PRESENTE Y FUTURO DEL TRATAMIENTO DEL CNCER DE















- Slides: 38

PRESENTE Y FUTURO DEL TRATAMIENTO DEL CÁNCER DE CÉRVIX DISEMINADO Dra. Ma Pilar Barretina Ginesta Institut Català d’Oncologia-Girona Madrid 28 octubre 2016

Index • • • Introduction Development of standard Chemotherapy Targeting angiogenesis and beyond Inmunotherapy Conclusions

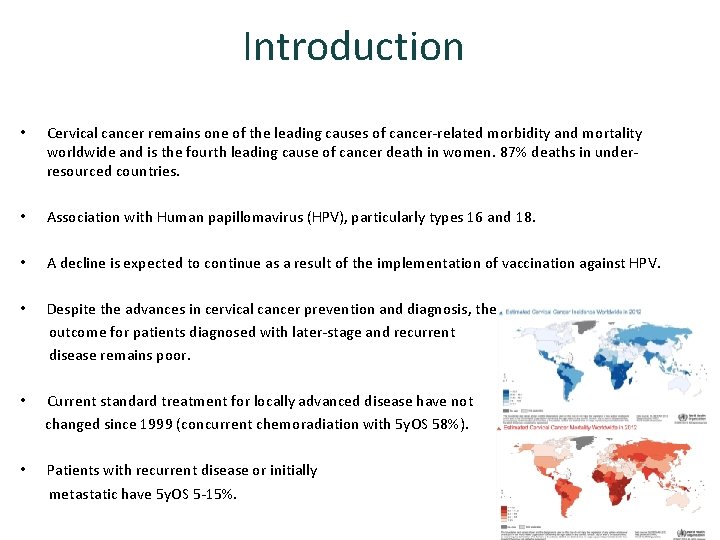
Introduction • Cervical cancer remains one of the leading causes of cancer-related morbidity and mortality worldwide and is the fourth leading cause of cancer death in women. 87% deaths in underresourced countries. • Association with Human papillomavirus (HPV), particularly types 16 and 18. • A decline is expected to continue as a result of the implementation of vaccination against HPV. • Despite the advances in cervical cancer prevention and diagnosis, the outcome for patients diagnosed with later-stage and recurrent disease remains poor. • Current standard treatment for locally advanced disease have not changed since 1999 (concurrent chemoradiation with 5 y. OS 58%). • Patients with recurrent disease or initially metastatic have 5 y. OS 5 -15%.

Development of standard chemotherapy

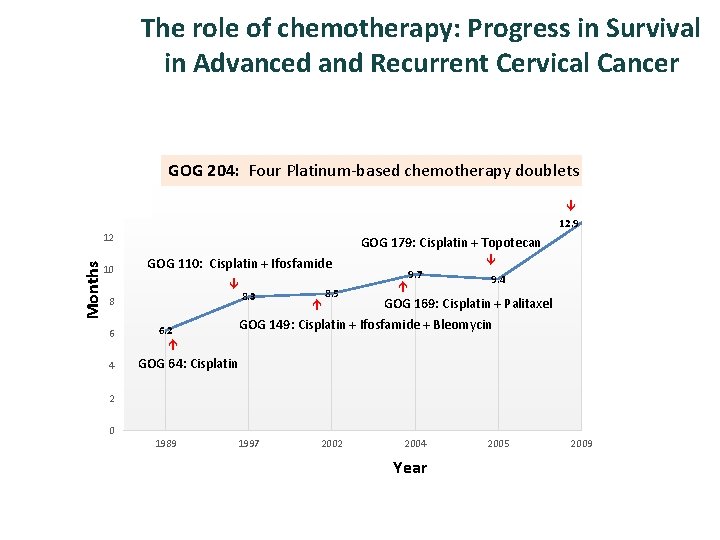
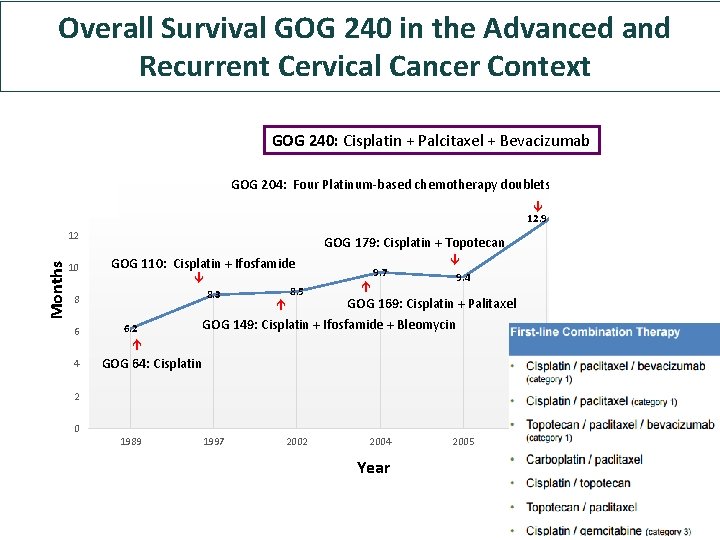
The role of chemotherapy: Progress in Survival in Advanced and Recurrent Cervical Cancer 18 16 GOG 204: Four Platinum-based chemotherapy doublets 14 17 12. 9 10 GOG 179: Cisplatin + Topotecan GOG 110: Cisplatin + Ifosfamide 8 6 4 8. 3 8. 5 9. 7 9. 4 GOG 169: Cisplatin + Palitaxel GOG 149: Cisplatin + Ifosfamide + Bleomycin 6. 2 Months 12 GOG 64: Cisplatin 2 0 1989 1997 2002 2004 Year 2005 2009 2013

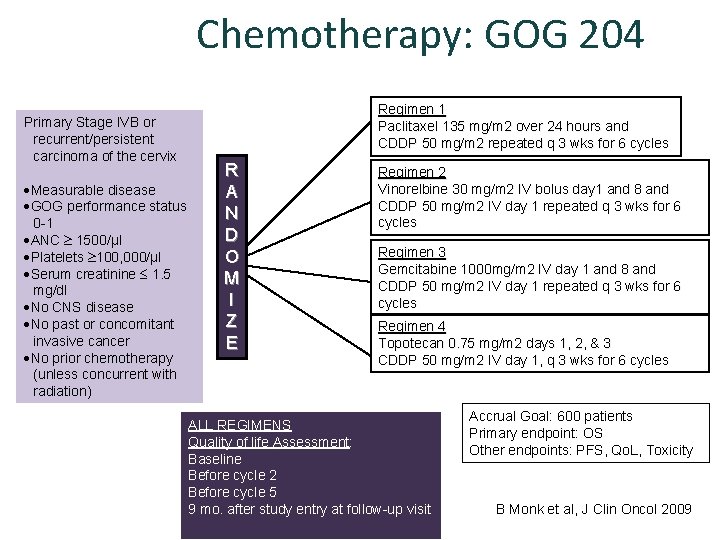
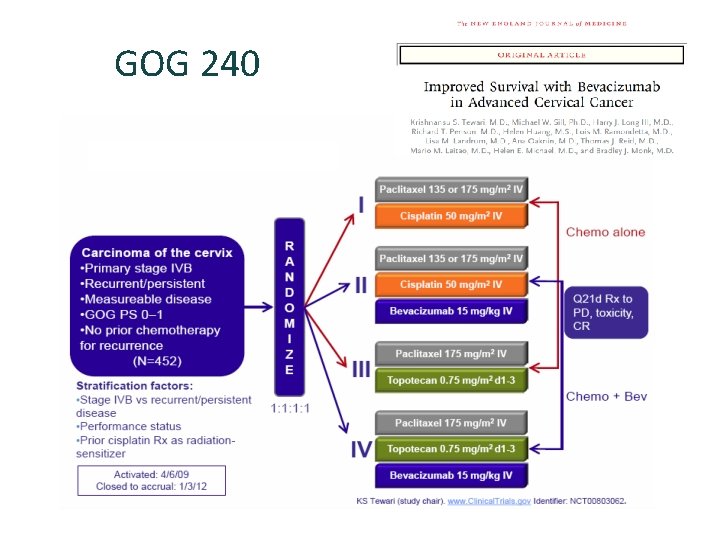
Chemotherapy: GOG 204 Primary Stage IVB or recurrent/persistent carcinoma of the cervix Measurable disease GOG performance status 0 -1 ANC 1500/µl Platelets 100, 000/µl Serum creatinine 1. 5 mg/dl No CNS disease No past or concomitant invasive cancer No prior chemotherapy (unless concurrent with radiation) Regimen 1 Paclitaxel 135 mg/m 2 over 24 hours and CDDP 50 mg/m 2 repeated q 3 wks for 6 cycles R A N D O M I Z E Regimen 2 Vinorelbine 30 mg/m 2 IV bolus day 1 and 8 and CDDP 50 mg/m 2 IV day 1 repeated q 3 wks for 6 cycles Regimen 3 Gemcitabine 1000 mg/m 2 IV day 1 and 8 and CDDP 50 mg/m 2 IV day 1 repeated q 3 wks for 6 cycles Regimen 4 Topotecan 0. 75 mg/m 2 days 1, 2, & 3 CDDP 50 mg/m 2 IV day 1, q 3 wks for 6 cycles ALL REGIMENS Quality of life Assessment: Baseline Before cycle 2 Before cycle 5 9 mo. after study entry at follow-up visit Accrual Goal: 600 patients Primary endpoint: OS Other endpoints: PFS, Qo. L, Toxicity B Monk et al, J Clin Oncol 2009

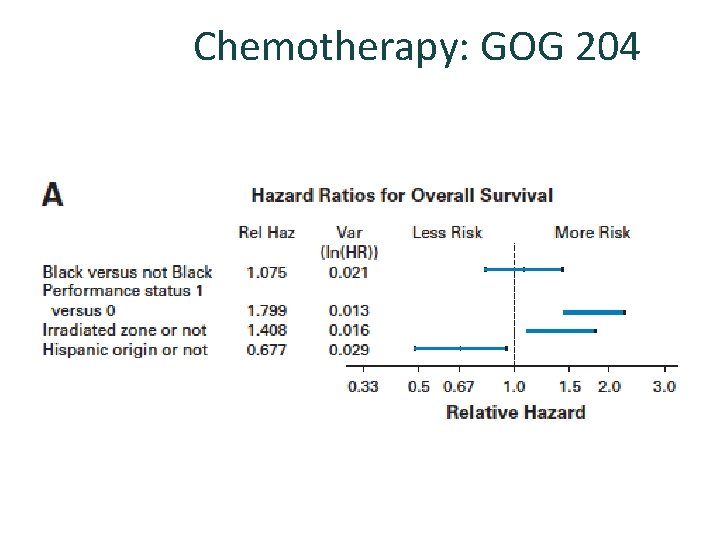
Chemotherapy: GOG 204 No statistically significant differences among the 4 arms in terms of PFS, OS, RR. Tend to benefit for the cisplatin-paclitaxel arm. Quality of life Monk BJ et al. J Clin Oncol 2009

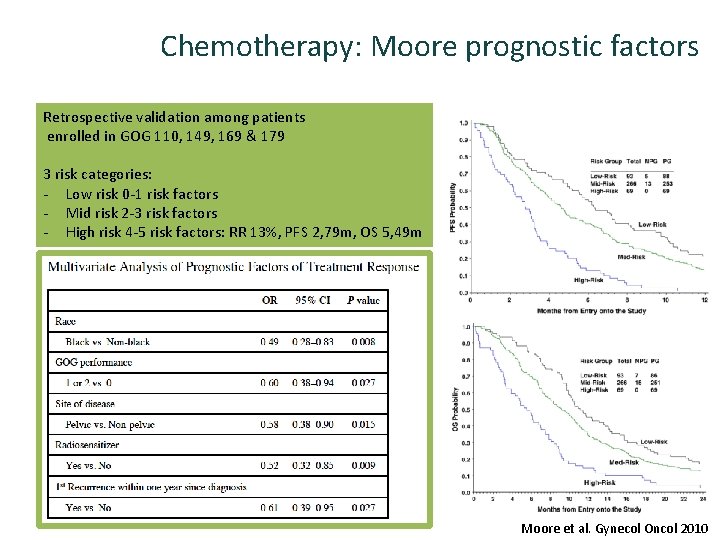
Chemotherapy: Moore prognostic factors Retrospective validation among patients enrolled in GOG 110, 149, 169 & 179 3 risk categories: - Low risk 0 -1 risk factors - Mid risk 2 -3 risk factors - High risk 4 -5 risk factors: RR 13%, PFS 2, 79 m, OS 5, 49 m Moore et al. Gynecol Oncol 2010

Chemotherapy: GOG 204

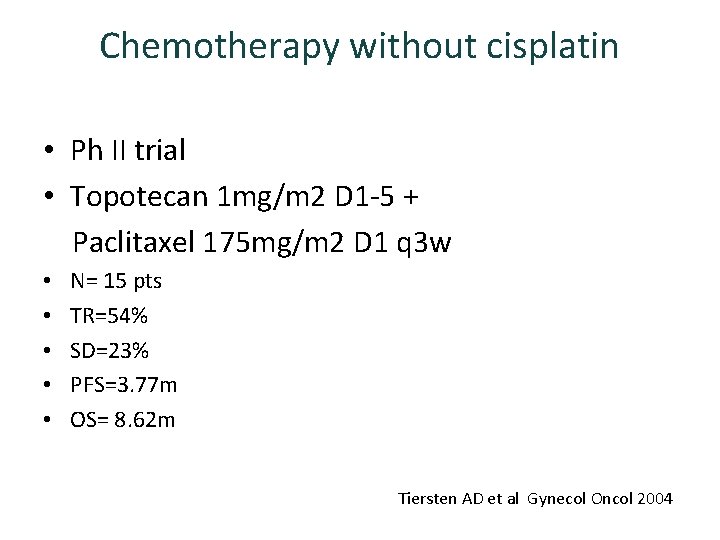
Chemotherapy without cisplatin • Ph II trial • Topotecan 1 mg/m 2 D 1 -5 + Paclitaxel 175 mg/m 2 D 1 q 3 w • • • N= 15 pts TR=54% SD=23% PFS=3. 77 m OS= 8. 62 m Tiersten AD et al Gynecol Oncol 2004

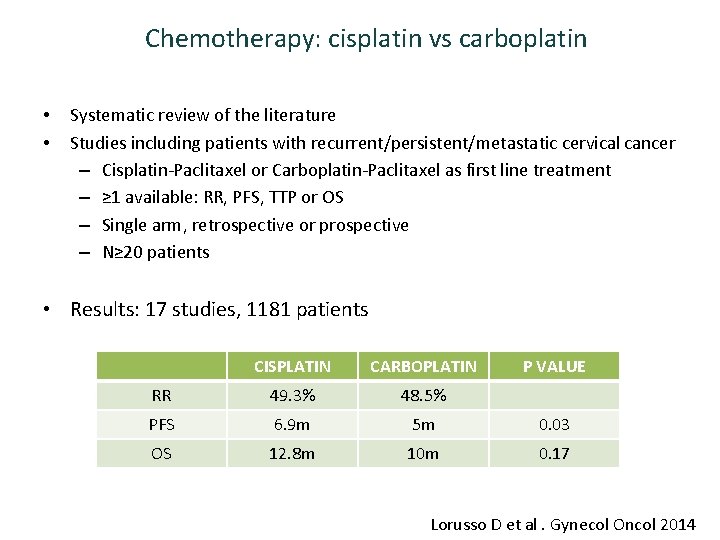
Chemotherapy: cisplatin vs carboplatin • • Systematic review of the literature Studies including patients with recurrent/persistent/metastatic cervical cancer – Cisplatin-Paclitaxel or Carboplatin-Paclitaxel as first line treatment – ≥ 1 available: RR, PFS, TTP or OS – Single arm, retrospective or prospective – N≥ 20 patients • Results: 17 studies, 1181 patients CISPLATIN CARBOPLATIN P VALUE RR 49. 3% 48. 5% PFS 6. 9 m 5 m 0. 03 OS 12. 8 m 10 m 0. 17 Lorusso D et al. Gynecol Oncol 2014

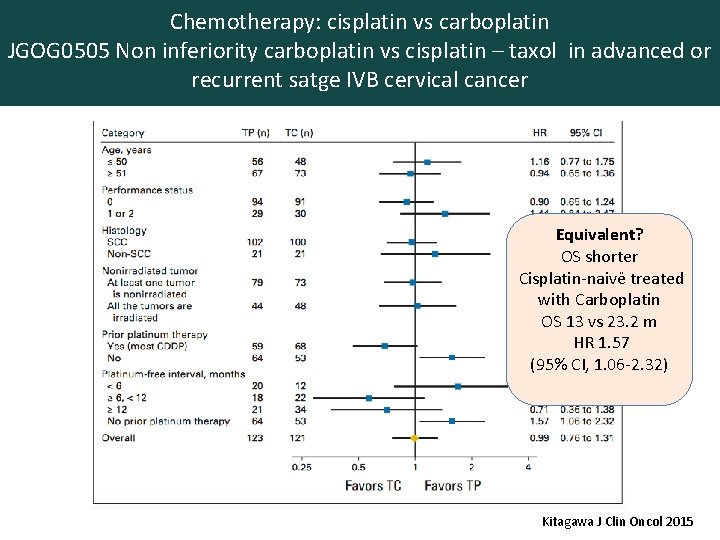
Chemotherapy: cisplatin vs carboplatin JGOG 0505 Non inferiority carboplatin vs cisplatin – taxol in advanced or recurrent satge IVB cervical cancer Equivalent? OS shorter Cisplatin-naivë treated with Carboplatin OS 13 vs 23. 2 m HR 1. 57 (95% CI, 1. 06 -2. 32) N=253 50% 1 st line, 50% 2 ond line with 80 -85% prior irradiation Kitagawa J Clin Oncol 2015

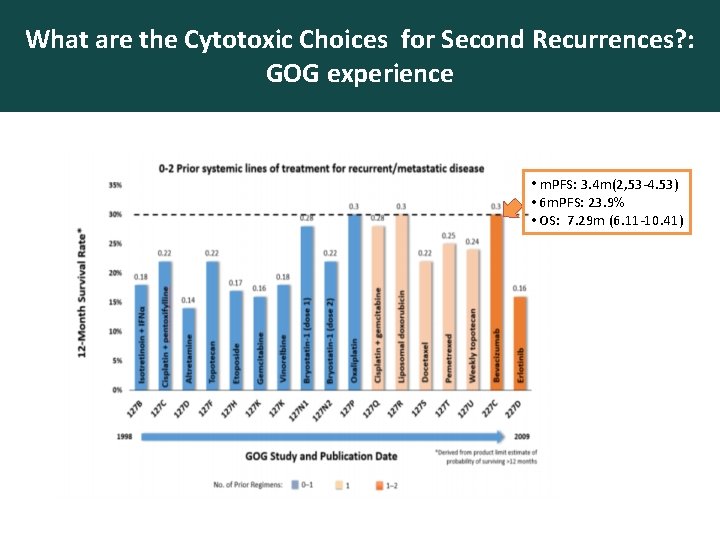
What are the Cytotoxic Choices for Second Recurrences? : GOG experience • m. PFS: 3. 4 m(2, 53 -4. 53) • 6 m. PFS: 23. 9% • OS: 7. 29 m (6. 11 -10. 41)

TARGETING ANGIOGENESIS

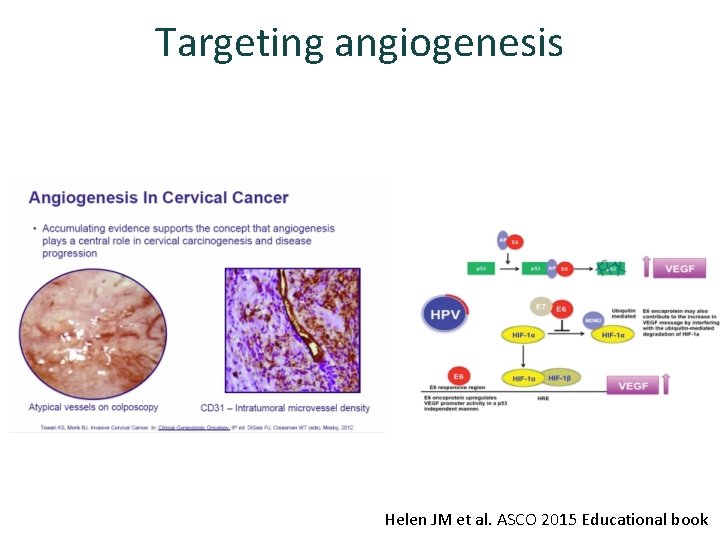
Targeting angiogenesis Helen JM et al. ASCO 2015 Educational book

GOG 240

GOG 240: final OS 1. 0 Events, n (%) Proportion Surviving 0. 8 Median OS, months No Bev (n=225) Bevacizumab (n=227) 178 (79) 170 (75) 13. 3 16. 8 HR=0. 765 (95% CI, 0. 62– 0. 95) Improvements in ORR, PFS and OS due to the P=. 0068 addition of bevacizumab did not worsen patients quality of life (FACT-Cx TOI scores) 0. 6 0. 4 0. 2 0. 0 0 1 227 2 225 12 24 142 114 75 54 36 Months on Study 30 17 Tewari et al, NEJM 2014, ESMO 2014 48 60 6 2 0 0 Penson RT et al Lancet Oncol 2015

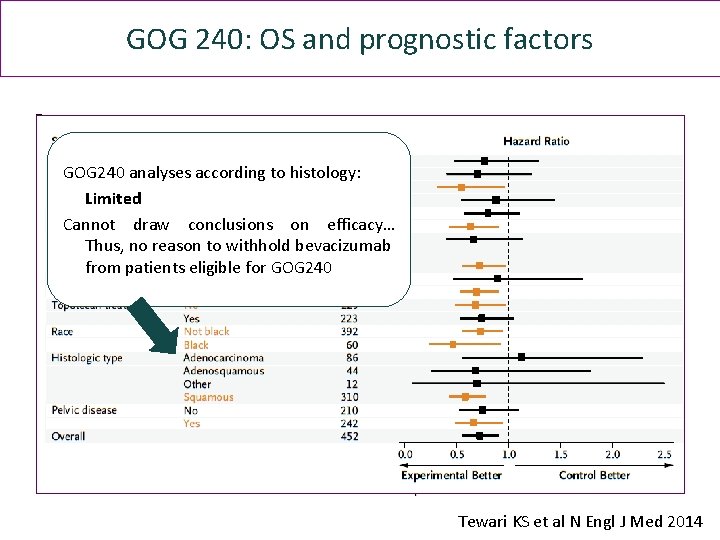
GOG 240: OS and prognostic factors GOG 240 analyses according to histology: Limited Cannot draw conclusions on efficacy… Thus, no reason to withhold bevacizumab from patients eligible for GOG 240 Tewari KS et al N Engl J Med 2014

GOG 240: Toxicity Increased incidence GI fistulae with Bevacizumab - patients with prior pelvic RT (8. 2% vs 0. 9%) Tewari KS et al N Engl J Med 2014

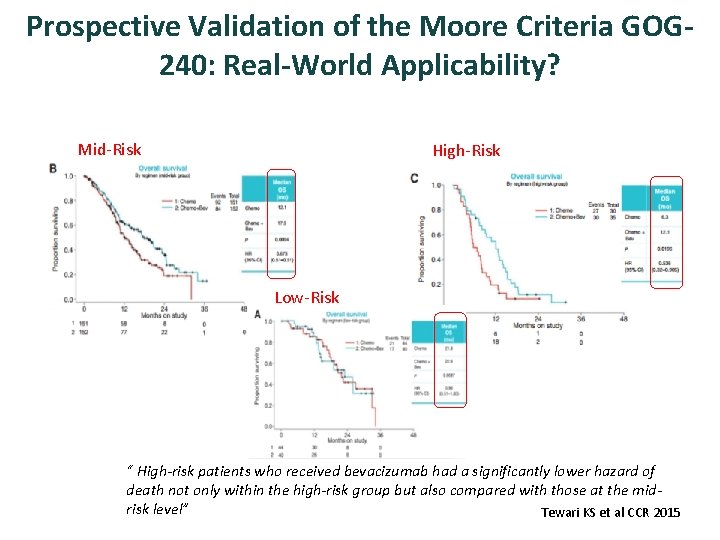
Prospective Validation of the Moore Criteria GOG 240: Real-World Applicability? Mid-Risk High-Risk Low-Risk “ High-risk patients who received bevacizumab had a significantly lower hazard of death not only within the high-risk group but also compared with those at the midrisk level” Tewari KS et al CCR 2015

GOG 240: Cisplatin + Palcitaxel + Bevacizumab 18 16 Overall Survival GOG 240 in the Advanced and Recurrent Cervical Cancer Context 17 GOG 204: Four Platinum-based chemotherapy doublets 14 12. 9 10 GOG 179: Cisplatin + Topotecan GOG 110: Cisplatin + Ifosfamide 8. 3 8 6 4 6. 2 8. 5 9. 7 9. 4 GOG 169: Cisplatin + Palitaxel GOG 149: Cisplatin + Ifosfamide + Bleomycin Months 12 GOG 64: Cisplatin 2 0 1989 1997 2002 2004 Year 2005 2009 2013

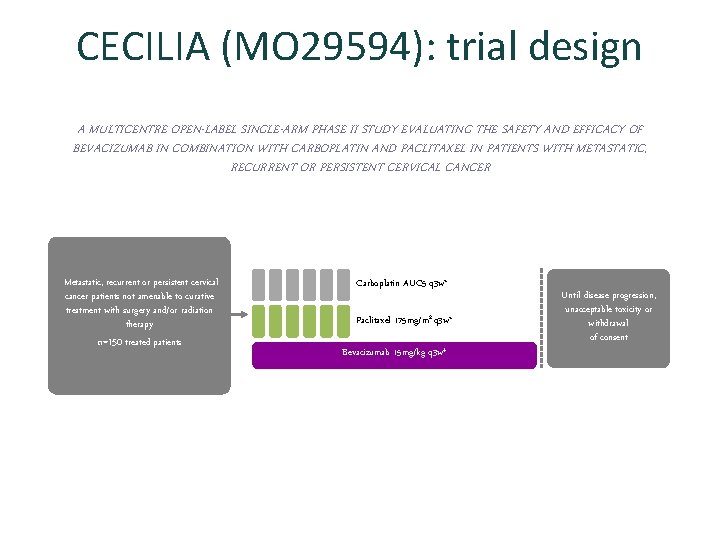
CECILIA (MO 29594): trial design A MULTICENTRE OPEN-LABEL SINGLE-ARM PHASE II STUDY EVALUATING THE SAFETY AND EFFICACY OF BEVACIZUMAB IN COMBINATION WITH CARBOPLATIN AND PACLITAXEL IN PATIENTS WITH METASTATIC, RECURRENT OR PERSISTENT CERVICAL CANCER Metastatic, recurrent or persistent cervical cancer patients not amenable to curative treatment with surgery and/or radiation therapy n=150 treated patients Carboplatin AUC 5 q 3 w* Paclitaxel 175 mg/m 2 q 3 w* Bevacizumab 15 mg/kg q 3 w‡ Until disease progression, unacceptable toxicity or withdrawal of consent

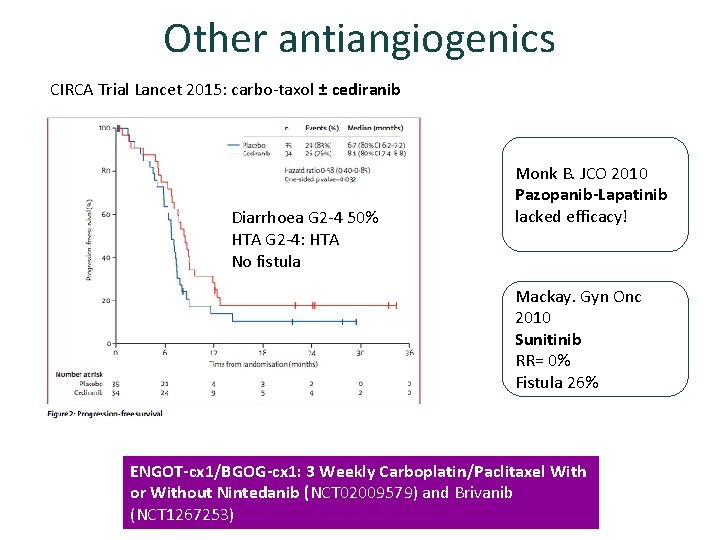
Other antiangiogenics CIRCA Trial Lancet 2015: carbo-taxol ± cediranib Diarrhoea G 2 -4 50% HTA G 2 -4: HTA No fistula Monk B. JCO 2010 Pazopanib-Lapatinib lacked efficacy! Mackay. Gyn Onc 2010 Sunitinib RR= 0% Fistula 26% ENGOT-cx 1/BGOG-cx 1: 3 Weekly Carboplatin/Paclitaxel With or Without Nintedanib (NCT 02009579) and Brivanib (NCT 1267253)

BEYOND ANGIOGENESIS

MOLECULAR PROFILING Gene SCC (N=80) ADC (N=40) P value PIK 3 CA 37, 5% 25% 0. 33 PTEN loss 13. 0% 3. 6% 0. 32 KRAS 0 17. 5% 0. 01 EGFR 7. 5% 0 0. 24 Wright et al Cancer 2013 Al Ojesina et al Nature 2014

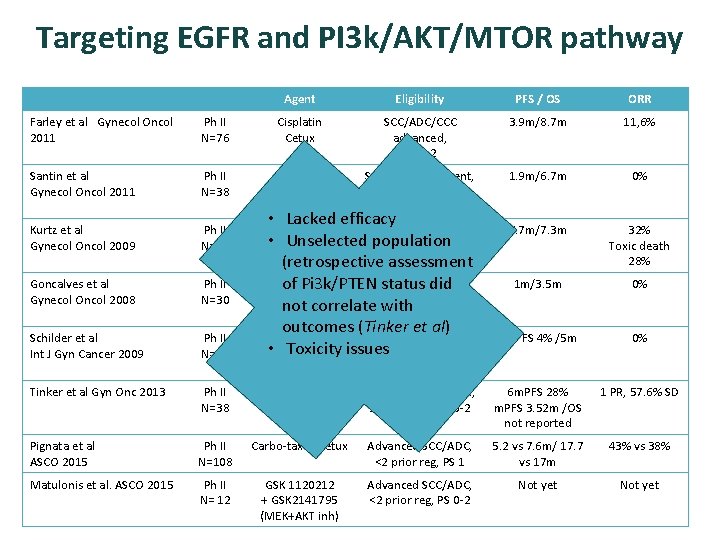
Targeting EGFR and PI 3 k/AKT/MTOR pathway Agent Eligibility PFS / OS ORR Farley et al Gynecol Oncol 2011 Ph II N=76 Cisplatin Cetux SCC/ADC/CCC advanced, PS 0 -2 3. 9 m/8. 7 m 11, 6% Santin et al Gynecol Oncol 2011 Ph II N=38 Cetux SCC/ADC, Recurrent, 1 -2 prior reg, PS 0 -2 1. 9 m/6. 7 m 0% Kurtz et al Gynecol Oncol 2009 Ph II N=19 5. 7 m/7. 3 m 32% Toxic death 28% Goncalves et al Gynecol Oncol 2008 Ph II N=30 1 m/3. 5 m 0% Schilder et al Int J Gyn Cancer 2009 Ph II N=28 6 m. PFS 4% /5 m 0% Tinker et al Gyn Onc 2013 Ph II N=38 Temsirolimus SCC, ADC, Advanced, ≤ 1 prior reg, PS 0 -2 6 m. PFS 28% m. PFS 3. 52 m /OS not reported 1 PR, 57. 6% SD Pignata et al ASCO 2015 Ph II N=108 Carbo-taxol±cetux Advanced SCC/ADC, <2 prior reg, PS 1 5. 2 vs 7. 6 m/ 17. 7 vs 17 m 43% vs 38% Matulonis et al. ASCO 2015 Ph II N= 12 GSK 1120212 + GSK 2141795 (MEK+AKT inh) Advanced SCC/ADC, <2 prior reg, PS 0 -2 Not yet • Lacked efficacy Cis-Topotecan SCC, Advanced, • Unselected population Cetux PS 0 -2 (retrospective assessment Gefitinib SCC, Advanced, ≥ 1 of Pi 3 k/PTEN status did prior reg, PS 0 -2 not correlate with outcomes (Tinker et al) Erlotinib SCC, Advanced, ≥ 2 • Toxicity issues prior reg, PS 0 -2

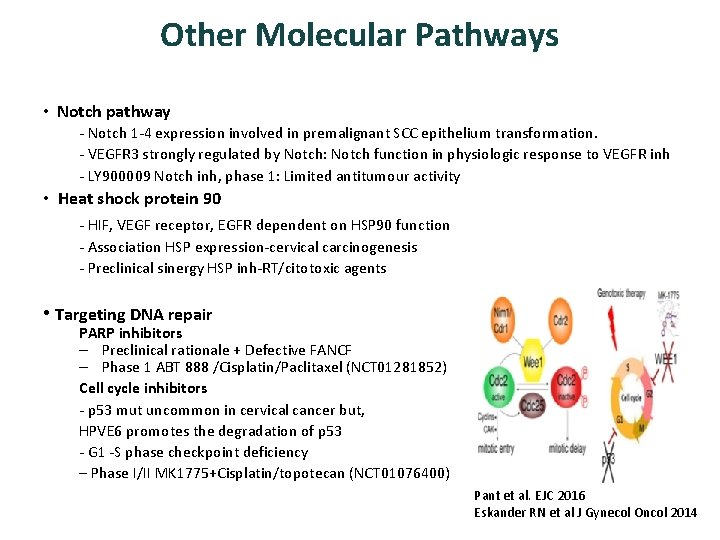
Other Molecular Pathways • Notch pathway - Notch 1 -4 expression involved in premalignant SCC epithelium transformation. - VEGFR 3 strongly regulated by Notch: Notch function in physiologic response to VEGFR inh - LY 900009 Notch inh, phase 1: Limited antitumour activity • Heat shock protein 90 - HIF, VEGF receptor, EGFR dependent on HSP 90 function - Association HSP expression-cervical carcinogenesis - Preclinical sinergy HSP inh-RT/citotoxic agents • Targeting DNA repair PARP inhibitors – Preclinical rationale + Defective FANCF – Phase 1 ABT 888 /Cisplatin/Paclitaxel (NCT 01281852) Cell cycle inhibitors - p 53 mut uncommon in cervical cancer but, HPVE 6 promotes the degradation of p 53 - G 1 -S phase checkpoint deficiency – Phase I/II MK 1775+Cisplatin/topotecan (NCT 01076400) Pant et al. EJC 2016 Eskander RN et al J Gynecol Oncol 2014

Targeting immune system • CD 8+cytotoxic T cell-response to E 6 and E 7 • CD 4+ T cell-response to HPV • Local immune supression in HPVtumors regulated by Tregs, inhibitory cytokines. . • CXRT: tumor antigen release and immune underperformance • Improved outcome associated with the presence of TILs 1 - Vaccine based therapy 2 - Adoptive T cell Therapy 3 - Immune checkpoints inhibition

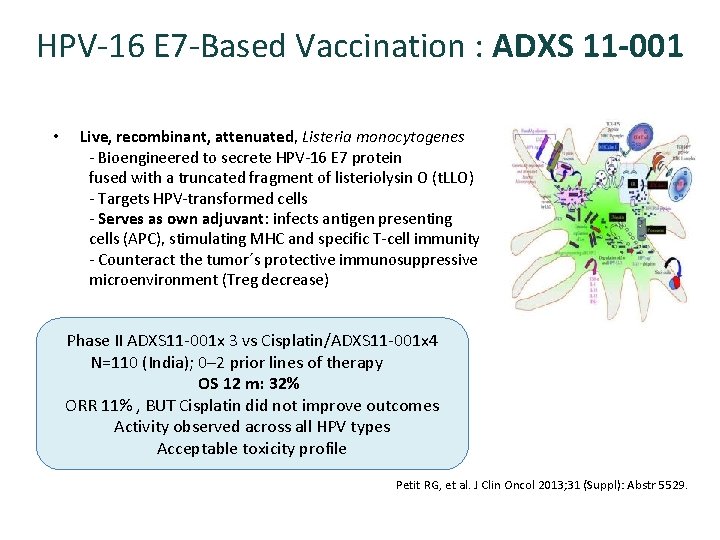
HPV-16 E 7 -Based Vaccination : ADXS 11 -001 • Live, recombinant, attenuated, Listeria monocytogenes - Bioengineered to secrete HPV-16 E 7 protein fused with a truncated fragment of listeriolysin O (t. LLO) - Targets HPV-transformed cells - Serves as own adjuvant: infects antigen presenting cells (APC), stimulating MHC and specific T-cell immunity - Counteract the tumor´s protective immunosuppressive microenvironment (Treg decrease) Phase II ADXS 11 -001 x 3 vs Cisplatin/ADXS 11 -001 x 4 N=110 (India); 0– 2 prior lines of therapy OS 12 m: 32% ORR 11% , BUT Cisplatin did not improve outcomes Activity observed across all HPV types Acceptable toxicity profile Petit RG, et al. J Clin Oncol 2013; 31 (Suppl): Abstr 5529.

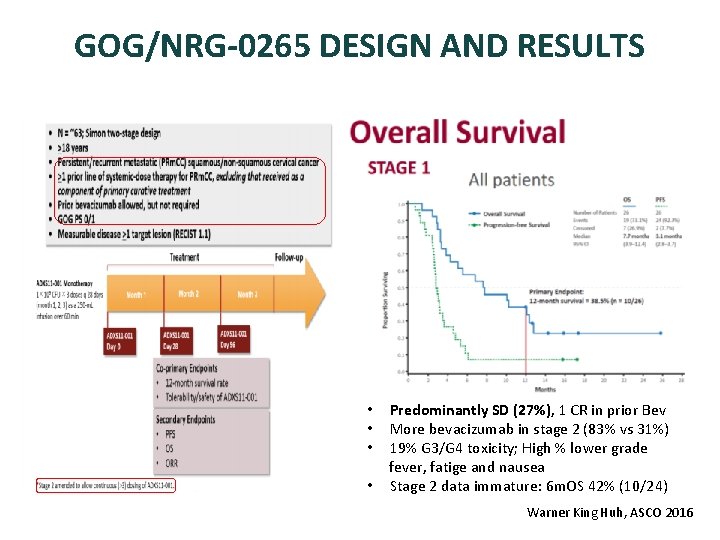
GOG/NRG-0265 DESIGN AND RESULTS • Predominantly SD (27%), 1 CR in prior Bev • More bevacizumab in stage 2 (83% vs 31%) • 19% G 3/G 4 toxicity; High % lower grade fever, fatige and nausea • Stage 2 data immature: 6 m. OS 42% (10/24) Warner King Huh, ASCO 2016

Therapeutic vaccination: VGX 300 • Vaccine that targets type 16&18 HPV • Efficacy shown for CIN 2/3 (Trimble CL et al The Lancet 2015) • Phase I/IIA, Open-Label, Safety, Tolerability, and Immunogenicity Study of INO-3112 Delivered by Electroporation (EP) in Women With Cervical Cancer After Chemoradiation for Newly Diagnosed Disease or Therapy for Recurrent and/or Persistent Disease (NCT 02172911)

Adoptive immunoterapy: tumor infiltrating lymphocytes 1. Isolation and ex vivo expansion of HPVTILs 2. Non-myeloablative condition followed by high bolus aldesleukin 3. Single infusion of HPV-TILs - HPV reactivity observed in 6 of 8 patients - 3/9 responses (1 PR, 2 CR) - Durable remision >18 months - Expansion cervical cancer cohort of patients. Stevanovic et al J Clin Oncol 2015

Adoptive immunoterapy with tumor infiltrating lymphocytes

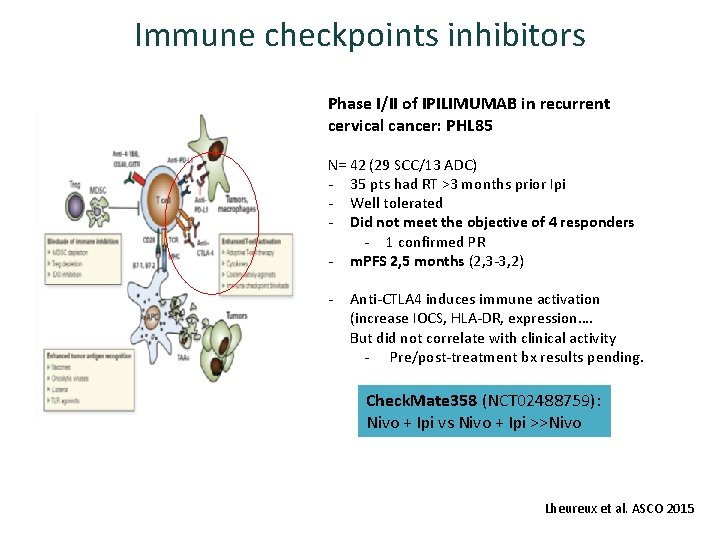
Immune checkpoints inhibitors Phase I/II of IPILIMUMAB in recurrent cervical cancer: PHL 85 N= 42 (29 SCC/13 ADC) - 35 pts had RT >3 months prior Ipi - Well tolerated - Did not meet the objective of 4 responders - 1 confirmed PR - m. PFS 2, 5 months (2, 3 -3, 2) - Anti-CTLA 4 induces immune activation (increase IOCS, HLA-DR, expression…. But did not correlate with clinical activity - Pre/post-treatment bx results pending. Check. Mate 358 (NCT 02488759): Nivo + Ipi vs Nivo + Ipi >>Nivo Lheureux et al. ASCO 2015

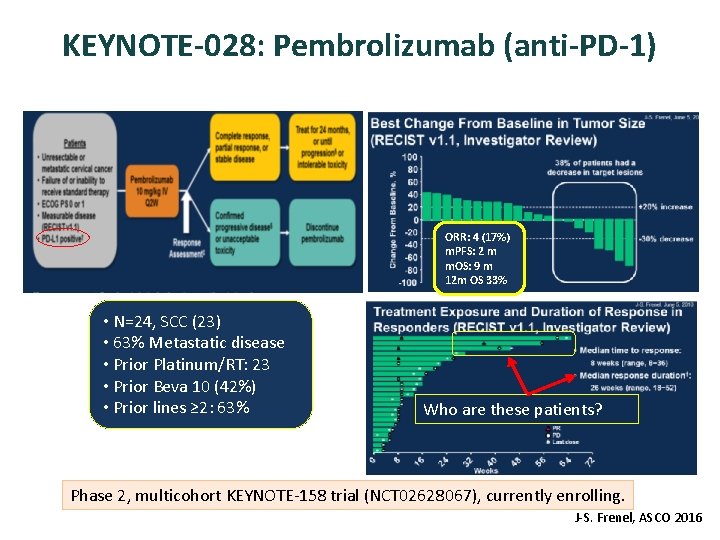
KEYNOTE-028: Pembrolizumab (anti-PD-1) ORR: 4 (17%) m. PFS: 2 m m. OS: 9 m 12 m OS 33% • N=24, SCC (23) • 63% Metastatic disease • Prior Platinum/RT: 23 • Prior Beva 10 (42%) • Prior lines ≥ 2: 63% Who are these patients? Phase 2, multicohort KEYNOTE-158 trial (NCT 02628067), currently enrolling. J-S. Frenel, ASCO 2016

CONCLUSIONS • Advanced and recurrent cervical cancer remains a major problem. Hope for failing incidence with better acces to screening programs and profilactic vaccines. • Bevacizumab in combination with cisplatin based chemotherapy is the first targeted therapy to show an overall survival benefit for these patients and is the standard first line therapy. There is no standard second line. • There are many rationale potentially interesting targets under investigation due to better knowledge of cervical cancer biology. • Inmunotherapy represents a promising area. • Joining resources and expanding collaboration should help as to define novel biomarkers to predict response and stablish best sequence/combination of treatments to improve results.

Gracias por vuestra atención

 Gog 240
Gog 240 Cncer
Cncer Verbo ir
Verbo ir Presente perfecto
Presente perfecto Presente de indicativo irregulares
Presente de indicativo irregulares Modo indicativo, subjuntivo e imperativo
Modo indicativo, subjuntivo e imperativo Present simple y present continuous
Present simple y present continuous Verbo ir
Verbo ir Verbos pasado presente y futuro en inglés
Verbos pasado presente y futuro en inglés Verbos bailar
Verbos bailar Imachikkuna
Imachikkuna Pasado perfecto de speak
Pasado perfecto de speak Presente continuo y presente simple
Presente continuo y presente simple No recibiras presente porque el presente ciega
No recibiras presente porque el presente ciega Be en presente progresivo
Be en presente progresivo Tecnologías al final del tubo ejemplos
Tecnologías al final del tubo ejemplos Fases de la enfermedad periodontal
Fases de la enfermedad periodontal Verbo to will
Verbo to will Future perfect estructura
Future perfect estructura Dinero en el tiempo
Dinero en el tiempo El futuro irregulares
El futuro irregulares Gerencia del futuro
Gerencia del futuro I sette saperi di morin
I sette saperi di morin Dario guarascio
Dario guarascio Pnsd sintesi
Pnsd sintesi Verbo cantare in tutti i modi
Verbo cantare in tutti i modi Congiuntivo essere avere
Congiuntivo essere avere Sífilis embarazo tratamiento
Sífilis embarazo tratamiento Tratamiento de crisis hipertensiva
Tratamiento de crisis hipertensiva Relaciones anatomicas de la glandula tiroides
Relaciones anatomicas de la glandula tiroides Tromboxitopenia
Tromboxitopenia Ppd positiva
Ppd positiva Joroba de hampton tep
Joroba de hampton tep Bronquiectasias tratamiento
Bronquiectasias tratamiento Pseudohipokalemia
Pseudohipokalemia Sindrome anticolinergico tratamiento
Sindrome anticolinergico tratamiento Disalimentacion
Disalimentacion Tratamiento primario acortado de la tuberculosis
Tratamiento primario acortado de la tuberculosis Rickettsias y clamidias
Rickettsias y clamidias