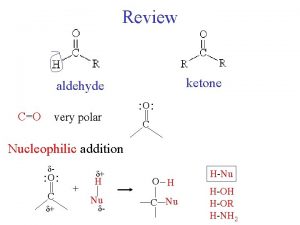
Chapter 7 2 Reactions of Alkyl Halides Nucleophilic

![Kinetics of Nucleophilic Substitution Rate = d[CH 3 Br]/dt = k[CH 3 Br][OH-1] This Kinetics of Nucleophilic Substitution Rate = d[CH 3 Br]/dt = k[CH 3 Br][OH-1] This](https://slidetodoc.com/presentation_image_h/f6f2e008a59b28ac1d4451623128fa14/image-8.jpg)










- Slides: 75

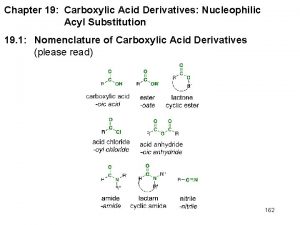
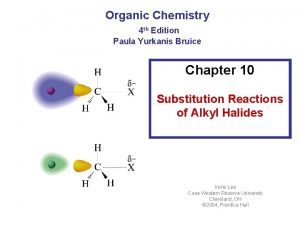
Chapter 7 -2. Reactions of Alkyl Halides: Nucleophilic Substitutions Based on Mc. Murry’s Organic Chemistry, 6 th edition

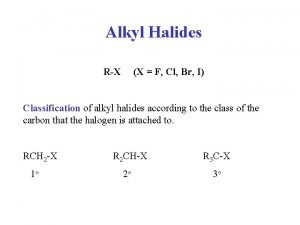
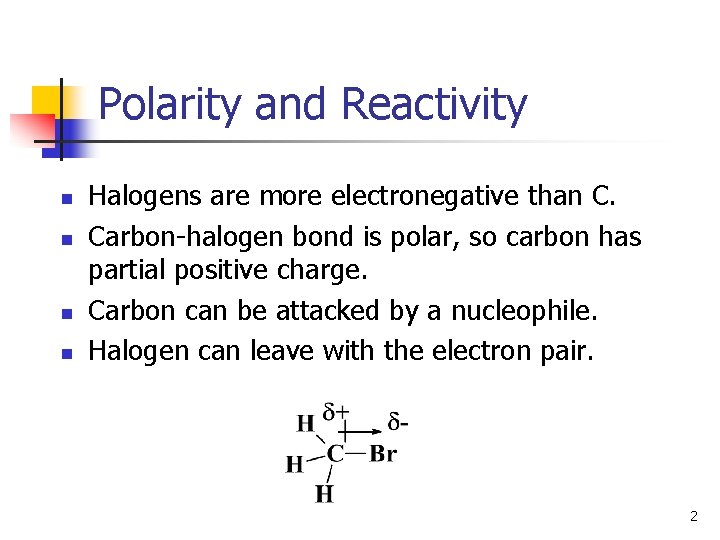
Polarity and Reactivity n n Halogens are more electronegative than C. Carbon-halogen bond is polar, so carbon has partial positive charge. Carbon can be attacked by a nucleophile. Halogen can leave with the electron pair. 2

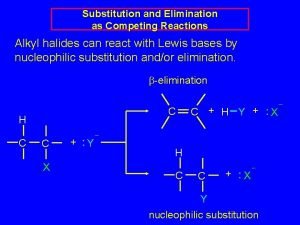
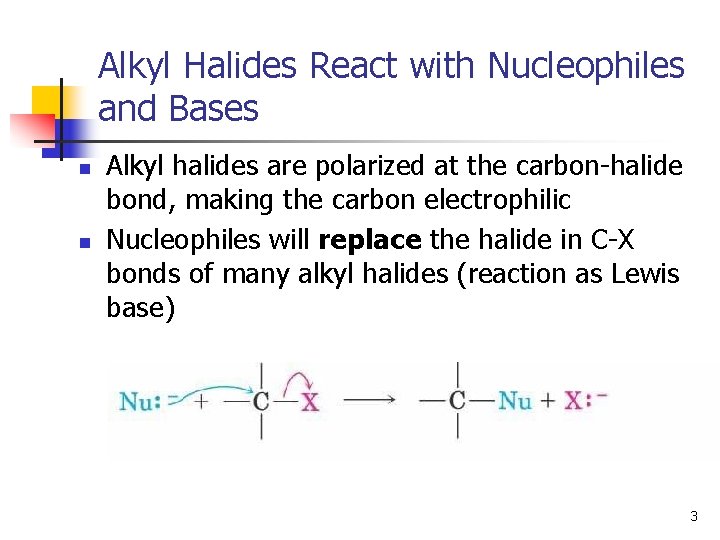
Alkyl Halides React with Nucleophiles and Bases n n Alkyl halides are polarized at the carbon-halide bond, making the carbon electrophilic Nucleophiles will replace the halide in C-X bonds of many alkyl halides (reaction as Lewis base) 3

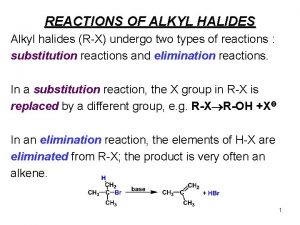
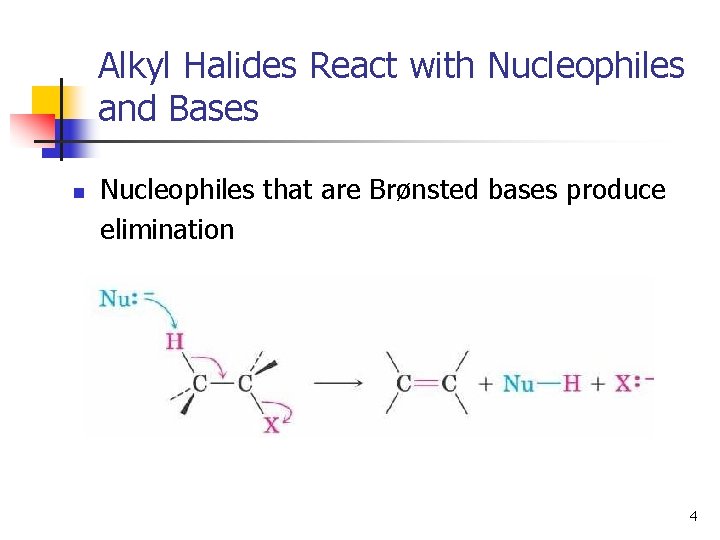
Alkyl Halides React with Nucleophiles and Bases n Nucleophiles that are Brønsted bases produce elimination 4

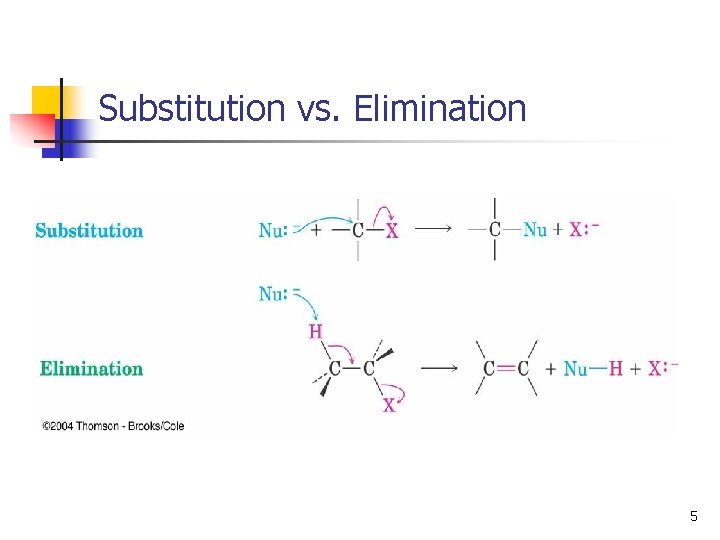
Substitution vs. Elimination 5

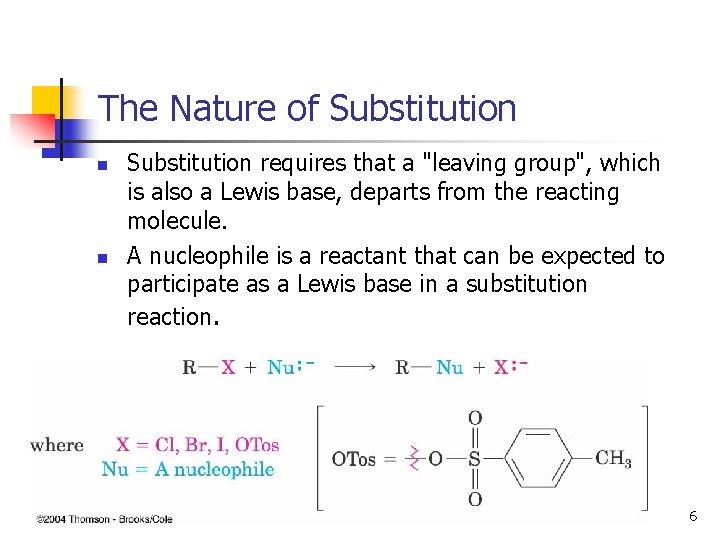
The Nature of Substitution n n Substitution requires that a "leaving group", which is also a Lewis base, departs from the reacting molecule. A nucleophile is a reactant that can be expected to participate as a Lewis base in a substitution reaction. 6

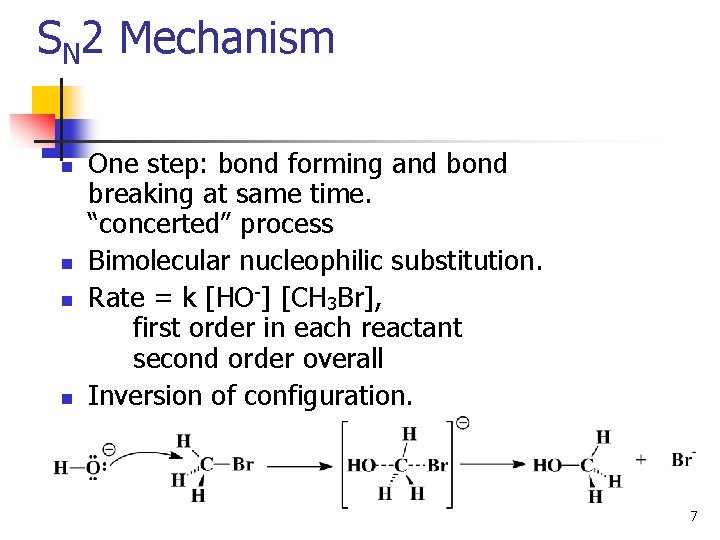
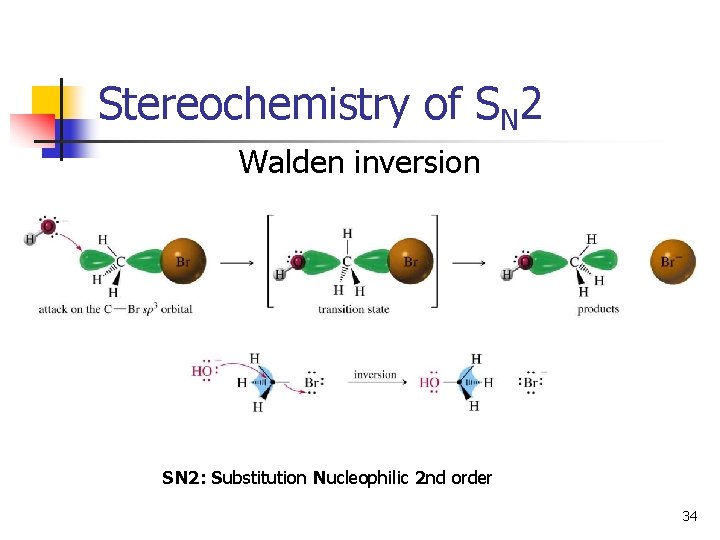
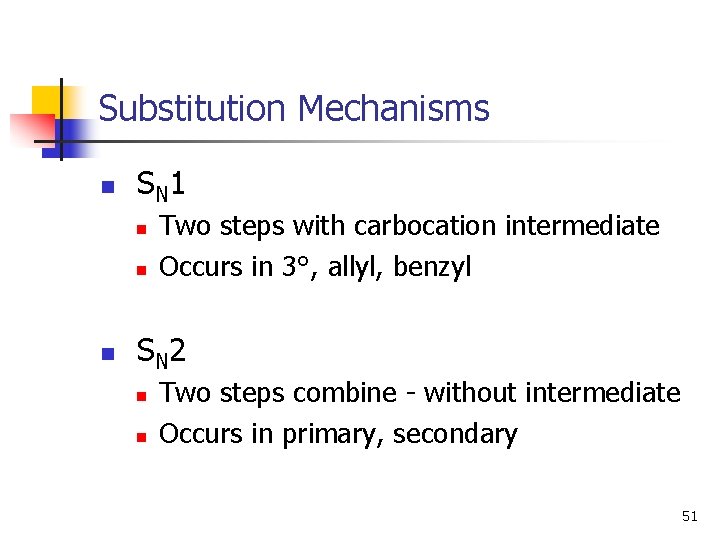
SN 2 Mechanism n n One step: bond forming and bond breaking at same time. “concerted” process Bimolecular nucleophilic substitution. Rate = k [HO-] [CH 3 Br], first order in each reactant second order overall Inversion of configuration. 7
![Kinetics of Nucleophilic Substitution Rate dCH 3 Brdt kCH 3 BrOH1 This Kinetics of Nucleophilic Substitution Rate = d[CH 3 Br]/dt = k[CH 3 Br][OH-1] This](https://slidetodoc.com/presentation_image_h/f6f2e008a59b28ac1d4451623128fa14/image-8.jpg)
Kinetics of Nucleophilic Substitution Rate = d[CH 3 Br]/dt = k[CH 3 Br][OH-1] This reaction is second order: two concentrations appear in the rate law SN 2: Substitution Nucleophilic 2 nd order 8

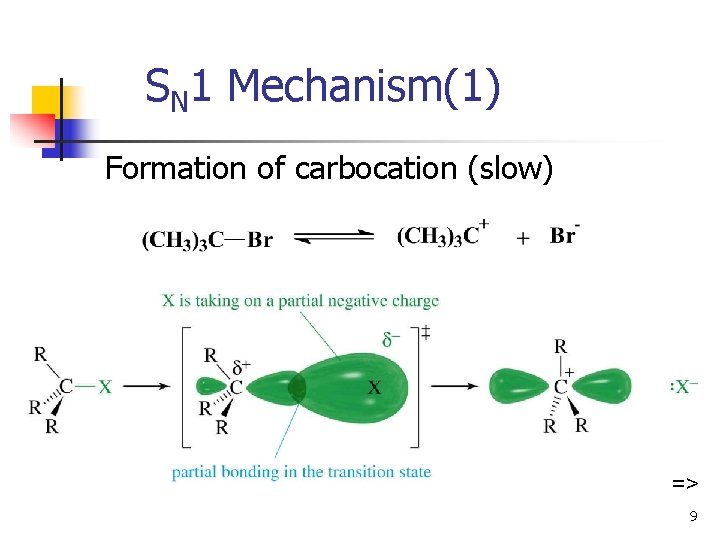
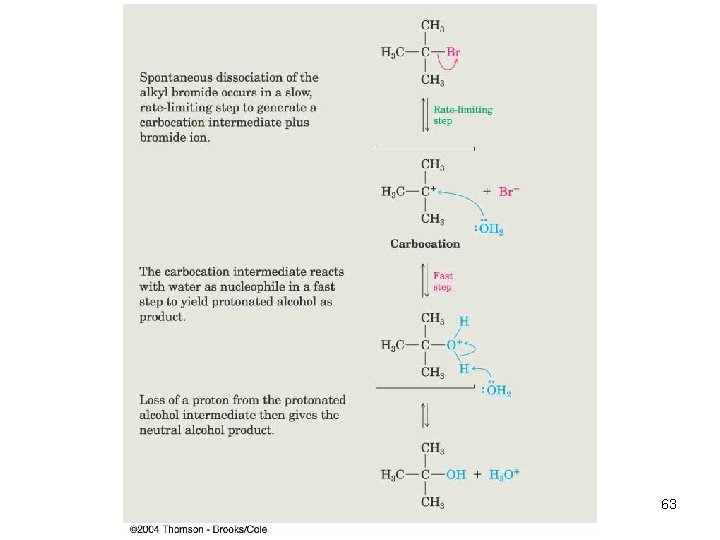
SN 1 Mechanism(1) Formation of carbocation (slow) => 9

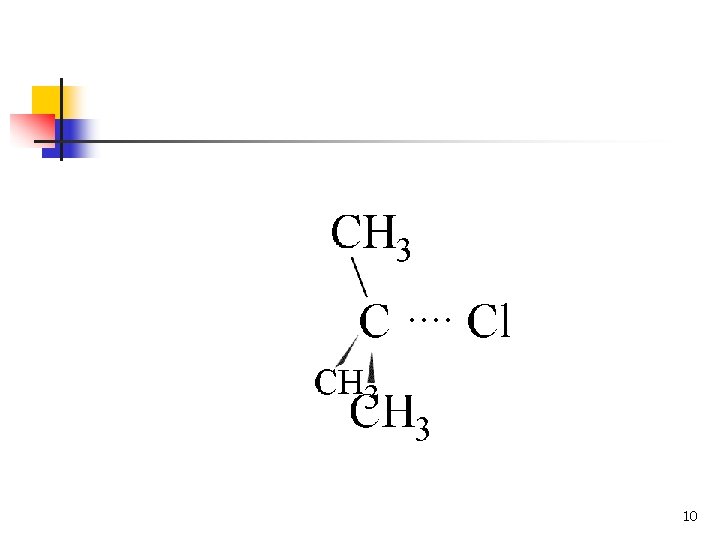
10

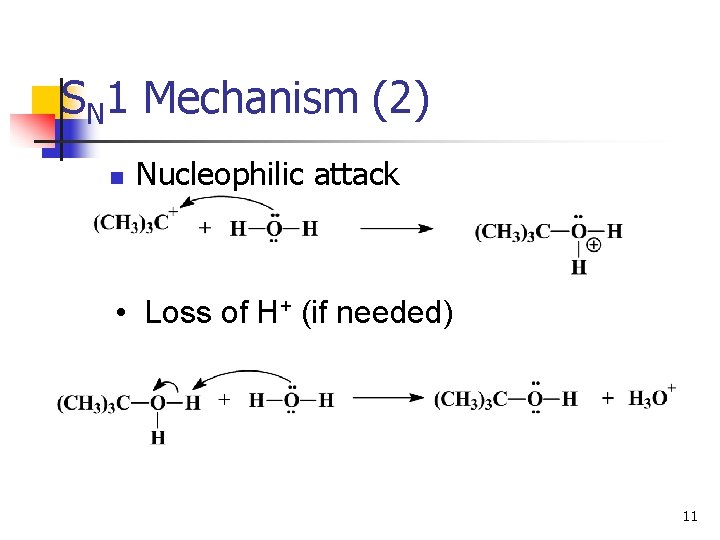
SN 1 Mechanism (2) n Nucleophilic attack • Loss of H+ (if needed) 11

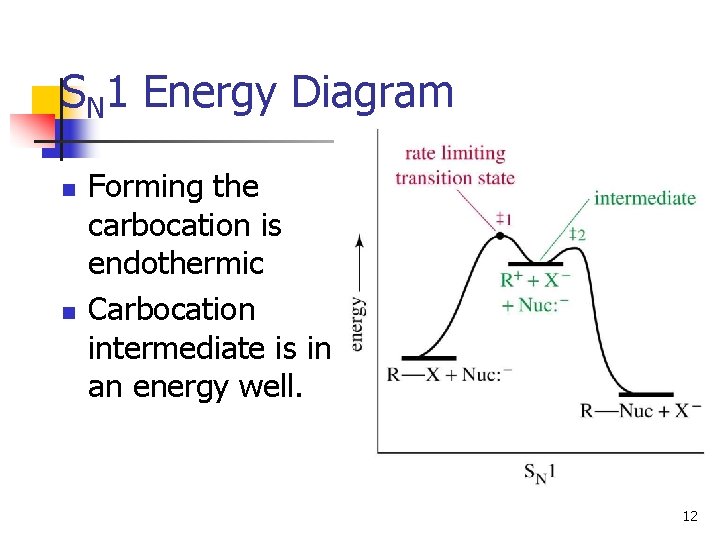
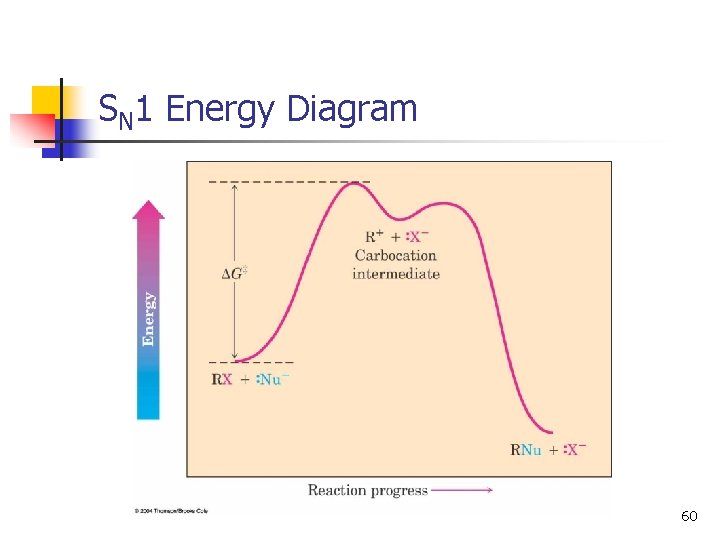
SN 1 Energy Diagram n n Forming the carbocation is endothermic Carbocation intermediate is in an energy well. 12

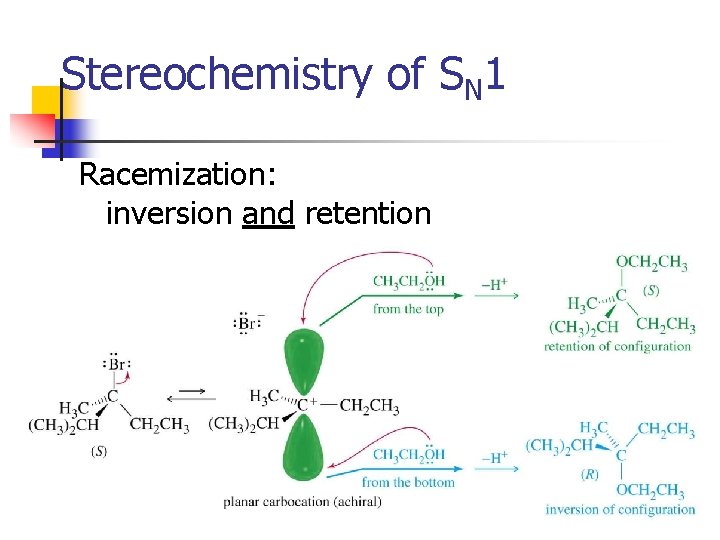
SN 1 Mechanism n n Unimolecular nucleophilic substitution. Two step reaction with carbocation intermediate. Rate = k [RX] first order in the alkyl halide zero order in the nucleophile. Racemization occurs. 13

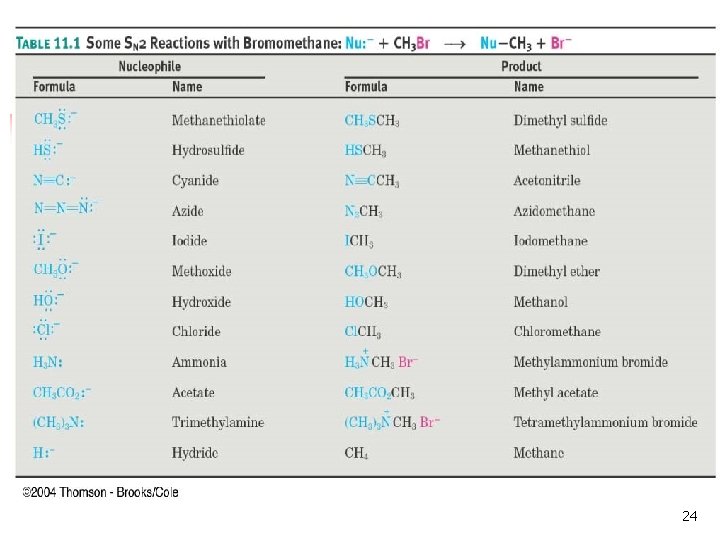
Factors affecting the rates of SN Reactions Concentration n Nature of the alkyl group n Nature of the nucleophile n Nature of the leaving group n Nature of the solvent n 14

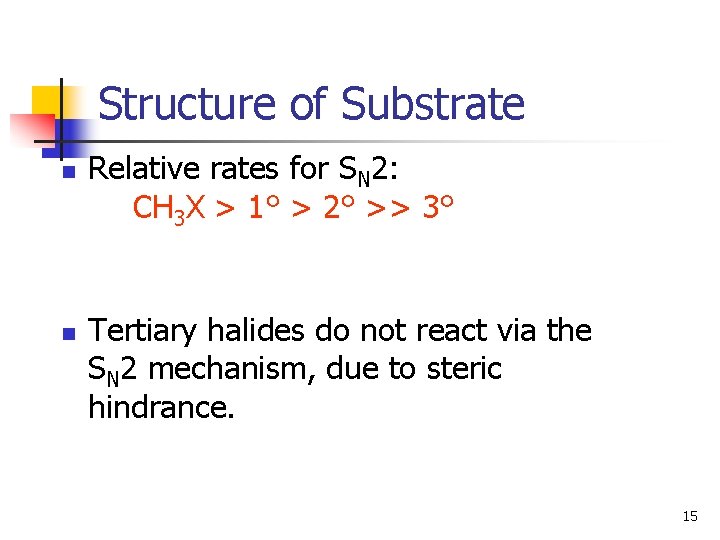
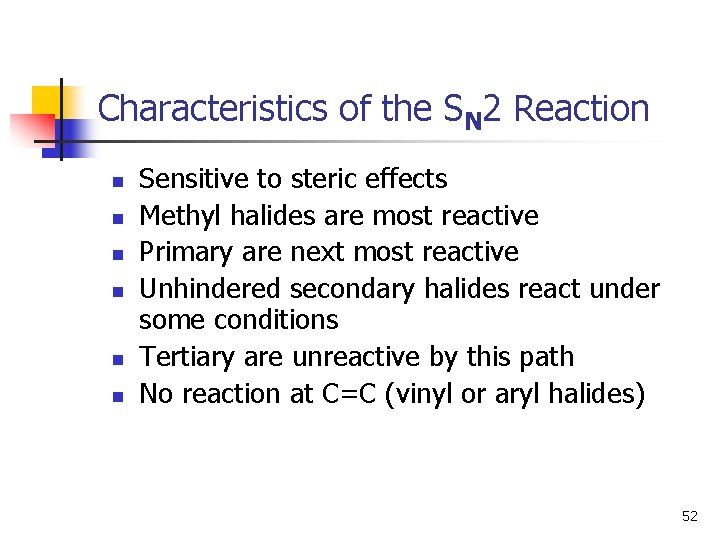
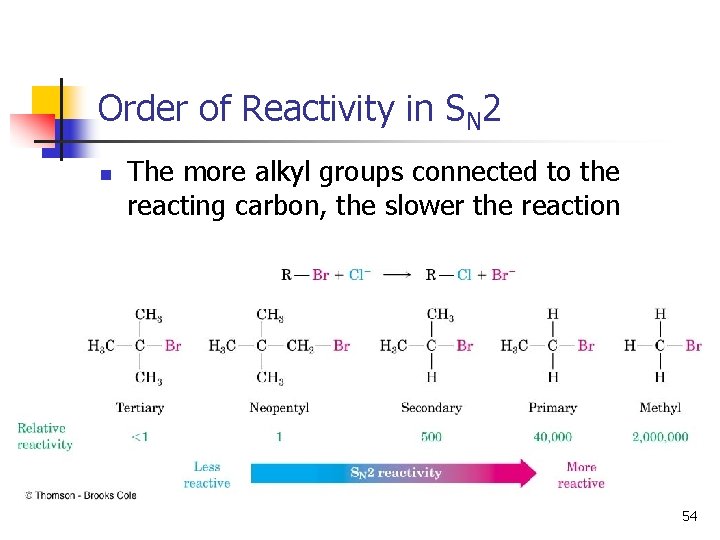
Structure of Substrate n n Relative rates for SN 2: CH 3 X > 1° > 2° >> 3° Tertiary halides do not react via the SN 2 mechanism, due to steric hindrance. 15

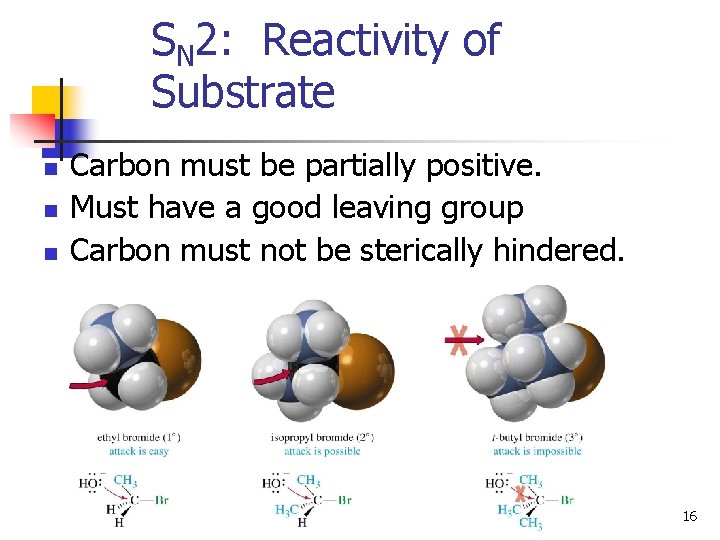
SN 2: Reactivity of Substrate n n n Carbon must be partially positive. Must have a good leaving group Carbon must not be sterically hindered. 16

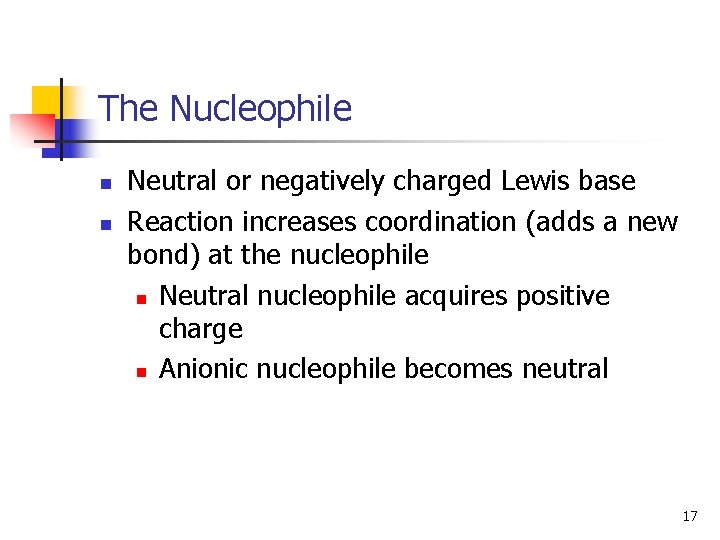
The Nucleophile n n Neutral or negatively charged Lewis base Reaction increases coordination (adds a new bond) at the nucleophile n Neutral nucleophile acquires positive charge n Anionic nucleophile becomes neutral 17

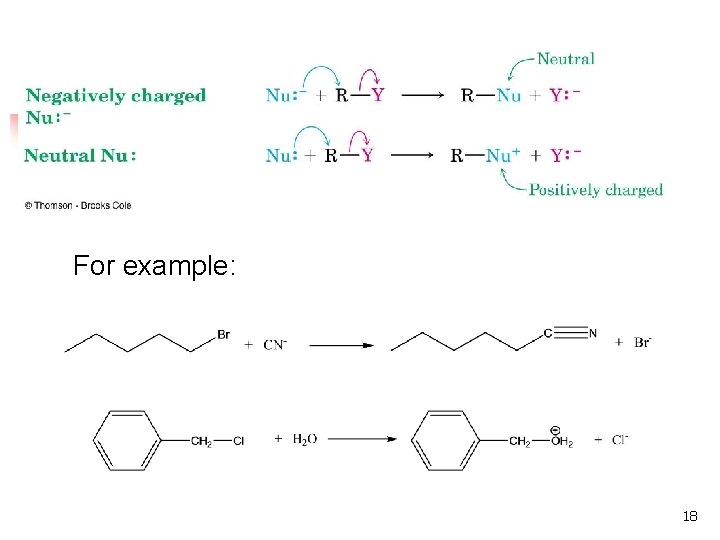
For example: 18

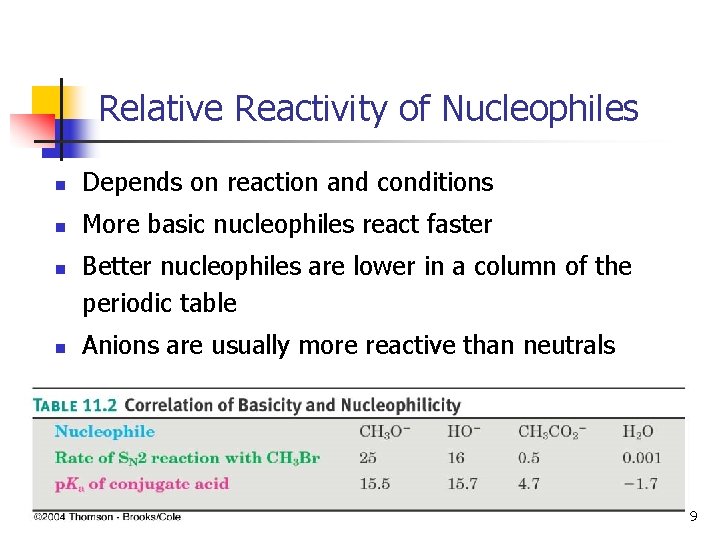
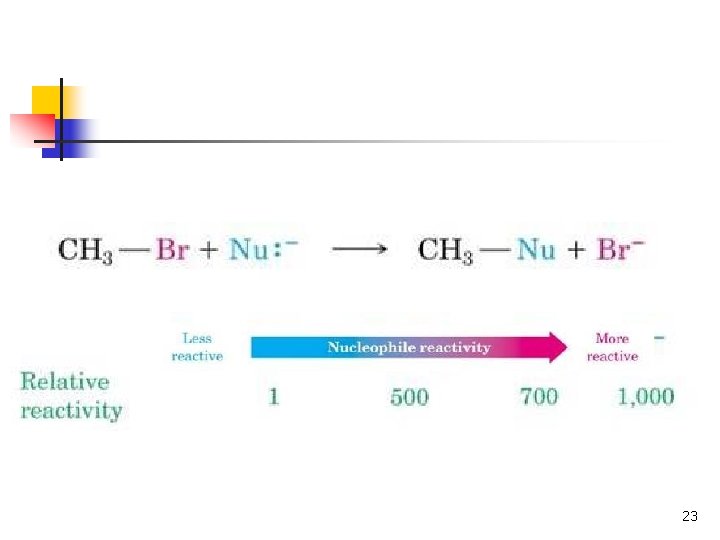
Relative Reactivity of Nucleophiles n Depends on reaction and conditions n More basic nucleophiles react faster n n Better nucleophiles are lower in a column of the periodic table Anions are usually more reactive than neutrals 19

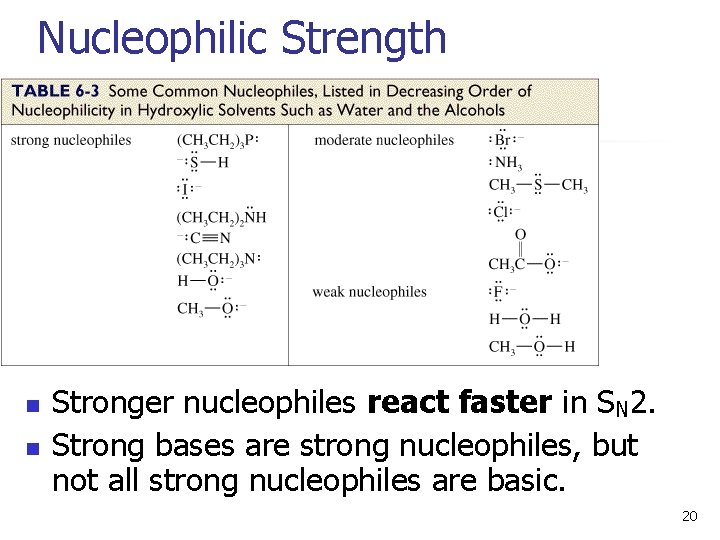
Nucleophilic Strength n n Stronger nucleophiles react faster in SN 2. Strong bases are strong nucleophiles, but not all strong nucleophiles are basic. 20

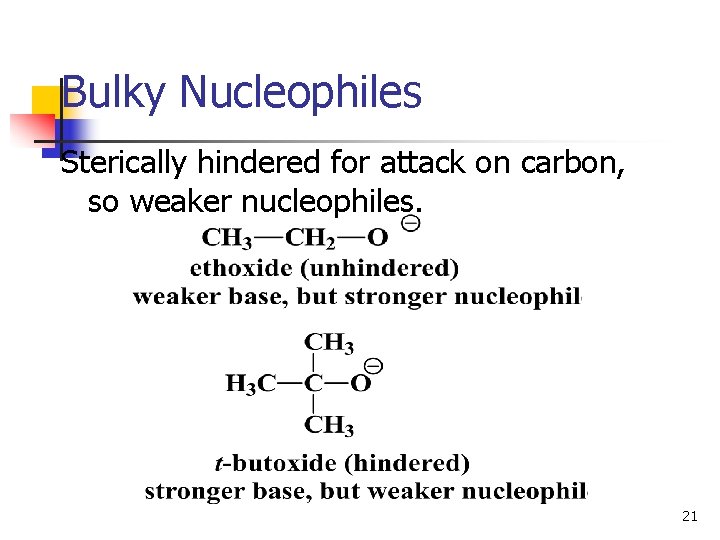
Bulky Nucleophiles Sterically hindered for attack on carbon, so weaker nucleophiles. 21

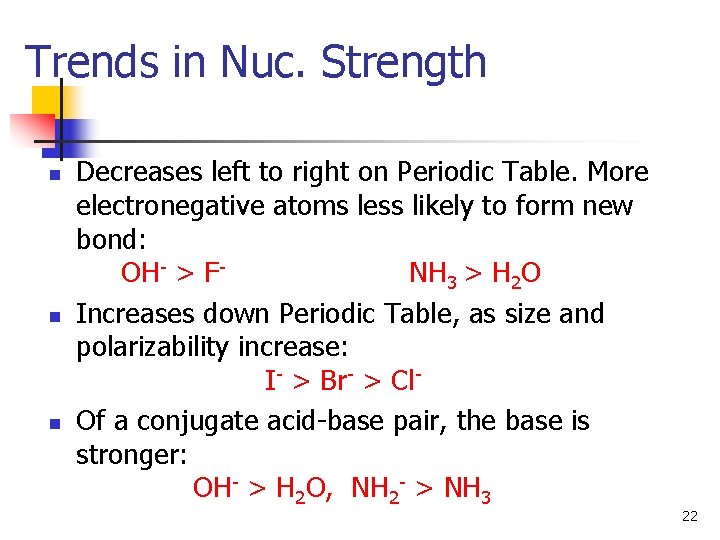
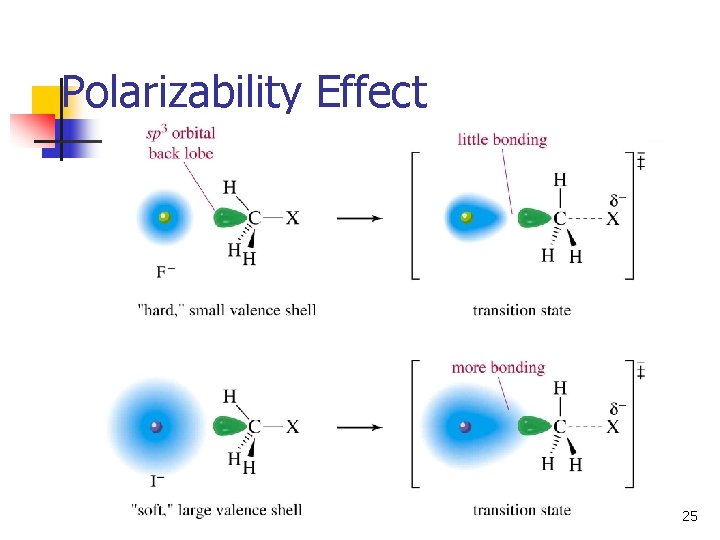
Trends in Nuc. Strength n n n Decreases left to right on Periodic Table. More electronegative atoms less likely to form new bond: OH- > FNH 3 > H 2 O Increases down Periodic Table, as size and polarizability increase: I- > Br- > Cl. Of a conjugate acid-base pair, the base is stronger: OH- > H 2 O, NH 2 - > NH 3 22

23

24

Polarizability Effect 25

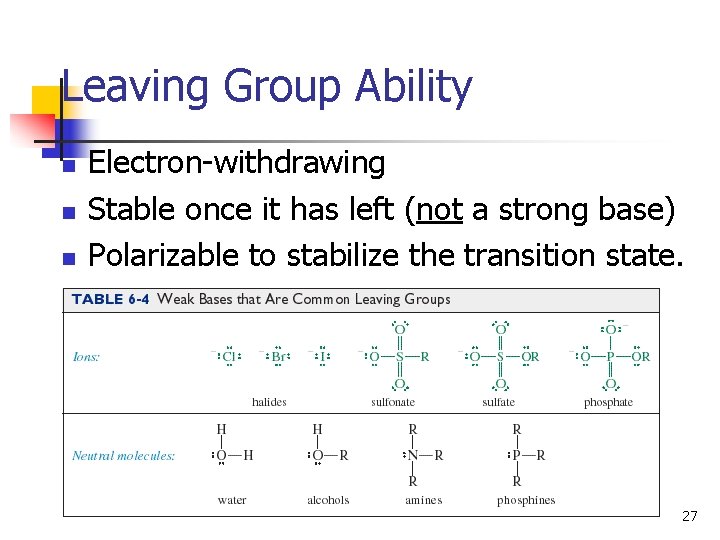
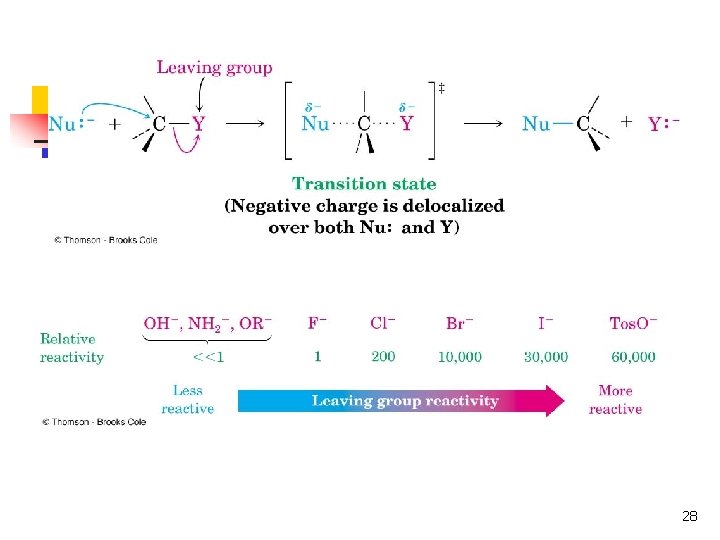
The Leaving Group n n n A good leaving group reduces the energy of activation of a reaction Stable anions that are weak bases (conjugate bases of strong acids) are usually excellent leaving groups Stronger bases (conjugate bases of weaker acids) are usually poorer leaving groups 26

Leaving Group Ability n n n Electron-withdrawing Stable once it has left (not a strong base) Polarizable to stabilize the transition state. 27

28

Poor Leaving Groups n If a group is very basic or very small, it does not undergo nucleophilic substitution. 29

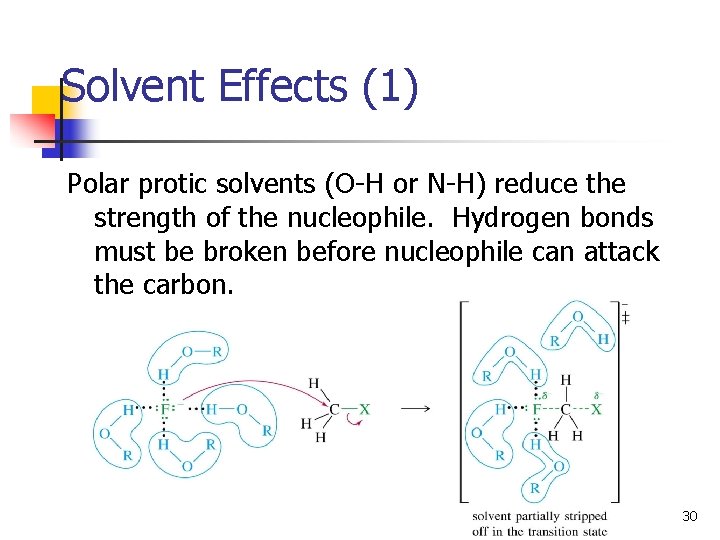
Solvent Effects (1) Polar protic solvents (O-H or N-H) reduce the strength of the nucleophile. Hydrogen bonds must be broken before nucleophile can attack the carbon. 30

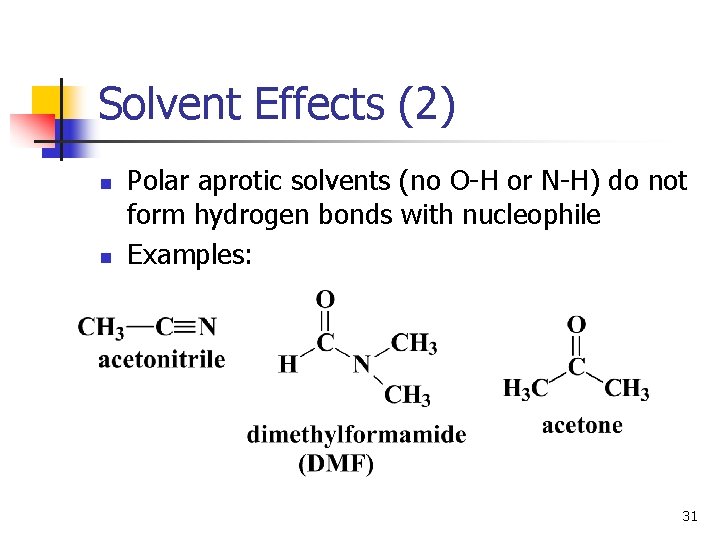
Solvent Effects (2) n n Polar aprotic solvents (no O-H or N-H) do not form hydrogen bonds with nucleophile Examples: 31

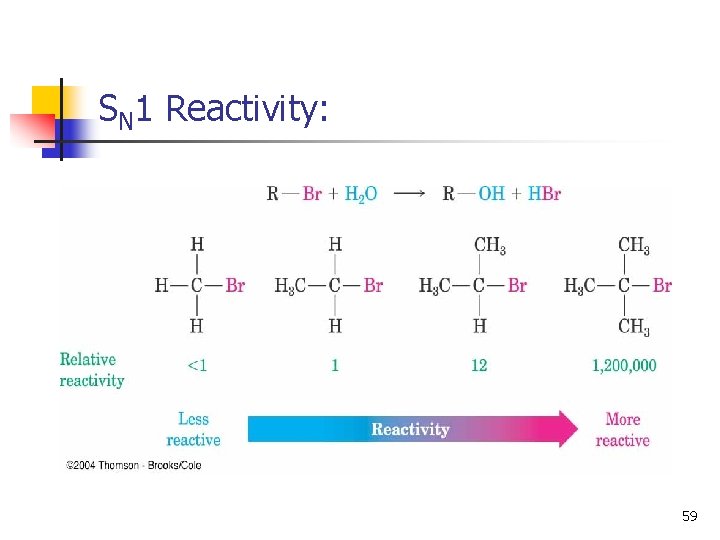
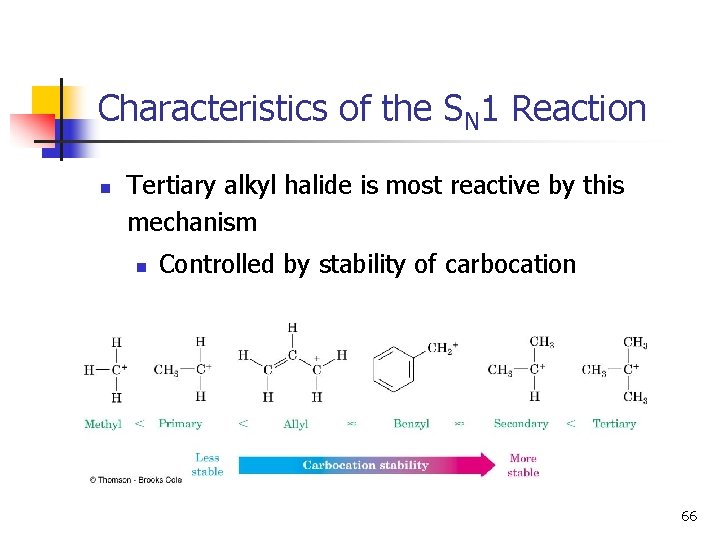
Rates of SN 1 Reactions n 3° > 2° > 1° >> CH 3 X n n Order follows stability of carbocations (opposite to SN 2) More stable ion requires less energy to form Better leaving group, faster reaction (like SN 2) Polar protic solvent best: It solvates ions strongly with hydrogen bonding. 32

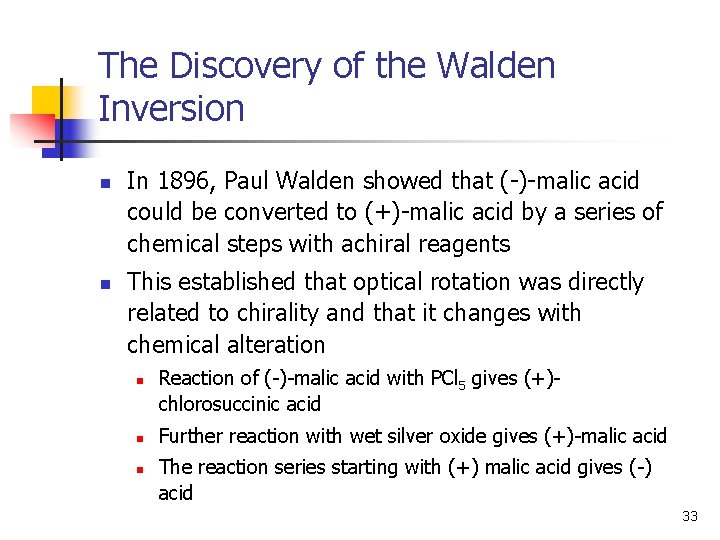
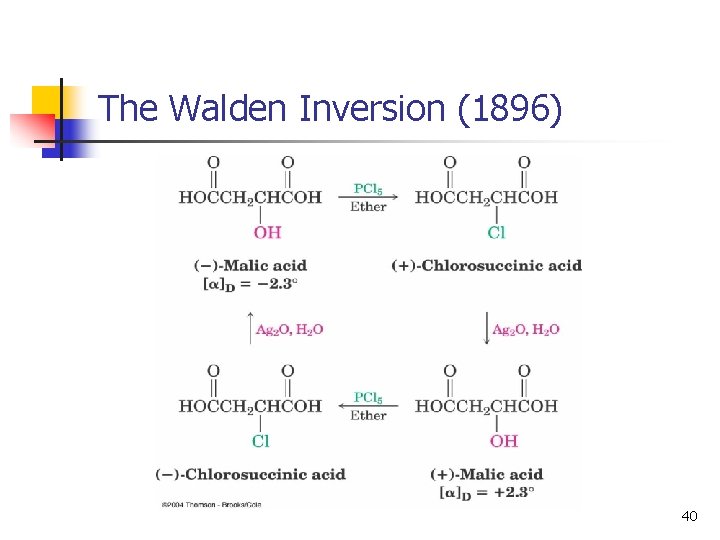
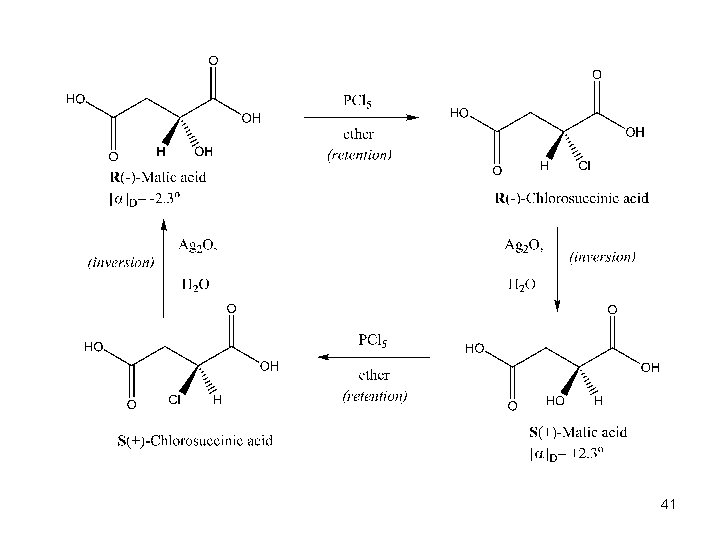
The Discovery of the Walden Inversion n n In 1896, Paul Walden showed that (-)-malic acid could be converted to (+)-malic acid by a series of chemical steps with achiral reagents This established that optical rotation was directly related to chirality and that it changes with chemical alteration n Reaction of (-)-malic acid with PCl 5 gives (+)chlorosuccinic acid Further reaction with wet silver oxide gives (+)-malic acid The reaction series starting with (+) malic acid gives (-) acid 33

Stereochemistry of SN 2 Walden inversion SN 2: Substitution Nucleophilic 2 nd order 34

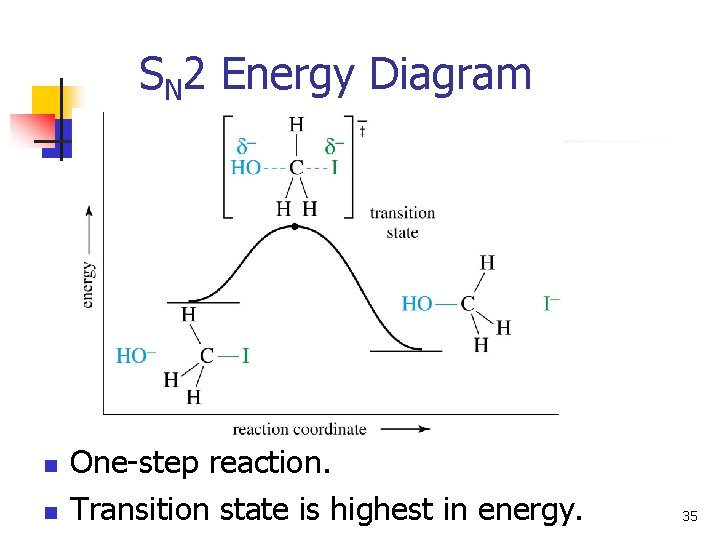
SN 2 Energy Diagram n n One-step reaction. Transition state is highest in energy. 35

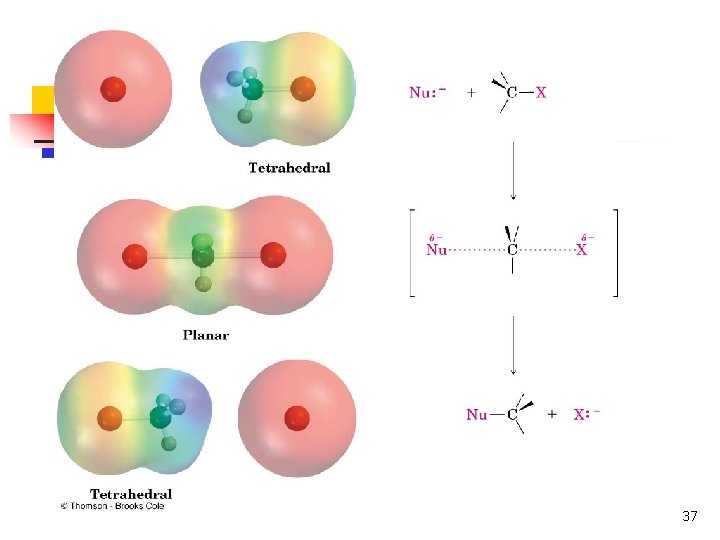
SN 2 Transition State n n The transition state of an SN 2 reaction has a planar arrangement of the carbon atom and the remaining three groups Hybridization is sp 2 36

37

Stereochemistry of SN 1 Racemization: inversion and retention => 38

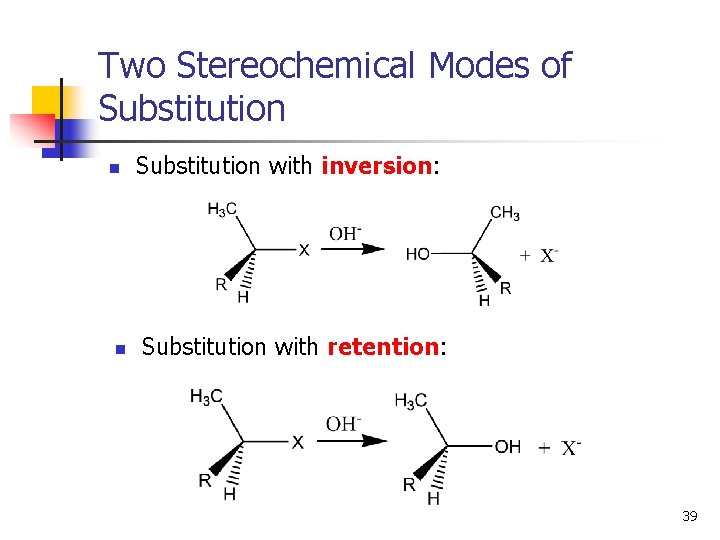
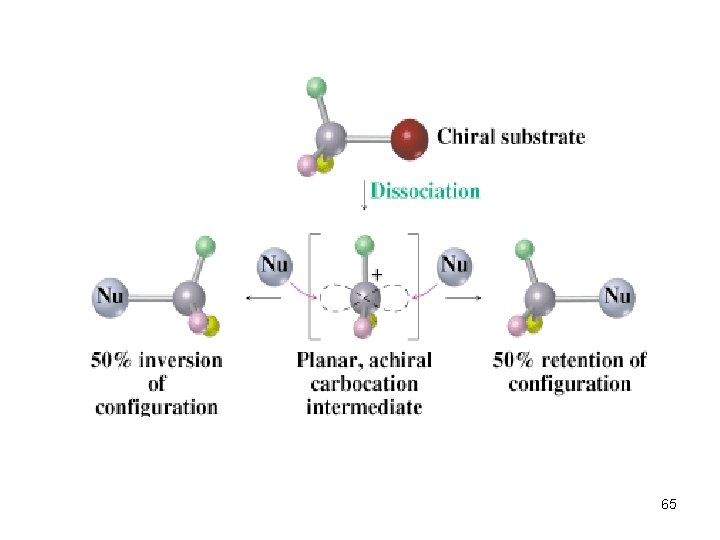
Two Stereochemical Modes of Substitution n n Substitution with inversion: Substitution with retention: 39

The Walden Inversion (1896) 40

41

Significance of the Walden Inversion n n The reactions involve substitution at the chiral center Therefore, nucleophilic substitution can invert the configuration at a chirality center 42

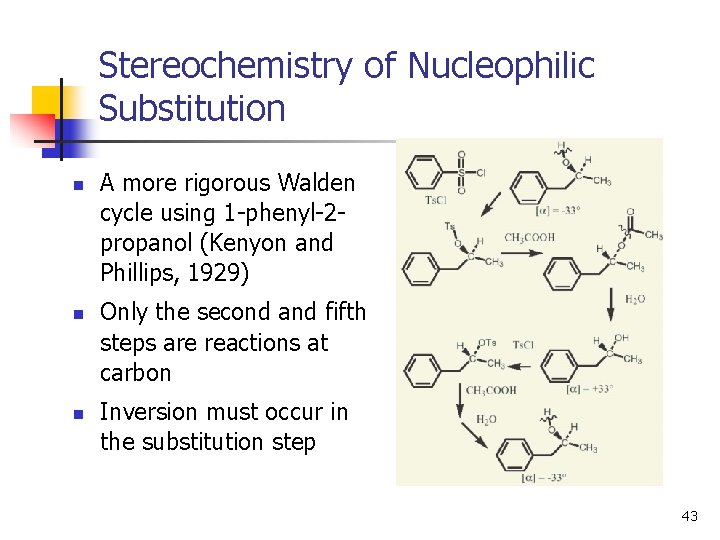
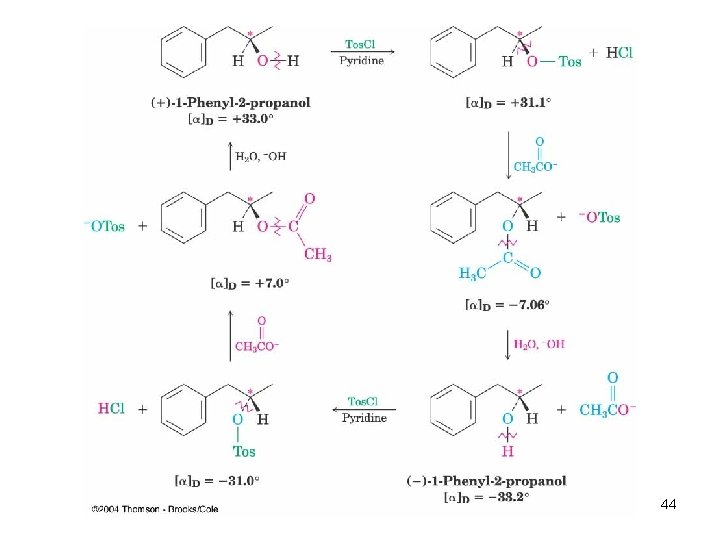
Stereochemistry of Nucleophilic Substitution n A more rigorous Walden cycle using 1 -phenyl-2 propanol (Kenyon and Phillips, 1929) Only the second and fifth steps are reactions at carbon Inversion must occur in the substitution step 43

44

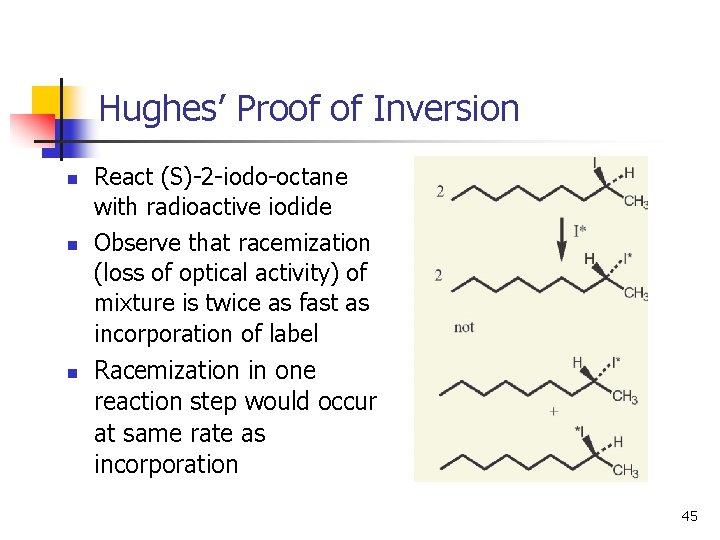
Hughes’ Proof of Inversion n React (S)-2 -iodo-octane with radioactive iodide Observe that racemization (loss of optical activity) of mixture is twice as fast as incorporation of label Racemization in one reaction step would occur at same rate as incorporation 45

Hughes’ Proof of Inversion 46

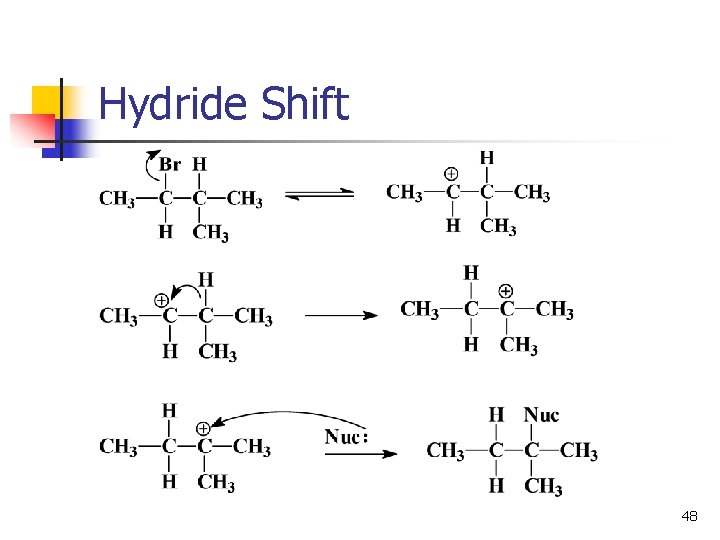
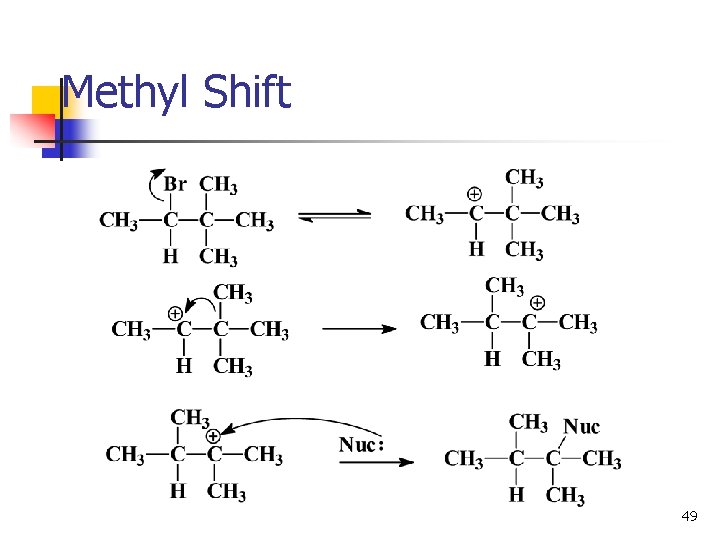
Rearrangements n n n Carbocations can rearrange to form a more stable carbocation. Hydride shift: H- on adjacent carbon bonds with C+. Methyl shift: CH 3 - moves from adjacent carbon if no H’s are available. 47

Hydride Shift 48

Methyl Shift 49

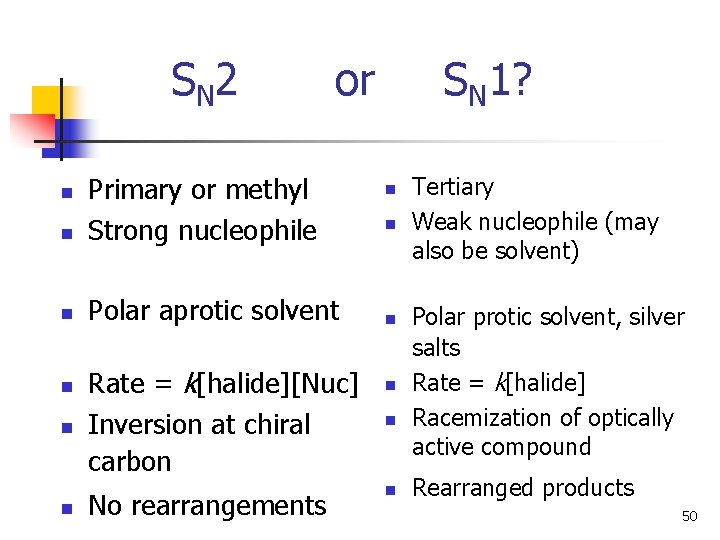
S N 2 or n Primary or methyl Strong nucleophile n Polar aprotic solvent n n Rate = k[halide][Nuc] Inversion at chiral carbon No rearrangements SN 1? n n n Tertiary Weak nucleophile (may also be solvent) Polar protic solvent, silver salts Rate = k[halide] Racemization of optically active compound Rearranged products 50

Substitution Mechanisms n S N 1 n n n Two steps with carbocation intermediate Occurs in 3°, allyl, benzyl S N 2 n n Two steps combine - without intermediate Occurs in primary, secondary 51

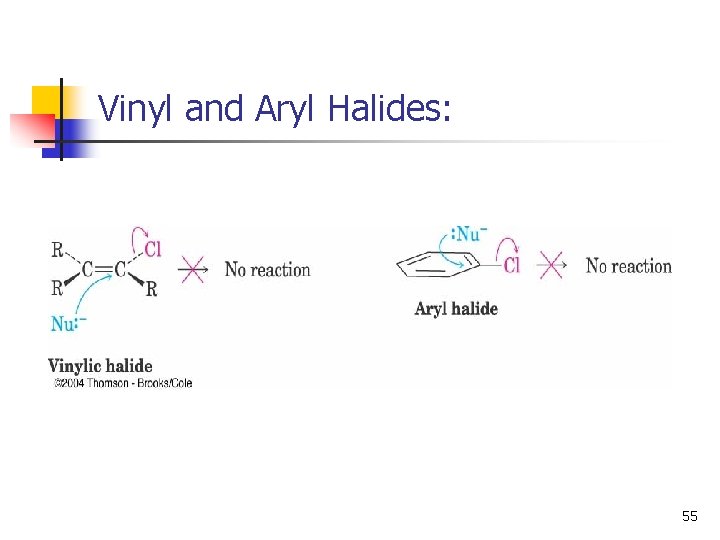
Characteristics of the SN 2 Reaction n n n Sensitive to steric effects Methyl halides are most reactive Primary are next most reactive Unhindered secondary halides react under some conditions Tertiary are unreactive by this path No reaction at C=C (vinyl or aryl halides) 52

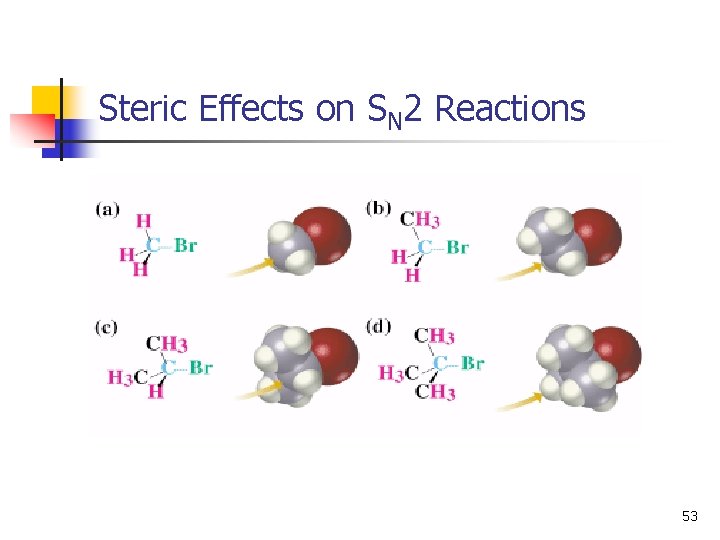
Steric Effects on SN 2 Reactions 53

Order of Reactivity in SN 2 n The more alkyl groups connected to the reacting carbon, the slower the reaction 54

Vinyl and Aryl Halides: 55

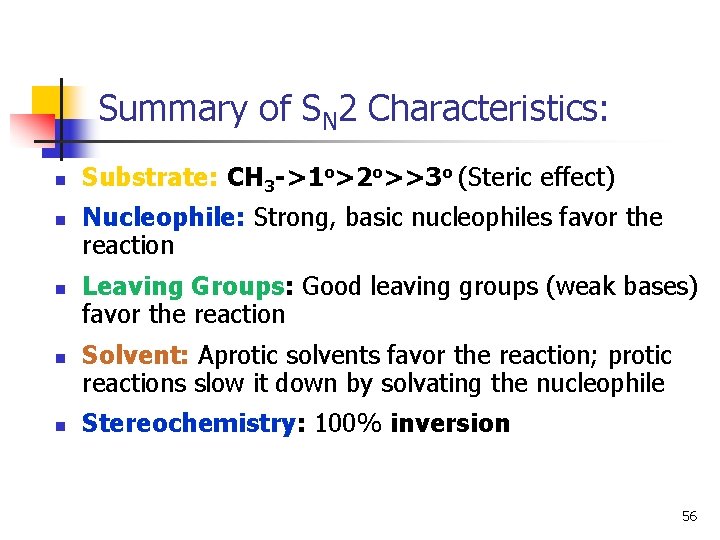
Summary of SN 2 Characteristics: n n n Substrate: CH 3 ->1 o>2 o>>3 o (Steric effect) Nucleophile: Strong, basic nucleophiles favor the reaction Leaving Groups: Good leaving groups (weak bases) favor the reaction Solvent: Aprotic solvents favor the reaction; protic reactions slow it down by solvating the nucleophile Stereochemistry: 100% inversion 56

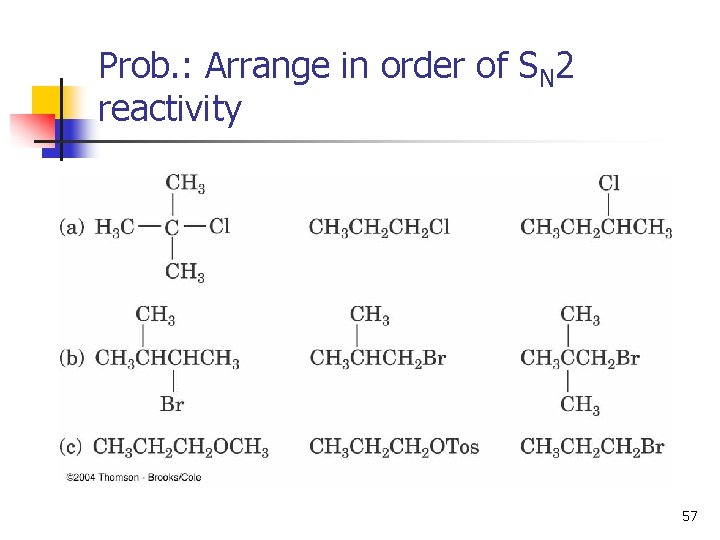
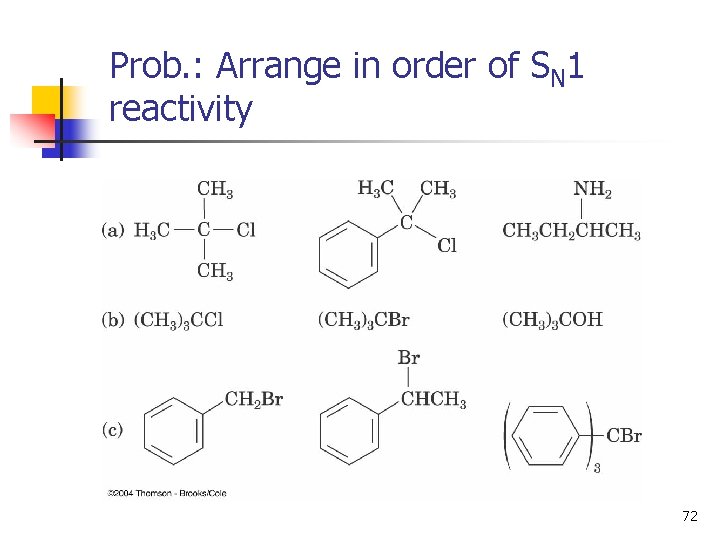
Prob. : Arrange in order of SN 2 reactivity 57

The SN 1 Reaction n Tertiary alkyl halides react rapidly in protic solvents by a mechanism that involves departure of the leaving group prior to the addition of the nucleophile. Reaction occurs in two distinct steps, while SN 2 occurs in one step (concerted). Rate-determining step is formation of carbocation: 58

SN 1 Reactivity: 59

SN 1 Energy Diagram 60

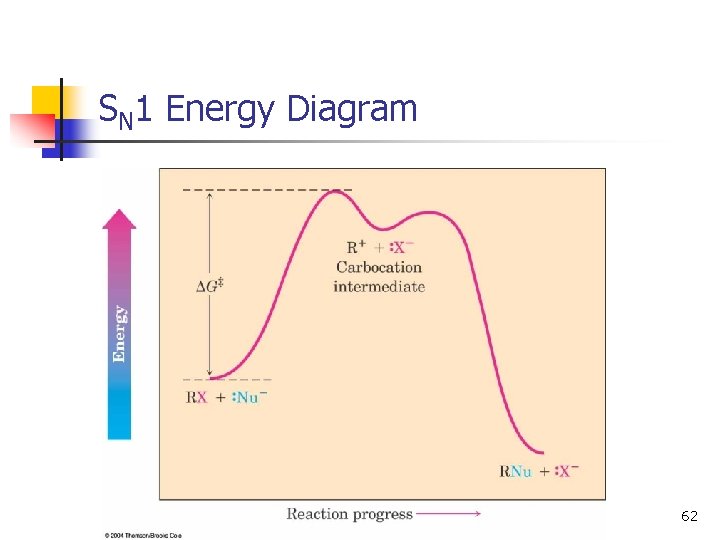
Rate-Limiting Step n n n The overall rate of a reaction is controlled by the rate of the slowest step The rate depends on the concentration of the species and the rate constant of the step The step with the largest energy of activation is the rate-limiting or rate-determining step. 61

SN 1 Energy Diagram 62

63

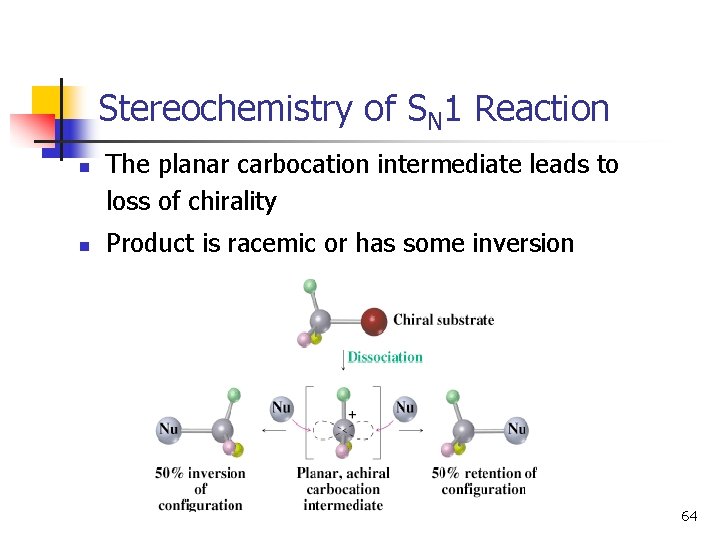
Stereochemistry of SN 1 Reaction n n The planar carbocation intermediate leads to loss of chirality Product is racemic or has some inversion 64

65

Characteristics of the SN 1 Reaction n Tertiary alkyl halide is most reactive by this mechanism n Controlled by stability of carbocation 66

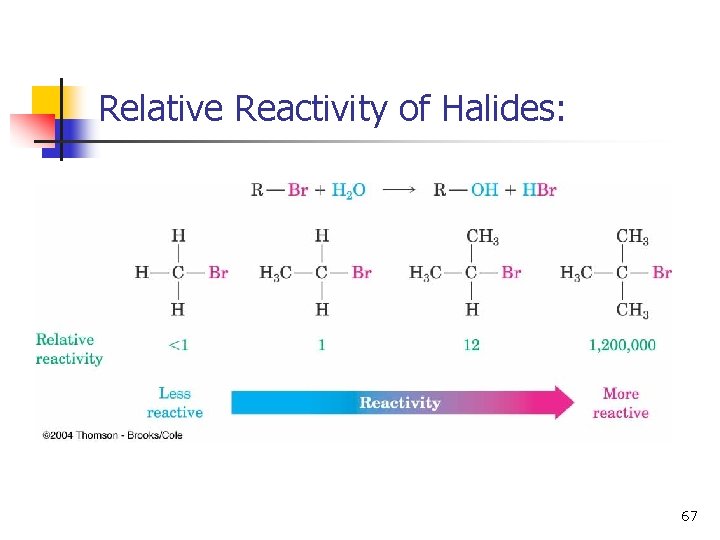
Relative Reactivity of Halides: 67

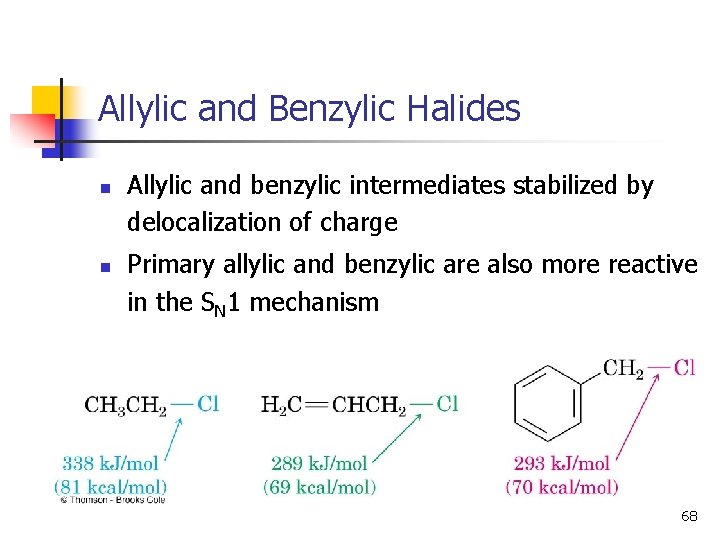
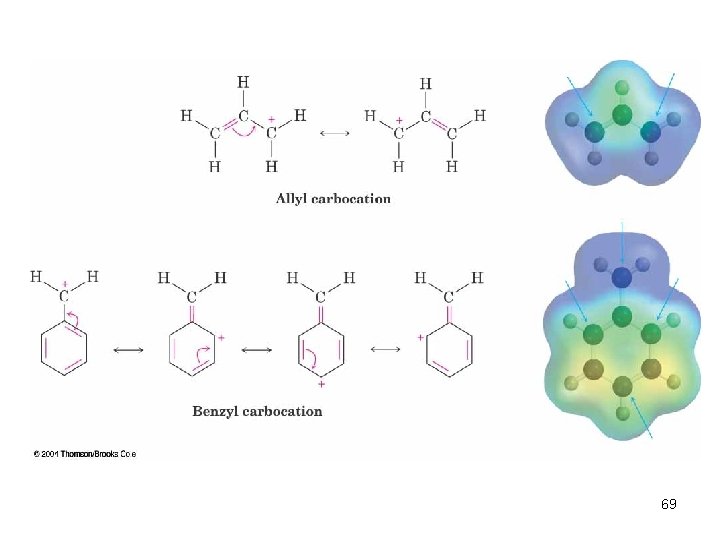
Allylic and Benzylic Halides n n Allylic and benzylic intermediates stabilized by delocalization of charge Primary allylic and benzylic are also more reactive in the SN 1 mechanism 68

69

Effect of Leaving Group on SN 1 n Critically dependent on leaving group n n n Reactivity: the larger halides ions are better leaving groups In acid, OH of an alcohol is protonated and leaving group is H 2 O, which is still less reactive than halide p-Toluensulfonate (Tos. O-) is an excellent leaving group 70

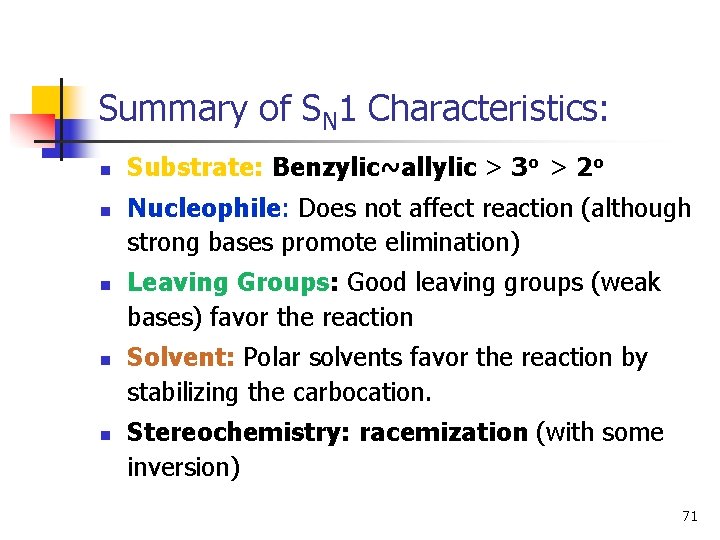
Summary of SN 1 Characteristics: n n n Substrate: Benzylic~allylic > 3 o > 2 o Nucleophile: Does not affect reaction (although strong bases promote elimination) Leaving Groups: Good leaving groups (weak bases) favor the reaction Solvent: Polar solvents favor the reaction by stabilizing the carbocation. Stereochemistry: racemization (with some inversion) 71

Prob. : Arrange in order of SN 1 reactivity 72

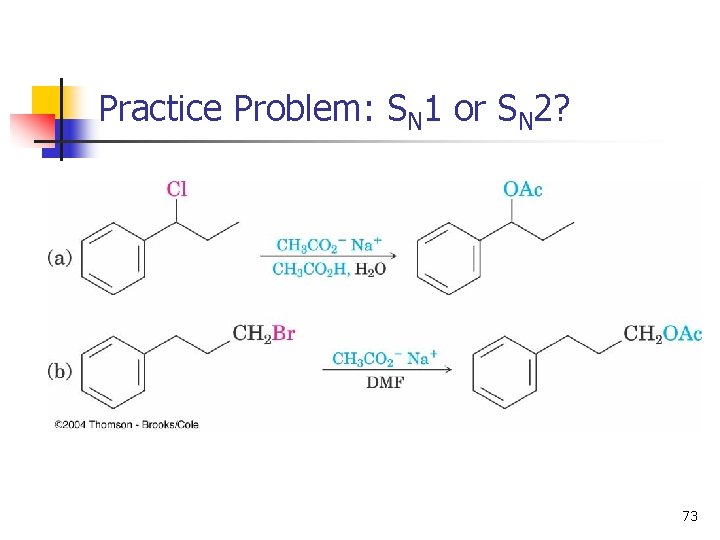
Practice Problem: SN 1 or SN 2? 73

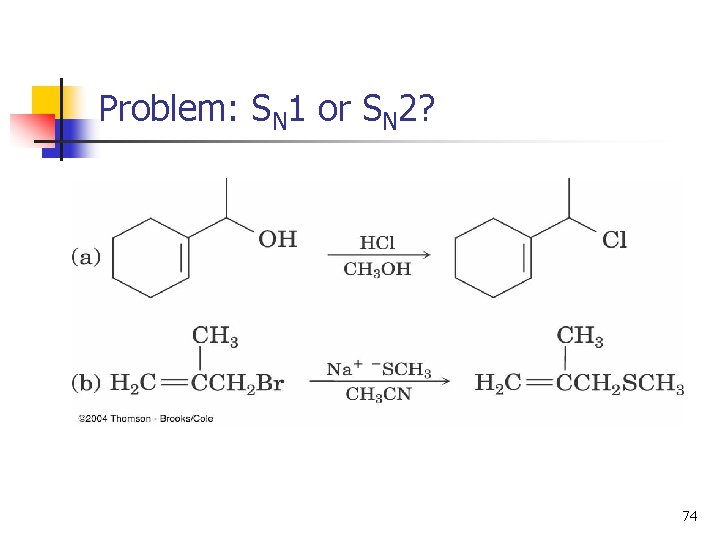
Problem: SN 1 or SN 2? 74

Chapter 7 -2, Questions 27, 29, 31, 32, 34, 39, 46, 47 75
 E1 reaction of alkyl halides
E1 reaction of alkyl halides Nucleophilic substitution of alkyl halides
Nucleophilic substitution of alkyl halides Nucleophilic substitution of alkyl halides
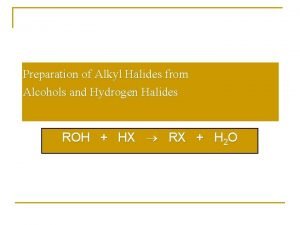
Nucleophilic substitution of alkyl halides Alcohol from alkyl halide
Alcohol from alkyl halide Reaction of alkyl halides
Reaction of alkyl halides Alkyl halide examples
Alkyl halide examples Alkyl halide reactions
Alkyl halide reactions Hydrogen halide
Hydrogen halide Naming alkyl halides
Naming alkyl halides B2h6/diglyme
B2h6/diglyme Properties of alkyl halides
Properties of alkyl halides Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Oxidation half reaction
Oxidation half reaction Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemistry unit 5 reactions balancing reactions worksheet
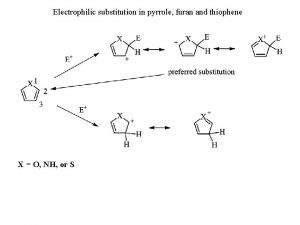
Chemistry unit 5 reactions balancing reactions worksheet Electrophilic substitution in pyrrole
Electrophilic substitution in pyrrole Nucleophilic acyl substitution mechanism
Nucleophilic acyl substitution mechanism Nucleophilic substitution of carboxylic acids
Nucleophilic substitution of carboxylic acids Aminolysis
Aminolysis Carboxylic acids and their derivatives
Carboxylic acids and their derivatives Nucleophilic addition
Nucleophilic addition Arrow pushing patterns
Arrow pushing patterns Unimolecular nucleophilic substitution
Unimolecular nucleophilic substitution Electrophilic substitution of quinoline
Electrophilic substitution of quinoline Nucleophilic addition
Nucleophilic addition Bruice
Bruice Isoquinoline nucleophilic substitution
Isoquinoline nucleophilic substitution Unimolecular substitution
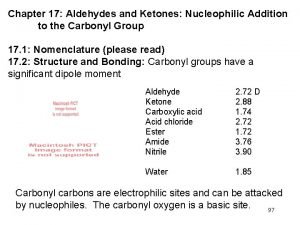
Unimolecular substitution Aldehydes and ketones nucleophilic addition
Aldehydes and ketones nucleophilic addition Polyhalide
Polyhalide Reactivity group 7
Reactivity group 7 2 methylpropene + hbr
2 methylpropene + hbr Addition of hydrogen halides to alkynes
Addition of hydrogen halides to alkynes Aryl vs vinyl
Aryl vs vinyl Properties of aryl halides
Properties of aryl halides Halides examples
Halides examples Test for halide
Test for halide Addition of hydrogen halides
Addition of hydrogen halides Halides minerals
Halides minerals Chapter 10 chapter assessment chemical reactions answers
Chapter 10 chapter assessment chemical reactions answers Chapter 9 chemical reactions study guide
Chapter 9 chemical reactions study guide Isomers of hexane structure
Isomers of hexane structure Primary amine ir spectrum
Primary amine ir spectrum Primary alkyl halide
Primary alkyl halide Alcohol to alkyl halide
Alcohol to alkyl halide Halide
Halide What are active metals
What are active metals Propaandizuur
Propaandizuur Nonbonding vs antibonding
Nonbonding vs antibonding General formula for alkyl group
General formula for alkyl group Alcohol to chloride
Alcohol to chloride Alkyl silane
Alkyl silane Cyclopentane condensed structural formula
Cyclopentane condensed structural formula Alkyl group
Alkyl group Alkyl tail
Alkyl tail Oxidation reduction reactions chapter 19 review
Oxidation reduction reactions chapter 19 review Chapter 9 chemical reactions
Chapter 9 chemical reactions Chemistry chapter 8 review chemical equations and reactions
Chemistry chapter 8 review chemical equations and reactions Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chapter 20 worksheet redox
Chapter 20 worksheet redox Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Predict the products of the following reactions.
Predict the products of the following reactions. Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chapter 19 review oxidation reduction reactions answers
Chapter 19 review oxidation reduction reactions answers Synthesis reaction
Synthesis reaction Types of reactions
Types of reactions Chapter 4 reactions in aqueous solutions
Chapter 4 reactions in aqueous solutions Concentrated solution
Concentrated solution Chapter 4 reactions in aqueous solutions worksheet answers
Chapter 4 reactions in aqueous solutions worksheet answers Chapter 4 reactions in aqueous solutions
Chapter 4 reactions in aqueous solutions What are active metals
What are active metals Fast reactions
Fast reactions Stoichiometry mole island diagram
Stoichiometry mole island diagram