Preparation of Alkyl Halides from Alcohols and Hydrogen

- Slides: 12

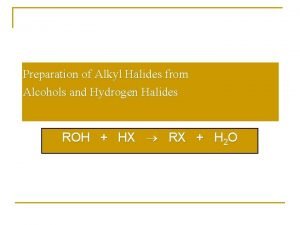
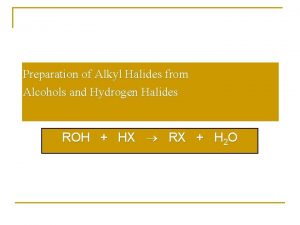
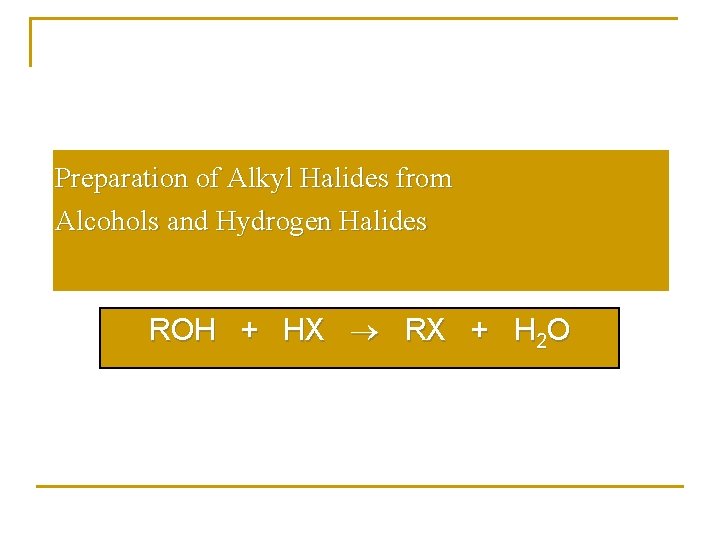
Preparation of Alkyl Halides from Alcohols and Hydrogen Halides ROH + HX RX + H 2 O

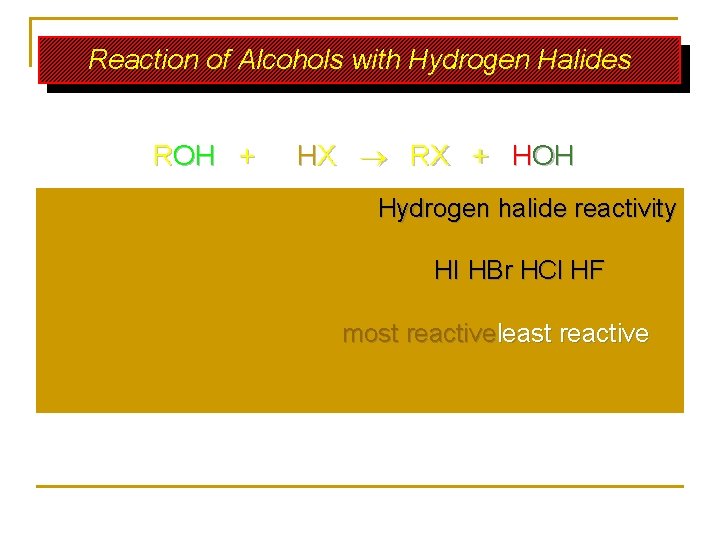
Reaction of Alcohols with Hydrogen Halides ROH + HX RX + HOH Hydrogen halide reactivity HI HBr HCl HF most reactiveleast reactive

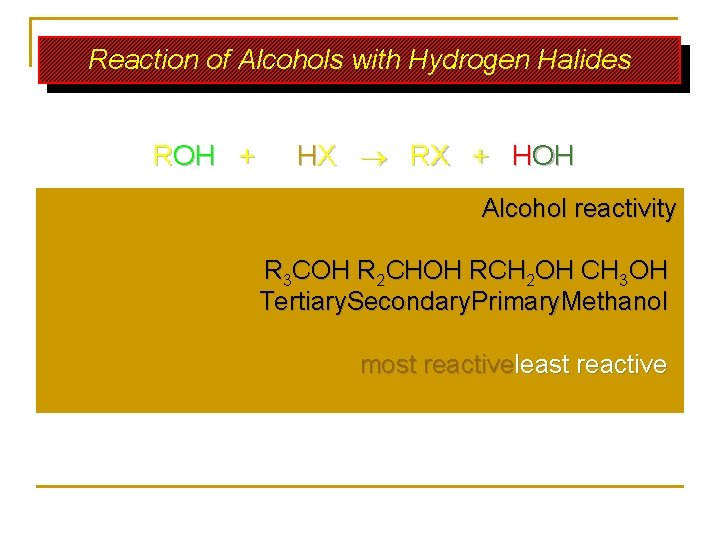
Reaction of Alcohols with Hydrogen Halides ROH + HX RX + HOH Alcohol reactivity R 3 COH R 2 CHOH RCH 2 OH CH 3 OH Tertiary. Secondary. Primary. Methanol most reactiveleast reactive

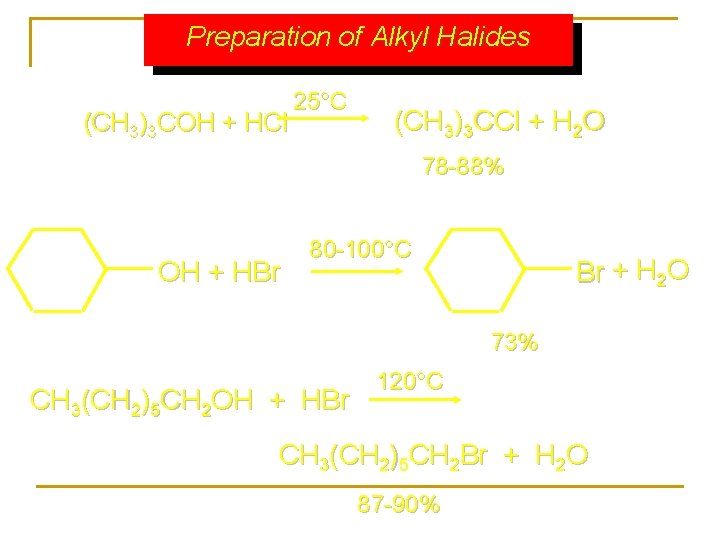
Preparation of Alkyl Halides (CH 3)3 COH + HCl 25°C (CH 3)3 CCl + H 2 O 78 -88% OH + HBr 80 -100°C Br + H 2 O 73% CH 3(CH 2)5 CH 2 OH + HBr 120°C CH 3(CH 2)5 CH 2 Br + H 2 O 87 -90%

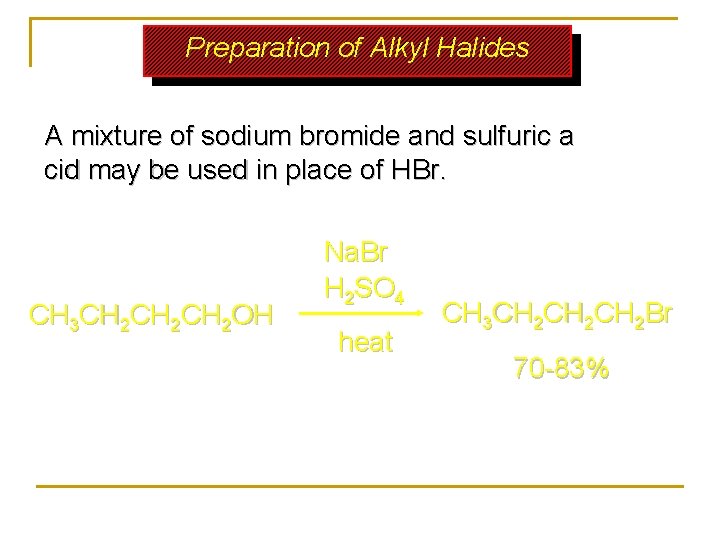
Preparation of Alkyl Halides A mixture of sodium bromide and sulfuric a cid may be used in place of HBr. CH 3 CH 2 CH 2 OH Na. Br H 2 SO 4 heat CH 3 CH 2 CH 2 Br 70 -83%

Mechanism of the Reaction of Alcohols with Hydrogen Halides

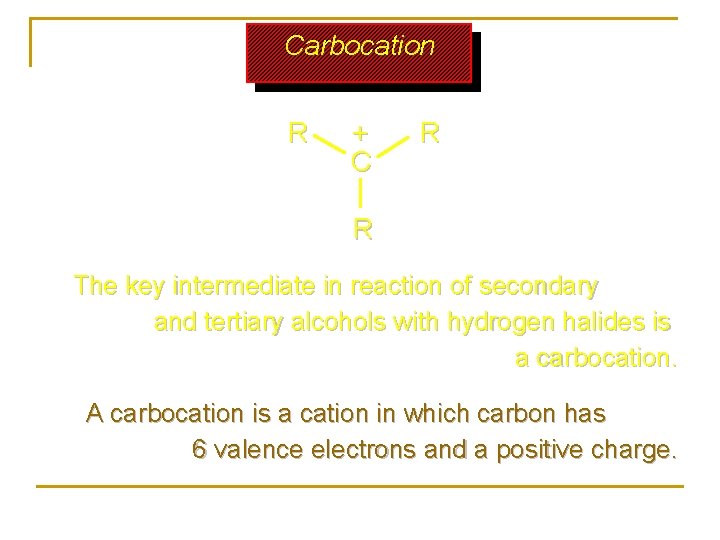
Carbocation R + C R R The key intermediate in reaction of secondary and tertiary alcohols with hydrogen halides is a carbocation. A carbocation is a cation in which carbon has 6 valence electrons and a positive charge.

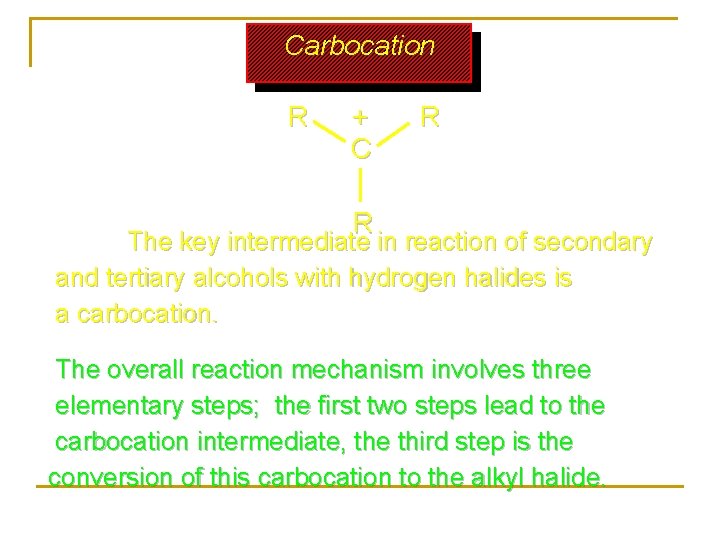
Carbocation R + C R R The key intermediate in reaction of secondary and tertiary alcohols with hydrogen halides is a carbocation. The overall reaction mechanism involves three elementary steps; the first two steps lead to the carbocation intermediate, the third step is the conversion of this carbocation to the alkyl halide.

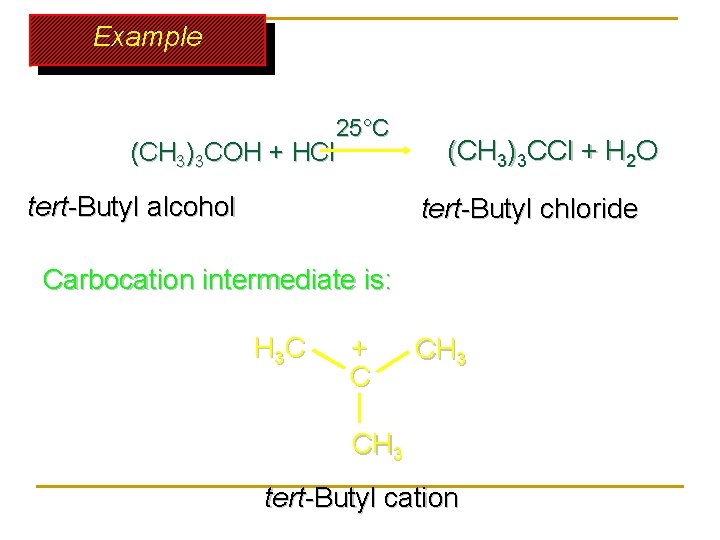
Example 25°C (CH 3)3 COH + HCl tert-Butyl alcohol (CH 3)3 CCl + H 2 O tert-Butyl chloride Carbocation intermediate is: H 3 C + C CH 3 tert-Butyl cation

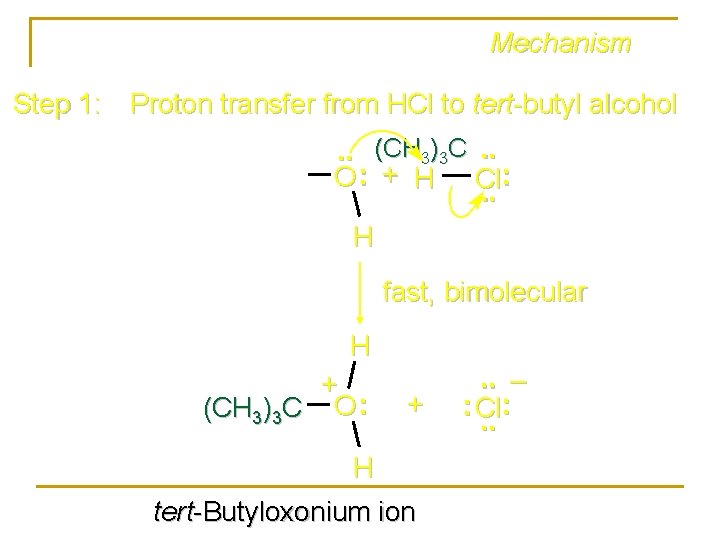
Mechanism Step 1: Proton transfer from HCl to tert-butyl alcohol. . (CH 3)3 C. . O : + H Cl: . . H fast, bimolecular H + (CH 3)3 C O : + H tert-Butyloxonium ion . . – : Cl: . .

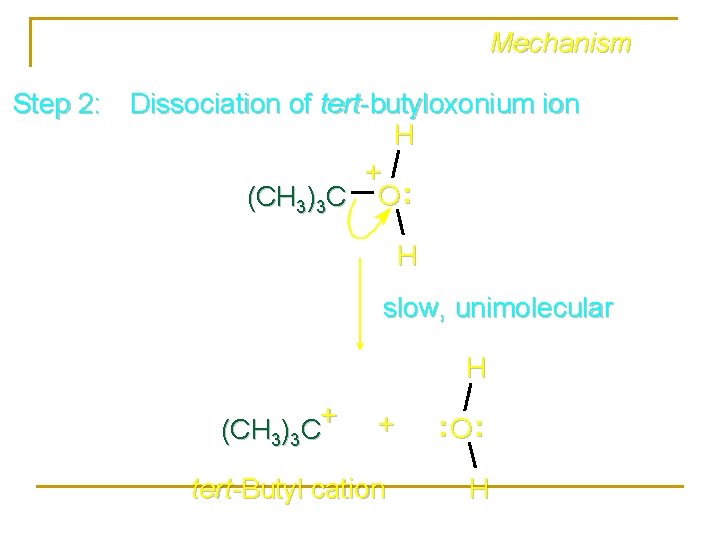
Mechanism Step 2: Dissociation of tert-butyloxonium ion H + (CH 3)3 C O : H slow, unimolecular H + (CH 3)3 C + tert-Butyl cation : O: H

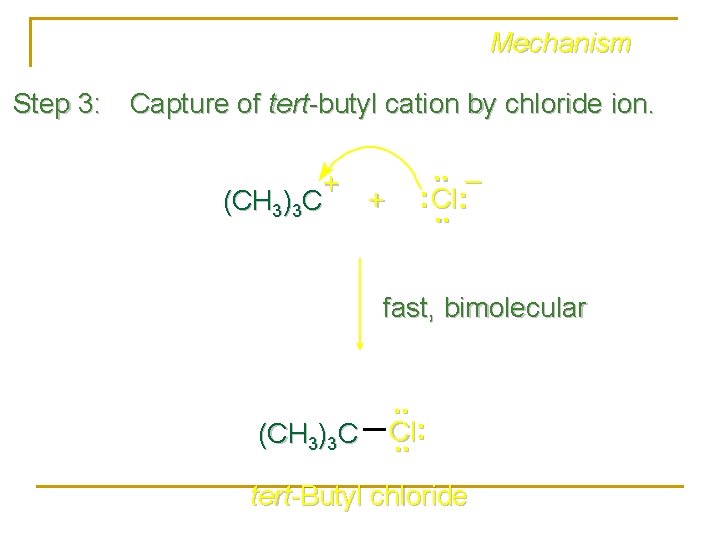
Mechanism Step 3: Capture of tert-butyl cation by chloride ion. + (CH 3)3 C + . . – : Cl: . . fast, bimolecular (CH 3)3 C . . Cl. . : tert-Butyl chloride
 Preparation of alkyl halides from alcohols
Preparation of alkyl halides from alcohols Solvents for sn2 reactions
Solvents for sn2 reactions Factors affecting nucleophilic substitution reaction
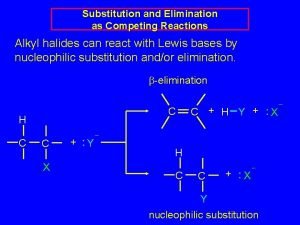
Factors affecting nucleophilic substitution reaction Nucleophilic substitution of alkyl halides
Nucleophilic substitution of alkyl halides Naming alkyl halides
Naming alkyl halides Nucleophilic substitution of alkyl halides
Nucleophilic substitution of alkyl halides Alcohol synthesis from alkyl halide
Alcohol synthesis from alkyl halide Name the following alkyl halides:
Name the following alkyl halides: Alkyl halide reactions
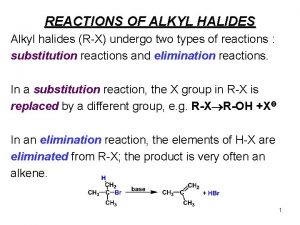
Alkyl halide reactions Alkyl halide
Alkyl halide Nomenclature of alkyl halides
Nomenclature of alkyl halides Oxymercuration demercuration of alkynes
Oxymercuration demercuration of alkynes Is acid catalyzed hydration syn or anti
Is acid catalyzed hydration syn or anti