Chapter 15 Alcohols Diols and Thiols Copyright The


















- Slides: 57

Chapter 15 Alcohols, Diols, and Thiols Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

Sources of Alcohols

Sources of Alcohols Reactions discussed in earlier chapters (Table 15. 1) Hydration of alkenes Hydroboration-oxidation of alkenes Hydrolysis of alkyl halides Syntheses using Grignard reagents Organolithium reagents

Sources of Alcohols New methods in Chapter 15 Reduction of aldehydes and ketones Reduction of carboxylic acids Reaction of Grignard reagents with epoxides Diols by hydroxylation of alkenes

Preparation of Alcohols by Reduction of Aldehydes and Ketones

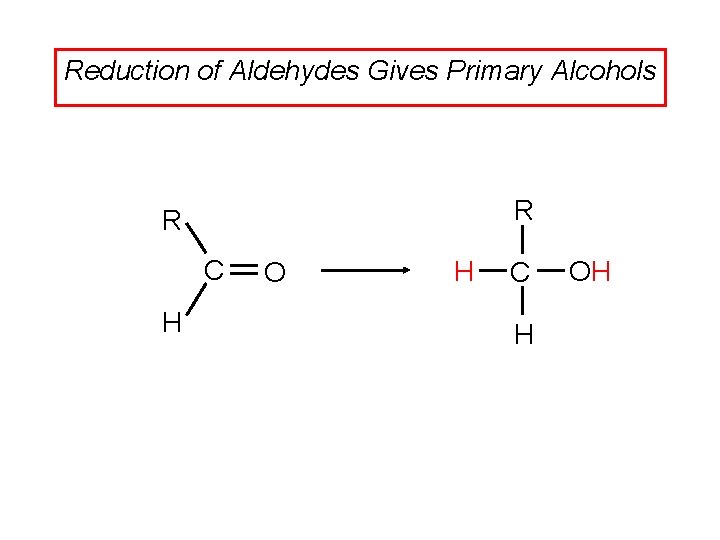
Reduction of Aldehydes Gives Primary Alcohols R R C H O H C H OH

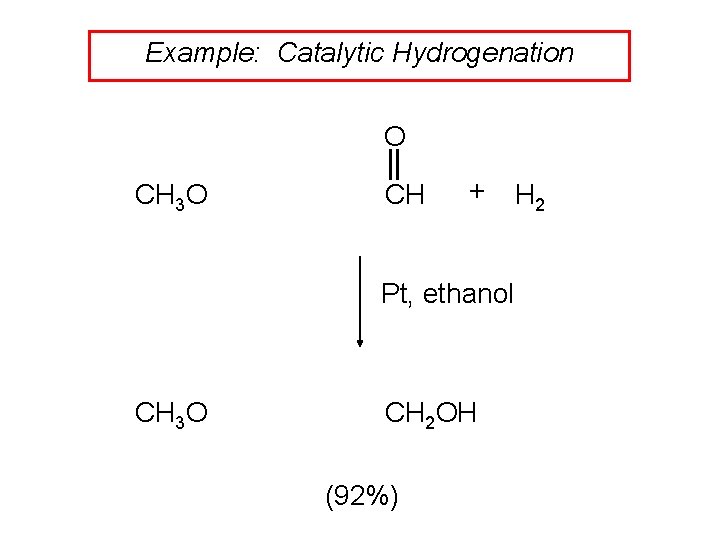
Example: Catalytic Hydrogenation O CH 3 O CH + H 2 Pt, ethanol CH 3 O CH 2 OH (92%)

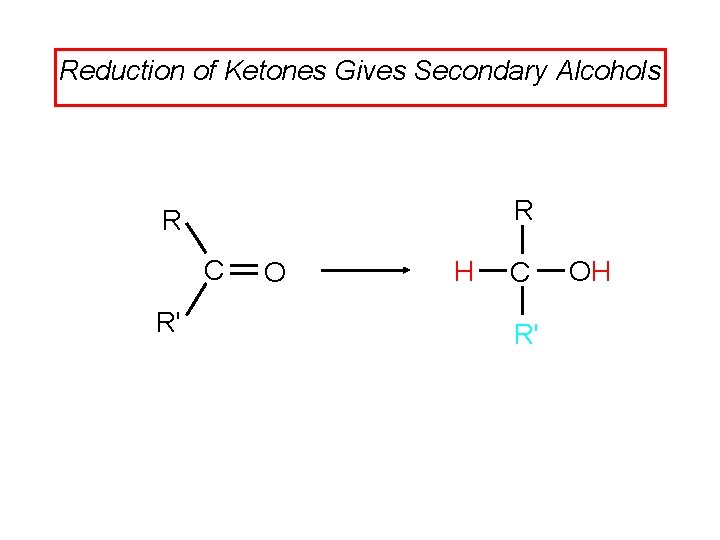
Reduction of Ketones Gives Secondary Alcohols R R C R' O H C R' OH

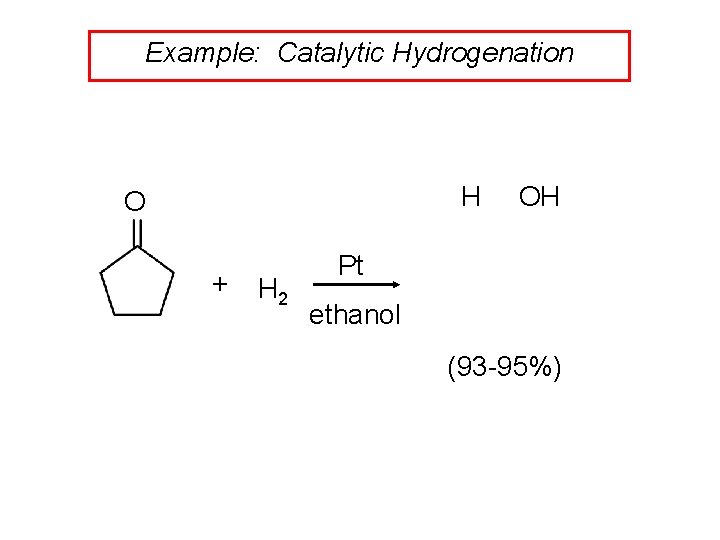
Example: Catalytic Hydrogenation H O + H 2 OH Pt ethanol (93 -95%)

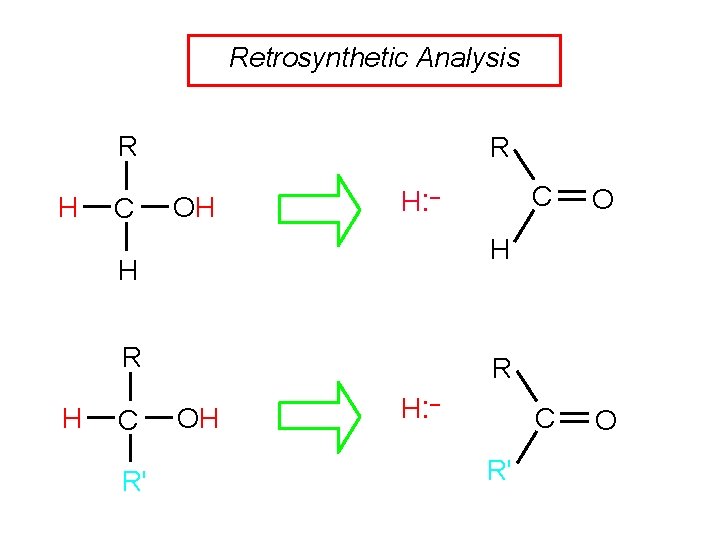
Retrosynthetic Analysis R H C R OH H: – R C R' O C O H H H C R OH H: – R'

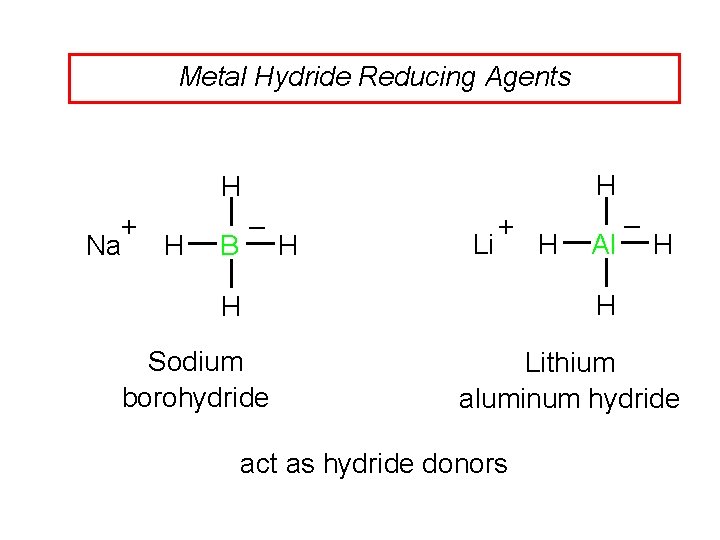
Metal Hydride Reducing Agents H H + Na H – B H Li + Al H H H Sodium borohydride H – Lithium aluminum hydride act as hydride donors

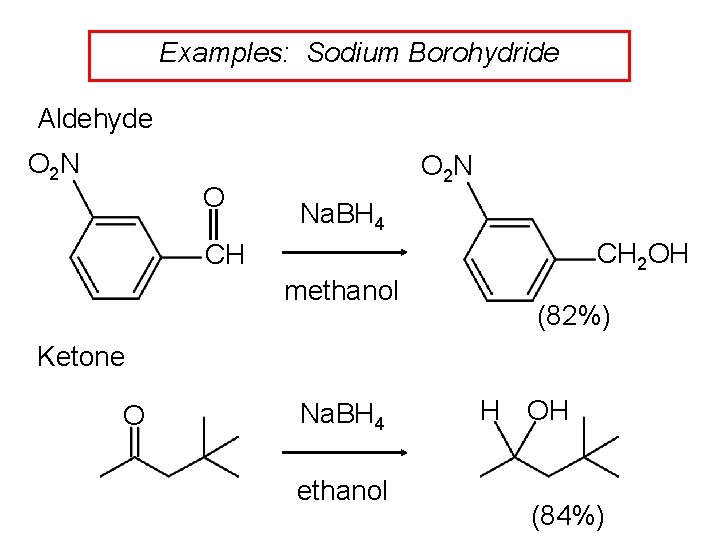
Examples: Sodium Borohydride Aldehyde O 2 N O O 2 N Na. BH 4 CH 2 OH CH methanol (82%) Ketone O Na. BH 4 ethanol H OH (84%)

Lithium Aluminum Hydride More reactive than sodium borohydride. Cannot use water, ethanol, methanol etc. as solvents. Diethyl ether is most commonly used solvent.

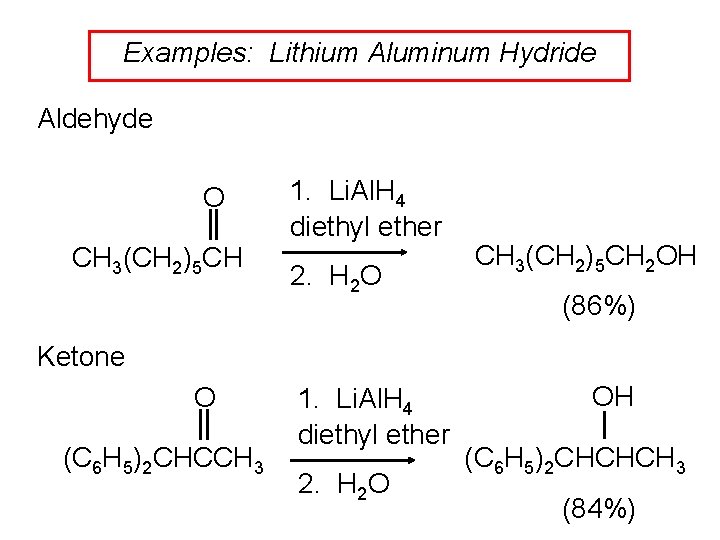
Examples: Lithium Aluminum Hydride Aldehyde O CH 3(CH 2)5 CH 1. Li. Al. H 4 diethyl ether 2. H 2 O CH 3(CH 2)5 CH 2 OH (86%) Ketone O (C 6 H 5)2 CHCCH 3 1. Li. Al. H 4 diethyl ether 2. H 2 O OH (C 6 H 5)2 CHCHCH 3 (84%)

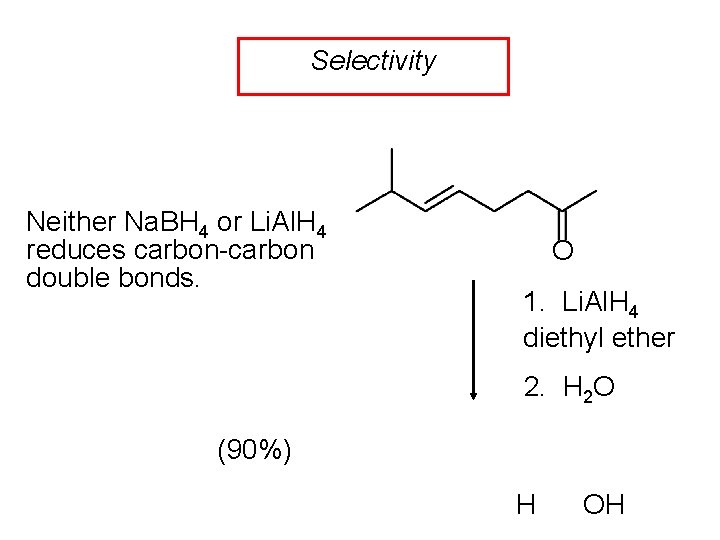
Selectivity Neither Na. BH 4 or Li. Al. H 4 reduces carbon-carbon double bonds. O 1. Li. Al. H 4 diethyl ether 2. H 2 O (90%) H OH

Preparation of Alcohols By Reduction of Carboxylic Acids

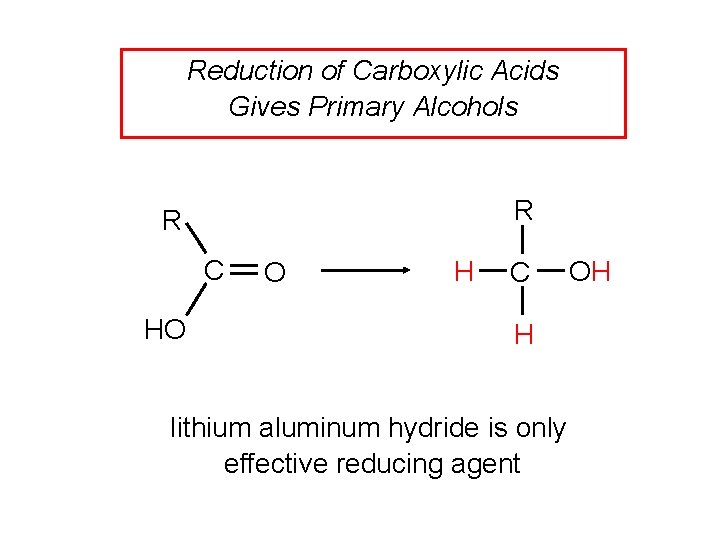
Reduction of Carboxylic Acids Gives Primary Alcohols R R C HO O H C H lithium aluminum hydride is only effective reducing agent OH

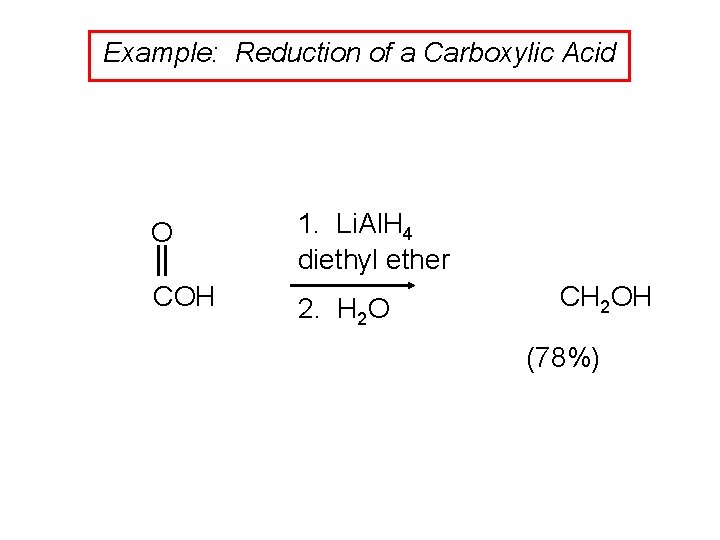
Example: Reduction of a Carboxylic Acid O 1. Li. Al. H 4 diethyl ether COH 2. H 2 O CH 2 OH (78%)

Preparation of Alcohols From Epoxides

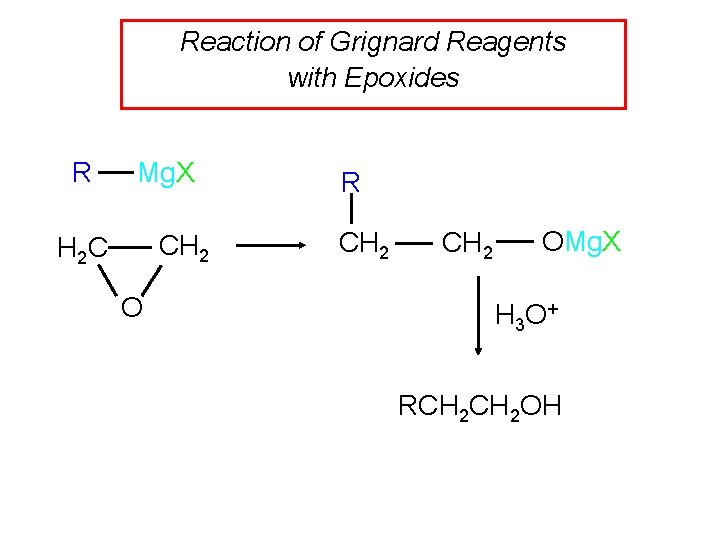
Reaction of Grignard Reagents with Epoxides R Mg. X CH 2 H 2 C O R CH 2 OMg. X H 3 O + RCH 2 OH

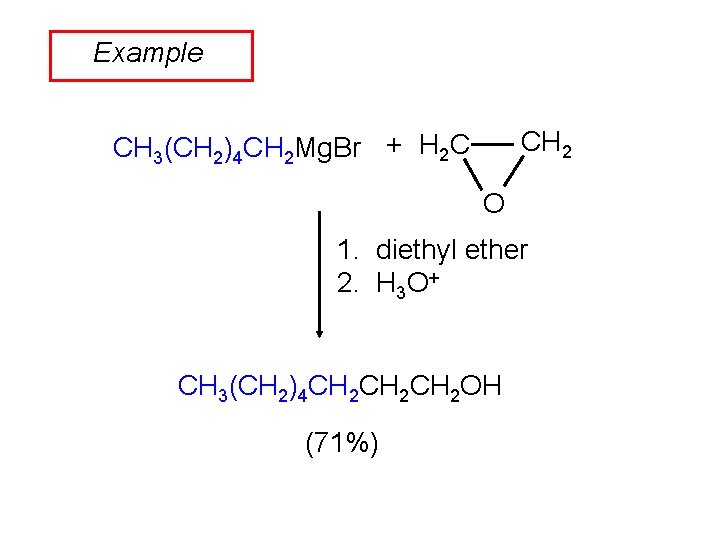
Example CH 2 CH 3(CH 2)4 CH 2 Mg. Br + H 2 C O 1. diethyl ether 2. H 3 O+ CH 3(CH 2)4 CH 2 CH 2 OH (71%)

Preparation of Diols

Diols are Prepared by. . . Reactions used to prepare alcohols Hydroxylation of alkenes

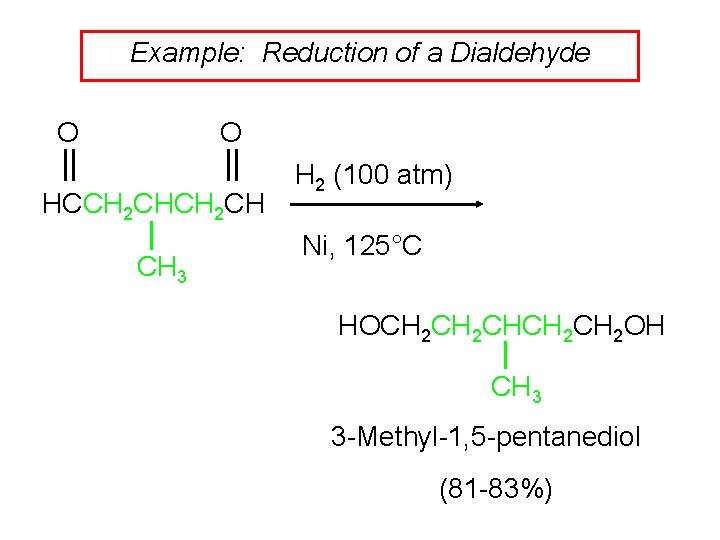
Example: Reduction of a Dialdehyde O O HCCH 2 CH CH 3 H 2 (100 atm) Ni, 125°C HOCH 2 CHCH 2 OH CH 3 3 -Methyl-1, 5 -pentanediol (81 -83%)

Hydroxylation of Alkenes Gives Vicinal Diols Vicinal diols have hydroxyl groups on adjacent carbons. Ethylene glycol (HOCH 2 OH) is most familiar example.

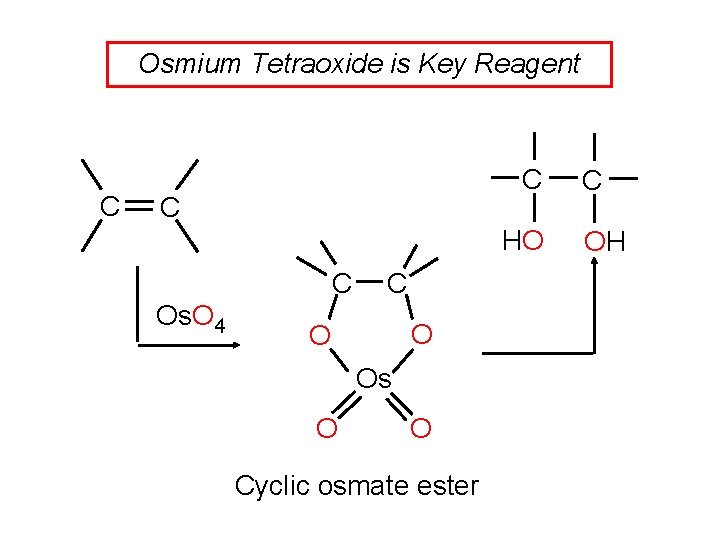
Osmium Tetraoxide is Key Reagent C C C HO C Os. O 4 C O O Os O O Cyclic osmate ester C OH

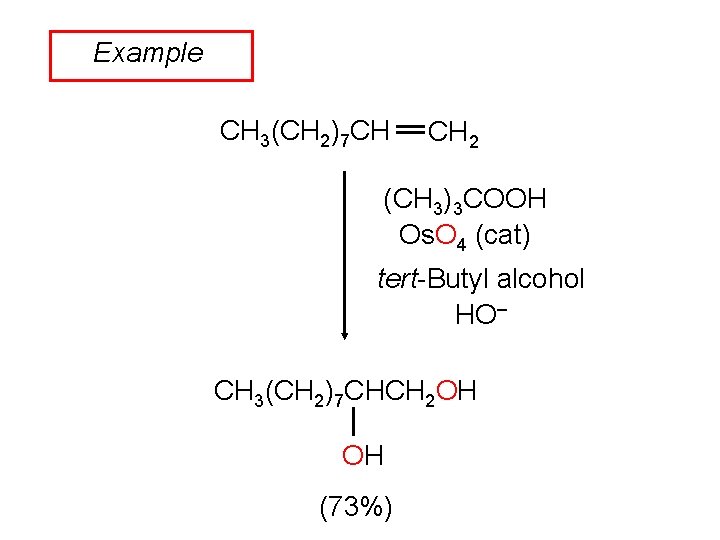
Example CH 3(CH 2)7 CH CH 2 (CH 3)3 COOH Os. O 4 (cat) tert-Butyl alcohol HO– CH 3(CH 2)7 CHCH 2 OH OH (73%)

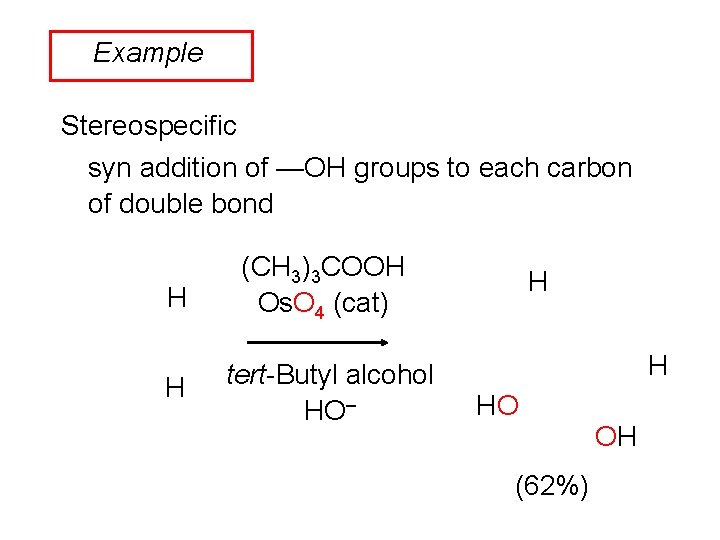
Example Stereospecific syn addition of —OH groups to each carbon of double bond H (CH 3)3 COOH Os. O 4 (cat) H tert-Butyl alcohol HO– H H HO (62%) OH

Reactions of Alcohols: A Review and a Preview Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

Table 15. 2 Review of Reactions of Alcohols Reaction with hydrogen halides Reaction with thionyl chloride Reaction with phosphorous trihalides Acid-catalyzed dehydration Conversion to p-toluenesulfonate esters

New Reactions of Alcohols in This Chapter Conversion to ethers Esterification Oxidation Cleavage of vicinal diols

Conversion of Alcohols to Ethers

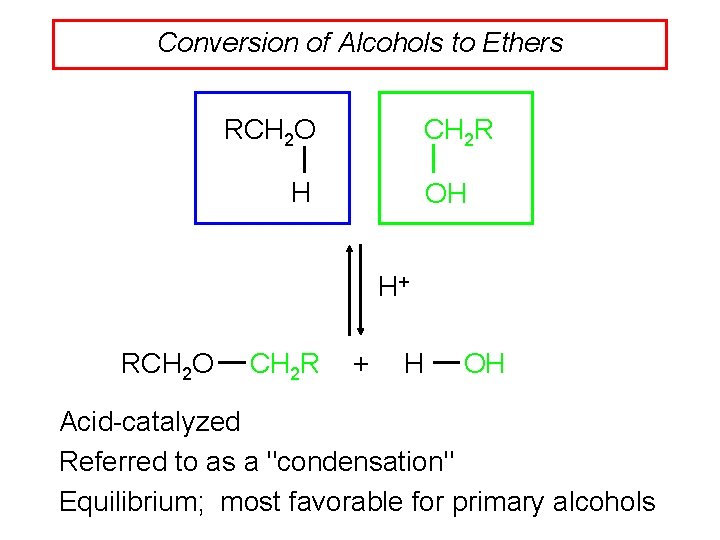
Conversion of Alcohols to Ethers RCH 2 O CH 2 R H OH H+ RCH 2 O CH 2 R + H OH Acid-catalyzed Referred to as a "condensation" Equilibrium; most favorable for primary alcohols

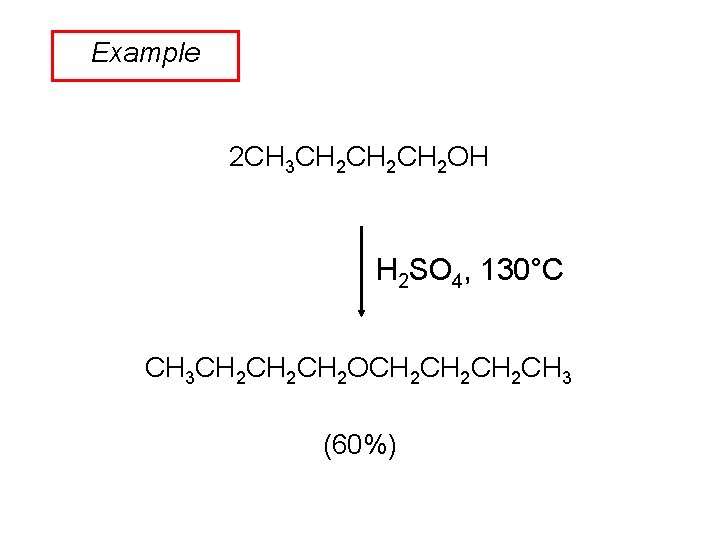
Example 2 CH 3 CH 2 CH 2 OH H 2 SO 4, 130°C CH 3 CH 2 CH 2 OCH 2 CH 2 CH 3 (60%)

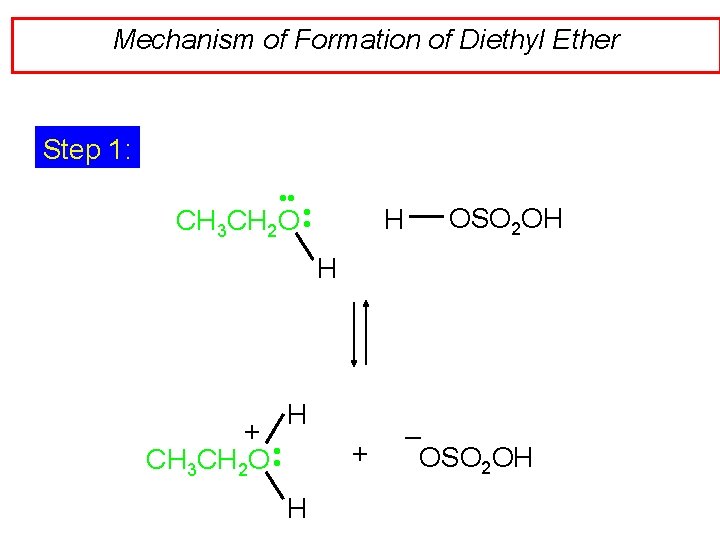
Mechanism of Formation of Diethyl Ether Step 1: • • CH 3 CH 2 O • • H OSO 2 OH H + CH 3 CH 2 O • • H + H – OSO 2 OH

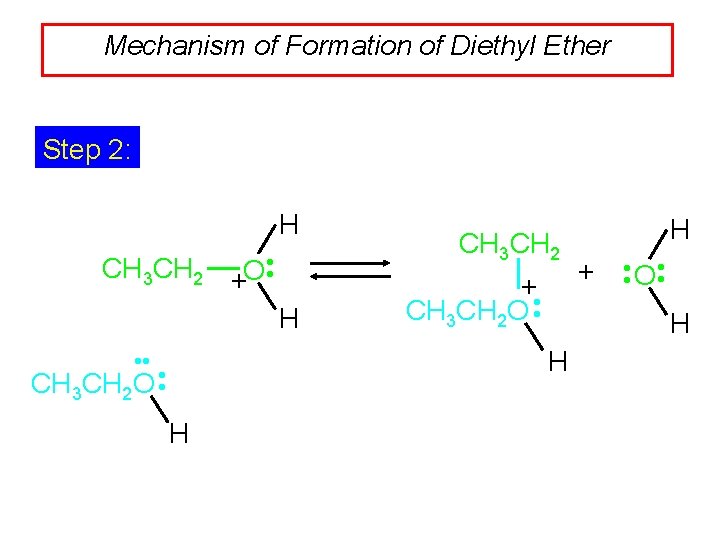
Mechanism of Formation of Diethyl Ether Step 2: H CH 3 CH 2 +O • • H CH 3 CH 2 + CH 3 CH 2 O • • H + • • O • • H H • • H

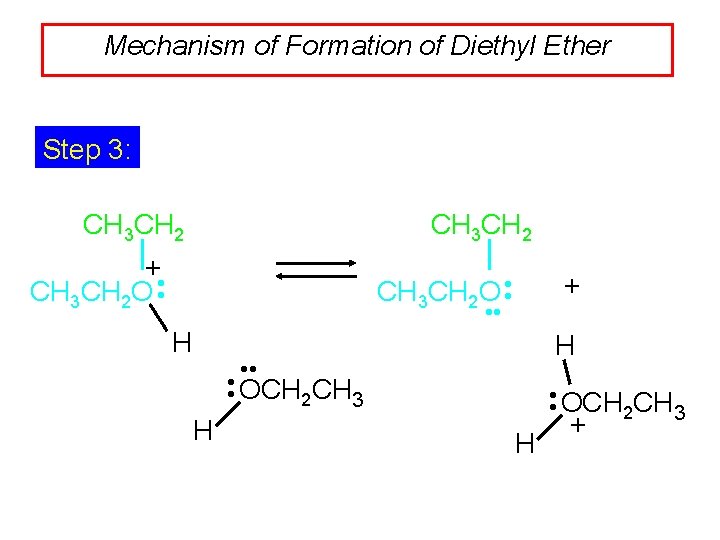
Mechanism of Formation of Diethyl Ether Step 3: CH 3 CH 2 + CH 3 CH 2 O • • + • • H H • • OCH CH 2 3 H +

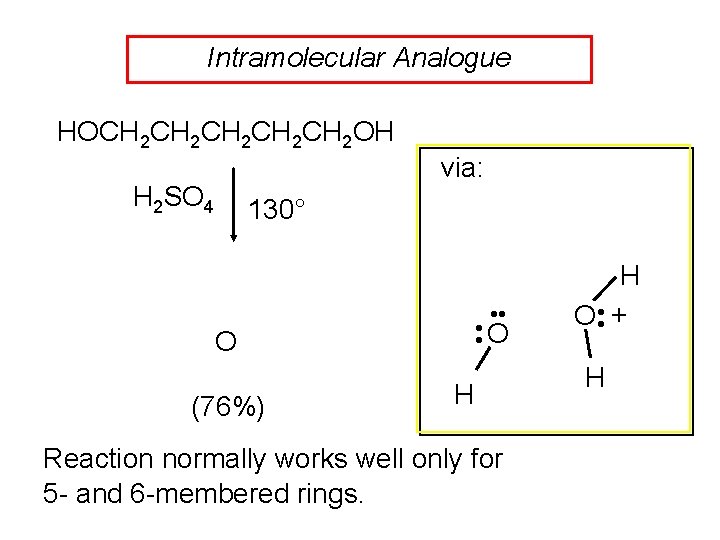
Intramolecular Analogue HOCH 2 CH 2 CH 2 OH H 2 SO 4 via: 130° • • O O (76%) H Reaction normally works well only for 5 - and 6 -membered rings. H O • • + H

Esterification

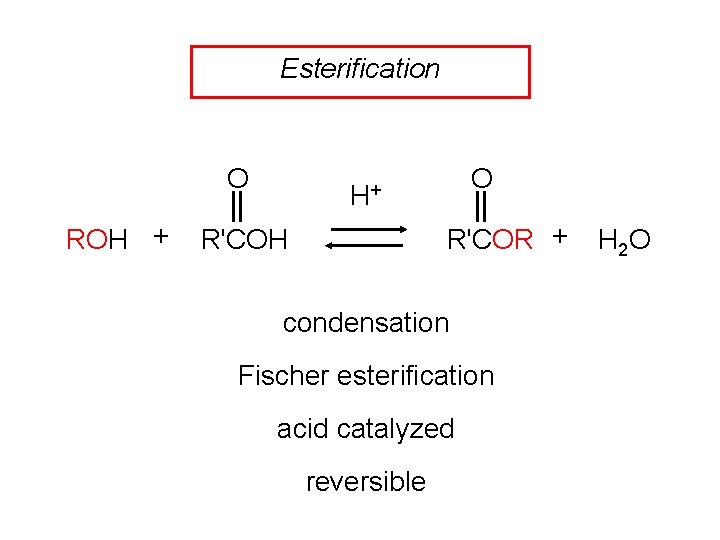
Esterification O ROH + O H+ R'COR + R'COH condensation Fischer esterification acid catalyzed reversible H 2 O

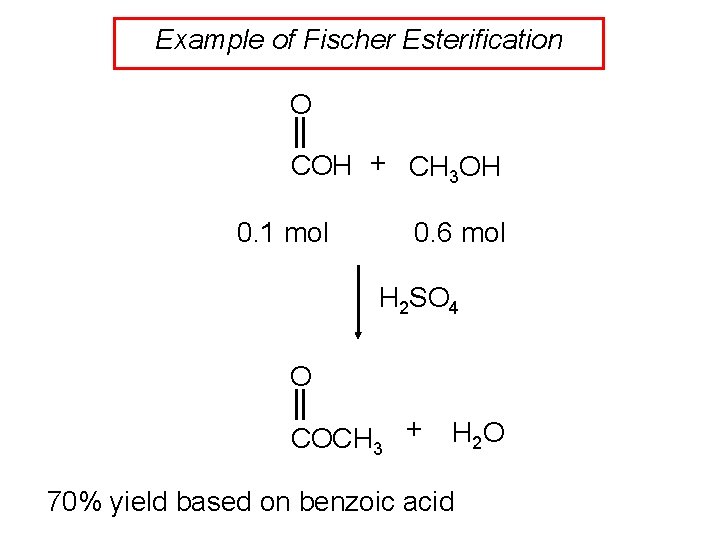
Example of Fischer Esterification O COH + CH 3 OH 0. 1 mol 0. 6 mol H 2 SO 4 O COCH 3 + H 2 O 70% yield based on benzoic acid

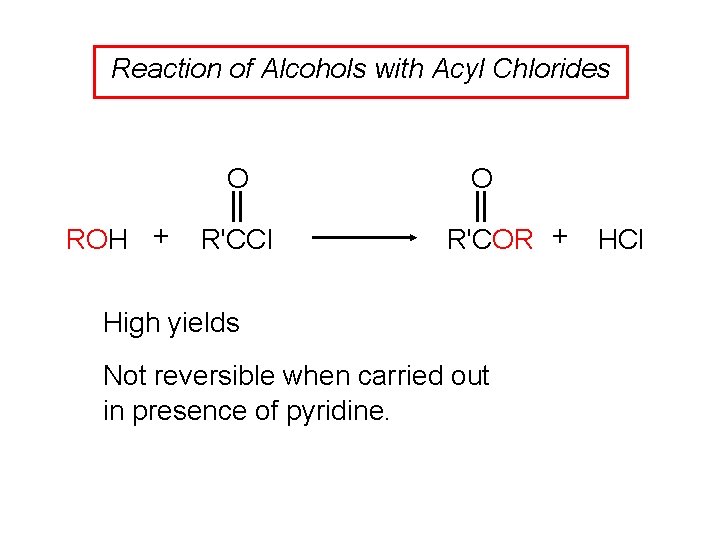
Reaction of Alcohols with Acyl Chlorides O ROH + R'CCl O R'COR + High yields Not reversible when carried out in presence of pyridine. HCl

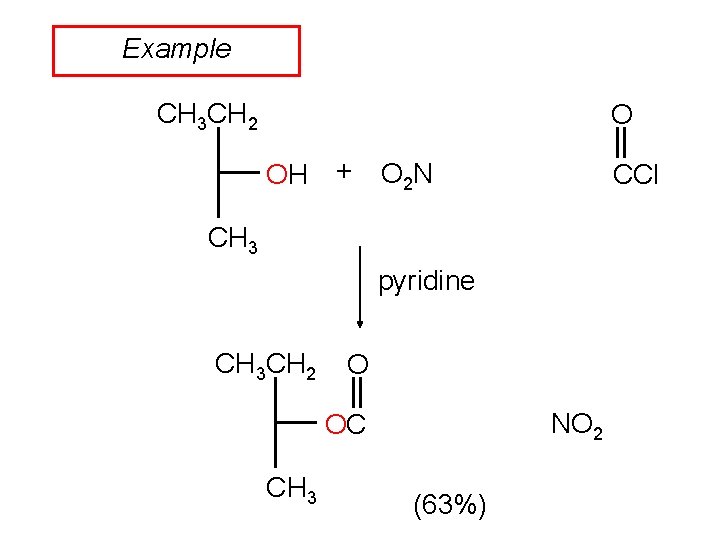
Example CH 3 CH 2 O OH + O 2 N CCl CH 3 pyridine CH 3 CH 2 O NO 2 OC CH 3 (63%)

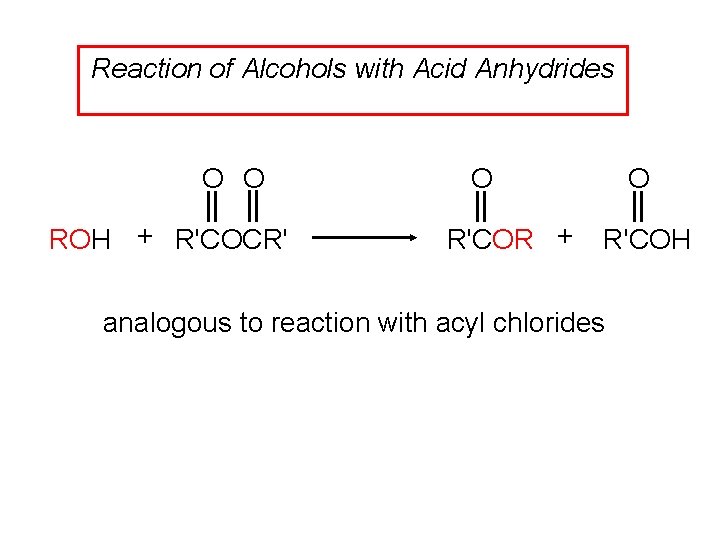
Reaction of Alcohols with Acid Anhydrides O O ROH + R'COCR' O R'COR + O R'COH analogous to reaction with acyl chlorides

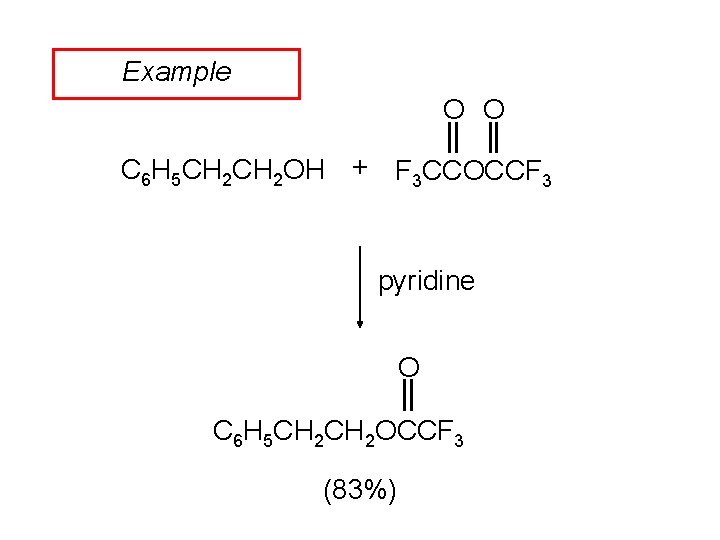
Example O O C 6 H 5 CH 2 OH + F 3 CCOCCF 3 pyridine O C 6 H 5 CH 2 OCCF 3 (83%)

Oxidation of Alcohols

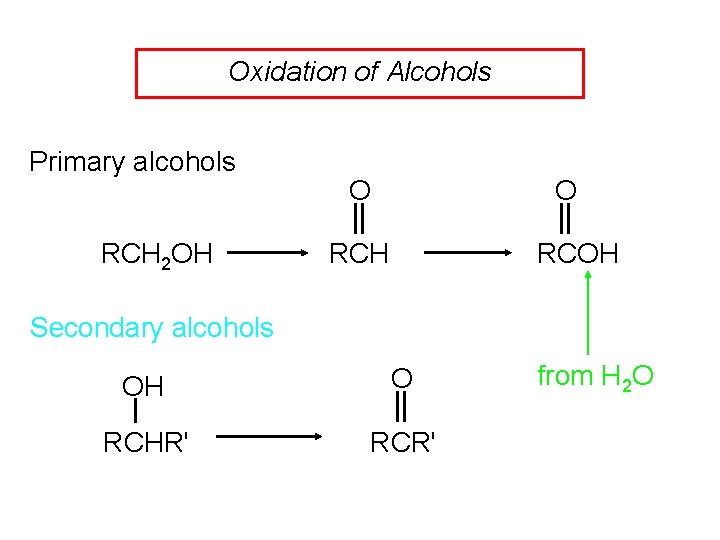
Oxidation of Alcohols Primary alcohols RCH 2 OH O O RCH RCOH Secondary alcohols OH O RCHR' RCR' from H 2 O

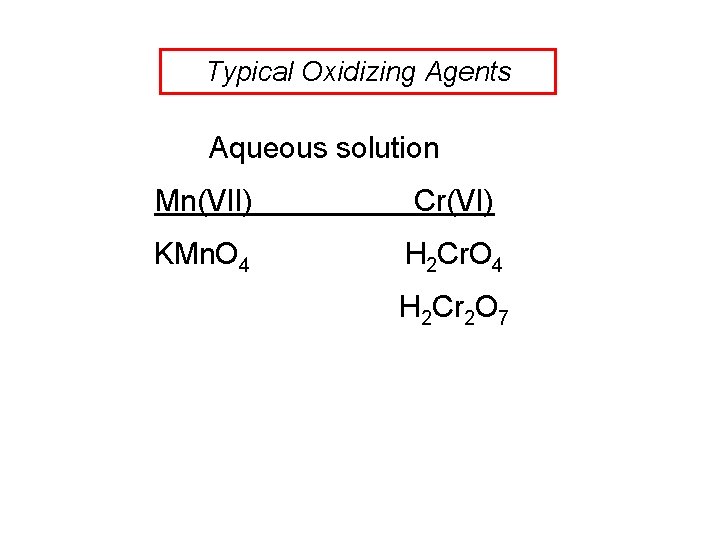
Typical Oxidizing Agents Aqueous solution Mn(VII) Cr(VI) KMn. O 4 H 2 Cr 2 O 7

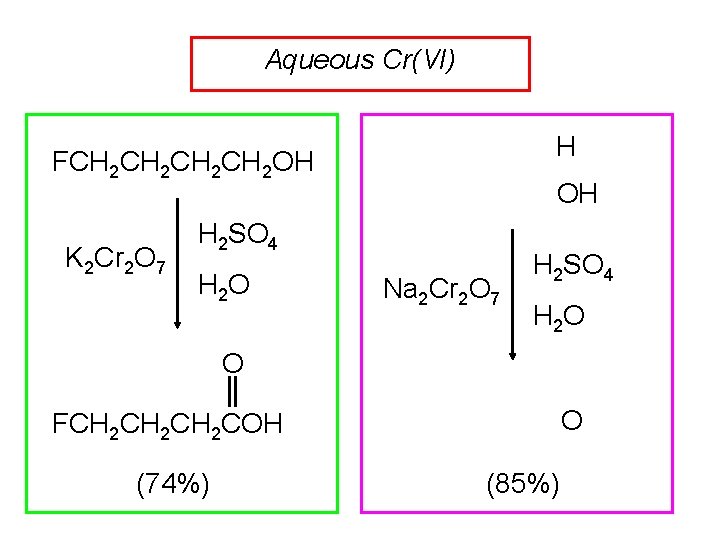
Aqueous Cr(VI) H FCH 2 CH 2 OH K 2 Cr 2 O 7 OH H 2 SO 4 H 2 O Na 2 Cr 2 O 7 H 2 SO 4 H 2 O O O FCH 2 CH 2 COH (74%) (85%)

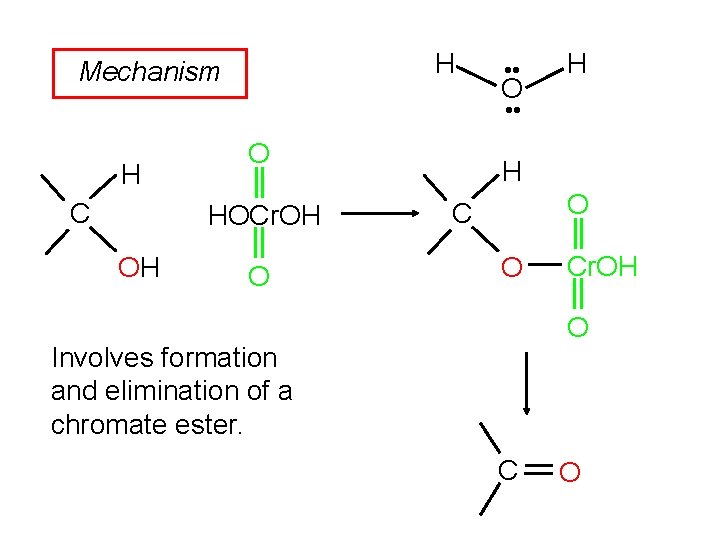
H Mechanism • • O H • • H C O HOCr. OH OH O C O Cr. OH O Involves formation and elimination of a chromate ester. C O

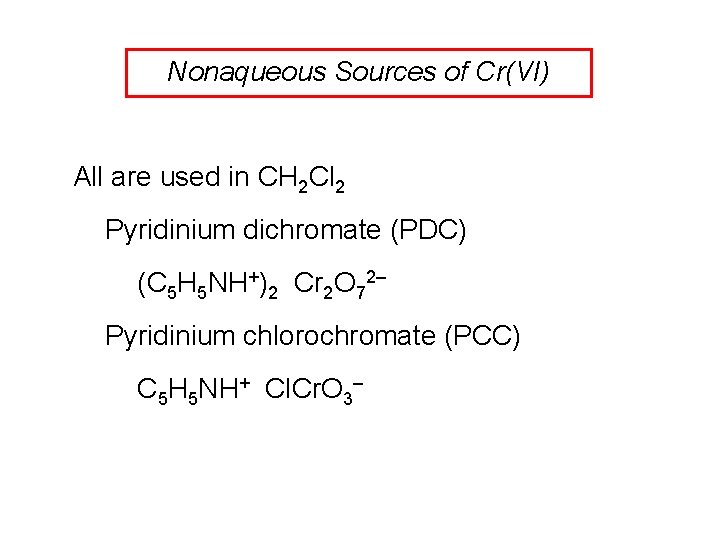
Nonaqueous Sources of Cr(VI) All are used in CH 2 Cl 2 Pyridinium dichromate (PDC) (C 5 H 5 NH+)2 Cr 2 O 72– Pyridinium chlorochromate (PCC) C 5 H 5 NH+ Cl. Cr. O 3–

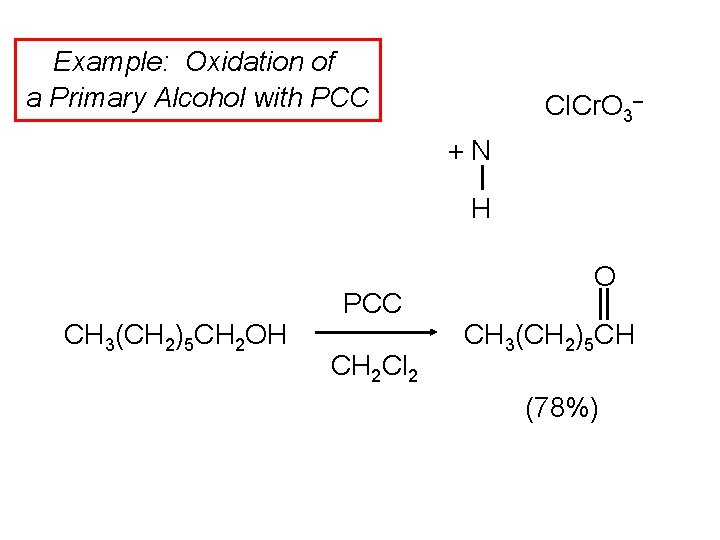
Example: Oxidation of a Primary Alcohol with PCC Cl. Cr. O 3– +N H CH 3(CH 2)5 CH 2 OH PCC CH 2 Cl 2 O CH 3(CH 2)5 CH (78%)

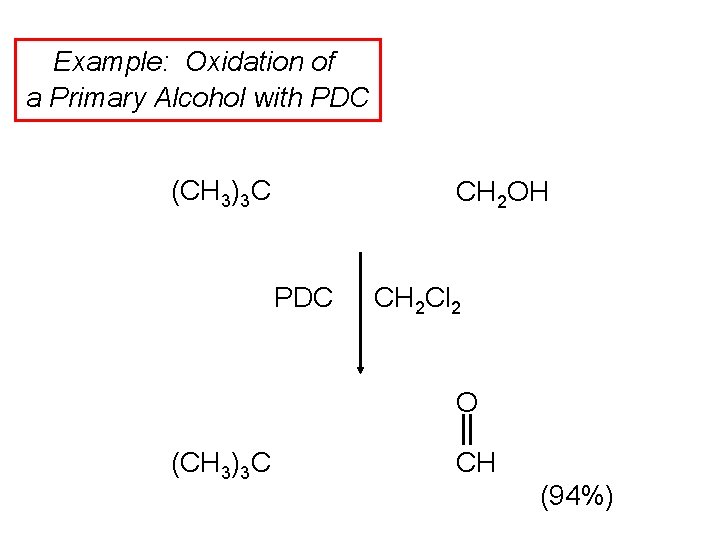
Example: Oxidation of a Primary Alcohol with PDC (CH 3)3 C CH 2 OH PDC CH 2 Cl 2 O (CH 3)3 C CH (94%)

Oxidative Cleavage of Vicinal Diols

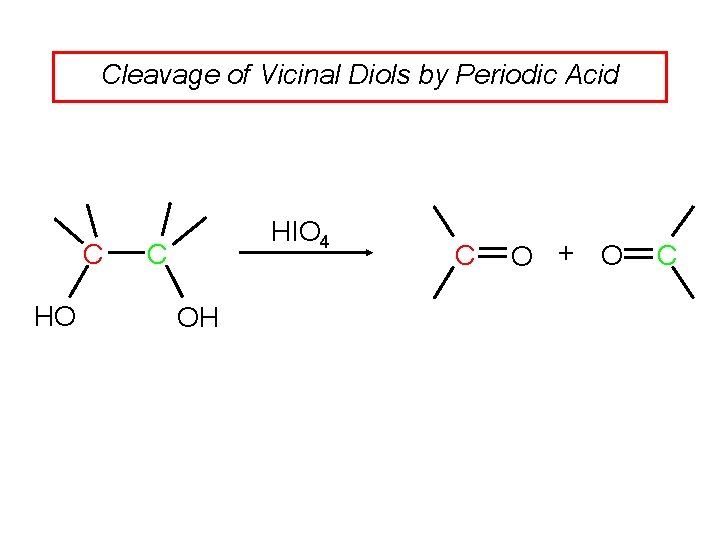
Cleavage of Vicinal Diols by Periodic Acid C HO HIO 4 C OH C O + O C

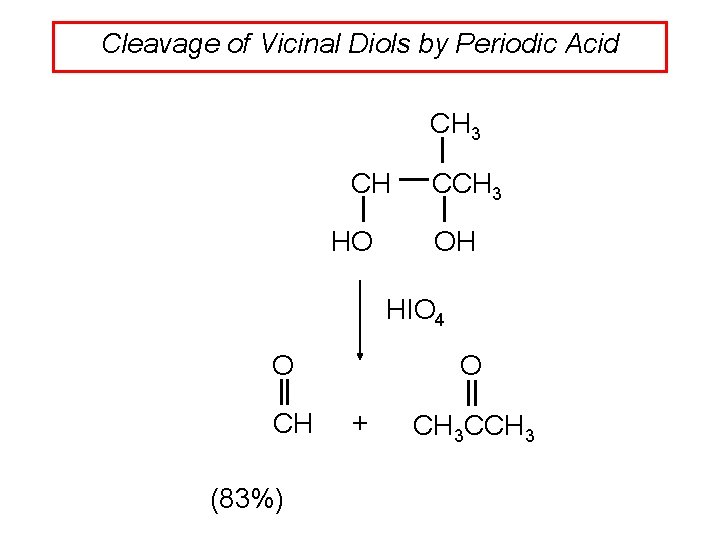
Cleavage of Vicinal Diols by Periodic Acid CH 3 CH HO CCH 3 OH HIO 4 O CH (83%) O + CH 3 CCH 3

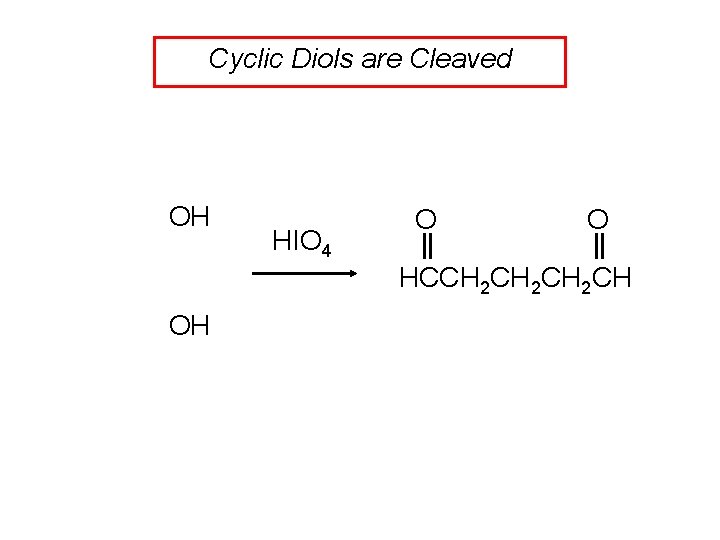
Cyclic Diols are Cleaved OH HIO 4 O O HCCH 2 CH 2 CH OH