Chapter 5 The Working Cell Power Point Lectures














- Slides: 97

Chapter 5 The Working Cell Power. Point Lectures Campbell Biology: Concepts & Connections, Eighth Edition REECE • TAYLOR • SIMON • DICKEY • HOGAN © 2015 Pearson Education, Inc. Lecture by Edward J. Zalisko

Introduction • The plasma membrane and its proteins enable cells to • survive and • function. • Aquaporins are membrane proteins that function as water channels. © 2015 Pearson Education, Inc.

Introduction • This chapter addresses how working cells use • membranes, • energy, and • enzymes. © 2015 Pearson Education, Inc.

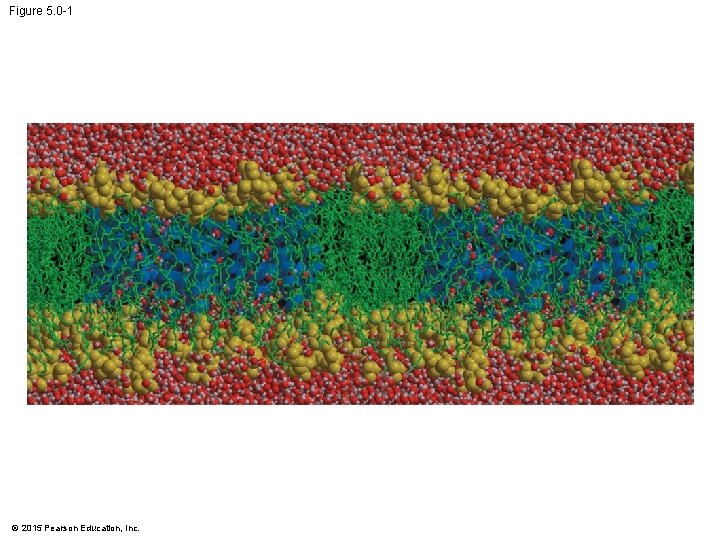
Figure 5. 0 -1 © 2015 Pearson Education, Inc.

MEMBRANE STRUCTURE AND FUNCTION © 2015 Pearson Education, Inc.

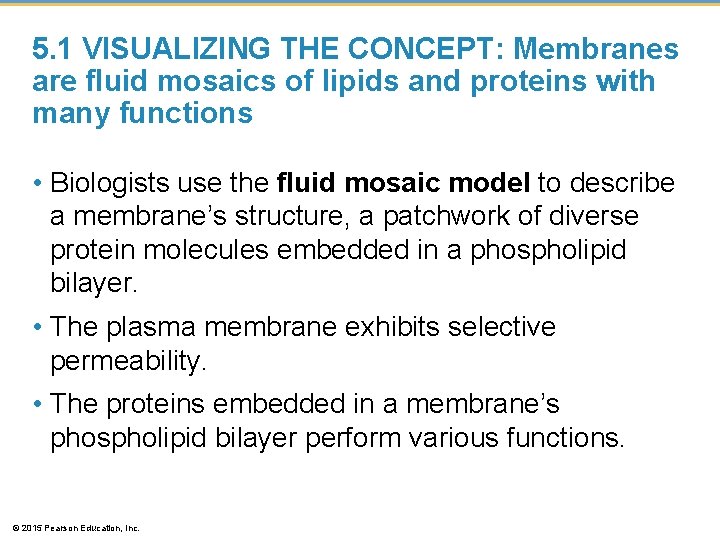
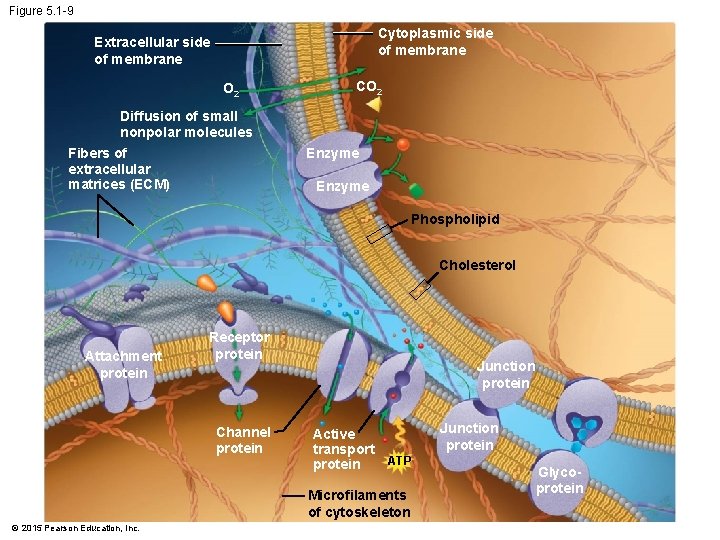
5. 1 VISUALIZING THE CONCEPT: Membranes are fluid mosaics of lipids and proteins with many functions • Biologists use the fluid mosaic model to describe a membrane’s structure, a patchwork of diverse protein molecules embedded in a phospholipid bilayer. • The plasma membrane exhibits selective permeability. • The proteins embedded in a membrane’s phospholipid bilayer perform various functions. © 2015 Pearson Education, Inc.

Figure 5. 1 Cytoplasmic side of membrane Extracellular side of membrane O 2 CO 2 Fibers of extracellular matrices (ECM) Phospholipid Cholesterol Membrane proteins Microfilaments of cytoskeleton © 2015 Pearson Education, Inc.

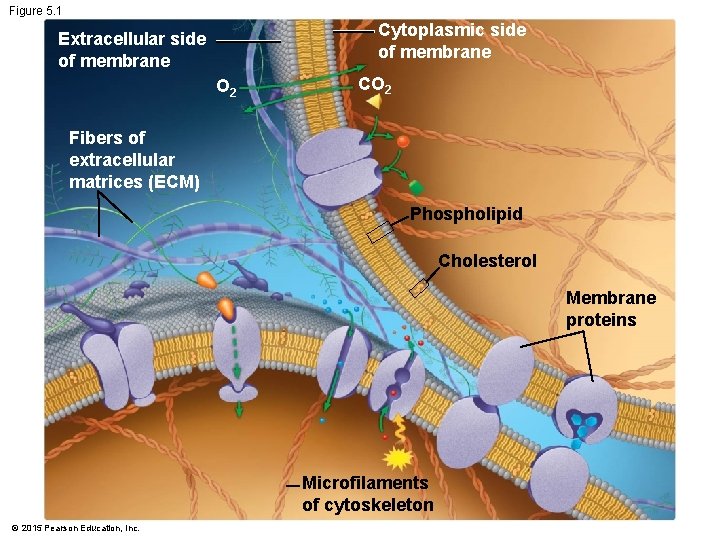
Figure 5. 1 -1 O 2 CO 2 Diffusion of small nonpolar molecules Enzyme Attachment protein Receptor protein Channel protein © 2015 Pearson Education, Inc. Junction protein Active transport protein Junction protein ATP Glycoprotein

Figure 5. 1 -2 O 2 CO 2 Diffusion of small nonpolar molecules © 2015 Pearson Education, Inc.

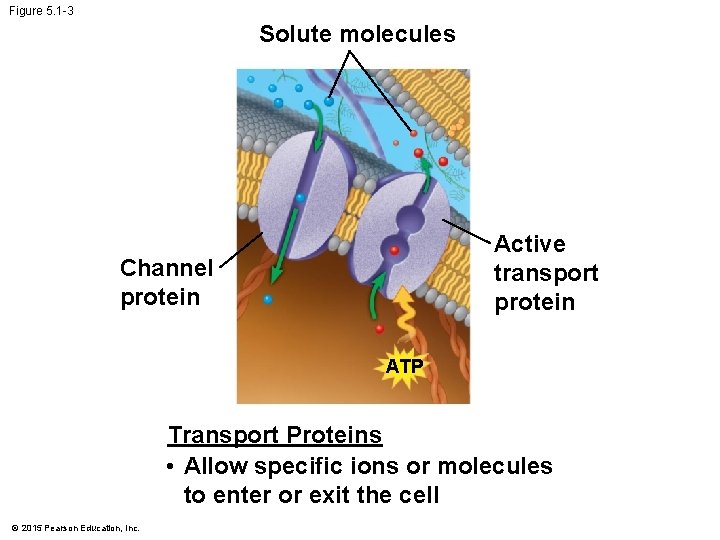
Figure 5. 1 -3 Solute molecules Active transport protein Channel protein ATP Transport Proteins • Allow specific ions or molecules to enter or exit the cell © 2015 Pearson Education, Inc.

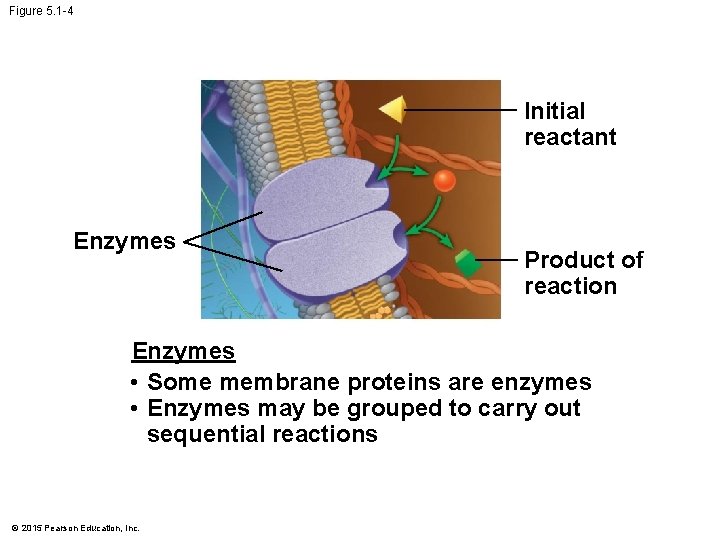
Figure 5. 1 -4 Initial reactant Enzymes Product of reaction Enzymes • Some membrane proteins are enzymes • Enzymes may be grouped to carry out sequential reactions © 2015 Pearson Education, Inc.

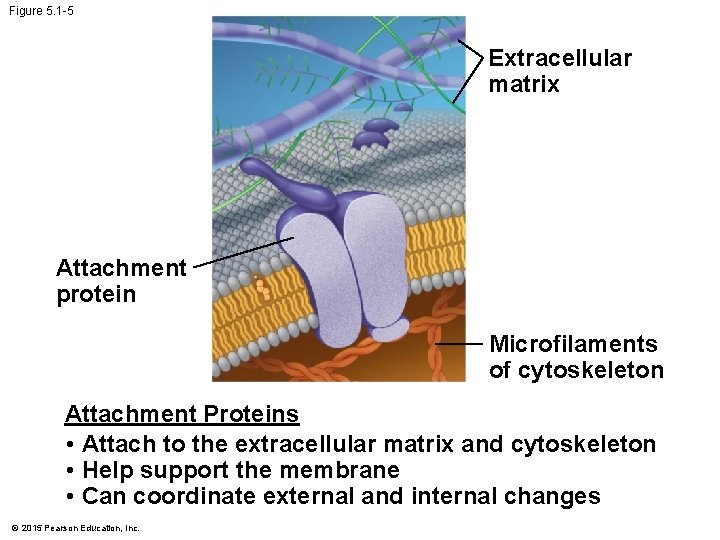
Figure 5. 1 -5 Extracellular matrix Attachment protein Microfilaments of cytoskeleton Attachment Proteins • Attach to the extracellular matrix and cytoskeleton • Help support the membrane • Can coordinate external and internal changes © 2015 Pearson Education, Inc.

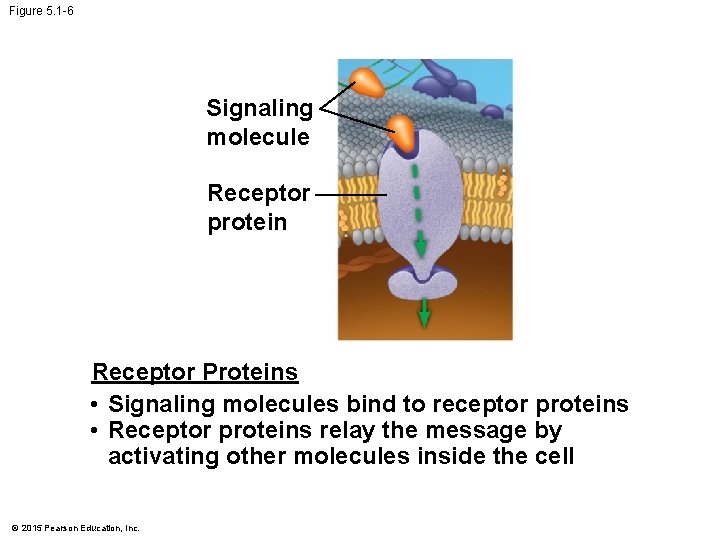
Figure 5. 1 -6 Signaling molecule Receptor protein Receptor Proteins • Signaling molecules bind to receptor proteins • Receptor proteins relay the message by activating other molecules inside the cell © 2015 Pearson Education, Inc.

Figure 5. 1 -7 Junction protein Junction Proteins • Form intercellular junctions that attach adjacent cells © 2015 Pearson Education, Inc.

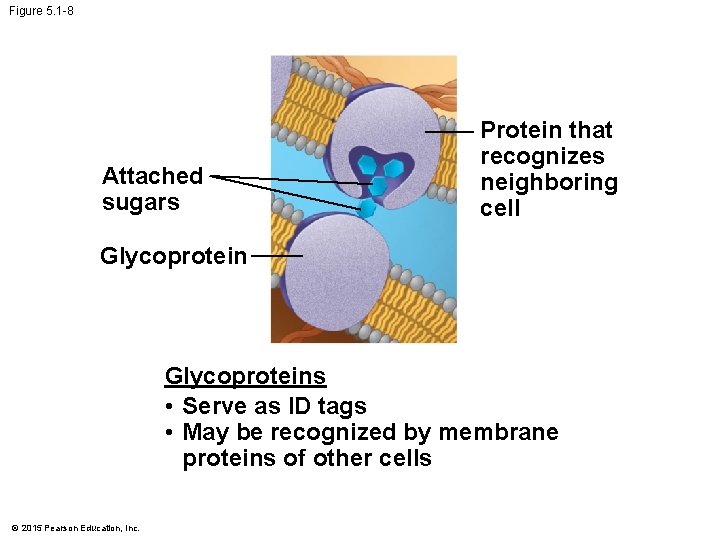
Figure 5. 1 -8 Attached sugars Protein that recognizes neighboring cell Glycoproteins • Serve as ID tags • May be recognized by membrane proteins of other cells © 2015 Pearson Education, Inc.

Figure 5. 1 -9 Cytoplasmic side of membrane Extracellular side of membrane O 2 CO 2 Diffusion of small nonpolar molecules Enzyme Fibers of extracellular matrices (ECM) Enzyme Phospholipid Cholesterol Attachment protein Receptor protein Channel protein Junction protein Active transport ATP protein Microfilaments of cytoskeleton © 2015 Pearson Education, Inc. Junction protein Glycoprotein

5. 2 EVOLUTION CONNECTION: The spontaneous formation of membranes was a critical step in the origin of life • Phospholipids, the key ingredient of biological membranes, spontaneously self-assemble into simple membranes. • The formation of membrane-enclosed collections of molecules was a critical step in the evolution of the first cells. © 2015 Pearson Education, Inc.

Figure 5. 2 Water-filled bubble made of phospholipids © 2015 Pearson Education, Inc.

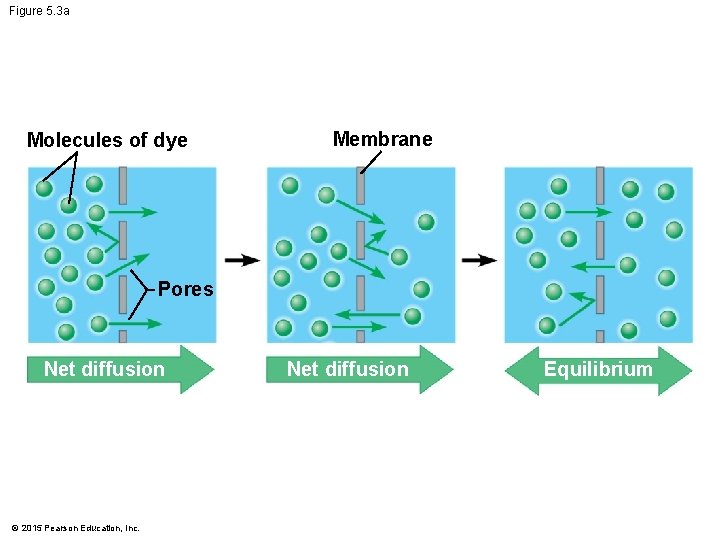
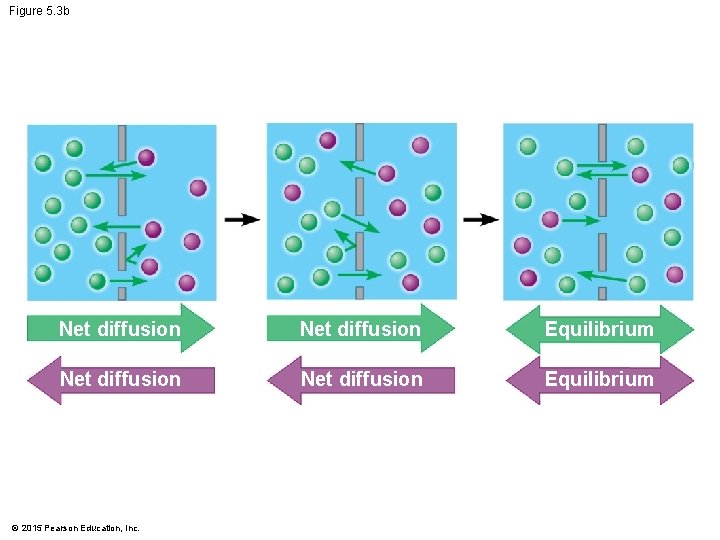
5. 3 Passive transport is diffusion across a membrane with no energy investment • Diffusion is the tendency of particles to spread out evenly in an available space. • Particles move from an area of more concentrated particles to an area where they are less concentrated. • This means that particles diffuse down their concentration gradient. • Eventually, the particles reach dynamic equilibrium, where there is no net change in concentration on either side of the membrane. © 2015 Pearson Education, Inc.

Figure 5. 3 a Molecules of dye Membrane Pores Net diffusion © 2015 Pearson Education, Inc. Net diffusion Equilibrium

Figure 5. 3 b Net diffusion Equilibrium © 2015 Pearson Education, Inc.

5. 3 Passive transport is diffusion across a membrane with no energy investment • Diffusion across a cell membrane does not require energy, so it is called passive transport. • Diffusion down concentration gradients is the sole means by which oxygen enters your cells and carbon dioxide passes out of cells. © 2015 Pearson Education, Inc.

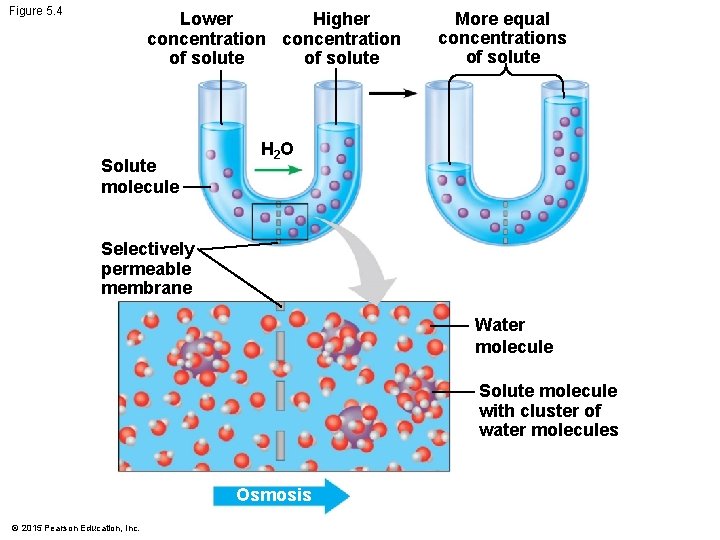
5. 4 Osmosis is the diffusion of water across a membrane • One of the most important substances that crosses membranes by passive transport is water. • The diffusion of water across a selectively permeable membrane is called osmosis. © 2015 Pearson Education, Inc.

5. 4 Osmosis is the diffusion of water across a membrane • If a membrane, permeable to water but not to a solute, separates two solutions with different concentrations of solute, water will cross the membrane, moving down its own concentration gradient, until the solute concentration on both sides is equal. © 2015 Pearson Education, Inc.

Figure 5. 4 Lower Higher concentration of solute Solute molecule More equal concentrations of solute H 2 O Selectively permeable membrane Water molecule Solute molecule with cluster of water molecules Osmosis © 2015 Pearson Education, Inc.

5. 5 Water balance between cells and their surroundings is crucial to organisms • Tonicity is a term that describes the ability of a surrounding solution to cause a cell to gain or lose water. • The tonicity of a solution mainly depends on its concentration of solutes relative to the concentration of solutes inside the cell. © 2015 Pearson Education, Inc.

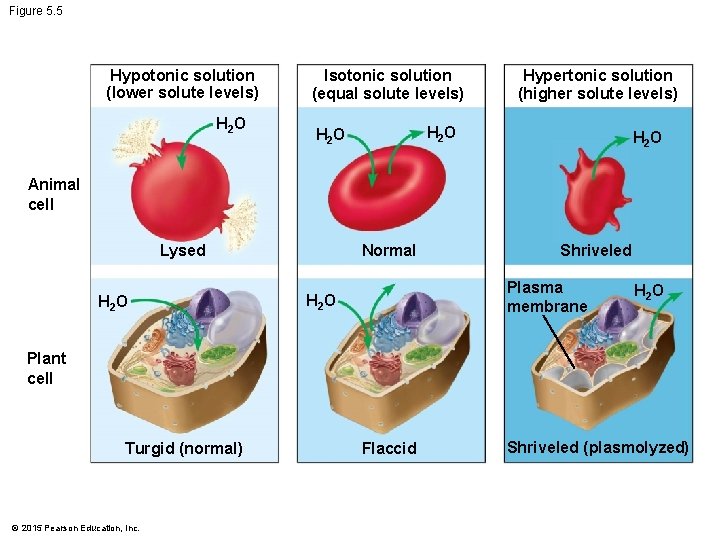
5. 5 Water balance between cells and their surroundings is crucial to organisms • How will animal cells be affected when placed into solutions of various tonicities? • In an isotonic solution, the concentration of solute is the same on both sides of a membrane, and the cell volume will not change. • In a hypotonic solution, the solute concentration is lower outside the cell, water molecules move into the cell, and the cell will expand may burst. • In a hypertonic solution, the solute concentration is higher outside the cell, water molecules move out of the cell, and the cell will shrink. © 2015 Pearson Education, Inc.

Figure 5. 5 Hypotonic solution (lower solute levels) H 2 O Isotonic solution (equal solute levels) Hypertonic solution (higher solute levels) H 2 O Animal cell Normal Lysed H 2 O Shriveled Plasma membrane H 2 O Plant cell Turgid (normal) © 2015 Pearson Education, Inc. Flaccid Shriveled (plasmolyzed)

5. 5 Water balance between cells and their surroundings is crucial to organisms • For an animal cell to survive in a hypotonic or hypertonic environment, it must engage in osmoregulation, the control of water balance. © 2015 Pearson Education, Inc.

5. 5 Water balance between cells and their surroundings is crucial to organisms • The cell walls of plant cells, prokaryotes, and fungi make water balance issues somewhat different. • The cell wall of a plant cell exerts pressure that prevents the cell from taking in too much water and bursting when placed in a hypotonic environment. • But in a hypertonic environment, plant and animal cells both shrivel. © 2015 Pearson Education, Inc.

Figure 5. 5 Hypotonic solution (lower solute levels) H 2 O Isotonic solution (equal solute levels) H 2 O Hypertonic solution (higher solute levels) Plasma H 2 O membrane Plant cell Turgid (normal) © 2015 Pearson Education, Inc. Flaccid Shriveled (plasmolyzed)

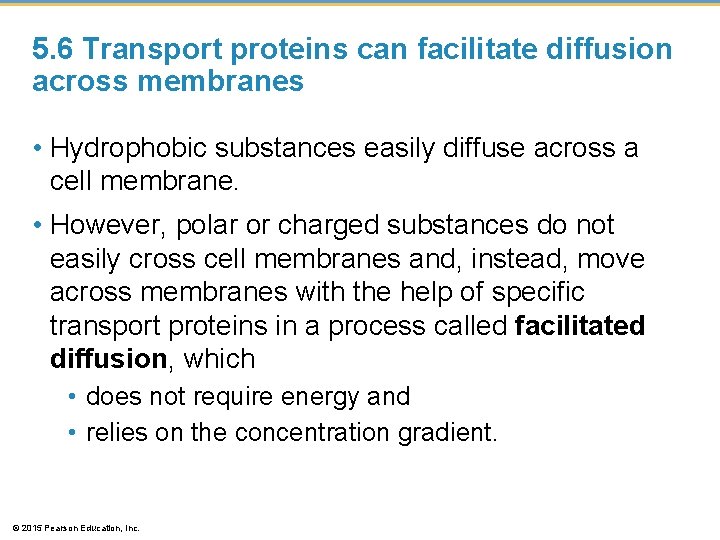
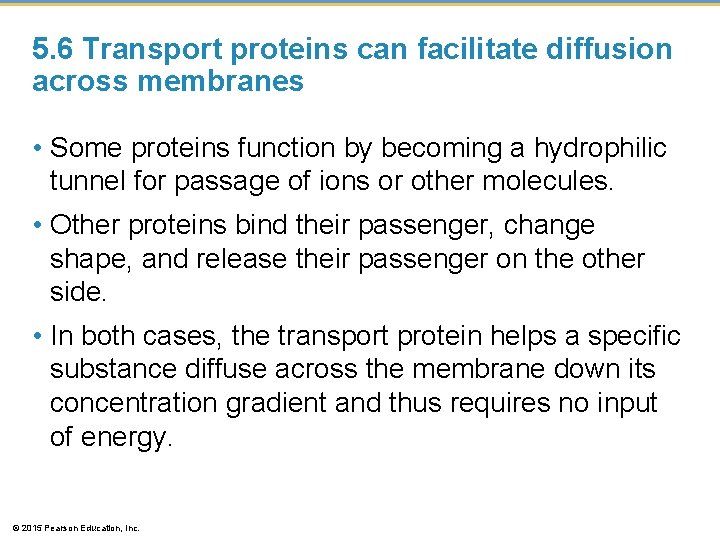
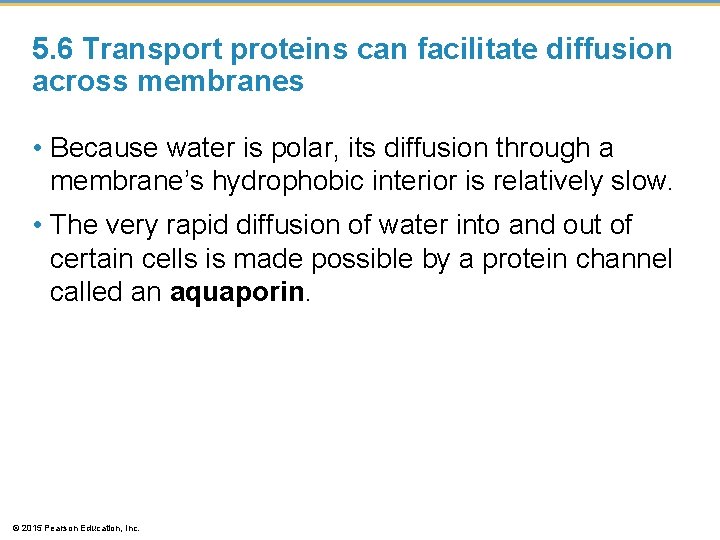
5. 6 Transport proteins can facilitate diffusion across membranes • Hydrophobic substances easily diffuse across a cell membrane. • However, polar or charged substances do not easily cross cell membranes and, instead, move across membranes with the help of specific transport proteins in a process called facilitated diffusion, which • does not require energy and • relies on the concentration gradient. © 2015 Pearson Education, Inc.

5. 6 Transport proteins can facilitate diffusion across membranes • Some proteins function by becoming a hydrophilic tunnel for passage of ions or other molecules. • Other proteins bind their passenger, change shape, and release their passenger on the other side. • In both cases, the transport protein helps a specific substance diffuse across the membrane down its concentration gradient and thus requires no input of energy. © 2015 Pearson Education, Inc.

5. 6 Transport proteins can facilitate diffusion across membranes • Because water is polar, its diffusion through a membrane’s hydrophobic interior is relatively slow. • The very rapid diffusion of water into and out of certain cells is made possible by a protein channel called an aquaporin. © 2015 Pearson Education, Inc.

5. 7 SCIENTIFIC THINKING: Research on another membrane protein led to the discovery of aquaporins • Dr. Peter Agre received the 2003 Nobel Prize in Chemistry for his discovery of aquaporins. • His research on the Rh protein used in blood typing led to this discovery. © 2015 Pearson Education, Inc.

Relative volume of representative eggs Figure 5. 7 © 2015 Pearson Education, Inc. 1. 4 Time of rupture 1. 3 RNA-injected eggs 1. 2 1. 1 Control eggs 1. 0 0 1 2 3 Time (min) 4 5

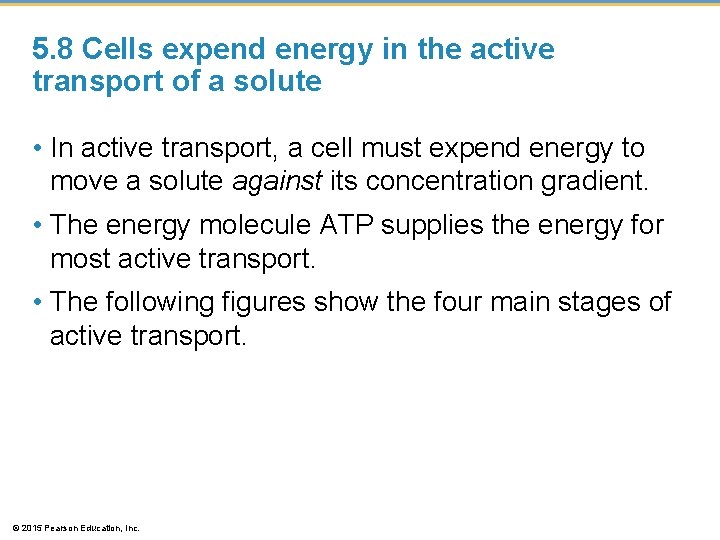
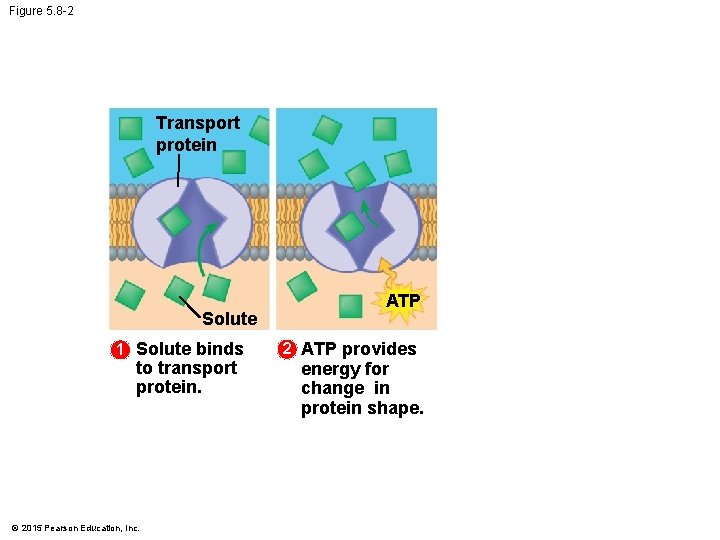
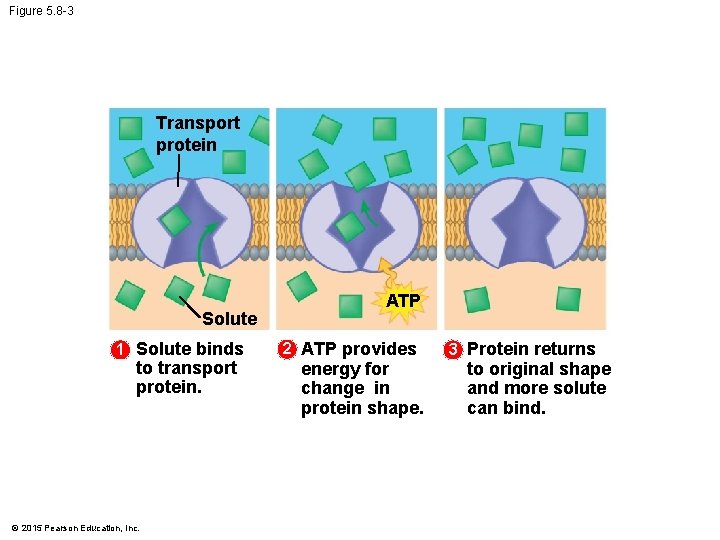
5. 8 Cells expend energy in the active transport of a solute • In active transport, a cell must expend energy to move a solute against its concentration gradient. • The energy molecule ATP supplies the energy for most active transport. • The following figures show the four main stages of active transport. © 2015 Pearson Education, Inc.

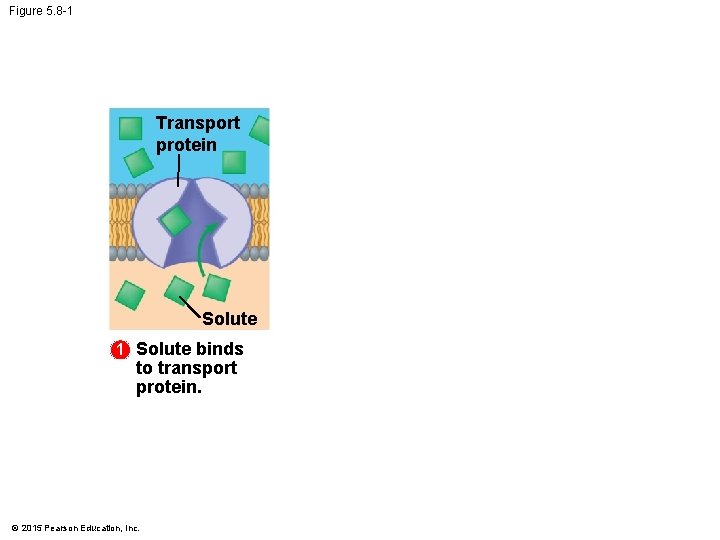
Figure 5. 8 -1 Transport protein Solute 1 Solute binds to transport protein. © 2015 Pearson Education, Inc.

Figure 5. 8 -2 Transport protein Solute 1 Solute binds to transport protein. © 2015 Pearson Education, Inc. ATP 2 ATP provides energy for change in protein shape.

Figure 5. 8 -3 Transport protein Solute 1 Solute binds to transport protein. © 2015 Pearson Education, Inc. ATP 2 ATP provides energy for change in protein shape. 3 Protein returns to original shape and more solute can bind.

5. 9 Exocytosis and endocytosis transport large molecules across membranes • A cell uses two mechanisms to move large molecules across membranes. 1. Exocytosis is used to export bulky molecules, such as proteins or polysaccharides. 2. Endocytosis is used to take in large molecules. • In both cases, material to be transported is packaged within a vesicle that fuses with the membrane. © 2015 Pearson Education, Inc.

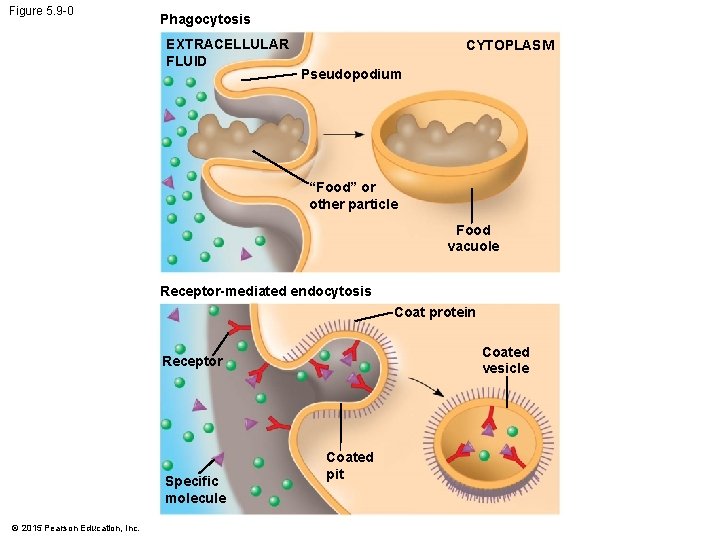
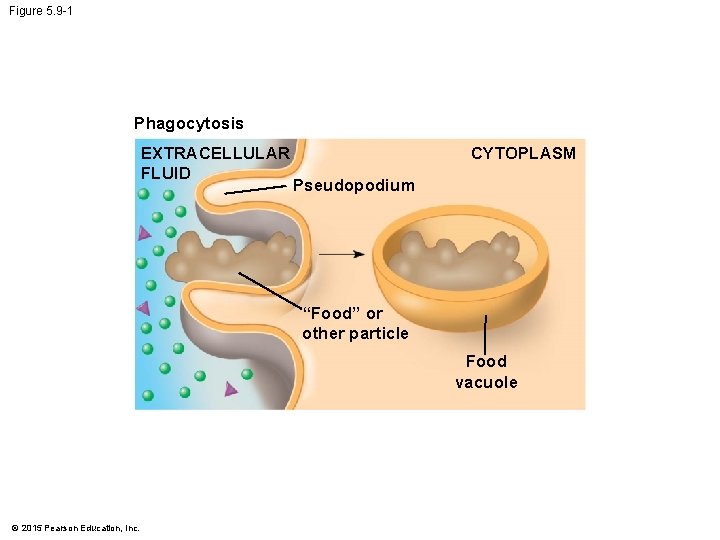
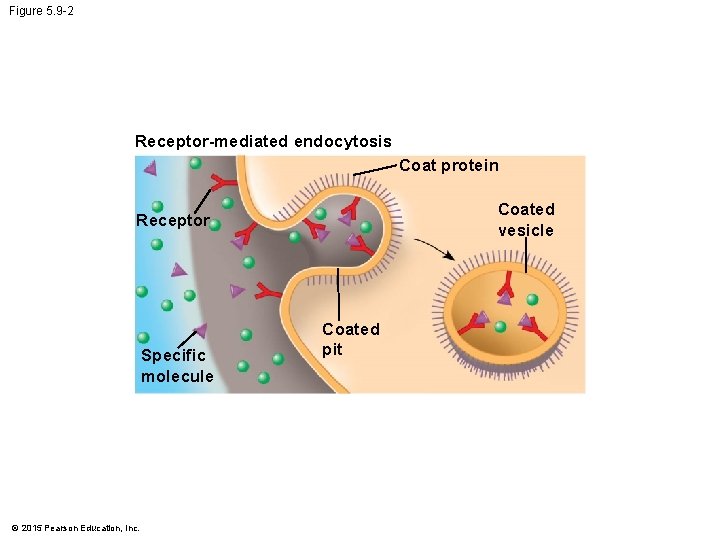
5. 9 Exocytosis and endocytosis transport large molecules across membranes • There are two kinds of endocytosis. 1. Phagocytosis is the engulfment of a particle by the cell wrapping cell membrane around it, forming a vacuole. 2. Receptor-mediated endocytosis uses membrane receptors for specific solutes. The region of the membrane with receptors pinches inward to form a vesicle. • Receptor-mediated endocytosis is used to take in cholesterol from the blood. © 2015 Pearson Education, Inc.

Figure 5. 9 -0 Phagocytosis EXTRACELLULAR FLUID CYTOPLASM Pseudopodium “Food” or other particle Food vacuole Receptor-mediated endocytosis Coat protein Coated vesicle Receptor Specific molecule © 2015 Pearson Education, Inc. Coated pit

Figure 5. 9 -1 Phagocytosis EXTRACELLULAR FLUID CYTOPLASM Pseudopodium “Food” or other particle Food vacuole © 2015 Pearson Education, Inc.

Figure 5. 9 -2 Receptor-mediated endocytosis Coat protein Coated vesicle Receptor Specific molecule © 2015 Pearson Education, Inc. Coated pit

ENERGY AND THE CELL © 2015 Pearson Education, Inc.

5. 10 Cells transform energy as they perform work • Cells are miniature chemical factories, housing thousands of chemical reactions. • Some of these chemical reactions release energy, and others require energy. © 2015 Pearson Education, Inc.

5. 10 Cells transform energy as they perform work • Energy is the capacity to cause change or to perform work. • There are two basic forms of energy. 1. Kinetic energy is the energy of motion. 2. Potential energy is energy that matter possesses as a result of its location or structure. © 2015 Pearson Education, Inc.

5. 10 Cells transform energy as they perform work • Thermal energy is a type of kinetic energy associated with the random movement of atoms or molecules. • Thermal energy in transfer from one object to another is called heat. • Light is also a type of kinetic energy; it can be harnessed to power photosynthesis. © 2015 Pearson Education, Inc.

5. 10 Cells transform energy as they perform work • Chemical energy is the • potential energy available for release in a chemical reaction and • the most important type of energy for living organisms to power the work of the cell. © 2015 Pearson Education, Inc.

5. 10 Cells transform energy as they perform work • Thermodynamics is the study of energy transformations that occur in a collection of matter. • The word system is used for the matter under study. • The word surroundings is used for everything outside the system; the rest of the universe. © 2015 Pearson Education, Inc.

5. 10 Cells transform energy as they perform work • Two laws govern energy transformations in organisms. • Per the first law of thermodynamics (also known as the law of energy conservation), energy in the universe is constant. • Per the second law of thermodynamics, energy conversions increase the disorder of the universe. • Entropy is the measure of disorder or randomness. © 2015 Pearson Education, Inc.

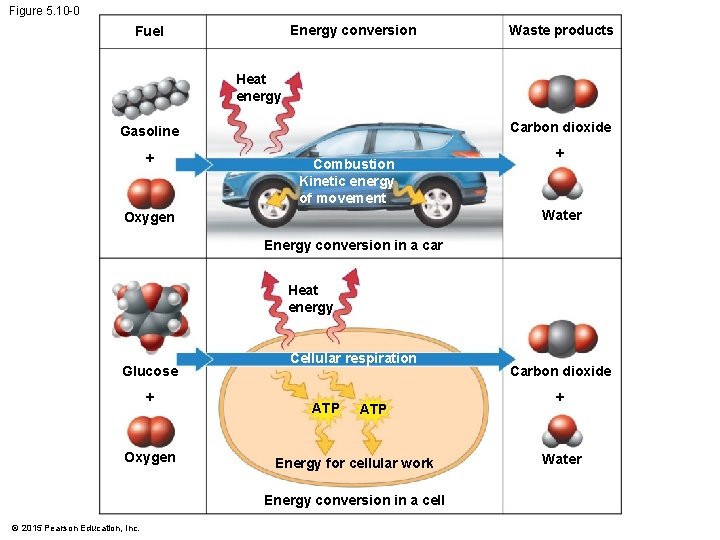
5. 10 Cells transform energy as they perform work • Automobile engines and cells use the same basic process to make the chemical energy of their fuel available for work. • In the car and cells, the waste products are carbon dioxide and water. • Cells use oxygen in reactions that release energy from fuel molecules. • In cellular respiration, the chemical energy stored in organic molecules is used to produce ATP, which the cell can use to perform work. © 2015 Pearson Education, Inc.

Figure 5. 10 -0 Energy conversion Fuel Waste products Heat energy Carbon dioxide Gasoline + Combustion Kinetic energy of movement + Water Oxygen Energy conversion in a car Heat energy Glucose + Oxygen Cellular respiration ATP Energy for cellular work Energy conversion in a cell © 2015 Pearson Education, Inc. Carbon dioxide + Water

5. 11 Chemical reactions either release or store energy • Chemical reactions either • release energy (exergonic reactions) or • require an input of energy and store energy (endergonic reactions). © 2015 Pearson Education, Inc.

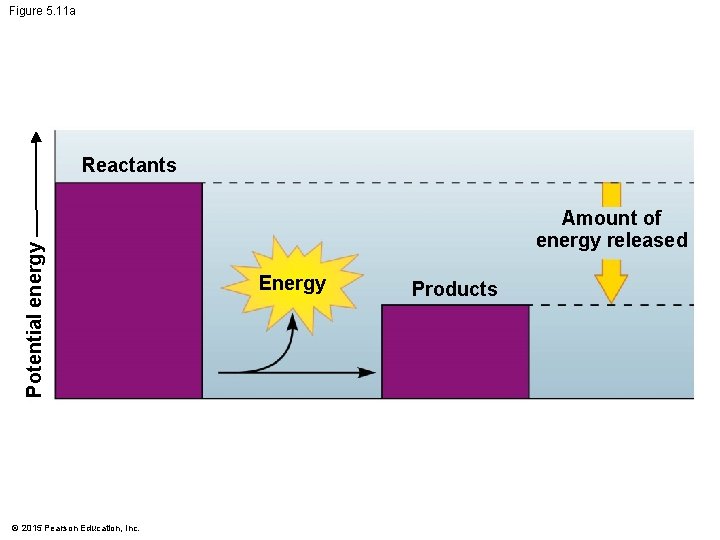
5. 11 Chemical reactions either release or store energy • Exergonic reactions release energy. • These reactions release the energy in covalent bonds of the reactants. • Burning wood releases the energy in glucose as heat and light. • Cellular respiration • involves many steps, • releases energy slowly, and • uses some of the released energy to produce ATP. © 2015 Pearson Education, Inc.

Figure 5. 11 a Potential energy Reactants © 2015 Pearson Education, Inc. Amount of energy released Energy Products

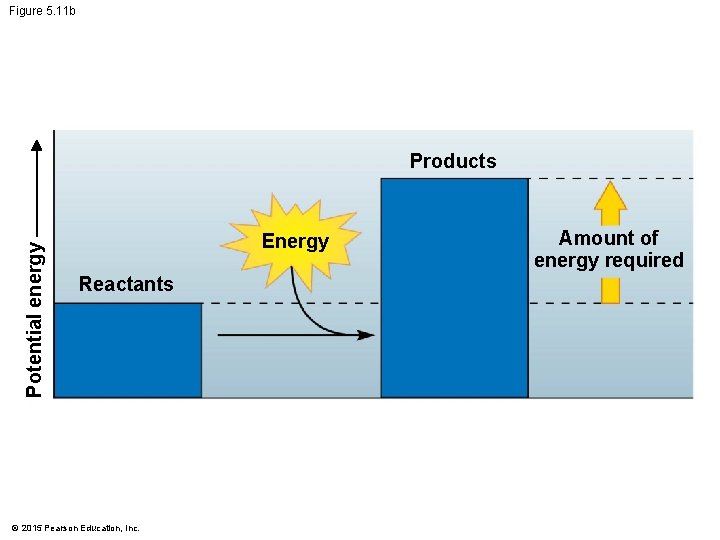
5. 11 Chemical reactions either release or store energy • An endergonic reaction • requires an input of energy and • yields products rich in potential energy. • Endergonic reactions • start with reactant molecules that contain relatively little potential energy but • end with products that contain more chemical energy. © 2015 Pearson Education, Inc.

Figure 5. 11 b Potential energy Products Energy Reactants © 2015 Pearson Education, Inc. Amount of energy required

5. 11 Chemical reactions either release or store energy • Photosynthesis is a type of endergonic process. • In photosynthesis, • energy-poor reactants (carbon dioxide and water) are used, • energy is absorbed from sunlight, and • energy-rich sugar molecules are produced. © 2015 Pearson Education, Inc.

5. 11 Chemical reactions either release or store energy • A living organism carries out thousands of endergonic and exergonic chemical reactions. • The total of an organism’s chemical reactions is called metabolism. • A metabolic pathway is a series of chemical reactions that either • builds a complex molecule or • breaks down a complex molecule into simpler compounds. © 2015 Pearson Education, Inc.

5. 11 Chemical reactions either release or store energy • Energy coupling uses the energy released from exergonic reactions to drive endergonic reactions, typically using the energy stored in ATP molecules. © 2015 Pearson Education, Inc.

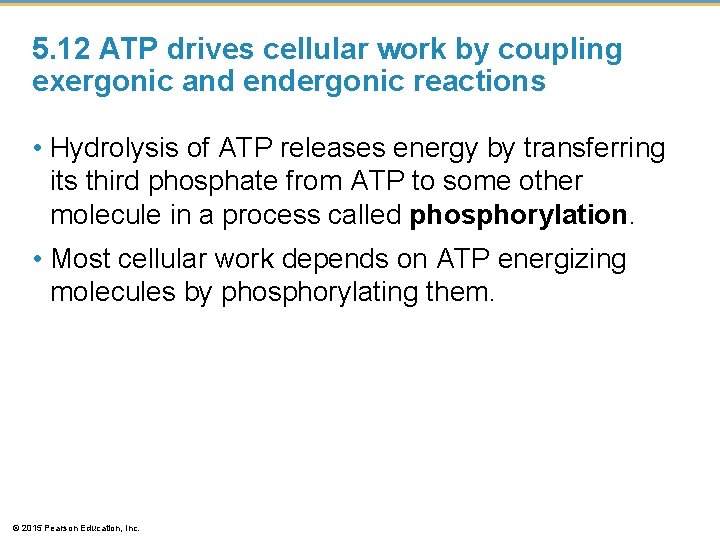
5. 12 ATP drives cellular work by coupling exergonic and endergonic reactions • ATP, adenosine triphosphate, powers nearly all forms of cellular work. • ATP consists of • adenosine and • a triphosphate tail of three phosphate groups. © 2015 Pearson Education, Inc.

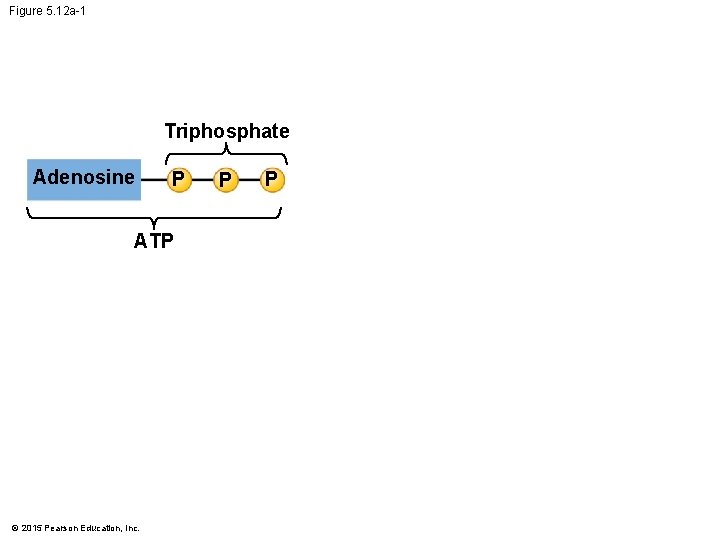
5. 12 ATP drives cellular work by coupling exergonic and endergonic reactions • Hydrolysis of ATP releases energy by transferring its third phosphate from ATP to some other molecule in a process called phosphorylation. • Most cellular work depends on ATP energizing molecules by phosphorylating them. © 2015 Pearson Education, Inc.

Figure 5. 12 a-1 Triphosphate Adenosine P ATP © 2015 Pearson Education, Inc. P P

Figure 5. 12 a-2 Triphosphate Adenosine P P P ATP Diphosphate H 2 O Adenosine ADP © 2015 Pearson Education, Inc. P Phosphate Energy

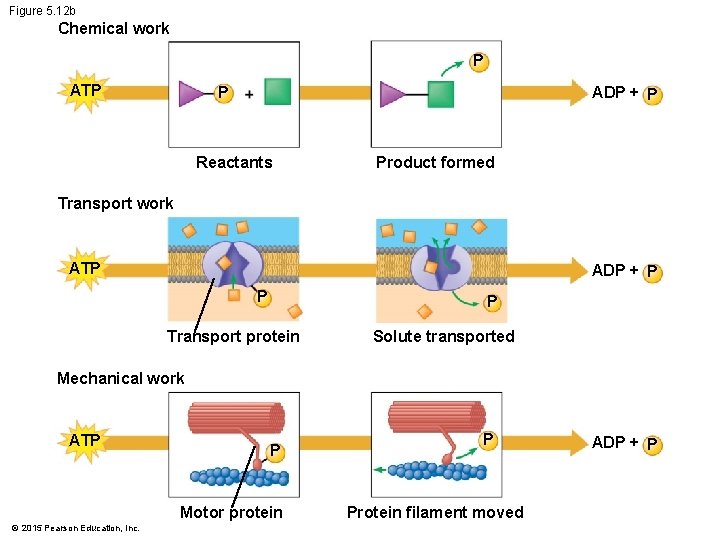
5. 12 ATP drives cellular work by coupling exergonic and endergonic reactions • There are three main types of cellular work: 1. chemical, 2. mechanical, and 3. transport. • ATP drives all three of these types of work. © 2015 Pearson Education, Inc.

Figure 5. 12 b Chemical work P ATP P ADP + P Reactants Product formed Transport work ATP ADP + P P P Transport protein Solute transported Mechanical work ATP P Motor protein © 2015 Pearson Education, Inc. P Protein filament moved ADP + P

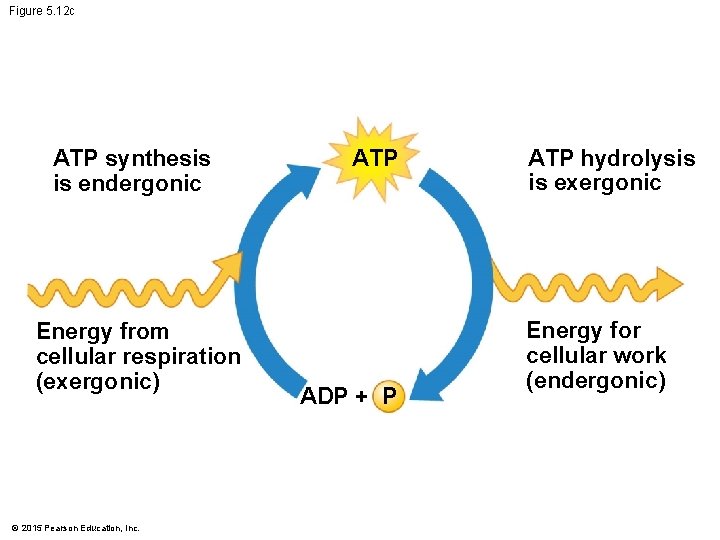
5. 12 ATP drives cellular work by coupling exergonic and endergonic reactions • A cell uses and regenerates ATP continuously. • In the ATP cycle, energy released in an exergonic reaction, such as the breakdown of glucose during cellular respiration, is used in an endergonic reaction to generate ATP from ADP. © 2015 Pearson Education, Inc.

Figure 5. 12 c ATP synthesis is endergonic Energy from cellular respiration (exergonic) © 2015 Pearson Education, Inc. ATP ADP + P ATP hydrolysis is exergonic Energy for cellular work (endergonic)

HOW ENZYMES FUNCTION © 2015 Pearson Education, Inc.

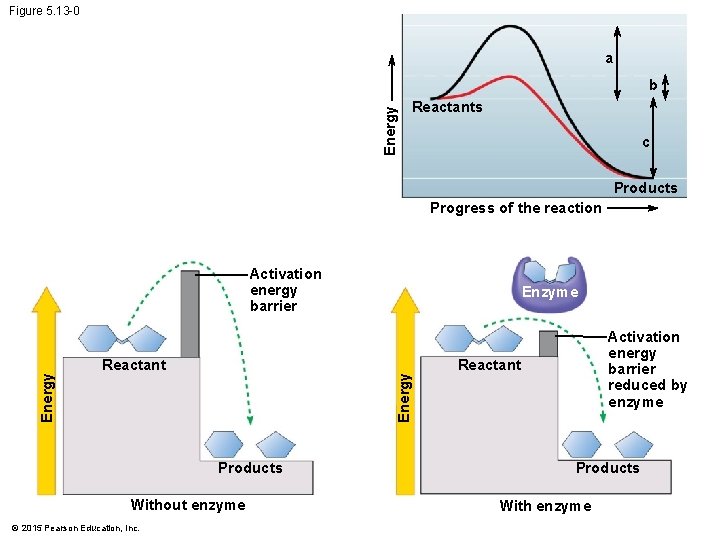
5. 13 Enzymes speed up the cell’s chemical reactions by lowering energy barriers • Although biological molecules possess much potential energy, it is not released spontaneously. • An energy barrier must be overcome before a chemical reaction can begin. • This energy is called the activation energy (because it activates the reactants). © 2015 Pearson Education, Inc.

5. 13 Enzymes speed up the cell’s chemical reactions by lowering energy barriers • We can think of activation energy as the amount of energy needed for a reactant molecule to move “uphill” to a higher-energy but an unstable state so that the “downhill” part of the reaction can begin. • One way to speed up a reaction is to add heat, which agitates atoms so that bonds break more easily and reactions can proceed, but too much heat will kill a cell. © 2015 Pearson Education, Inc.

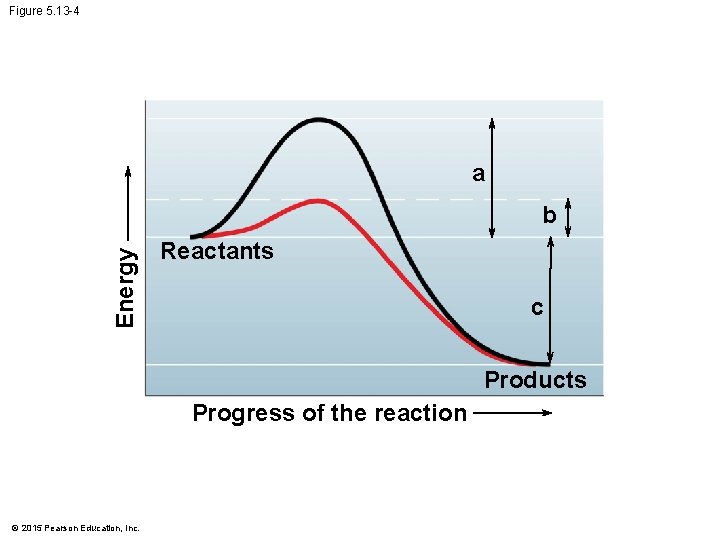
5. 13 Enzymes speed up the cell’s chemical reactions by lowering energy barriers • Enzymes • function as biological catalysts, • increase the rate of a reaction without being consumed by the reaction, and • are usually proteins (although some RNA molecules can function as enzymes). • Enzymes speed up a reaction by lowering the activation energy needed for a reaction to begin. © 2015 Pearson Education, Inc.

Figure 5. 13 -0 a Energy b Reactants c Products Progress of the reaction Reactant Products Without enzyme © 2015 Pearson Education, Inc. Enzyme Energy Activation energy barrier reduced by enzyme Reactant Products With enzyme

Figure 5. 13 -4 a Energy b Reactants c Products Progress of the reaction © 2015 Pearson Education, Inc.

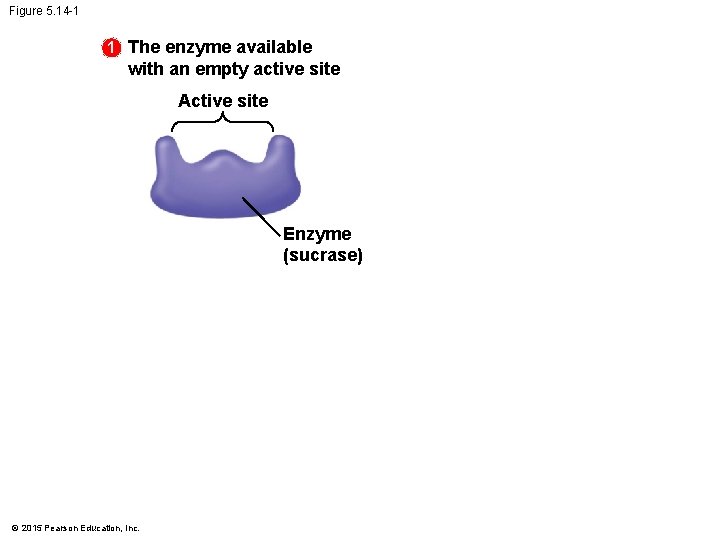
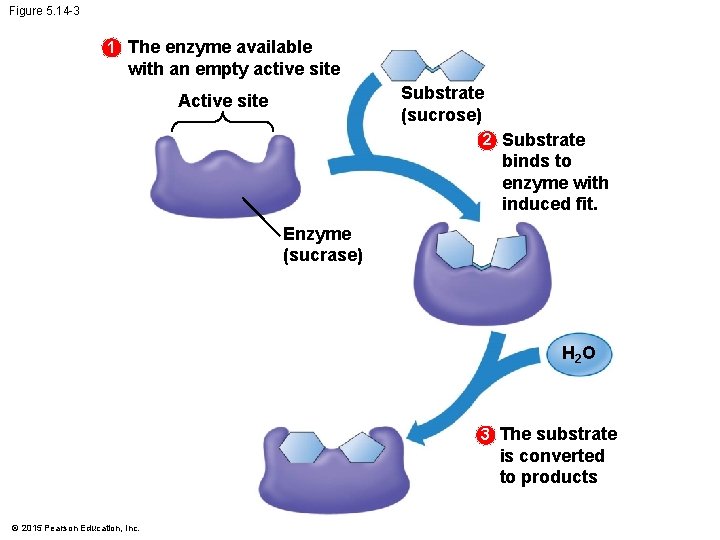
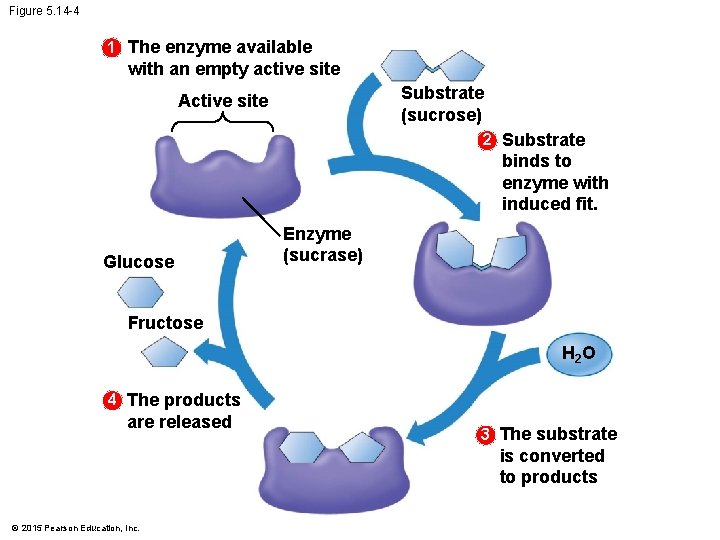
5. 14 A specific enzyme catalyzes each cellular reaction • An enzyme • is very selective in the reaction it catalyzes and • has a shape that determines the enzyme’s specificity. • The specific reactant that an enzyme acts on is called the enzyme’s substrate. • A substrate fits into a region of the enzyme called the active site. • Enzymes are specific because only specific substrate molecules fit into their active site. © 2015 Pearson Education, Inc.

5. 14 A specific enzyme catalyzes each cellular reaction • The following figure illustrates the catalytic cycle of an enzyme. © 2015 Pearson Education, Inc.

Figure 5. 14 -1 1 The enzyme available with an empty active site Active site Enzyme (sucrase) © 2015 Pearson Education, Inc.

Figure 5. 14 -2 1 The enzyme available with an empty active site Substrate (sucrose) Active site 2 Substrate binds to enzyme with induced fit. Enzyme (sucrase) © 2015 Pearson Education, Inc.

Figure 5. 14 -3 1 The enzyme available with an empty active site Substrate (sucrose) Active site 2 Substrate binds to enzyme with induced fit. Enzyme (sucrase) H 2 O 3 The substrate is converted to products © 2015 Pearson Education, Inc.

Figure 5. 14 -4 1 The enzyme available with an empty active site Substrate (sucrose) Active site 2 Substrate binds to enzyme with induced fit. Glucose Enzyme (sucrase) Fructose H 2 O 4 The products are released 3 The substrate is converted to products © 2015 Pearson Education, Inc.

5. 14 A specific enzyme catalyzes each cellular reaction • For every enzyme, there are optimal conditions under which it is most effective. • Temperature affects molecular motion. • An enzyme’s optimal temperature produces the highest rate of contact between the reactants and the enzyme’s active site. • Most human enzymes work best at 35– 40°C. • The optimal p. H for most enzymes is near neutrality. © 2015 Pearson Education, Inc.

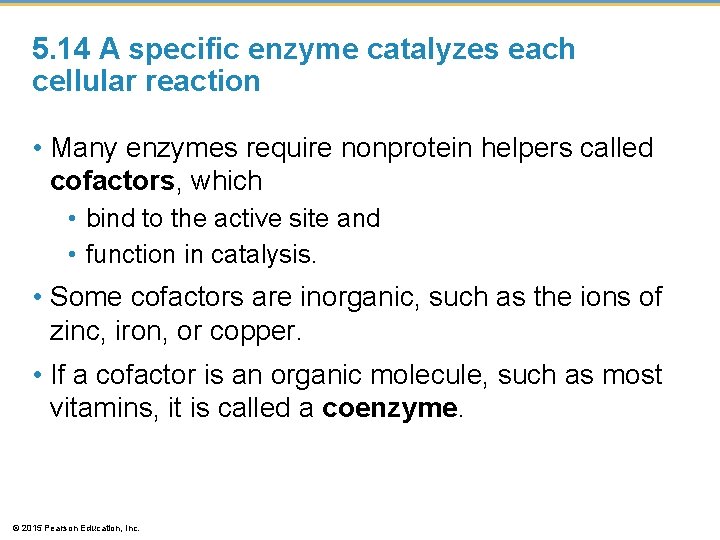
5. 14 A specific enzyme catalyzes each cellular reaction • Many enzymes require nonprotein helpers called cofactors, which • bind to the active site and • function in catalysis. • Some cofactors are inorganic, such as the ions of zinc, iron, or copper. • If a cofactor is an organic molecule, such as most vitamins, it is called a coenzyme. © 2015 Pearson Education, Inc.

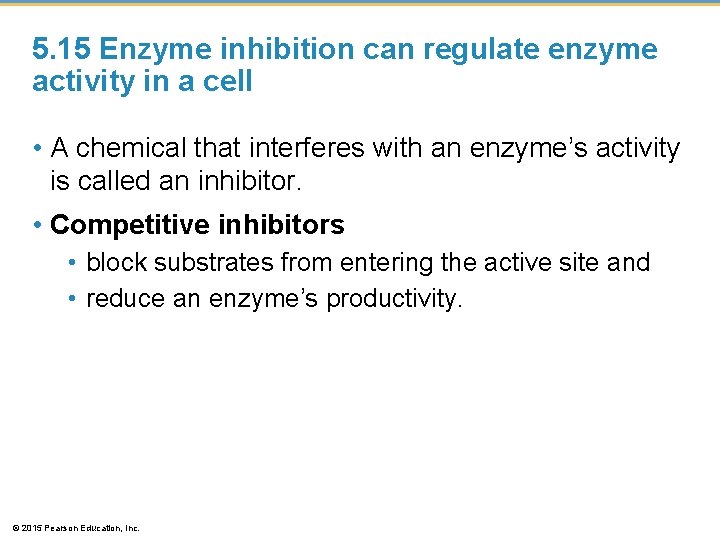
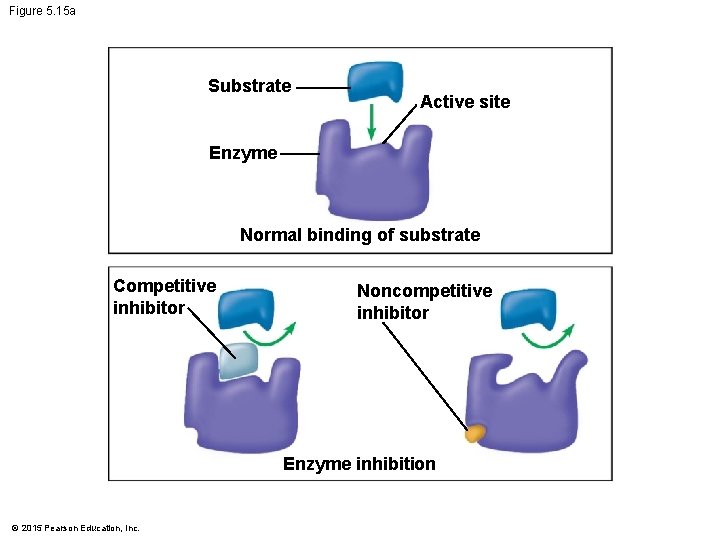
5. 15 Enzyme inhibition can regulate enzyme activity in a cell • A chemical that interferes with an enzyme’s activity is called an inhibitor. • Competitive inhibitors • block substrates from entering the active site and • reduce an enzyme’s productivity. © 2015 Pearson Education, Inc.

5. 15 Enzyme inhibition can regulate enzyme activity in a cell • Noncompetitive inhibitors • bind to the enzyme somewhere other than the active site, • change the shape of the active site, and • prevent the substrate from binding. © 2015 Pearson Education, Inc.

Figure 5. 15 a Substrate Active site Enzyme Normal binding of substrate Competitive inhibitor Noncompetitive inhibitor Enzyme inhibition © 2015 Pearson Education, Inc.

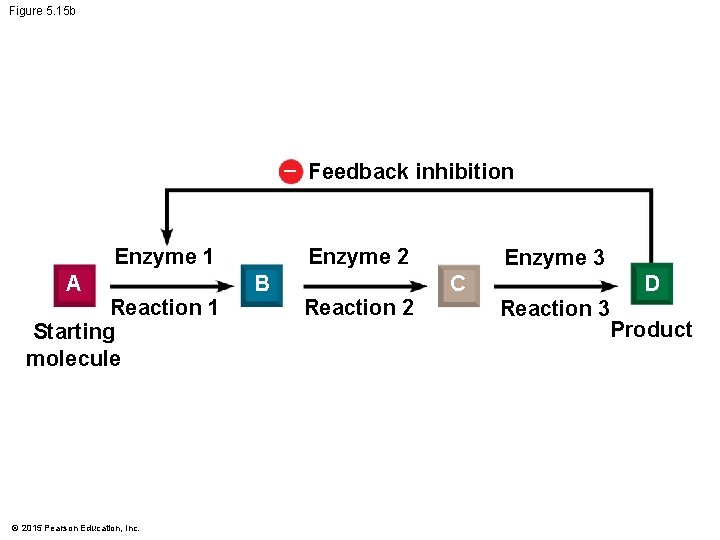
5. 15 Enzyme inhibition can regulate enzyme activity in a cell • Enzyme inhibitors are important in regulating cell metabolism. • In some reactions, the product may act as an inhibitor of one of the enzymes in the pathway that produced it. This is called feedback inhibition. © 2015 Pearson Education, Inc.

Figure 5. 15 b – Feedback inhibition Enzyme 2 Enzyme 1 A Reaction 1 Starting molecule © 2015 Pearson Education, Inc. B Reaction 2 Enzyme 3 C D Reaction 3 Product

5. 16 CONNECTION: Many drugs, pesticides, and poisons are enzyme inhibitors • Many beneficial drugs act as enzyme inhibitors, including • ibuprofen, which inhibits an enzyme involved in the production of prostaglandins (messenger molecules that increase the sensation of pain and inflammation), • some blood pressure medicines, • some antidepressants, • many antibiotics, and • protease inhibitors used to fight HIV. © 2015 Pearson Education, Inc.

Figure 5. 16 © 2015 Pearson Education, Inc.

5. 16 CONNECTION: Many drugs, pesticides, and poisons are enzyme inhibitors • Enzyme inhibitors have also been developed as • pesticides and • deadly poisons for chemical warfare. © 2015 Pearson Education, Inc.

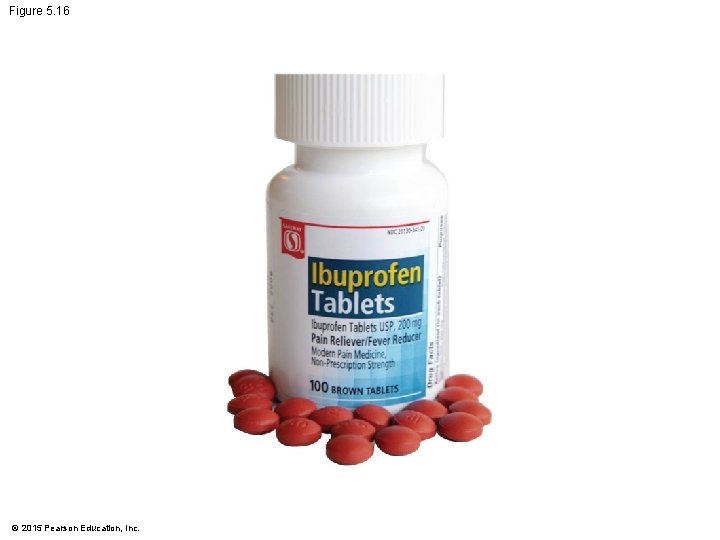
Figure 5. UN 01 Passive transport (requires no energy) Diffusion Facilitated diffusion Higher solute concentration Active transport (requires energy) Osmosis Higher free water concentration Higher solute concentration Solute Water Lower solute concentration © 2015 Pearson Education, Inc. Lower free water concentration ATP Lower solute concentration

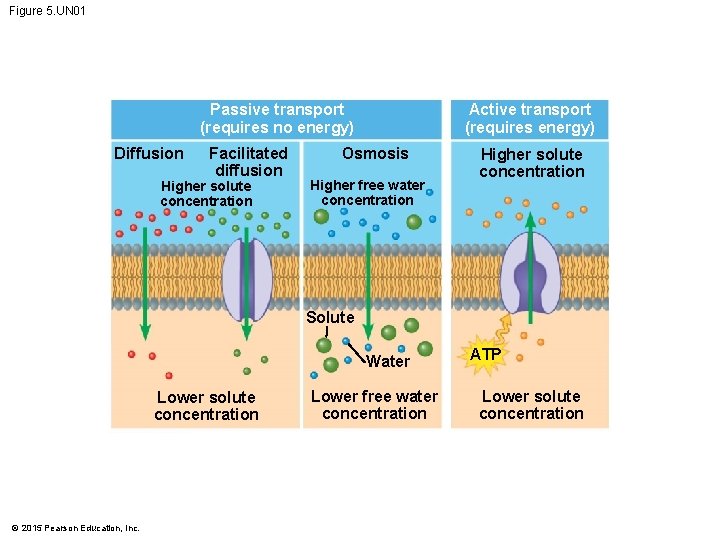
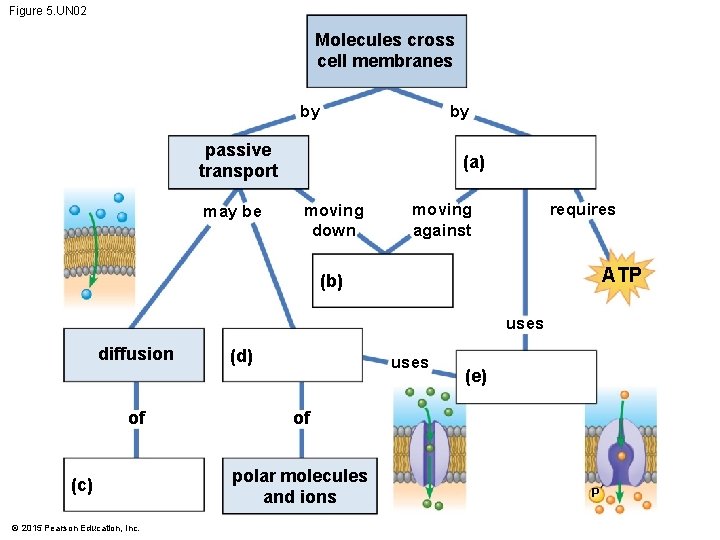
Figure 5. UN 02 Molecules cross cell membranes by by passive transport may be (a) moving down requires moving against ATP (b) uses diffusion of (c) © 2015 Pearson Education, Inc. (d) uses (e) of polar molecules and ions P

Figure 5. UN 03 c. b. a. d. f. e. © 2015 Pearson Education, Inc.

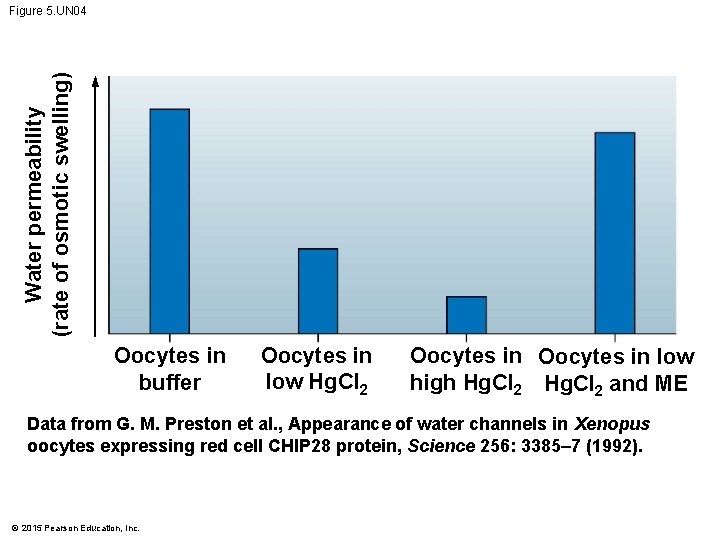
Water permeability (rate of osmotic swelling) Figure 5. UN 04 Oocytes in buffer Oocytes in low Hg. Cl 2 Oocytes in low high Hg. Cl 2 and ME Data from G. M. Preston et al. , Appearance of water channels in Xenopus oocytes expressing red cell CHIP 28 protein, Science 256: 3385– 7 (1992). © 2015 Pearson Education, Inc.

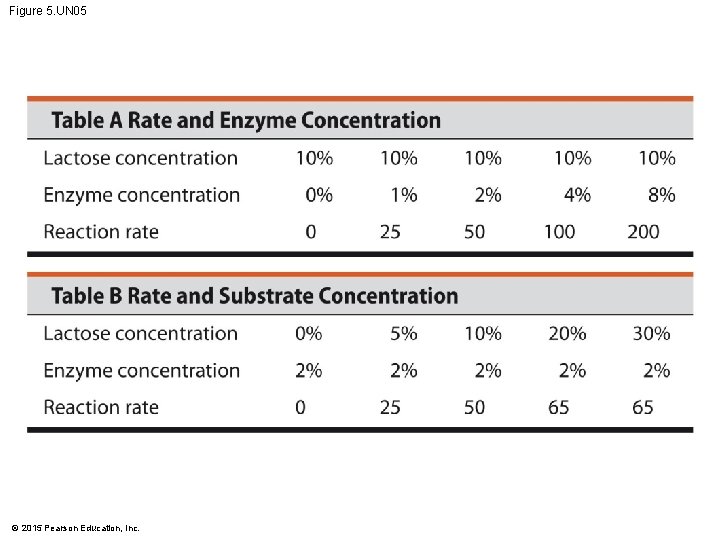
Figure 5. UN 05 © 2015 Pearson Education, Inc.