Physicochemical properties of fats and oil Jeroen Vereecken





- Slides: 54

Physicochemical properties of fats and oil Jeroen Vereecken, post doctoral researcher at Ghent University, Belgium Jeroen. vereecken@ugent. be Daniel J E Kalnin, project manager lipid structures at Surface Chemistry Institute, Sweden daniel. kalnin@yki. se

Outline • Definition of lipid classes and their properties • Importance of fat crystallization • Structural levels in fat crystallization – Primary crystallization • Thermodynamic driving force, • nucleation, • crystal growth, • polymorphism – Microstructural development – Macroscopic properties • How to measure? • Case study cocoa butter

Outline • Definition of lipid classes and their properties • Importance of fat crystallization • Structural levels in fat crystallization – Primary crystallization • Thermodynamic driving force, • nucleation, • crystal growth, • polymorphism – Microstructural development – Macroscopic properties • How to measure? • Case study cocoa butter

Lipids- Fats and Oils Definition: Molecules (of biological origin) containing more than 4 CH 2 groups as an aliphatic chain • There are different classes of lipids depending on functionality and chemistry • Their properties may or may not depend on water

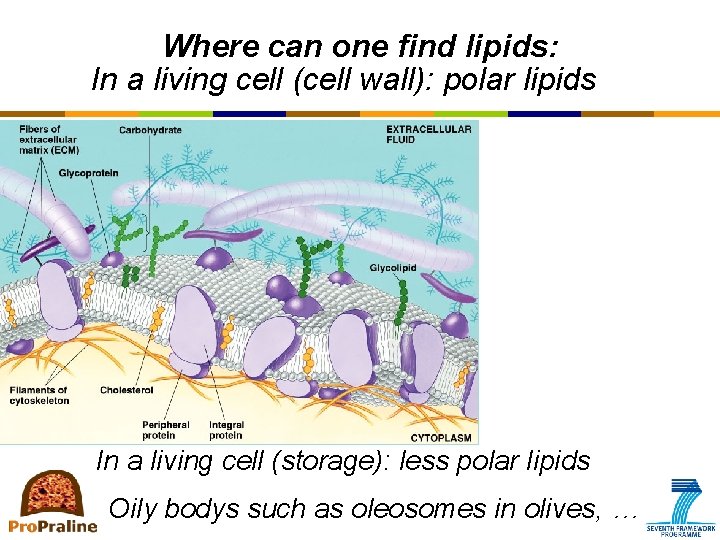
Where can one find lipids: In a living cell (cell wall): polar lipids In a living cell (storage): less polar lipids Oily bodys such as oleosomes in olives, …

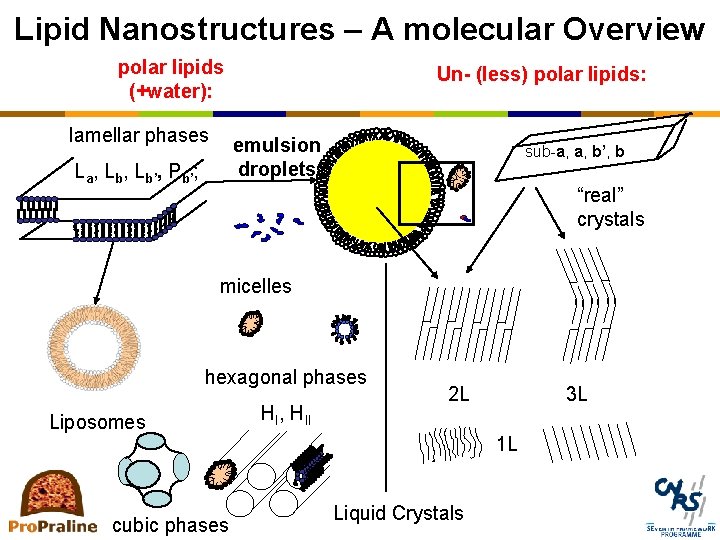
Lipid Nanostructures – A molecular Overview polar lipids (+water): lamellar phases Un- (less) polar lipids: emulsion droplets La, Lb’, Pb’; sub-a, a, b’, b “real” crystals micelles hexagonal phases Liposomes HI, HII 2 L 3 L 1 L cubic phases Liquid Crystals

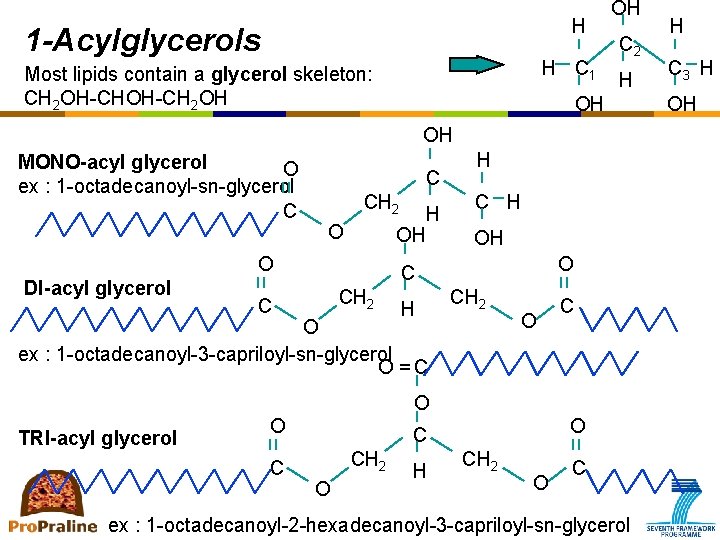
H 1 -Acylglycerols H C 1 Most lipids contain a glycerol skeleton: CH 2 OH-CH 2 OH OH C 2 H OH OH MONO-acyl glycerol O ex : 1 -octadecanoyl-sn-glycerol C C CH 2 O OH O DI-acyl glycerol H H C H OH O C CH 2 C H CH 2 O ex : 1 -octadecanoyl-3 -capriloyl-sn-glycerol O =C O TRI-acyl glycerol O C CH 2 O H CH 2 O C ex : 1 -octadecanoyl-2 -hexadecanoyl-3 -capriloyl-sn-glycerol H C 3 H OH

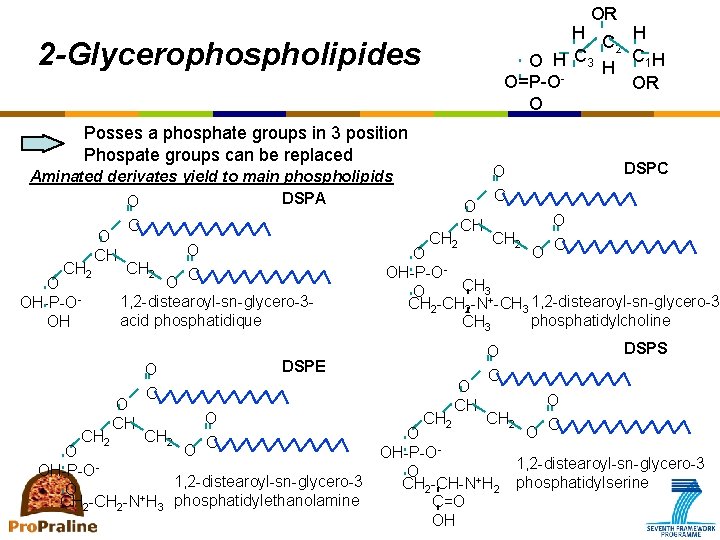
OR H H C 2 O H C 3 H C 1 H O=P-OOR O 2 -Glycerophospholipides Posses a phosphate groups in 3 position Phospate groups can be replaced Aminated derivates yield to main phospholipids DSPA O CH 2 O CH O OH-P-OOH CH 2 C O CH 2 O C 1, 2 -distearoyl-sn-glycero-3 acid phosphatidique O CH O O C O OH-P-O 1, 2 -distearoyl-sn-glycero-3 O CH 2 -N+H 3 phosphatidylethanolamine O O C CH 2 O OH-P-OCH 3 O CH 2 -N+-CH 3 1, 2 -distearoyl-sn-glycero-3 phosphatidylcholine CH 3 DSPE O C CH 2 O CH DSPC O CH DSPS O C O O C CH 2 O OH-P-O 1, 2 -distearoyl-sn-glycero-3 O CH 2 -CH-N+H 2 phosphatidylserine C=O OH

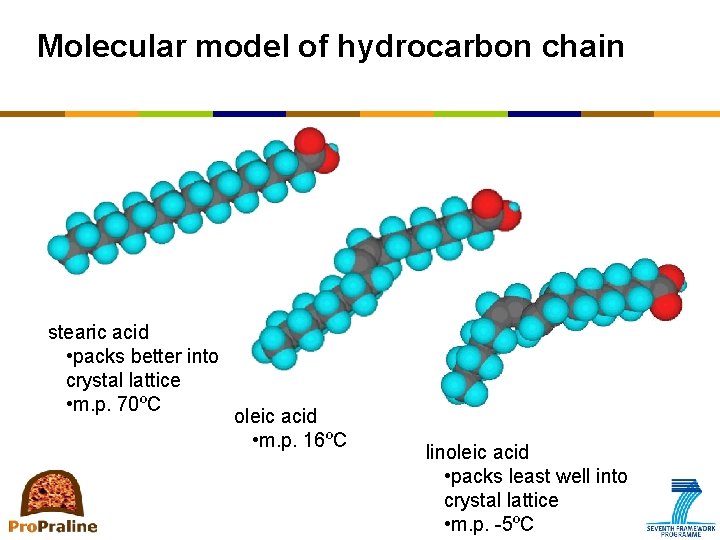
Molecular model of hydrocarbon chain stearic acid • packs better into crystal lattice • m. p. 70ºC oleic acid • m. p. 16ºC linoleic acid • packs least well into crystal lattice • m. p. -5ºC

Molecular model of lipids Group of Polar lipids Less-polar lipid

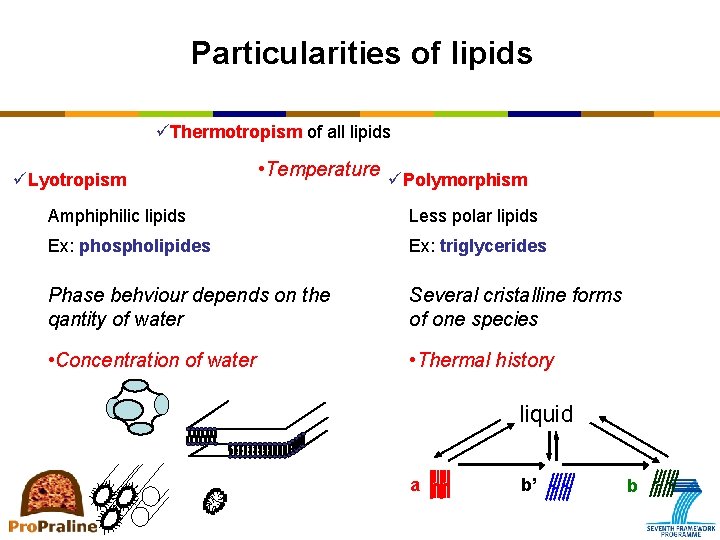
Particularities of lipids üThermotropism of all lipids üLyotropism • Temperature üPolymorphism Amphiphilic lipids Less polar lipids Ex: phospholipides Ex: triglycerides Phase behviour depends on the qantity of water Several cristalline forms of one species • Concentration of water • Thermal history liquid a b’ b

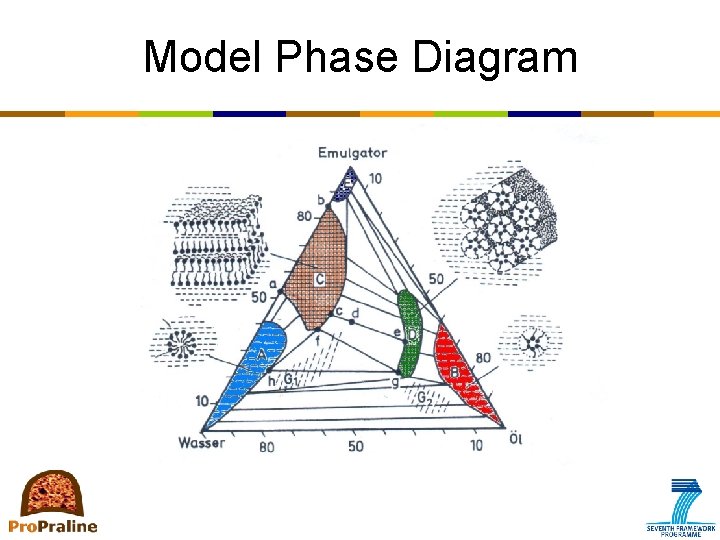
Model Phase Diagram

Outline • Definition of lipid classes • Importance of fat crystallization • Structural levels in fat crystallization – Primary crystallization • Thermodynamic driving force, • nucleation, • crystal growth, • polymorphism – Microstructural development – Macroscopic properties • How to measure? • Case study cocoa butter

Importance of fat crystallization • • Many food products contain fat Substantial amount of fat is present in the crystallized form Affects product structure and texture Determines product quality

Outline • Definition of lipid classes and their properties • Importance of fat crystallization • Structural levels in fat crystallization – Primary crystallization • Thermodynamic driving force, • nucleation, • crystal growth, • polymorphism – Microstructural development – Macroscopic properties • How to measure? • Case study cocoa butter

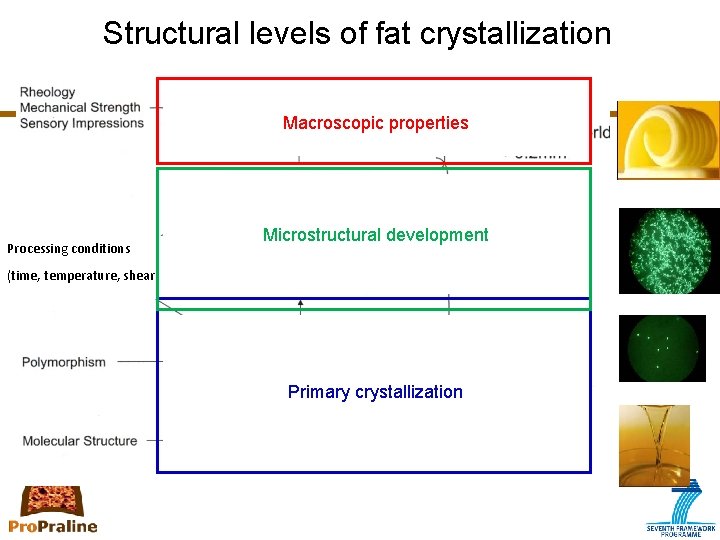
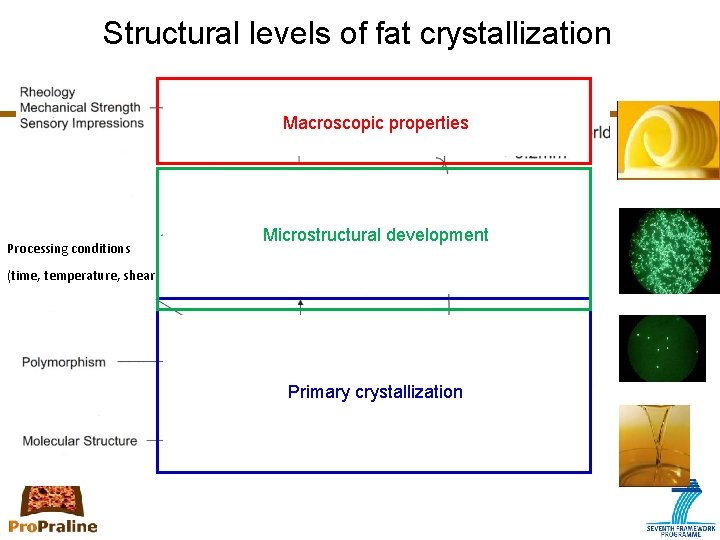
Structural levels of fat crystallization Macroscopic properties Processing conditions Microstructural development (time, temperature, shear) Primary crystallization

Primary crystallization: nucleation – = generation of a crystal nucleus by assembling growth units ⇒ critical activation-energy needed!!! – Primary homogeneous nucleation • = not catalyzed by foreign surfaces or existing fat crystals • Up to 30 K supercooling needed – Primary heterogeneous nucleation • = catalyzed by foreign surfaces (e. g. impeller blades etc. ) • Most frequent in natural fats and oils, enough impurities present – Secondary nucleation • = catalyzed by crystals of the crystallizing material ⇒ secundary nuclei • Very frequent in crystallization from solution and in industrial crystallizers

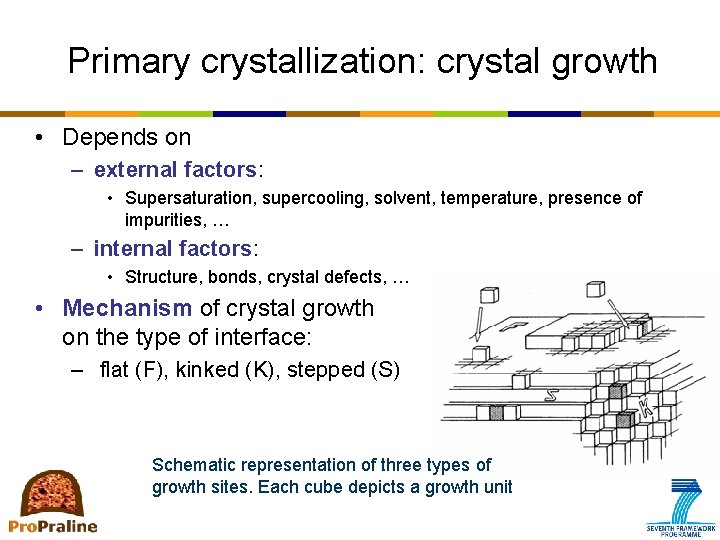
Primary crystallization: crystal growth • Depends on – external factors: • Supersaturation, supercooling, solvent, temperature, presence of impurities, … – internal factors: • Structure, bonds, crystal defects, … • Mechanism of crystal growth on the type of interface: – flat (F), kinked (K), stepped (S) Schematic representation of three types of growth sites. Each cube depicts a growth unit depends

Primary crystallization: crystal growth Palm stearine at 30°C

Primary crystallization: Polymorphism • = the existence of several crystalline phases with the same chemical composition that have a different structure, but yield identical liquid phases upon melting. • Polymorphic TRANSITION of less stable to more stable polymorphs • Importance: obtaining and maintaining (during storage) of specific macroscopic properties – e. g sandiness in margarine, fat bloom on chocolate

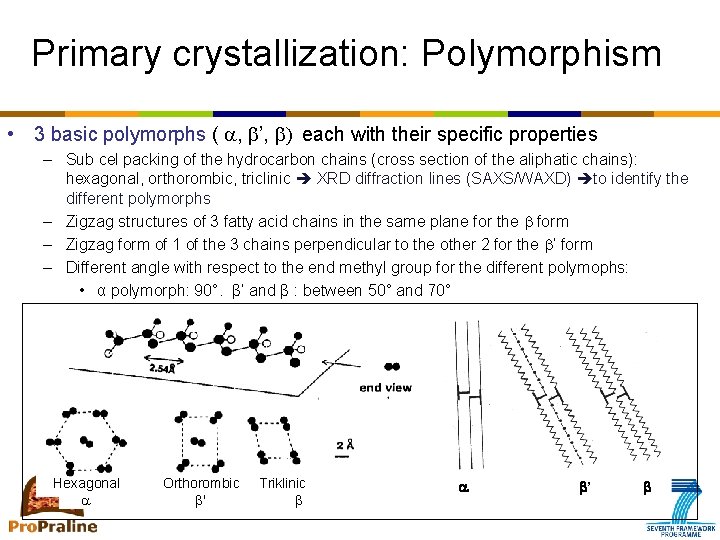
Primary crystallization: Polymorphism • 3 basic polymorphs ( a, ’, ) each with their specific properties – Sub cel packing of the hydrocarbon chains (cross section of the aliphatic chains): hexagonal, orthorombic, triclinic XRD diffraction lines (SAXS/WAXD) to identify the different polymorphs – Zigzag structures of 3 fatty acid chains in the same plane for the form – Zigzag form of 1 of the 3 chains perpendicular to the other 2 for the ’ form – Different angle with respect to the end methyl group for the different polymophs: • α polymorph: 90°. β’ and β : between 50° and 70° Hexagonal a Orthorombic ' Triklinic a b’ b

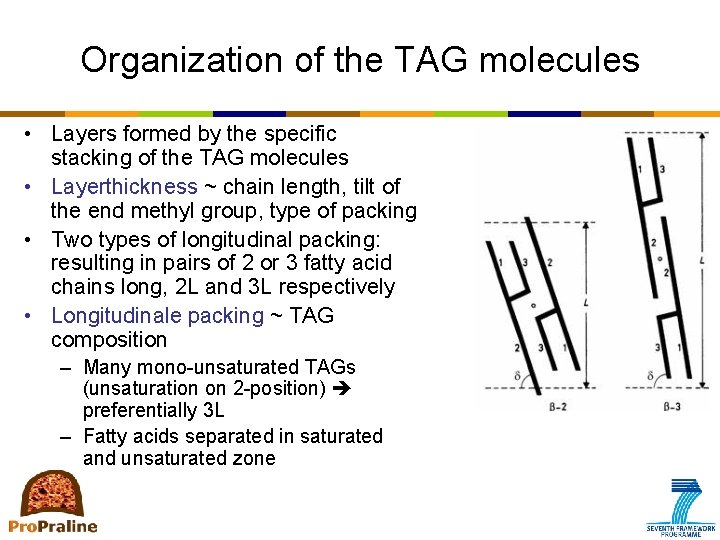
Organization of the TAG molecules • Layers formed by the specific stacking of the TAG molecules • Layerthickness ~ chain length, tilt of the end methyl group, type of packing • Two types of longitudinal packing: resulting in pairs of 2 or 3 fatty acid chains long, 2 L and 3 L respectively • Longitudinale packing ~ TAG composition – Many mono-unsaturated TAGs (unsaturation on 2 -position) preferentially 3 L – Fatty acids separated in saturated and unsaturated zone

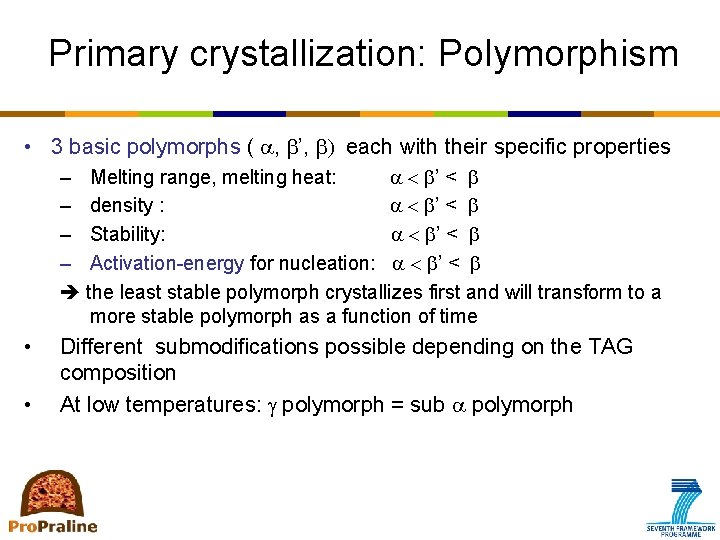
Primary crystallization: Polymorphism • 3 basic polymorphs ( a, ’, ) each with their specific properties – Melting range, melting heat: a < ’ < – density : a < ’ < – Stability: a < ’ < – Activation-energy for nucleation: a < ’ < the least stable polymorph crystallizes first and will transform to a more stable polymorph as a function of time • • Different submodifications possible depending on the TAG composition At low temperatures: g polymorph = sub a polymorph

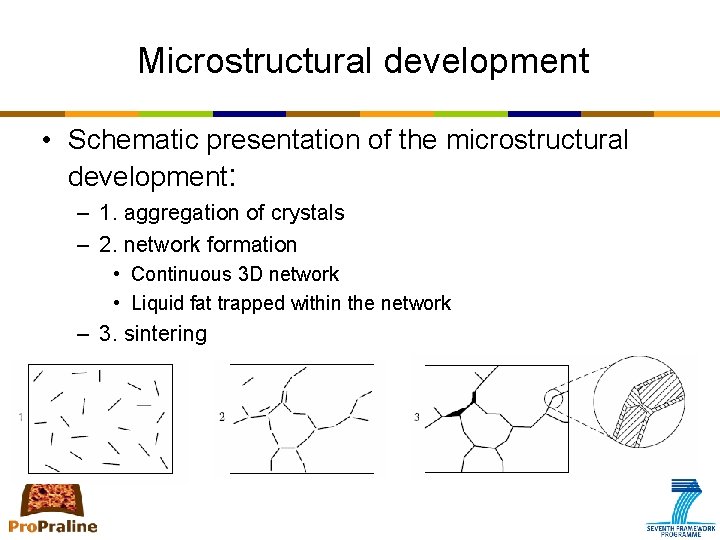
Microstructural development • Schematic presentation of the microstructural development: – 1. aggregation of crystals – 2. network formation • Continuous 3 D network • Liquid fat trapped within the network – 3. sintering

Microstructural development • Network properties depend on: – Number and size of the crystals – Interactions between the crystals – Presence of other components • Influencing factors – – Crystallization temperature Cooling rate Agitation Storage time

Macroscopic properties • How does the consumer experience the product? – – – – Smell Taste Appearance e. g. Fat bloom Mouth feel (cool sensation, no waxy taste) Melting in the hand, sticking to fingers Snap. . .

Outline • Definition of lipid classes and their properties • Importance of fat crystallization • Structural levels in fat crystallization – Primary crystallization • Thermodynamic driving force, • nucleation, • crystal growth, • polymorphism – Microstructural development – Macroscopic properties • How to measure? • Case study cocoa butter

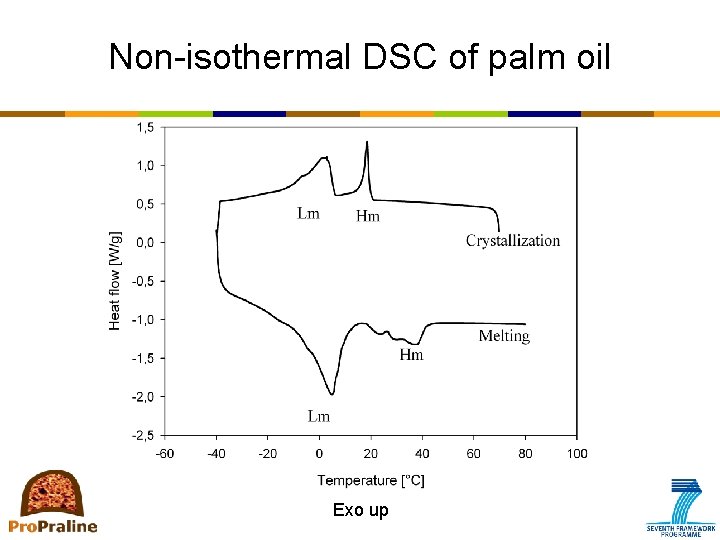
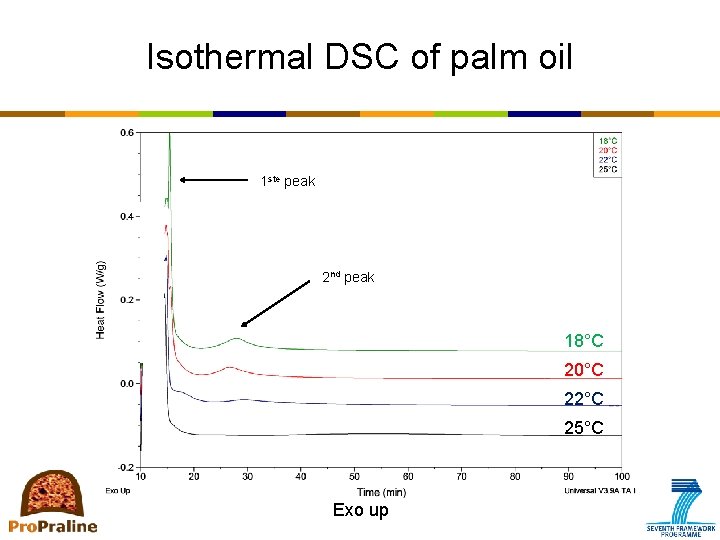
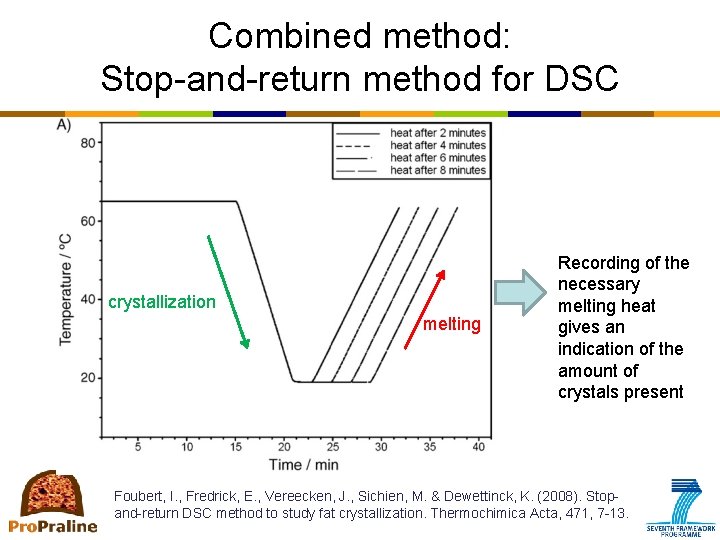
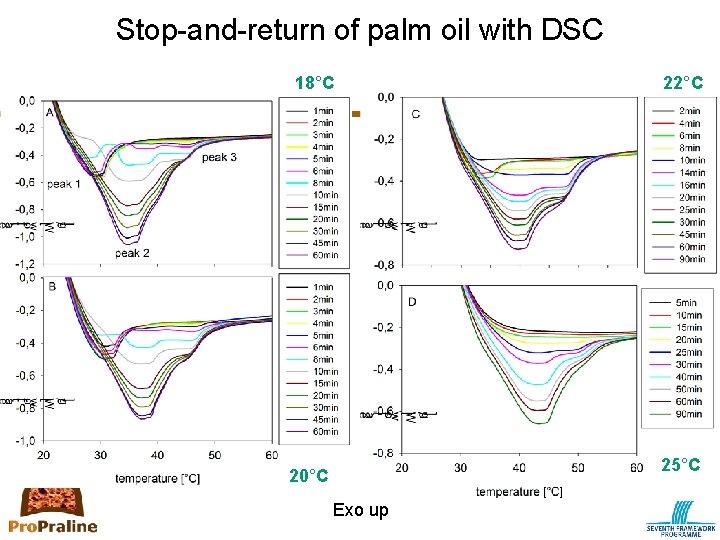
Primary crystallization: How to measure? • DSC (differential scanning calorimetry) – Measures the difference in heat flow between the sample (=fat) and the reference (=air) while both are subjected to the same temperature-time protocol – Provides information on processes that involve heat release (crystallization) or heat absorption (melting) – Types of analysis • Non-isothermal • Isothermal • Combined methods e. g. Stop-and-return – Types of information • Monitoring kinetics of crystallization • Recording melting profile; also provides an indication of polymorphism ⇒ stop-and-return method • No information on aggregation and microstructure

Non-isothermal DSC of palm oil Exo up

Isothermal DSC of palm oil 1 ste peak 2 nd peak 18°C 20°C 22°C 25°C Exo up

Combined method: Stop-and-return method for DSC crystallization melting Recording of the necessary melting heat gives an indication of the amount of crystals present Foubert, I. , Fredrick, E. , Vereecken, J. , Sichien, M. & Dewettinck, K. (2008). Stopand-return DSC method to study fat crystallization. Thermochimica Acta, 471, 7 -13.

Stop-and-return of palm oil with DSC 18°C 22°C 25°C 20°C Exo up

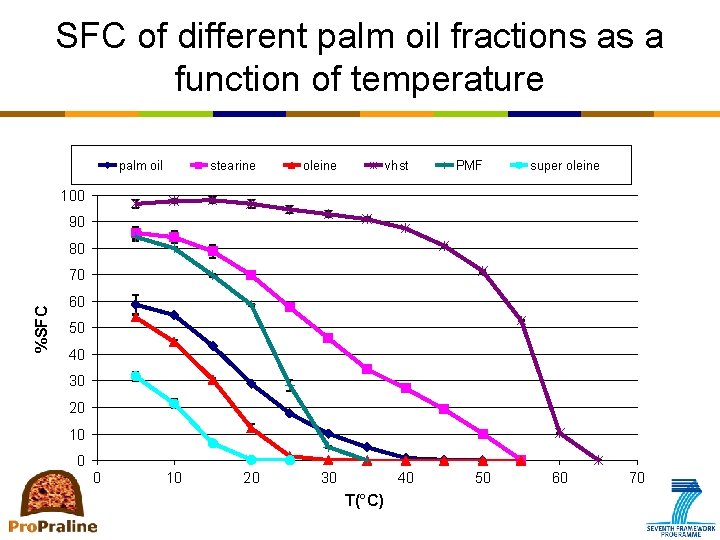
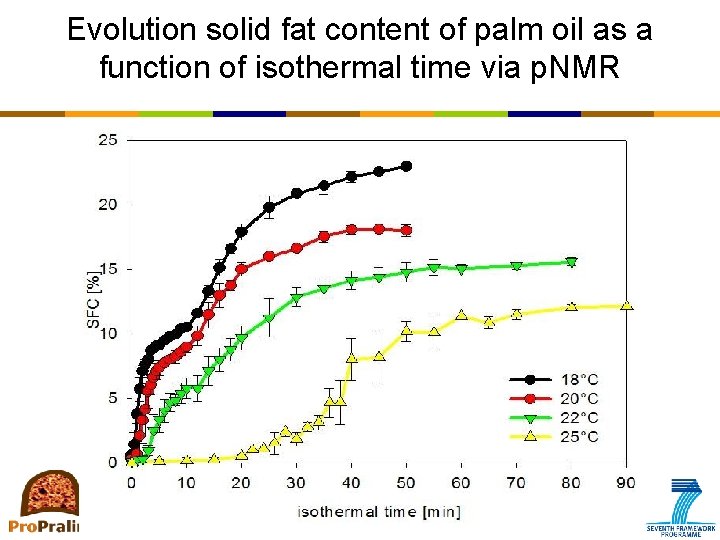
Primary crystallization: How to measure? • p. NMR (pulsed nuclear magnetic resonance) – Most frequently used: solid fat content as a function of time or temperature • SFC as a function of temperature: – equilibrium situation, no information on kinetics • SFC as a function of isothermal time – Information on kinetics – No information on aggregation or microstructure

SFC of different palm oil fractions as a function of temperature palm oil stearine oleine 20 30 vhst PMF super oleine 100 90 80 %SFC 70 60 50 40 30 20 10 0 0 10 40 T(°C) 50 60 70

Evolution solid fat content of palm oil as a function of isothermal time via p. NMR

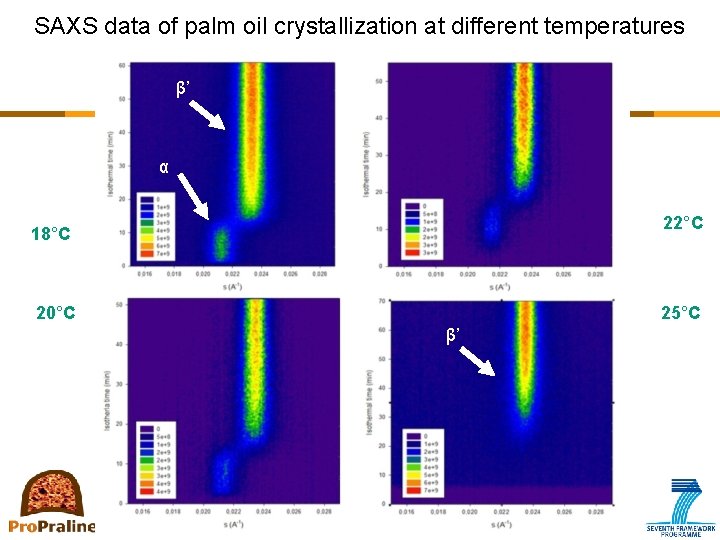
Primary crystallization: How to measure? • X-ray diffraction – Identifying the polymorphic form – Recording time: 1 frame per minute • Disadvantage when monitoring tme-resolved crystallization processes • Synchrotron radiation (e. g. ESRF Grenoble, MAXlab, Lund) – More intense X-rays recording time is limited to a few seconds

SAXS data of palm oil crystallization at different temperatures β’ α 22°C 18°C 20°C 25°C β’

Microstructure: how to measure? • Microscopy – Visual representation • Morphology, size, number of crystals • Aggregation and network formation – Several techniques • Polarized light microscopy • Confocal scanning laser microscopy • (Cryo-) Scanning electron microscopy • Rheology

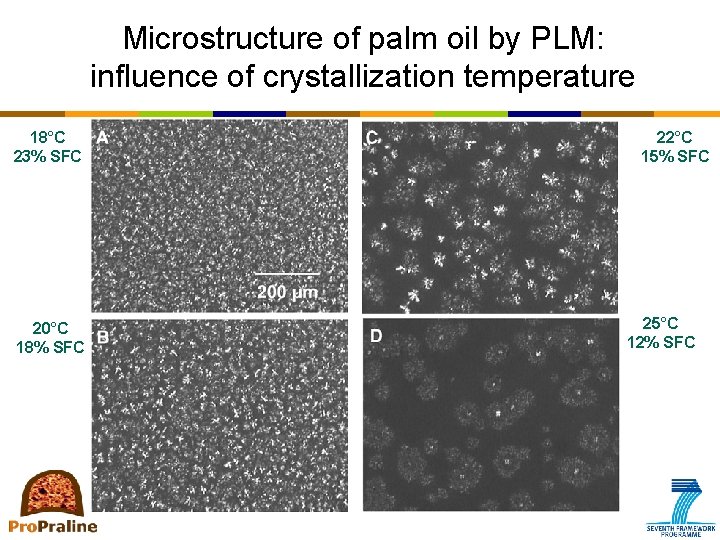
Microstructure of palm oil by PLM: influence of crystallization temperature 18°C 23% SFC 20°C 18% SFC 22°C 15% SFC 25°C 12% SFC

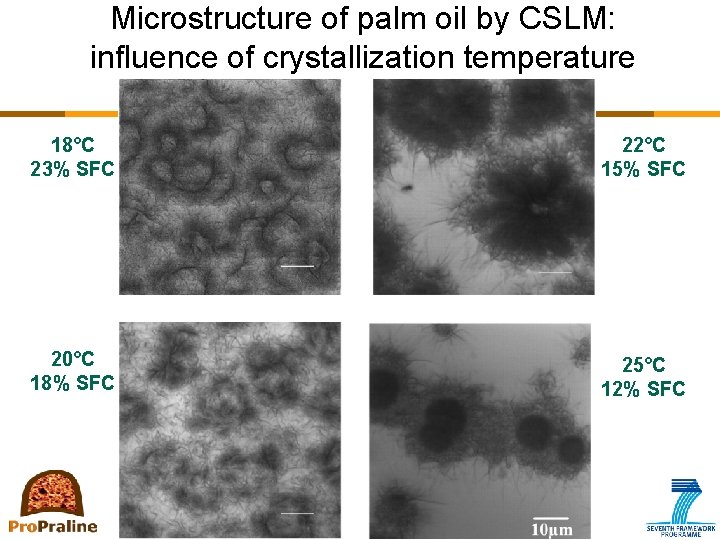
Microstructure of palm oil by CSLM: influence of crystallization temperature 18°C 23% SFC 22°C 15% SFC 20°C 18% SFC 25°C 12% SFC

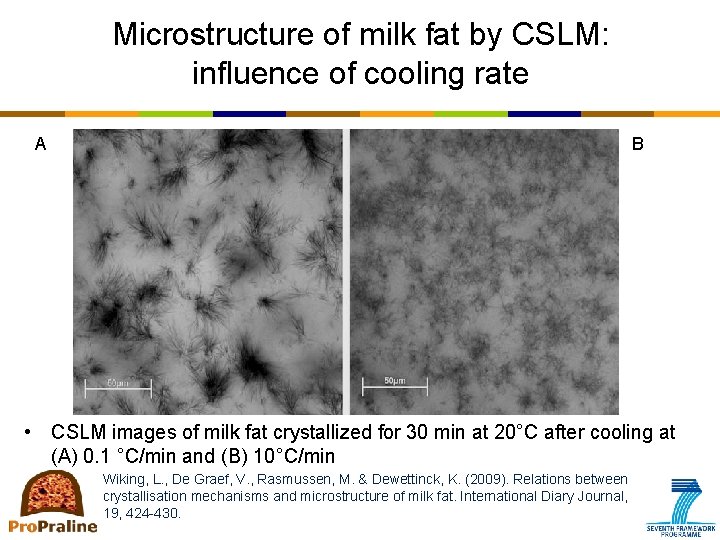
Microstructure of milk fat by CSLM: influence of cooling rate A B • CSLM images of milk fat crystallized for 30 min at 20°C after cooling at (A) 0. 1 °C/min and (B) 10°C/min Wiking, L. , De Graef, V. , Rasmussen, M. & Dewettinck, K. (2009). Relations between crystallisation mechanisms and microstructure of milk fat. International Diary Journal, 19, 424 -430.

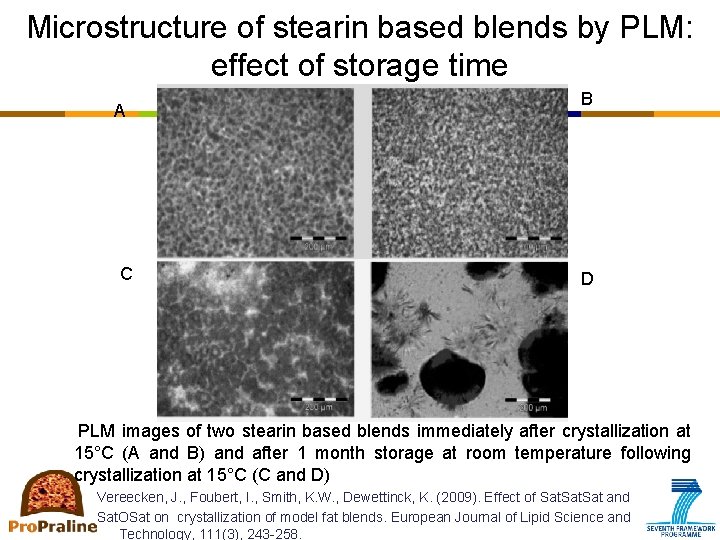
Microstructure of stearin based blends by PLM: effect of storage time A C B D PLM images of two stearin based blends immediately after crystallization at 15°C (A and B) and after 1 month storage at room temperature following crystallization at 15°C (C and D) Vereecken, J. , Foubert, I. , Smith, K. W. , Dewettinck, K. (2009). Effect of Sat and Sat. OSat on crystallization of model fat blends. European Journal of Lipid Science and Technology, 111(3), 243 -258.

Macroscopic properties: how to measure? • Texture analysis – Puncture test – Breaking test – Texture profile analysis test • Sensorial analysis – Taste panel

Outline • Definition of lipid classes and their properties • Importance of fat crystallization • Structural levels in fat crystallization – Primary crystallization • Thermodynamic driving force, • nucleation, • crystal growth, • polymorphism – Microstructural development – Macroscopic properties • How to measure? • Case study cocoa butter

Cocoa butter • Theobroma cacao : – Up to 6 m tall – Cocoa fruit: 20 cm – contains 30 -40 seeds (65% moisture) – Originally from tropical Amazon forest – Cultivated by • Mayas from Yucatan, Guatemala • Aztecs from Mexico – Production of aphrodisiacum

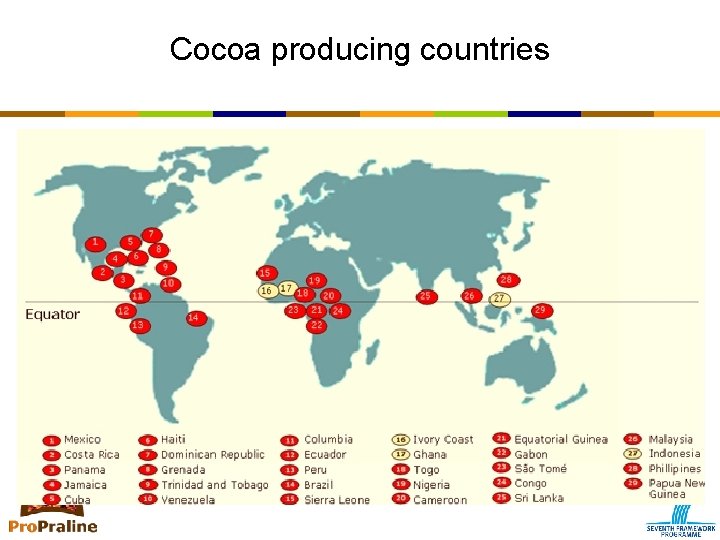
Cocoa producing countries

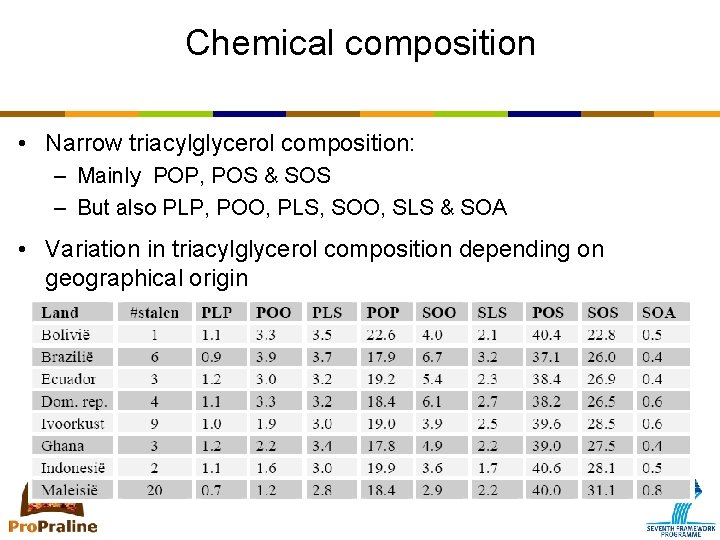
Chemical composition • Narrow triacylglycerol composition: – Mainly POP, POS & SOS – But also PLP, POO, PLS, SOO, SLS & SOA • Variation in triacylglycerol composition depending on geographical origin

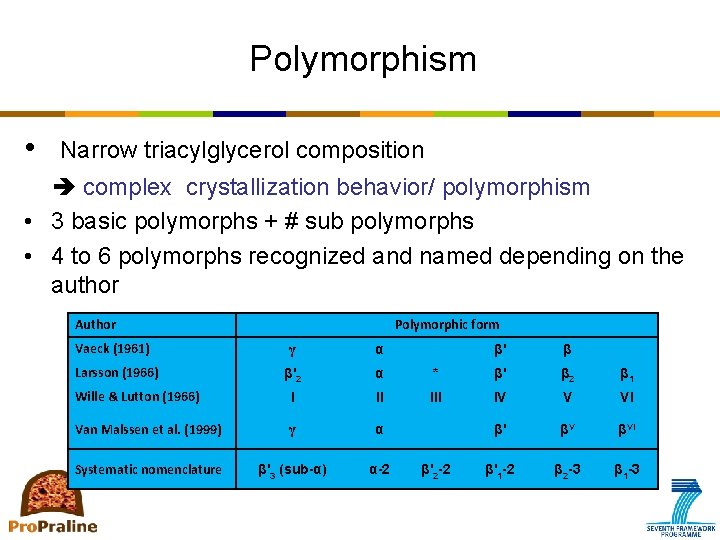
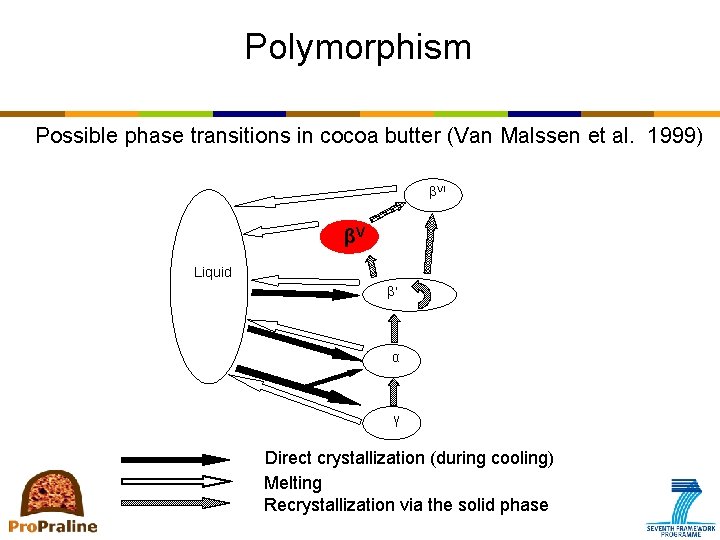
Polymorphism • Narrow triacylglycerol composition complex crystallization behavior/ polymorphism • 3 basic polymorphs + # sub polymorphs • 4 to 6 polymorphs recognized and named depending on the author Author Vaeck (1961) Polymorphic form γ α β'2 α Wille & Lutton (1966) I II Van Malssen et al. (1999) γ α Systematic nomenclature β'3 (sub-α) α-2 Larsson (1966) β' β * β' β 2 β 1 III IV V VI β' βV βVI β'1 -2 β 2 -3 β 1 -3 β'2 -2

Polymorphism Possible phase transitions in cocoa butter (Van Malssen et al. 1999) βVI βV Liquid ’ α γ Direct crystallization (during cooling) Melting Recrystallization via the solid phase

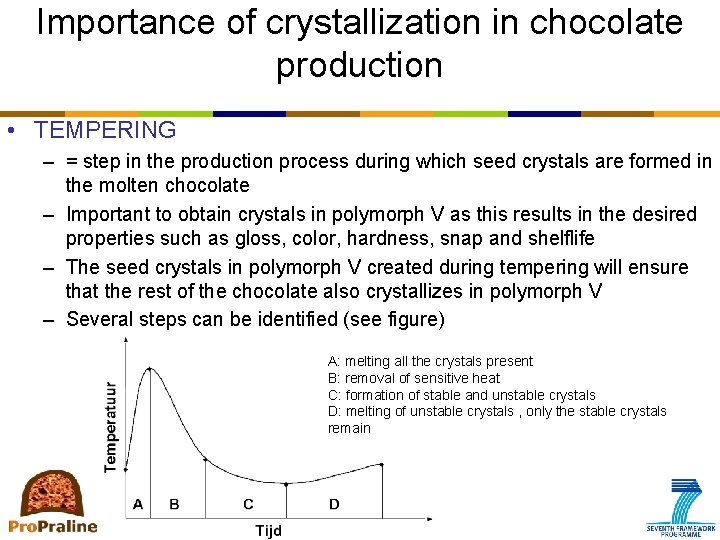
Importance of crystallization in chocolate production • TEMPERING – = step in the production process during which seed crystals are formed in the molten chocolate – Important to obtain crystals in polymorph V as this results in the desired properties such as gloss, color, hardness, snap and shelflife – The seed crystals in polymorph V created during tempering will ensure that the rest of the chocolate also crystallizes in polymorph V – Several steps can be identified (see figure) A: melting all the crystals present B: removal of sensitive heat C: formation of stable and unstable crystals D: melting of unstable crystals , only the stable crystals remain

Importance of crystallization in chocolate production • COOLING – Further crystallization based on seeds formed during tempering – Proper time-temperature program in cooling tunnel is important: gloss, sufficient hardening at the end of the cooling • STORAGE – Ongoing crystallization (depending on the cooling): crystallization heat can get trapped in the wrapping – Amongst others, polymorphic transition to form VI Fat bloom

Structural levels of fat crystallization Macroscopic properties Processing conditions Microstructural development (time, temperature, shear) Primary crystallization

Physicochemical properties of fats and oil Veerle De Graef, post doctoral researcher at Ghent University, Belgium veerle. degraef@ugent. be Daniel J E Kalnin, project manager lipid structures at Surface Chemistry Institute, Sweden daniel. kalnin@yki. se