Alkyl Halides Session 34 Alkyl Halides Are compounds






- Slides: 14

Alkyl Halides (Session 34)

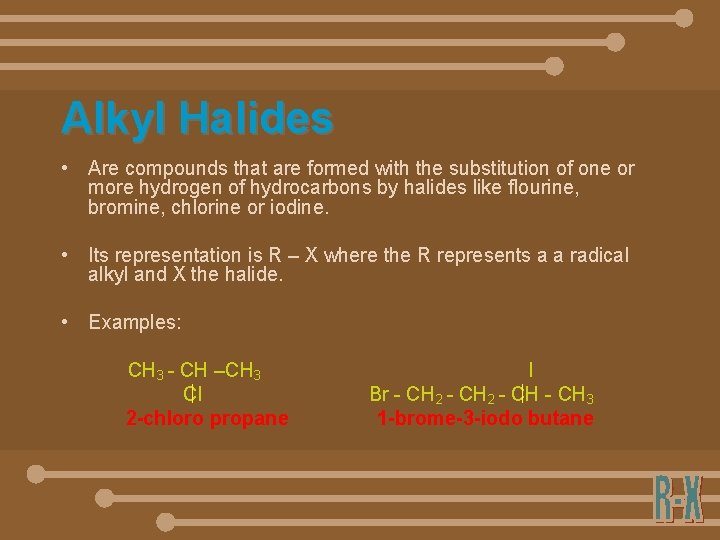
Alkyl Halides • Are compounds that are formed with the substitution of one or more hydrogen of hydrocarbons by halides like flourine, bromine, chlorine or iodine. • Its representation is R – X where the R represents a a radical alkyl and X the halide. • Examples: CH 3 - CH –CH 3 Cl 2 -chloro propane I Br - CH 2 - CH 3 1 -brome-3 -iodo butane

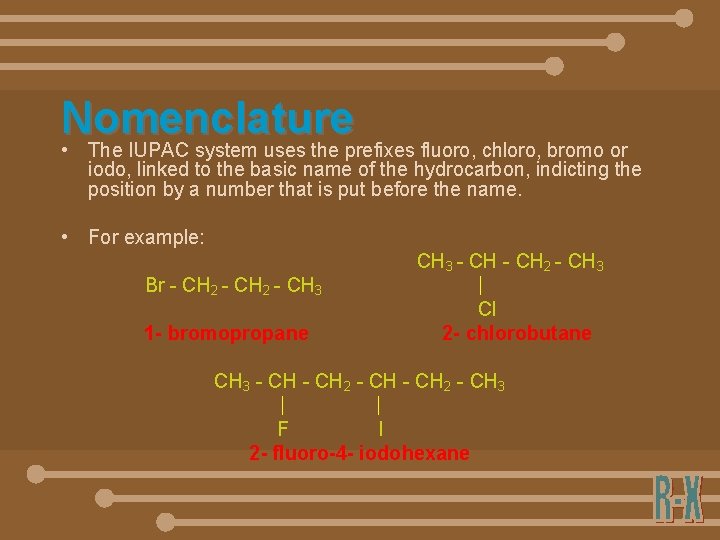
Nomenclature • The IUPAC system uses the prefixes fluoro, chloro, bromo or iodo, linked to the basic name of the hydrocarbon, indicting the position by a number that is put before the name. • For example: Br - CH 2 - CH 3 1 - bromopropane CH 3 - CH 2 - CH 3 Cl 2 - chlorobutane CH 3 - CH 2 - CH 3 F I 2 - fluoro-4 - iodohexane

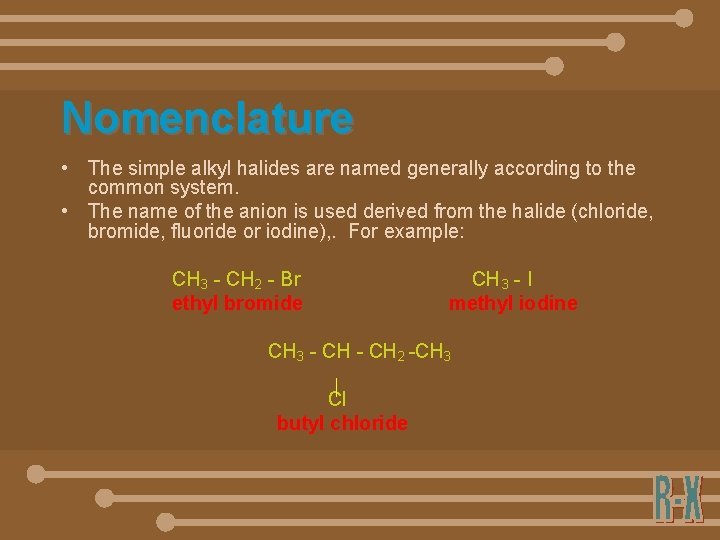
Nomenclature • The simple alkyl halides are named generally according to the common system. • The name of the anion is used derived from the halide (chloride, bromide, fluoride or iodine), . For example: CH 3 - CH 2 - Br ethyl bromide CH 3 - I methyl iodine CH 3 - CH 2 -CH 3 Cl butyl chloride

• Physical Properties The majority of R_X are liquids at room temperature with exception to three halomethanes (chloride, brome y fluorine) which are gases. • R-X present a dipolar moment due to it C-X link. • Their boiling points are higher compared with hydrocarbons with a similar molecular weight. • The boiling points increase when the size of the halide is increased. • There are insoluble in water. • The iodines and bromides of alkyl are more dense the water and the chlorides and fluorides are less dense than water.

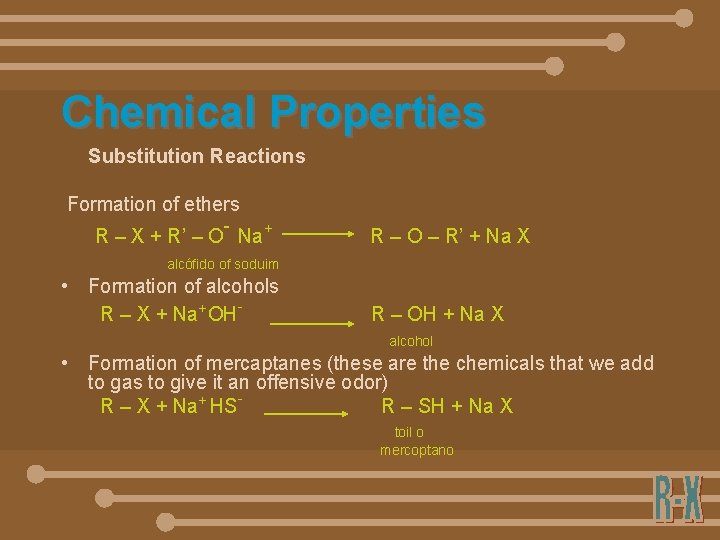
Chemical Properties Substitution Reactions Formation of ethers - R – X + R’ – O Na+ R – O – R’ + Na X alcófido of soduim • Formation of alcohols R – X + Na+OH- R – OH + Na X alcohol • Formation of mercaptanes (these are the chemicals that we add to gas to give it an offensive odor) R – X + Na+ HSR – SH + Na X toil o mercoptano

Importance and application of R-X • Anesthetics • Street lamps / Highway Lamps • Agents antihelminths (against intestinal parasites) Insecticides

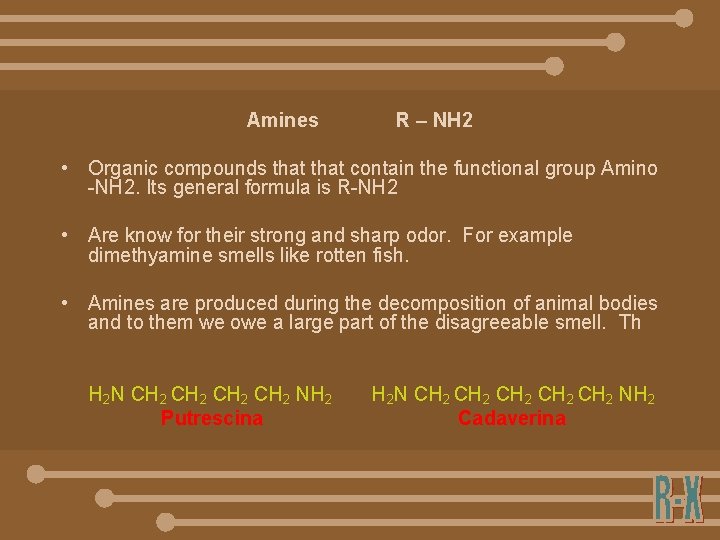
Amines R – NH 2 • Organic compounds that contain the functional group Amino -NH 2. Its general formula is R-NH 2 • Are know for their strong and sharp odor. For example dimethyamine smells like rotten fish. • Amines are produced during the decomposition of animal bodies and to them we owe a large part of the disagreeable smell. Th H 2 N CH 2 NH 2 Putrescina H 2 N CH 2 CH 2 NH 2 Cadaverina

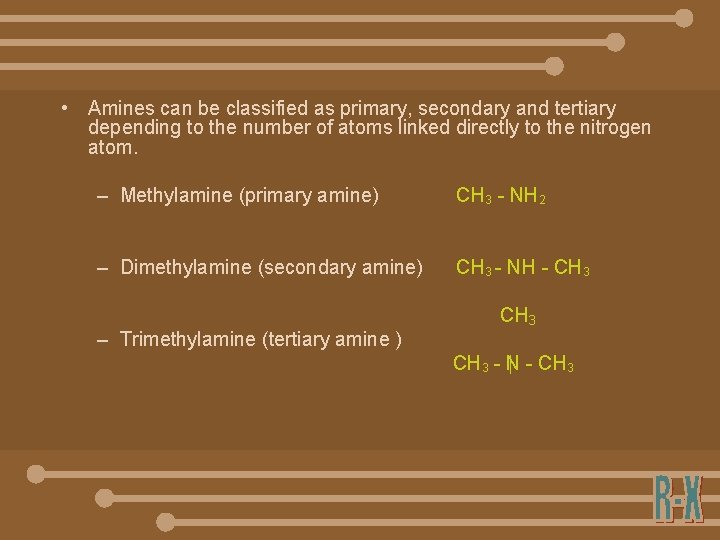
• Amines can be classified as primary, secondary and tertiary depending to the number of atoms linked directly to the nitrogen atom. – Methylamine (primary amine) CH 3 - NH 2 – Dimethylamine (secondary amine) CH 3 - NH - CH 3 – Trimethylamine (tertiary amine ) CH 3 - N - CH 3

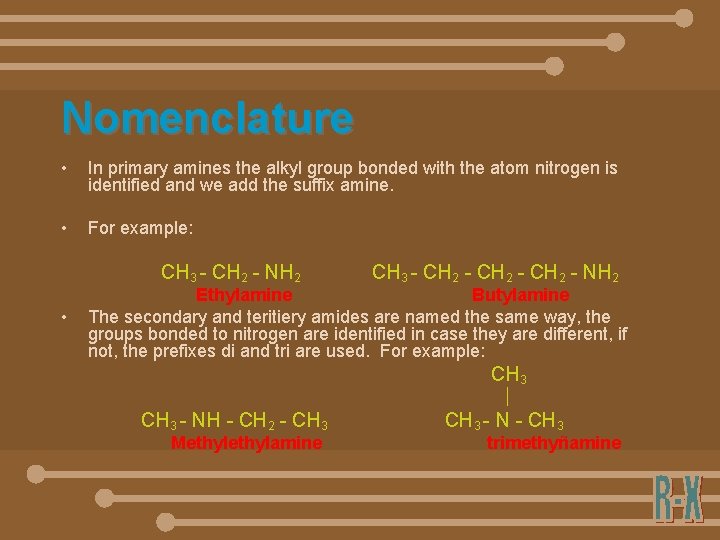
Nomenclature • In primary amines the alkyl group bonded with the atom nitrogen is identified and we add the suffix amine. • For example: CH 3 - CH 2 - NH 2 • CH 3 - CH 2 - NH 2 Ethylamine Butylamine The secondary and teritiery amides are named the same way, the groups bonded to nitrogen are identified in case they are different, if not, the prefixes di and tri are used. For example: CH 3 - NH - CH 2 - CH 3 Methylamine CH 3 - N - CH 3 trimethyñamine

Physical Properties • Amines of 1 or 4 carbon atoms are gases, the rest are liquid at room temperature. • Amines are polar compounds, the primary and secondary amines can form hydrogen bridges with water and other amines. • They have higher boiling points then alkenes with similar molecular weight. • Amines are short bonds of carbon atoms that are soluble in water.

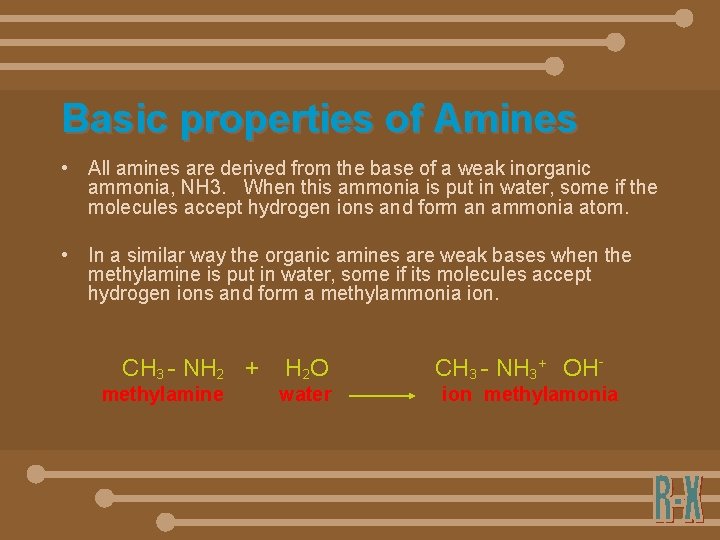
Basic properties of Amines • All amines are derived from the base of a weak inorganic ammonia, NH 3. When this ammonia is put in water, some if the molecules accept hydrogen ions and form an ammonia atom. • In a similar way the organic amines are weak bases when the methylamine is put in water, some if its molecules accept hydrogen ions and form a methylammonia ion. CH 3 - NH 2 + methylamine H 2 O water CH 3 - NH 3+ OHion methylamonia

Importance and application of Amines • Medicine (treatment for pathological depressions)

Bibliography • Bloomfield, M. (1997) Chemistry in Living Organisms. 1 st ed. Mexico: LIMUSA • Rakoff, H. et al. (1992) Fundamental Organic Chemistry. 1 st ed. Mexico: LIMUSA • Solomons, G. (1996) Fundaments of Organic Chemistry. 2 nd. ed. Mexico: LIMUSA