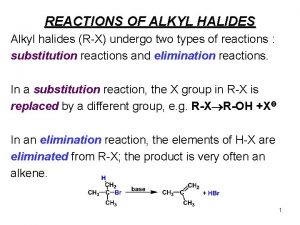
6 4 Electrophilic Addition of Hydrogen Halides to






- Slides: 22

6. 4 Electrophilic Addition of Hydrogen Halides to Alkenes

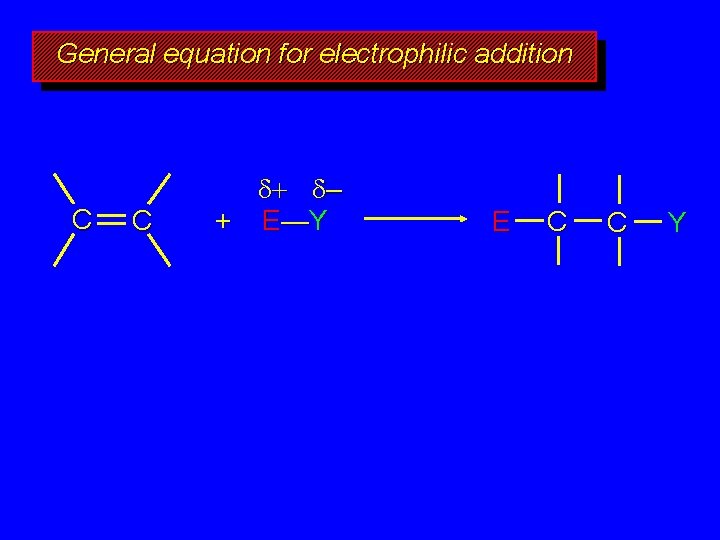
General equation for electrophilic addition C C – + E—Y E C C Y

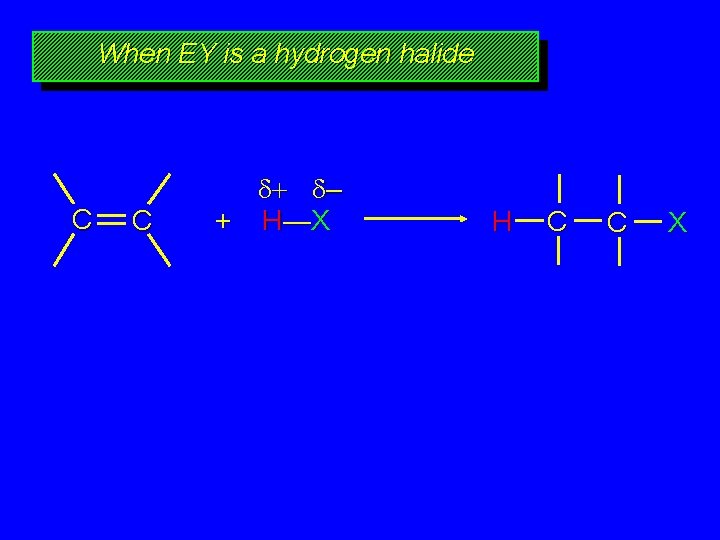
When EY is a hydrogen halide C C – + H—X H C C X

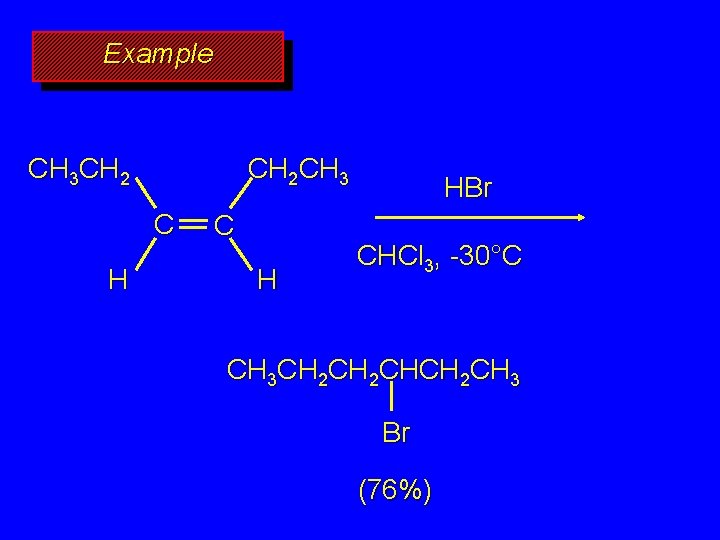
Example CH 2 CH 3 CH 2 C H HBr CHCl 3, -30°C CH 3 CH 2 CHCH 2 CH 3 Br (76%)

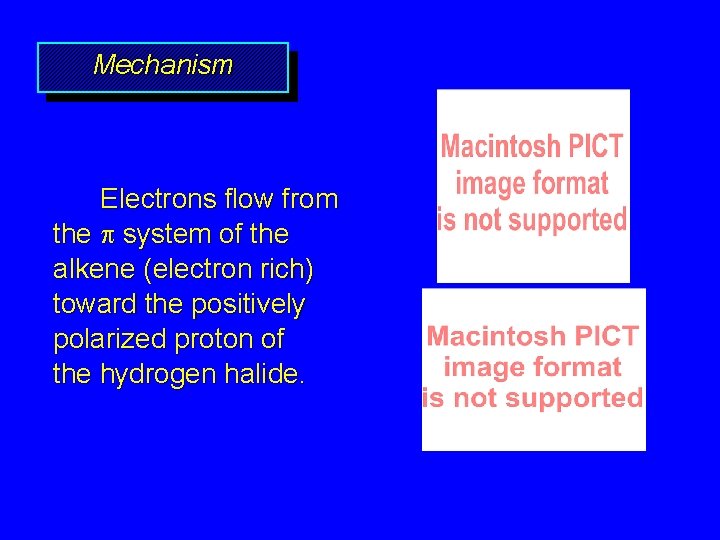
Mechanism Electrophilic addition of hydrogen halides to alkenes proceeds by rate-determining formation of a carbocation intermediate.

Mechanism Electrons flow from the system of the alkene (electron rich) toward the positively polarized proton of the hydrogen halide.

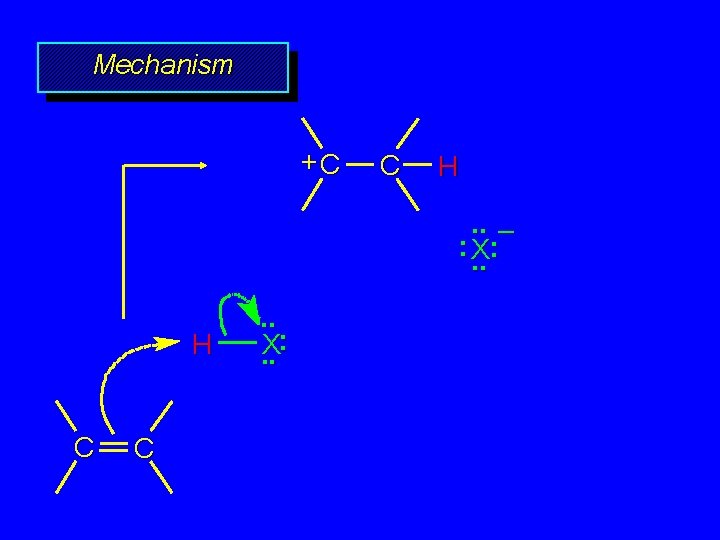
Mechanism +C C H. . – : X: . . H C C . . X: . .

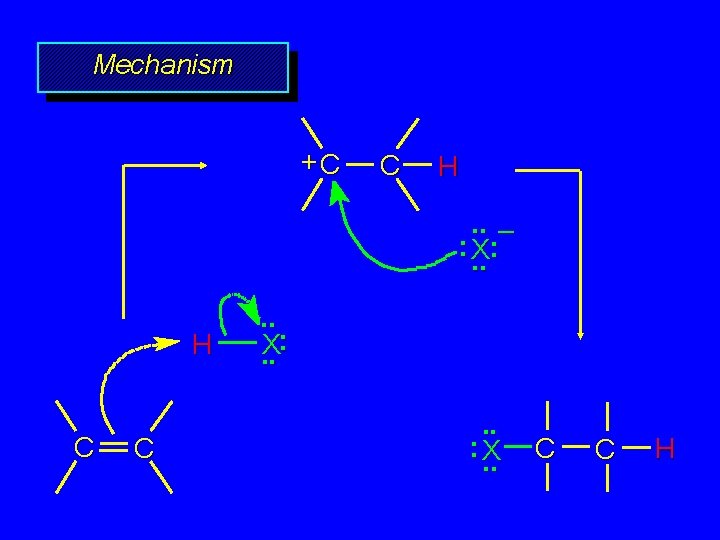
Mechanism +C C H. . – : X: . . H C C . . X: . . : X. . C C H

6. 5 Regioselectivity of Hydrogen Halide Addition: Markovnikov's Rule

Markovnikov's Rule When an unsymmetrically substituted alkene reacts with a hydrogen halide, the hydrogen adds to the carbon that has the greater number of hydrogen substituents, and the halogen adds to the carbon that has the fewer hydrogen substituents.

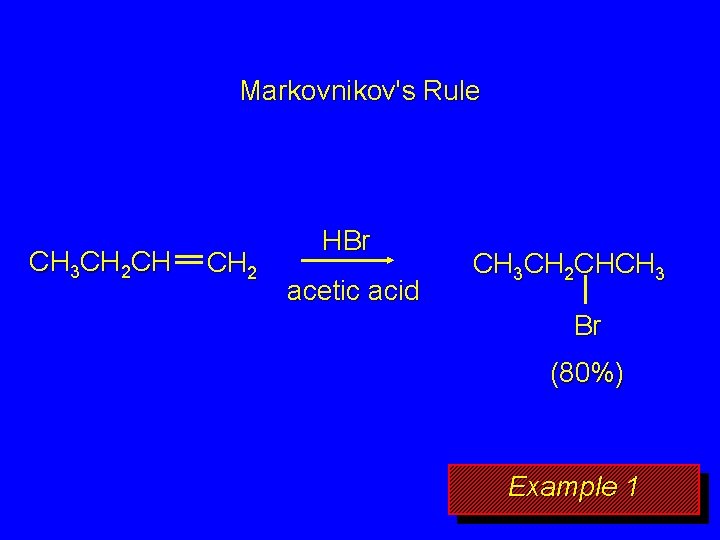
Markovnikov's Rule CH 3 CH 2 CH CH 2 HBr acetic acid CH 3 CH 2 CHCH 3 Br (80%) Example 1

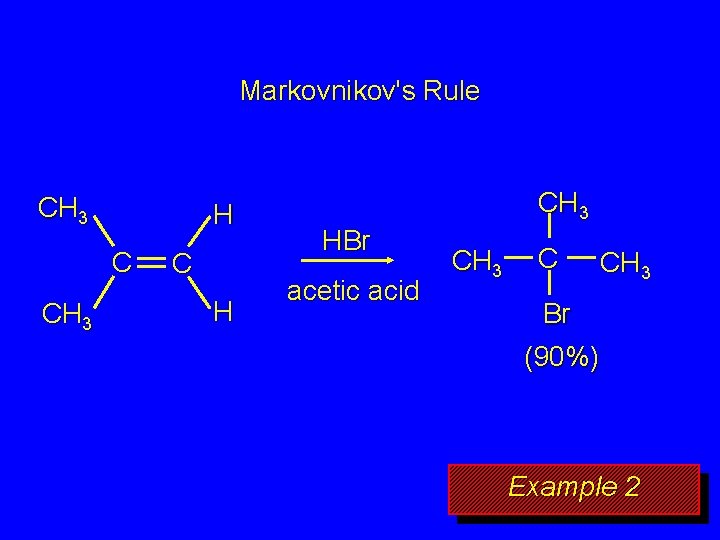
Markovnikov's Rule CH 3 H C CH 3 C H CH 3 HBr acetic acid CH 3 C CH 3 Br (90%) Example 2

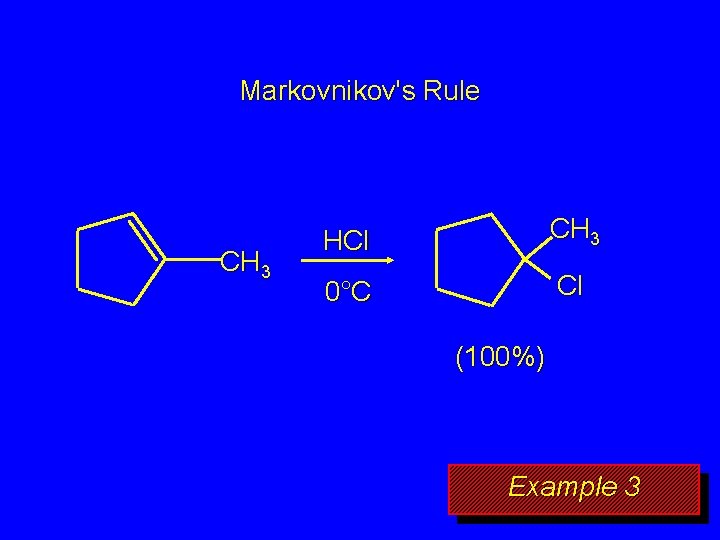
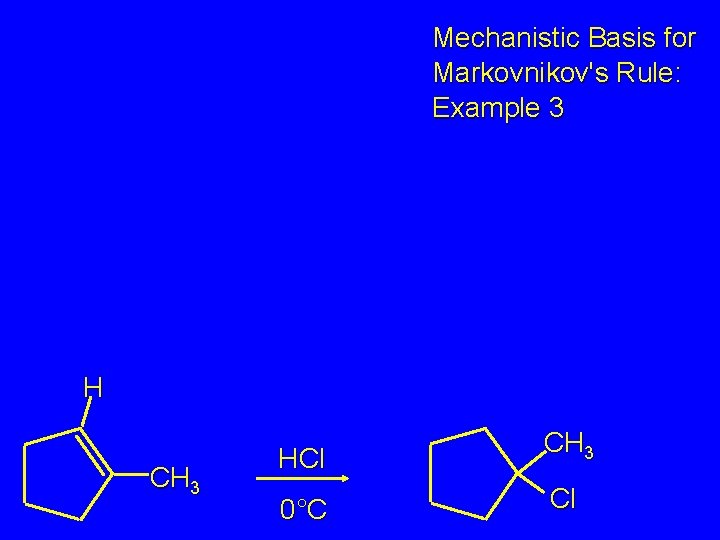
Markovnikov's Rule CH 3 HCl CH 3 0°C Cl (100%) Example 3

6. 6 Mechanistic Basis for Markovnikov's Rule Protonation of double bond occurs in direction that gives more stable of two possible carbocations.

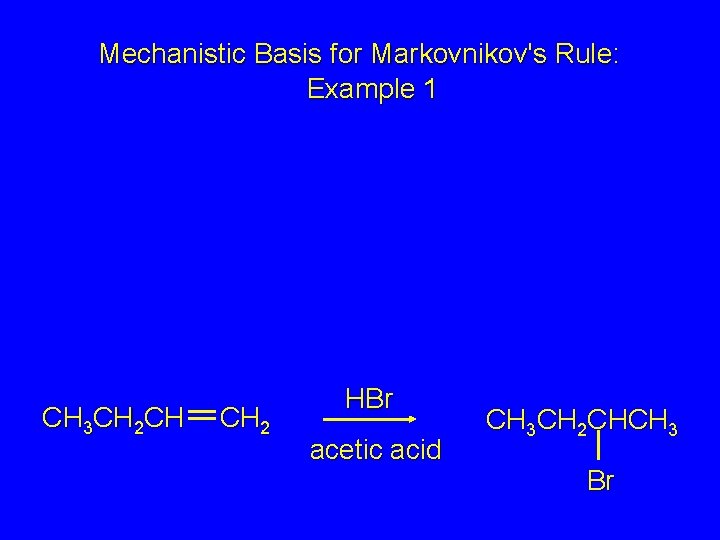
Mechanistic Basis for Markovnikov's Rule: Example 1 CH 3 CH 2 CH CH 2 HBr acetic acid CH 3 CH 2 CHCH 3 Br

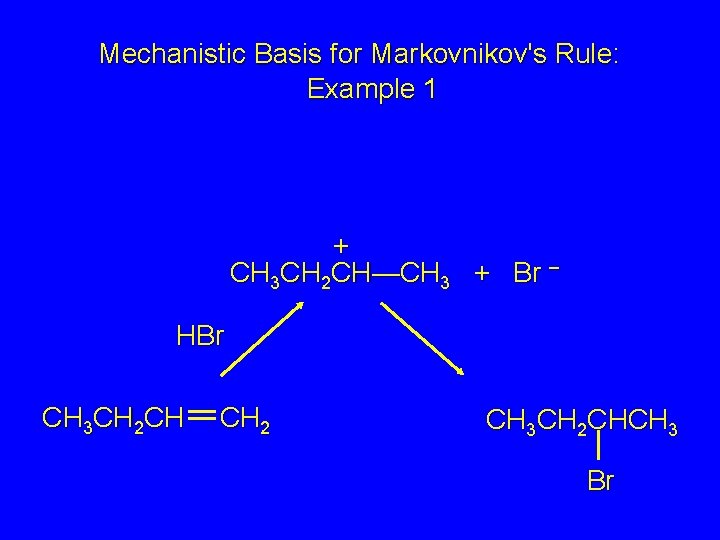
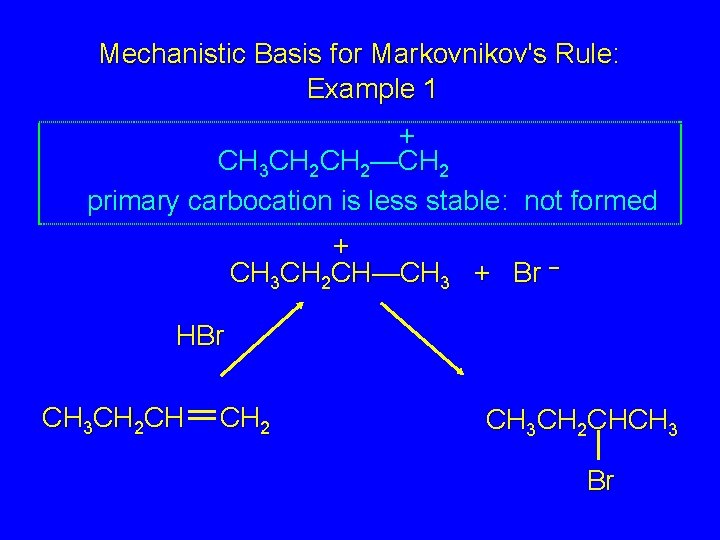
Mechanistic Basis for Markovnikov's Rule: Example 1 + CH 3 CH 2 CH—CH 3 + Br – HBr CH 3 CH 2 CH CH 2 CH 3 CH 2 CHCH 3 Br

Mechanistic Basis for Markovnikov's Rule: Example 1 + CH 3 CH 2—CH 2 primary carbocation is less stable: not formed + CH 3 CH 2 CH—CH 3 + Br – HBr CH 3 CH 2 CH CH 2 CH 3 CH 2 CHCH 3 Br

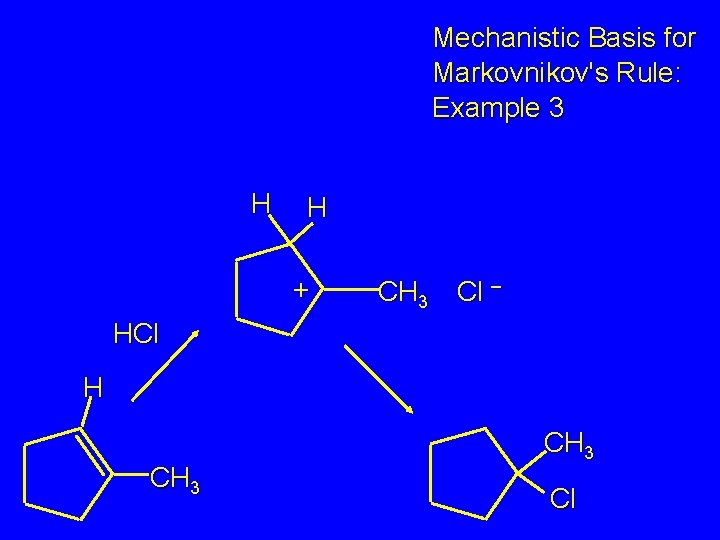
Mechanistic Basis for Markovnikov's Rule: Example 3 H CH 3 HCl CH 3 0°C Cl

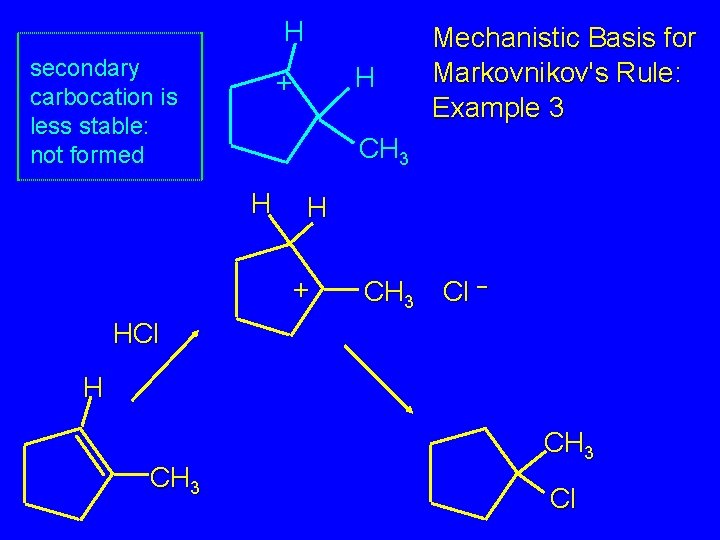
Mechanistic Basis for Markovnikov's Rule: Example 3 H H + CH 3 Cl – HCl H CH 3 Cl

H secondary carbocation is less stable: not formed H + Mechanistic Basis for Markovnikov's Rule: Example 3 CH 3 H H + CH 3 Cl – HCl H CH 3 Cl

6. 7 Carbocation Rearrangements in Hydrogen Halide Addition to Alkenes

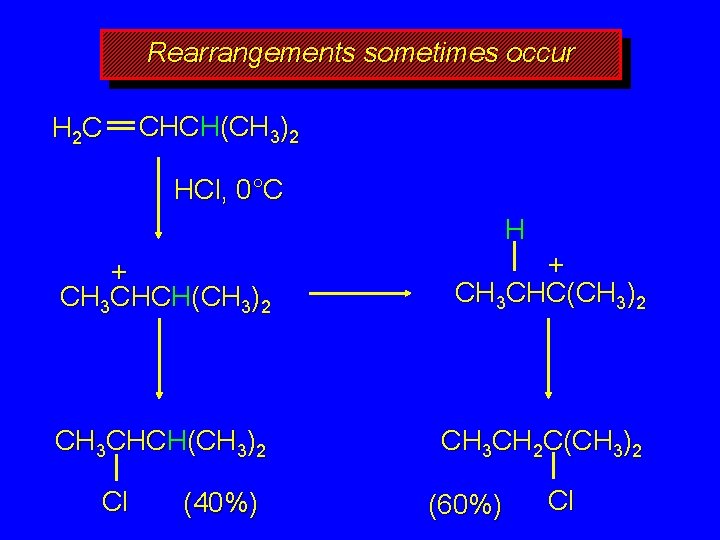
Rearrangements sometimes occur H 2 C CHCH(CH 3)2 HCl, 0°C H + CH 3 CHCH(CH 3)2 + CH 3 CHC(CH 3)2 CH 3 CHCH(CH 3)2 CH 3 CH 2 C(CH 3)2 Cl (40%) (60%) Cl
 Addition of halogens to alkenes
Addition of halogens to alkenes Addition of hydrogen halides
Addition of hydrogen halides 2 methylpropene with hbr
2 methylpropene with hbr Electrophilic addition hbr
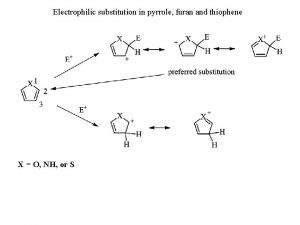
Electrophilic addition hbr Electrophilic substitution of pyrrole
Electrophilic substitution of pyrrole Cincin heterosiklik adalah
Cincin heterosiklik adalah Methyl group ortho para directing
Methyl group ortho para directing Benzene friedel crafts acylation
Benzene friedel crafts acylation Isoquinoline nucleophilic substitution
Isoquinoline nucleophilic substitution Difference between electrophilic and nucleophilic
Difference between electrophilic and nucleophilic P-bromonitrobenzene
P-bromonitrobenzene What makes something electrophilic
What makes something electrophilic Isoquinoline nucleophilic substitution
Isoquinoline nucleophilic substitution Alkene to alkyl halide
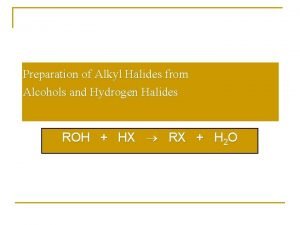
Alkene to alkyl halide Preparation of alkyl halides from alcohols
Preparation of alkyl halides from alcohols Halides minerals
Halides minerals Halides examples
Halides examples Nucleophilic substitution of alkyl halides
Nucleophilic substitution of alkyl halides Name the following alkyl halides
Name the following alkyl halides Polyhalide ions examples
Polyhalide ions examples Halide test
Halide test Vinyl and aryl halides
Vinyl and aryl halides Solvents for sn2 reactions
Solvents for sn2 reactions