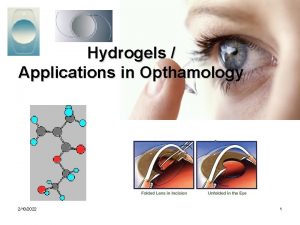
Hydrogels of Solid lipid Nanoparticles of Curcumin Presented












- Slides: 24

Hydrogels of Solid lipid Nanoparticles of Curcumin Presented by RUCHI CHAWLA ASSISTANT PROFESSOR IIT(BHU), VARANASI 5 th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems March 16 -18, 2015 Crowne Plaza, Dubai, UAE

Contents § Introduction § Experimental work § Preparation of SLNs § Analytical Method for Curcumin § Formulation characterization § Summary and conclusion § References

Introduction Hydrogels: Advantages of Hydrogels (Kopecek, 2009): Shape stability and softness similar to that of the soft surrounding tissues Chemical and biochemical stability Absence of extractables High permeability for water-soluble nutrients and metabolites across the biomaterial tissue-interface Convenient handling Easy application Excellent tissue biocompatibility due to their high water content

Curcumin Yellow spice derived from the roots, rhizome of Curcuma longa Adaptogen, bio-protectant, anti-bacterial, antioxidant and anti-inflammatory Chemically it is a mixture of three principal compounds (Strimpakos and Sharma, 2008): Curcumin (sometimes referred to as curcumin I), Demethoxycurcumin (curcumin II), and Bisdemethoxycurcumin (curcumin III) Limitations: Poor systemic bioavailability because of poor absorption and rapid systemic elimination via glucuronidation (Aggarwal and Sung, 2009). Hydrophobic compound with a high partition coefficient of 3. 2 and water solubility around 0. 6 μg/ml (Kurien et al. , 2007 and Patel et al. , 2009).

Why Solid lipid Nanoparticles? Small size Possibility of controlled rug release Increased drug stability High drug pay load Incorporation of lipophilic and hydrophilic drugs Biocompatibility Enhancement of bioavailability of the incorporated drugs (Mehnert and Mader, 2001) Enhanced penetration of drug into the skin from lipid nanoparticles, occlusion effect and film formation of lipid nanoparticles on the skin Larger surface area and contact point to adhere to the skin layer than that of microparticle. SLN can provide benefits of accumulation of drug in the skin strata to various skin diseases such as acne and eczema (Korting et al, 2007).

Experimental Work

Preparation of SLNs of Curcumin by nanoprecipitation method (Chorny et al. , 2002) Organic Phase (58. 5 ml acetone and 1. 5 ml dichloromethane) consisting of 600 mg of stearic acid and 9. 0 mg Curcumin Injected slowly with stirring at 1000 rpm Aqueous Phase (120 m. L) containing 0. 5, 1. 0 & 2. 0% w/v PVA Solvent removal by evaporation room temperature for 24 hours Evaporation of organic phase SLNs suspended in aqueous medium Ultracentrifugation at 10, 000 x g for 5 min Collection of SLNs

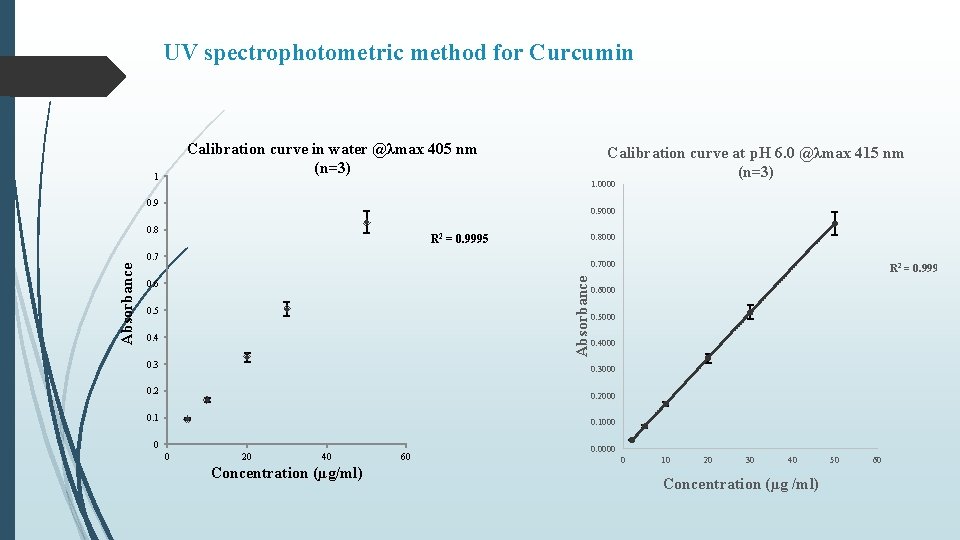
UV spectrophotometric method for Curcumin Calibration curve in water @λmax 405 nm (n=3) 1 Calibration curve at p. H 6. 0 @λmax 415 nm (n=3) 1. 0000 0. 9000 0. 8 R 2 = 0. 9995 0. 8000 0. 7000 Absorbance 0. 7 0. 6 0. 5 0. 4 0. 3 R 2 = 0. 9999 0. 6000 0. 5000 0. 4000 0. 3000 0. 2000 0. 1000 0 0 20 40 Concentration (µg/ml) 60 0. 0000 0 10 20 30 40 Concentration (µg /ml) 50 60

Characterization of SLNs

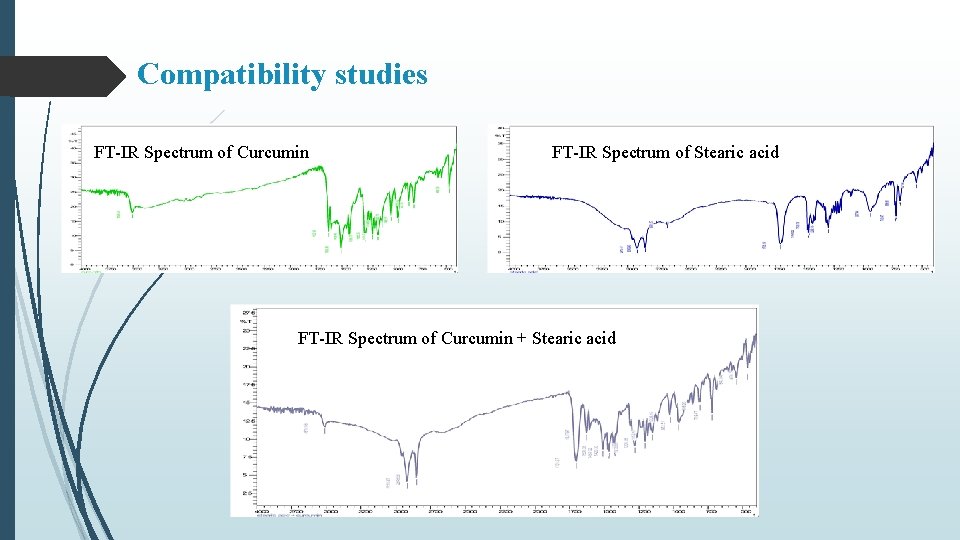
Compatibility studies FT-IR Spectrum of Curcumin FT-IR Spectrum of Stearic acid FT-IR Spectrum of Curcumin + Stearic acid

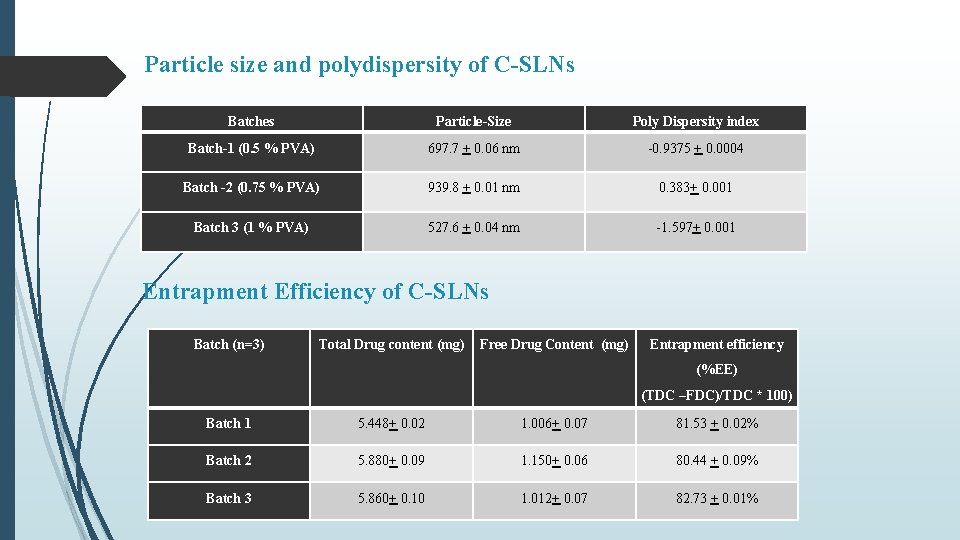
Particle size and polydispersity of C-SLNs Batches Particle-Size Poly Dispersity index Batch-1 (0. 5 % PVA) 697. 7 + 0. 06 nm -0. 9375 + 0. 0004 Batch -2 (0. 75 % PVA) 939. 8 + 0. 01 nm 0. 383+ 0. 001 Batch 3 (1 % PVA) 527. 6 + 0. 04 nm -1. 597+ 0. 001 Entrapment Efficiency of C-SLNs Batch (n=3) Total Drug content (mg) Free Drug Content (mg) Entrapment efficiency (%EE) (TDC –FDC)/TDC * 100) Batch 1 5. 448+ 0. 02 1. 006+ 0. 07 81. 53 + 0. 02% Batch 2 5. 880+ 0. 09 1. 150+ 0. 06 80. 44 + 0. 09% Batch 3 5. 860+ 0. 10 1. 012+ 0. 07 82. 73 + 0. 01%

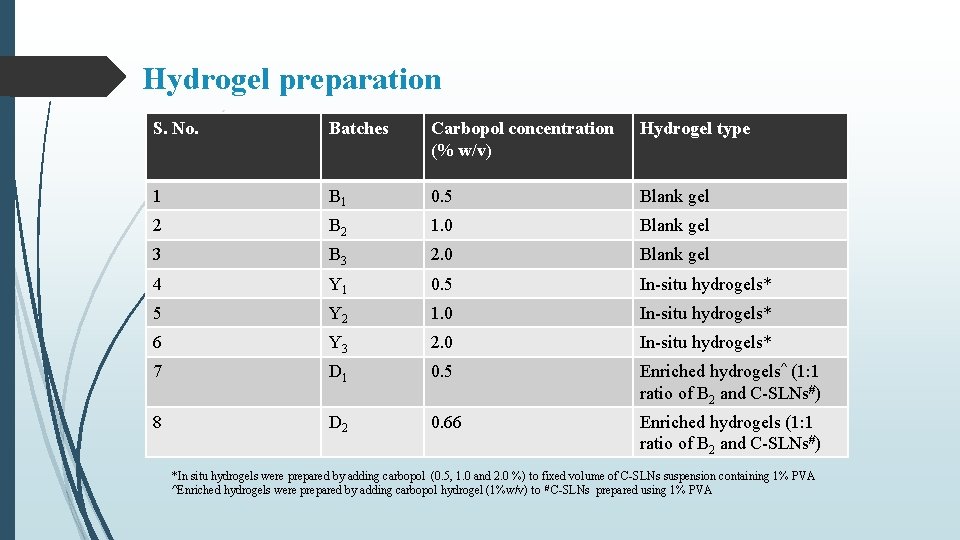
Hydrogel preparation S. No. Batches Carbopol concentration (% w/v) Hydrogel type 1 B 1 0. 5 Blank gel 2 B 2 1. 0 Blank gel 3 B 3 2. 0 Blank gel 4 Y 1 0. 5 In-situ hydrogels* 5 Y 2 1. 0 In-situ hydrogels* 6 Y 3 2. 0 In-situ hydrogels* 7 D 1 0. 5 Enriched hydrogels^ (1: 1 ratio of B 2 and C-SLNs#) 8 D 2 0. 66 Enriched hydrogels (1: 1 ratio of B 2 and C-SLNs#) *In situ hydrogels were prepared by adding carbopol (0. 5, 1. 0 and 2. 0 %) to fixed volume of C-SLNs suspension containing 1% PVA ^Enriched hydrogels were prepared by adding carbopol hydrogel (1%w/v) to # C-SLNs prepared using 1% PVA

Texture profile analysis Typical force-time plot of Texture analysis

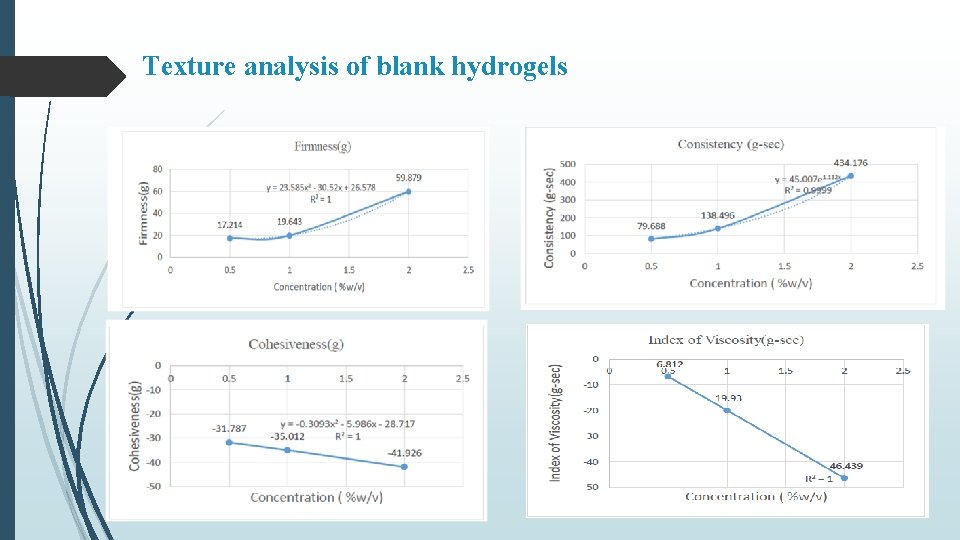
Texture analysis of blank hydrogels

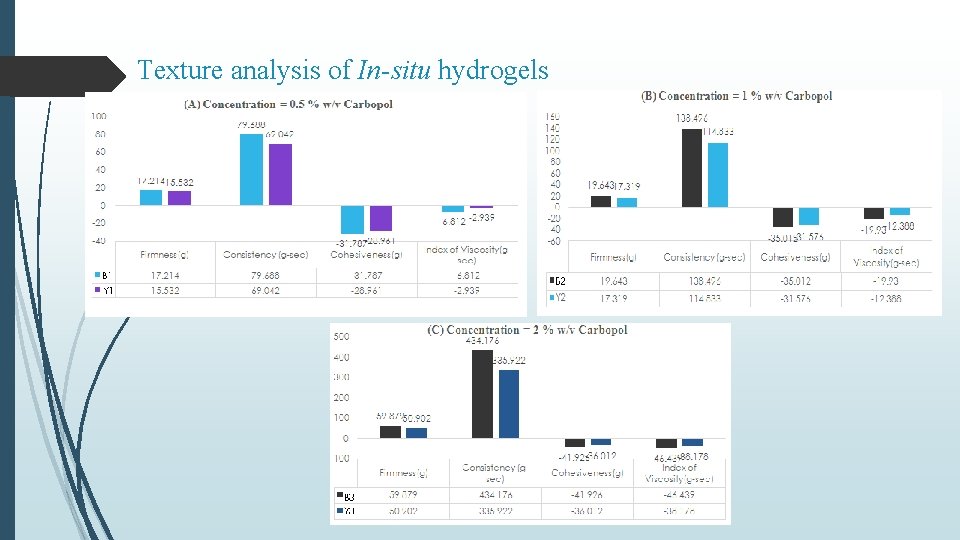
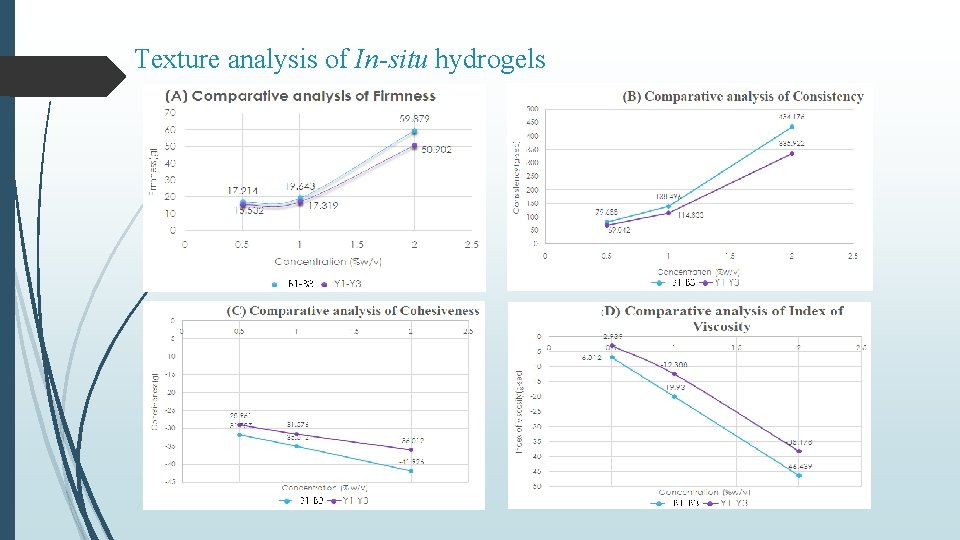
Texture analysis of In-situ hydrogels

Texture analysis of In-situ hydrogels

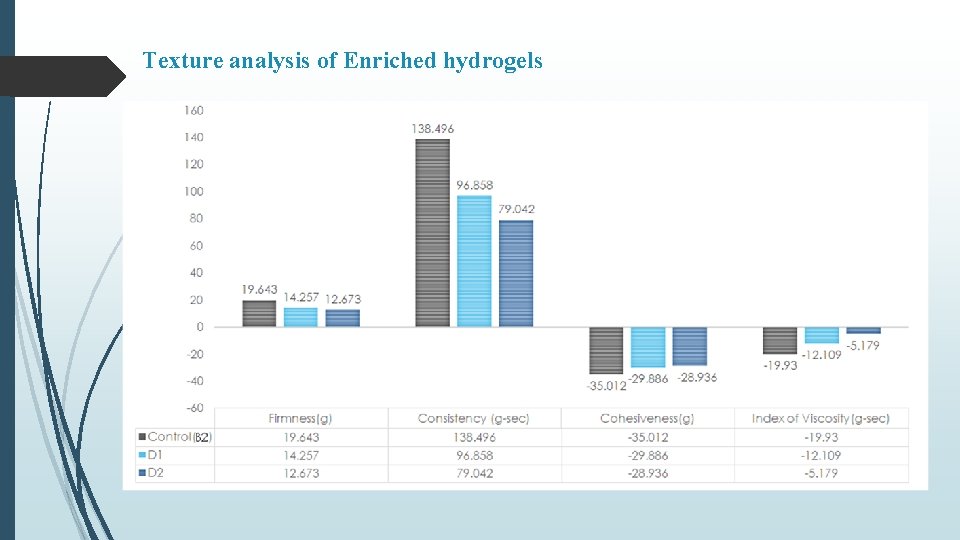
Texture analysis of Enriched hydrogels

Comparative Texture analysis

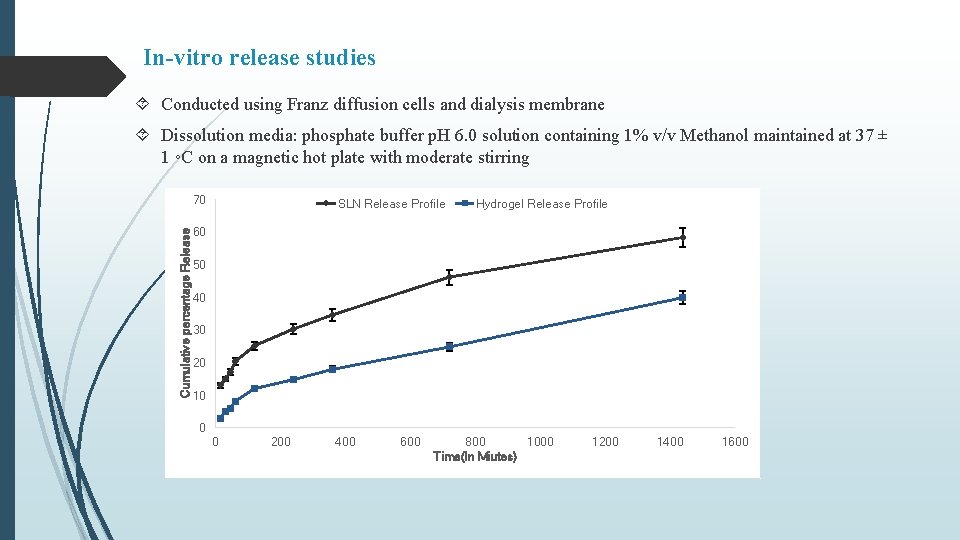
In-vitro release studies Conducted using Franz diffusion cells and dialysis membrane Dissolution media: phosphate buffer p. H 6. 0 solution containing 1% v/v Methanol maintained at 37 ± 1 ◦C on a magnetic hot plate with moderate stirring Cumulative percentage Release 70 SLN Release Profile Hydrogel Release Profile 60 50 40 30 20 10 0 0 200 400 600 800 1000 Time(In Miutes) 1200 1400 1600

In-vitro occlusion test 120 100 80 60 40 20 0 % water loss (relative to Control ) Occlusion Factor, F Control 100 Occlusion 0 Factor, F 0. 5 % Carbopol Gel 61. 3333333 % water loss 38. 6666665 (relative to Control ) 0. 5 % SLN-Gel 28. 83333332 71. 16666667

Summary & Conclusion Curcumin loaded SLNs were prepared by nano-precipitation technique using stearic acid as lipid and PVA as surfactant. Batch-3 (1% PVA) exhibited particle size of 527. 6 nm, PDI of -1. 597 and % Entrapment efficiency 82. 73% In vitro release from C-SLN enriched hydrogel showed controlled release of drug in comparison to C-SLNs upto 72 hr with approx. 58 % release within 24 hours. Occlusion test showed reduced water loss from carbopol and even lesser from enriched hydrogel. Stability studies over a period of 90 days, showed increase in firmness, cohesiveness and index of viscosity and decrease in consistency. In-situ hydrogels exhibited a concentration (carbopol) dependent increase in firmness, consistency, cohesiveness and viscosity, however, presence of C-SLNs significantly decreased (p < 0. 05) these values in comparison to blank hydrogels. Similar observations were made in enriched hydrogels Also, a significant difference (p < 0. 05) in hydrogel properties was observed between in-situ and enriched hydrogels indicating effect of SLNs on the swelling properties of Carbopol. Occlusive properties of in-situ hydrogels were better than enriched and blank hydrogels. In-situ hydrogels also exhibited uniform and extended release of curcumin, alongwith higher permeation characteristics. Better formulation characteristics of in-situ hydrogels might be because of homogenous deposition and gelling of carbopol around curcumin nanoparticles.

References: Strimpakos, A. S. , Sharma, R. A. (2008). Comprehensive invited review curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxidants & Redox Signaling 10, 511 -546. Kurien, B. T. , Singh, A. , Matsumoto, H. , Scofield, R. H. (2007). Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay and Drug Development Technologies 5, 567 -576. Patel, R. , Singh, S. K. , Singh, S. , Sheth, N. R. , Gendle, R. (2009). Development and characterization of curcumin loaded transfersome for transdermal delivery. Journal of Pharmaceutical Sciences and Research 1, 71 -80. Aggarwal, B. B. , Sung, B. (2009). Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends in Pharmacological Sciences 30, 85 -94. Mehnert, W. and Mader, K. (2001) Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. , 47, 165 -196. Yang, S. , Zhu, J. , Lu, Y. , Liang, B. , Yang C. (1999) Body distribution of camptothecin solid lipid nanoparticles after oral administration. Pharm. Res. , 16 , pp. 751– 757. Korting, M. S. , Mehnert, W. and Korting, H. C. (2007) lipid nanoparticles for improved topical applicationof drugs for skin disease, Adv Drug Del Review, 59, 427 -443. Chorny, M. , Fishbein, I. , Danenberg, H. D. , Golomb, G. (2002) Lipophilic drug loaded nanospheres prepared by nanoprecipitation: effect of formulation variables on size, drug recovery and release kinetics Journal of Controlled Release 83 389– 400.

23

Thank You
 Lipid nanoparticles
Lipid nanoparticles Curcumin breast cancer
Curcumin breast cancer Curcumin plus vestige
Curcumin plus vestige Enhancing thermal conductivity of fluids with nanoparticles
Enhancing thermal conductivity of fluids with nanoparticles Introduction about nanoparticles
Introduction about nanoparticles Nanoparticles
Nanoparticles Introduction of nanoparticles
Introduction of nanoparticles What is a nanoparticle gcse
What is a nanoparticle gcse Evaporation mixture examples
Evaporation mixture examples A solid solution example
A solid solution example Crystalline solid and amorphous solid
Crystalline solid and amorphous solid Covalent molecular and covalent network
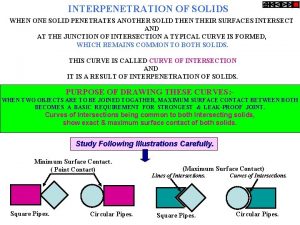
Covalent molecular and covalent network When a solid completely penetrates another solid
When a solid completely penetrates another solid Is cotton candy anisotropic
Is cotton candy anisotropic Crystalline vs non crystalline
Crystalline vs non crystalline Interpenetration of solids
Interpenetration of solids Crystalline solid and amorphous solid
Crystalline solid and amorphous solid Crystal solid and amorphous solid
Crystal solid and amorphous solid The kitchen presented by
The kitchen presented by Generic structure of spoof text
Generic structure of spoof text Bob cratchit quotes
Bob cratchit quotes Topic main idea and supporting details examples
Topic main idea and supporting details examples Revenge theme in romeo and juliet
Revenge theme in romeo and juliet How it's made presented by
How it's made presented by Nnn1996
Nnn1996