Lipid Management in Stroke Statin and Other Lipid







- Slides: 44

Lipid Management in Stroke : Statin and Other Lipid Modifying Agents Professor Pierre Amarenco INSERM U-698 and Paris-Diderot University Department of Neurology and Stroke Centre Bichat-Claude Bernard Hospital, Paris, France

INTERSTROKE: Population-attributable risk for common risk factors Risk factor Population-attributable risk, % (99% CI) Hypertension Smoking Waist-to-hip ratio (tertile 2 vs tertile 1) Dietary risk score (tertile 2 vs tertile 1) Regular physical activity Diabetes Alcohol intake Cardiac causes Ratio of apolipoprotein B to A 1 (tertile 2 vs tertile 1) Psychological factors • Stress • Depression 34. 6 (30. 4– 39. 1) 18. 9 (15. 3– 23. 1) 26. 5 (18. 8– 36. 0) 18. 8 (11. 2– 29. 7) 28. 5 (14. 5– 48. 5) 5. 0 (2. 6– 9. 5) 3. 8 (0. 9– 14. 4) 6. 7 (4. 8– 9. 1) 24. 9 (15. 7– 37. 1) 4. 6 (2. 1– 9. 6) 5. 2 (2. 7– 9. 8) *For the protective factor of physical activity, the population-attributable risks are provided for individuals who do not participate in regular physical activity. O'Donnell MJ et al. Lancet 2010; available at: http: //www. thelancet. com.

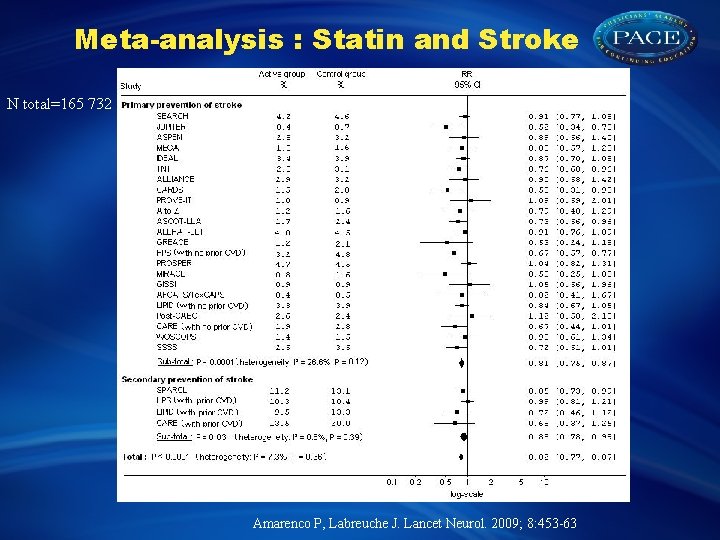
Meta-analysis : Statin and Stroke N total=165 732 Amarenco P, Labreuche J. Lancet Neurol. 2009; 8: 453 -63

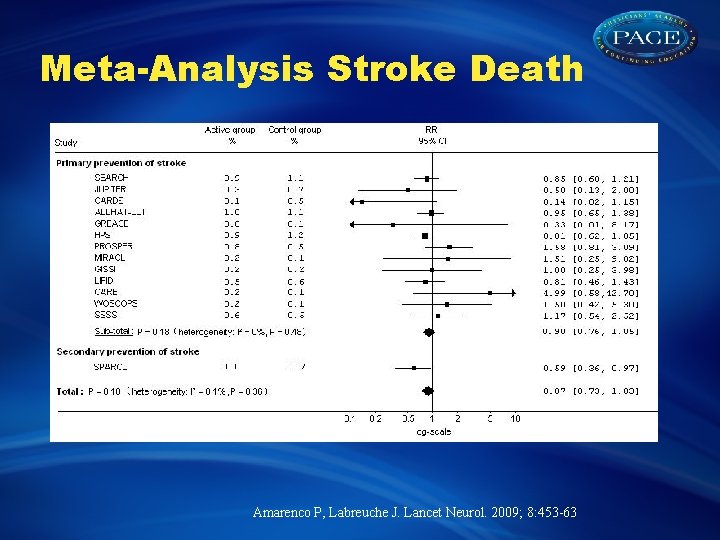
Meta-Analysis Stroke Death Amarenco P, Labreuche J. Lancet Neurol. 2009; 8: 453 -63

Meta-analysis Hemorrhagic stroke Amarenco P, Labreuche J. Lancet Neurol. 2009; 8: 453 -63

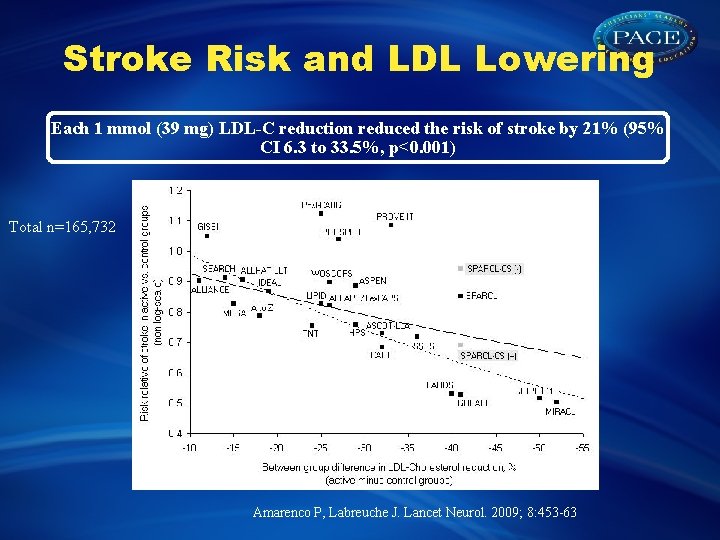
Stroke Risk and LDL Lowering Each 1 mmol (39 mg) LDL-C reduction reduced the risk of stroke by 21% (95% CI 6. 3 to 33. 5%, p<0. 001) Total n=165, 732 Amarenco P, Labreuche J. Lancet Neurol. 2009; 8: 453 -63

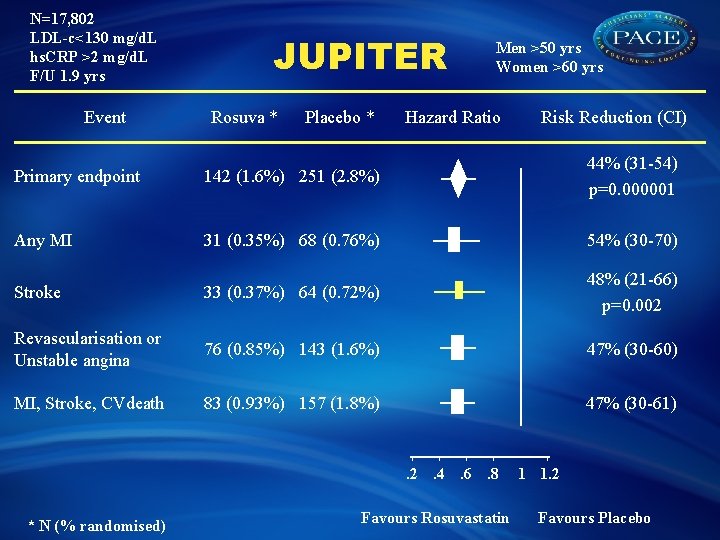
N=17, 802 LDL-c<130 mg/d. L hs. CRP >2 mg/d. L F/U 1. 9 yrs Event JUPITER Rosuva * Placebo * Men >50 yrs Women >60 yrs Hazard Ratio Risk Reduction (CI) Primary endpoint 142 (1. 6%) 251 (2. 8%) 44% (31 -54) p=0. 000001 Any MI 31 (0. 35%) 68 (0. 76%) 54% (30 -70) Stroke 33 (0. 37%) 64 (0. 72%) 48% (21 -66) p=0. 002 Revascularisation or Unstable angina 76 (0. 85%) 143 (1. 6%) 47% (30 -60) MI, Stroke, CVdeath 83 (0. 93%) 157 (1. 8%) 47% (30 -61) . 2. 4. 6. 8 * N (% randomised) Favours Rosuvastatin 1 1. 2 Favours Placebo

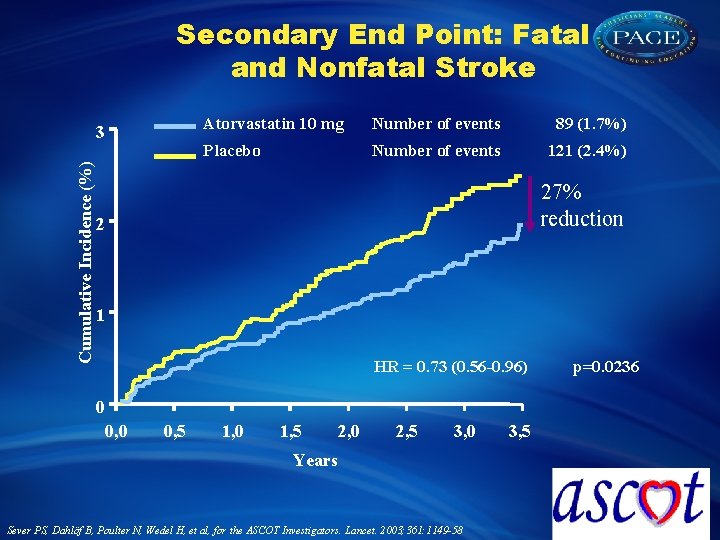
Secondary End Point: Fatal and Nonfatal Stroke Cumulative Incidence (%) 3 Atorvastatin 10 mg Number of events 89 (1. 7%) Placebo Number of events 121 (2. 4%) 27% reduction 2 1 HR = 0. 73 (0. 56 -0. 96) 0 0, 5 1, 0 1, 5 2, 0 2, 5 3, 0 Years Sever PS, Dahlöf B, Poulter N, Wedel H, et al, for the ASCOT Investigators. Lancet. 2003; 361: 1149 -58 3, 5 p=0. 0236

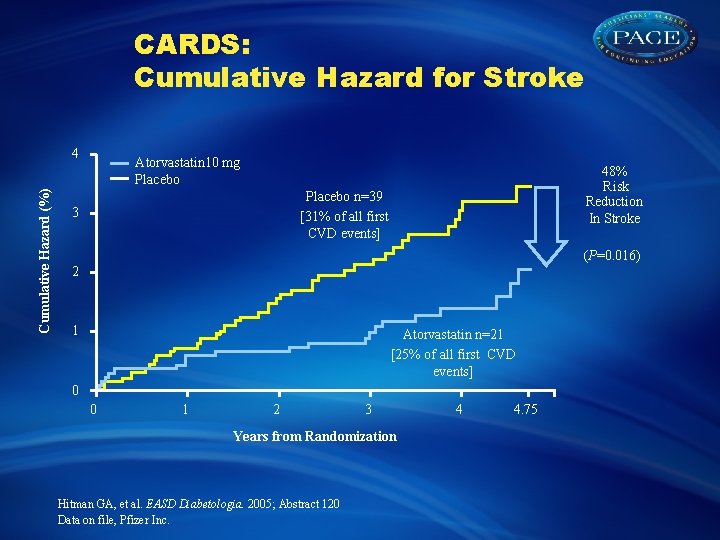
CARDS: Cumulative Hazard for Stroke Cumulative Hazard (%) 4 Atorvastatin 10 mg Placebo 48% Risk Reduction In Stroke Placebo n=39 [31% of all first CVD events] 3 (P=0. 016) 2 1 Atorvastatin n=21 [25% of all first CVD events] 0 0 1 2 3 Years from Randomization Hitman GA, et al. EASD Diabetologia. 2005; Abstract 120 Data on file, Pfizer Inc. 4 4. 75

Pleiotropic Effects Studied Parameter Within the Plaque Control Group n=13 Pravastatin Group n=11 P Value 23. 9% 8. 2% <0. 001 22% 13. 3% <0. 001 Macrophage contain 25. 3% 15. 3% <0. 05 T-Cell count 23. 4% 11. 2% <0. 05 SMC 16. 9% 24. 3% <0. 05 32% 17. 7% <0. 05 Lipid contain (Oil Red O) Ox-LDL (NA 59) Apoptotic Cells (TUNEL) Crisby et al. Circulation 2001

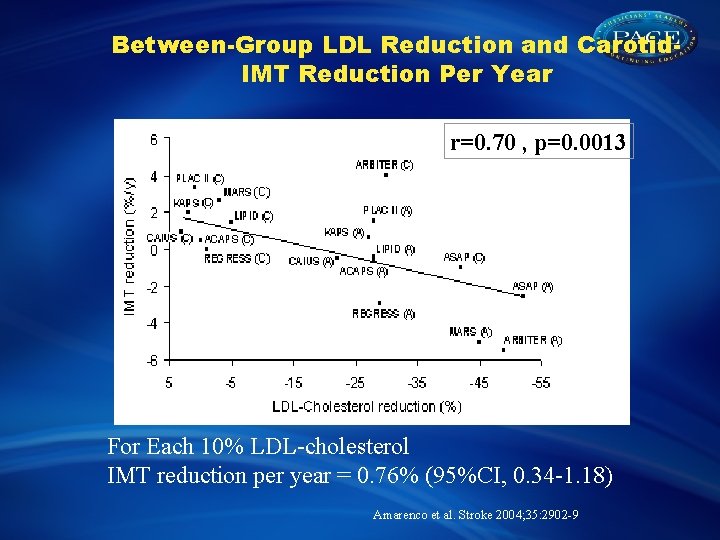
Between-Group LDL Reduction and Carotid. IMT Reduction Per Year r=0. 70 , p=0. 0013 For Each 10% LDL-cholesterol IMT reduction per year = 0. 76% (95%CI, 0. 34 -1. 18) Amarenco et al. Stroke 2004; 35: 2902 -9

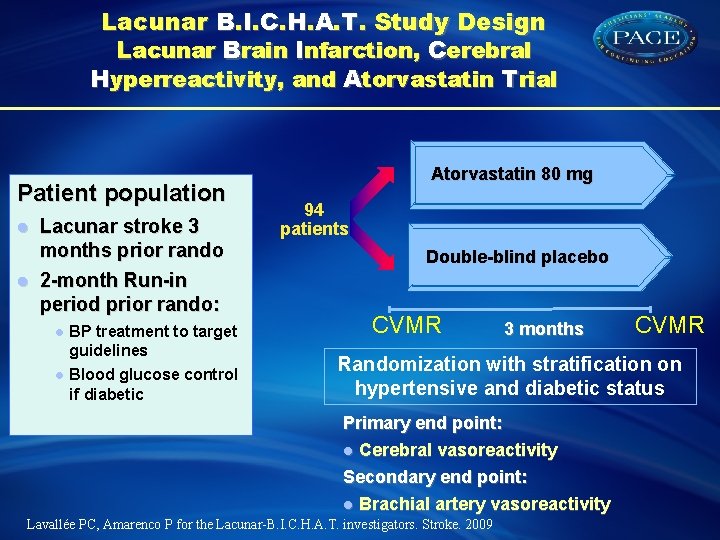
Lacunar B. I. C. H. A. T. Study Design Lacunar Brain Infarction, Cerebral Hyperreactivity, and Atorvastatin Trial Patient population l l Lacunar stroke 3 months prior rando 2 -month Run-in period prior rando: l l BP treatment to target guidelines Blood glucose control if diabetic Atorvastatin 80 mg 94 patients Double-blind placebo CVMR 3 months CVMR Randomization with stratification on hypertensive and diabetic status Primary end point: l Cerebral vasoreactivity Secondary end point: l Brachial artery vasoreactivity Lavallée PC, Amarenco P for the Lacunar-B. I. C. H. A. T. investigators. Stroke. 2009

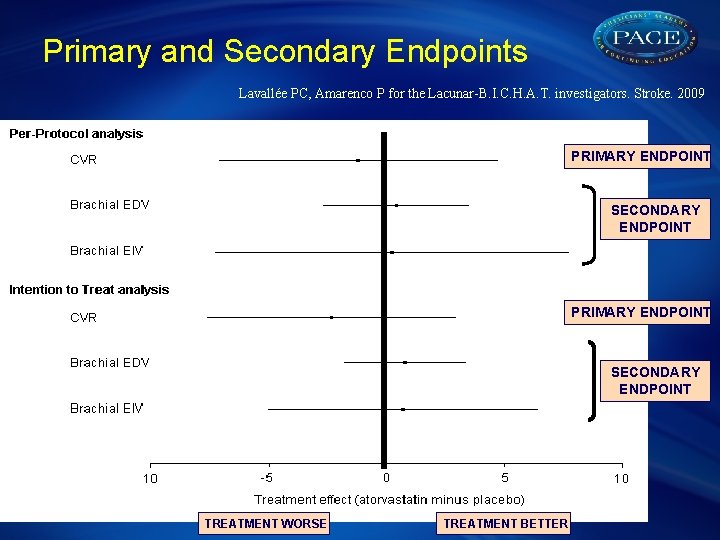
Primary and Secondary Endpoints Lavallée PC, Amarenco P for the Lacunar-B. I. C. H. A. T. investigators. Stroke. 2009 PRIMARY ENDPOINT SECONDARY ENDPOINT TREATMENT WORSE TREATMENT BETTER

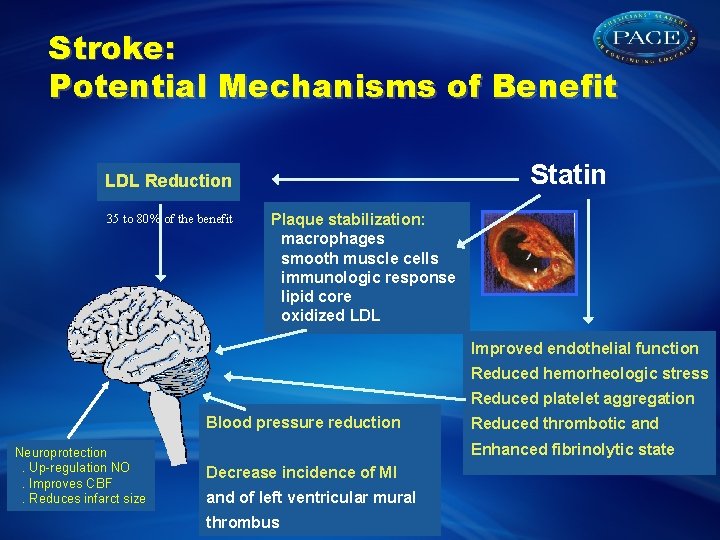
Stroke: Potential Mechanisms of Benefit Statin LDL Reduction 35 to 80% of the benefit Plaque stabilization: macrophages smooth muscle cells immunologic response lipid core oxidized LDL Improved endothelial function Reduced hemorheologic stress Reduced platelet aggregation Blood pressure reduction Neuroprotection. Up-regulation NO. Improves CBF. Reduces infarct size Reduced thrombotic and Enhanced fibrinolytic state Decrease incidence of MI and of left ventricular mural thrombus

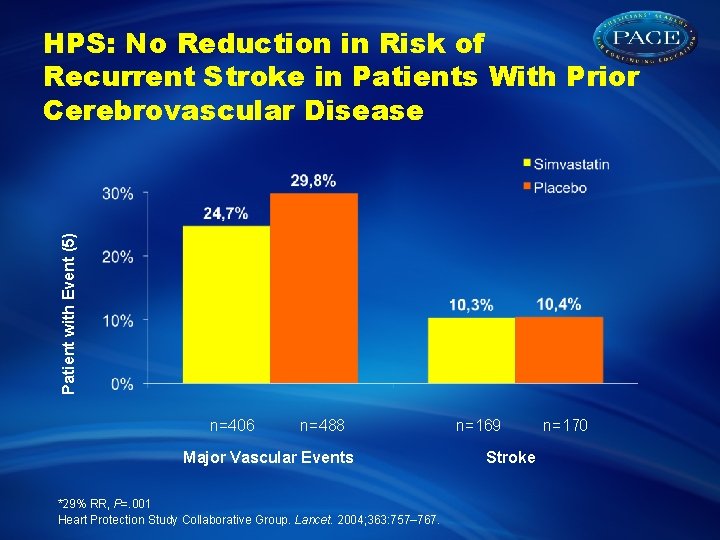
Patient with Event (5) HPS: No Reduction in Risk of Recurrent Stroke in Patients With Prior Cerebrovascular Disease n=406 n=488 Major Vascular Events *29% RR, P=. 001 Heart Protection Study Collaborative Group. Lancet. 2004; 363: 757– 767. n=169 Stroke n=170

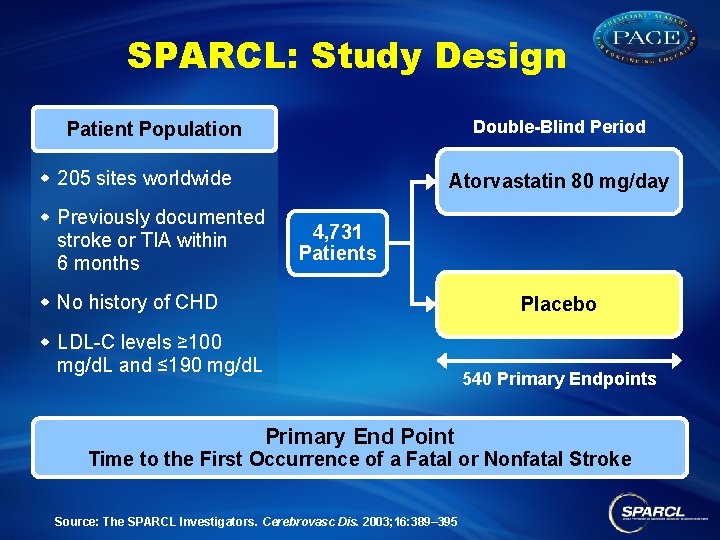
SPARCL: Study Design Double-Blind Period Patient Population w 205 sites worldwide Atorvastatin 80 mg/day w Previously documented stroke or TIA within 6 months 4, 731 Patients w No history of CHD Placebo w LDL-C levels ≥ 100 mg/d. L and ≤ 190 mg/d. L 540 Primary Endpoints Primary End Point Time to the First Occurrence of a Fatal or Nonfatal Stroke Source: The SPARCL Investigators. Cerebrovasc Dis. 2003; 16: 389– 395

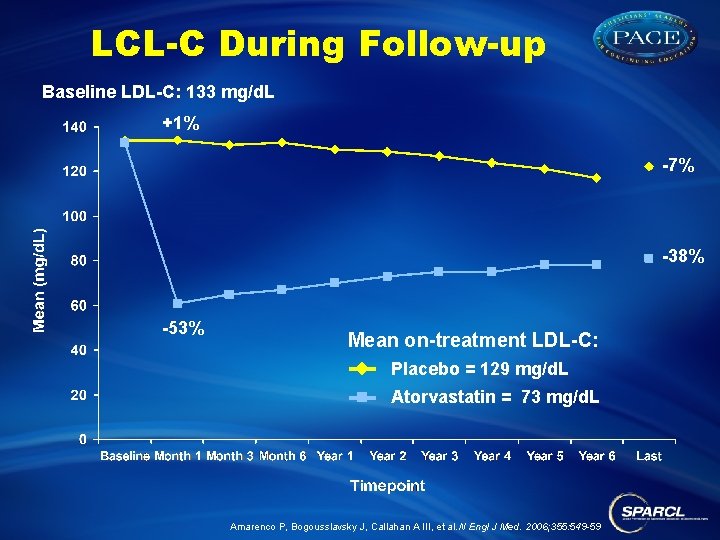
LCL-C During Follow-up Baseline LDL-C: 133 mg/d. L +1% -7% -38% -53% Mean on-treatment LDL-C: Placebo = 129 mg/d. L Atorvastatin = 73 mg/d. L Amarenco P, Bogousslavsky J, Callahan A III, et al. N Engl J Med. 2006; 355: 549 -59

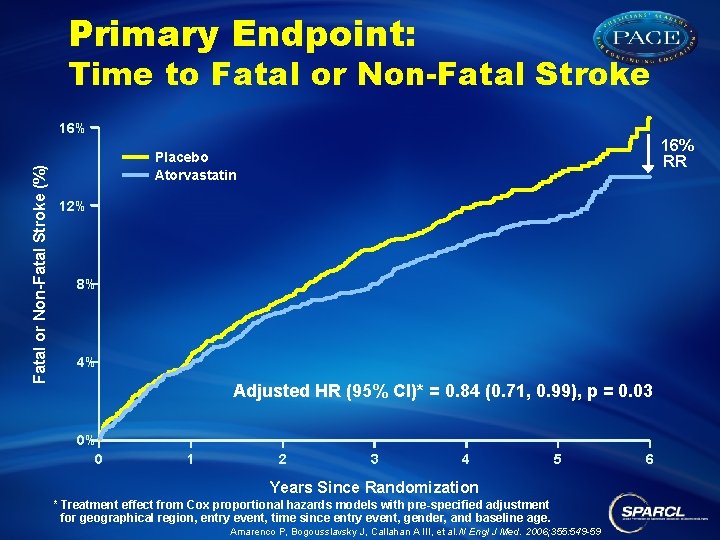
Primary Endpoint: Time to Fatal or Non-Fatal Stroke (%) 16% RR Placebo Atorvastatin 12% 8% 4% Adjusted HR (95% CI)* = 0. 84 (0. 71, 0. 99), p = 0. 03 0% 0 1 2 3 4 5 Years Since Randomization * Treatment effect from Cox proportional hazards models with pre-specified adjustment for geographical region, entry event, time since entry event, gender, and baseline age. Amarenco P, Bogousslavsky J, Callahan A III, et al. N Engl J Med. 2006; 355: 549 -59 6

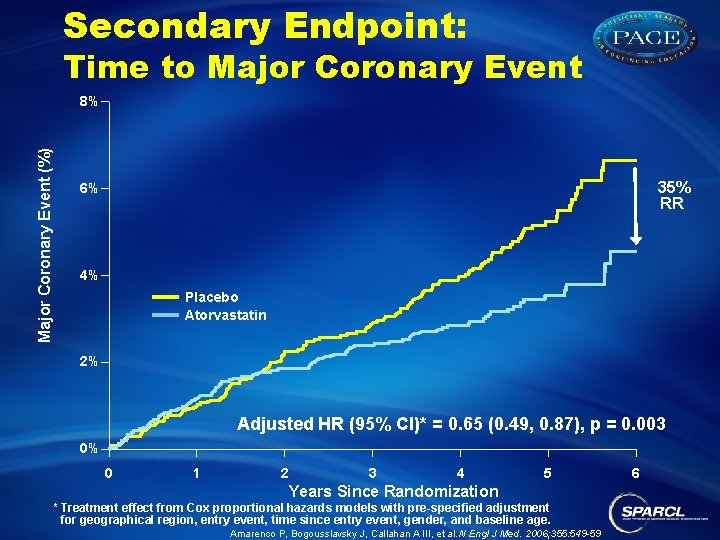
Secondary Endpoint: Time to Major Coronary Event (%) 8% 35% RR 6% 4% Placebo Atorvastatin 2% Adjusted HR (95% CI)* = 0. 65 (0. 49, 0. 87), p = 0. 003 0% 0 1 2 3 4 5 Years Since Randomization * Treatment effect from Cox proportional hazards models with pre-specified adjustment for geographical region, entry event, time since entry event, gender, and baseline age. Amarenco P, Bogousslavsky J, Callahan A III, et al. N Engl J Med. 2006; 355: 549 -59 6

Gender: Stroke Outcomes Pre-specified adjustment for region, entry event, time since entry event and age Goldstein LB, Amarenco P, Callahan A III, al. . Stroke. 2008; 39: 2444 -48

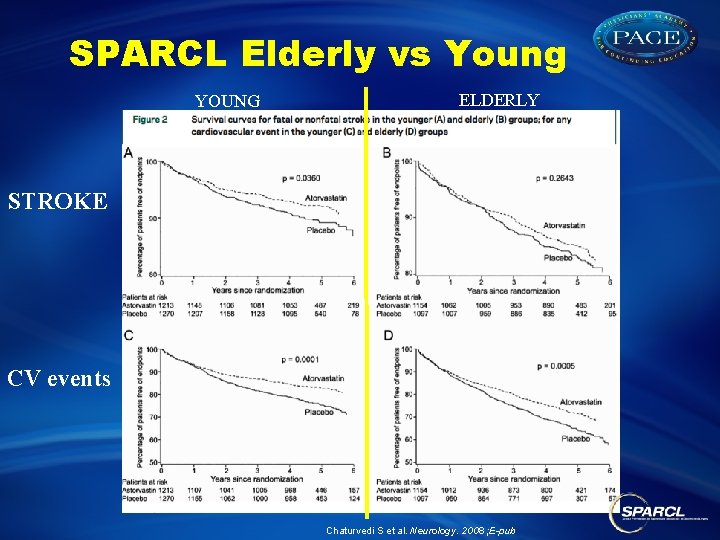
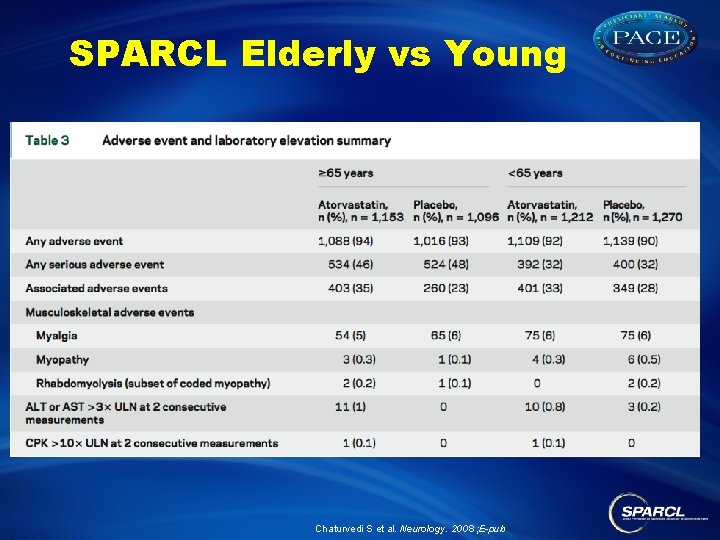
SPARCL Elderly vs Young YOUNG ELDERLY STROKE CV events Chaturvedi S et al. Neurology. 2008 ; E-pub

SPARCL Elderly vs Young Chaturvedi S et al. Neurology. 2008 ; E-pub

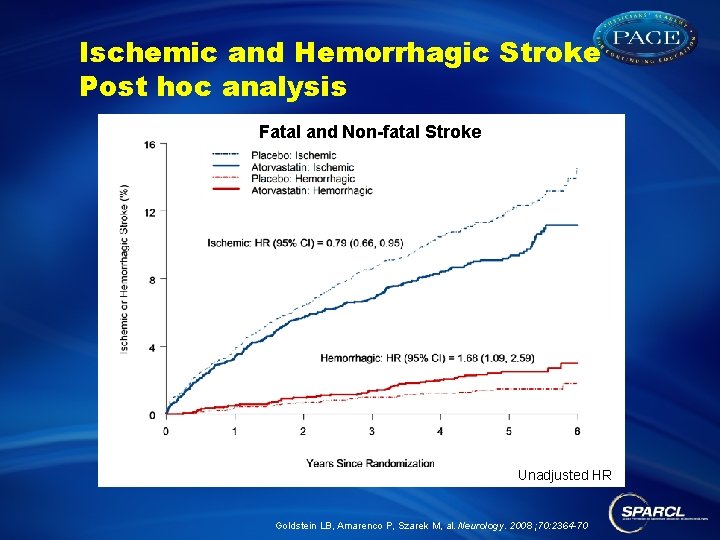
Ischemic and Hemorrhagic Stroke Post hoc analysis Fatal and Non-fatal Stroke Unadjusted HR Goldstein LB, Amarenco P, Szarek M, al. Neurology. 2008 ; 70: 2364 -70

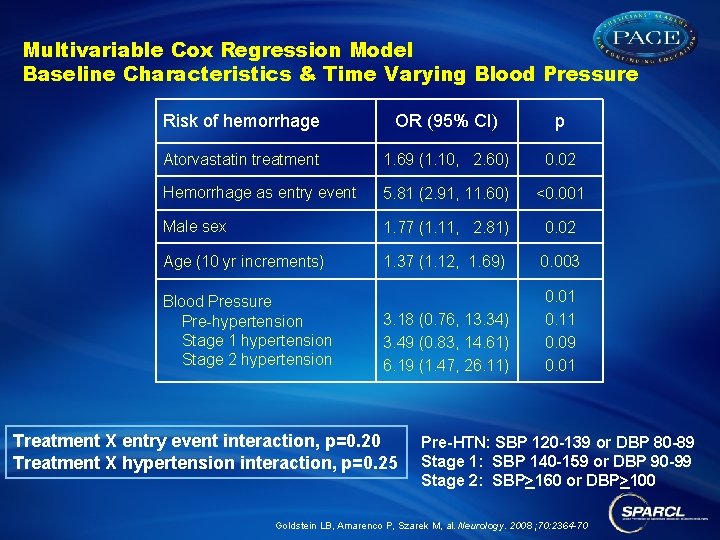
Multivariable Cox Regression Model Baseline Characteristics & Time Varying Blood Pressure Risk of hemorrhage OR (95% CI) p Atorvastatin treatment 1. 69 (1. 10, 2. 60) 0. 02 Hemorrhage as entry event 5. 81 (2. 91, 11. 60) <0. 001 Male sex 1. 77 (1. 11, 2. 81) 0. 02 Age (10 yr increments) 1. 37 (1. 12, 1. 69) 0. 003 3. 18 (0. 76, 13. 34) 3. 49 (0. 83, 14. 61) 6. 19 (1. 47, 26. 11) 0. 01 0. 11 0. 09 0. 01 Blood Pressure Pre-hypertension Stage 1 hypertension Stage 2 hypertension Treatment X entry event interaction, p=0. 20 Treatment X hypertension interaction, p=0. 25 Pre-HTN: SBP 120 -139 or DBP 80 -89 Stage 1: SBP 140 -159 or DBP 90 -99 Stage 2: SBP>160 or DBP>100 Goldstein LB, Amarenco P, Szarek M, al. Neurology. 2008 ; 70: 2364 -70

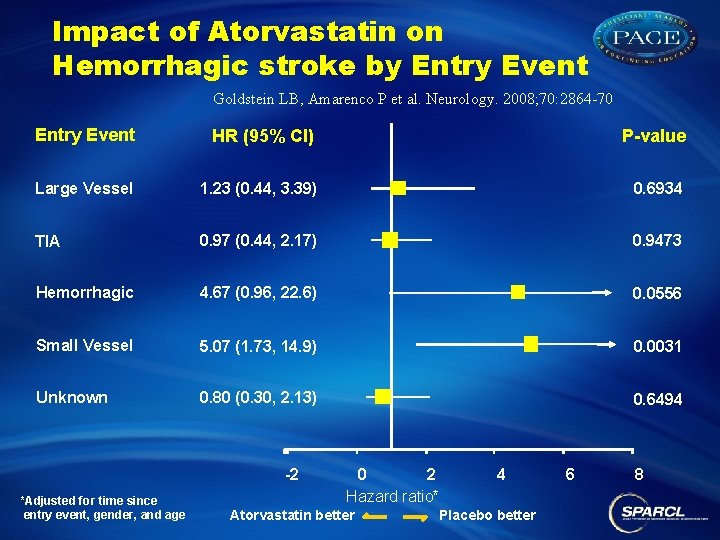
Impact of Atorvastatin on Hemorrhagic stroke by Entry Event Goldstein LB, Amarenco P et al. Neurology. 2008; 70: 2864 -70 Entry Event HR (95% CI) P-value Large Vessel 1. 23 (0. 44, 3. 39) 0. 6934 TIA 0. 97 (0. 44, 2. 17) 0. 9473 Hemorrhagic 4. 67 (0. 96, 22. 6) 0. 0556 Small Vessel 5. 07 (1. 73, 14. 9) 0. 0031 Unknown 0. 80 (0. 30, 2. 13) 0. 6494 -2 *Adjusted for time since entry event, gender, and age 0 2 4 Hazard ratio* Atorvastatin better Placebo better 6 8

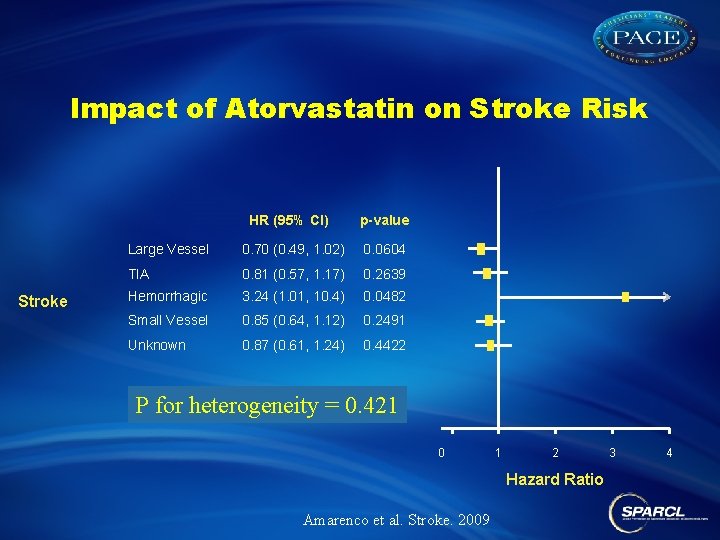
Impact of Atorvastatin on Stroke Risk HR (95% CI) Stroke p-value Large Vessel 0. 70 (0. 49, 1. 02) 0. 0604 TIA 0. 81 (0. 57, 1. 17) 0. 2639 Hemorrhagic 3. 24 (1. 01, 10. 4) 0. 0482 Small Vessel 0. 85 (0. 64, 1. 12) 0. 2491 Unknown 0. 87 (0. 61, 1. 24) 0. 4422 P for heterogeneity = 0. 421 0 1 2 Hazard Ratio Amarenco et al. Stroke. 2009 3 4

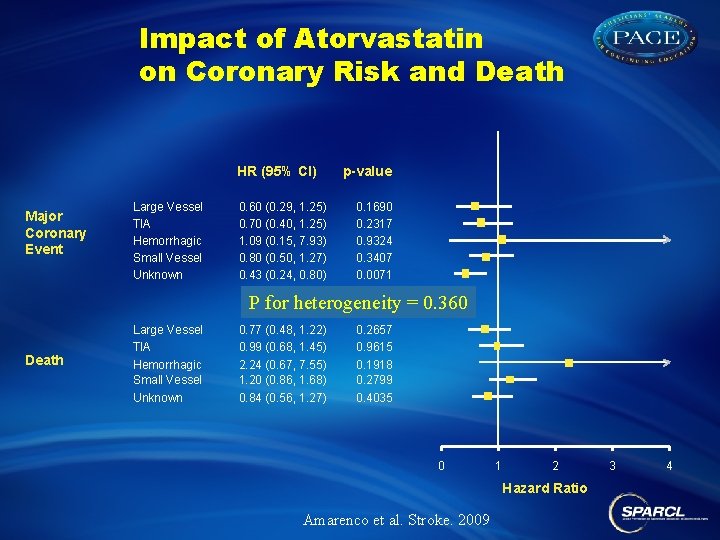
Impact of Atorvastatin on Coronary Risk and Death HR (95% CI) Major Coronary Event Large Vessel TIA Hemorrhagic Small Vessel Unknown 0. 60 (0. 29, 1. 25) 0. 70 (0. 40, 1. 25) 1. 09 (0. 15, 7. 93) 0. 80 (0. 50, 1. 27) 0. 43 (0. 24, 0. 80) p-value 0. 1690 0. 2317 0. 9324 0. 3407 0. 0071 P for heterogeneity = 0. 360 Death Large Vessel TIA Hemorrhagic Small Vessel Unknown 0. 77 (0. 48, 1. 22) 0. 99 (0. 68, 1. 45) 2. 24 (0. 67, 7. 55) 1. 20 (0. 86, 1. 68) 0. 84 (0. 56, 1. 27) 0. 2657 0. 9615 0. 1918 0. 2799 0. 4035 0 1 2 Hazard Ratio Amarenco et al. Stroke. 2009 3 4

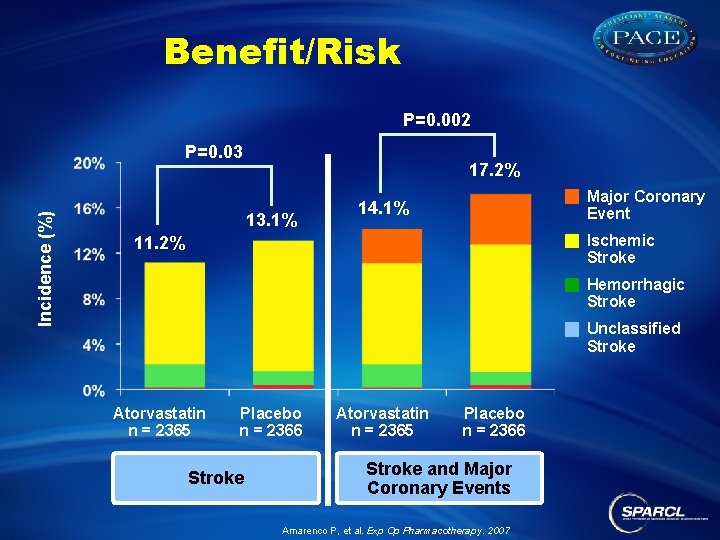
Benefit/Risk P=0. 002 Incidence (%) P=0. 03 17. 2% 13. 1% Major Coronary Event 14. 1% Ischemic Stroke 11. 2% Hemorrhagic Stroke Unclassified Stroke Atorvastatin n = 2365 Placebo n = 2366 Stroke and Major Coronary Events Amarenco P, et al. Exp Op Pharmacotherapy. 2007

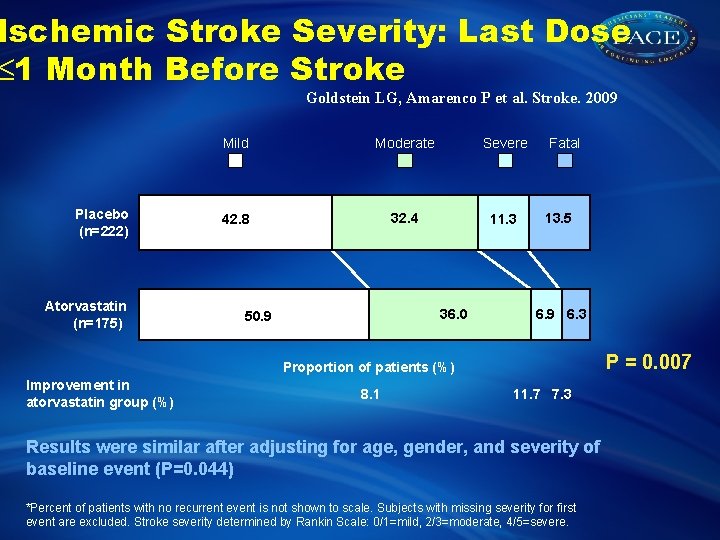
Ischemic Stroke Severity: Last Dose 1 Month Before Stroke Goldstein LG, Amarenco P et al. Stroke. 2009 Placebo (n=222) Atorvastatin (n=175) Mild Moderate Severe 42. 8 32. 4 11. 3 36. 0 50. 9 Fatal 13. 5 6. 9 6. 3 P = 0. 007 Proportion of patients (%) Improvement in atorvastatin group (%) 8. 1 11. 7 7. 3 Results were similar after adjusting for age, gender, and severity of baseline event (P=0. 044) *Percent of patients with no recurrent event is not shown to scale. Subjects with missing severity for first event are excluded. Stroke severity determined by Rankin Scale: 0/1=mild, 2/3=moderate, 4/5=severe.

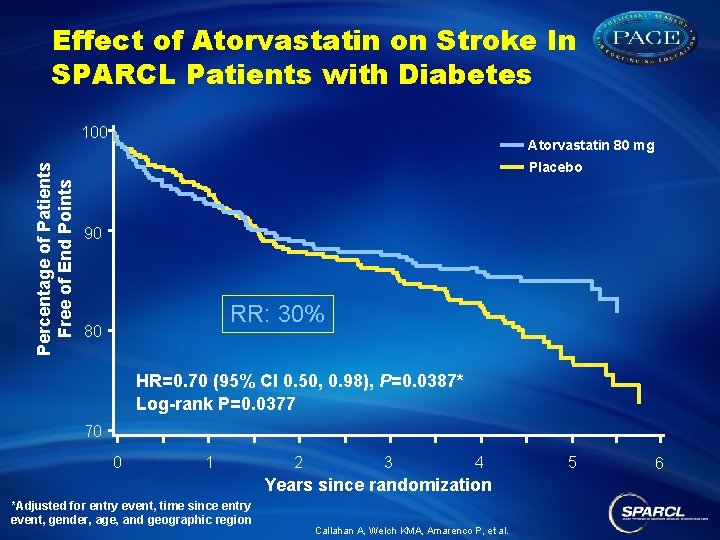
Effect of Atorvastatin on Stroke In SPARCL Patients with Diabetes Percentage of Patients Free of End Points 100 Atorvastatin 80 mg Placebo 90 RR: 30% 80 HR=0. 70 (95% CI 0. 50, 0. 98), P=0. 0387* Log-rank P=0. 0377 70 0 1 2 3 4 Years since randomization *Adjusted for entry event, time since entry event, gender, age, and geographic region Callahan A, Welch KMA, Amarenco P, et al. 5 6

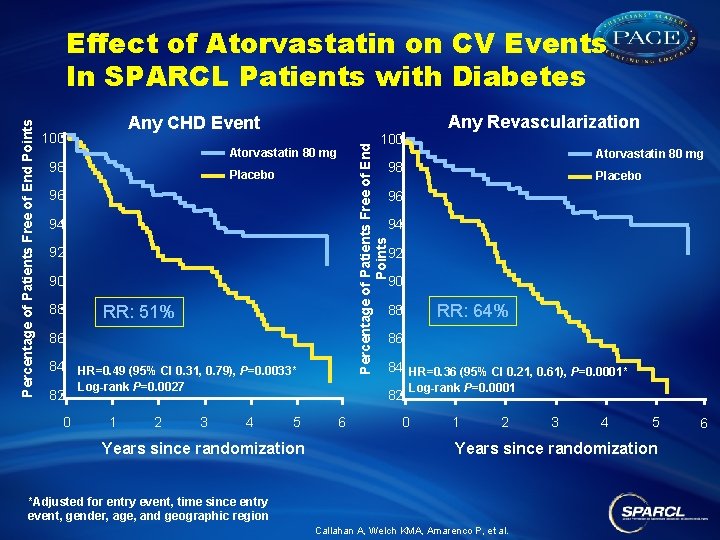
Any CHD Event 100 Atorvastatin 80 mg 98 94 92 92 90 90 RR: 51% RR: 64% 88 86 86 84 HR=0. 49 (95% CI 0. 31, 0. 79), P=0. 0033* 0 Placebo 96 94 82 Atorvastatin 80 mg 98 Placebo 96 88 Any Revascularization 100 Percentage of Patients Free of End Points Effect of Atorvastatin on CV Events In SPARCL Patients with Diabetes 84 HR=0. 36 (95% CI 0. 21, 0. 61), P=0. 0001* Log-rank P=0. 0027 1 2 82 3 4 5 Years since randomization 6 Log-rank P=0. 0001 0 1 2 3 4 5 Years since randomization *Adjusted for entry event, time since entry event, gender, age, and geographic region Callahan A, Welch KMA, Amarenco P, et al. 6

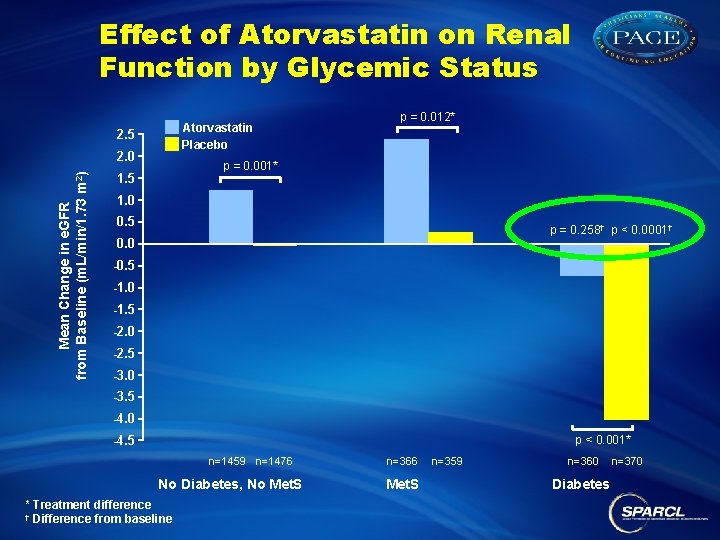
Effect of Atorvastatin on Renal Function by Glycemic Status Atorvastatin Placebo 2. 5 Mean Change in e. GFR from Baseline (m. L/min/1. 73 m 2) 2. 0 p = 0. 012* p = 0. 001* 1. 5 1. 0 0. 5 p = 0. 258† p < 0. 0001† 0. 0 -0. 5 -1. 0 -1. 5 -2. 0 -2. 5 -3. 0 -3. 5 -4. 0 -4. 5 p < 0. 001* n=1459 n=1476 No Diabetes, No Met. S * Treatment difference † Difference from baseline n=366 Met. S n=359 n=360 Diabetes n=370

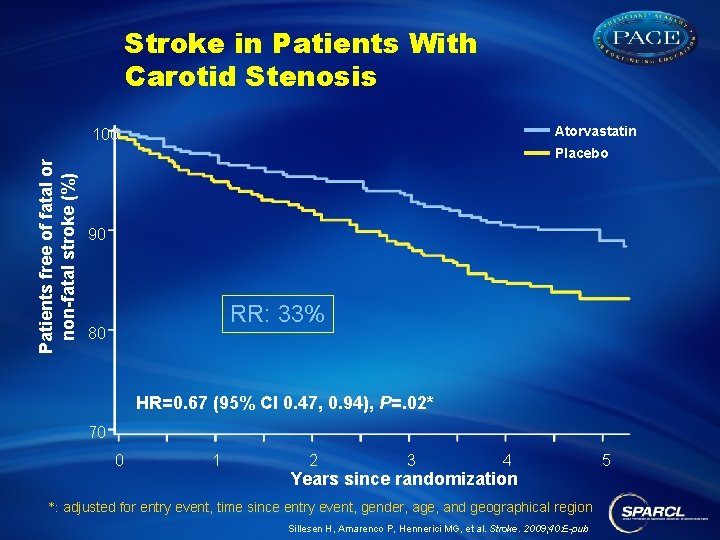
Stroke in Patients With Carotid Stenosis Atorvastatin Patients free of fatal or non-fatal stroke (%) 100 Placebo 90 RR: 33% 80 HR=0. 67 (95% CI 0. 47, 0. 94), P=. 02* 70 0 1 2 3 4 Years since randomization *: adjusted for entry event, time since entry event, gender, age, and geographical region Sillesen H, Amarenco P, Hennerici MG, et al. Stroke. 2009; 40: E-pub 5

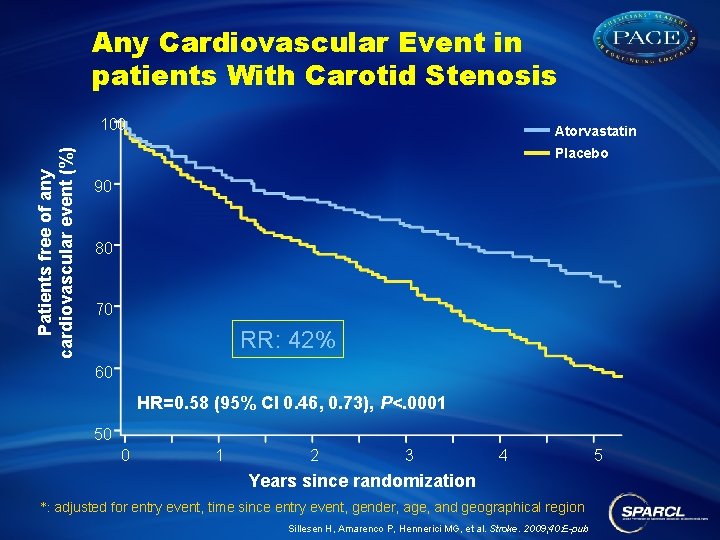
Any Cardiovascular Event in patients With Carotid Stenosis Patients free of any cardiovascular event (%) 100 Atorvastatin Placebo 90 80 70 RR: 42% 60 HR=0. 58 (95% CI 0. 46, 0. 73), P<. 0001 50 0 1 2 3 4 Years since randomization *: adjusted for entry event, time since entry event, gender, age, and geographical region Sillesen H, Amarenco P, Hennerici MG, et al. Stroke. 2009; 40: E-pub 5

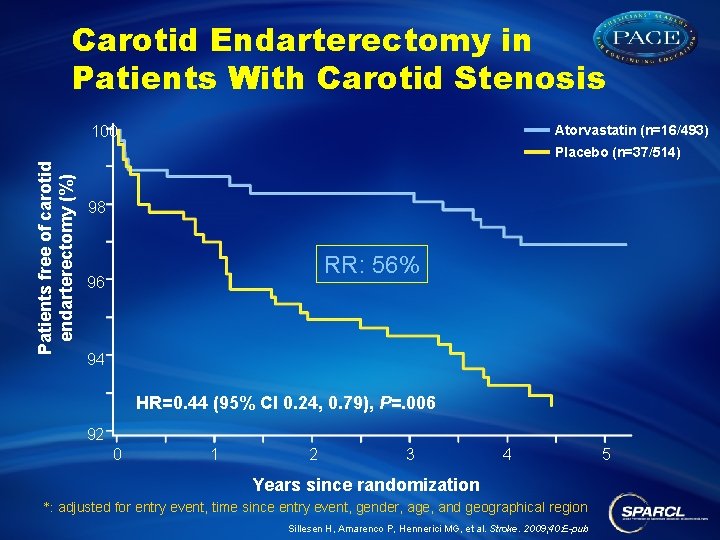
Carotid Endarterectomy in Patients With Carotid Stenosis Atorvastatin (n=16/493) 100 Patients free of carotid endarterectomy (%) Placebo (n=37/514) 98 RR: 56% 96 94 HR=0. 44 (95% CI 0. 24, 0. 79), P=. 006 92 0 1 2 3 4 Years since randomization *: adjusted for entry event, time since entry event, gender, age, and geographical region Sillesen H, Amarenco P, Hennerici MG, et al. Stroke. 2009; 40: E-pub 5

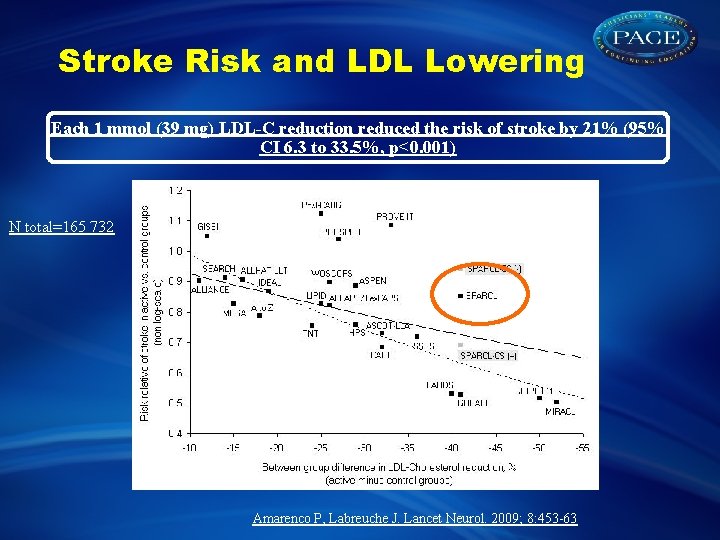
Stroke Risk and LDL Lowering Each 1 mmol (39 mg) LDL-C reduction reduced the risk of stroke by 21% (95% CI 6. 3 to 33. 5%, p<0. 001) N total=165 732 Amarenco P, Labreuche J. Lancet Neurol. 2009; 8: 453 -63

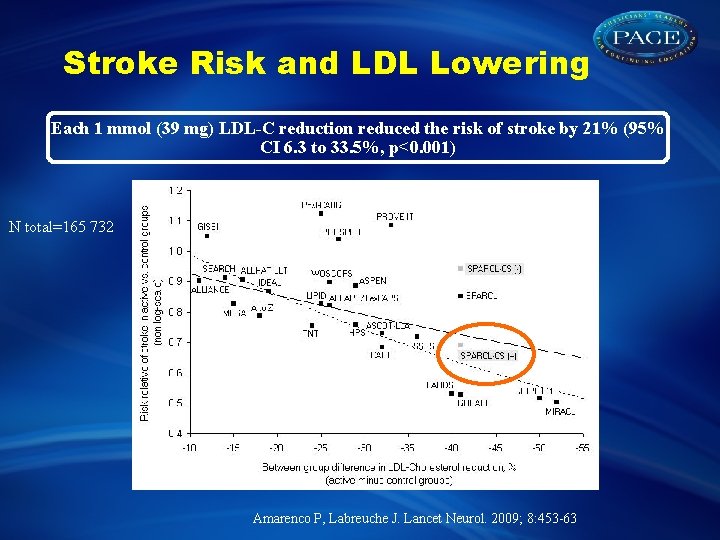
Stroke Risk and LDL Lowering Each 1 mmol (39 mg) LDL-C reduction reduced the risk of stroke by 21% (95% CI 6. 3 to 33. 5%, p<0. 001) N total=165 732 Amarenco P, Labreuche J. Lancet Neurol. 2009; 8: 453 -63

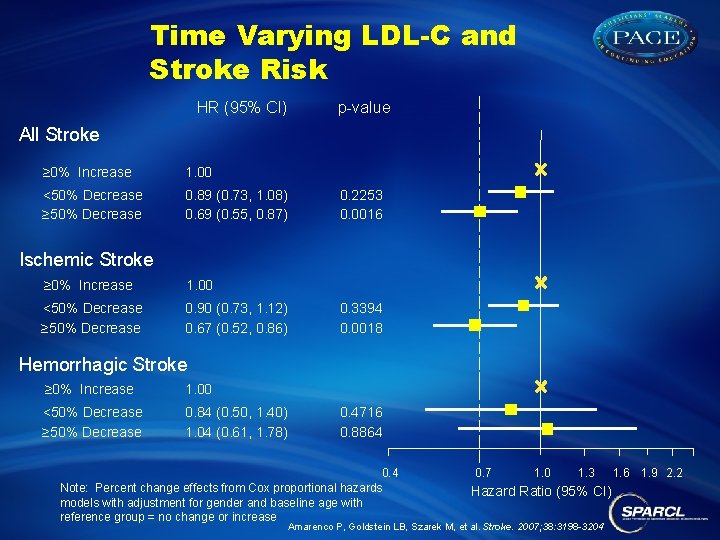
Time Varying LDL-C and Stroke Risk HR (95% CI) p-value All Stroke ≥ 0% Increase 1. 00 <50% Decrease ≥ 50% Decrease 0. 89 (0. 73, 1. 08) 0. 69 (0. 55, 0. 87) 0. 2253 0. 0016 Ischemic Stroke ≥ 0% Increase 1. 00 <50% Decrease ≥ 50% Decrease 0. 90 (0. 73, 1. 12) 0. 67 (0. 52, 0. 86) 0. 3394 0. 0018 Hemorrhagic Stroke ≥ 0% Increase 1. 00 <50% Decrease ≥ 50% Decrease 0. 84 (0. 50, 1. 40) 1. 04 (0. 61, 1. 78) 0. 4716 0. 8864 0. 4 Note: Percent change effects from Cox proportional hazards models with adjustment for gender and baseline age with reference group = no change or increase 0. 7 1. 0 1. 3 Hazard Ratio (95% CI) Amarenco P, Goldstein LB, Szarek M, et al. Stroke. 2007; 38: 3198 -3204 1. 6 1. 9 2. 2

Time Varying LDL-C and Stroke Risk HR (95% CI) p-value All Stroke ≥ 100 mg/d. L 1. 00 70 to < 100 mg/d. L < 70 mg/d. L 1. 01 (0. 81, 1. 27) 0. 72 (0. 59, 0. 89) 0. 9076 0. 0016 0. 4 0. 7 1. 0 1. 3 Hazard Ratio (95% CI) Note: Nominal value effects from Cox proportional hazards models with adjustment for gender and baseline age with reference group = no change or increase Amarenco P, Goldstein LB, Szarek M, et al. Stroke. 2007; 38: 3198 -3204 1. 6 1. 9 2. 2

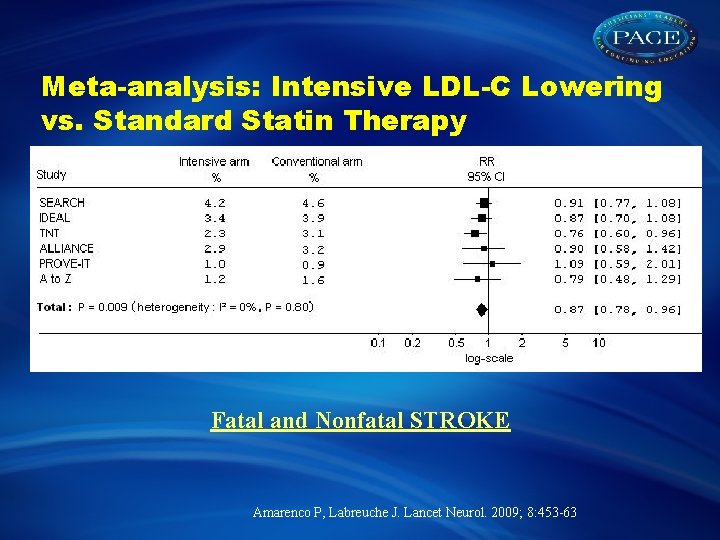
Meta-analysis: Intensive LDL-C Lowering vs. Standard Statin Therapy Fatal and Nonfatal STROKE Amarenco P, Labreuche J. Lancet Neurol. 2009; 8: 453 -63

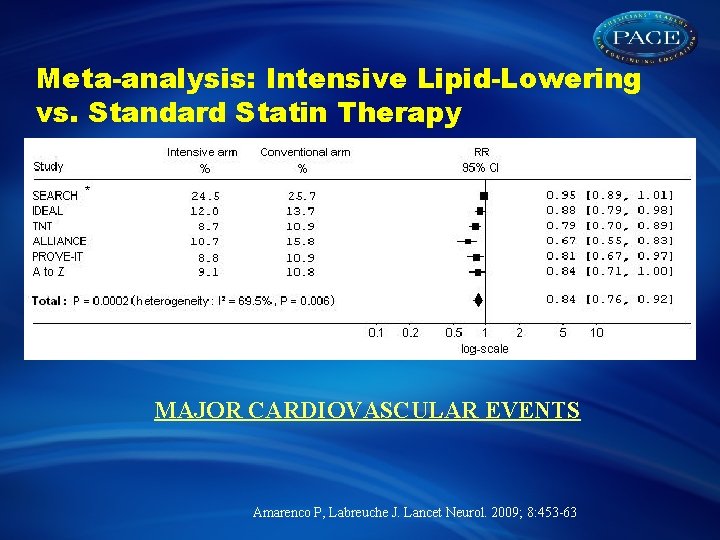
Meta-analysis: Intensive Lipid-Lowering vs. Standard Statin Therapy MAJOR CARDIOVASCULAR EVENTS Amarenco P, Labreuche J. Lancet Neurol. 2009; 8: 453 -63

Mean Lipids and BP During Follow-up in Atorvastatin and Placebo Groups TG 140. 0 Blood Pressure (mm Hg) 150 Lipids (mg/dl) 125 LDL 100 75 50 HDL 25 0 0 3 6 12 18 24 30 36 42 48 54 60 66 72 LO 139. 5 SBP 139. 0 138. 5 138. 0 137. 5 137. 0 82. 0 81. 5 81. 0 80. 5 80. 0 1. 0 0. 0 DBP 0 36 Time (months) Solid lines = atorvastatin 80 mg group; dashed lines = placebo group Amarenco P, Goldstein LB, Messig M, et al. Stroke. 2009 LO = last observation 12 18 24 30 36 42 Time (months) 48 54 60 66 72 LO

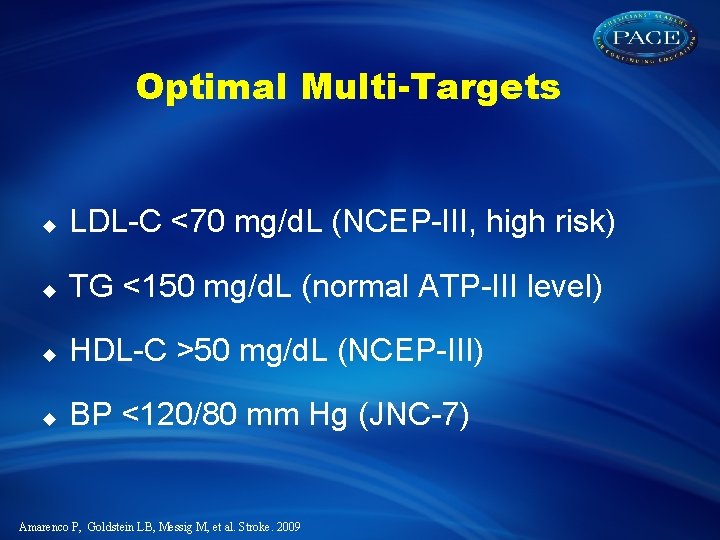
Optimal Multi-Targets u LDL-C <70 mg/d. L (NCEP-III, high risk) u TG <150 mg/d. L (normal ATP-III level) u HDL-C >50 mg/d. L (NCEP-III) u BP <120/80 mm Hg (JNC-7) Amarenco P, Goldstein LB, Messig M, et al. Stroke. 2009

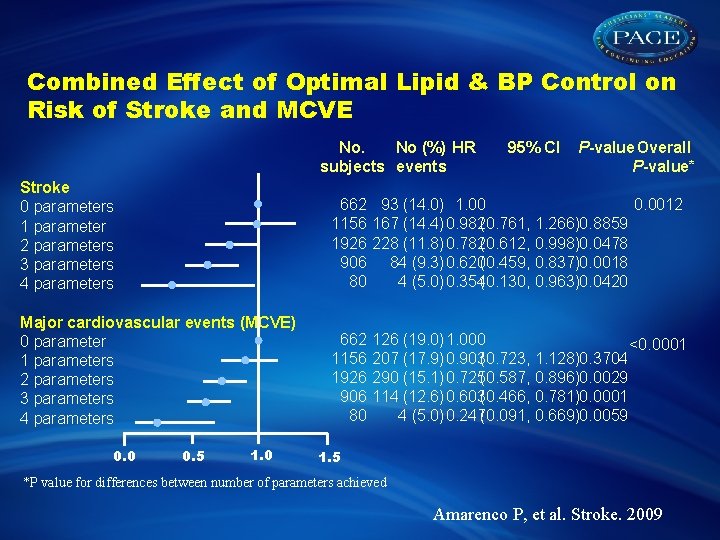
Combined Effect of Optimal Lipid & BP Control on Risk of Stroke and MCVE No. No (%) HR subjects events 95% CI P-value Overall P-value* Stroke 0 parameters 1 parameter 2 parameters 3 parameters 4 parameters 662 93 (14. 0) 1. 00 0. 0012 1156 167 (14. 4) 0. 982(0. 761, 1. 266)0. 8859 1926 228 (11. 8) 0. 782(0. 612, 0. 998)0. 0478 906 84 (9. 3) 0. 620(0. 459, 0. 837)0. 0018 80 4 (5. 0) 0. 354(0. 130, 0. 963)0. 0420 Major cardiovascular events (MCVE) 0 parameter 1 parameters 2 parameters 3 parameters 4 parameters 662 1156 1926 906 80 0. 5 1. 0 126 (19. 0) 1. 000 <0. 0001 207 (17. 9) 0. 903(0. 723, 1. 128)0. 3704 290 (15. 1) 0. 725(0. 587, 0. 896)0. 0029 114 (12. 6) 0. 603(0. 466, 0. 781)0. 0001 4 (5. 0) 0. 247(0. 091, 0. 669)0. 0059 1. 5 *P value for differences between number of parameters achieved Amarenco P, et al. Stroke. 2009
 Statin mechanism of action
Statin mechanism of action Diabetes statin guidelines 2020
Diabetes statin guidelines 2020 Kolestiramin etki mekanizması
Kolestiramin etki mekanizması Anterior stroke vs posterior stroke
Anterior stroke vs posterior stroke Nursing management of stroke
Nursing management of stroke Nursing management of stroke
Nursing management of stroke Nursing management of stroke
Nursing management of stroke Other initiated self repair example
Other initiated self repair example Lipid digestion and absorption
Lipid digestion and absorption Stroke and turn clinic
Stroke and turn clinic Stroke culture method
Stroke culture method Composite media in microbiology
Composite media in microbiology Muscle refractory period
Muscle refractory period Heart wall
Heart wall Cardiac output and stroke volume
Cardiac output and stroke volume Anaerobic gaspak
Anaerobic gaspak Explain stab culture and stroke culture
Explain stab culture and stroke culture Guidelines for adult stroke rehabilitation and recovery
Guidelines for adult stroke rehabilitation and recovery Cvds
Cvds Disadvantages picture
Disadvantages picture Role of steroid hormone
Role of steroid hormone Carbohydrate vs lipid structure
Carbohydrate vs lipid structure Triacylglycerol synthesis
Triacylglycerol synthesis Dichromate test for lipids
Dichromate test for lipids Classification of lipids
Classification of lipids Fats, oils, and waxes
Fats, oils, and waxes Elements found in lipid
Elements found in lipid Oxidation of lipids
Oxidation of lipids Alpha lipid lifeline walmart
Alpha lipid lifeline walmart Jenis lipid
Jenis lipid Lipid rancidity
Lipid rancidity Prolactin target
Prolactin target Peta konsep karbohidrat kimia
Peta konsep karbohidrat kimia Lipid nanoparticles
Lipid nanoparticles Lipid soluble hormone
Lipid soluble hormone Lipid soluble hormones examples
Lipid soluble hormones examples Alpha lipid milk benefits
Alpha lipid milk benefits Classify each as a carbohydrate, protein, or lipid.
Classify each as a carbohydrate, protein, or lipid. Classify each as a carbohydrate, protein, or lipid.
Classify each as a carbohydrate, protein, or lipid. Lipid soluble hormones examples
Lipid soluble hormones examples Structure of storage lipids
Structure of storage lipids Lipid digestion
Lipid digestion Lipid emulsion
Lipid emulsion Naming triglycerides
Naming triglycerides Nitrogen-containing base
Nitrogen-containing base