Chapter 3 Alcohol and Alkyl Halides Alkyl Halides



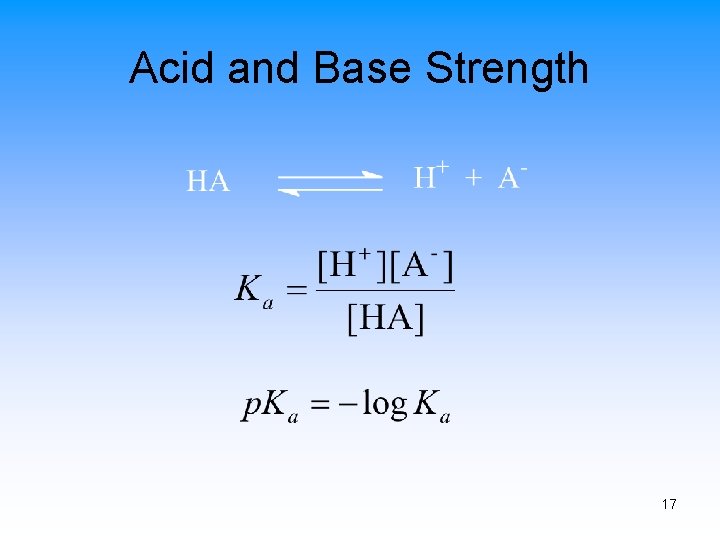


- Slides: 41

Chapter 3. Alcohol and Alkyl Halides

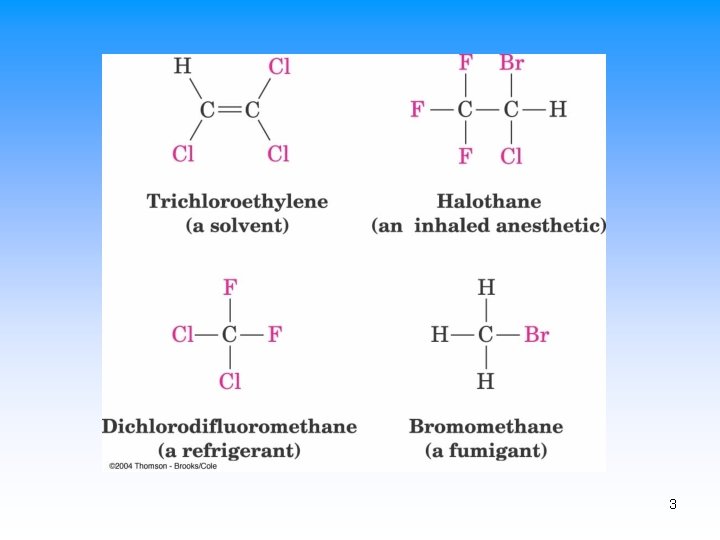
Alkyl Halides • An organic compound containing at least one carbonhalogen bond (C-X) – X (F, Cl, Br, I) replaces H • Can contain many C-X bonds • Properties and some uses – Fire-resistant solvents – Refrigerants – Pharmaceuticals and precursors 2

3

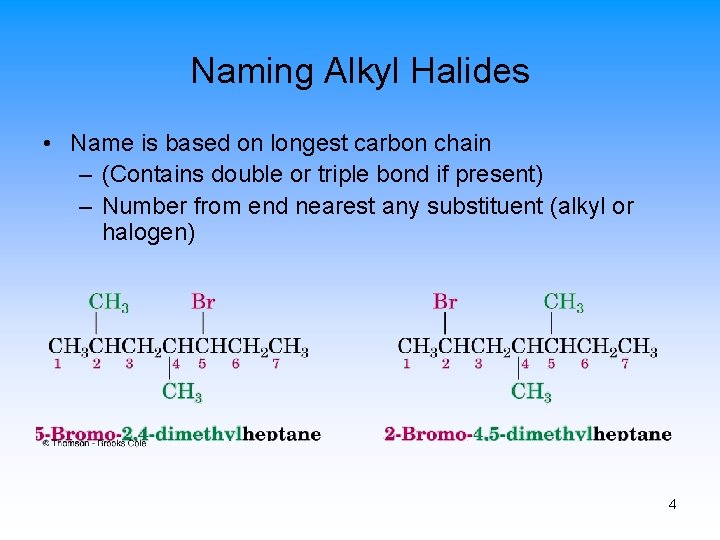
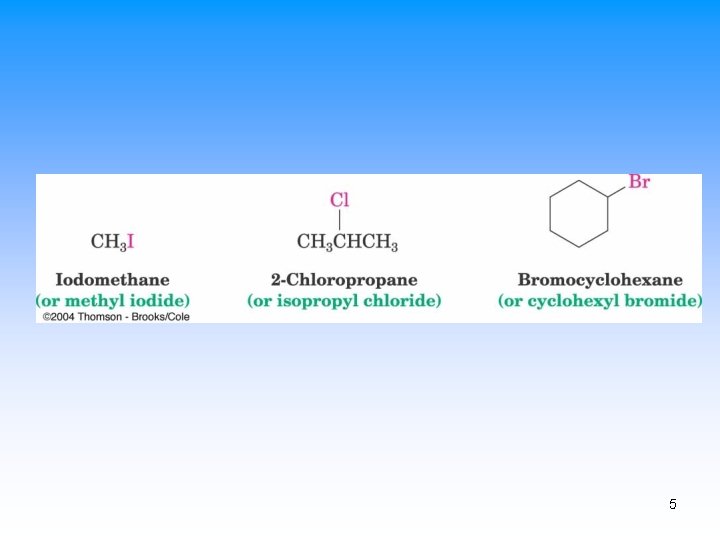
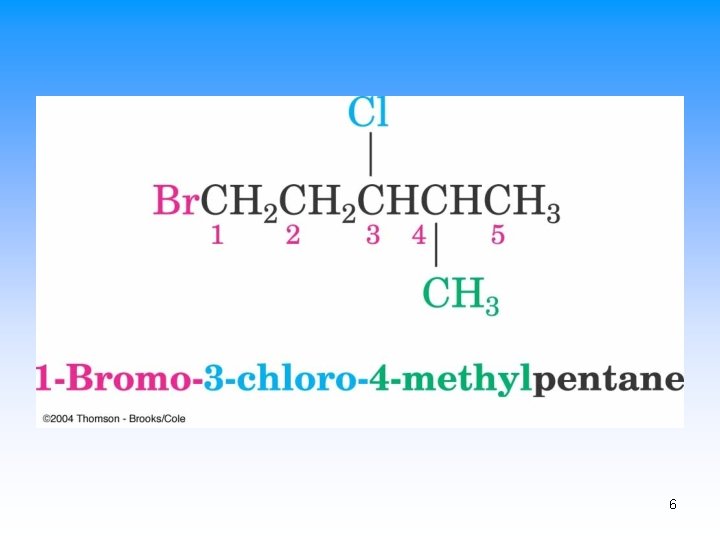
Naming Alkyl Halides • Name is based on longest carbon chain – (Contains double or triple bond if present) – Number from end nearest any substituent (alkyl or halogen) 4

5

6

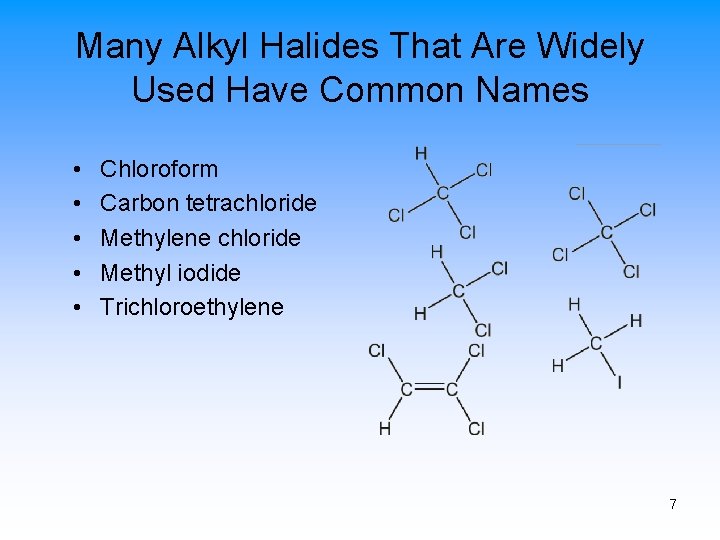
Many Alkyl Halides That Are Widely Used Have Common Names • • • Chloroform Carbon tetrachloride Methylene chloride Methyl iodide Trichloroethylene 7

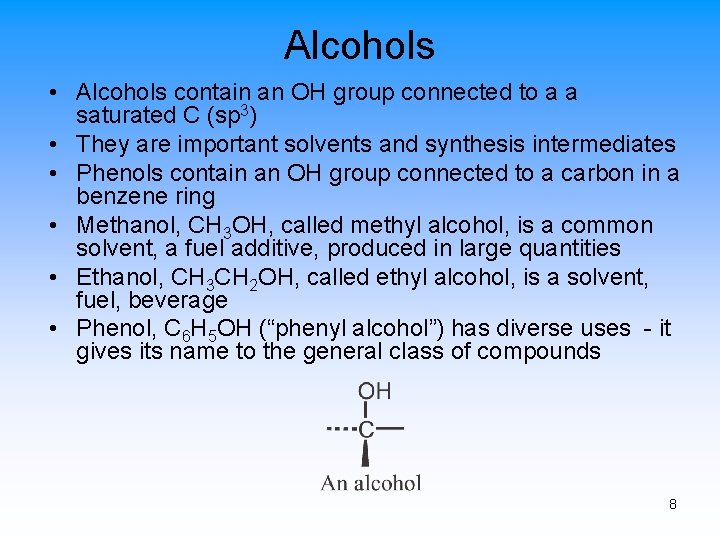
Alcohols • Alcohols contain an OH group connected to a a saturated C (sp 3) • They are important solvents and synthesis intermediates • Phenols contain an OH group connected to a carbon in a benzene ring • Methanol, CH 3 OH, called methyl alcohol, is a common solvent, a fuel additive, produced in large quantities • Ethanol, CH 3 CH 2 OH, called ethyl alcohol, is a solvent, fuel, beverage • Phenol, C 6 H 5 OH (“phenyl alcohol”) has diverse uses - it gives its name to the general class of compounds 8

Naming Alcohols • General classifications of alcohols based on substitution on C to which OH is attached • Methyl (C has 3 H’s), Primary (1°) (C has two H’s, one R), secondary (2°) (C has one H, two R’s), tertiary (3°) (C has no H, 3 R’s), 9

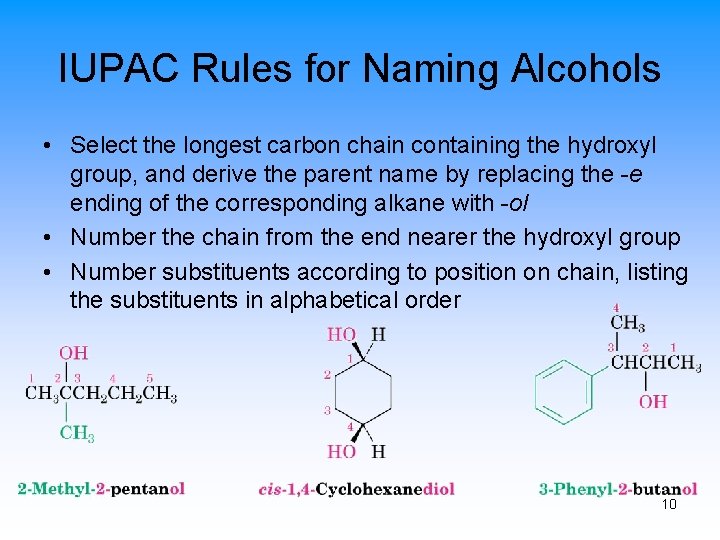
IUPAC Rules for Naming Alcohols • Select the longest carbon chain containing the hydroxyl group, and derive the parent name by replacing the -e ending of the corresponding alkane with -ol • Number the chain from the end nearer the hydroxyl group • Number substituents according to position on chain, listing the substituents in alphabetical order 10

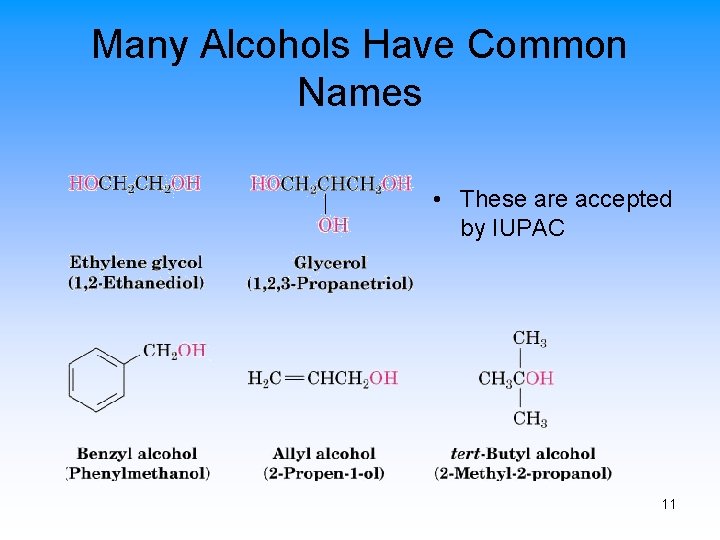
Many Alcohols Have Common Names • These are accepted by IUPAC 11

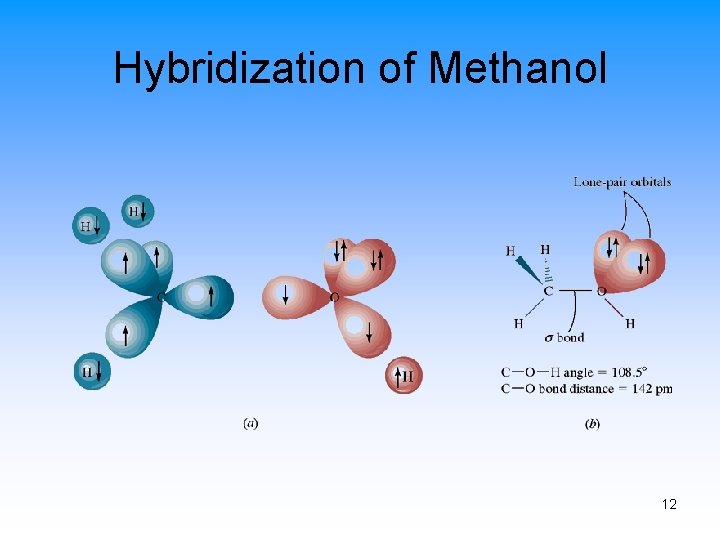
Hybridization of Methanol 12

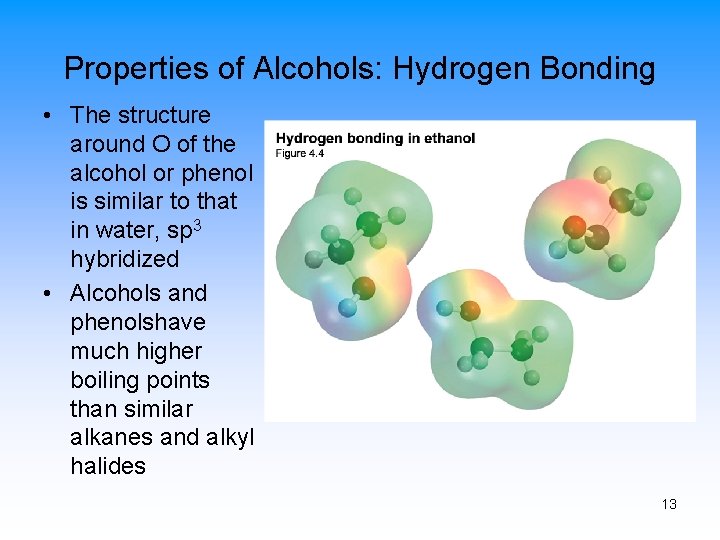
Properties of Alcohols: Hydrogen Bonding • The structure around O of the alcohol or phenol is similar to that in water, sp 3 hybridized • Alcohols and phenolshave much higher boiling points than similar alkanes and alkyl halides 13

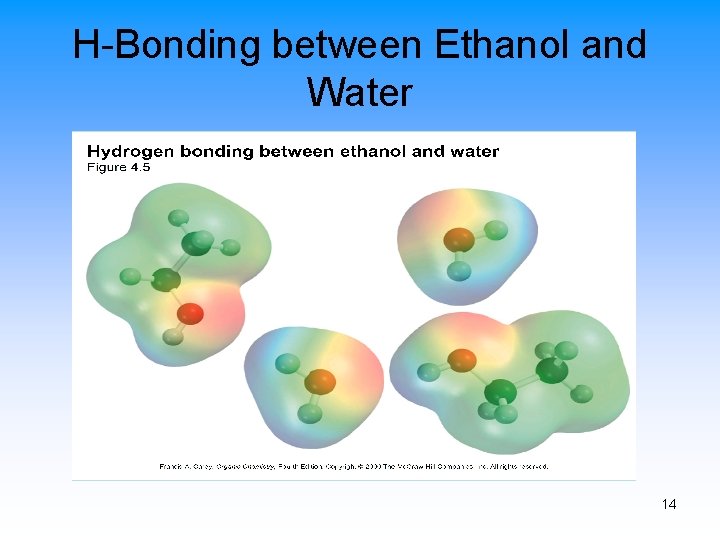
H-Bonding between Ethanol and Water 14

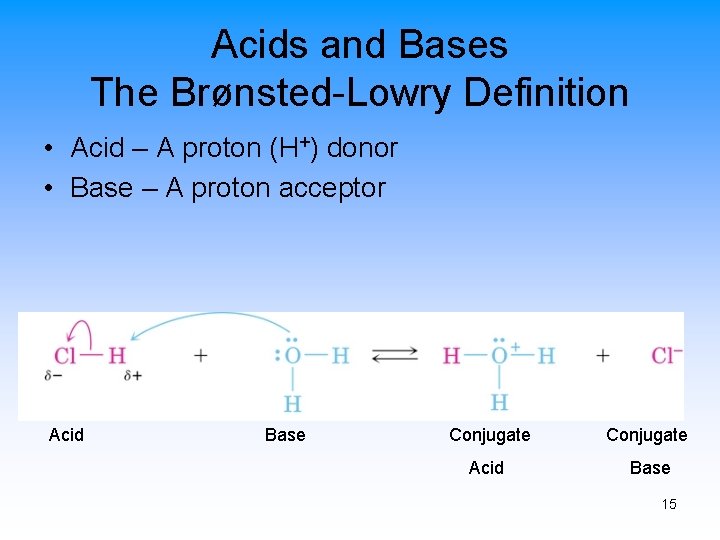
Acids and Bases The Brønsted-Lowry Definition • Acid – A proton (H+) donor • Base – A proton acceptor Acid Base Conjugate Acid Base 15

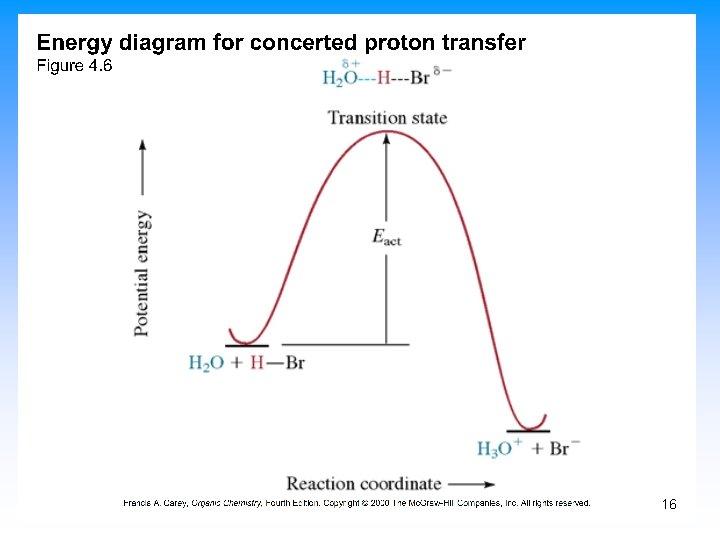
16
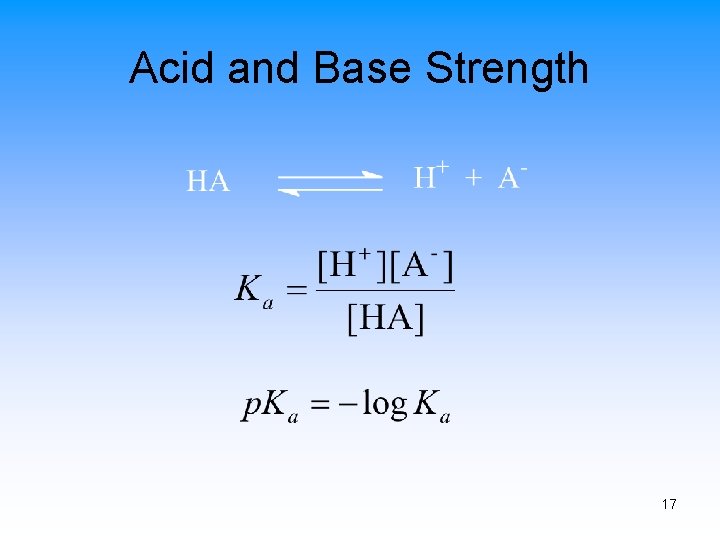
Acid and Base Strength 17

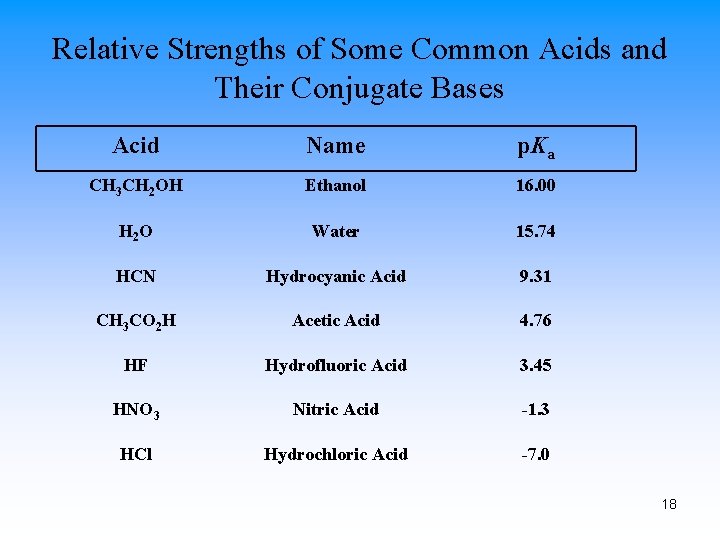
Relative Strengths of Some Common Acids and Their Conjugate Bases Acid Name p. Ka CH 3 CH 2 OH Ethanol 16. 00 H 2 O Water 15. 74 HCN Hydrocyanic Acid 9. 31 CH 3 CO 2 H Acetic Acid 4. 76 HF Hydrofluoric Acid 3. 45 HNO 3 Nitric Acid -1. 3 HCl Hydrochloric Acid -7. 0 18

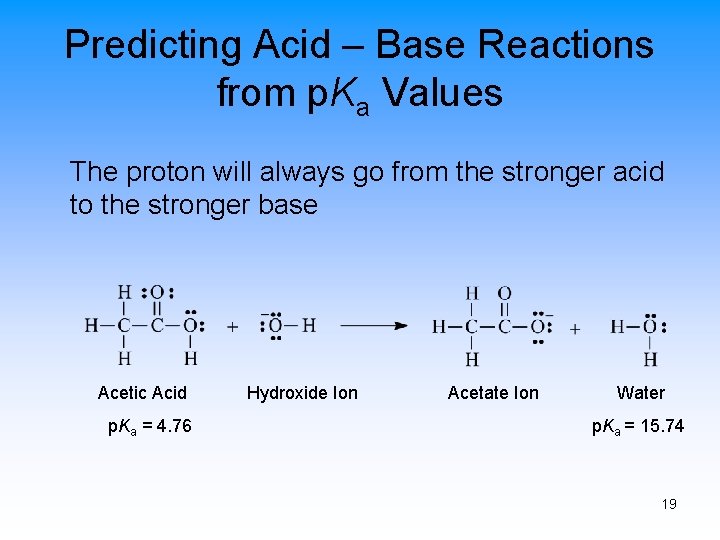
Predicting Acid – Base Reactions from p. Ka Values The proton will always go from the stronger acid to the stronger base Acetic Acid p. Ka = 4. 76 Hydroxide Ion Acetate Ion Water p. Ka = 15. 74 19

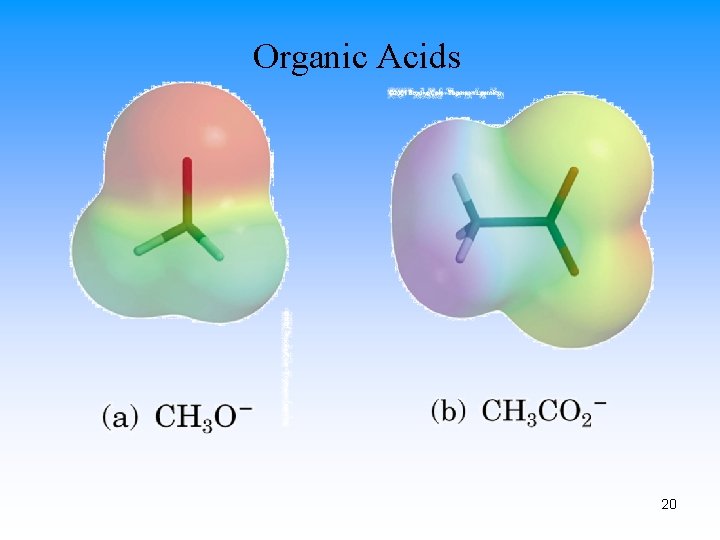
Organic Acids 20

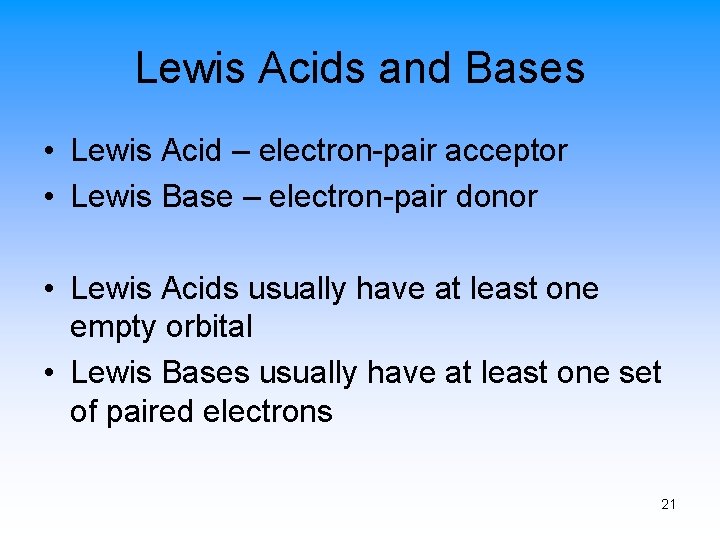
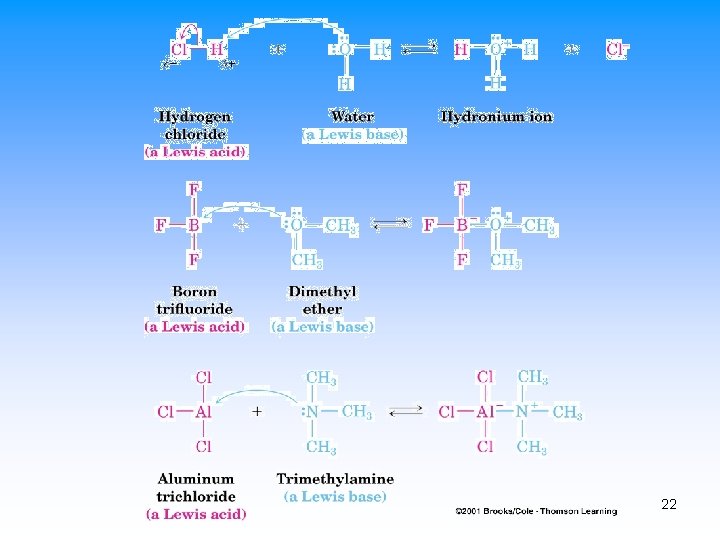
Lewis Acids and Bases • Lewis Acid – electron-pair acceptor • Lewis Base – electron-pair donor • Lewis Acids usually have at least one empty orbital • Lewis Bases usually have at least one set of paired electrons 21

22

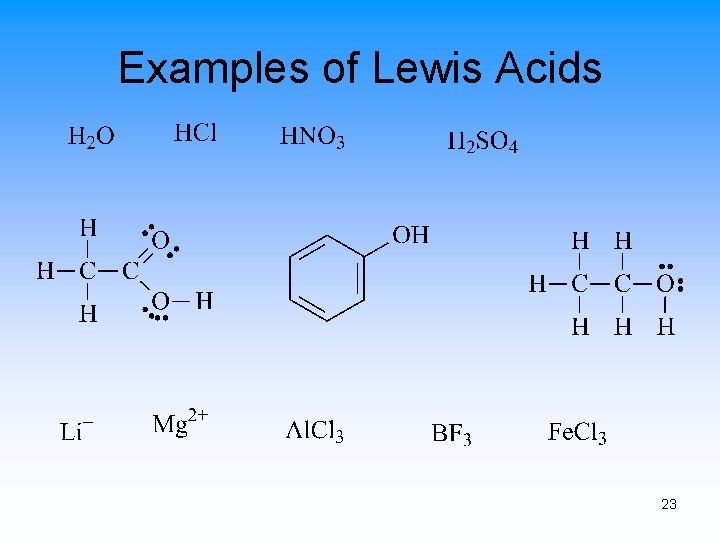
Examples of Lewis Acids 23

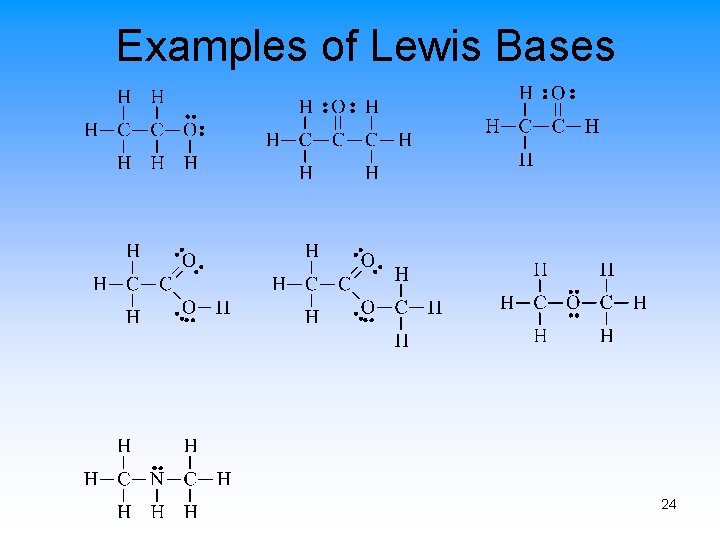
Examples of Lewis Bases 24

Kinds of Organic Reactions • In general, we look at what occurs and try to learn how it happens • Common patterns describe the changes – Addition reactions – two molecules combine – Elimination reactions – one molecule splits into two – Substitution – parts from two molecules exchange – Rearrangement reactions – a molecule undergoes changes in the way its atoms are connected 25

How Organic Reactions Occur: Mechanisms • In a clock the hands move but the mechanism behind the face is what causes the movement • In an organic reaction, we see the transformation that has occurred. The mechanism describes the steps behind the changes that we can observe • Reactions occur in defined steps that lead from reactant to product 26

Steps in Mechanisms • We classify the types of steps in a sequence • A step involves either the formation or breaking of a covalent bond • Steps can occur in individually or in combination with other steps • When several steps occur at the same time they are said to be concerted 27

Types of Steps in Reaction Mechanisms • Formation of a covalent bond – Homogenic or heterogenic • Breaking of a covalent bond – Homogenic or heterogenic • Oxidation of a functional group • Reduction of a functional group 28

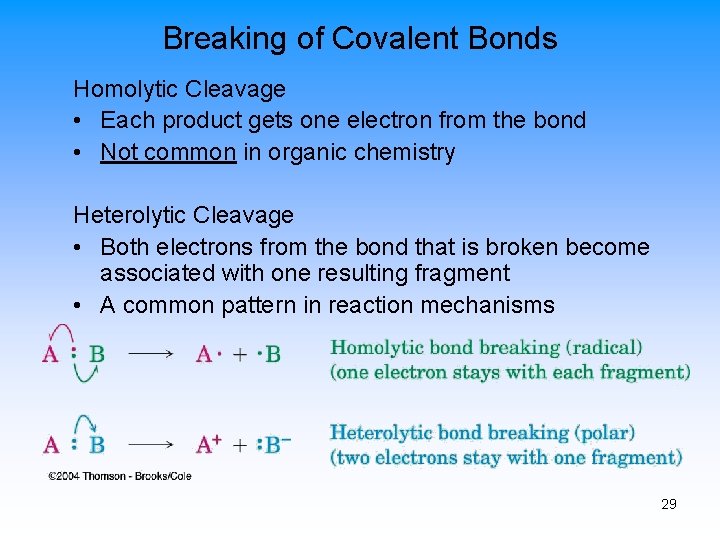
Breaking of Covalent Bonds Homolytic Cleavage • Each product gets one electron from the bond • Not common in organic chemistry Heterolytic Cleavage • Both electrons from the bond that is broken become associated with one resulting fragment • A common pattern in reaction mechanisms 29

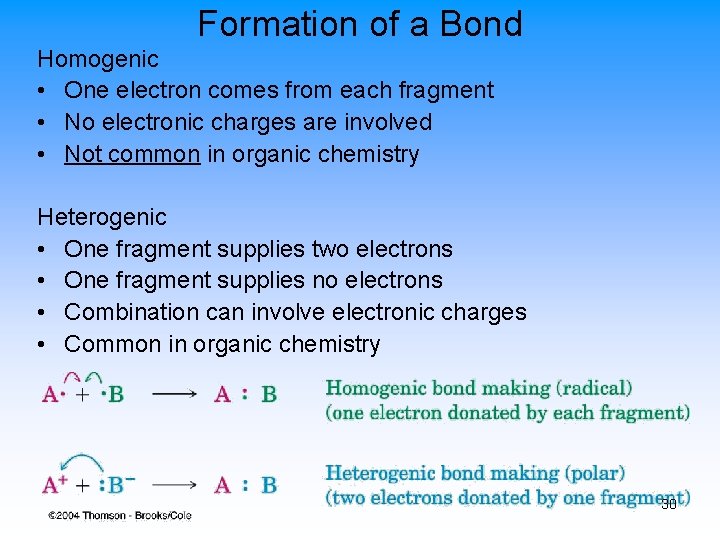
Formation of a Bond Homogenic • One electron comes from each fragment • No electronic charges are involved • Not common in organic chemistry Heterogenic • One fragment supplies two electrons • One fragment supplies no electrons • Combination can involve electronic charges • Common in organic chemistry 30

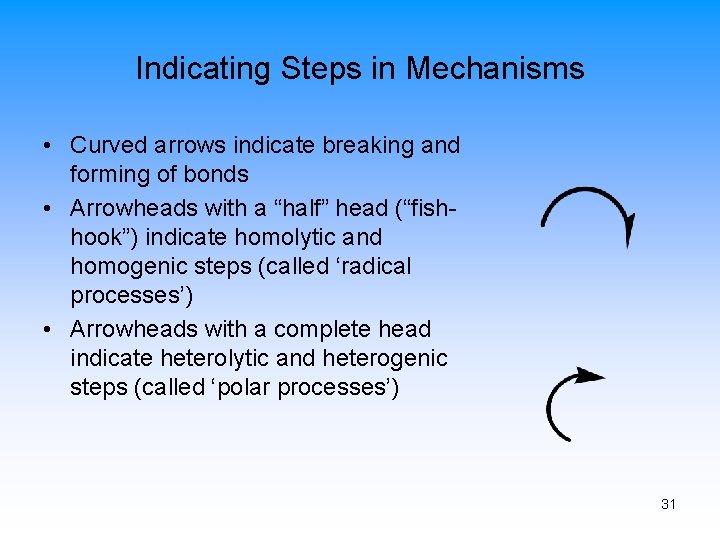
Indicating Steps in Mechanisms • Curved arrows indicate breaking and forming of bonds • Arrowheads with a “half” head (“fishhook”) indicate homolytic and homogenic steps (called ‘radical processes’) • Arrowheads with a complete head indicate heterolytic and heterogenic steps (called ‘polar processes’) 31

5. 6 Using Curved Arrows in Polar Reaction Mechanisms • Curved arrows are a way to keep track of changes in bonding in polar reaction • The arrows track “electron movement” • Electrons always move in pairs • Charges change during the reaction • One curved arrow corresponds to one step in a reaction mechanism 32

5. 4 Polar Reactions and How They Occur • Molecules can contain local unsymmetrical electron distributions due to differences in electronegativities • This causes a partial negative charge on an atom and a compensating partial positive charge on an adjacent atom • The more electronegative atom has the greater electron density 33

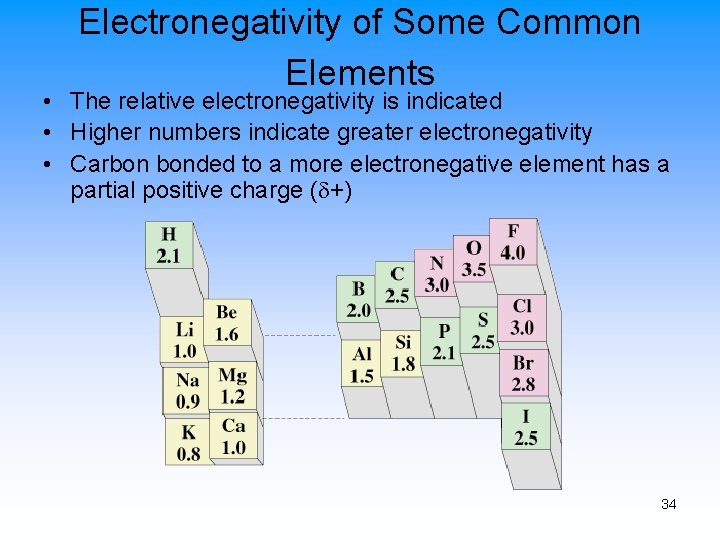
Electronegativity of Some Common Elements • The relative electronegativity is indicated • Higher numbers indicate greater electronegativity • Carbon bonded to a more electronegative element has a partial positive charge ( +) 34

Polarizability • Polarization is a change in electron distribution as a response to change in electronic nature of the surroundings • Polarizability is the tendency to undergo polarization • Polar reactions occur between regions of high electron density and regions of low electron density 35

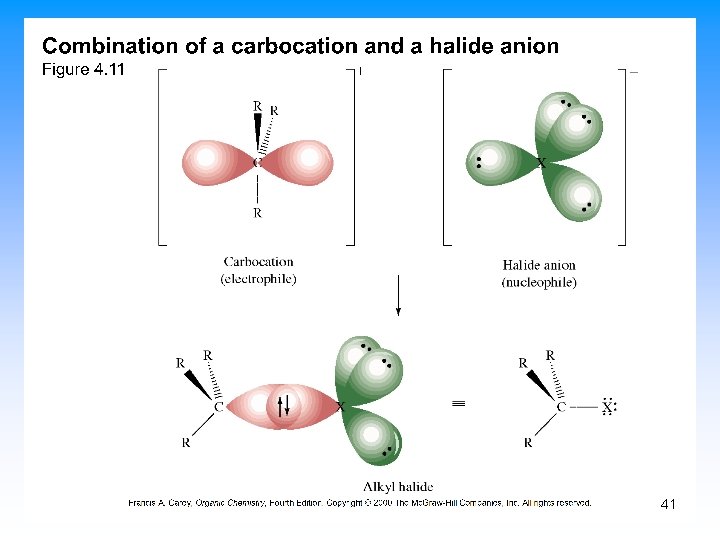
Generalized Polar Reactions • An electrophile, an electron-poor species, combines with a nucleophile, an electron-rich species • An electrophile is a Lewis acid • A nucleophile is a Lewis base • The combination is indicate with a curved arrow from nucleophile to electrophile 36

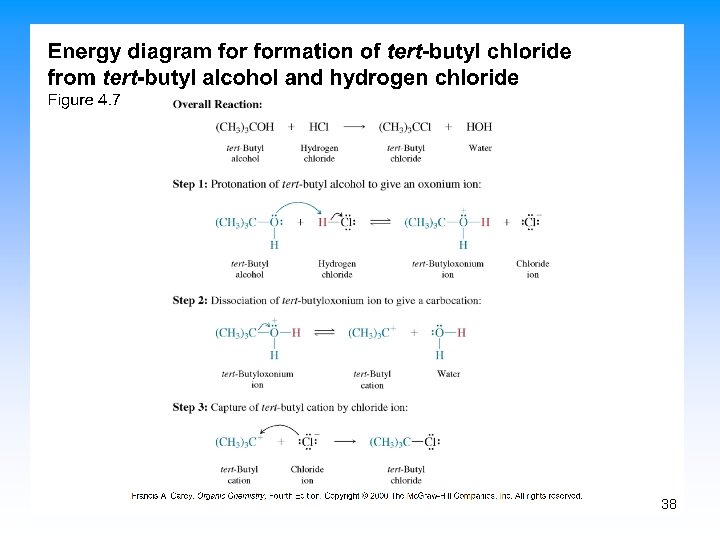
10. 7 Preparing Alkyl Halides from Alcohols • Reaction of tertiary C-OH with HX is fast and effective – Add HCl or HBr gas into ether solution of tertiary alcohol • Primary and secondary alcohols react very slowly and often rearrange, so alternative methods are used 37

38

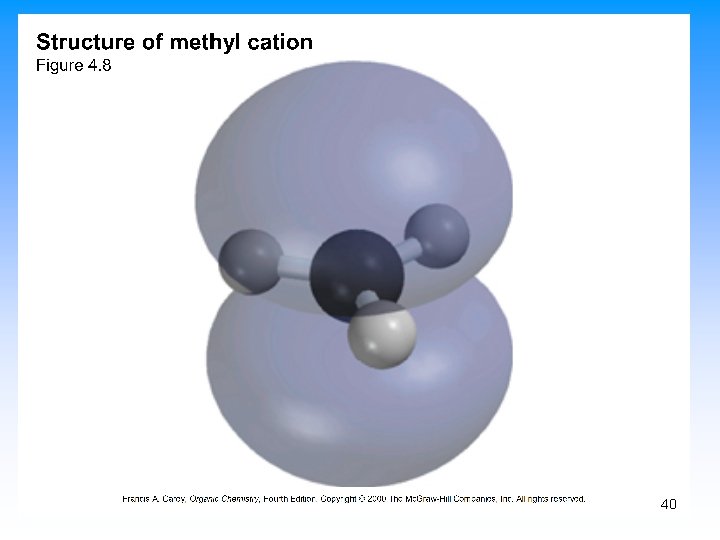
5. 10 Describing a Reaction: Intermediates • If a reaction occurs in more than one step, it must involve species that are neither the reactant nor the final product • These are called reaction intermediates or simply “intermediates” • Each step has its own free energy of activation • The complete diagram for the reaction shows the free energy changes associated with an intermediate 39

40

41