HYDROGEN ENERGY Hydrogen storage Hydrogen Uses of hydrogen















- Slides: 24

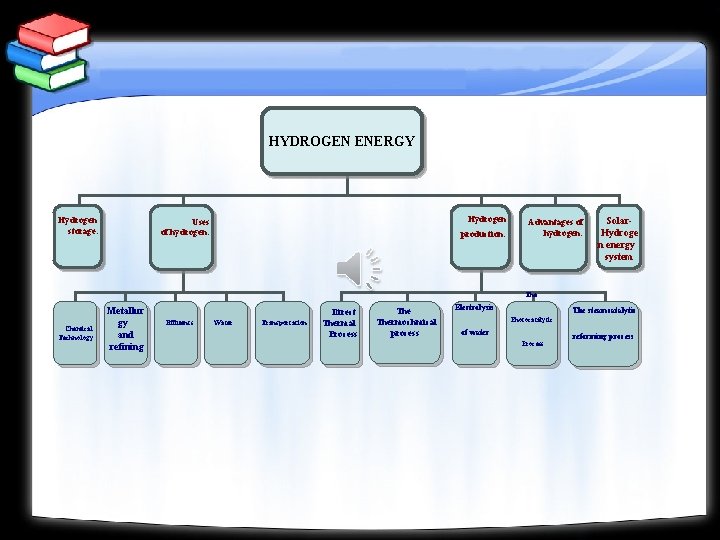
HYDROGEN ENERGY Hydrogen storage: Hydrogen Uses of hydrogen: production: Advantages of hydrogen: Solar. Hydroge n energy system The Chemical Technology Metallur gy and refining Effluents Water Transportation Direct Thermal Process Thermochmical process Electrolysis The steamcatalytic Photocatalytic of water Process reforming process

INTRODUCTION Producing hydrogen for storing and transmitting solar energy seems the proper solution to recent difficulties of energy shortage and environmental pollution. The availability and the economic advantages of using hydrocarbons directly as heat, leaves no chance for the utilization of hydrogen as energy source. However, hydrogen is potentially a more flexible energy carrier and energy storage medium. It can be converted to other forms of energy needed by the users such as mechanical, electrical, and thermal with high efficiency. Hydrogen has good combustion characteristics and is non polluting because upon combustion, the only products are heat and water vapor. It can be used to fire boilers, run gas turbines of power fuel cells. It could also be used for ground air transportation.

Hydrogen storage and transfer : Hydrogen is easily stored and transported in many forms. Hydrogen can be stored as compressed gas or in liquid form at -253°C in large cryogenic containers above ground or in large containers in rock covers. Hydrogen can also be stored underground in gaseous form in large geologeically formed reservoirs. During recent years, the use of metal hydrides for hydrogen storage has been under development. Metal hydride storage systems offer a low cost and safe hydrogen storage. It is possible to pack more hydrogen in a metal hydride than in the same volume of liquid hydrogen.

Ammonia can be used as portable store and back up source of hydrogen. The process of forming ammonia from hydrogen is relatively easy. On the receiving areas, breaking down the ammonia to yield hydrogen is also relatively easy. As for the transformation of hydrogen, evacuated, doublewall, insulated containers which have been mounted on trailer trucks, or barges can be used. It is possible to use natural gas pipelines for transmission of hydrogen gas

Hydrogen production using solar energy: The basic methods known for hydrogen production are: Direct Thermal Process: . 1 The dissociation of water into hydrogen and oxygen will start if water is heated up to about 3000 K or above. Water decomposition reaction is:

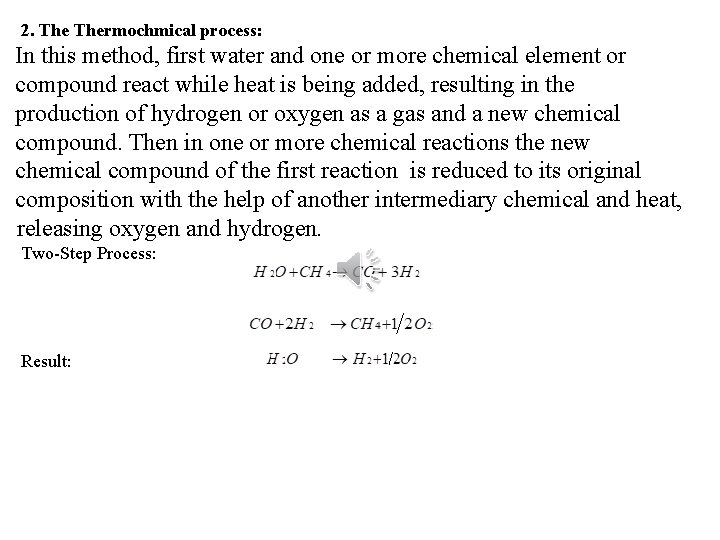
2. Thermochmical process: In this method, first water and one or more chemical element or compound react while heat is being added, resulting in the production of hydrogen or oxygen as a gas and a new chemical compound. Then in one or more chemical reactions the new chemical compound of the first reaction is reduced to its original composition with the help of another intermediary chemical and heat, releasing oxygen and hydrogen. Two-Step Process: Result:



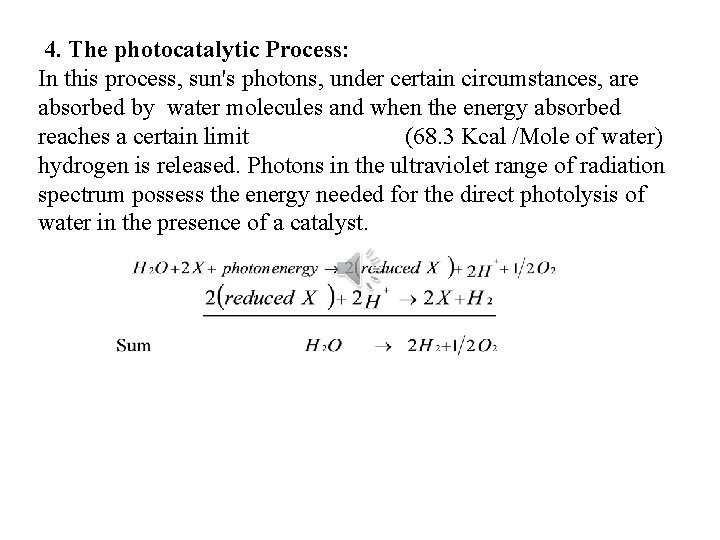
4. The photocatalytic Process: In this process, sun's photons, under certain circumstances, are absorbed by water molecules and when the energy absorbed reaches a certain limit (68. 3 Kcal /Mole of water) hydrogen is released. Photons in the ultraviolet range of radiation spectrum possess the energy needed for the direct photolysis of water in the presence of a catalyst.



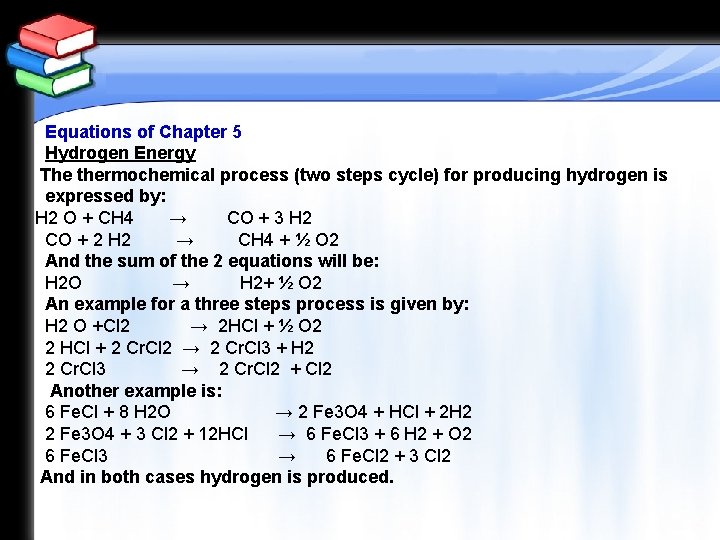
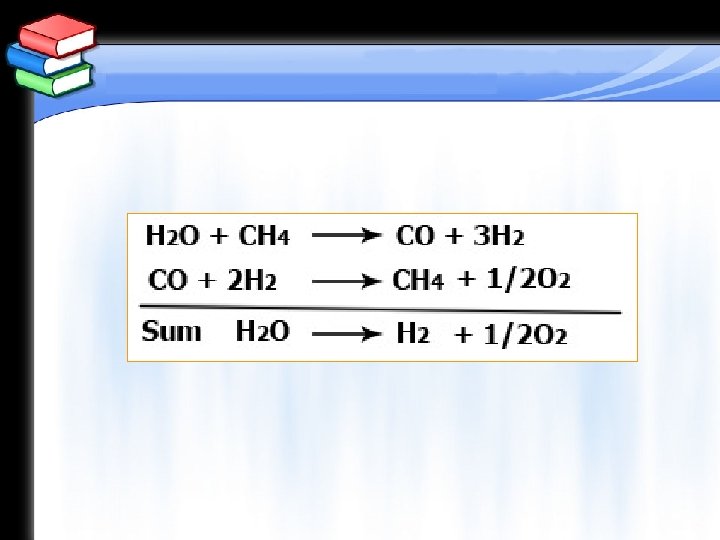
Equations of Chapter 5 Hydrogen Energy The thermochemical process (two steps cycle) for producing hydrogen is expressed by: H 2 O + CH 4 → CO + 3 H 2 CO + 2 H 2 → CH 4 + ½ O 2 And the sum of the 2 equations will be: H 2 O → H 2+ ½ O 2 An example for a three steps process is given by: H 2 O +Cl 2 → 2 HCl + ½ O 2 2 HCl + 2 Cr. Cl 2 → 2 Cr. Cl 3 + H 2 2 Cr. Cl 3 → 2 Cr. Cl 2 + Cl 2 Another example is: 6 Fe. Cl + 8 H 2 O → 2 Fe 3 O 4 + HCl + 2 H 2 2 Fe 3 O 4 + 3 Cl 2 + 12 HCl → 6 Fe. Cl 3 + 6 H 2 + O 2 6 Fe. Cl 3 → 6 Fe. Cl 2 + 3 Cl 2 And in both cases hydrogen is produced.

Using a photo- catalyst, hydrogen could be produced through a photocatalytic process as follows: H 2 O +2 X + photoenergy → 2(reduced X) + 2 H+ + ½ O 2 2 (reduced X) + 2 H+ → 2 X + H 2 and the sum will be: H 2 O → 2 H 2 + ½ O 2

Water could be electrically electrolyized to produce hydrogen (which is liberated at the cathode according to the equation: 4 H 2 O + 4 e- → 2 H 2 + 4 OH- At the anode, the following pocess takes place: 4 OH- → 2 H 2 O + O 2 + 4 e- Thus, the overall process is as follows: 2 H 2 O + electrical energy → 2 H 2 + O 2

Using a photo- catalyst, hydrogen could be produced through a photocatalytic process as follows: H 2 O +2 X + photoenergy → 2(reduced X) + 2 H+ + ½ O 2 2 (reduced X) + 2 H+ → 2 X + H 2 and the sum will be: H 2 O → 2 H 2 + ½ O 2

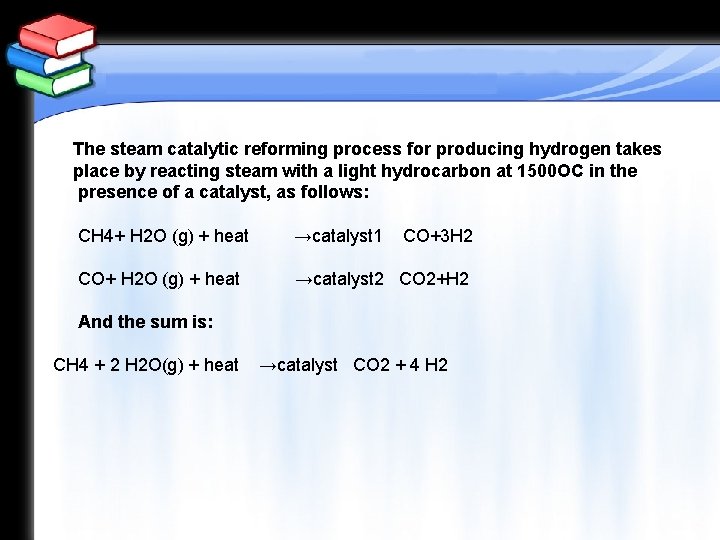
The steam catalytic reforming process for producing hydrogen takes place by reacting steam with a light hydrocarbon at 1500 OC in the presence of a catalyst, as follows: CH 4+ H 2 O (g) + heat →catalyst 1 CO+3 H 2 CO+ H 2 O (g) + heat →catalyst 2 CO 2+H 2 And the sum is: CH 4 + 2 H 2 O(g) + heat →catalyst CO 2 + 4 H 2








Summary Hydrogen energy This chapter presents the importance o hydrogen as an energy source. The advantages of hydrogen when used as a fuel are given. It is also illustrated the means of hydrogen storage and transfer. Other uses of hydrogen are given. Such uses are: in chemical technology, in metallurgy and refining, in effluent and water treatment and in transportation. The different means of producing hydrogen using solar energy are illustrated. These means are: direct thermal process, thermochemical process, electrolysis of water, the photocatalytic process and the steam catalytic reforming process. Advantages of hydrogen as an energy source are also illustrated in the present chapter.