Reduction (%) 0 Rosuvastatin (mg) Atorvastatin (mg) Simvastatin (mg) 10 20 40 80 Pravastatin (mg) 10 20 40 -5 -10 -8. 2 -7. 7 -11. 9 -15 -20 -25 -30 -14. 8 -17. 6 -19. 8 * -20 -23. 7 ** -26. 1 † -22. 6 -26. 8 -28. 2 *P<. 002 vs pravastatin 10 mg, 20 mg. **P<. 002 vs simvastatin 40 mg; pravastatin 20 mg, 40 mg. †P<. 002 vs simvastatin 40 mg; pravastatin 40 mg. Jones et al. Am J Cardiol. 2003; 93: 152 -160. -18. 2 -13. 2

Percentage Change From Baseline in HDL-C at Week 6 by Dose (ITT) HDL-C Increase (%) 12 † ** 9. 6 9. 5 10 8 * 7. 7 6. 8 6. 0 5. 7 6 4. 8 5. 3 4. 4 4 3. 2 2. 1 2 0 5. 6 5. 2 10 20 40 80 Rosuvastatin Atorvastatin (mg) 10 20 40 80 Simvastatin (mg) 10 20 40 Pravastatin (mg) ITT = intention-to-treat. *P<. 002 vs pravastatin 10 mg. **P<. 002 vs atorvastatin 20 mg, 40 mg, 80 mg; simvastatin 40 mg; pravastatin 20 mg, 40 mg. †P<. 002 vs atorvastatin 40 mg, 80 mg; simvastatin 40 mg; pravastatin 40 mg. Jones et al. Am J Cardiol. 2003; 93: 152 -160.

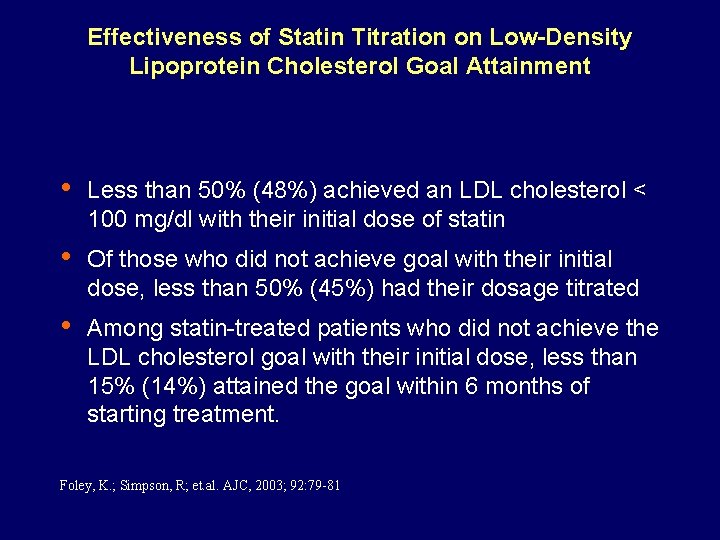
Effectiveness of Statin Titration on Low-Density Lipoprotein Cholesterol Goal Attainment • Less than 50% (48%) achieved an LDL cholesterol < 100 mg/dl with their initial dose of statin • Of those who did not achieve goal with their initial dose, less than 50% (45%) had their dosage titrated • Among statin-treated patients who did not achieve the LDL cholesterol goal with their initial dose, less than 15% (14%) attained the goal within 6 months of starting treatment. Foley, K. ; Simpson, R; et. al. AJC, 2003; 92: 79 -81

Core Components of Statin Safety • • Muscle-related adverse events (AEs) Liver-related AEs

Incidence of Statin-Associated Rhabdomyolysis • The incidence of statin-associated rhabdomyolysis across large, randomized, controlled statin trials is <0. 1% • The reported incidence of fatal rhabdomyolysis with statins is extremely rare: – 0. 15 deaths per 1 million prescriptions which is a weighted average of 0. 18 for simvastatin and lovastatin; 0. 06 for atorvastatin and pravastatin; and 0. 00 for fluvastatin and rosuvastatin

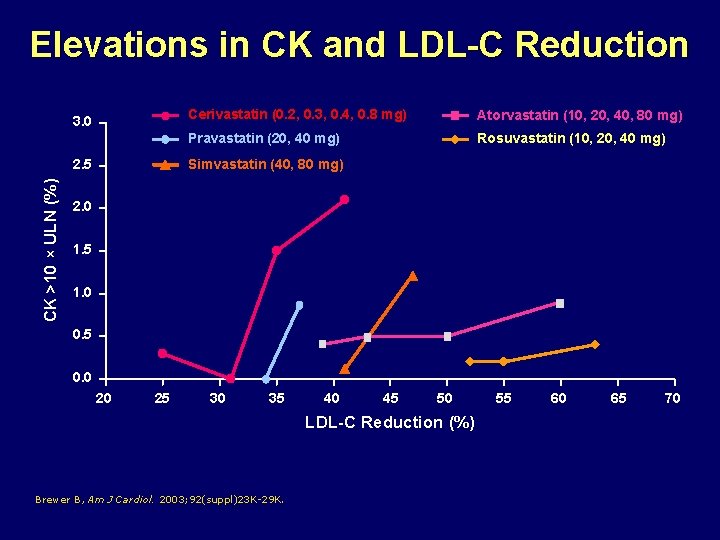
Elevations in CK and LDL-C Reduction 3. 0 CK >10 × ULN (%) 2. 5 Cerivastatin (0. 2, 0. 3, 0. 4, 0. 8 mg) Atorvastatin (10, 20, 40, 80 mg) Pravastatin (20, 40 mg) Rosuvastatin (10, 20, 40 mg) Simvastatin (40, 80 mg) 2. 0 1. 5 1. 0 0. 5 0. 0 20 25 30 35 40 45 50 LDL-C Reduction (%) Brewer B, Am J Cardiol. 2003; 92(suppl)23 K-29 K. 55 60 65 70

Statin-Associated Transaminase Elevations • Progression to liver failure due to statins is exceedingly rare • Reversal of transaminase elevation is frequently noted with a reduction in statin dose – Elevations do not often recur with either readministration or selection of another statin • Statins have not been shown to worsen the outcome in persons with chronic transaminase elevations due to hepatitis B or C • Statin therapy may actually improve transaminase elevations in individuals with fatty livers. Pasternak et al. ACC/AHA/NHLBI Advisory on the Use and Safety of Statins. Circulation. 2002; 106: 1024 -1028.

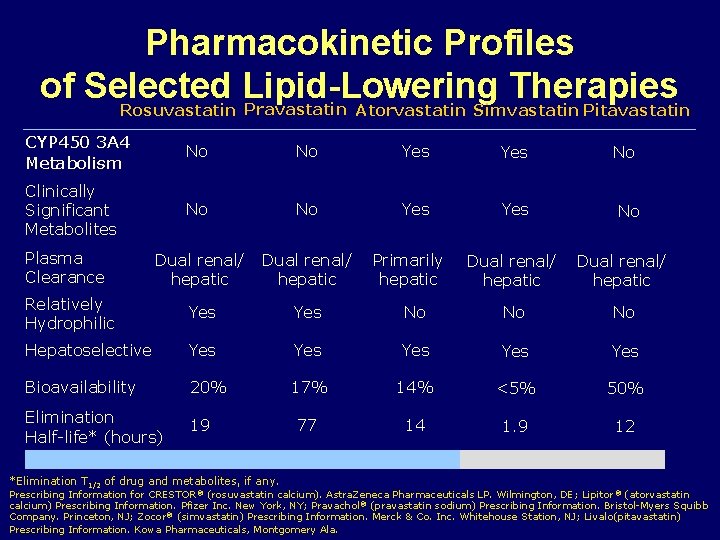
Pharmacokinetic Profiles of Selected Lipid-Lowering Therapies Rosuvastatin Pravastatin Atorvastatin Simvastatin Pitavastatin CYP 450 3 A 4 Metabolism No No Yes Clinically Significant Metabolites No No Yes Dual renal/ hepatic Relatively Hydrophilic Yes Hepatoselective Yes Bioavailability 20% Elimination Half-life* (hours) 19 Plasma Clearance *Elimination T 1/2 of drug and metabolites, if any. Primarily hepatic No No Dual renal/ hepatic No No No Yes Yes 17% 14% <5% 50% 77 14 1. 9 12 Prescribing Information for CRESTOR ® (rosuvastatin calcium). Astra. Zeneca Pharmaceuticals LP. Wilmington, DE; Lipitor ® (atorvastatin calcium) Prescribing Information. Pfizer Inc. New York, NY; Pravachol® (pravastatin sodium) Prescribing Information. Bristol-Myers Squibb Company. Princeton, NJ; Zocor ® (simvastatin) Prescribing Information. Merck & Co. Inc. Whitehouse Station, NJ; Livalo(pitavastatin) Prescribing Information. Kowa Pharmaceuticals, Montgomery Ala.

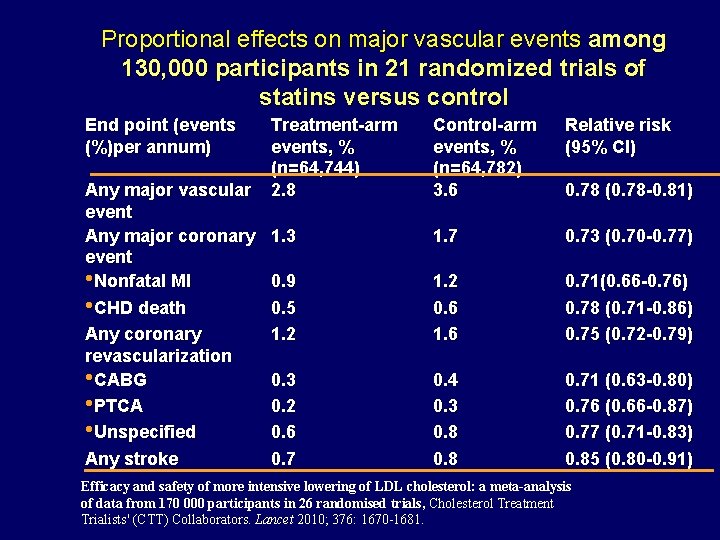
Proportional effects on major vascular events among 130, 000 participants in 21 randomized trials of statins versus control End point (events (%)per annum) Treatment-arm events, % (n=64, 744) Any major vascular 2. 8 event Any major coronary 1. 3 event • Nonfatal MI 0. 9 • CHD death 0. 5 Any coronary 1. 2 revascularization • CABG 0. 3 • PTCA 0. 2 • Unspecified 0. 6 Any stroke 0. 7 Control-arm events, % (n=64, 782) 3. 6 Relative risk (95% CI) 1. 7 0. 73 (0. 70 -0. 77) 1. 2 0. 6 1. 6 0. 71(0. 66 -0. 76) 0. 78 (0. 71 -0. 86) 0. 75 (0. 72 -0. 79) 0. 4 0. 3 0. 8 0. 71 (0. 63 -0. 80) 0. 76 (0. 66 -0. 87) 0. 77 (0. 71 -0. 83) 0. 85 (0. 80 -0. 91) 0. 78 (0. 78 -0. 81) Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials, Cholesterol Treatment Trialists' (CTT) Collaborators. Lancet 2010; 376: 1670 -1681.

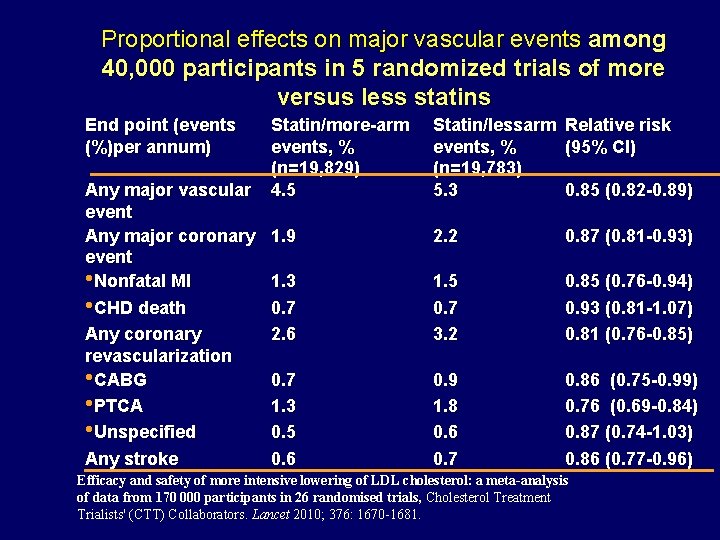
Proportional effects on major vascular events among 40, 000 participants in 5 randomized trials of more versus less statins End point (events (%)per annum) Statin/more-arm events, % (n=19, 829) Any major vascular 4. 5 event Any major coronary 1. 9 event • Nonfatal MI 1. 3 • CHD death 0. 7 Any coronary 2. 6 revascularization • CABG 0. 7 • PTCA 1. 3 • Unspecified 0. 5 Any stroke 0. 6 Statin/lessarm Relative risk events, % (95% CI) (n=19, 783) 5. 3 0. 85 (0. 82 -0. 89) 2. 2 0. 87 (0. 81 -0. 93) 1. 5 0. 7 3. 2 0. 85 (0. 76 -0. 94) 0. 93 (0. 81 -1. 07) 0. 81 (0. 76 -0. 85) 0. 9 1. 8 0. 6 0. 7 0. 86 (0. 75 -0. 99) 0. 76 (0. 69 -0. 84) 0. 87 (0. 74 -1. 03) 0. 86 (0. 77 -0. 96) Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials, Cholesterol Treatment Trialists' (CTT) Collaborators. Lancet 2010; 376: 1670 -1681.

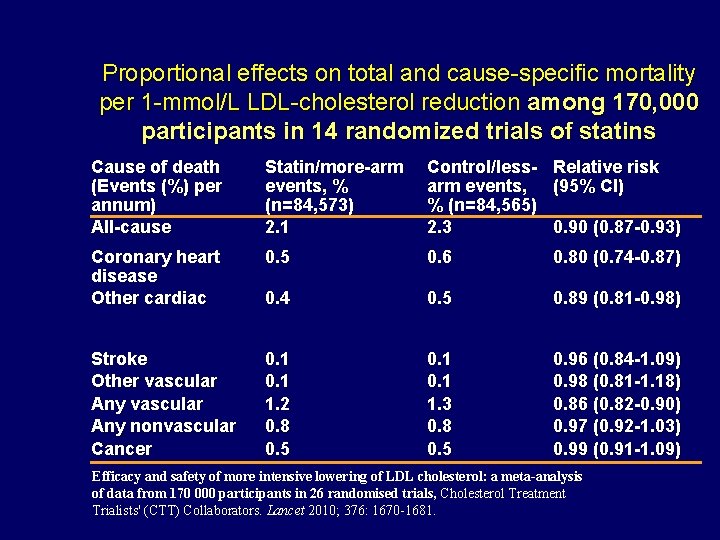
Proportional effects on total and cause-specific mortality per 1 -mmol/L LDL-cholesterol reduction among 170, 000 participants in 14 randomized trials of statins Cause of death (Events (%) per annum) All-cause Statin/more-arm events, % (n=84, 573) 2. 1 Control/lessarm events, % (n=84, 565) 2. 3 Relative risk (95% CI) Coronary heart disease Other cardiac 0. 5 0. 6 0. 80 (0. 74 -0. 87) 0. 4 0. 5 0. 89 (0. 81 -0. 98) Stroke Other vascular Any nonvascular Cancer 0. 1 1. 2 0. 8 0. 5 0. 1 1. 3 0. 8 0. 5 0. 96 (0. 84 -1. 09) 0. 98 (0. 81 -1. 18) 0. 86 (0. 82 -0. 90) 0. 97 (0. 92 -1. 03) 0. 99 (0. 91 -1. 09) 0. 90 (0. 87 -0. 93) Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials, Cholesterol Treatment Trialists' (CTT) Collaborators. Lancet 2010; 376: 1670 -1681.

No significant excess of rhabdomyolysis among 90, 056 participants in 14 randomized trials of statins treated for about 5 years Statin n(%) 9(0. 023) Control n(%) 6(0. 015) 5 year excess risk = 0. 01%(p=0. 4) Cholesterol Treatment Trialists' (CTT) Collaborators. Lancet 2005; 366: 1267 -1278

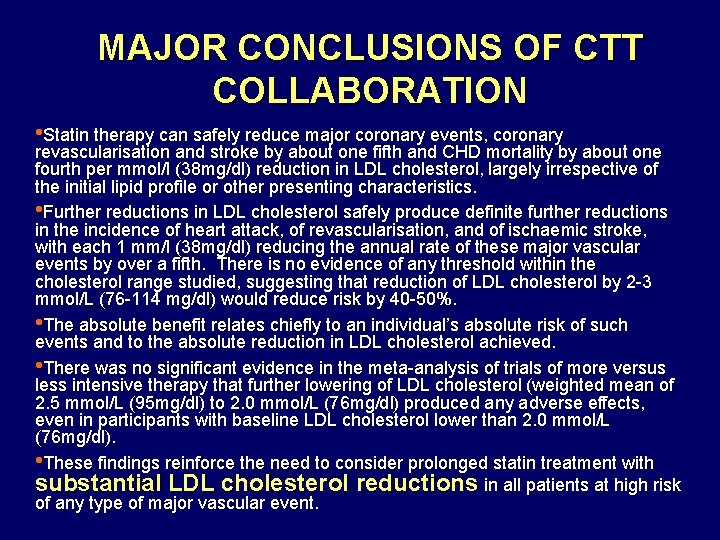
MAJOR CONCLUSIONS OF CTT COLLABORATION • Statin therapy can safely reduce major coronary events, coronary revascularisation and stroke by about one fifth and CHD mortality by about one fourth per mmol/l (38 mg/dl) reduction in LDL cholesterol, largely irrespective of the initial lipid profile or other presenting characteristics. • Further reductions in LDL cholesterol safely produce definite further reductions in the incidence of heart attack, of revascularisation, and of ischaemic stroke, with each 1 mm/l (38 mg/dl) reducing the annual rate of these major vascular events by over a fifth. There is no evidence of any threshold within the cholesterol range studied, suggesting that reduction of LDL cholesterol by 2 -3 mmol/L (76 -114 mg/dl) would reduce risk by 40 -50%. • The absolute benefit relates chiefly to an individual’s absolute risk of such events and to the absolute reduction in LDL cholesterol achieved. • There was no significant evidence in the meta-analysis of trials of more versus less intensive therapy that further lowering of LDL cholesterol (weighted mean of 2. 5 mmol/L (95 mg/dl) to 2. 0 mmol/L (76 mg/dl) produced any adverse effects, even in participants with baseline LDL cholesterol lower than 2. 0 mmol/L (76 mg/dl). • These findings reinforce the need to consider prolonged statin treatment with substantial LDL cholesterol reductions in all patients at high risk of any type of major vascular event.

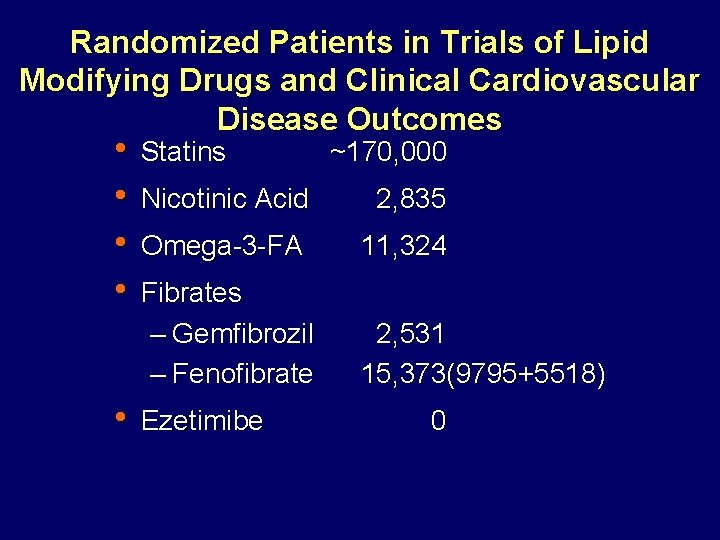
Randomized Patients in Trials of Lipid Modifying Drugs and Clinical Cardiovascular Disease Outcomes • • • Statins ~170, 000 Nicotinic Acid 2, 835 Omega-3 -FA 11, 324 Fibrates – Gemfibrozil – Fenofibrate 2, 531 15, 373(9795+5518) Ezetimibe 0

SHARP: Major conclusions Among 8384 patients with chronic renal disease randomized to simvastatin + ezetimibe or placebo for 4. 9 years there were • No increases in risks of myopathy, liver, biliary disorders, cancer, or nonvascular mortality • • No substantial effects in kidney disease progression • Similar proportional reductions in all subgroups (including among dialysis and non-dialysis patients) • No direct comparisons of whether ezetimibe adds to the benefits of the statin A significant 17% reduction in major atherosclerotic events with 2/3 compliance to simvastatin +ezetimibe

ETHICAL CONSIDERATIONS Ethical considerations surrounding statins include whether To treat or not to treat Evidence based doses of a statin should be the first choice of drug therapy for virtually all patients who require pharmacologic therapy for the management of their lipids as an adjunct to therapeutic lifestyle changes


French Fries 20 years ago 210 calories 2. 4 ounces Today 610 calories How many calories are 6. 9 ounces in these fries? Calorie difference: 400 Calories How to burn* 400 calories: Walk 2 hour 20 minutes *Based on 130 -pound person.

Darwinism and Risk of Cardiovascular Disease

Walking the Dog

A bit of cultural news …. . After a 2 year loan to the United States, David returns to Italy

…Proud Sponsors

Trends Among US Adolescents Smoking (22%-30%) Obesity Physical Inactivity Type 2 Diabetes Hennekens CH. Circulation. 1998; 97: 1095.

The United States is the fattest society in the world and likely to be the fattest in the history of the world. Professor Charles H. Hennekens New York Times January 1, 1999

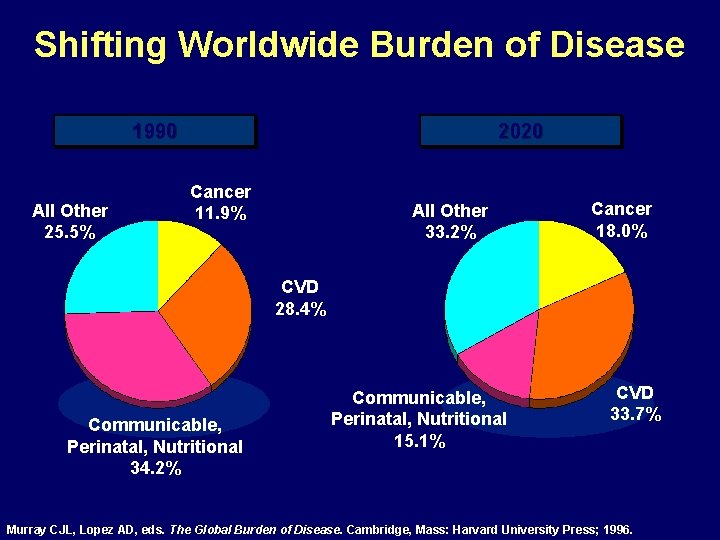
Shifting Worldwide Burden of Disease 1990 All Other 25. 5% 2020 Cancer 11. 9% All Other 33. 2% Cancer 18. 0% CVD 28. 4% Communicable, Perinatal, Nutritional 34. 2% Communicable, Perinatal, Nutritional 15. 1% CVD 33. 7% Murray CJL, Lopez AD, eds. The Global Burden of Disease. Cambridge, Mass: Harvard University Press; 1996.

Reasons for Worldwide Increase in Cardiovascular Disease Malnutrition Infection Smoking BMI Hennekens CH. Circulation. 1998; 97: 1095 -1102.

2005: Beijing, China vs US Beijing US Cigarette smoking in men 58% Same as 1950 Body Mass Index Weight in Kg/ Height in m 2 24. 8 Same as 1990

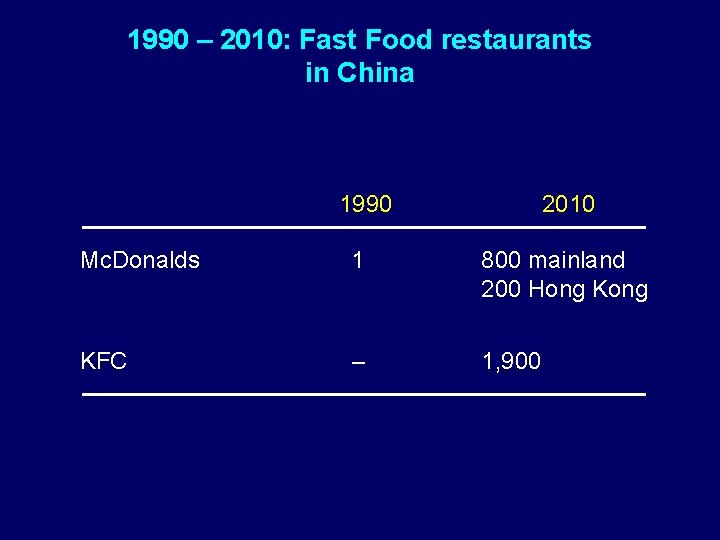
1990 – 2010: Fast Food restaurants in China 1990 2010 Mc. Donalds 1 800 mainland 200 Hong KFC – 1, 900

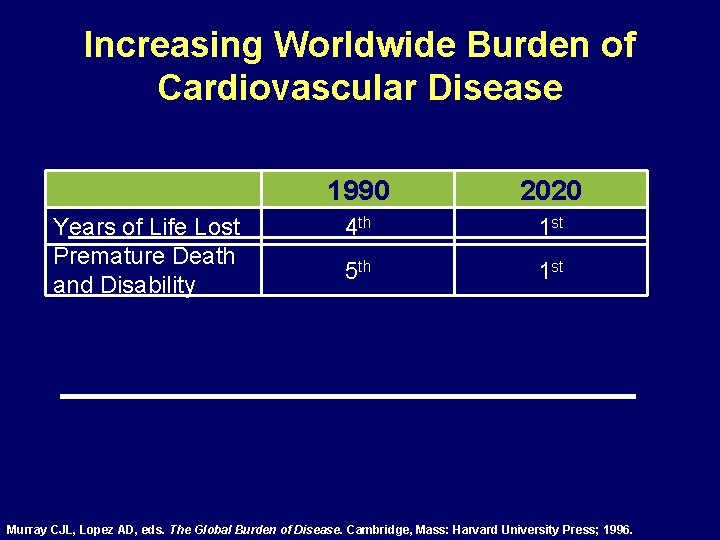
Increasing Worldwide Burden of Cardiovascular Disease Years of Life Lost Premature Death and Disability 1990 2020 4 th 1 st 5 th 1 st Murray CJL, Lopez AD, eds. The Global Burden of Disease. Cambridge, Mass: Harvard University Press; 1996.

PUBLIC HEALTH CONSIDERATIONS • Based on these considerations, the World Health Organization has projected that, within the next decade, cardiovascular disease will become the leading cause of death and disability in the world

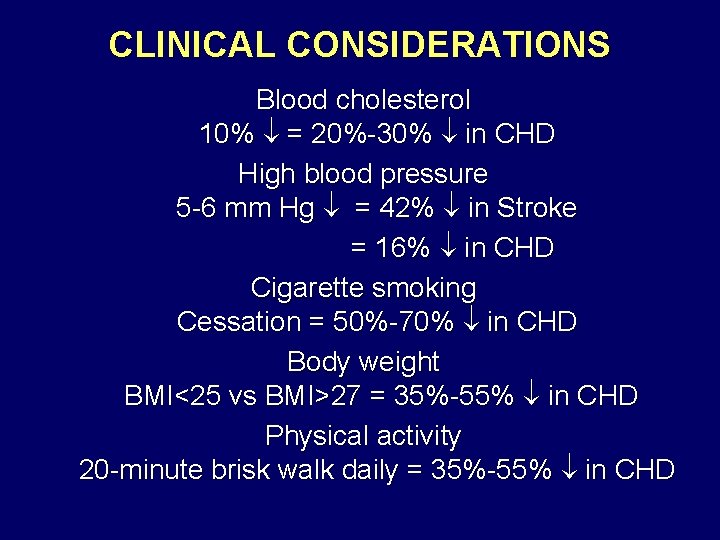
CLINICAL CONSIDERATIONS Blood cholesterol 10% = 20%-30% in CHD High blood pressure 5 -6 mm Hg = 42% in Stroke = 16% in CHD Cigarette smoking Cessation = 50%-70% in CHD Body weight BMI<25 vs BMI>27 = 35%-55% in CHD Physical activity 20 -minute brisk walk daily = 35%-55% in CHD


GOALS OF HEALTH CARE PROVIDERS AND ACADEMIC RESEARCHERS Health Care Providers: Make clinical decisions based on the totality of evidence not dependence on particular subgroups of particular studies. Academic Researchers: Avoid misstatements of benefit to risk ratios which may increase publicity, promotions and grant support in the short run but confuse colleagues and frighten patients and make it more difficult to conduct high quality research ( COX-2 inhibitors and glitazones) Health Care Providers and Academic Researchers Maximize benefit and minimize risk which is not to be confused with avoidance of risk.

When the totality of evidence is incomplete it is appropriate to remain uncertain. Hennekens CH, De. Mets D: The need for large scale randomized evidence without undue emphasis on small trials, their meta-analyses or subgroup analyses JAMA 2009; 302: 2361 -2362.

Don’t let the perfect be the enemy of the possible

“We must all hang together or assuredly we shall hang separately. ” Benjamin Franklin July 4, 1776

 Primary prevention secondary prevention tertiary prevention
Primary prevention secondary prevention tertiary prevention Lipid digestion and absorption
Lipid digestion and absorption Stately motility of clostridium
Stately motility of clostridium Interception interruption modification and fabrication
Interception interruption modification and fabrication Pre and post modification
Pre and post modification Modified roots
Modified roots Pros and cons of genetic engineering
Pros and cons of genetic engineering Pros and cons of biotechnology
Pros and cons of biotechnology øa section through a leaf
øa section through a leaf Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Chụp tư thế worms-breton
Chụp tư thế worms-breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Các môn thể thao bắt đầu bằng tiếng đua
Các môn thể thao bắt đầu bằng tiếng đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Phép trừ bù
Phép trừ bù độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thứ tự các dấu thăng giáng ở hóa biểu
Thứ tự các dấu thăng giáng ở hóa biểu Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Số nguyên tố là
Số nguyên tố là Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Steroid hormones include
Steroid hormones include Glycogen vs starch
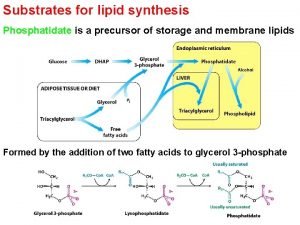
Glycogen vs starch Triacylglycerol synthesis
Triacylglycerol synthesis Qualitative test for lipids objective
Qualitative test for lipids objective Are lipids nonpolar
Are lipids nonpolar Fats, oils, and waxes
Fats, oils, and waxes Element found in lipids
Element found in lipids Apa itu tbhq
Apa itu tbhq Clever and complicated
Clever and complicated Jenis lipid
Jenis lipid Lipid rancidity
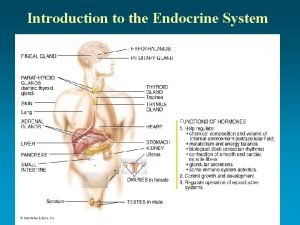
Lipid rancidity Lipid soluble hormones examples
Lipid soluble hormones examples Contoh peta konsep glikolisis
Contoh peta konsep glikolisis Lipid nanoparticles
Lipid nanoparticles Lipid soluble hormones examples
Lipid soluble hormones examples Lipid soluble hormones examples
Lipid soluble hormones examples Alpha lipid colostrum new zealand
Alpha lipid colostrum new zealand Classify each as a carbohydrate, protein, or lipid.
Classify each as a carbohydrate, protein, or lipid. Classify each as a carbohydrate protein or lipid
Classify each as a carbohydrate protein or lipid What are the lipid soluble hormones
What are the lipid soluble hormones Storage lipid structure
Storage lipid structure Lipid digestion
Lipid digestion Lipid emulsion
Lipid emulsion Lipid monomer
Lipid monomer Nitrogen-containing base
Nitrogen-containing base Is hagfish slime a lipid
Is hagfish slime a lipid Lipid blast
Lipid blast Definition of lipids
Definition of lipids Aterial
Aterial 3 funtions of lipids
3 funtions of lipids Lipofuskin
Lipofuskin Apa itu lipid
Apa itu lipid Insoluble def
Insoluble def