6 7 Nucleophiles and Electrophiles A major focus









- Slides: 36

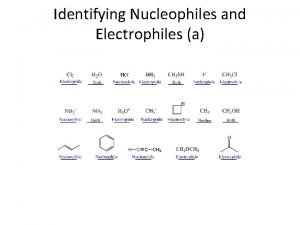
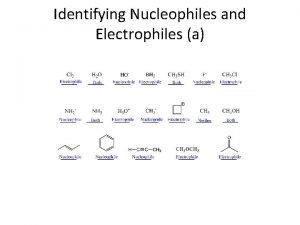
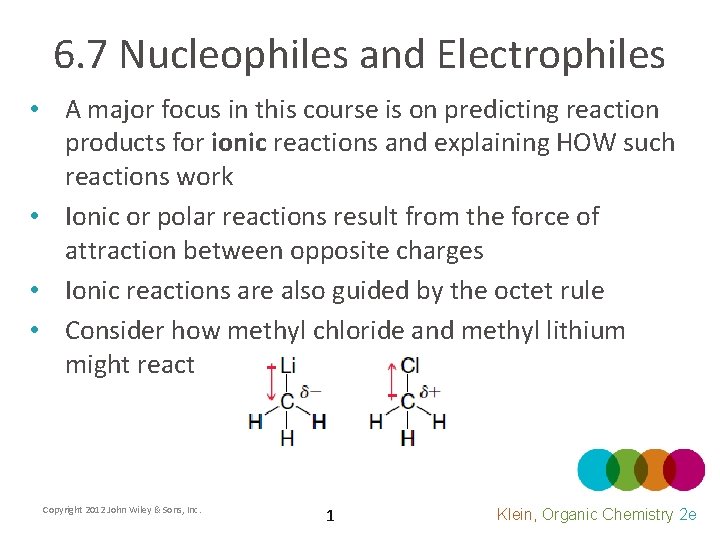
6. 7 Nucleophiles and Electrophiles • A major focus in this course is on predicting reaction products for ionic reactions and explaining HOW such reactions work • Ionic or polar reactions result from the force of attraction between opposite charges • Ionic reactions are also guided by the octet rule • Consider how methyl chloride and methyl lithium might react Copyright 2012 John Wiley & Sons, Inc. 1 Klein, Organic Chemistry 2 e

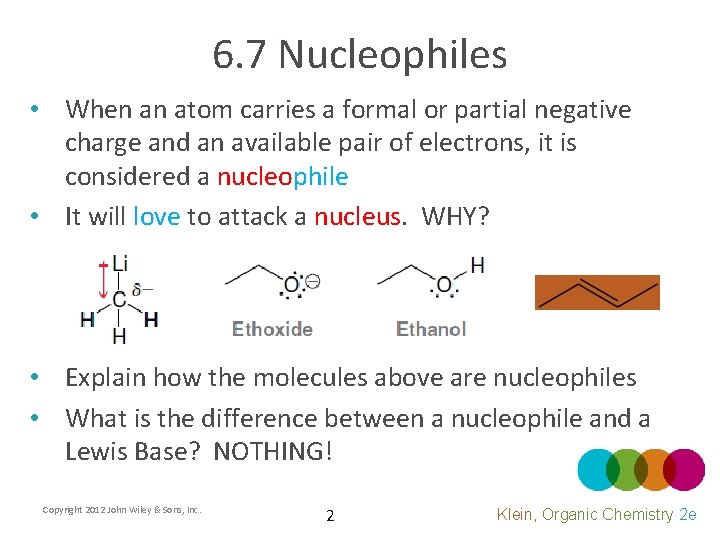
6. 7 Nucleophiles • When an atom carries a formal or partial negative charge and an available pair of electrons, it is considered a nucleophile • It will love to attack a nucleus. WHY? • Explain how the molecules above are nucleophiles • What is the difference between a nucleophile and a Lewis Base? NOTHING! Copyright 2012 John Wiley & Sons, Inc. 2 Klein, Organic Chemistry 2 e

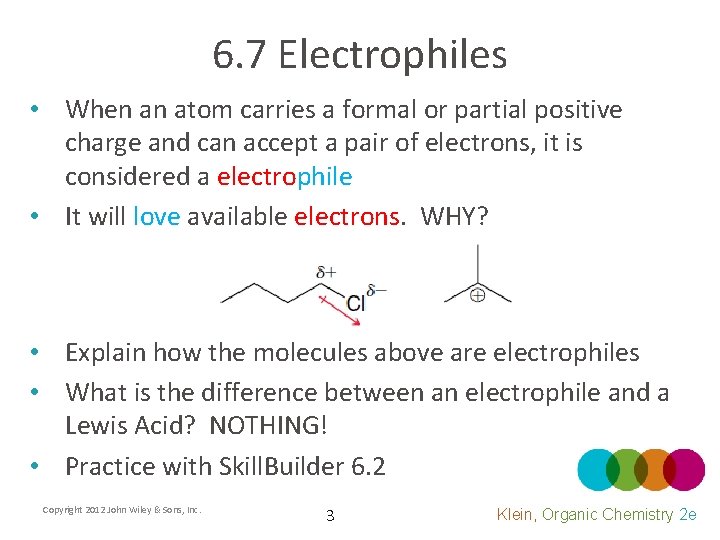
6. 7 Electrophiles • When an atom carries a formal or partial positive charge and can accept a pair of electrons, it is considered a electrophile • It will love available electrons. WHY? • Explain how the molecules above are electrophiles • What is the difference between an electrophile and a Lewis Acid? NOTHING! • Practice with Skill. Builder 6. 2 Copyright 2012 John Wiley & Sons, Inc. 3 Klein, Organic Chemistry 2 e

6. 7 Electrophiles • Label all of the nucleophilic and electrophilic sites on the following molecule Copyright 2012 John Wiley & Sons, Inc. 4 Klein, Organic Chemistry 2 e

6. 8 Mechanisms and Arrow Pushing • We use arrows to show electrons move when bonds break and form • It will be a huge benefit in this course to master the skill of arrow pushing • There are four main ways that electrons move in ionic reactions 1. 2. 3. 4. Nucleophilic Attack Loss of a Leaving Group Proton Transfers (Acid/Base) Rearrangements Copyright 2012 John Wiley & Sons, Inc. 5 Klein, Organic Chemistry 2 e

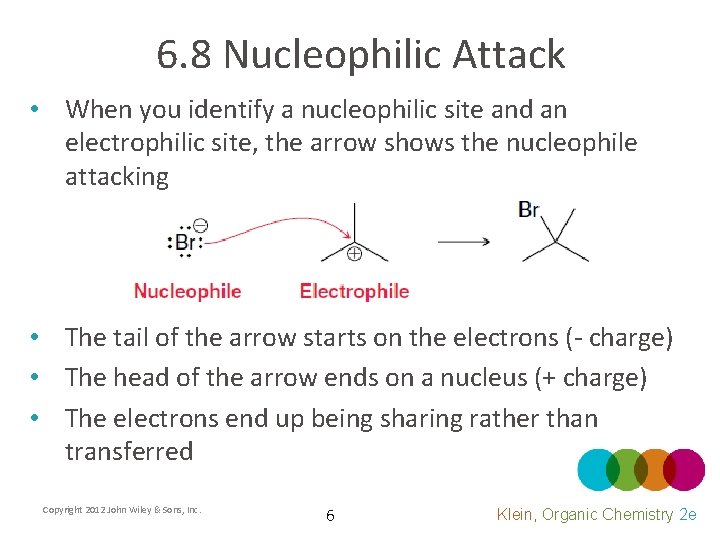
6. 8 Nucleophilic Attack • When you identify a nucleophilic site and an electrophilic site, the arrow shows the nucleophile attacking • The tail of the arrow starts on the electrons (- charge) • The head of the arrow ends on a nucleus (+ charge) • The electrons end up being sharing rather than transferred Copyright 2012 John Wiley & Sons, Inc. 6 Klein, Organic Chemistry 2 e

6. 8 Nucleophilic Attack • Nucleophilic attack may appear to occur in two steps • The alcohol is the nucleophile in this example. It attacks a carbon with a δ+ charge • The second arrow shows the flow of negative charge. WHY is it necessary? • The second arrow could be thought of as a resonance arrow. HOW? Copyright 2012 John Wiley & Sons, Inc. 7 Klein, Organic Chemistry 2 e

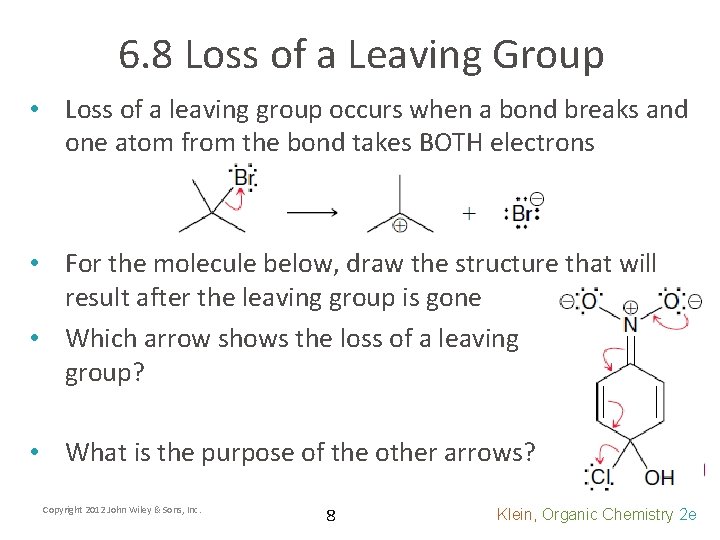
6. 8 Loss of a Leaving Group • Loss of a leaving group occurs when a bond breaks and one atom from the bond takes BOTH electrons • For the molecule below, draw the structure that will result after the leaving group is gone • Which arrow shows the loss of a leaving group? • What is the purpose of the other arrows? Copyright 2012 John Wiley & Sons, Inc. 8 Klein, Organic Chemistry 2 e

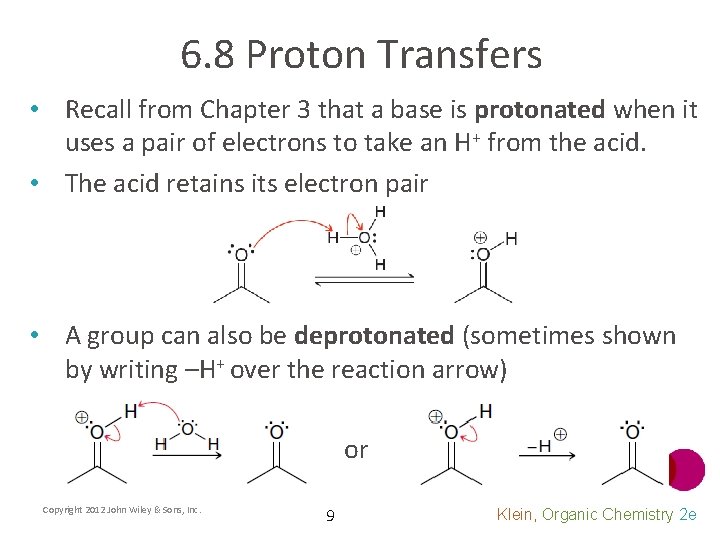
6. 8 Proton Transfers • Recall from Chapter 3 that a base is protonated when it uses a pair of electrons to take an H+ from the acid. • The acid retains its electron pair • A group can also be deprotonated (sometimes shown by writing –H+ over the reaction arrow) or Copyright 2012 John Wiley & Sons, Inc. 9 Klein, Organic Chemistry 2 e

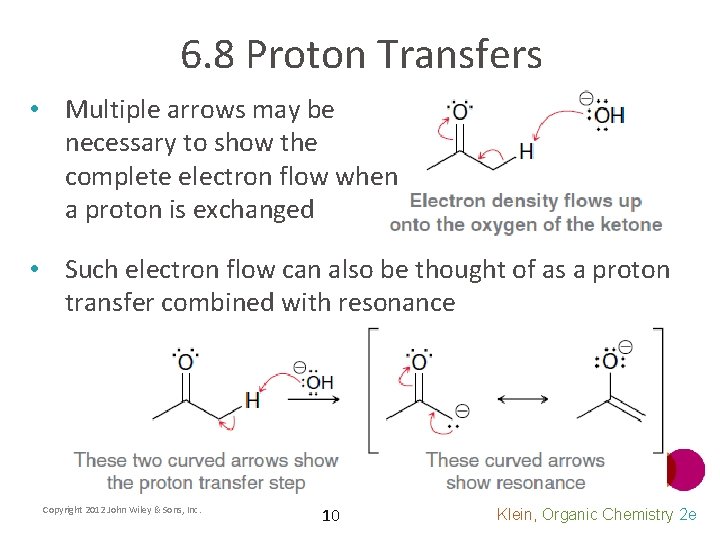
6. 8 Proton Transfers • Multiple arrows may be necessary to show the complete electron flow when a proton is exchanged • Such electron flow can also be thought of as a proton transfer combined with resonance Copyright 2012 John Wiley & Sons, Inc. 10 Klein, Organic Chemistry 2 e

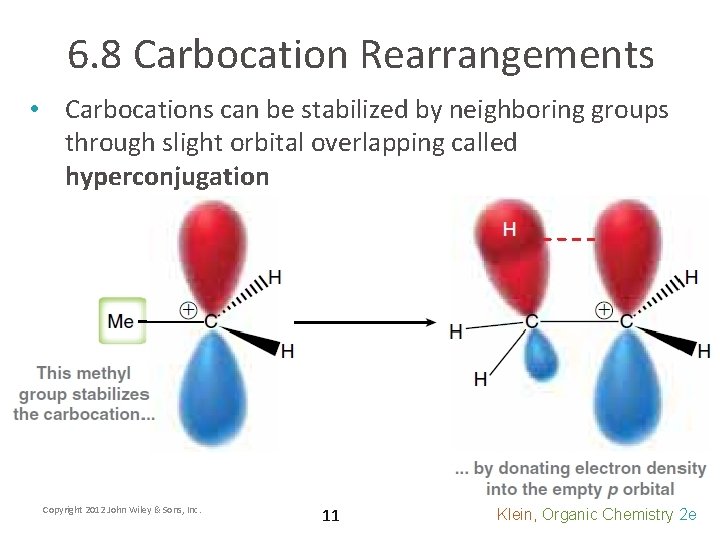
6. 8 Carbocation Rearrangements • Carbocations can be stabilized by neighboring groups through slight orbital overlapping called hyperconjugation Copyright 2012 John Wiley & Sons, Inc. 11 Klein, Organic Chemistry 2 e

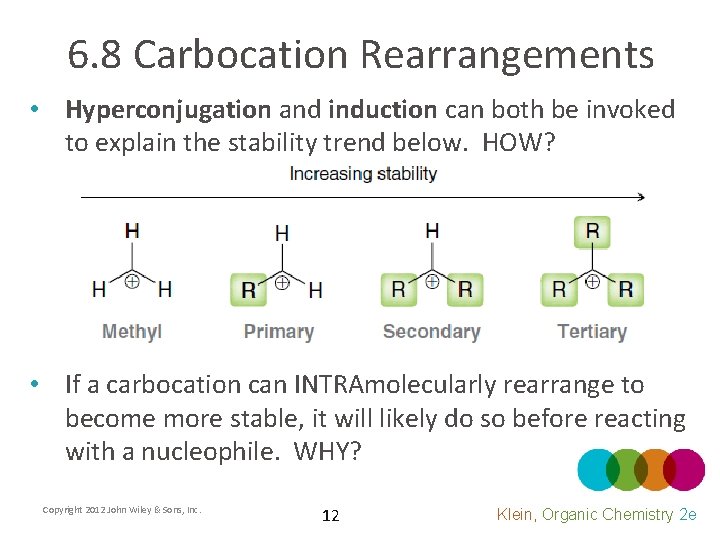
6. 8 Carbocation Rearrangements • Hyperconjugation and induction can both be invoked to explain the stability trend below. HOW? • If a carbocation can INTRAmolecularly rearrange to become more stable, it will likely do so before reacting with a nucleophile. WHY? Copyright 2012 John Wiley & Sons, Inc. 12 Klein, Organic Chemistry 2 e

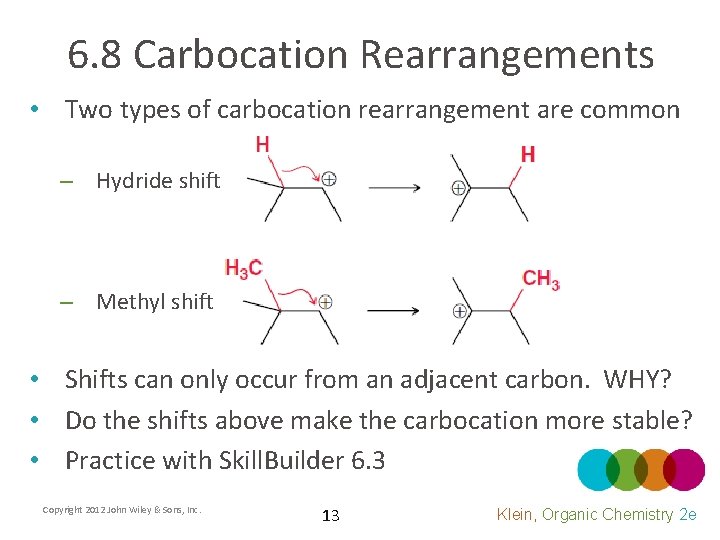
6. 8 Carbocation Rearrangements • Two types of carbocation rearrangement are common – Hydride shift – Methyl shift • Shifts can only occur from an adjacent carbon. WHY? • Do the shifts above make the carbocation more stable? • Practice with Skill. Builder 6. 3 Copyright 2012 John Wiley & Sons, Inc. 13 Klein, Organic Chemistry 2 e

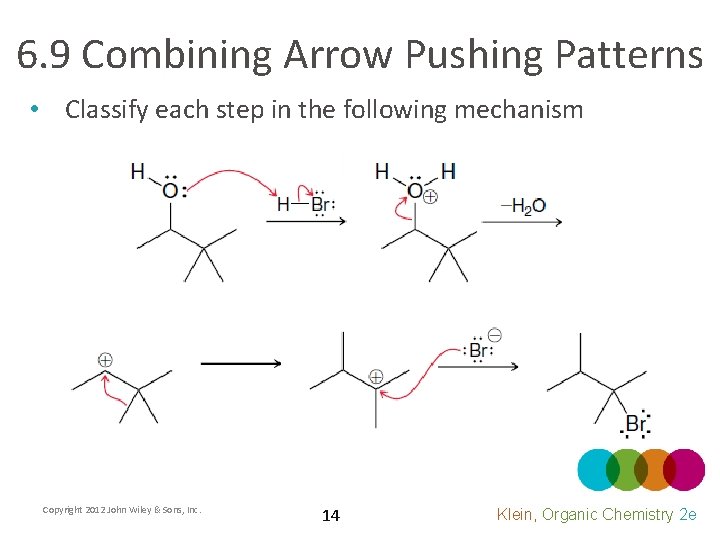
6. 9 Combining Arrow Pushing Patterns • Classify each step in the following mechanism Copyright 2012 John Wiley & Sons, Inc. 14 Klein, Organic Chemistry 2 e

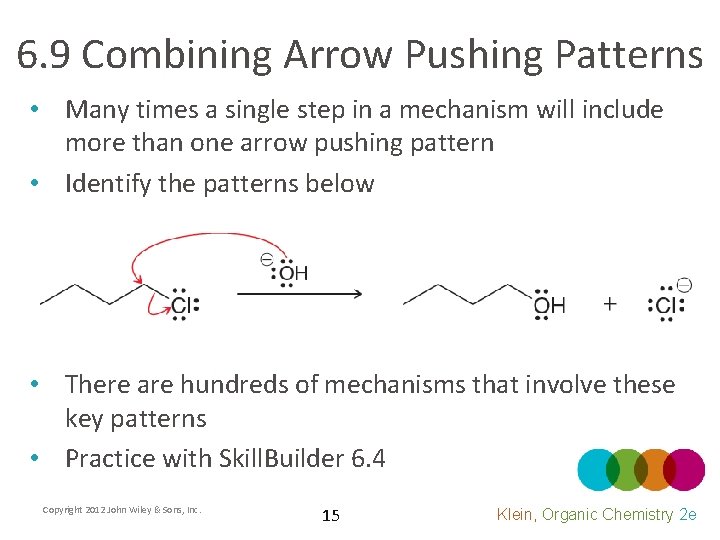
6. 9 Combining Arrow Pushing Patterns • Many times a single step in a mechanism will include more than one arrow pushing pattern • Identify the patterns below • There are hundreds of mechanisms that involve these key patterns • Practice with Skill. Builder 6. 4 Copyright 2012 John Wiley & Sons, Inc. 15 Klein, Organic Chemistry 2 e

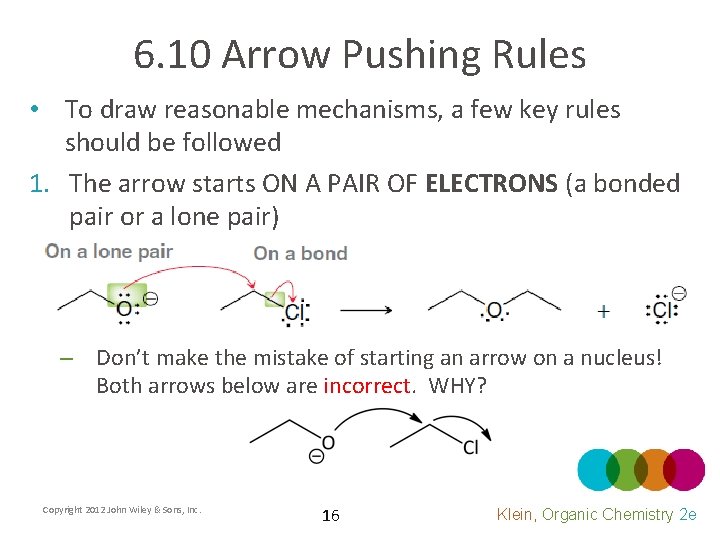
6. 10 Arrow Pushing Rules • To draw reasonable mechanisms, a few key rules should be followed 1. The arrow starts ON A PAIR OF ELECTRONS (a bonded pair or a lone pair) – Don’t make the mistake of starting an arrow on a nucleus! Both arrows below are incorrect. WHY? Copyright 2012 John Wiley & Sons, Inc. 16 Klein, Organic Chemistry 2 e

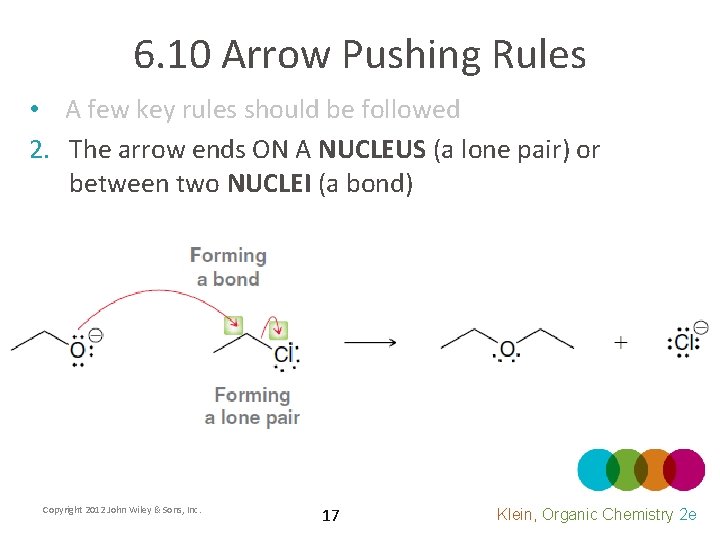
6. 10 Arrow Pushing Rules • A few key rules should be followed 2. The arrow ends ON A NUCLEUS (a lone pair) or between two NUCLEI (a bond) Copyright 2012 John Wiley & Sons, Inc. 17 Klein, Organic Chemistry 2 e

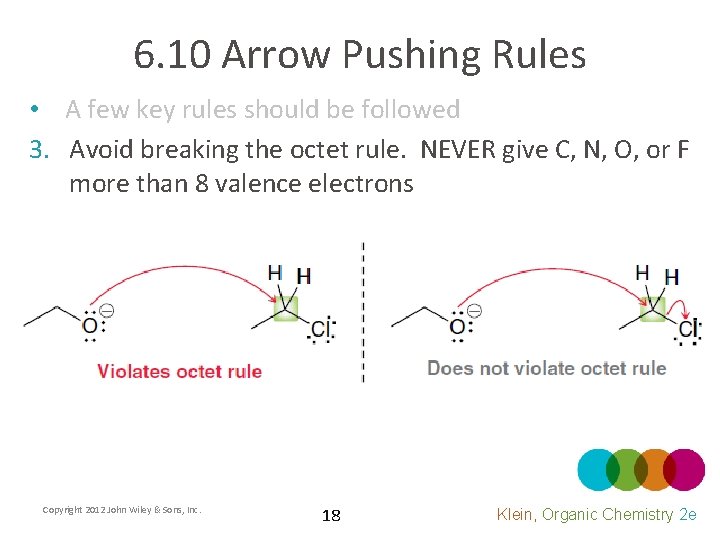
6. 10 Arrow Pushing Rules • A few key rules should be followed 3. Avoid breaking the octet rule. NEVER give C, N, O, or F more than 8 valence electrons Copyright 2012 John Wiley & Sons, Inc. 18 Klein, Organic Chemistry 2 e

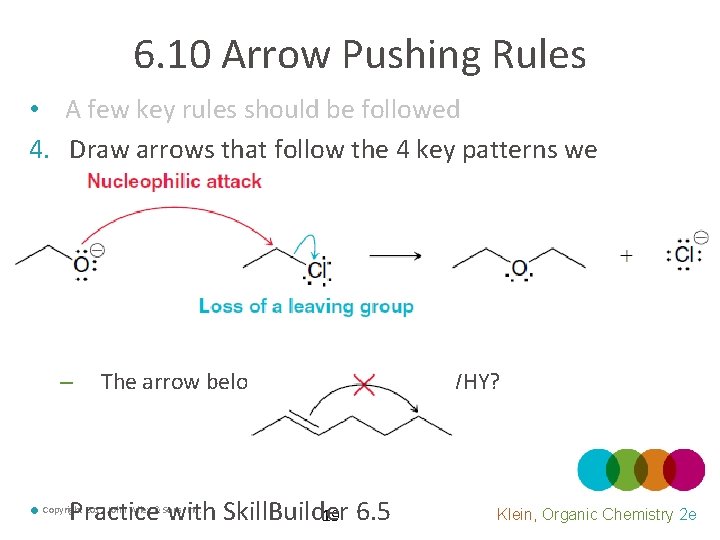
6. 10 Arrow Pushing Rules • A few key rules should be followed 4. Draw arrows that follow the 4 key patterns we outlined – • The arrow below is unreasonable. WHY? Practice with Skill. Builder 19 6. 5 Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 2 e

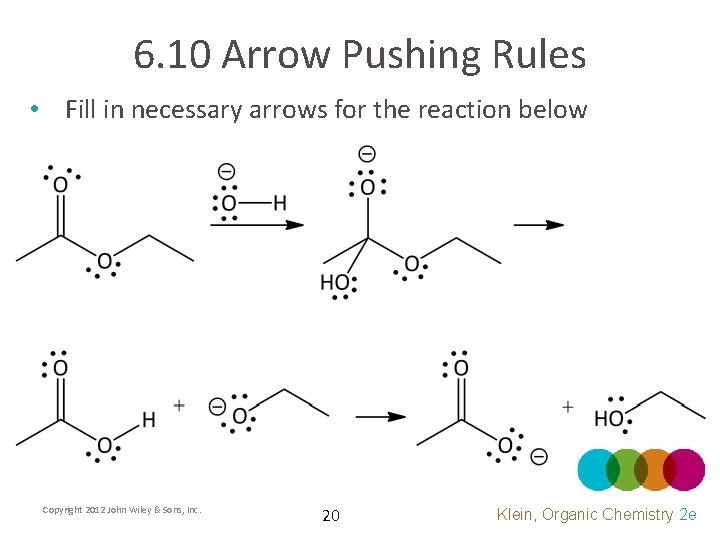
6. 10 Arrow Pushing Rules • Fill in necessary arrows for the reaction below Copyright 2012 John Wiley & Sons, Inc. 20 Klein, Organic Chemistry 2 e

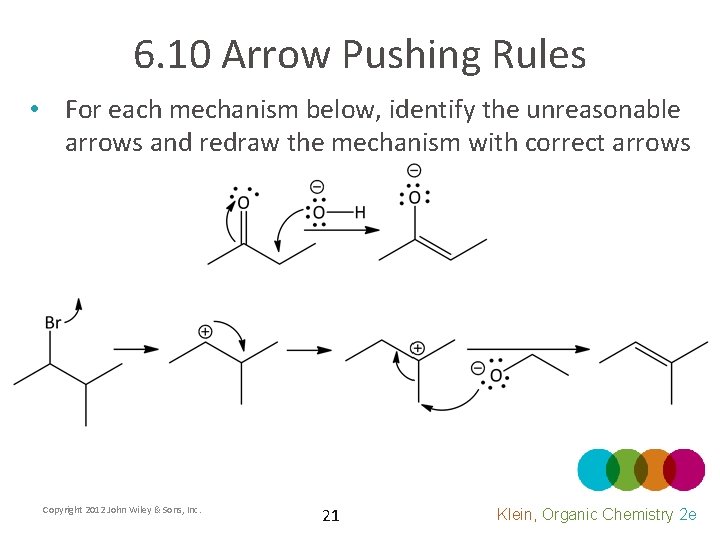
6. 10 Arrow Pushing Rules • For each mechanism below, identify the unreasonable arrows and redraw the mechanism with correct arrows Copyright 2012 John Wiley & Sons, Inc. 21 Klein, Organic Chemistry 2 e

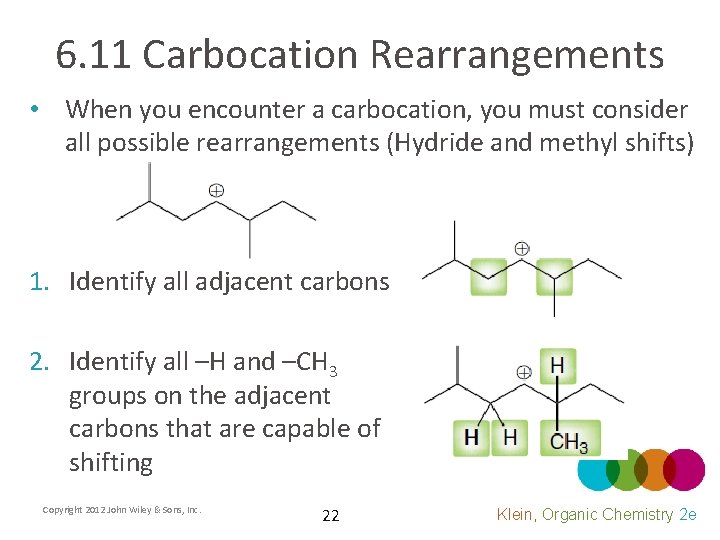
6. 11 Carbocation Rearrangements • When you encounter a carbocation, you must consider all possible rearrangements (Hydride and methyl shifts) 1. Identify all adjacent carbons 2. Identify all –H and –CH 3 groups on the adjacent carbons that are capable of shifting Copyright 2012 John Wiley & Sons, Inc. 22 Klein, Organic Chemistry 2 e

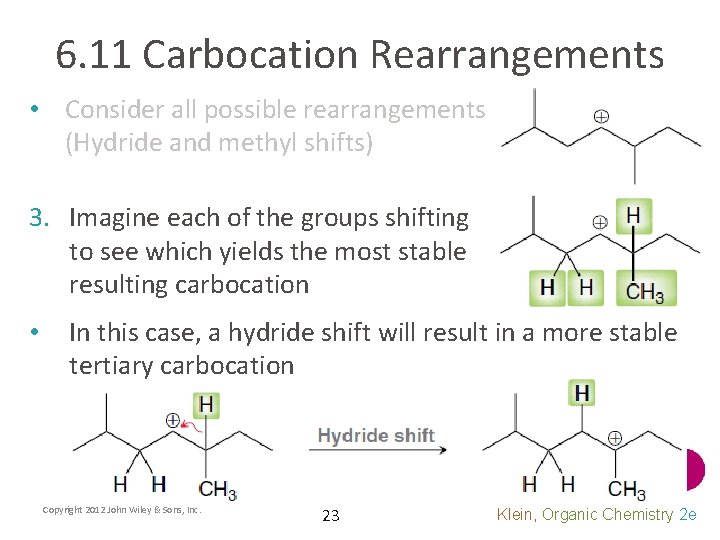
6. 11 Carbocation Rearrangements • Consider all possible rearrangements (Hydride and methyl shifts) 3. Imagine each of the groups shifting to see which yields the most stable resulting carbocation • In this case, a hydride shift will result in a more stable tertiary carbocation Copyright 2012 John Wiley & Sons, Inc. 23 Klein, Organic Chemistry 2 e

6. 11 Carbocation Rearrangements • Complete the same analysis for the molecule below 1. Identify all adjacent carbons 2. Identify all –H and –CH 3 groups capable of shifting 3. Determine which shift yields the most stable carbocation • Recall that allylic carbocations are especially stable • Practice with Skill. Builder 6. 6 Copyright 2012 John Wiley & Sons, Inc. 24 Klein, Organic Chemistry 2 e

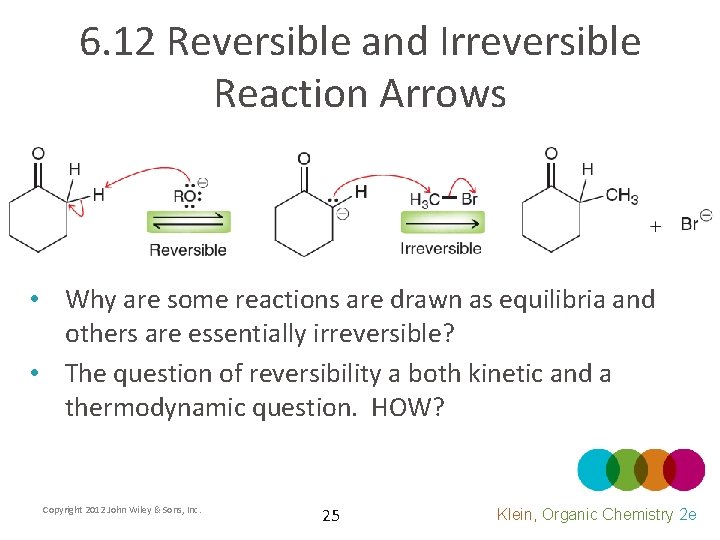
6. 12 Reversible and Irreversible Reaction Arrows • Why are some reactions are drawn as equilibria and others are essentially irreversible? • The question of reversibility a both kinetic and a thermodynamic question. HOW? Copyright 2012 John Wiley & Sons, Inc. 25 Klein, Organic Chemistry 2 e

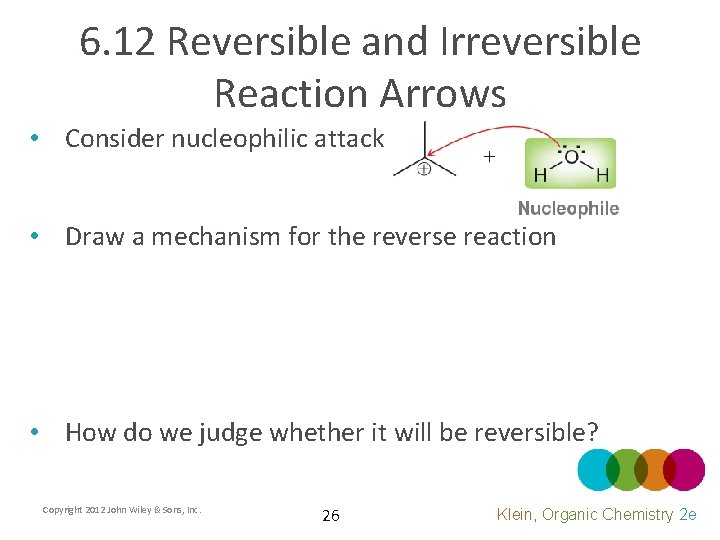
6. 12 Reversible and Irreversible Reaction Arrows • Consider nucleophilic attack • Draw a mechanism for the reverse reaction • How do we judge whether it will be reversible? Copyright 2012 John Wiley & Sons, Inc. 26 Klein, Organic Chemistry 2 e

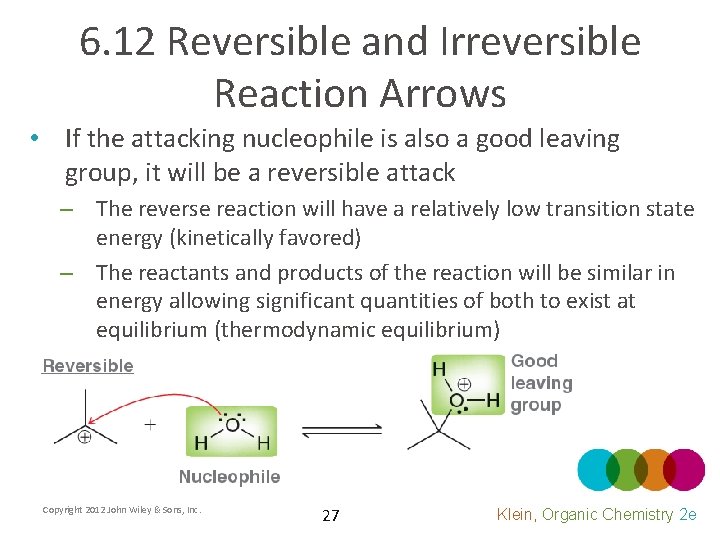
6. 12 Reversible and Irreversible Reaction Arrows • If the attacking nucleophile is also a good leaving group, it will be a reversible attack – The reverse reaction will have a relatively low transition state energy (kinetically favored) – The reactants and products of the reaction will be similar in energy allowing significant quantities of both to exist at equilibrium (thermodynamic equilibrium) Copyright 2012 John Wiley & Sons, Inc. 27 Klein, Organic Chemistry 2 e

6. 12 Reversible and Irreversible Reaction Arrows • If the attacking nucleophile is a poor leaving group, it will essentially be an irreversible attack – The reverse reaction will have a relatively HIGH transition state energy (kinetically disfavored) – The products will be much lower in energy so an insignificant quantity of reactant will remain at equilibrium Copyright 2012 John Wiley & Sons, Inc. 28 Klein, Organic Chemistry 2 e

6. 12 Reversible and Irreversible Reaction Arrows • Consider loss or a leaving group • Draw a mechanism for the reverse reaction • How do we judge whether it will be reversible? Copyright 2012 John Wiley & Sons, Inc. 29 Klein, Organic Chemistry 2 e

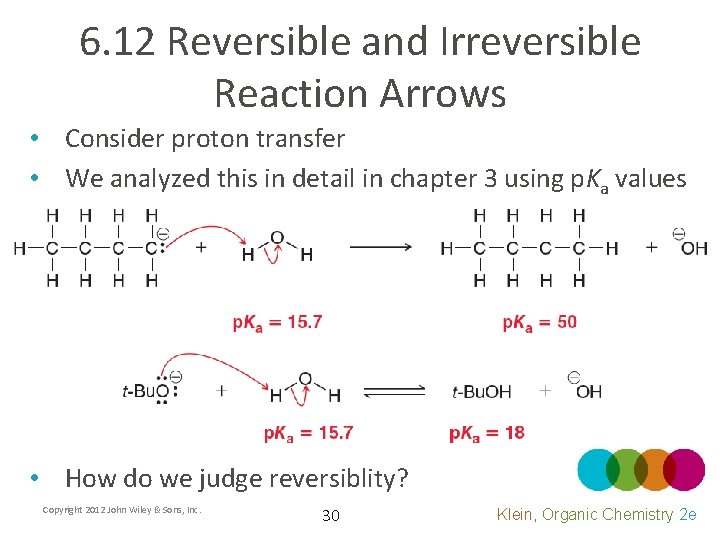
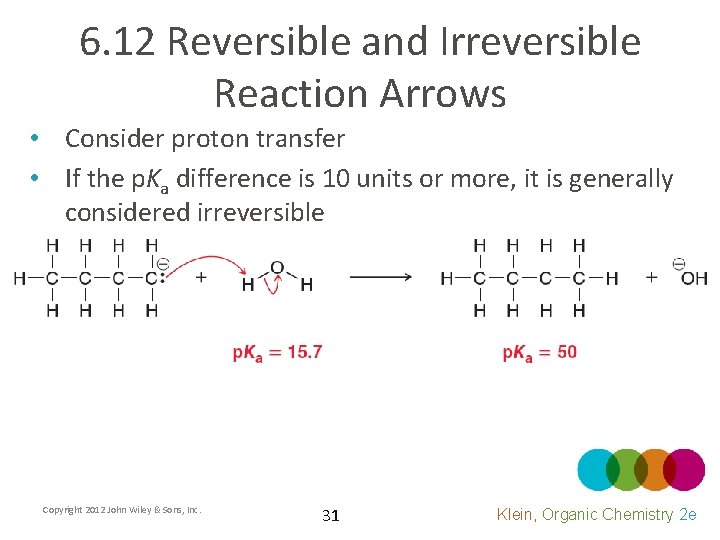
6. 12 Reversible and Irreversible Reaction Arrows • Consider proton transfer • We analyzed this in detail in chapter 3 using p. Ka values • How do we judge reversiblity? Copyright 2012 John Wiley & Sons, Inc. 30 Klein, Organic Chemistry 2 e

6. 12 Reversible and Irreversible Reaction Arrows • Consider proton transfer • If the p. Ka difference is 10 units or more, it is generally considered irreversible Copyright 2012 John Wiley & Sons, Inc. 31 Klein, Organic Chemistry 2 e

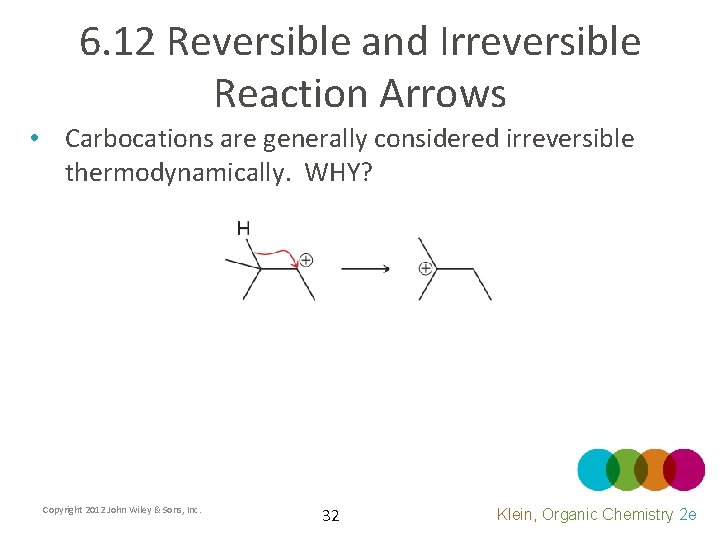
6. 12 Reversible and Irreversible Reaction Arrows • Carbocations are generally considered irreversible thermodynamically. WHY? Copyright 2012 John Wiley & Sons, Inc. 32 Klein, Organic Chemistry 2 e

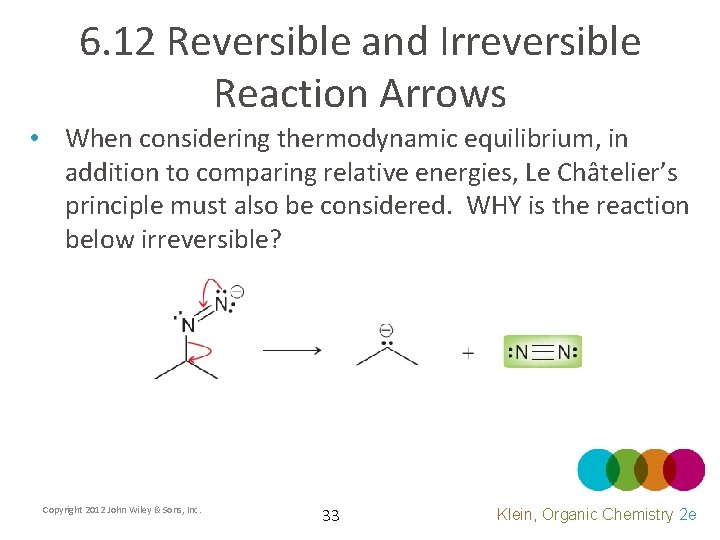
6. 12 Reversible and Irreversible Reaction Arrows • When considering thermodynamic equilibrium, in addition to comparing relative energies, Le Châtelier’s principle must also be considered. WHY is the reaction below irreversible? Copyright 2012 John Wiley & Sons, Inc. 33 Klein, Organic Chemistry 2 e

Study Guide for sections 6. 7 -6. 12 DAY 13, Terms to know: Sections 6. 7 -6. 12 nucleophile, electrophile, Nucleophilic Attack, Loss of a Leaving Group, Proton Transfers (Acid/Base), Rearrangements, hyperconjugation DAY 13, Specific outcomes and skills that may be tested on exam 2: Sections 6. 7 -6. 12 Specific outcomes and skills that may be tested on exam 2: • Given a molecule, be able to identify all of the nucleophilic sites and rank them in terms of their strength as a nucleophile. • Given a molecule, be able to identify all of the electrophilic sites and rank them in terms of their strength as an electrophile. • Given reaction arrows, be able to identify Nucleophilic Attack, Loss of a Leaving Group, Proton Transfers (Acid/Base), and carbocation Rearrangements. • Given one of the following terms: Nucleophilic Attack, Loss of a Leaving Group, Proton Transfers (Acid/Base), carbocation Rearrangement, be able to draw a reaction and correct arrows showing the corresponding electron movement. • Be able to list the rules for arrow pushing and use them properly when drawing mechanisms. • NEVER draw arrows that would give an atom in the 2 nd row of the periodic table more than 8 valence electrons, and never draw an atom in the 2 nd row of the periodic table with more than 8 valence electrons. • Be able to explain how the intramolecular nature of carbocation rearrangements allows them to occur with relatively fast reaction rates. • Given a reaction mechanism with arrows drawn incorrectly, be able to identify the incorrect arrows and replace them with reasonable arrows. Klein, Organic. Chemistry 2 e 2 e Klein, Organic 34

Practice Problems for sections 6. 7 -6. 12 Complete these problems outside of class until you are confident you have learned the SKILLS in this section outlined on the study guide and we will review some of them next class period. 6. 8 6. 9 6. 10 6. 11 6. 12 6. 13 6. 14 6. 16 6. 17 6. 18 6. 19 6. 30 6. 32 6. 33 6. 34 6. 36 6. 39 6. 40 6. 42 6. 43 6. 44 6. 45 6. 47 6. 48 6. 49 35 Klein, Organic. Chemistry 2 e 2 e Klein, Organic

Day 14: EXAM 2 Prep for Day 15 Must Watch videos: https: //www. youtube. com/watch? v=h 9 AIDOf 6 t. M 8, https: //www. youtube. com/watch? v=3 K 0 i 3 g. QQf. L 4, https: //www. youtube. com/watch? v=Q 3 m. Lbf. Sz. Ypk (SN 2 reactions) https: //www. youtube. com/watch? v=Oopqm. Yn. QLy. Q, https: //www. youtube. com/watch? v=yn. Upwvt. CU 9 o, https: //www. youtube. com/watch? v=e. QWpoijsx. I 4 (SN 1 reactions) https: //www. youtube. com/watch? v=Tn. Y 1 S 5 Id. Vq. I (SN 2 and SN 1 animations) Other helpful videos: https: //www. youtube. com/watch? v=ji. Jd. NWz. ETSk (naming alkyl halides) https: //www. youtube. com/watch? v=h 5 xva. P 6 b. IZI and https: //www. youtube. com/watch? v=Jmc. Vg. E 2 WKBE (SN 2 and SN 1 animations) http: //ps. uci. edu/content/chem-51 a-organic-chemistry (lectures 22 -23) Read sections 7. 1 -7. 5 36 Klein, Organic. Chemistry 2 e 2 e Klein, Organic
 How to identify nucleophile and electrophile
How to identify nucleophile and electrophile Common name of alkene
Common name of alkene Integrated cost leadership/differentiation strategy
Integrated cost leadership/differentiation strategy Timex cost leadership strategy
Timex cost leadership strategy Prolepsis
Prolepsis Actor focus vs object focus
Actor focus vs object focus Focus group advantages and disadvantages
Focus group advantages and disadvantages Advantages of focus groups
Advantages of focus groups Focus mode and diffuse mode
Focus mode and diffuse mode Core focus on proportions and probability answer key
Core focus on proportions and probability answer key Focus group advantages and disadvantages
Focus group advantages and disadvantages Pros and cons of focus groups
Pros and cons of focus groups Robert longo
Robert longo Tilting
Tilting Focus group advantages and disadvantages
Focus group advantages and disadvantages Developing competitive advantage and strategic focus
Developing competitive advantage and strategic focus Difference between focus and concentration
Difference between focus and concentration Coso cube 2013
Coso cube 2013 Focus group advantages and disadvantages
Focus group advantages and disadvantages Focus and epicenter of an earthquake
Focus and epicenter of an earthquake Romanticism principles
Romanticism principles Earthquake diagram labeled
Earthquake diagram labeled Dominance and focus in a work
Dominance and focus in a work Blue ocean strategy focus divergence and compelling tagline
Blue ocean strategy focus divergence and compelling tagline What are connectors
What are connectors Focus and epicenter of an earthquake
Focus and epicenter of an earthquake Significance of light
Significance of light Uwsom explore and focus
Uwsom explore and focus When a driver's awareness and focus drift from
When a driver's awareness and focus drift from Literary focus transcendentalism and romanticism answer key
Literary focus transcendentalism and romanticism answer key Mnemosyne meaning
Mnemosyne meaning Focus and coherence
Focus and coherence Focus divergence tagline
Focus divergence tagline Lesson 6 language focus
Lesson 6 language focus Unit 2 12 language focus
Unit 2 12 language focus Customer focus examples
Customer focus examples What is fault
What is fault