SHARPLESS ASYMMETRIC EPOXIDATION Chapter 6 ALKYL HALIDES NUCLEOPHILIC






- Slides: 14

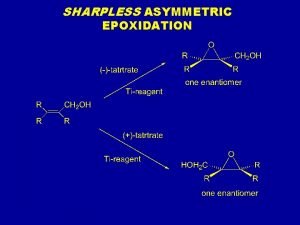
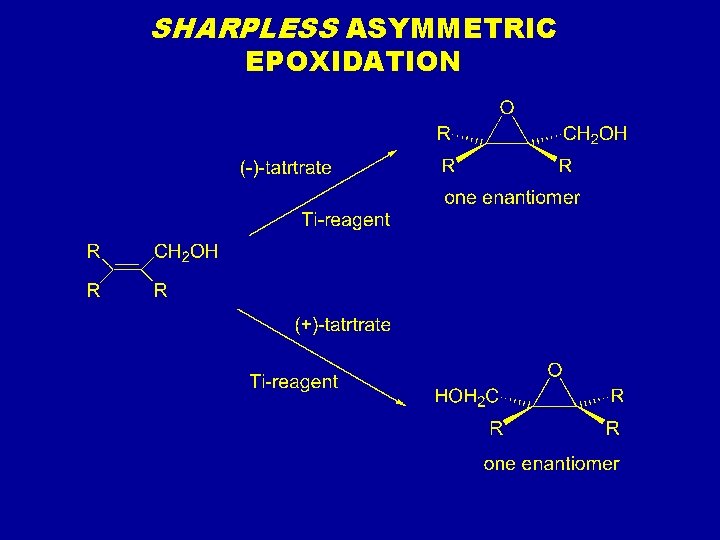
SHARPLESS ASYMMETRIC EPOXIDATION

Chapter 6 ALKYL HALIDES: NUCLEOPHILIC SUBSTITUTION AND ELIMINATION Chapter 6: Alkyl Halides: Nucleophilic Substitution and Elimination

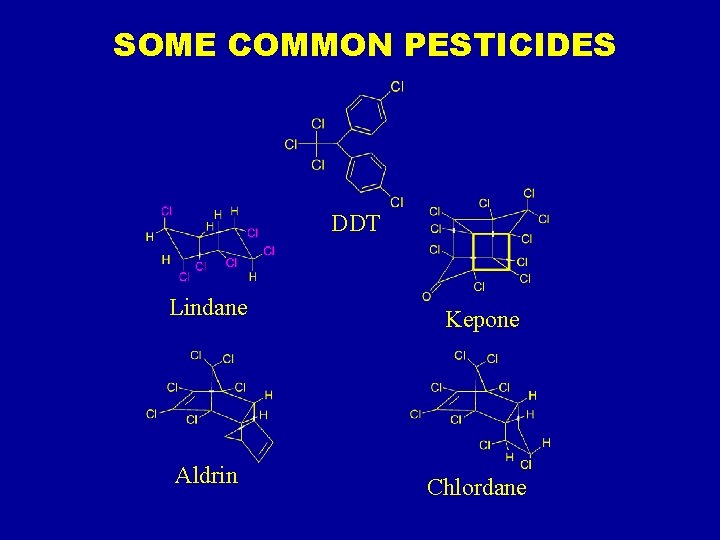
SOME COMMON PESTICIDES DDT Lindane Kepone Aldrin Chlordane

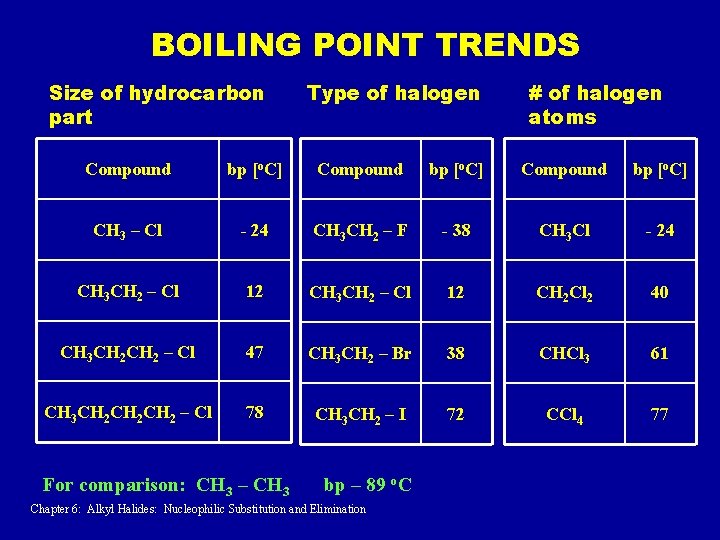
BOILING POINT TRENDS Size of hydrocarbon part Type of halogen # of halogen atoms Compound bp [o. C] CH 3 – Cl - 24 CH 3 CH 2 – F - 38 CH 3 Cl - 24 CH 3 CH 2 – Cl 12 CH 2 Cl 2 40 CH 3 CH 2 – Cl 47 CH 3 CH 2 – Br 38 CHCl 3 61 CH 3 CH 2 CH 2 – Cl 78 CH 3 CH 2 – I 72 CCl 4 77 For comparison: CH 3 – CH 3 bp – 89 o. C Chapter 6: Alkyl Halides: Nucleophilic Substitution and Elimination

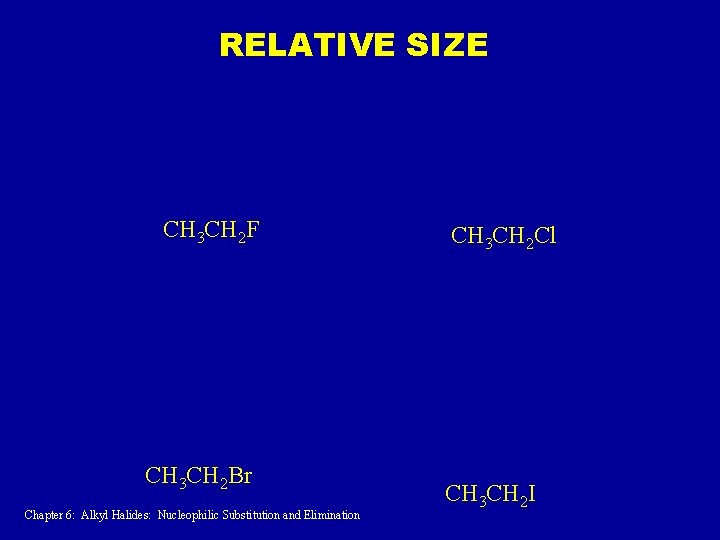
RELATIVE SIZE CH 3 CH 2 F CH 3 CH 2 Br Chapter 6: Alkyl Halides: Nucleophilic Substitution and Elimination CH 3 CH 2 Cl CH 3 CH 2 I

COMPARISON OF SN 1 AND SN 2 REACTIONS 1. Effect of Nucleophile: A. SN 2: Strong nucleophiles B. SN 1: Irrelevant, since nucleophile does not participate in rate-determining step 2. Effect of Substrate: A. SN 2: CH 3 > 1 o > 2 o (3 o is sterically too hindered) B. SN 1: 3 o > 2 o (1 o or CH 3 do not easily form cations) 3. Effect of Solvent: A. SN 2: Promoted by polar aprotic solvents or non-polar solvents B. SN 1: Promoted by polar protic solvents

COMPARISON OF SN 1 AND SN 2 REACTIONS 4. Leaving Group Effect: A. Both SN 2 and SN 1 require good leaving groups – higher electronegativity and polarizability, weak bases 5. Kinetics: A. SN 2: A bimolecular reaction: Rate = k[RX][Nuc] B. SN 1: A monomolecular reaction: Rate = k[RX] 6. Stereochemistry: A. SN 2: Inversion of configuration B. SN 1: Complete or partial racemization 7. Rearrangements: A. SN 2: Rearrangements not possible (because it is a concerted process) B. SN 1: Rearrangements are common (because the intermediate cation often rearranges to a more stable cation)

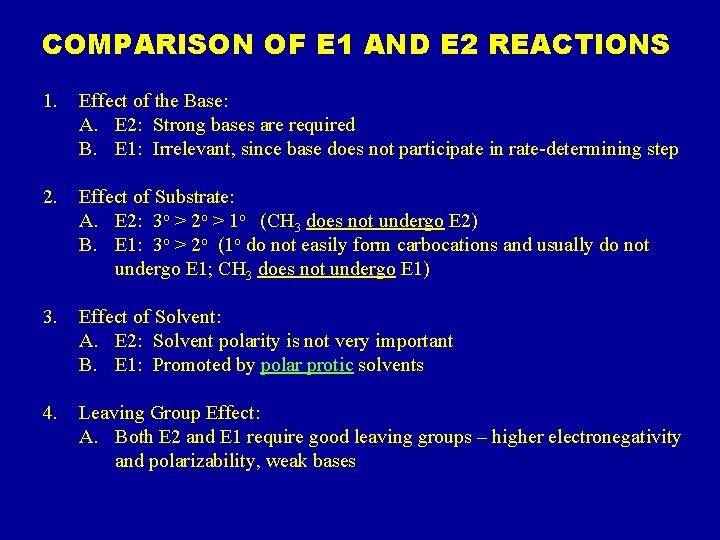
COMPARISON OF E 1 AND E 2 REACTIONS 1. Effect of the Base: A. E 2: Strong bases are required B. E 1: Irrelevant, since base does not participate in rate-determining step 2. Effect of Substrate: A. E 2: 3 o > 2 o > 1 o (CH 3 does not undergo E 2) B. E 1: 3 o > 2 o (1 o do not easily form carbocations and usually do not undergo E 1; CH 3 does not undergo E 1) 3. Effect of Solvent: A. E 2: Solvent polarity is not very important B. E 1: Promoted by polar protic solvents 4. Leaving Group Effect: A. Both E 2 and E 1 require good leaving groups – higher electronegativity and polarizability, weak bases

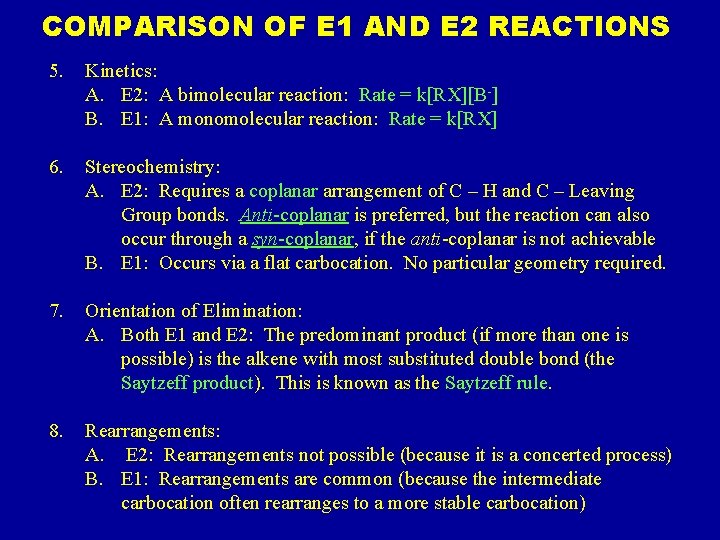
COMPARISON OF E 1 AND E 2 REACTIONS 5. Kinetics: A. E 2: A bimolecular reaction: Rate = k[RX][B-] B. E 1: A monomolecular reaction: Rate = k[RX] 6. Stereochemistry: A. E 2: Requires a coplanar arrangement of C – H and C – Leaving Group bonds. Anti-coplanar is preferred, but the reaction can also occur through a syn-coplanar, if the anti-coplanar is not achievable B. E 1: Occurs via a flat carbocation. No particular geometry required. 7. Orientation of Elimination: A. Both E 1 and E 2: The predominant product (if more than one is possible) is the alkene with most substituted double bond (the Saytzeff product). This is known as the Saytzeff rule. 8. Rearrangements: A. E 2: Rearrangements not possible (because it is a concerted process) B. E 1: Rearrangements are common (because the intermediate carbocation often rearranges to a more stable carbocation)

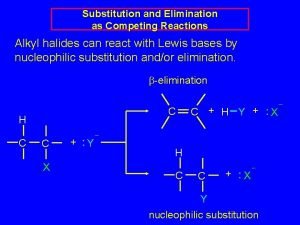
SUBSTITUTION VERSUS ELIMINATION 1. Strong nucleophile and a methyl substrate: SN 2 reaction. 2. Strong nucleophile (base) and primary substrate (1 o): An SN 2 reaction is most likely. Some E 2 product might be obtained as well. 3. Strong nucleophile (base) and secondary substrate (2 o): Both SN 2 and E 2 reactions will occur and a mixture of substitution and elimination products is likely. Difficult to predict whether SN 2 or E 2 will predominate. 4. Strong nucleophile (base) and tertiary substrate (3 o): An E 2 reaction. SN 2 does not occur since substrate is too sterically hindered.

SUBSTITUTION VERSUS ELIMINATION 5. Weak nucleophile (base) and methyl substrate: No reaction. 6. Weak nucleophile (base) and primary substrate (1 o): No reaction. EXCEPTION: If the primary substrate can undergo a concerted process of ionization + rearrangement to give a more stable carbocation (as is the case with neopentyl substrates), then the outcome is a mixture of SN 1 and E 1. 7. Weak nucleophile (base) and secondary substrate (2 o): Both SN 1 and E 1 will occur and a mixture of substitution and elimination products is likely. 8. Weak nucleophile (base) and tertiary substrate (3 o): Both SN 1 and E 1.

SUBSTITUTION VERSUS ELIMINATION ONE GENERAL CONCLUSION: With strong nucleophiles (bases) the reactions occur via bimolecular mechanism: SN 2 or E 2. With weak nucleophiles (bases) the reactions occur via monomolecular mechanism: SN 1 or E 1.

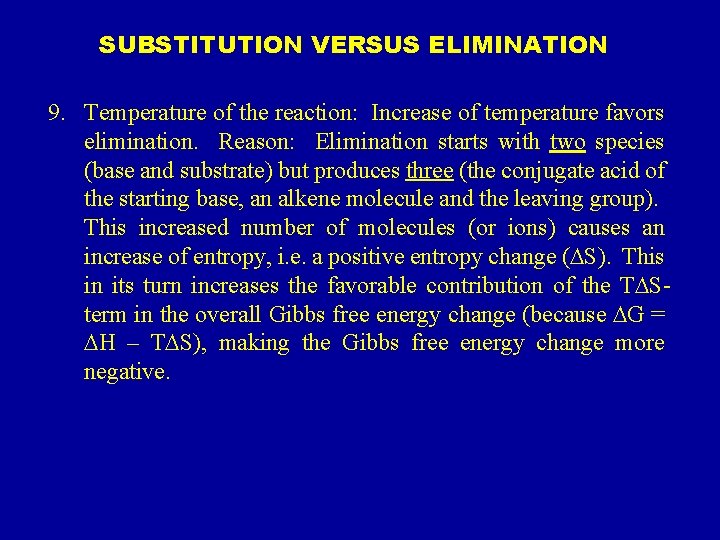
SUBSTITUTION VERSUS ELIMINATION 9. Temperature of the reaction: Increase of temperature favors elimination. Reason: Elimination starts with two species (base and substrate) but produces three (the conjugate acid of the starting base, an alkene molecule and the leaving group). This increased number of molecules (or ions) causes an increase of entropy, i. e. a positive entropy change (DS). This in its turn increases the favorable contribution of the TDSterm in the overall Gibbs free energy change (because DG = DH – TDS), making the Gibbs free energy change more negative.

SUBSTITUTION VERSUS ELIMINATION 10. Steric bulk of the nucleophile (base): In concerted reactions (SN 2 or E 2) the nucleophile (base) participates in the ratedetermining step. It has to approach closely the molecule and attack the electrophilic carbon center (then it is a nucleophile) or the proton at an adjacent carbon (then it is a base). Approaching closely the carbon center is more sensitive to changes of size. That is why the share (percentage) of elimination increases with an increasing size of the nucleophile (base).
 Sharpless asymmetric epoxidation
Sharpless asymmetric epoxidation Antiperiplanar
Antiperiplanar Nucleophilicity trend
Nucleophilicity trend Nucleophilic substitution of alkyl halides
Nucleophilic substitution of alkyl halides Alkyl halide examples
Alkyl halide examples Reaction of alkyl halides
Reaction of alkyl halides Alkyl halide examples
Alkyl halide examples Alkyl halide examples
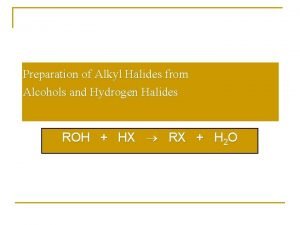
Alkyl halide examples Preparation of alkyl halides from alcohols
Preparation of alkyl halides from alcohols Naming alkyl halides
Naming alkyl halides Bulky base
Bulky base Nomenclature of alkyl halides
Nomenclature of alkyl halides Idroborazione ossidazione
Idroborazione ossidazione Epossidazione di sharpless
Epossidazione di sharpless Electrophilic substitution of pyrrole
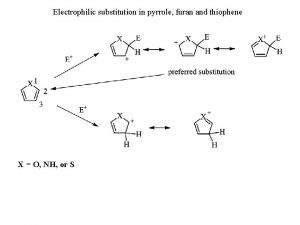
Electrophilic substitution of pyrrole