Chapter 5 The Working Cell Power Point Lectures






- Slides: 44

Chapter 5 The Working Cell Power. Point® Lectures for Campbell Essential Biology, Fourth Edition – Eric Simon, Jane Reece, and Jean Dickey Campbell Essential Biology with Physiology, Third Edition – Eric Simon, Jane Reece, and Jean Dickey Lectures by Chris C. Romero, updated by Edward J. Zalisko © 2010 Pearson Education, Inc.

Biology and Society: Natural Nanotechnology • Cells control their chemical environment using – Energy – Enzymes – The plasma membrane • Cell-based nanotechnology may be used to power microscopic robots. © 2010 Pearson Education, Inc.

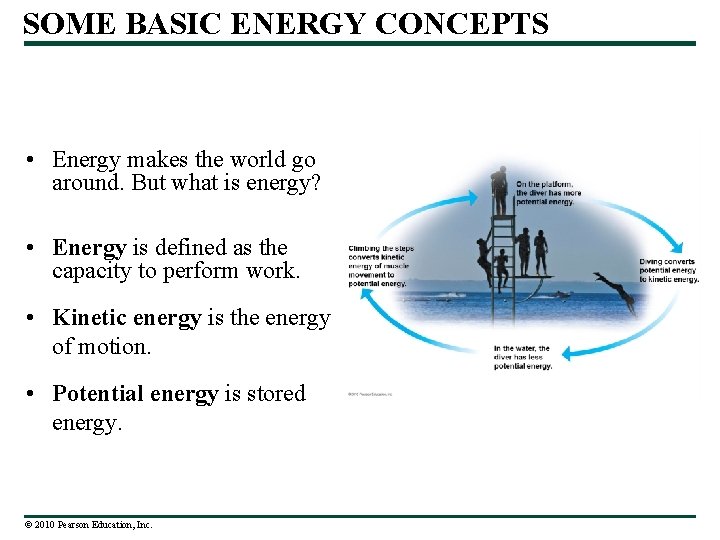
SOME BASIC ENERGY CONCEPTS • Energy makes the world go around. But what is energy? • Energy is defined as the capacity to perform work. • Kinetic energy is the energy of motion. • Potential energy is stored energy. © 2010 Pearson Education, Inc.

• Machines and organisms can transform kinetic energy to potential energy and vice versa. • In all such energy transformations, total energy is conserved. – Energy cannot be created or destroyed. – This is the principle of conservation of energy. • Every energy conversion releases some randomized energy in the form of heat. • Heat is a – Type of kinetic energy – Product of all energy conversions • Scientists use the term entropy as a measure of disorder, or randomness. All energy conversions increase the entropy of the universe. © 2010 Pearson Education, Inc.

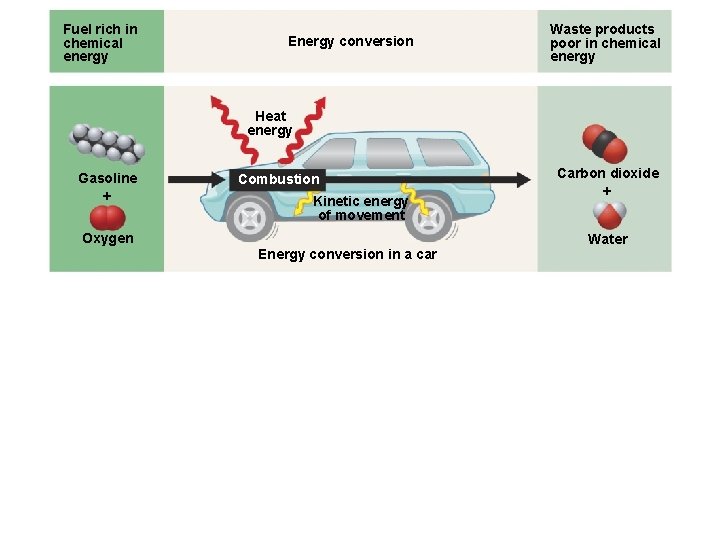
Chemical Energy • Molecules store varying amounts of potential energy in the arrangement of their atoms. • Organic compounds are relatively rich in such chemical energy. • Living cells and automobile engines use the same basic process to make chemical energy do work. • Cellular respiration is the energy-releasing chemical breakdown of fuel molecules that provides energy for cells to do work. • Humans convert about 40% of the energy in food to useful work, such as the contraction of muscles. • A calorie is the amount of energy that raises the temperature of one gram of water by 1 degree Celsius. • Food Calories are kilocalories, equal to 1, 000 calories. © 2010 Pearson Education, Inc.

Fuel rich in chemical energy Energy conversion Waste products poor in chemical energy Heat energy Gasoline Combustion Kinetic energy of movement Oxygen Energy conversion in a car Carbon dioxide Water Heat energy Cellular respiration Food Oxygen ATP Energy for cellular work Carbon dioxide Water Energy conversion in a cell Figure 5. 2

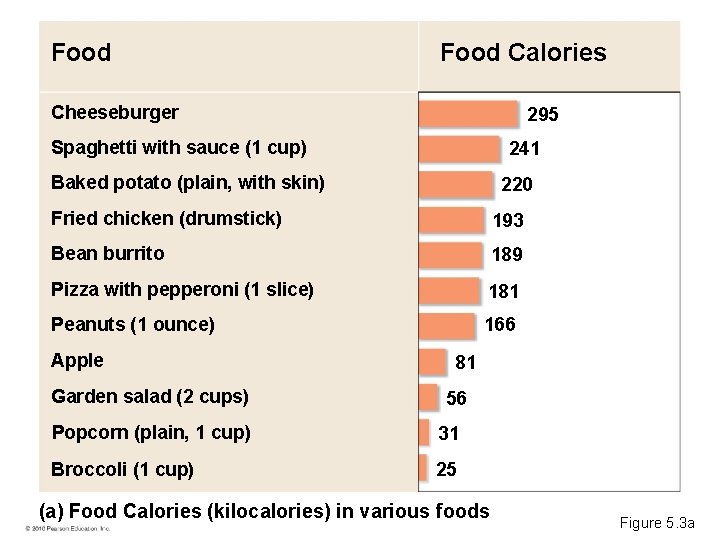
Food Calories Cheeseburger 295 Spaghetti with sauce (1 cup) 241 Baked potato (plain, with skin) 220 Fried chicken (drumstick) 193 Bean burrito 189 Pizza with pepperoni (1 slice) 181 Peanuts (1 ounce) 166 Apple Garden salad (2 cups) 81 56 Popcorn (plain, 1 cup) 31 Broccoli (1 cup) 25 (a) Food Calories (kilocalories) in various foods Figure 5. 3 a

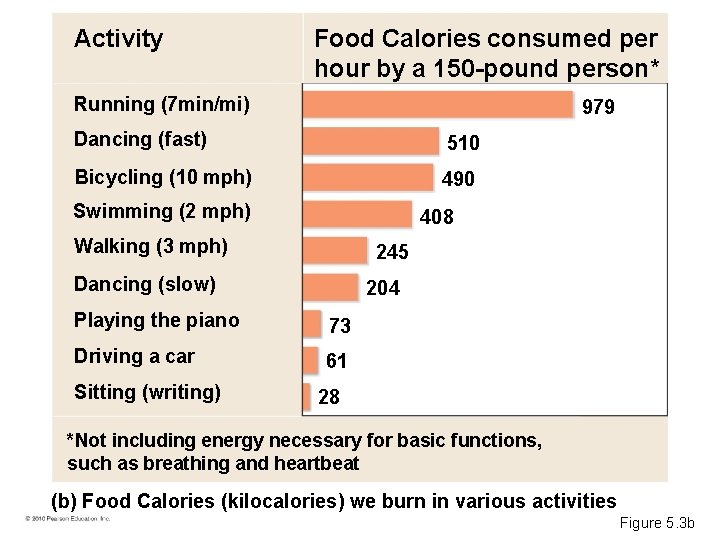
Activity Food Calories consumed per hour by a 150 -pound person* Running (7 min/mi) 979 Dancing (fast) 510 Bicycling (10 mph) 490 Swimming (2 mph) 408 Walking (3 mph) 245 Dancing (slow) 204 Playing the piano 73 Driving a car 61 Sitting (writing) 28 *Not including energy necessary for basic functions, such as breathing and heartbeat (b) Food Calories (kilocalories) we burn in various activities Figure 5. 3 b

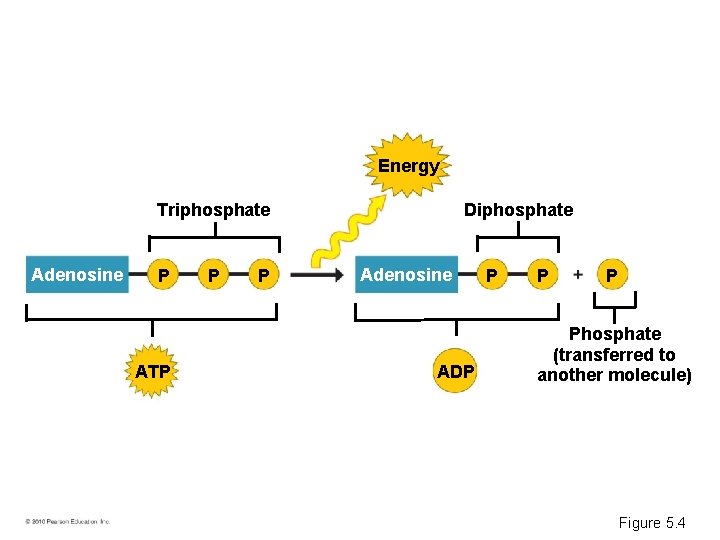
ATP AND CELLULAR WORK • Chemical energy is – Released by the breakdown of organic molecules during cellular respiration – Used to generate molecules of ATP • ATP – Acts like an energy shuttle – Stores energy obtained from food – Releases it later as needed • ATP (adenosine triphosphate) – Consists of adenosine plus a tail of three phosphate groups – Is broken down to ADP and a phosphate group, releasing energy © 2010 Pearson Education, Inc.

Energy Triphosphate Adenosine P ATP P P Diphosphate Adenosine ADP P Phosphate (transferred to another molecule) Figure 5. 4

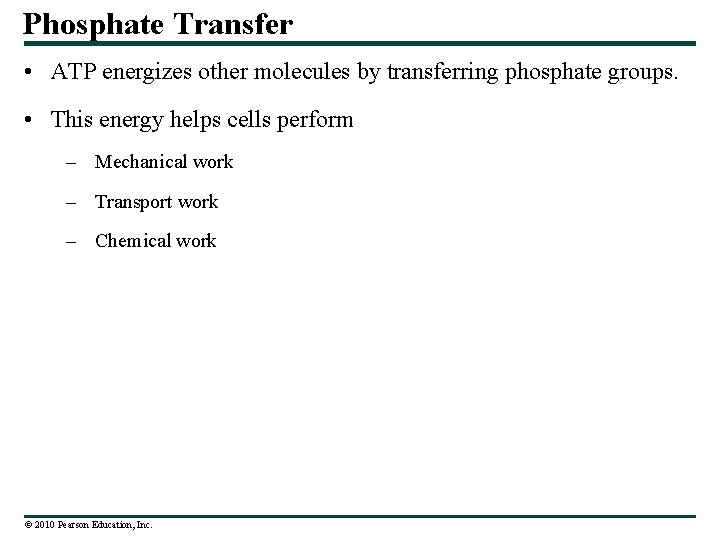
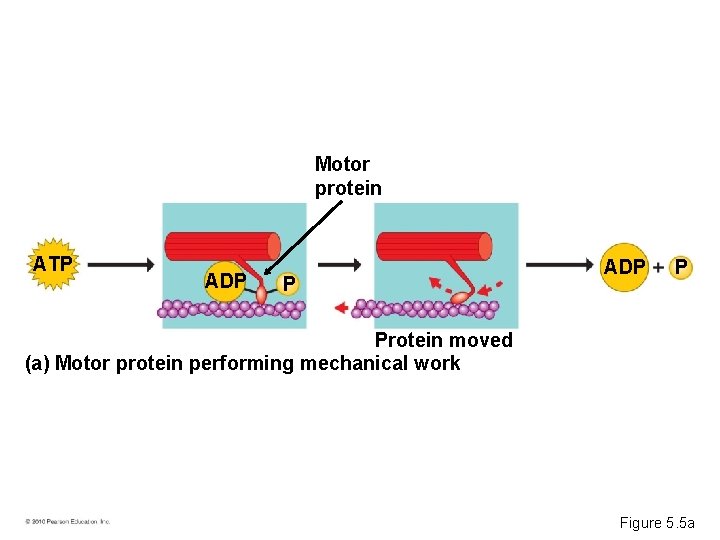
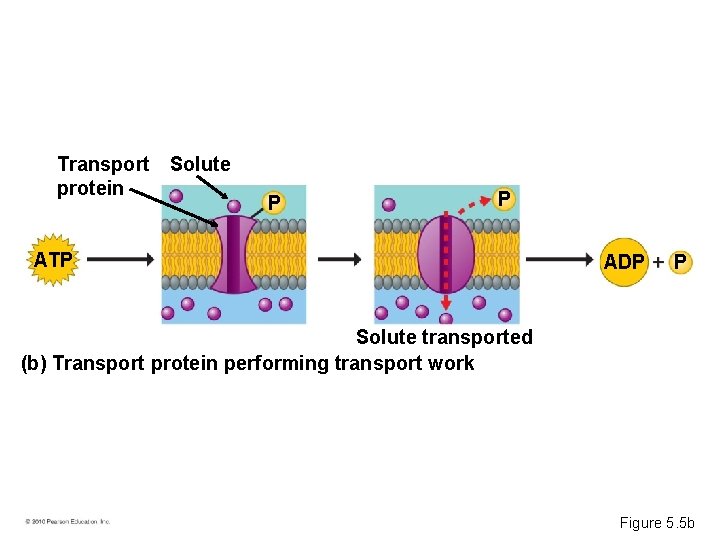
Phosphate Transfer • ATP energizes other molecules by transferring phosphate groups. • This energy helps cells perform – Mechanical work – Transport work – Chemical work © 2010 Pearson Education, Inc.

Motor protein ATP ADP P Protein moved (a) Motor protein performing mechanical work Figure 5. 5 a

Transport Solute protein P P ATP ADP P Solute transported (b) Transport protein performing transport work Figure 5. 5 b

P ATP X Y ADP P Y Reactants Product made (c) Chemical reactants performing chemical work Figure 5. 5 c

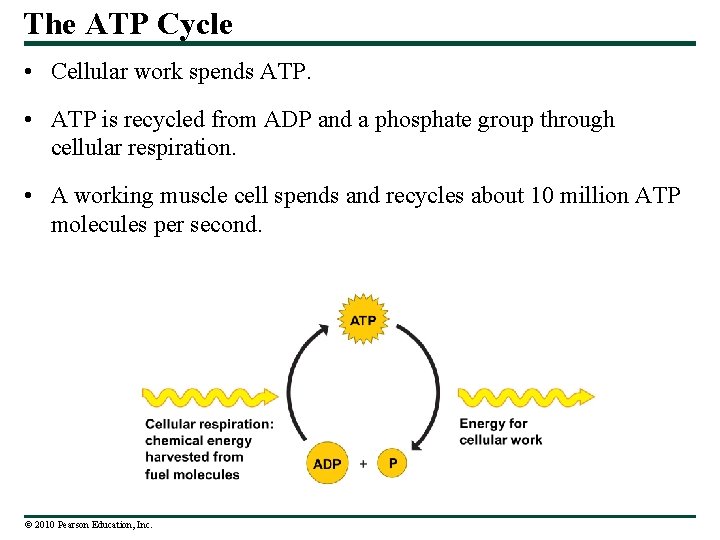
The ATP Cycle • Cellular work spends ATP. • ATP is recycled from ADP and a phosphate group through cellular respiration. • A working muscle cell spends and recycles about 10 million ATP molecules per second. © 2010 Pearson Education, Inc.

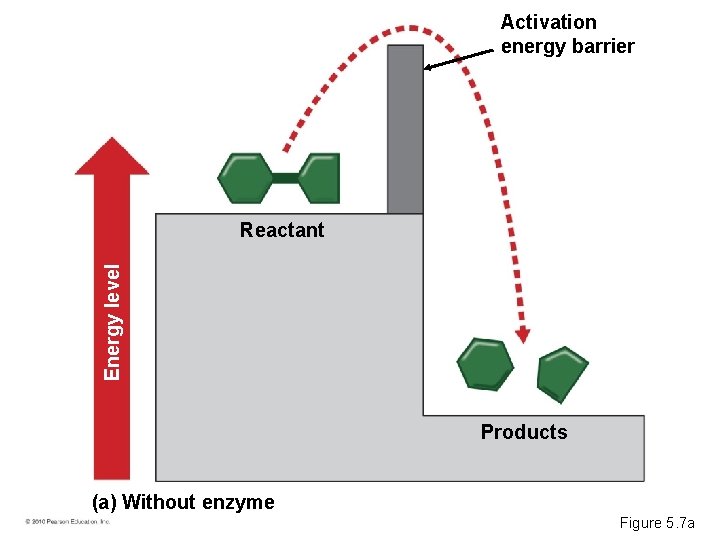
ENZYMES • Metabolism is the total of all chemical reactions in an organism. • Most metabolic reactions require the assistance of enzymes, proteins that speed up chemical reactions. • Activation energy – Activates the reactants – Triggers a chemical reaction • Enzymes lower the activation energy for chemical reactions. © 2010 Pearson Education, Inc.

Activation energy barrier Energy level Reactant Products (a) Without enzyme Figure 5. 7 a

Enzyme Activation energy barrier reduced by enzyme Energy level Reactant Products (b) With enzyme Figure 5. 7 b

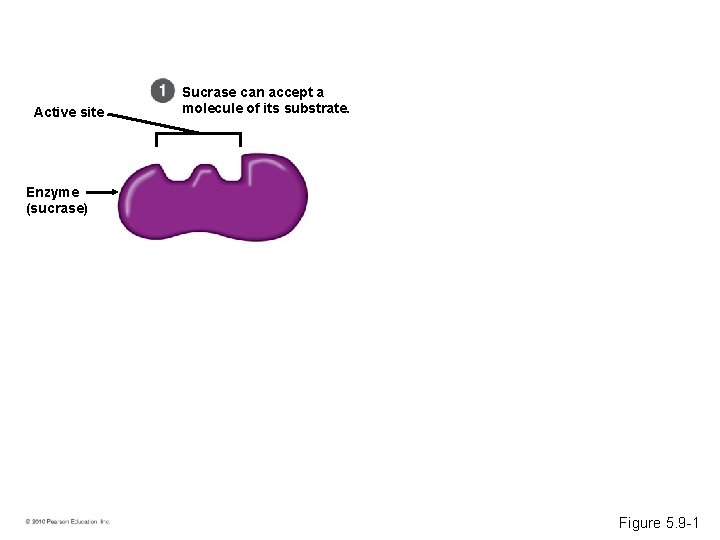
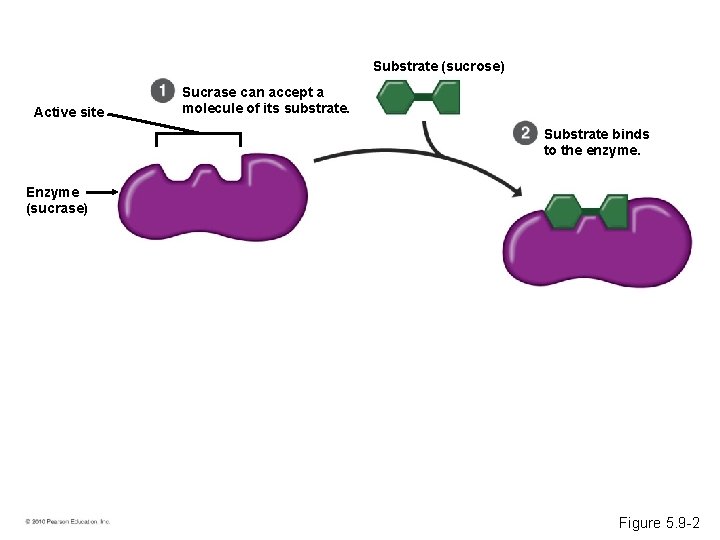
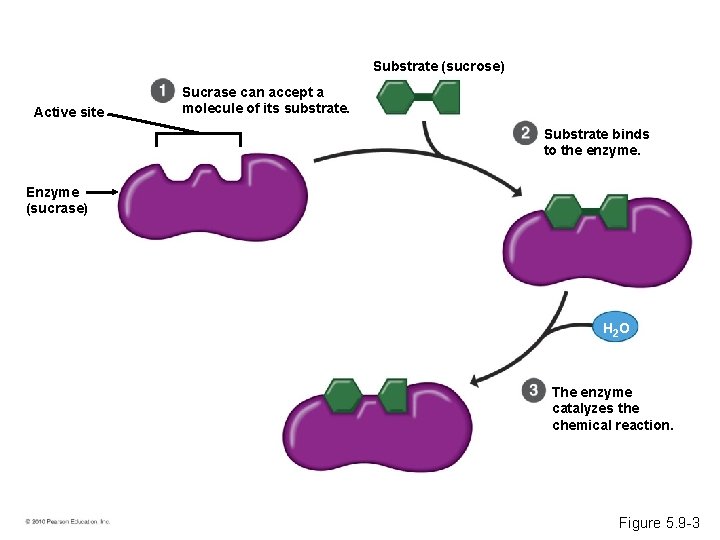
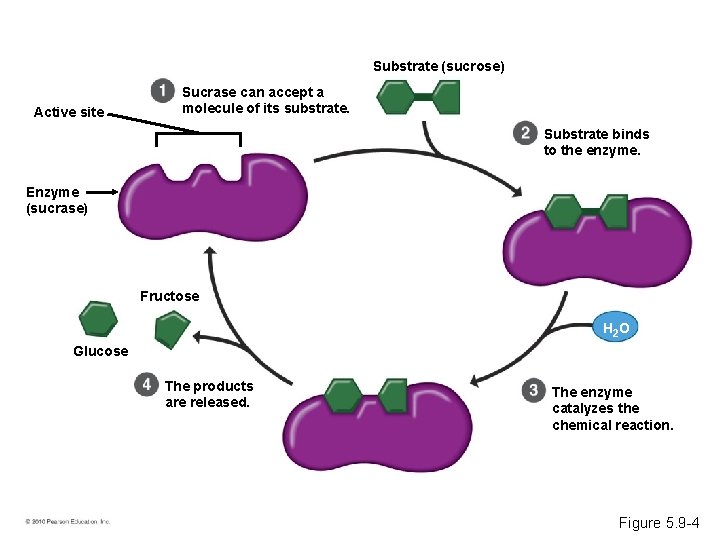
Induced Fit • Every enzyme is very selective, catalyzing a specific reaction. • Each enzyme recognizes a substrate, a specific reactant molecule. – The active site fits to the substrate, and the enzyme changes shape slightly. – This interaction is called induced fit. • Enzymes can function over and over again, a key characteristic of enzymes. © 2010 Pearson Education, Inc.

Active site Sucrase can accept a molecule of its substrate. Enzyme (sucrase) Figure 5. 9 -1

Substrate (sucrose) Active site Sucrase can accept a molecule of its substrate. Substrate binds to the enzyme. Enzyme (sucrase) Figure 5. 9 -2

Substrate (sucrose) Active site Sucrase can accept a molecule of its substrate. Substrate binds to the enzyme. Enzyme (sucrase) H 2 O The enzyme catalyzes the chemical reaction. Figure 5. 9 -3

Substrate (sucrose) Active site Sucrase can accept a molecule of its substrate. Substrate binds to the enzyme. Enzyme (sucrase) Fructose H 2 O Glucose The products are released. The enzyme catalyzes the chemical reaction. Figure 5. 9 -4

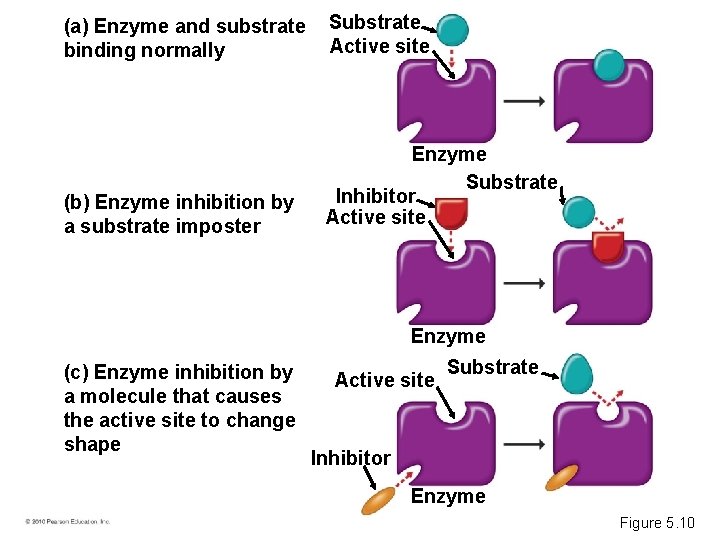
(a) Enzyme and substrate binding normally Substrate Active site (b) Enzyme inhibition by a substrate imposter Enzyme Substrate Inhibitor Active site Enzyme (c) Enzyme inhibition by a molecule that causes the active site to change shape Active site Substrate Inhibitor Enzyme Figure 5. 10

• Some products of a reaction may inhibit the enzyme required for its production. – This is called feedback regulation. – It prevents the cell from wasting resources. • Many antibiotics work by inhibiting enzymes of disease-causing bacteria. © 2010 Pearson Education, Inc.

MEMBRANE FUNCTION • Working cells must control the flow of materials to and from the environment. • Membrane proteins perform many functions. • Transport proteins – Are located in membranes – Regulate the passage of materials into and out of the cell © 2010 Pearson Education, Inc.

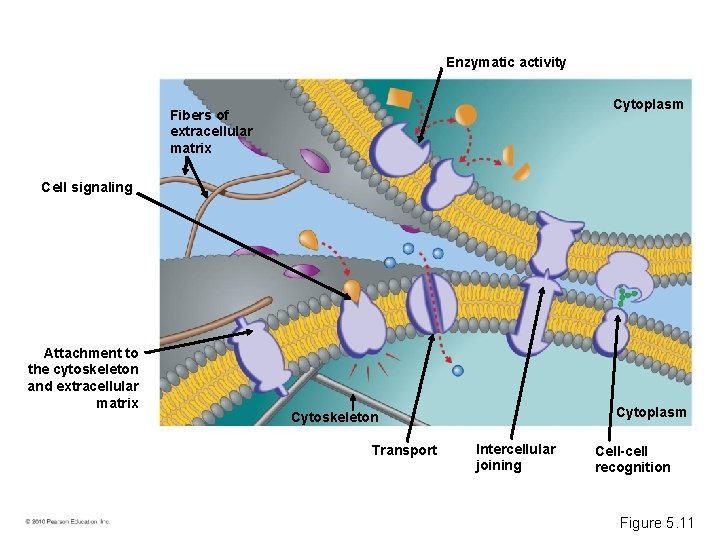
Enzymatic activity Cytoplasm Fibers of extracellular matrix Cell signaling Attachment to the cytoskeleton and extracellular matrix Cytoplasm Cytoskeleton Transport Intercellular joining Cell-cell recognition Figure 5. 11

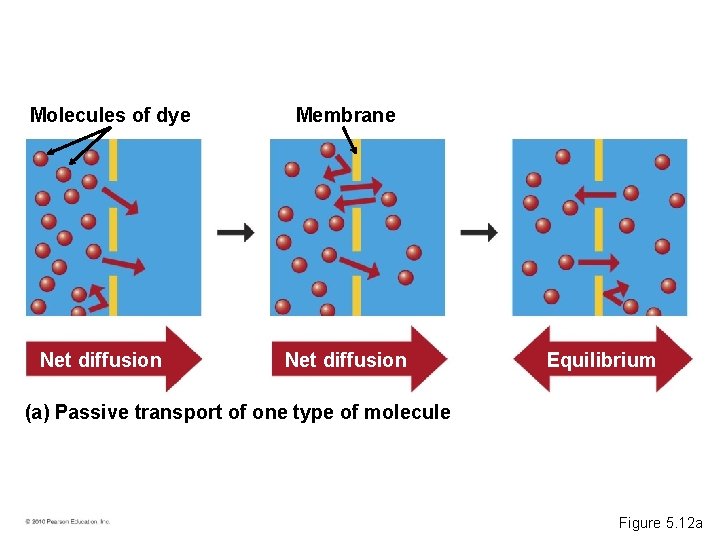
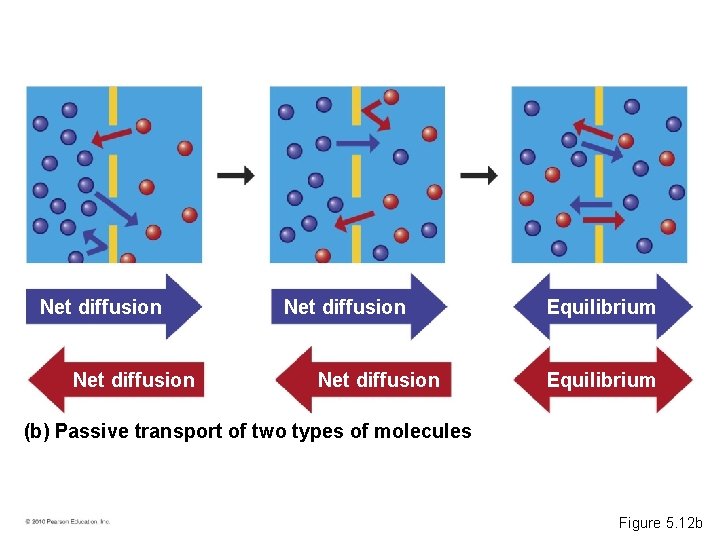
Passive Transport: Diffusion across Membranes • Molecules contain heat energy that causes them to vibrate and wander randomly. • Diffusion is the tendency for molecules of any substance to spread out into the available space. • Passive transport is the diffusion of a substance across a membrane without the input of energy. • Diffusion is an example of passive transport. • Substances diffuse down their concentration gradient, a region in which the substance’s density changes. © 2010 Pearson Education, Inc.

Molecules of dye Net diffusion Membrane Net diffusion Equilibrium (a) Passive transport of one type of molecule Figure 5. 12 a

Net diffusion Equilibrium (b) Passive transport of two types of molecules Figure 5. 12 b

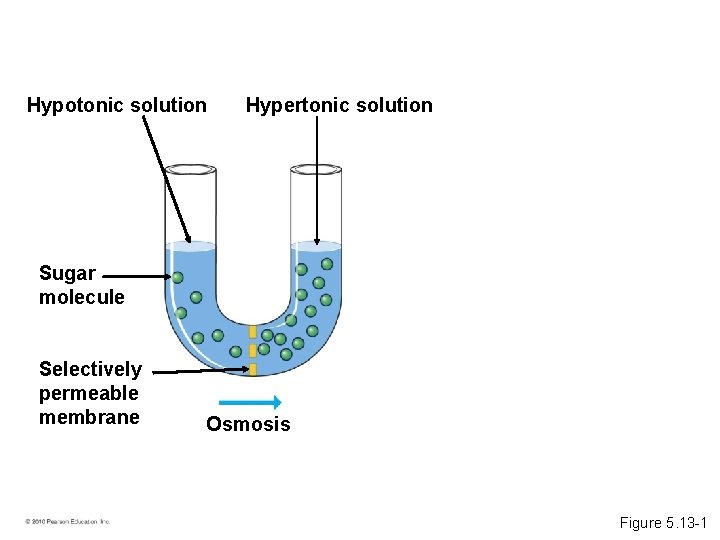
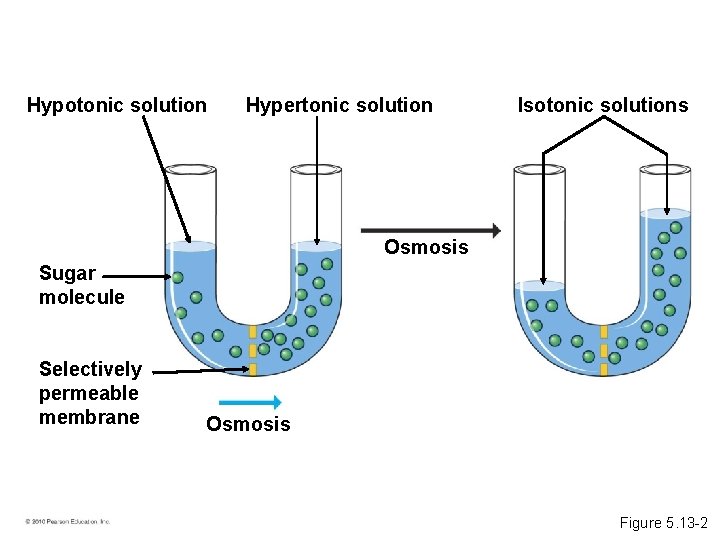
• Some substances do not cross membranes spontaneously. – These substances can be transported via facilitated diffusion. – Specific transport proteins act as selective corridors. – No energy input is needed. • The diffusion of water across a selectively permeable membrane is osmosis. © 2010 Pearson Education, Inc.

Hypotonic solution Hypertonic solution Sugar molecule Selectively permeable membrane Osmosis Figure 5. 13 -1

Hypotonic solution Hypertonic solution Isotonic solutions Osmosis Sugar molecule Selectively permeable membrane Osmosis Figure 5. 13 -2

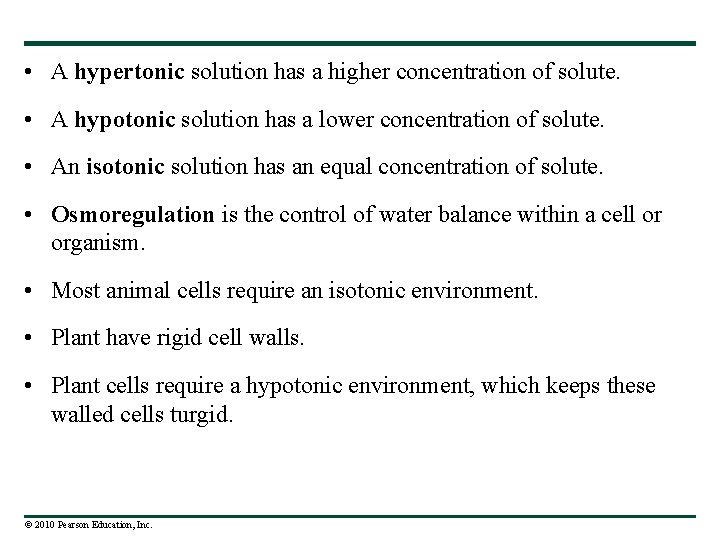
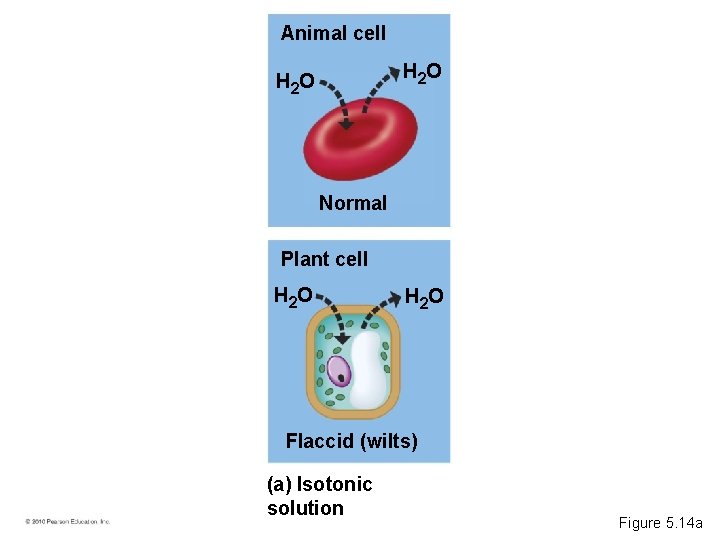
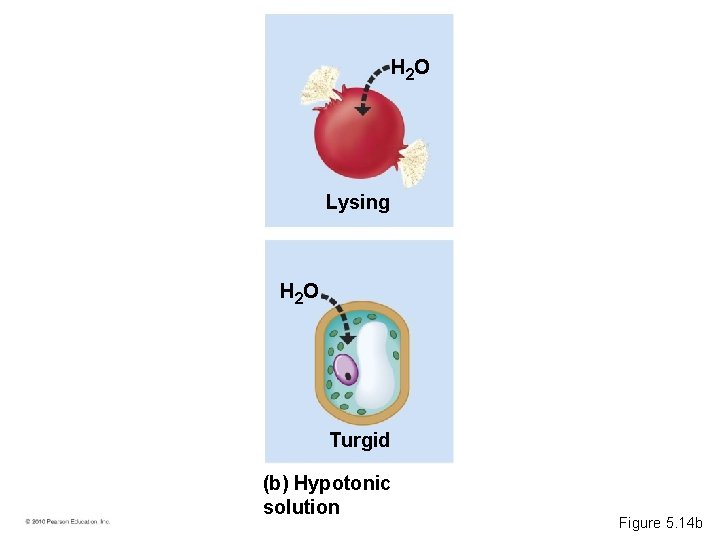
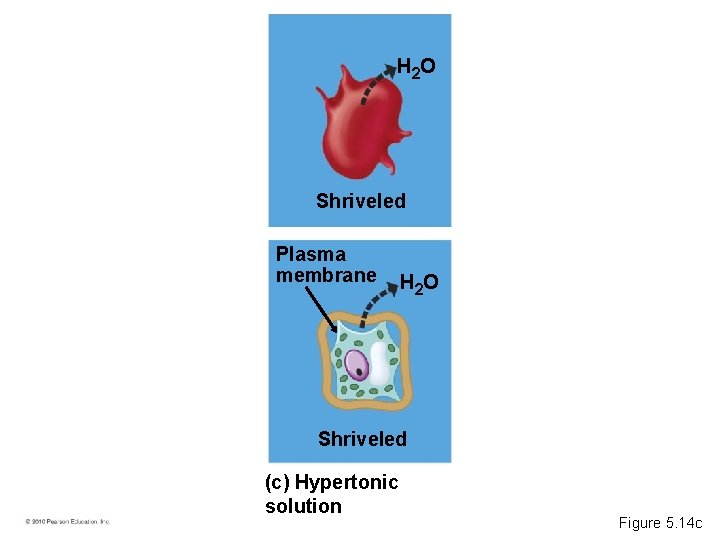
• A hypertonic solution has a higher concentration of solute. • A hypotonic solution has a lower concentration of solute. • An isotonic solution has an equal concentration of solute. • Osmoregulation is the control of water balance within a cell or organism. • Most animal cells require an isotonic environment. • Plant have rigid cell walls. • Plant cells require a hypotonic environment, which keeps these walled cells turgid. © 2010 Pearson Education, Inc.

Animal cell H 2 O Normal Plant cell H 2 O Flaccid (wilts) (a) Isotonic solution Figure 5. 14 a

H 2 O Lysing H 2 O Turgid (b) Hypotonic solution Figure 5. 14 b

H 2 O Shriveled Plasma membrane H 2 O Shriveled (c) Hypertonic solution Figure 5. 14 c

• As a plant cell loses water, – It shrivels. – Its plasma membrane may pull away from the cell wall in the process of plasmolysis, which usually kills the cell. Figure 5. 15

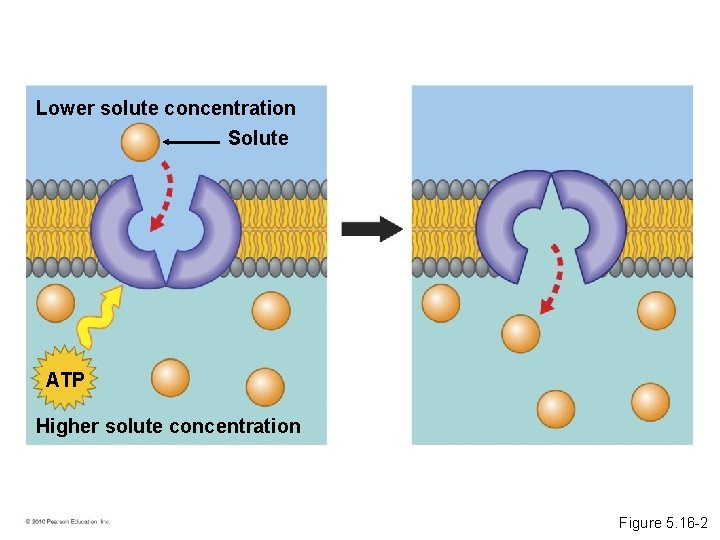
Lower solute concentration Solute • Active transport requires energy to move molecules across a membrane. ATP Higher solute concentration Figure 5. 16 -1

Lower solute concentration Solute ATP Higher solute concentration Figure 5. 16 -2

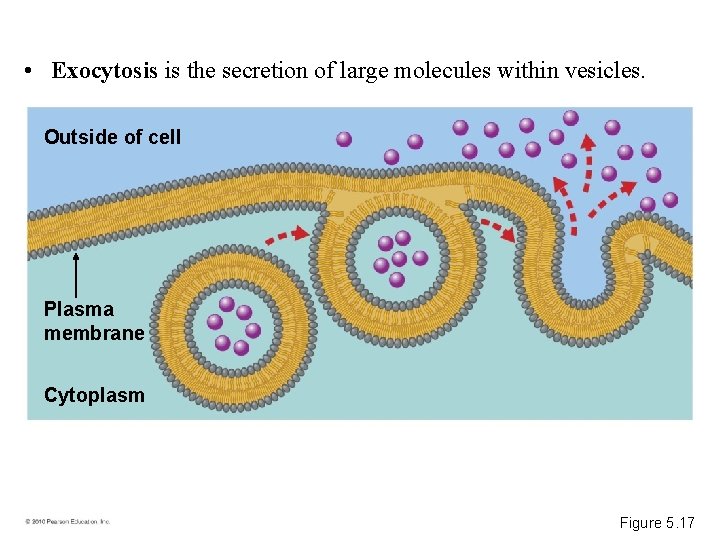
• Exocytosis is the secretion of large molecules within vesicles. Outside of cell Plasma membrane Cytoplasm Figure 5. 17

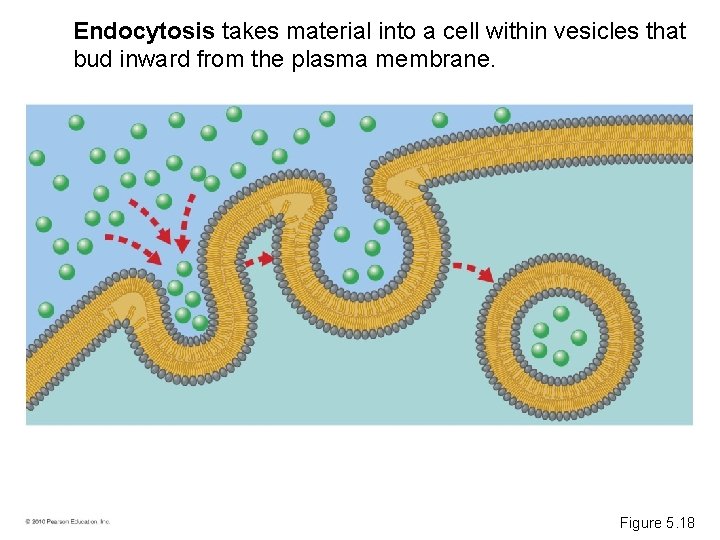
Endocytosis takes material into a cell within vesicles that bud inward from the plasma membrane. Figure 5. 18

• There are three types of endocytosis: – Phagocytosis (“cellular eating”); a cell engulfs a particle and packages it within a food vacuole – Pinocytosis (“cellular drinking”); a cell “gulps” droplets of fluid by forming tiny vesicles – Receptor-mediated endocytosis; a cell takes in very specific molecules © 2010 Pearson Education, Inc.

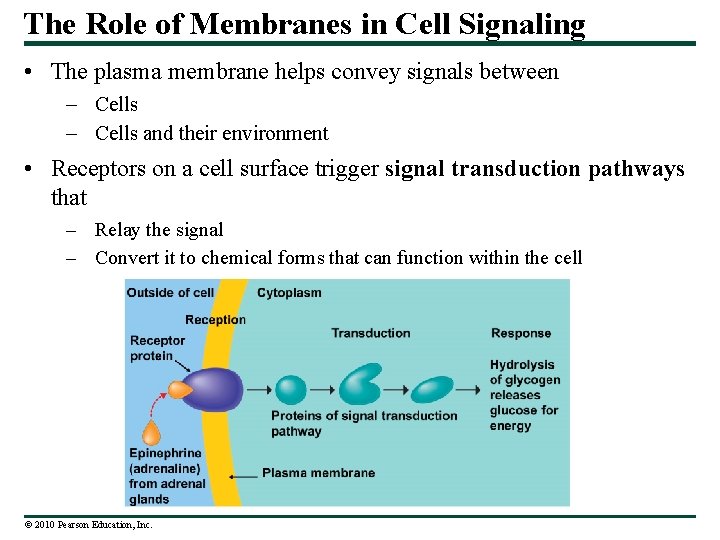
The Role of Membranes in Cell Signaling • The plasma membrane helps convey signals between – Cells and their environment • Receptors on a cell surface trigger signal transduction pathways that – Relay the signal – Convert it to chemical forms that can function within the cell © 2010 Pearson Education, Inc.