Reactions of Alcohols Oxidation RX Ether and Ester




- Slides: 60

Reactions of Alcohols Oxidation R-X, Ether, and Ester Preparation Protection of Alcohols Synthesis The Logic of Mechanisms

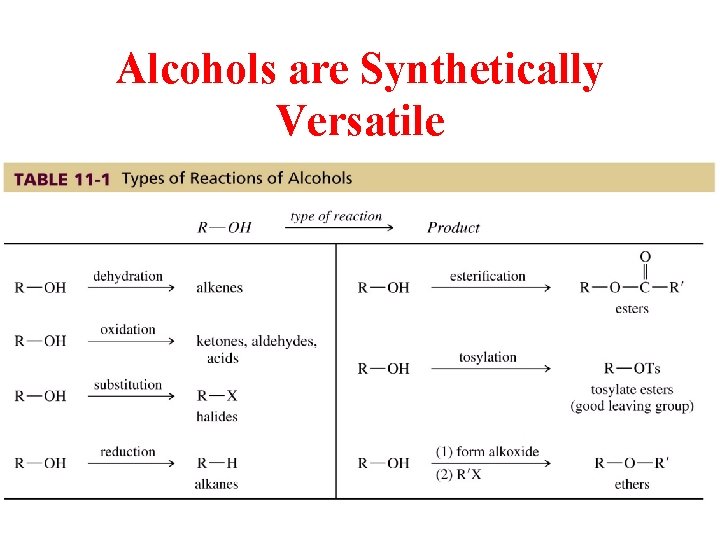
Alcohols are Synthetically Versatile

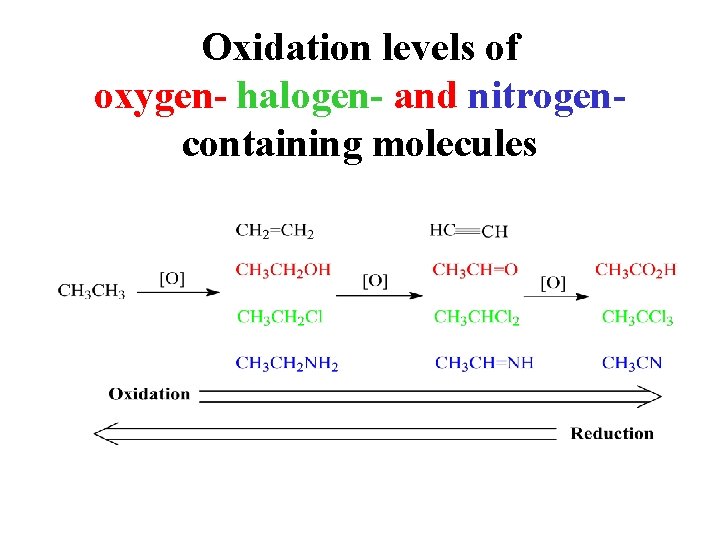
Oxidation levels of oxygen- halogen- and nitrogencontaining molecules

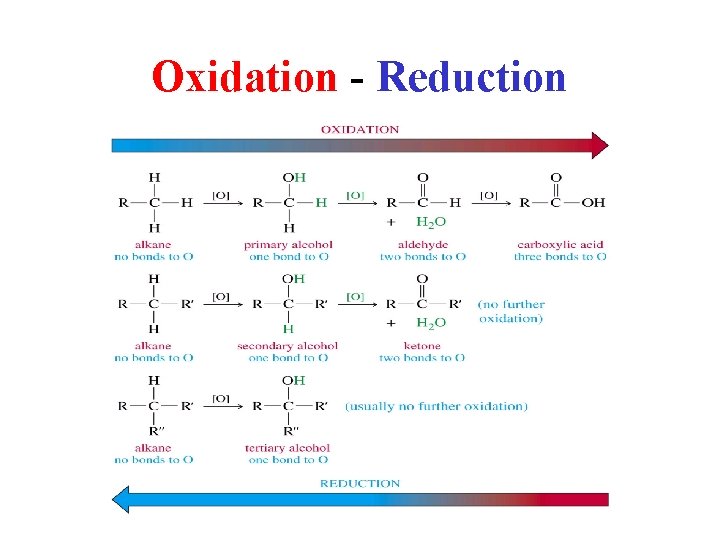
Oxidation - Reduction

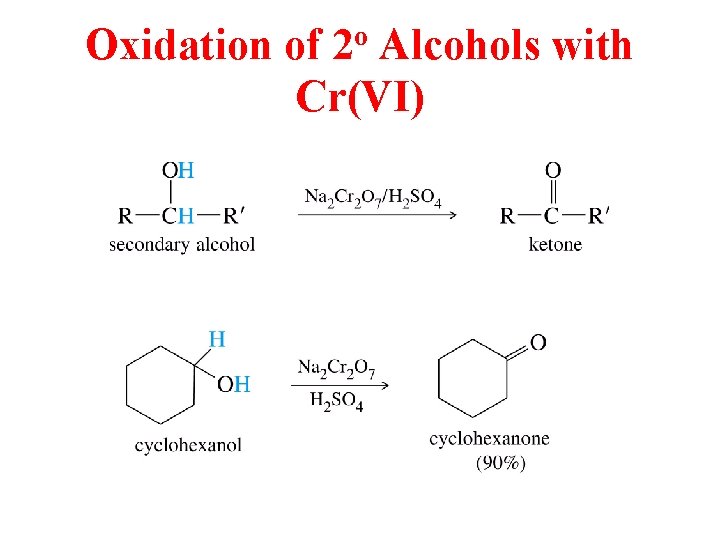
Oxidation of 2 o Alcohols with Cr(VI)

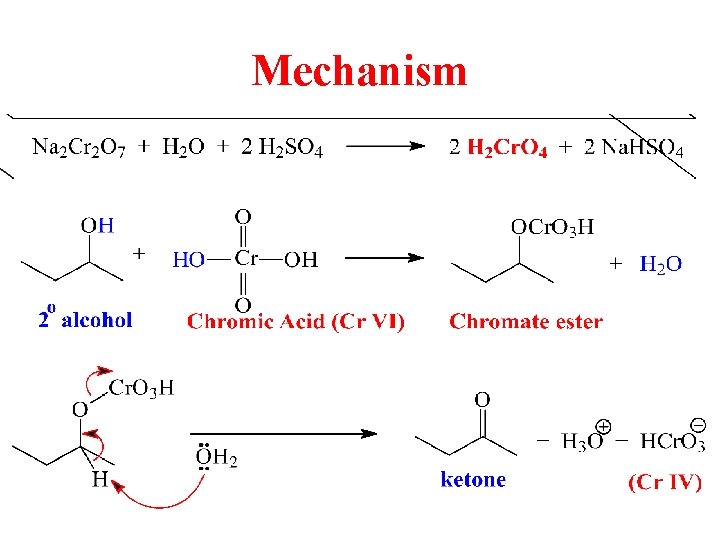
Mechanism

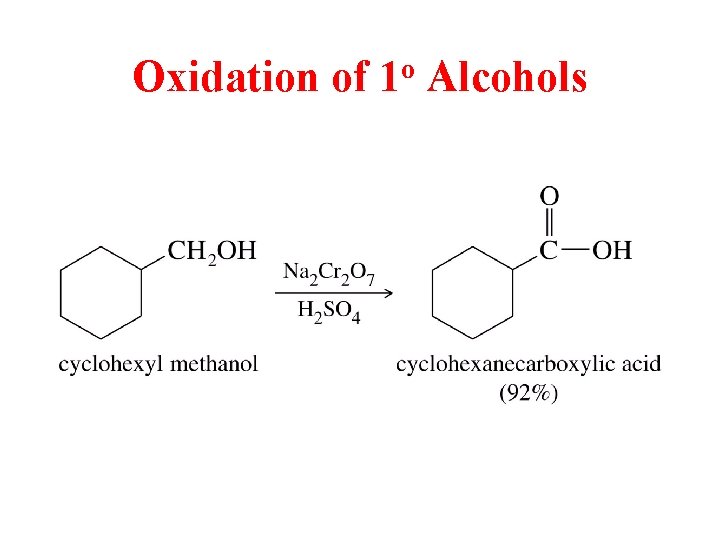
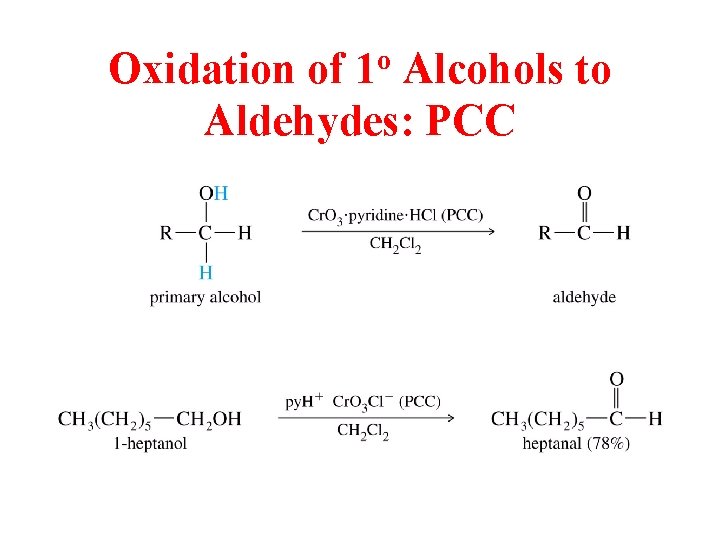
Oxidation of 1 o Alcohols

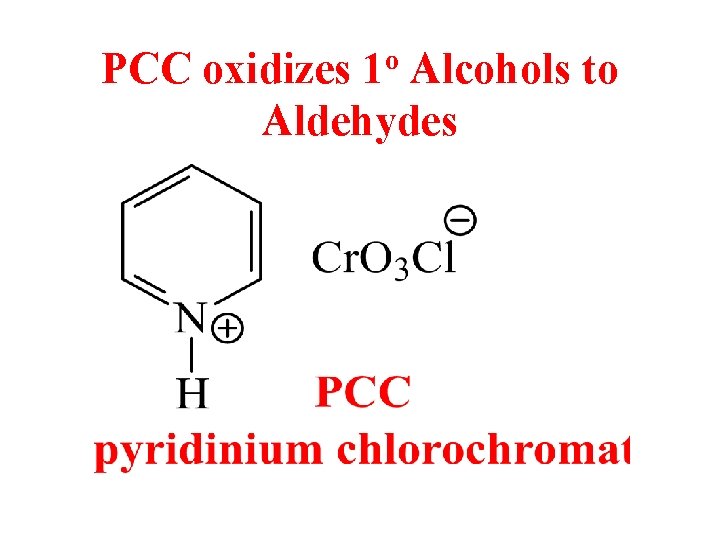
PCC oxidizes 1 o Alcohols to Aldehydes

Pyridinium Chlorochromate (PCC) • PCC is a complex of chromium trioxide, pyridine, and HCl. • Oxidizes primary alcohols to aldehydes. • Oxidizes secondary alcohols to ketones.

Oxidation of 1 o Alcohols to Aldehydes: PCC

3° Alcohols Cannot Be Oxidized • Carbon does not have hydrogen, so oxidation is difficult and involves the breakage of a C—C bond. • Chromic acid test is for primary and secondary alcohols because tertiary alcohols do not react. Orange color of Cr(VII) turns green - Cr(III); 3 o alcohol is not oxidized, therefore no color change.

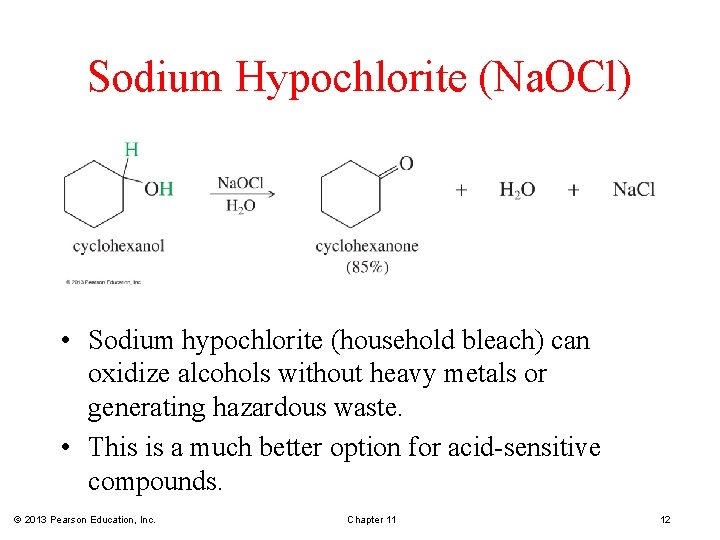
Sodium Hypochlorite (Na. OCl) • Sodium hypochlorite (household bleach) can oxidize alcohols without heavy metals or generating hazardous waste. • This is a much better option for acid-sensitive compounds. © 2013 Pearson Education, Inc. Chapter 11 12

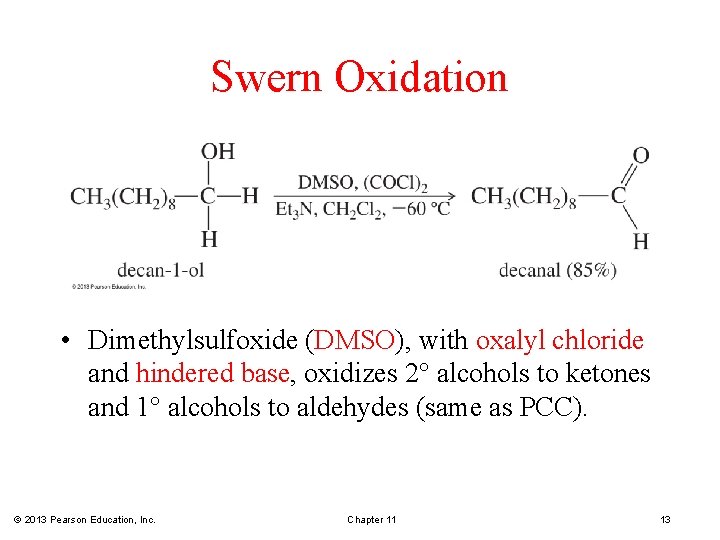
Swern Oxidation • Dimethylsulfoxide (DMSO), with oxalyl chloride and hindered base, oxidizes 2 alcohols to ketones and 1 alcohols to aldehydes (same as PCC). © 2013 Pearson Education, Inc. Chapter 11 13

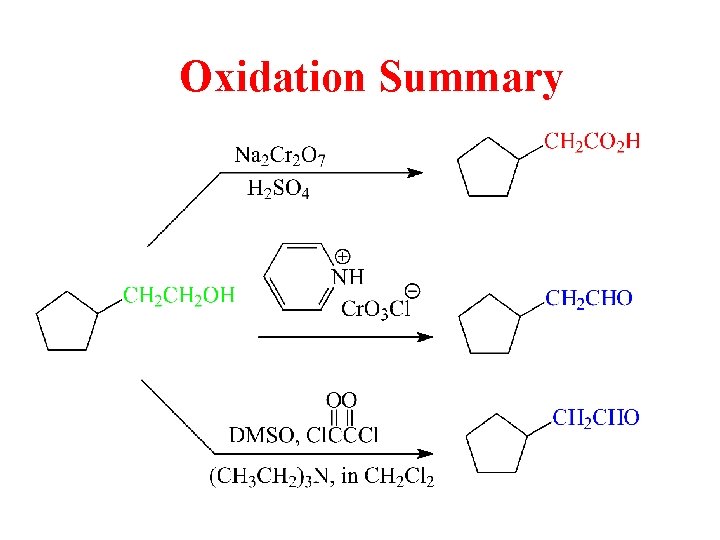
Oxidation Summary

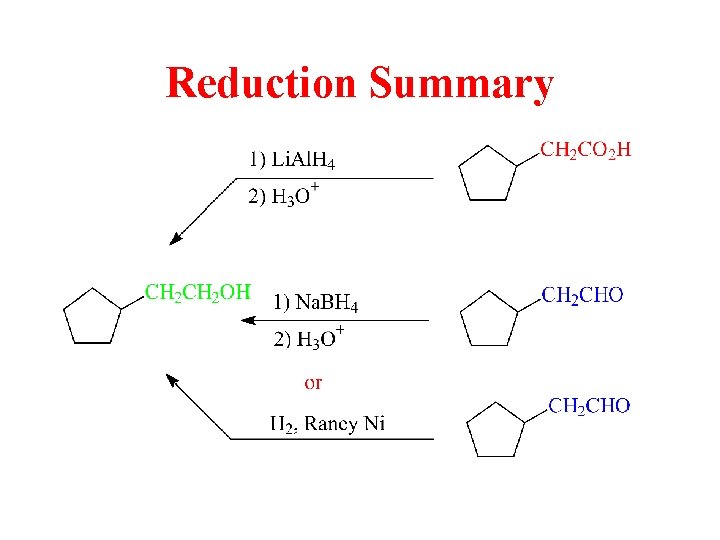
Reduction Summary

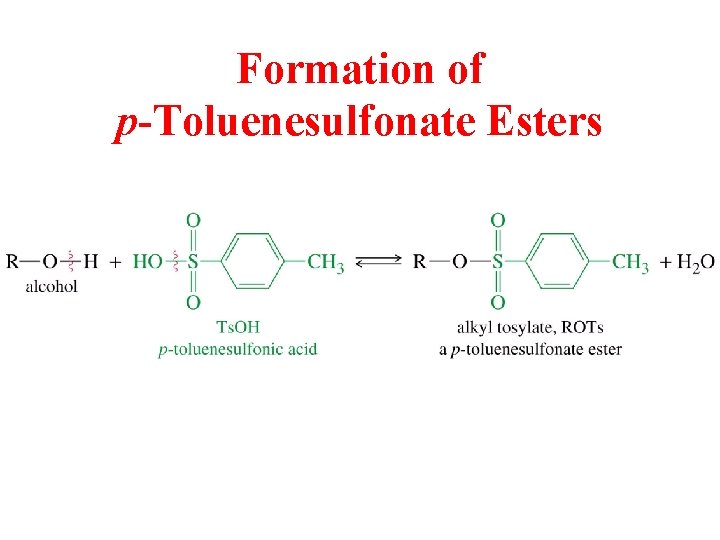
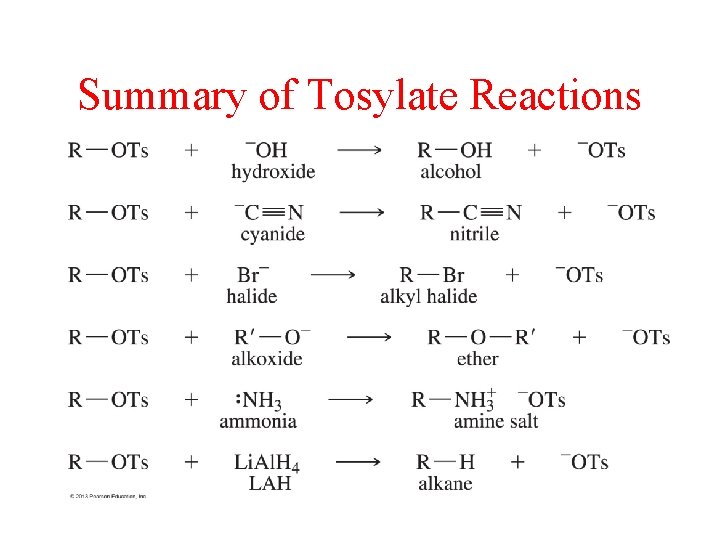
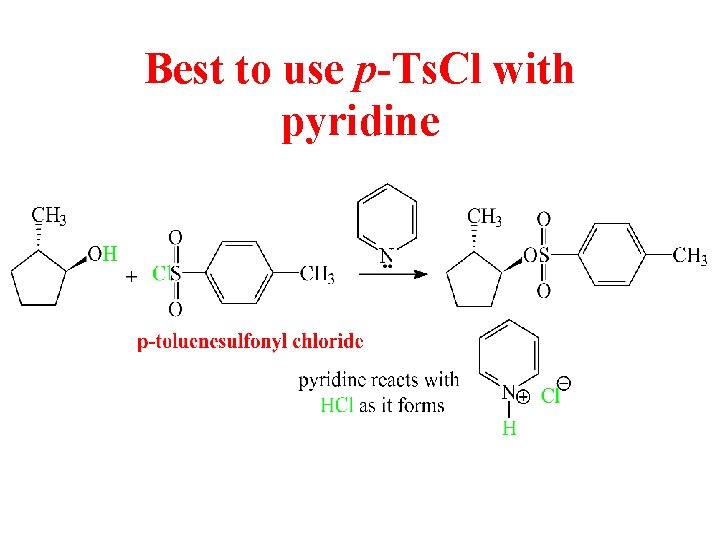
Conversion of Alcohol into a Leaving Group • Form Tosylate (p-Ts. Cl, pyridine) • Use strong acid (H 3 O+) • Convert to Alkyl Halide (HX, SOCl 2, PBr 3)

Formation of p-Toluenesulfonate Esters

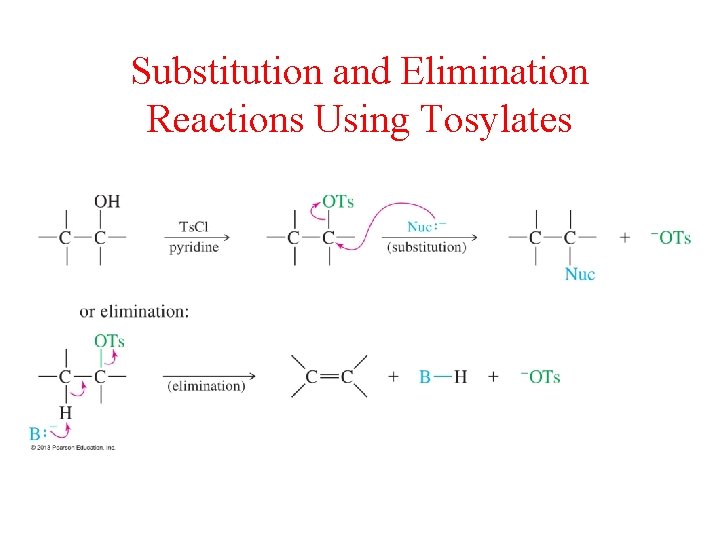
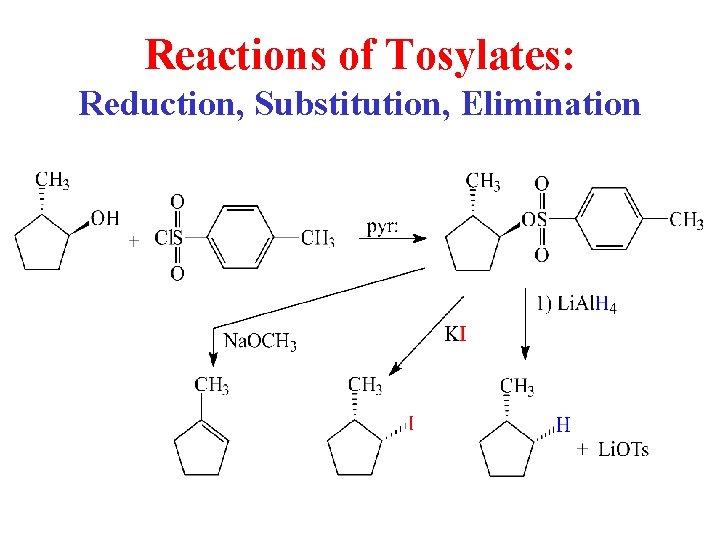
Substitution and Elimination Reactions Using Tosylates

Summary of Tosylate Reactions

Best to use p-Ts. Cl with pyridine

Reactions of Tosylates: Reduction, Substitution, Elimination

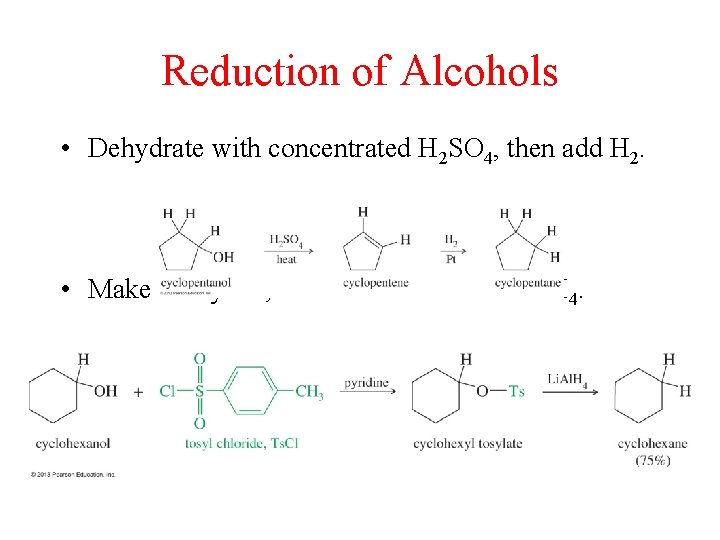
Reduction of Alcohols • Dehydrate with concentrated H 2 SO 4, then add H 2. • Make a tosylate, then reduce it with Li. Al. H 4.

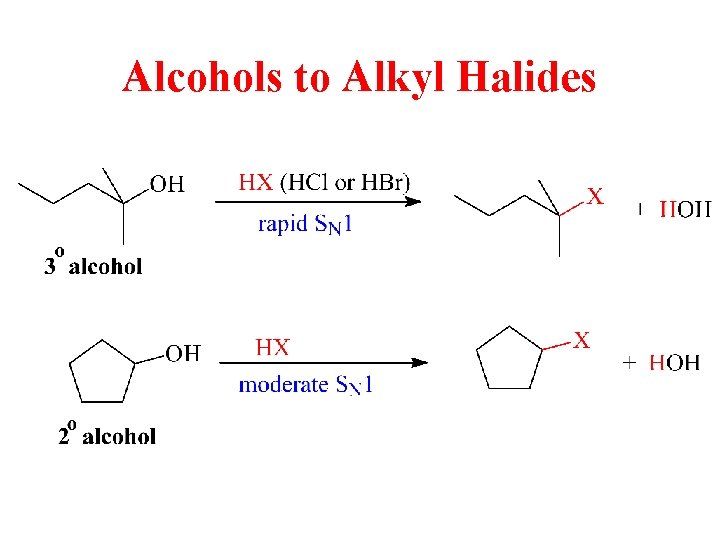
Alcohols to Alkyl Halides

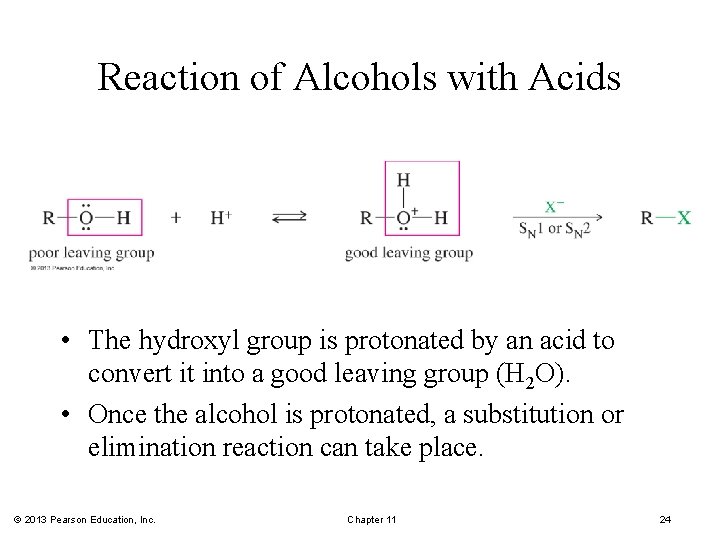
Reaction of Alcohols with Acids • The hydroxyl group is protonated by an acid to convert it into a good leaving group (H 2 O). • Once the alcohol is protonated, a substitution or elimination reaction can take place. © 2013 Pearson Education, Inc. Chapter 11 24

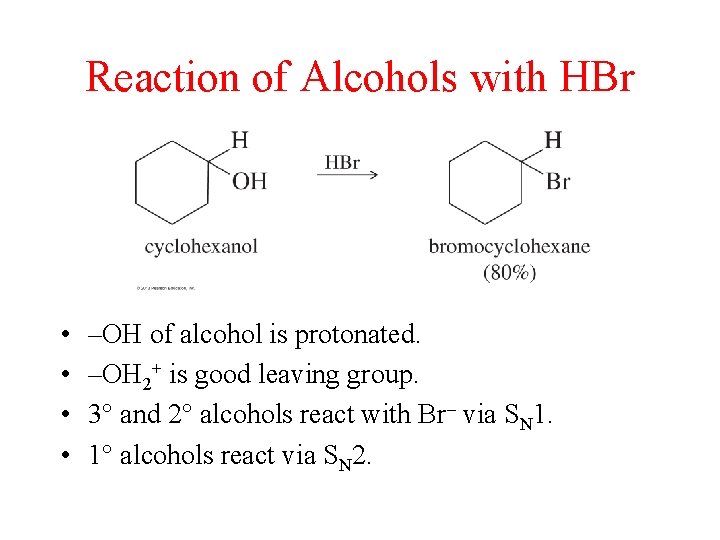
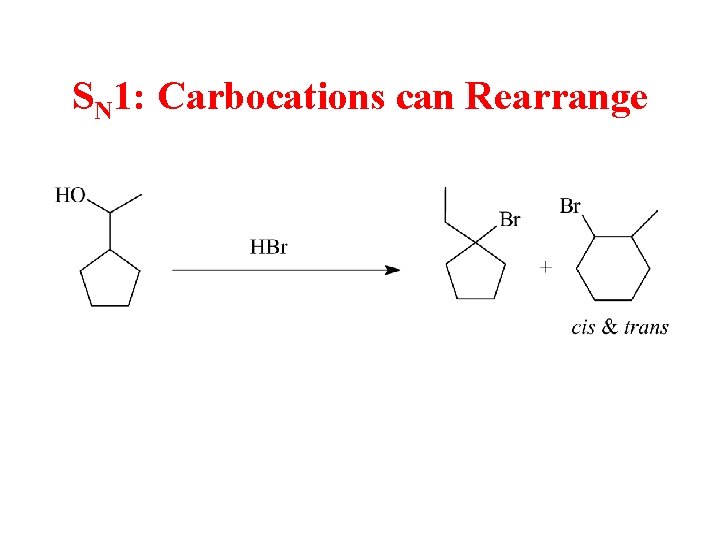
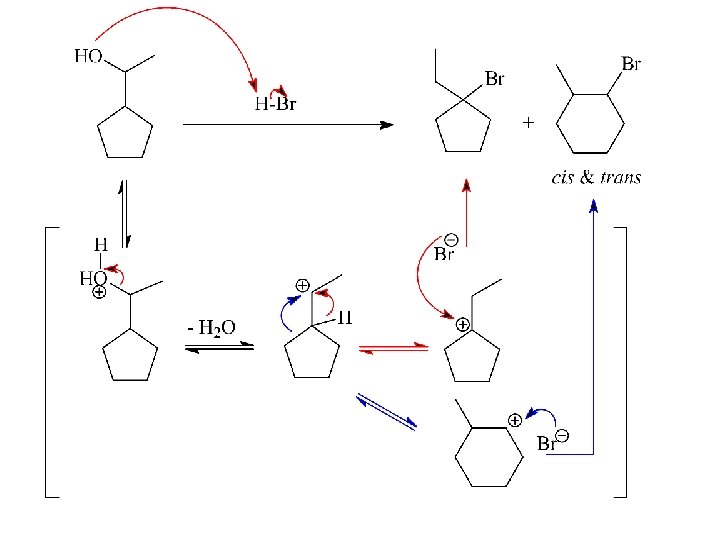
Reaction of Alcohols with HBr • • –OH of alcohol is protonated. –OH 2+ is good leaving group. 3° and 2° alcohols react with Br– via SN 1. 1° alcohols react via SN 2.

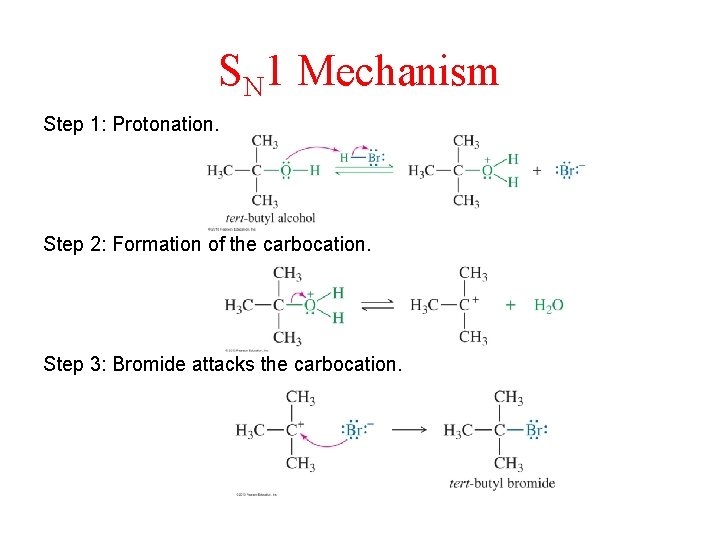
SN 1 Mechanism Step 1: Protonation. Step 2: Formation of the carbocation. Step 3: Bromide attacks the carbocation.

SN 1: Carbocations can Rearrange


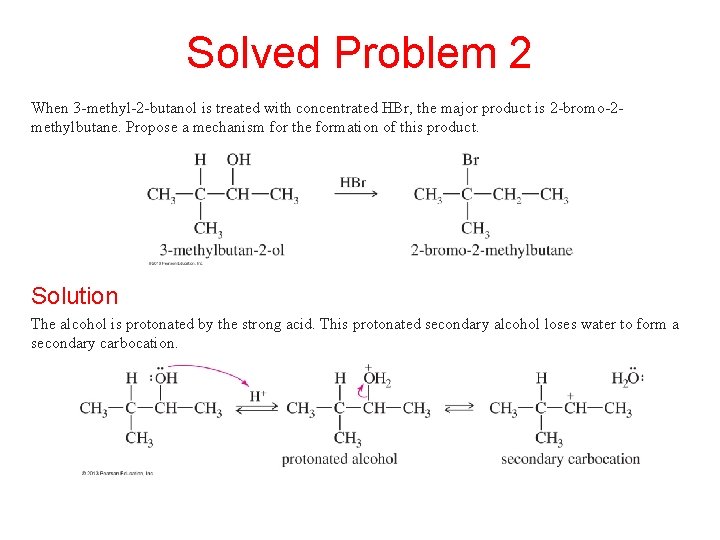
Solved Problem 2 When 3 -methyl-2 -butanol is treated with concentrated HBr, the major product is 2 -bromo-2 methylbutane. Propose a mechanism for the formation of this product. Solution The alcohol is protonated by the strong acid. This protonated secondary alcohol loses water to form a secondary carbocation.

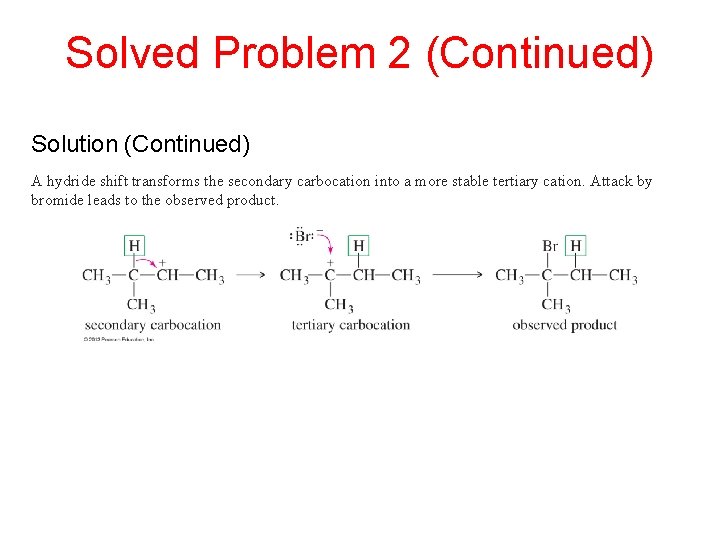
Solved Problem 2 (Continued) Solution (Continued) A hydride shift transforms the secondary carbocation into a more stable tertiary cation. Attack by bromide leads to the observed product.

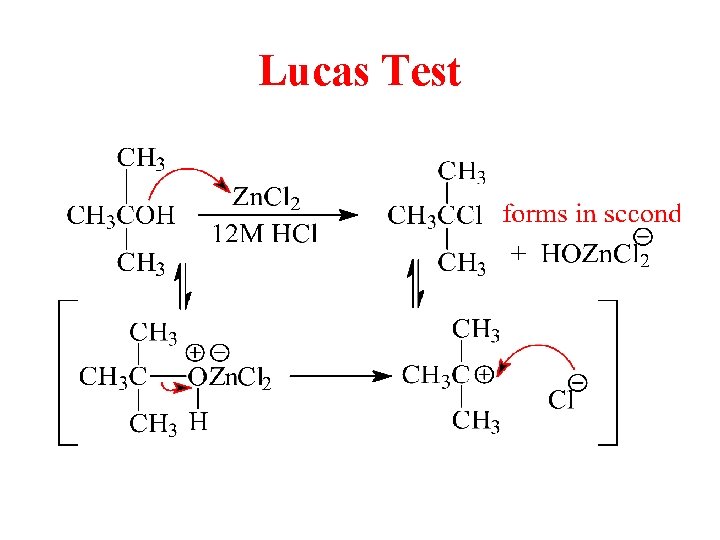
Lucas Test

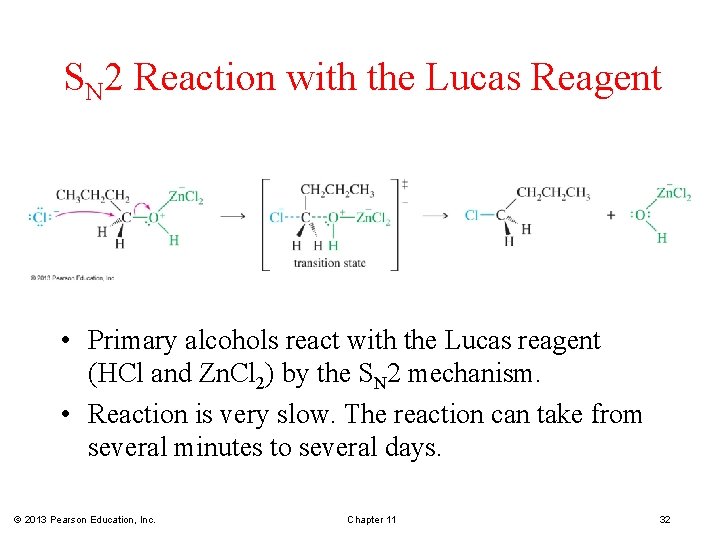
SN 2 Reaction with the Lucas Reagent • Primary alcohols react with the Lucas reagent (HCl and Zn. Cl 2) by the SN 2 mechanism. • Reaction is very slow. The reaction can take from several minutes to several days. © 2013 Pearson Education, Inc. Chapter 11 32

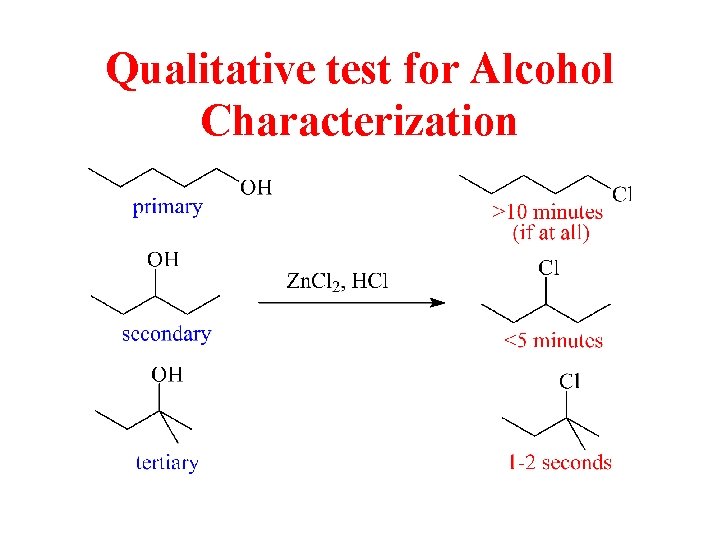
Qualitative test for Alcohol Characterization

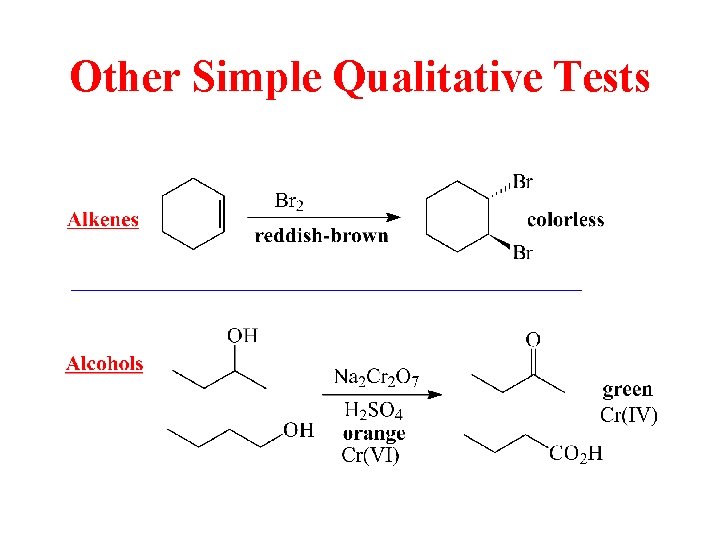
Other Simple Qualitative Tests

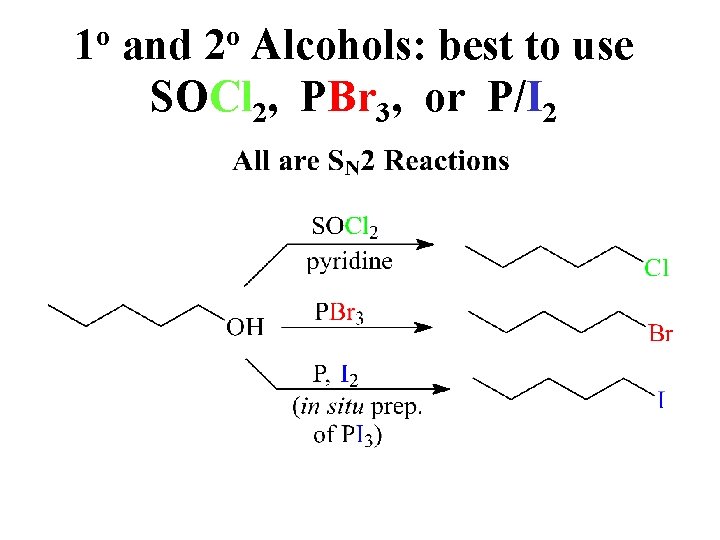
1 o and 2 o Alcohols: best to use SOCl 2, PBr 3, or P/I 2

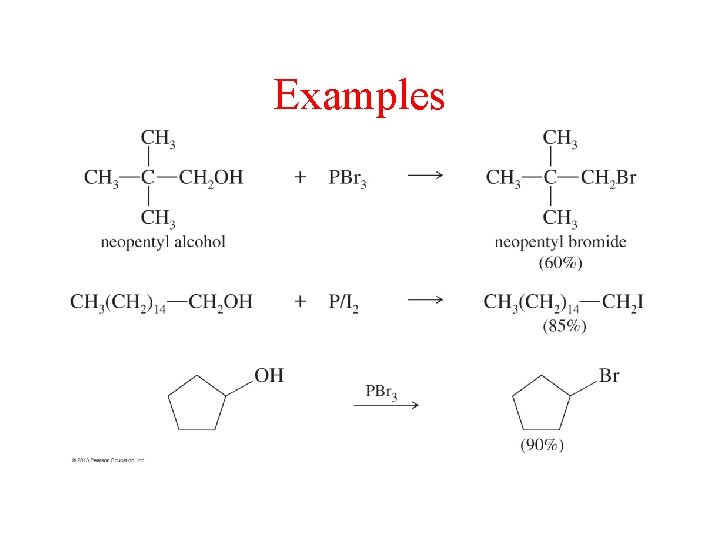
Examples

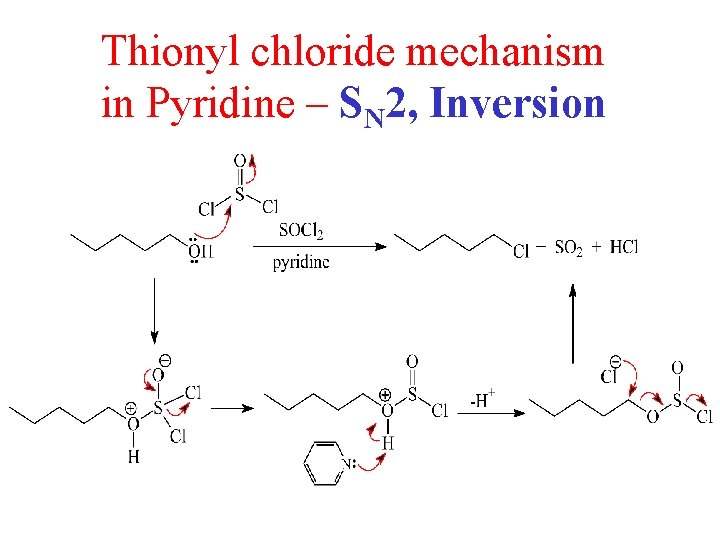
Thionyl chloride mechanism in Pyridine – SN 2, Inversion

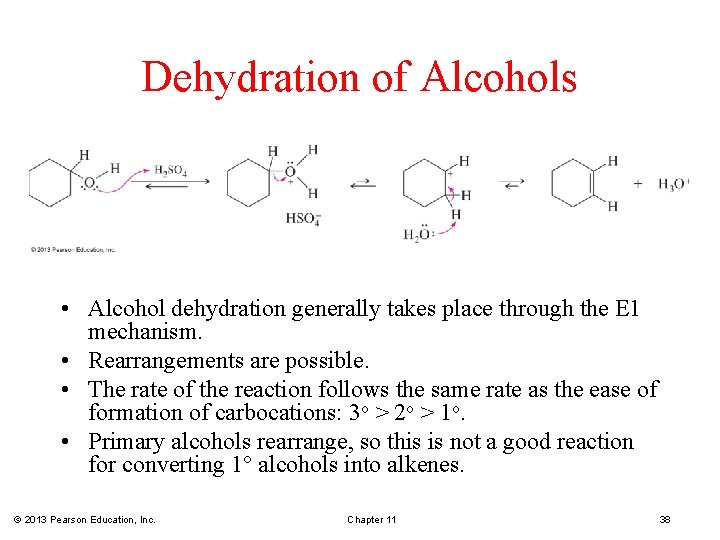
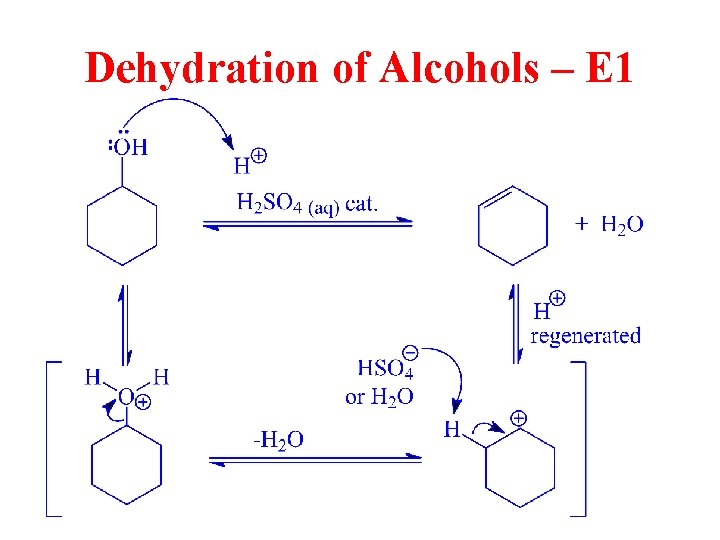
Dehydration of Alcohols • Alcohol dehydration generally takes place through the E 1 mechanism. • Rearrangements are possible. • The rate of the reaction follows the same rate as the ease of formation of carbocations: 3 o > 2 o > 1 o. • Primary alcohols rearrange, so this is not a good reaction for converting 1° alcohols into alkenes. © 2013 Pearson Education, Inc. Chapter 11 38

Dehydration of Alcohols – E 1

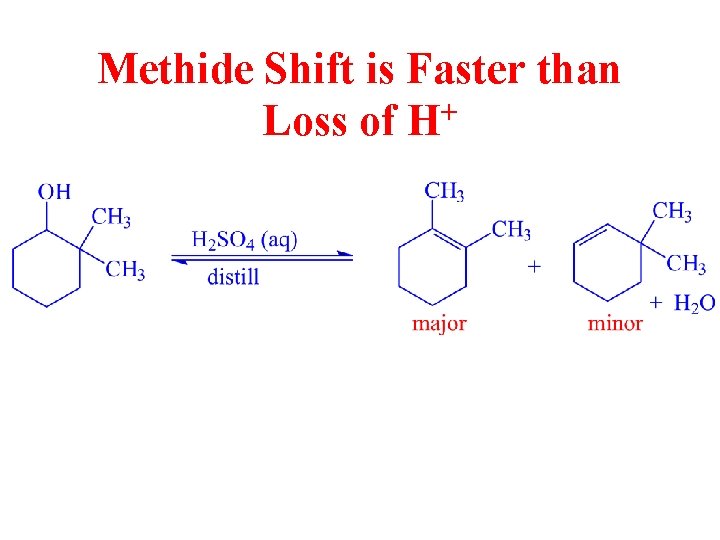
Methide Shift is Faster than Loss of H+

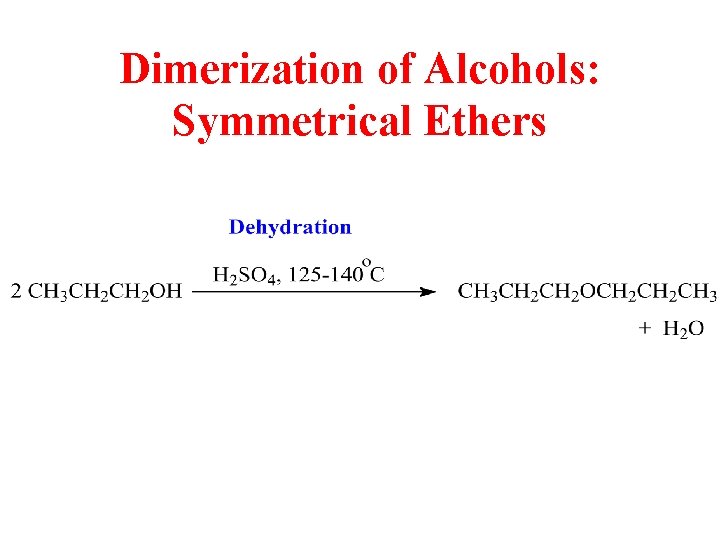
Dimerization of Alcohols: Symmetrical Ethers

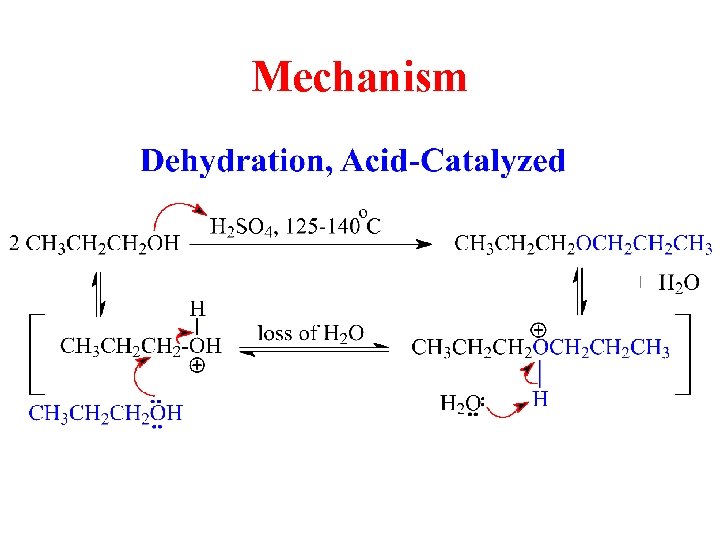
Mechanism

Esterification • • • Fischer: Alcohol + carboxylic acid Tosylate esters Sulfate esters Nitrate esters Phosphate esters

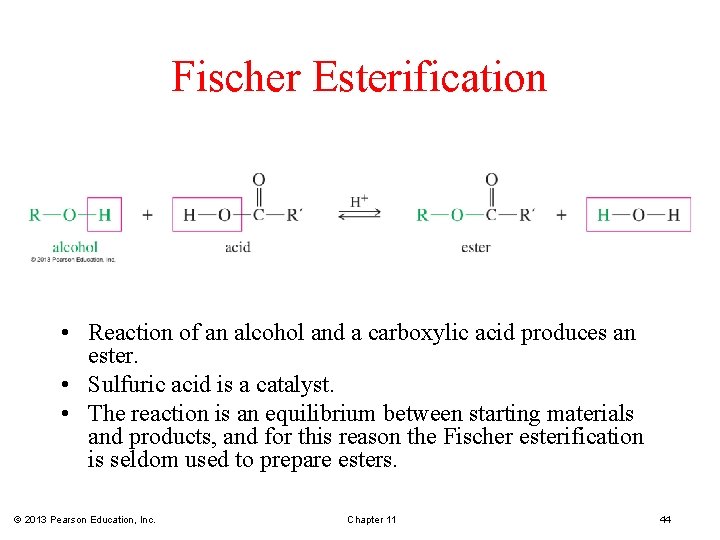
Fischer Esterification • Reaction of an alcohol and a carboxylic acid produces an ester. • Sulfuric acid is a catalyst. • The reaction is an equilibrium between starting materials and products, and for this reason the Fischer esterification is seldom used to prepare esters. © 2013 Pearson Education, Inc. Chapter 11 44

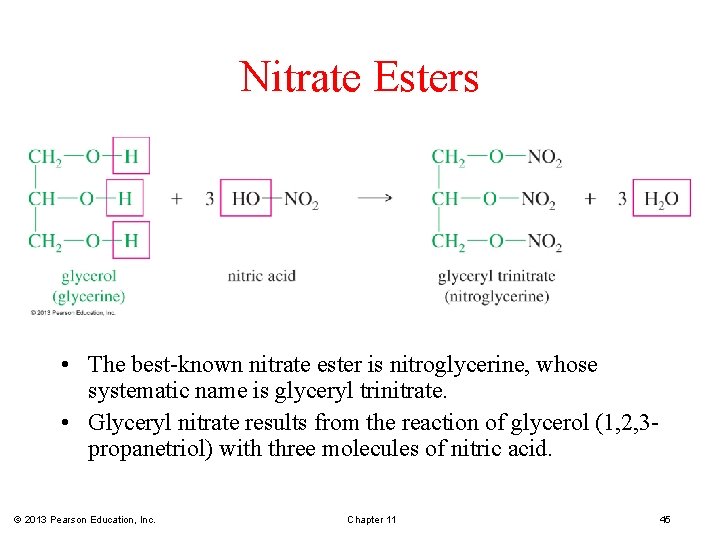
Nitrate Esters • The best-known nitrate ester is nitroglycerine, whose systematic name is glyceryl trinitrate. • Glyceryl nitrate results from the reaction of glycerol (1, 2, 3 propanetriol) with three molecules of nitric acid. © 2013 Pearson Education, Inc. Chapter 11 45

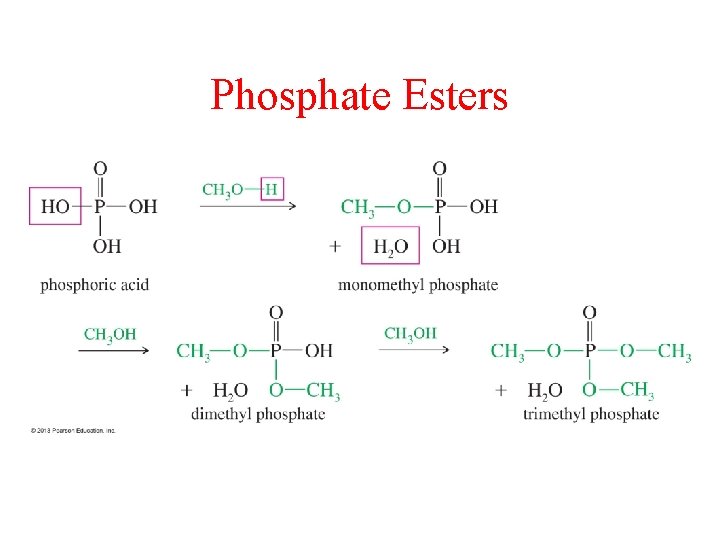
Phosphate Esters

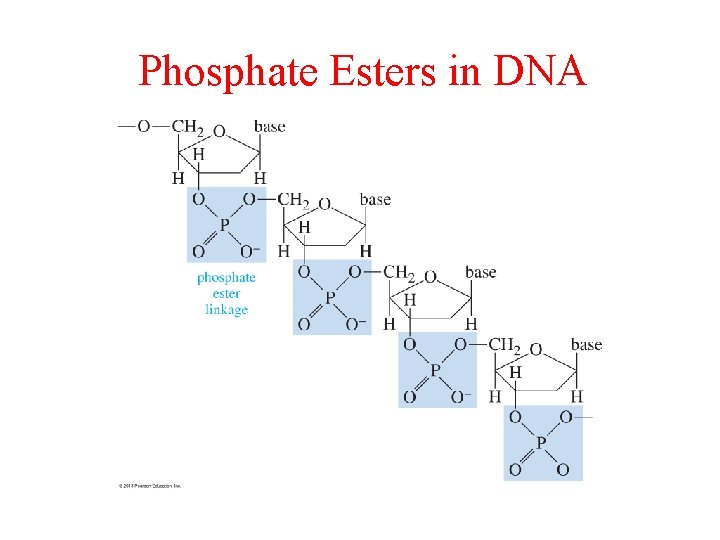
Phosphate Esters in DNA

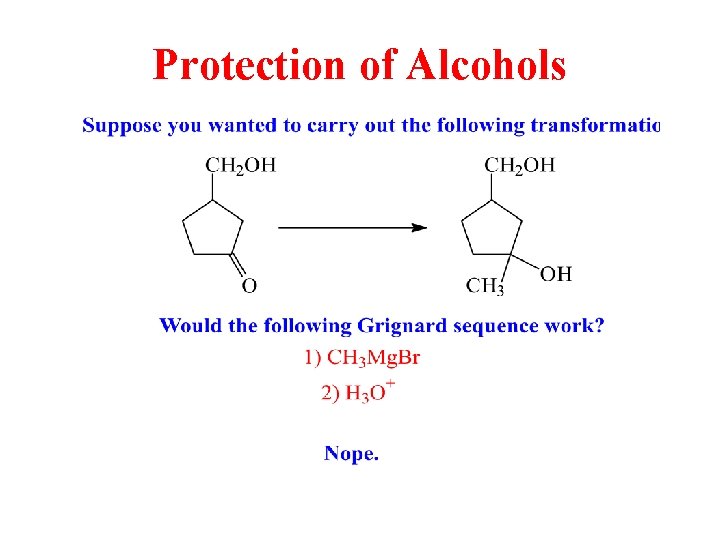
Protection of Alcohols

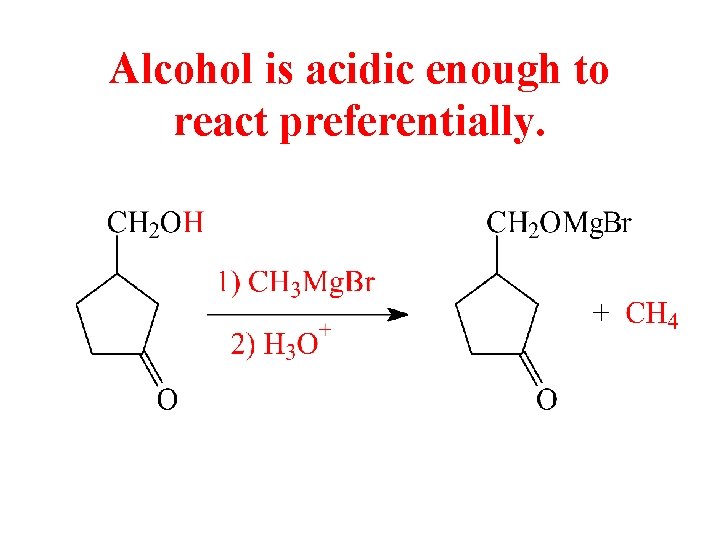
Alcohol is acidic enough to react preferentially.

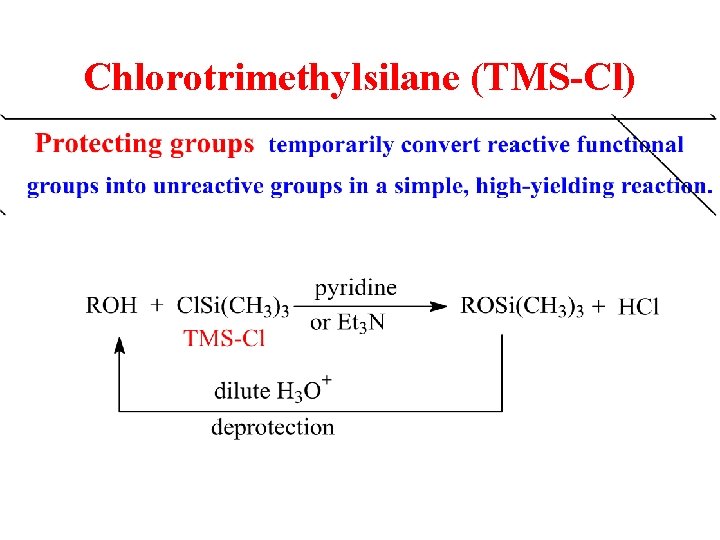
Chlorotrimethylsilane (TMS-Cl)

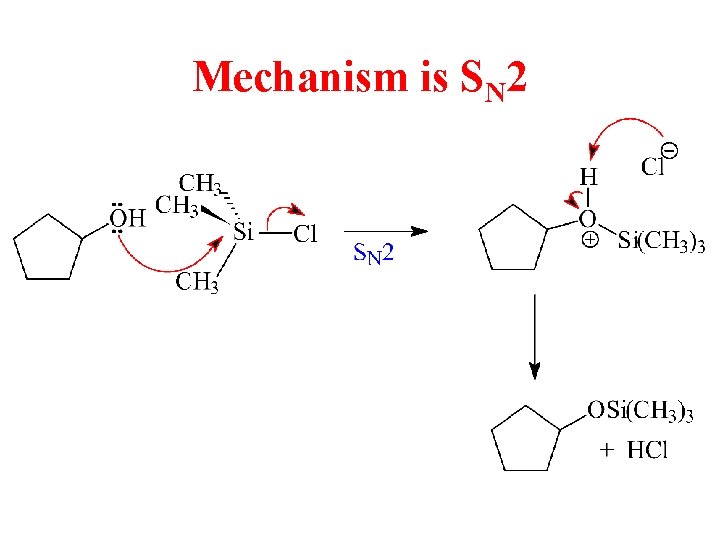
Mechanism is SN 2

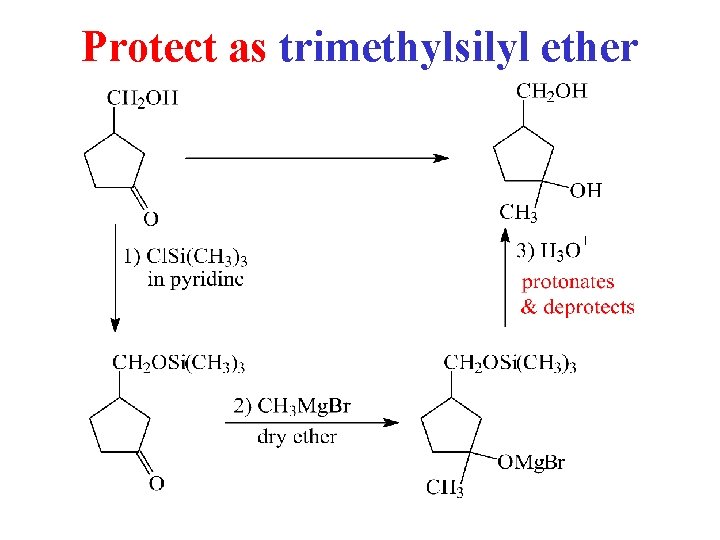
Protect as trimethylsilyl ether

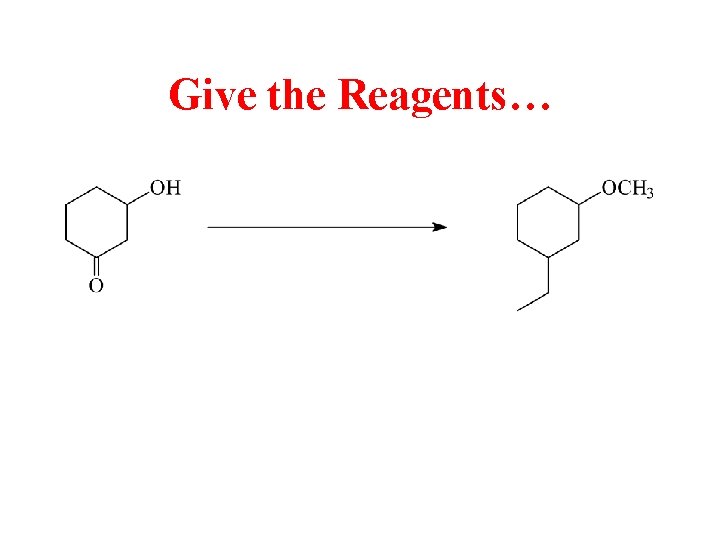
Give the Reagents…

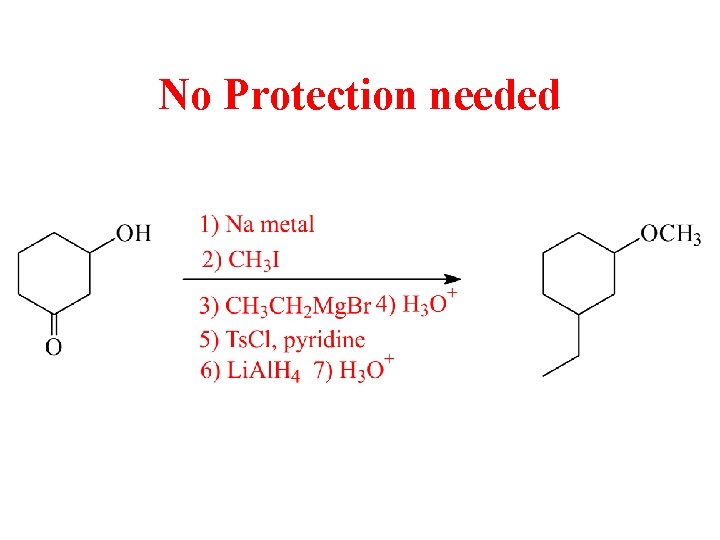
No Protection needed

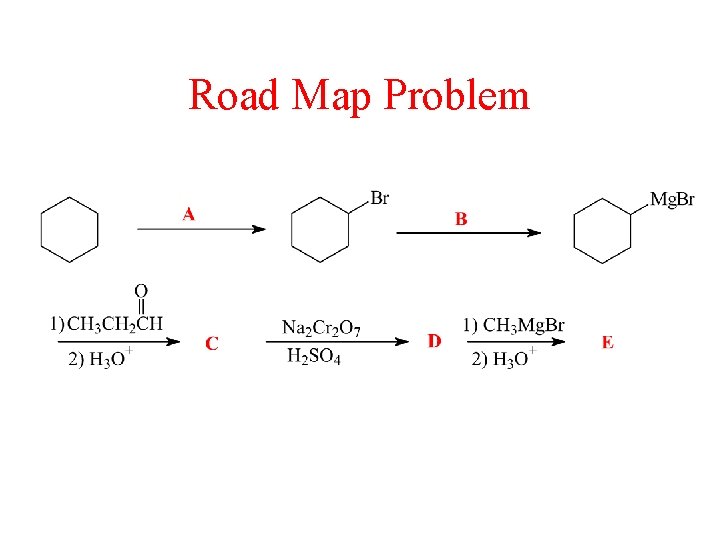
Road Map Problem

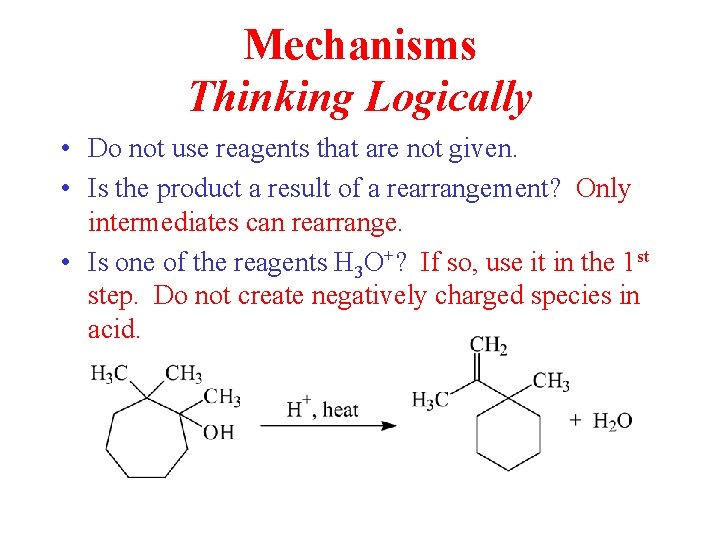
Mechanisms Thinking Logically • Do not use reagents that are not given. • Is the product a result of a rearrangement? Only intermediates can rearrange. • Is one of the reagents H 3 O+? If so, use it in the 1 st step. Do not create negatively charged species in acid.

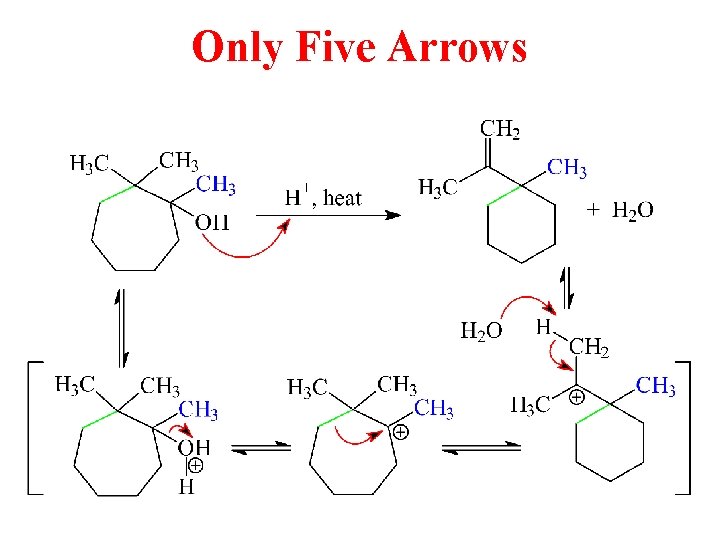
Only Five Arrows

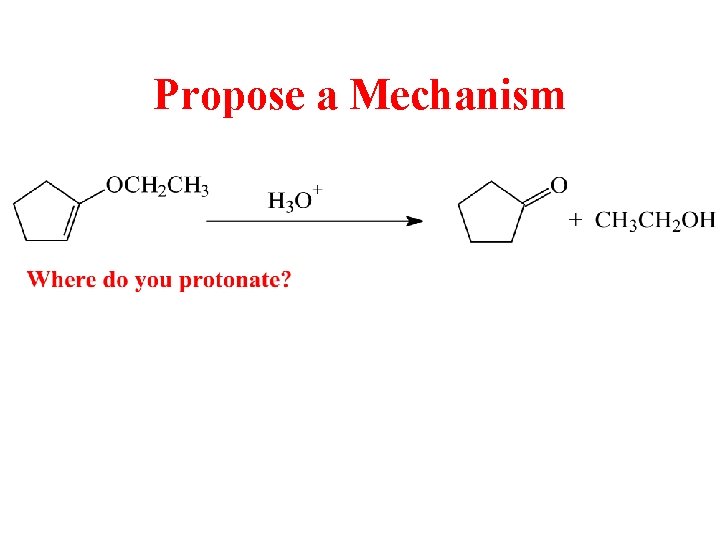
Propose a Mechanism

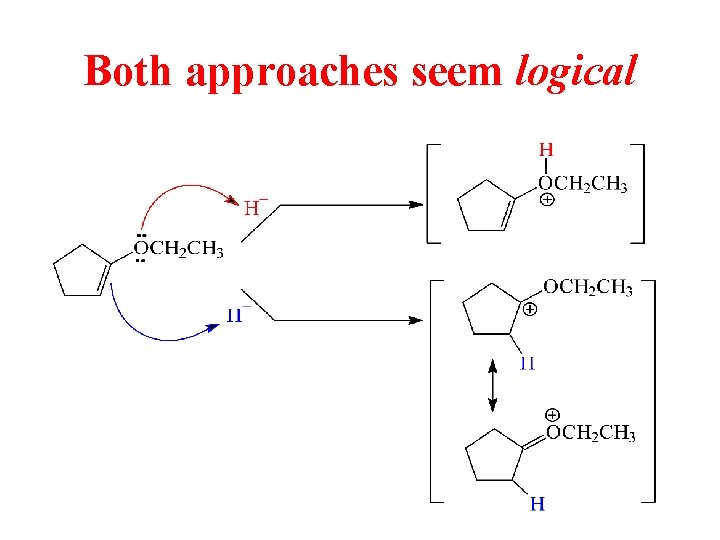
Both approaches seem logical

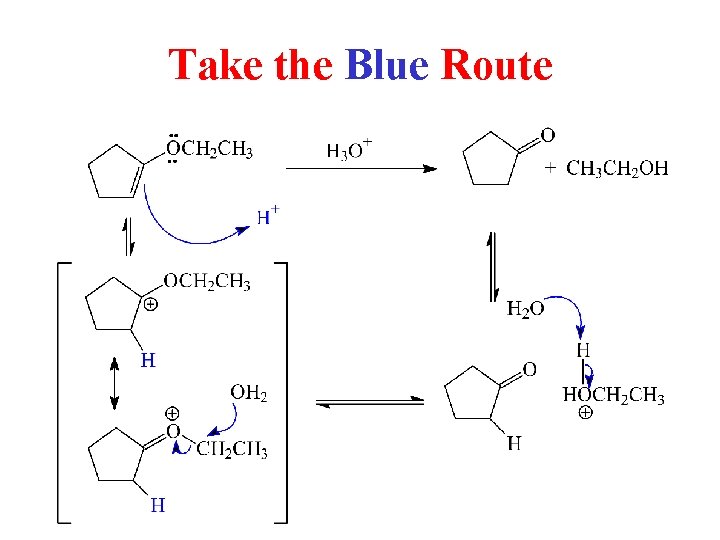
Take the Blue Route
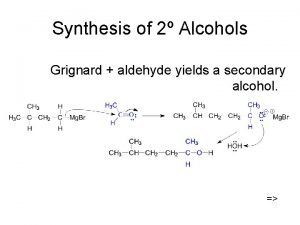
 Secondary alcohol oxidation
Secondary alcohol oxidation Tertiary alcohol synthesis
Tertiary alcohol synthesis Chemsheets
Chemsheets Oxidation examples
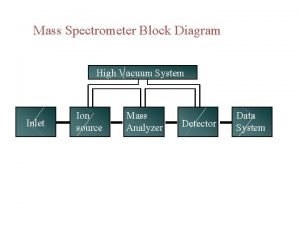
Oxidation examples Deal-grove model
Deal-grove model Chapter 19 redox reactions study guide answers
Chapter 19 redox reactions study guide answers Chapter 20 worksheet redox
Chapter 20 worksheet redox Oxidation and reduction in galvanic cells
Oxidation and reduction in galvanic cells Cathode and anode reduction and oxidation
Cathode and anode reduction and oxidation Chapter 19 review oxidation-reduction reactions answers
Chapter 19 review oxidation-reduction reactions answers Oxidation–reduction reactions
Oxidation–reduction reactions Leo the lion says ger
Leo the lion says ger Redox
Redox Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Example of redox reaction
Example of redox reaction Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Alcohols phenols thiols and ethers
Alcohols phenols thiols and ethers These are alcohols containing cppp nucleus?
These are alcohols containing cppp nucleus? Formation of alcohols
Formation of alcohols Lucas test mechanism
Lucas test mechanism Lucas test for alcohols
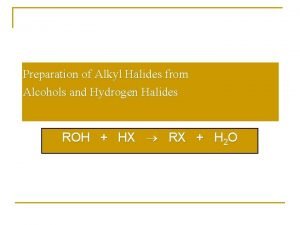
Lucas test for alcohols Preparation of alkyl halides from alcohols
Preparation of alkyl halides from alcohols Nomenclature
Nomenclature Sp
Sp Names of sugar alcohols
Names of sugar alcohols Alcohols nomenclature
Alcohols nomenclature Naming alkyl halides
Naming alkyl halides Alcohols nomenclature
Alcohols nomenclature Propan-1-ol oxidation
Propan-1-ol oxidation What does pcc ch2cl2 do
What does pcc ch2cl2 do Lucas reaction
Lucas reaction Upac name
Upac name Ir c
Ir c Petroleum ether composition
Petroleum ether composition 4-heptanone
4-heptanone Intermolecular forces formaldehyde
Intermolecular forces formaldehyde Aldehyde functional group
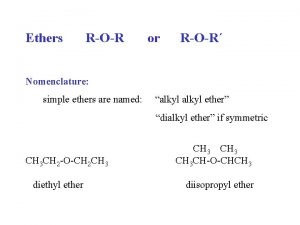
Aldehyde functional group R-o-r ether
R-o-r ether Ether naming
Ether naming Reaction of anhydride with alcohol
Reaction of anhydride with alcohol Ether naming
Ether naming Alcohol to ether mechanism
Alcohol to ether mechanism Naming ether
Naming ether Ether synthesis
Ether synthesis Dry ether example
Dry ether example Naming ethers
Naming ethers Dipropyl ether structure
Dipropyl ether structure Ether 2:12
Ether 2:12 Formal ether sedimentation technique
Formal ether sedimentation technique Diethyl ether uses
Diethyl ether uses Ether oxygen
Ether oxygen Petroleum ether nfpa
Petroleum ether nfpa Ether 12 4
Ether 12 4 Ether
Ether Etheraccess
Etheraccess Giardia lamblia
Giardia lamblia Nomenclature of ether
Nomenclature of ether Diphenyl ether
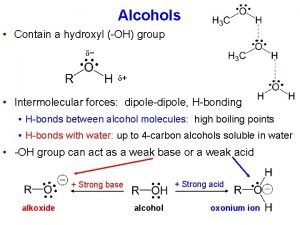
Diphenyl ether What is the most important physical property of alcohol?
What is the most important physical property of alcohol?