Indicaciones de quimioterapia adyuvante en cncer de endometrio




















- Slides: 64

Indicaciones de quimioterapia adyuvante en cáncer de endometrio Nuria Lainez Milagro Complejo Hospitalario de Navarra Complejo hospitalario de Navarra

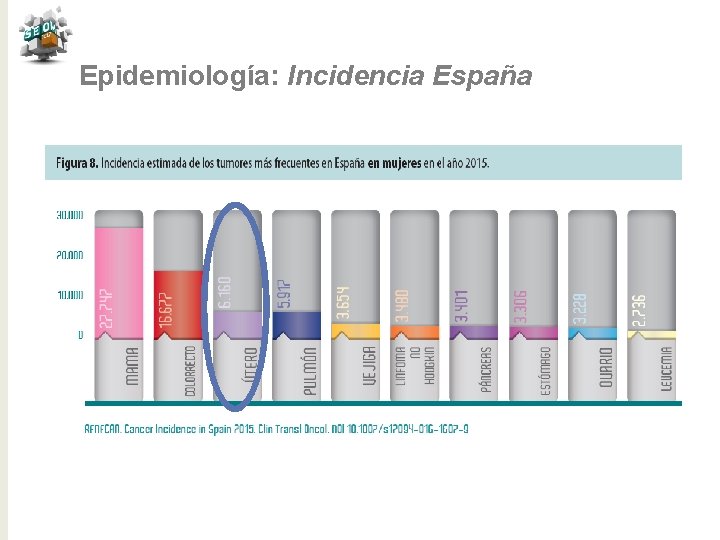
Epidemiología: Incidencia España

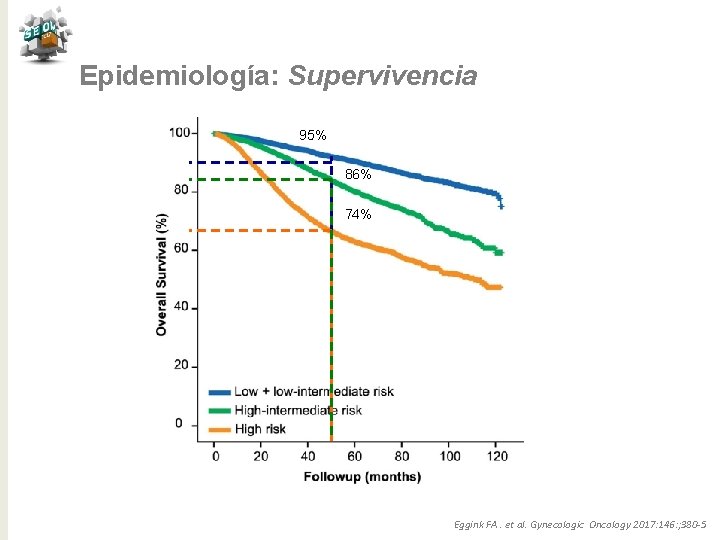
Epidemiología: Supervivencia 95% 86% 74% Eggink FA. et al. Gynecologic Oncology 2017: 146: ; 380 -5

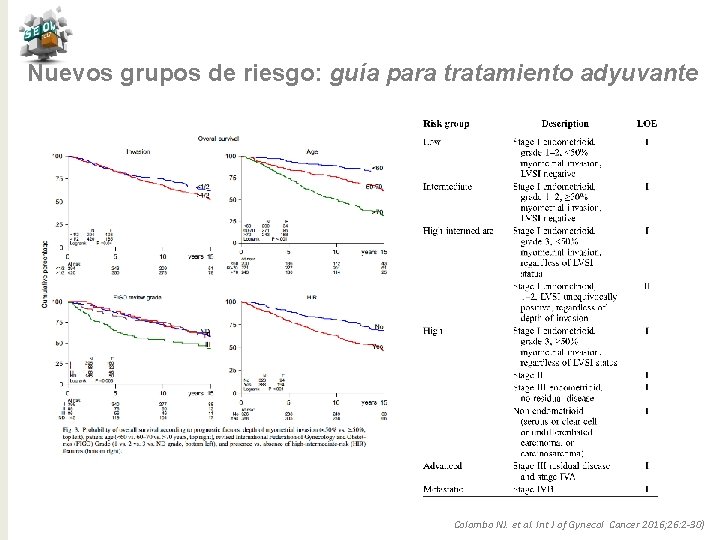
Nuevos grupos de riesgo: guía para tratamiento adyuvante Colombo NJ. et al. Int J of Gynecol Cancer 2016; 26: 2 -30)

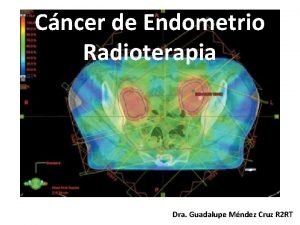
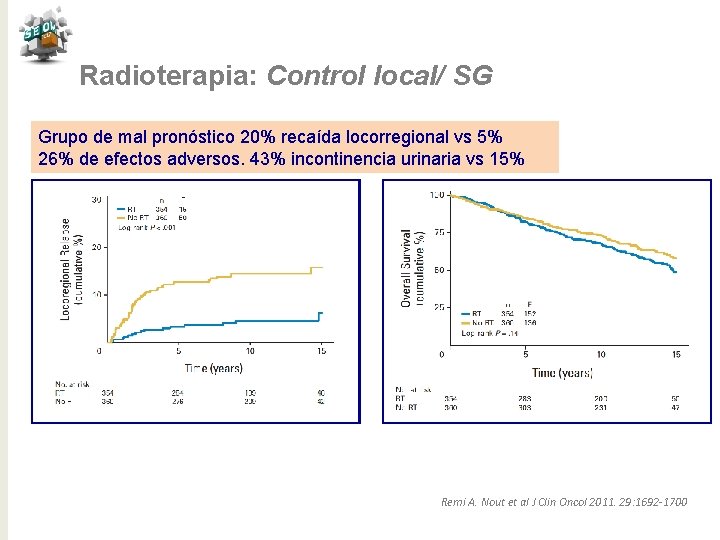
Radioterapia: Control local/ SG Grupo de mal pronóstico 20% recaída locorregional vs 5% 26% de efectos adversos. 43% incontinencia urinaria vs 15% Remi A. Nout et al J Clin Oncol 2011. 29: 1692 -1700

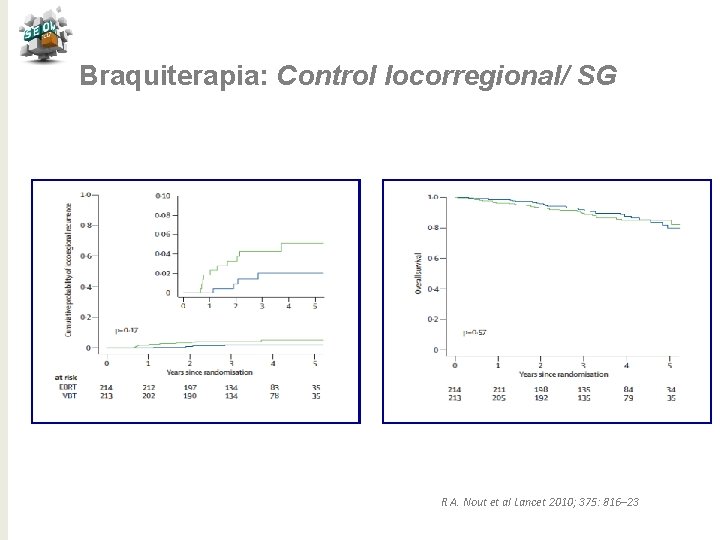
Braquiterapia: Control locorregional/ SG R A. Nout et al Lancet 2010; 375: 816– 23

Radioterapia: Control locorregional/ SG R A. Nout et al Lancet 2010; 375: 816– 23

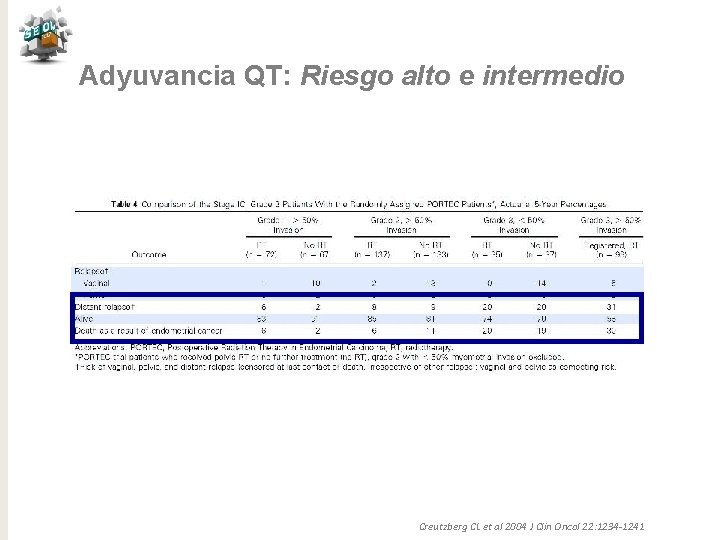
Adyuvancia QT: Riesgo alto e intermedio Creutzberg CL et al 2004 J Clin Oncol 22: 1234 -1241

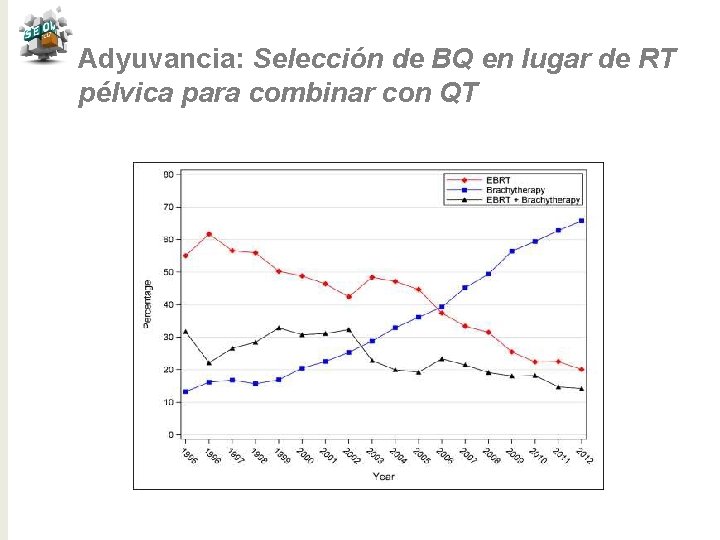
Adyuvancia: Selección de BQ en lugar de RT pélvica para combinar con QT

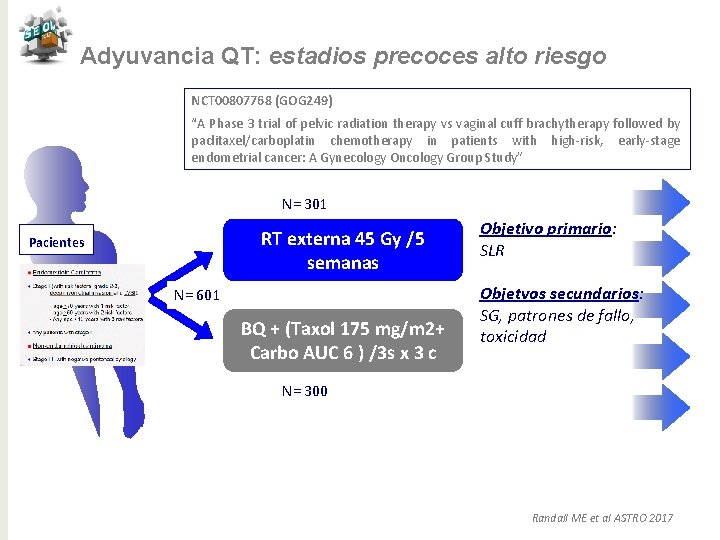
Adyuvancia QT: estadios precoces alto riesgo NCT 00807768 (GOG 249) “A Phase 3 trial of pelvic radiation therapy vs vaginal cuff brachytherapy followed by paclitaxel/carboplatin chemotherapy in patients with high-risk, early-stage endometrial cancer: A Gynecology Oncology Group Study” N= 301 RT externa 45 Gy /5 semanas Pacientes N= 601 BQ + (Taxol 175 mg/m 2+ Carbo AUC 6 ) /3 s x 3 c Objetivo primario: SLR Objetvos secundarios: SG, patrones de fallo, toxicidad N= 300 Randall ME et al ASTRO 2017

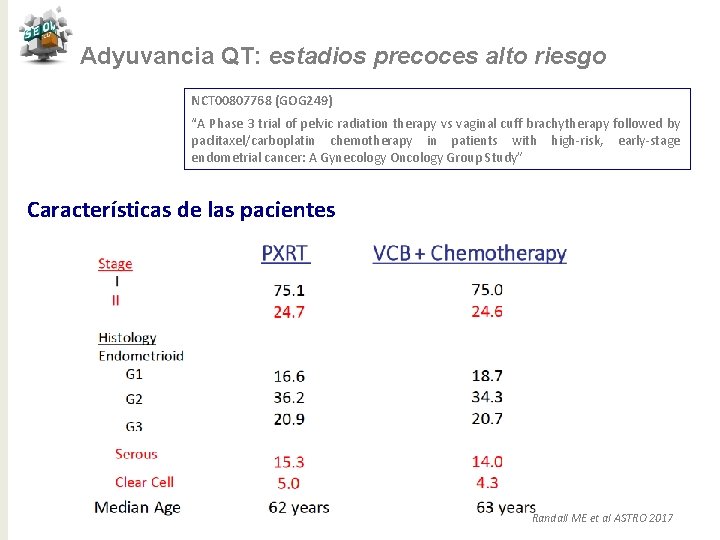
Adyuvancia QT: estadios precoces alto riesgo NCT 00807768 (GOG 249) “A Phase 3 trial of pelvic radiation therapy vs vaginal cuff brachytherapy followed by paclitaxel/carboplatin chemotherapy in patients with high-risk, early-stage endometrial cancer: A Gynecology Oncology Group Study” Características de las pacientes Randall ME et al ASTRO 2017

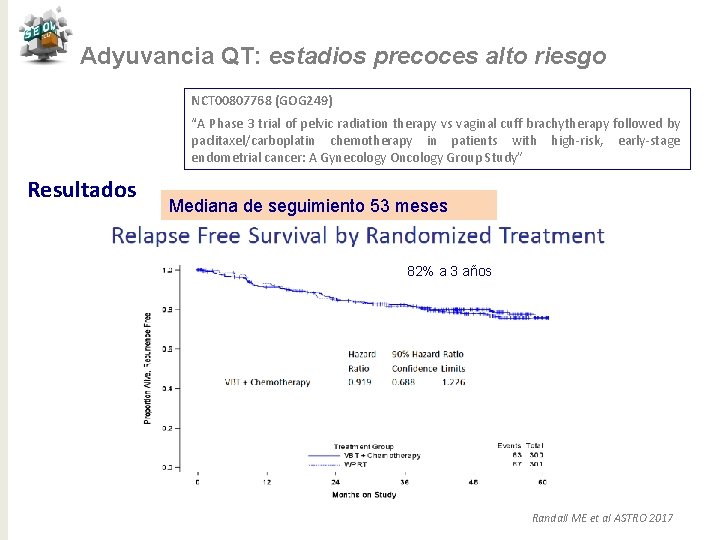
Adyuvancia QT: estadios precoces alto riesgo NCT 00807768 (GOG 249) “A Phase 3 trial of pelvic radiation therapy vs vaginal cuff brachytherapy followed by paclitaxel/carboplatin chemotherapy in patients with high-risk, early-stage endometrial cancer: A Gynecology Oncology Group Study” Resultados Mediana de seguimiento 53 meses 82% a 3 años Randall ME et al ASTRO 2017

Adyuvancia QT: estadios precoces alto riesgo NCT 00807768 (GOG 249) “A Phase 3 trial of pelvic radiation therapy vs vaginal cuff brachytherapy followed by paclitaxel/carboplatin chemotherapy in patients with high-risk, early-stage endometrial cancer: A Gynecology Oncology Group Study” Resultados 91% vs 98% a 3 años Randall ME et al ASTRO 2017

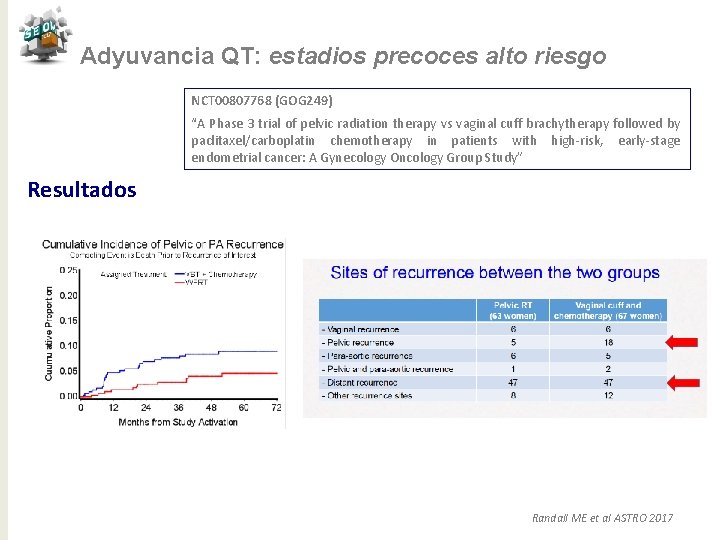
Adyuvancia QT: estadios precoces alto riesgo NCT 00807768 (GOG 249) “A Phase 3 trial of pelvic radiation therapy vs vaginal cuff brachytherapy followed by paclitaxel/carboplatin chemotherapy in patients with high-risk, early-stage endometrial cancer: A Gynecology Oncology Group Study” Resultados Randall ME et al ASTRO 2017

Adyuvancia QT: estadios precoces alto riesgo NCT 00807768 (GOG 249) “A Phase 3 trial of pelvic radiation therapy vs vaginal cuff brachytherapy followed by paclitaxel/carboplatin chemotherapy in patients with high-risk, early-stage endometrial cancer: A Gynecology Oncology Group Study” Resultados Randall ME et al ASTRO 2017

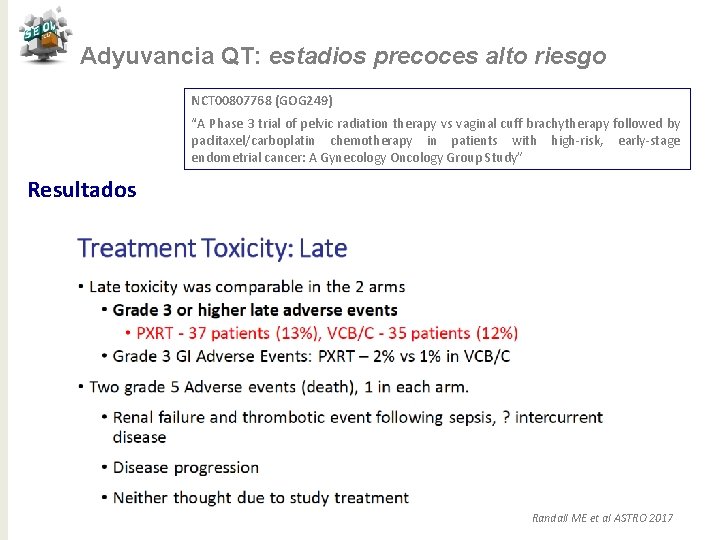
Adyuvancia QT: estadios precoces alto riesgo NCT 00807768 (GOG 249) “A Phase 3 trial of pelvic radiation therapy vs vaginal cuff brachytherapy followed by paclitaxel/carboplatin chemotherapy in patients with high-risk, early-stage endometrial cancer: A Gynecology Oncology Group Study” Resultados Randall ME et al ASTRO 2017

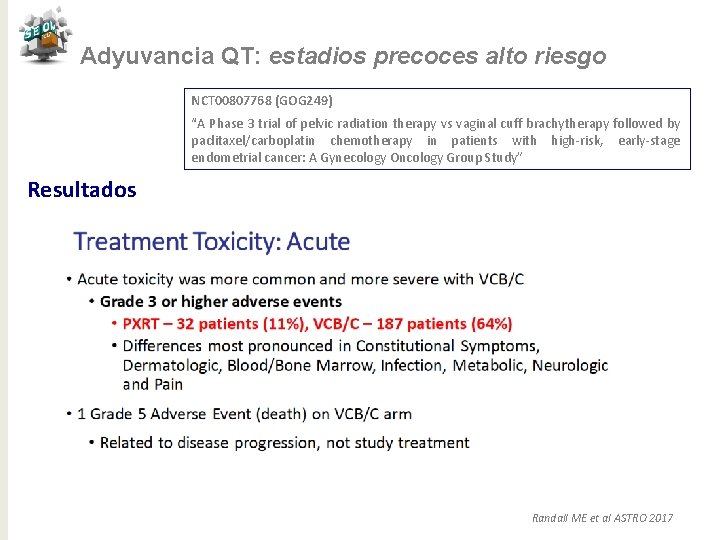
Adyuvancia QT: estadios precoces alto riesgo NCT 00807768 (GOG 249) “A Phase 3 trial of pelvic radiation therapy vs vaginal cuff brachytherapy followed by paclitaxel/carboplatin chemotherapy in patients with high-risk, early-stage endometrial cancer: A Gynecology Oncology Group Study” Conclusiones ü El estudio no demuestra superioridad de BQ/QT frente a RT ü Toxicidad aguda mayor en rama experimental ü Recaída ganglionar más frecuente en rama experimental ü RT sola sigue siendo un tratamiento apropiado para las pacientes con tumores en estadíos precoces de alto riesgo ( la modalidad de RT se ha de individualizar en base a factores pronósticos) Randall ME et al ASTRO 2017

Adyuvancia QT: estadios precoces alto riesgo

Radioterapia vs Quimioterapia 19

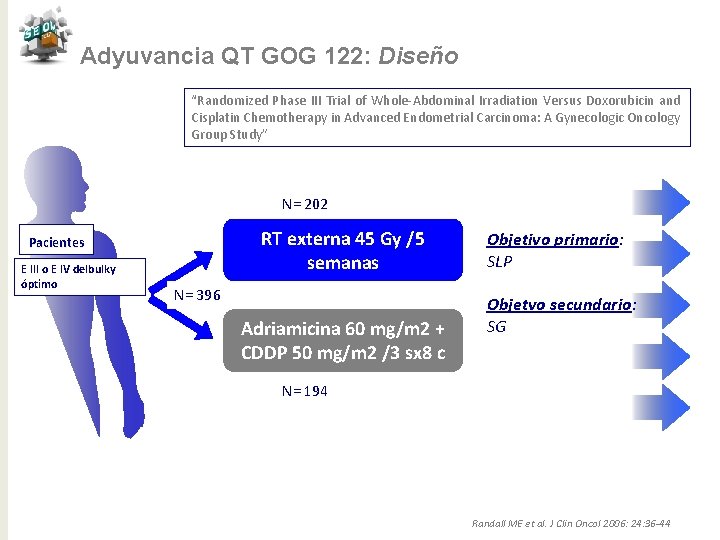
Adyuvancia QT GOG 122: Diseño “Randomized Phase III Trial of Whole-Abdominal Irradiation Versus Doxorubicin and Cisplatin Chemotherapy in Advanced Endometrial Carcinoma: A Gynecologic Oncology Group Study” N= 202 RT externa 45 Gy /5 semanas Pacientes E III o E IV delbulky óptimo N= 396 Adriamicina 60 mg/m 2 + CDDP 50 mg/m 2 /3 sx 8 c Objetivo primario: SLP Objetvo secundario: SG N= 194 Randall ME et al. J Clin Oncol 2006: 24: 36 -44

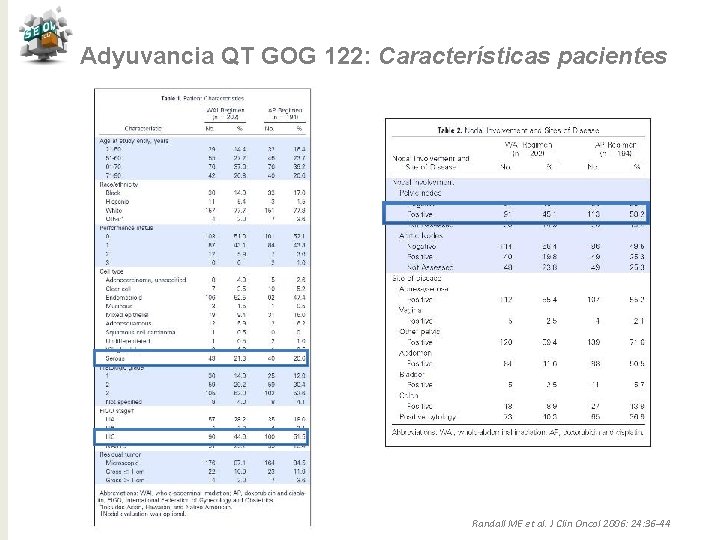
Adyuvancia QT GOG 122: Características pacientes Randall ME et al. J Clin Oncol 2006: 24: 36 -44

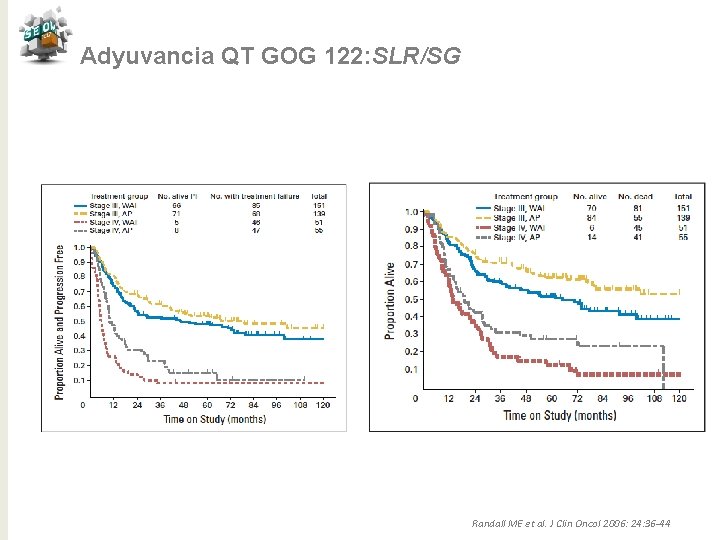
Adyuvancia QT GOG 122: SLR /SG Randall ME et al. J Clin Oncol 2006: 24: 36 -44

Adyuvancia QT GOG 122: SLR/SG Randall ME et al. J Clin Oncol 2006: 24: 36 -44

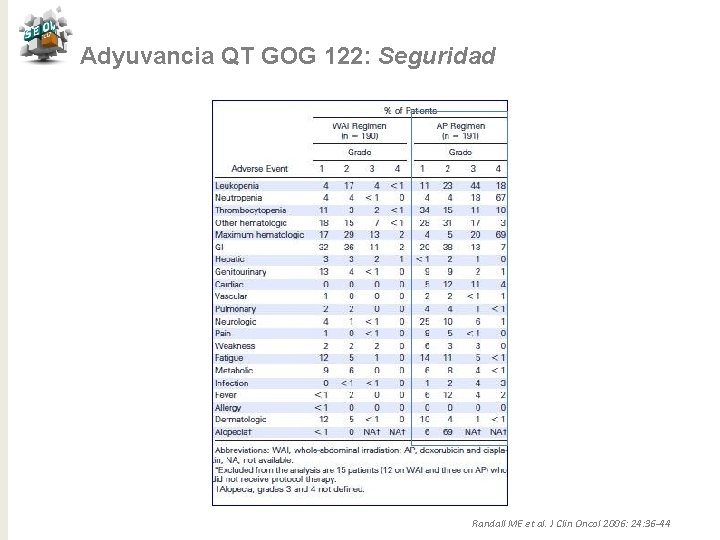
Adyuvancia QT GOG 122: Seguridad Randall ME et al. J Clin Oncol 2006: 24: 36 -44

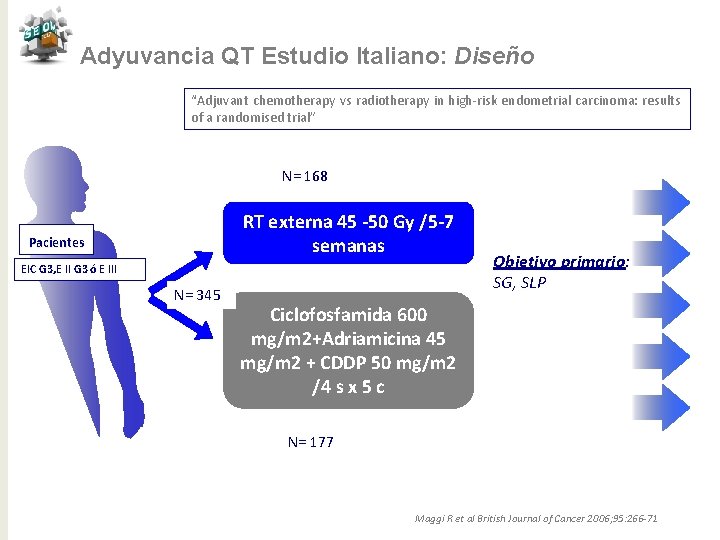
Adyuvancia QT Estudio Italiano: Diseño “Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial” N= 168 RT externa 45 -50 Gy /5 -7 semanas Pacientes EIC G 3, E II G 3 ó E III N= 345 Objetivo primario: SG, SLP Ciclofosfamida 600 mg/m 2+Adriamicina 45 mg/m 2 + CDDP 50 mg/m 2 /4 s x 5 c N= 177 Maggi R et al British Journal of Cancer 2006; 95: 266 -71

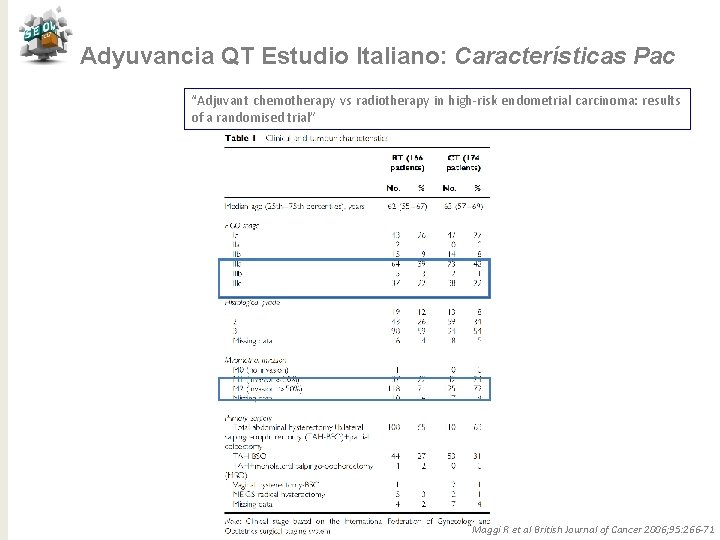
Adyuvancia QT Estudio Italiano: Características Pac “Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial” Maggi R et al British Journal of Cancer 2006; 95: 266 -71

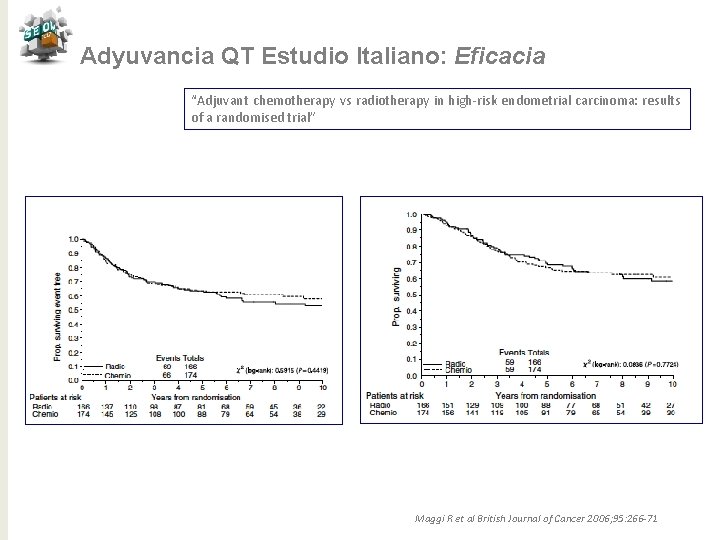
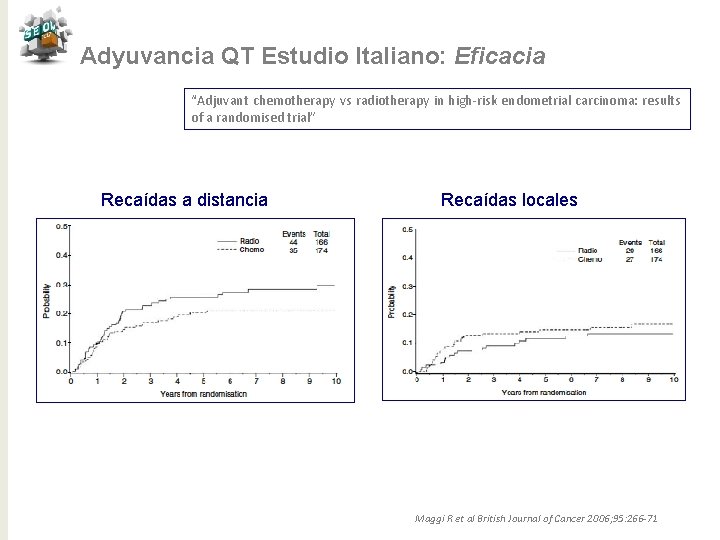
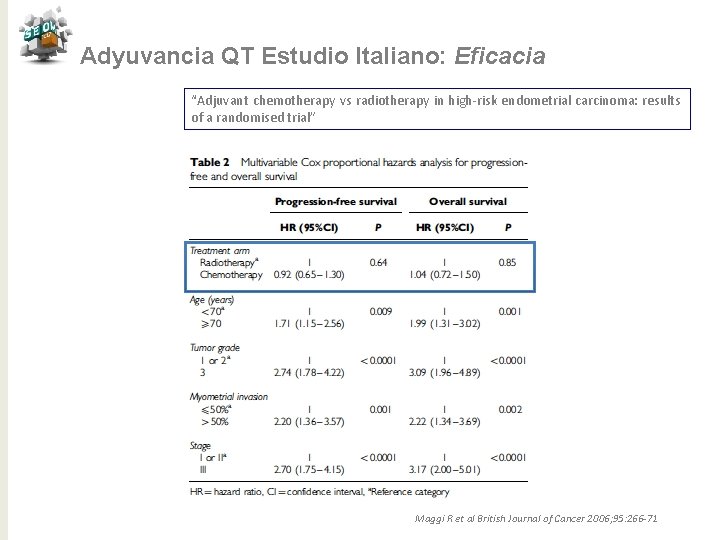
Adyuvancia QT Estudio Italiano: Eficacia “Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial” Maggi R et al British Journal of Cancer 2006; 95: 266 -71

Adyuvancia QT Estudio Italiano: Eficacia “Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial” Recaídas a distancia Recaídas locales Maggi R et al British Journal of Cancer 2006; 95: 266 -71

Adyuvancia QT Estudio Italiano: Eficacia “Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial” Maggi R et al British Journal of Cancer 2006; 95: 266 -71

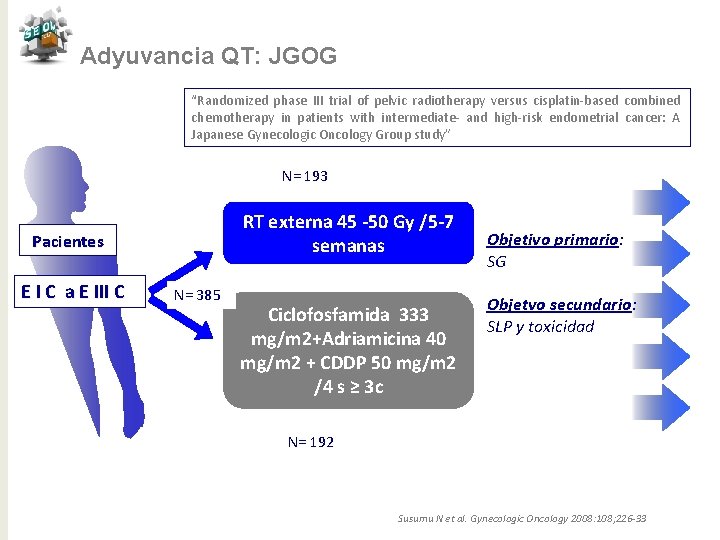
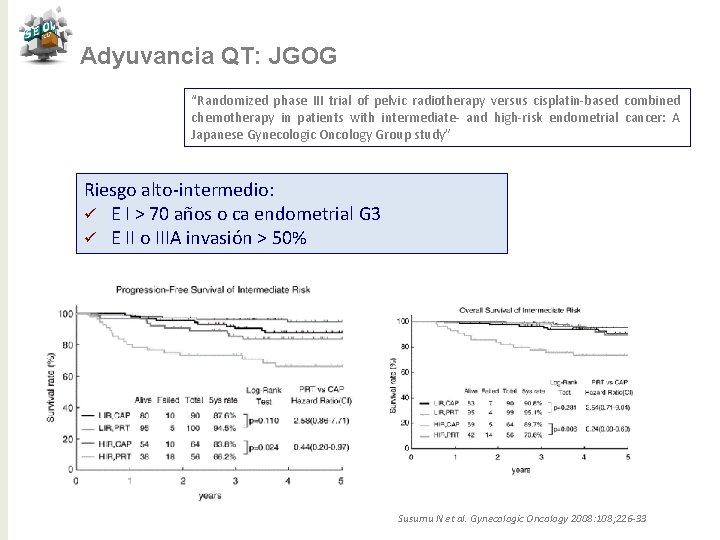
Adyuvancia QT: JGOG “Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: A Japanese Gynecologic Oncology Group study” N= 193 RT externa 45 -50 Gy /5 -7 semanas Pacientes E I C a E III C N= 385 Ciclofosfamida 333 mg/m 2+Adriamicina 40 mg/m 2 + CDDP 50 mg/m 2 /4 s ≥ 3 c Objetivo primario: SG Objetvo secundario: SLP y toxicidad N= 192 Susumu N et al. Gynecologic Oncology 2008: 108; 226 -33

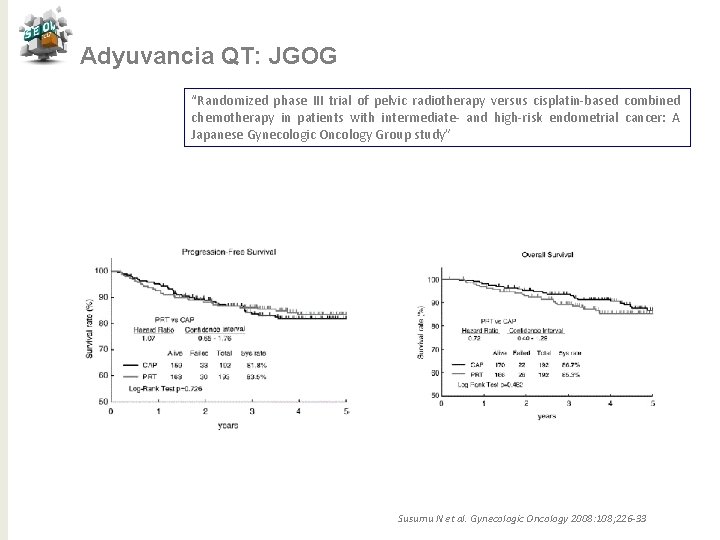
Adyuvancia QT: JGOG “Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: A Japanese Gynecologic Oncology Group study” Susumu N et al. Gynecologic Oncology 2008: 108; 226 -33

Adyuvancia QT: JGOG “Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: A Japanese Gynecologic Oncology Group study” Riesgo alto-intermedio: ü E I > 70 años o ca endometrial G 3 ü E II o IIIA invasión > 50% Susumu N et al. Gynecologic Oncology 2008: 108; 226 -33

Radioterapia vs Quimioradio 33

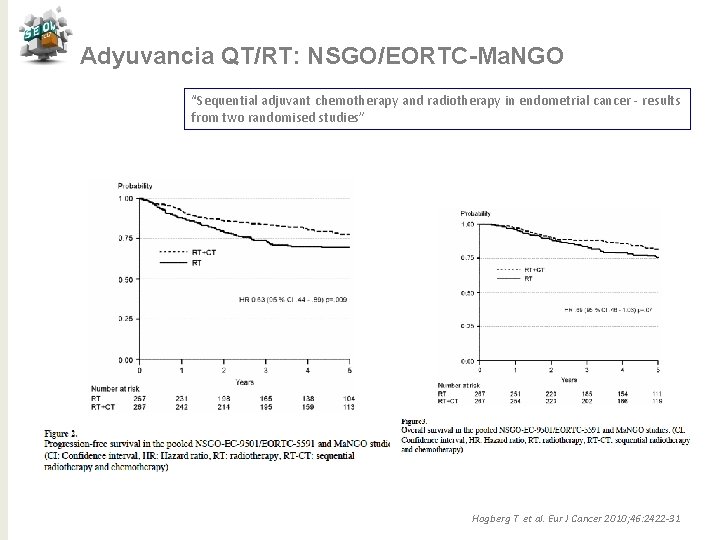
Adyuvancia QT/RT: NSGO/EORTC-Ma. NGO “Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer - results from two randomised studies” N 534 Figo I-IIIA alto riesgo Hogberg T et al. Eur J Cancer 2010; 46: 2422 -31

Adyuvancia QT/RT: NSGO/EORTC-Ma. NGO “Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer - results from two randomised studies” Hogberg T et al. Eur J Cancer 2010; 46: 2422 -31

Adyuvancia QT: Racional para uso taxanos • Adriamicina + platino ha sido el regimen estándar en cáncer de endometrio • GOG 177: Primera línea superioridad de añadir taxol a ciaplatino + adriamicina • GOG 209: demuestra que taxol+ platino+ adriamicina es igual a carboplatino + taxol

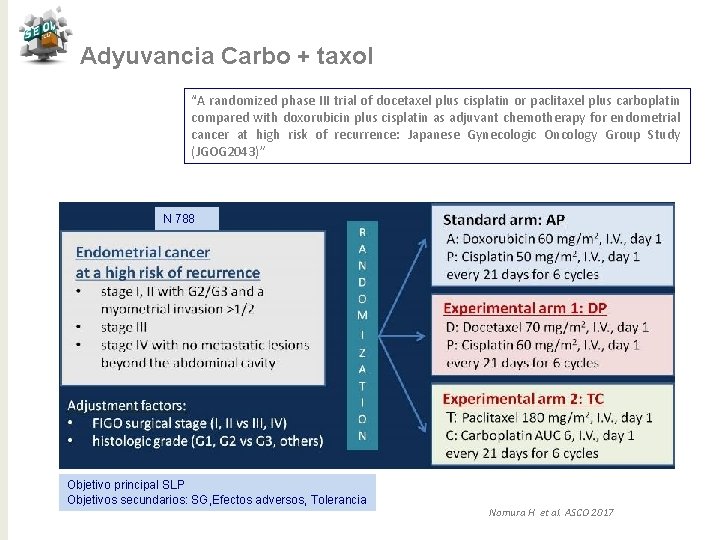
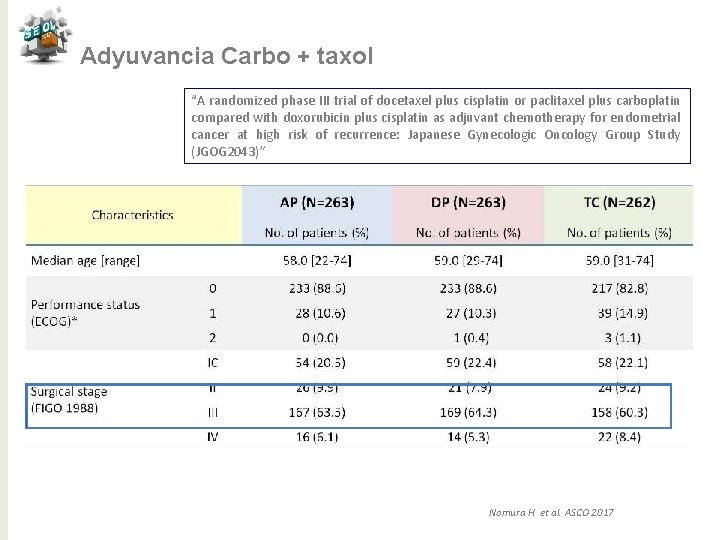
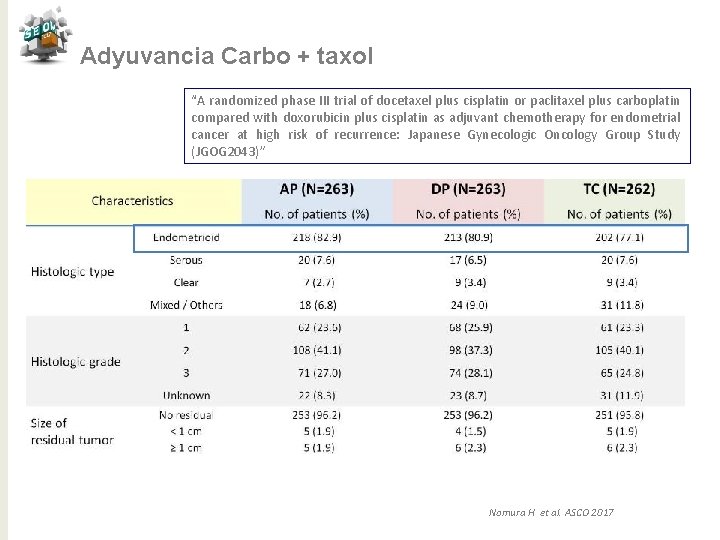
Adyuvancia Carbo + taxol “A randomized phase III trial of docetaxel plus cisplatin or paclitaxel plus carboplatin compared with doxorubicin plus cisplatin as adjuvant chemotherapy for endometrial cancer at high risk of recurrence: Japanese Gynecologic Oncology Group Study (JGOG 2043)” N 788 Objetivo principal SLP Objetivos secundarios: SG, Efectos adversos, Tolerancia Nomura H et al. ASCO 2017

Adyuvancia Carbo + taxol “A randomized phase III trial of docetaxel plus cisplatin or paclitaxel plus carboplatin compared with doxorubicin plus cisplatin as adjuvant chemotherapy for endometrial cancer at high risk of recurrence: Japanese Gynecologic Oncology Group Study (JGOG 2043)” Nomura H et al. ASCO 2017

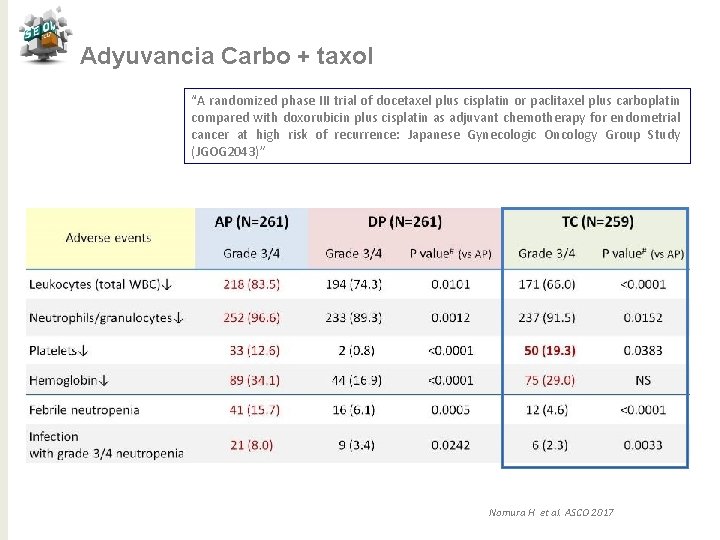
Adyuvancia Carbo + taxol “A randomized phase III trial of docetaxel plus cisplatin or paclitaxel plus carboplatin compared with doxorubicin plus cisplatin as adjuvant chemotherapy for endometrial cancer at high risk of recurrence: Japanese Gynecologic Oncology Group Study (JGOG 2043)” Nomura H et al. ASCO 2017

Adyuvancia Carbo + taxol “A randomized phase III trial of docetaxel plus cisplatin or paclitaxel plus carboplatin compared with doxorubicin plus cisplatin as adjuvant chemotherapy for endometrial cancer at high risk of recurrence: Japanese Gynecologic Oncology Group Study (JGOG 2043)” Nomura H et al. ASCO 2017

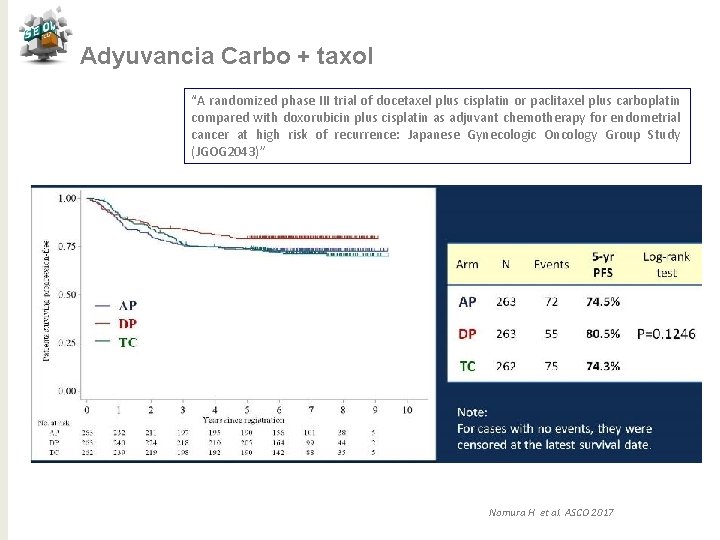
Adyuvancia Carbo + taxol “A randomized phase III trial of docetaxel plus cisplatin or paclitaxel plus carboplatin compared with doxorubicin plus cisplatin as adjuvant chemotherapy for endometrial cancer at high risk of recurrence: Japanese Gynecologic Oncology Group Study (JGOG 2043)” Nomura H et al. ASCO 2017

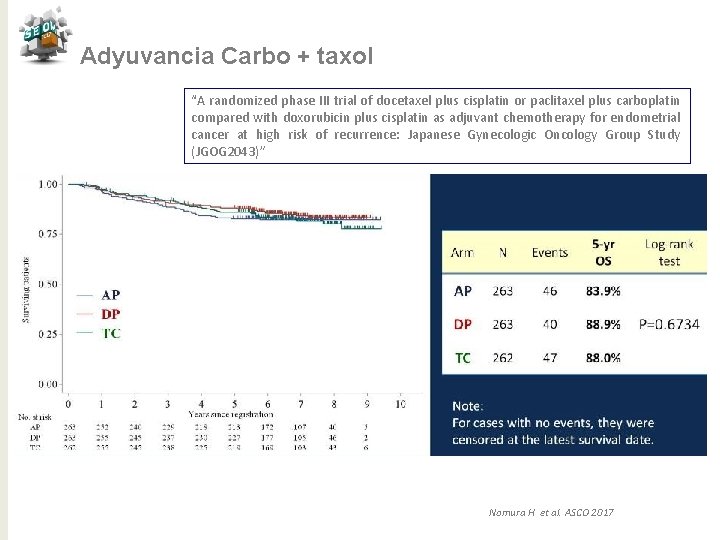
Adyuvancia Carbo + taxol “A randomized phase III trial of docetaxel plus cisplatin or paclitaxel plus carboplatin compared with doxorubicin plus cisplatin as adjuvant chemotherapy for endometrial cancer at high risk of recurrence: Japanese Gynecologic Oncology Group Study (JGOG 2043)” Nomura H et al. ASCO 2017

Adyuvancia Carbo + taxol “A randomized phase III trial of docetaxel plus cisplatin or paclitaxel plus carboplatin compared with doxorubicin plus cisplatin as adjuvant chemotherapy for endometrial cancer at high risk of recurrence: Japanese Gynecologic Oncology Group Study (JGOG 2043)” Nomura H et al. ASCO 2017

Quimioradio+QT vs Quimioterapia 44

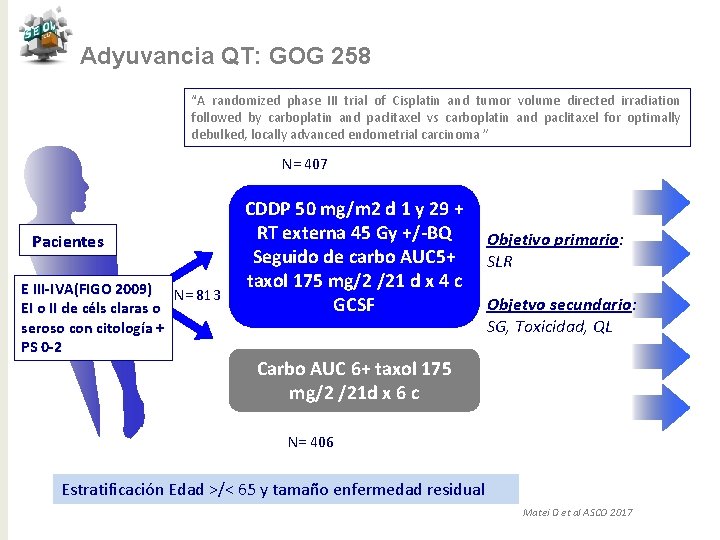
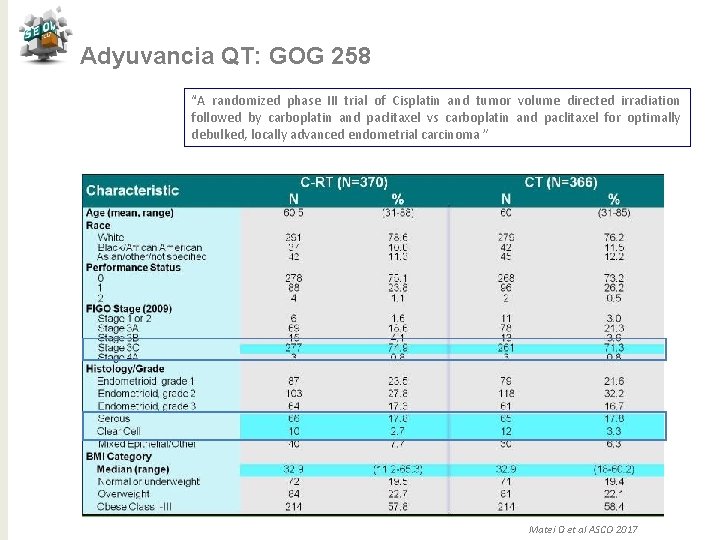
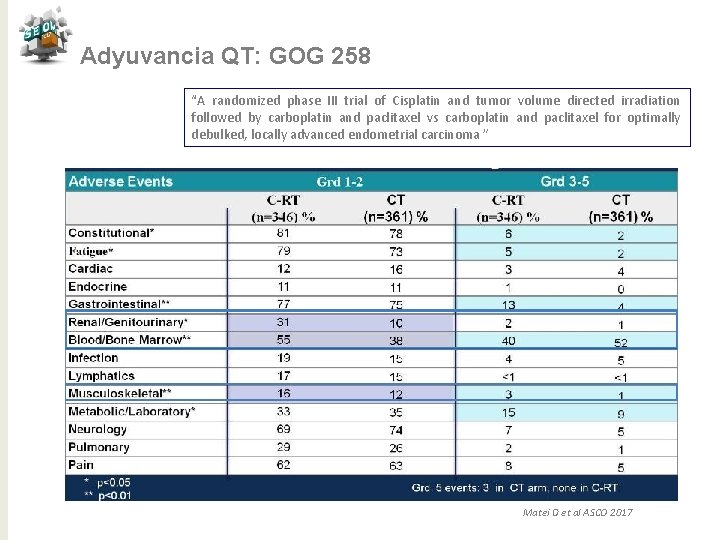
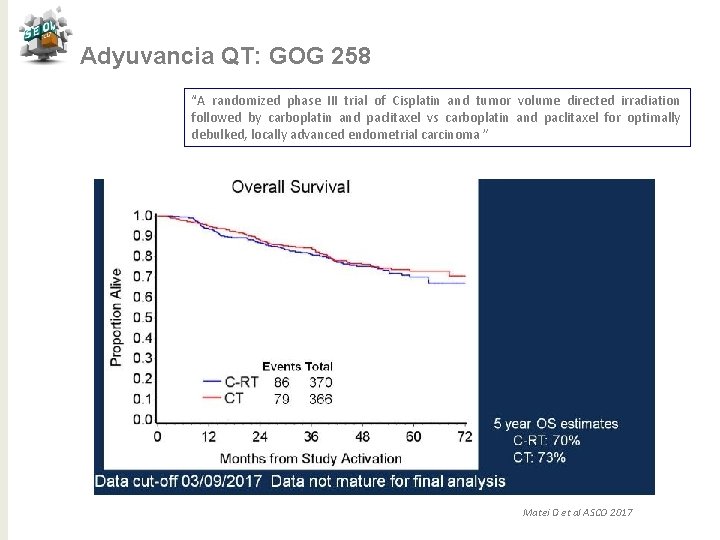
Adyuvancia QT: GOG 258 “A randomized phase III trial of Cisplatin and tumor volume directed irradiation followed by carboplatin and paclitaxel vs carboplatin and paclitaxel for optimally debulked, locally advanced endometrial carcinoma ” N= 407 Pacientes E III-IVA(FIGO 2009) N= 813 EI o II de céls claras o seroso con citología + PS 0 -2 CDDP 50 mg/m 2 d 1 y 29 + RT externa 45 Gy +/-BQ Seguido de carbo AUC 5+ taxol 175 mg/2 /21 d x 4 c GCSF Objetivo primario: SLR Objetvo secundario: SG, Toxicidad, QL Carbo AUC 6+ taxol 175 mg/2 /21 d x 6 c N= 406 Estratificación Edad >/< 65 y tamaño enfermedad residual Matei D et al ASCO 2017

Adyuvancia QT: GOG 258 “A randomized phase III trial of Cisplatin and tumor volume directed irradiation followed by carboplatin and paclitaxel vs carboplatin and paclitaxel for optimally debulked, locally advanced endometrial carcinoma ” Matei D et al ASCO 2017

Adyuvancia QT: GOG 258 “A randomized phase III trial of Cisplatin and tumor volume directed irradiation followed by carboplatin and paclitaxel vs carboplatin and paclitaxel for optimally debulked, locally advanced endometrial carcinoma ” Matei D et al ASCO 2017

Adyuvancia QT: GOG 258 “A randomized phase III trial of Cisplatin and tumor volume directed irradiation followed by carboplatin and paclitaxel vs carboplatin and paclitaxel for optimally debulked, locally advanced endometrial carcinoma ” Matei D et al ASCO 2017

Adyuvancia QT: GOG 258 “A randomized phase III trial of Cisplatin and tumor volume directed irradiation followed by carboplatin and paclitaxel vs carboplatin and paclitaxel for optimally debulked, locally advanced endometrial carcinoma ” Matei D et al ASCO 2017

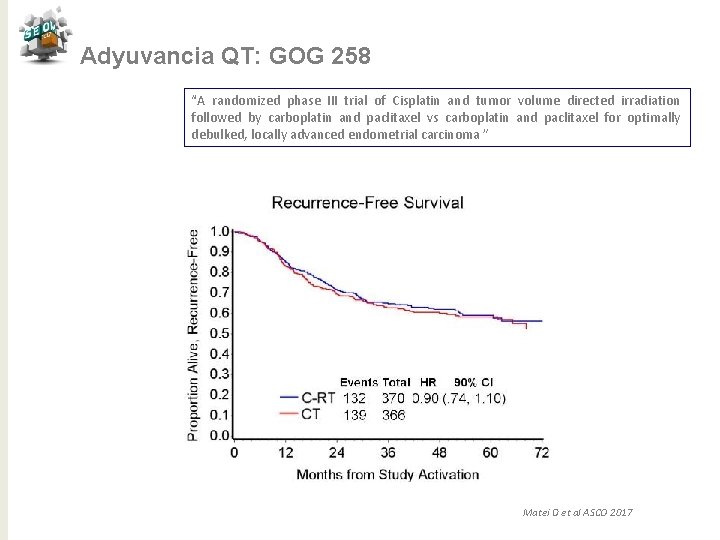
Adyuvancia QT: GOG 258 “A randomized phase III trial of Cisplatin and tumor volume directed irradiation followed by carboplatin and paclitaxel vs carboplatin and paclitaxel for optimally debulked, locally advanced endometrial carcinoma ” Conclusiones ü QT-RT no demuestra superioridad comparado con QT (HR 0. 9, CI 0. 74 -1. 1) ü Toxicidad aguda similar ambas ramas ü QT-RT reduce la incidencia de recaídas vaginales, pélvicas y paraaórticas con respecto a QT ü La recaída a distancia es más frecuente en QT-RT vs QT ü Los resultados en SG y QL se reportarán en el futuro

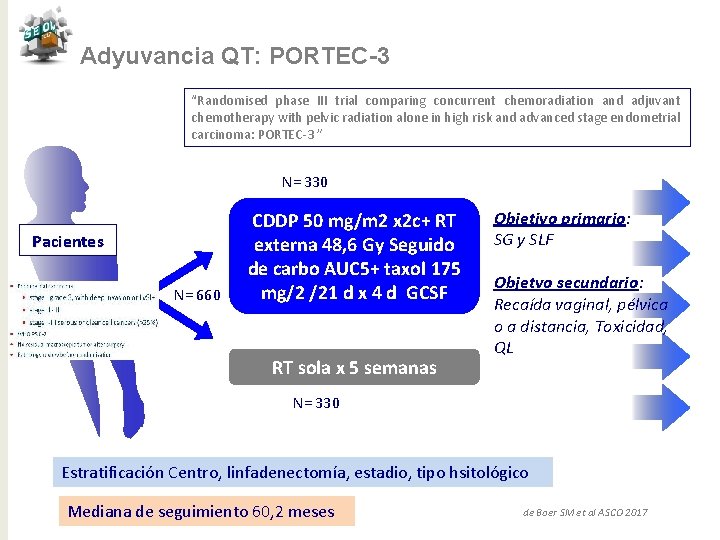
Adyuvancia QT: PORTEC-3 “Randomised phase III trial comparing concurrent chemoradiation and adjuvant chemotherapy with pelvic radiation alone in high risk and advanced stage endometrial carcinoma: PORTEC-3 ” N= 330 Pacientes N= 660 CDDP 50 mg/m 2 x 2 c+ RT externa 48, 6 Gy Seguido de carbo AUC 5+ taxol 175 mg/2 /21 d x 4 d GCSF RT sola x 5 semanas Objetivo primario: SG y SLF Objetvo secundario: Recaída vaginal, pélvica o a distancia, Toxicidad, QL N= 330 Estratificación Centro, linfadenectomía, estadio, tipo hsitológico Mediana de seguimiento 60, 2 meses de Boer SM et al ASCO 2017

Adyuvancia QT: PORTEC-3 “Randomised phase III trial comparing concurrent chemoradiation and adjuvant chemotherapy with pelvic radiation alone in high risk and advanced stage endometrial carcinoma: PORTEC-3 ” de Boer SM et al ASCO 2017

Adyuvancia QT: PORTEC-3 “Randomised phase III trial comparing concurrent chemoradiation and adjuvant chemotherapy with pelvic radiation alone in high risk and advanced stage endometrial carcinoma: PORTEC-3 ” de Boer SM et al ASCO 2017

Adyuvancia QT: PORTEC-3 “Randomised phase III trial comparing concurrent chemoradiation and adjuvant chemotherapy with pelvic radiation alone in high risk and advanced stage endometrial carcinoma: PORTEC-3 ” de Boer SM et al ASCO 2017

Adyuvancia QT: PORTEC-3 “Randomised phase III trial comparing concurrent chemoradiation and adjuvant chemotherapy with pelvic radiation alone in high risk and advanced stage endometrial carcinoma: PORTEC-3 ” de Boer SM et al ASCO 2017

Adyuvancia QT: PORTEC-3 “Randomised phase III trial comparing concurrent chemoradiation and adjuvant chemotherapy with pelvic radiation alone in high risk and advanced stage endometrial carcinoma: PORTEC-3 ” de Boer SM et al ASCO 2017

Adyuvancia QT: PORTEC-3 “Randomised phase III trial comparing concurrent chemoradiation and adjuvant chemotherapy with pelvic radiation alone in high risk and advanced stage endometrial carcinoma: PORTEC-3 ” de Boer SM et al ASCO 2017

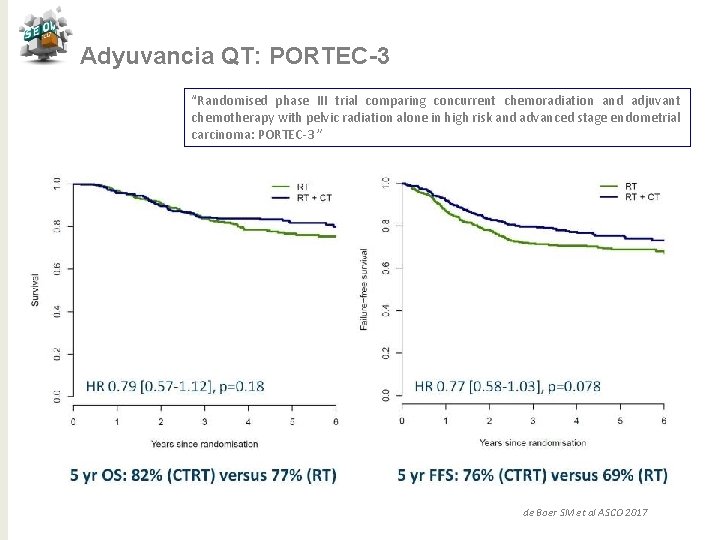
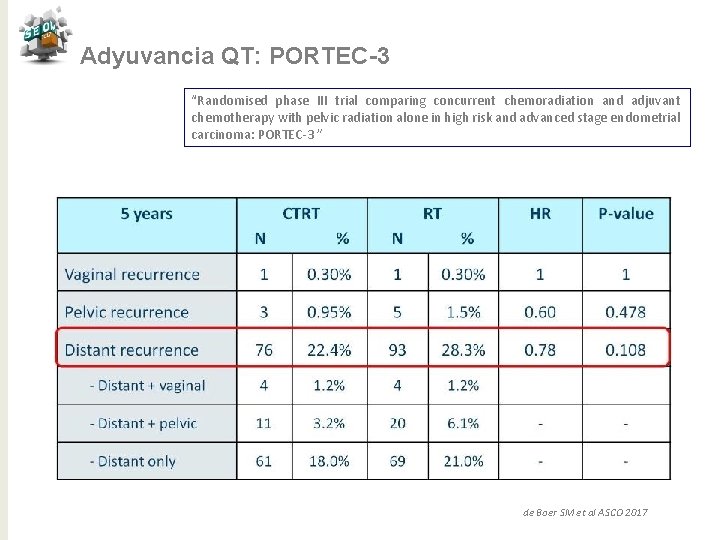
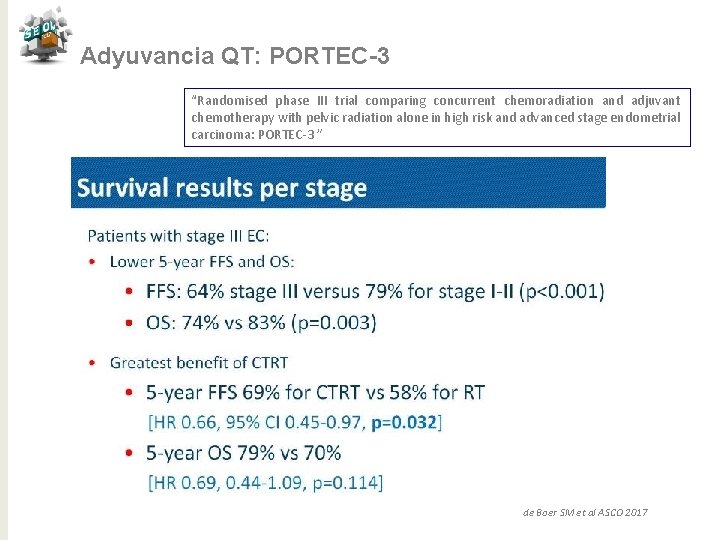
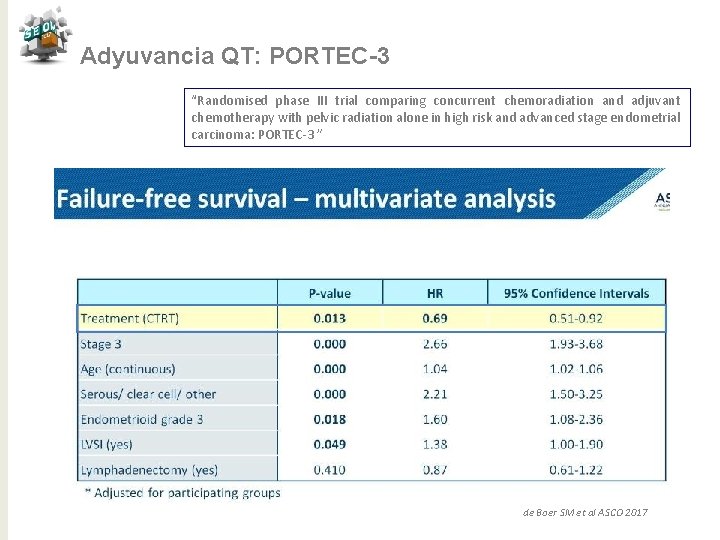
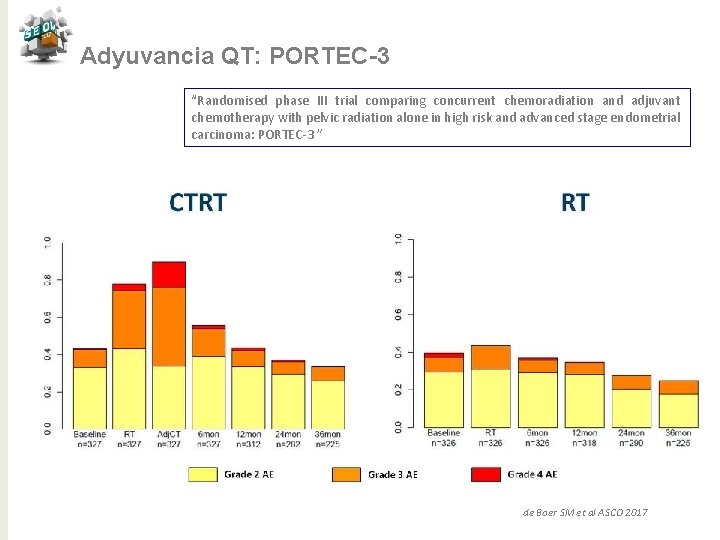
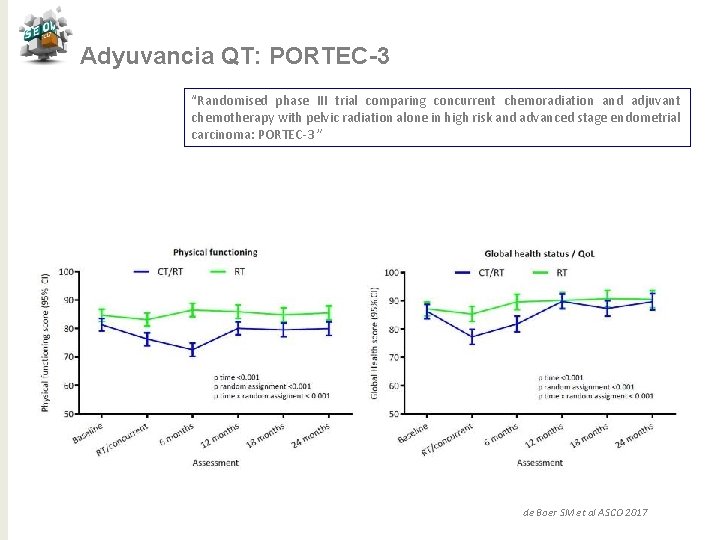
Adyuvancia QT: PORTEC-3 “Randomised phase III trial comparing concurrent chemoradiation and adjuvant chemotherapy with pelvic radiation alone in high risk and advanced stage endometrial carcinoma: PORTEC-3 ” Conclusiones QTRT vs RT para alto riesgo ü Tendencia de mejora en SV a 5 años con reducción 7% (SLF) y 5% (SG) ü Beneficio significativo del 11% en SLF con QTRT para E III ü No puede ser recomendado para E I-II ü Mayor toxicidad para QTRT en los primeros 12 meses ü Necesitamos > seguimiento para SG de Boer SM et al ASCO 2017 j





Conclusiones ü No indicación de rutina para tratamiento sistémico en pacientes con estadios iniciales de alto riesgo ü En estadios III y IV A indicación de QT adyuvante ( aumento SG y SLP) o RT QT frente a RT exclusiva ( Aumento SLR y SGCE) ü Pendiente de resultados de SG para indicación de QT/RT QT ü En tumores serosos y de células claras tratamiento QT adyuvante también en estadios precoces

Gracias por su atención!!!!! Nuria Lainez Milagro Complejo hospitalario de Navarra
 Cncer
Cncer Gog 240
Gog 240 Induccin
Induccin Folfiri esquema
Folfiri esquema Capta rh
Capta rh Quimioterapia abvd caída pelo
Quimioterapia abvd caída pelo Polipi endometriali
Polipi endometriali Ispessimento endometrio in menopausa senza sanguinamento
Ispessimento endometrio in menopausa senza sanguinamento Menopausa sintomi mestruazioni
Menopausa sintomi mestruazioni Reação cortical
Reação cortical Criterios de amsterdam cancer de colon
Criterios de amsterdam cancer de colon Pliche palmate
Pliche palmate Sonoisterografia polipo
Sonoisterografia polipo Tamaño normal del endometrio
Tamaño normal del endometrio Ciclo emorragico
Ciclo emorragico Dibujo de indicaciones
Dibujo de indicaciones Npt y npp
Npt y npp Surfactante pulmonar indicaciones
Surfactante pulmonar indicaciones Causas de cetoacidosis diabetica
Causas de cetoacidosis diabetica Resorte de coffin
Resorte de coffin Dibujo de indicaciones
Dibujo de indicaciones Gastrostomia indicaciones
Gastrostomia indicaciones Paracentesis tecnica
Paracentesis tecnica Alcalosis mixta
Alcalosis mixta Espirometría valores
Espirometría valores Accion terapeutica del paracetamol
Accion terapeutica del paracetamol Mecanismo de accion de la azitromicina
Mecanismo de accion de la azitromicina Indicaciones de dai
Indicaciones de dai Prospecto de celecoxib
Prospecto de celecoxib Indicaciones de cesarea relativas y absolutas
Indicaciones de cesarea relativas y absolutas L
L Indicaciones para un instructivo en modo imperativo
Indicaciones para un instructivo en modo imperativo Cherney incision
Cherney incision Dorsosacra
Dorsosacra Indicaciones de levosimendan
Indicaciones de levosimendan Signos de sobrecarga derecha
Signos de sobrecarga derecha Dibujo de borrador
Dibujo de borrador Resincronizacion cardiaca indicaciones
Resincronizacion cardiaca indicaciones Mantenedor de espacio indicaciones
Mantenedor de espacio indicaciones Yeso inguinopédico indicaciones
Yeso inguinopédico indicaciones Transfusion plaquetas indicaciones
Transfusion plaquetas indicaciones Indicaciones de nutricion enteral
Indicaciones de nutricion enteral Indicaciones de ejercicios pasivos
Indicaciones de ejercicios pasivos Indicaciones episiotomia
Indicaciones episiotomia Posición trendelenburg indicaciones
Posición trendelenburg indicaciones Tamaño de tubo endotraqueal
Tamaño de tubo endotraqueal Furosemida
Furosemida Dibujo de indicaciones
Dibujo de indicaciones Atigente
Atigente Yeso inguinopédico indicaciones
Yeso inguinopédico indicaciones Inotrópicos
Inotrópicos Items de apareamiento ejemplos
Items de apareamiento ejemplos Ajuste oclusal por desgaste seletivo
Ajuste oclusal por desgaste seletivo