CHEMISTRY OF BENZENE ELECTROPHILIC AROMATIC SUBSTITUTION CHEM 2425

CHEMISTRY OF BENZENE: ELECTROPHILIC AROMATIC SUBSTITUTION CHEM 2425 Chapter 16

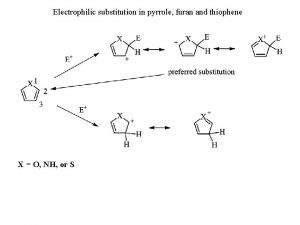
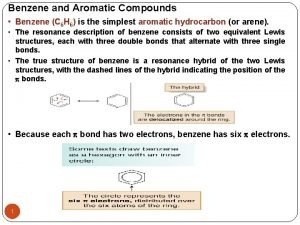
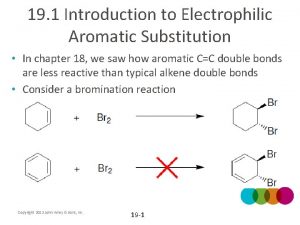
Benzene Reactivity • Unsaturated, but doesn’t behave like alkene • Alkenes: • Benzene: • Reaction is called electrophilic aromatic substitution

Energy Diagram • Aromatic (substitution) product is more stable than product from addition across double bond

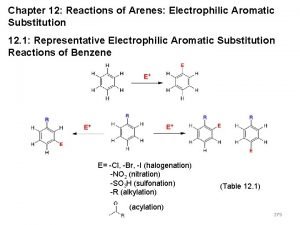
Reactions of Aromatic Compounds Electrophilic aromatic substitution mechanism II. Halogenation III. Sulfonation IV. Nitration V. Friedel-Crafts Alkylation and Acylation VI. Reactions of Benzene Substituents VII. EAS on Substituted Benzenes VIII. Synthesis I.

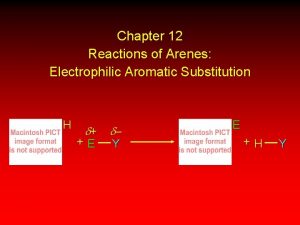
Electrophilic Aromatic Substitution • Aromatic ring (nucleophile) + electrophile

REACTIONS OF AROMATIC COMPOUNDS. Electrophilic Aromatic Substitution TNT, useful compound! Ibuprofen (Motrin, Advil), useful compound!

Electrophilic Aromatic Substitution • Mechanism:
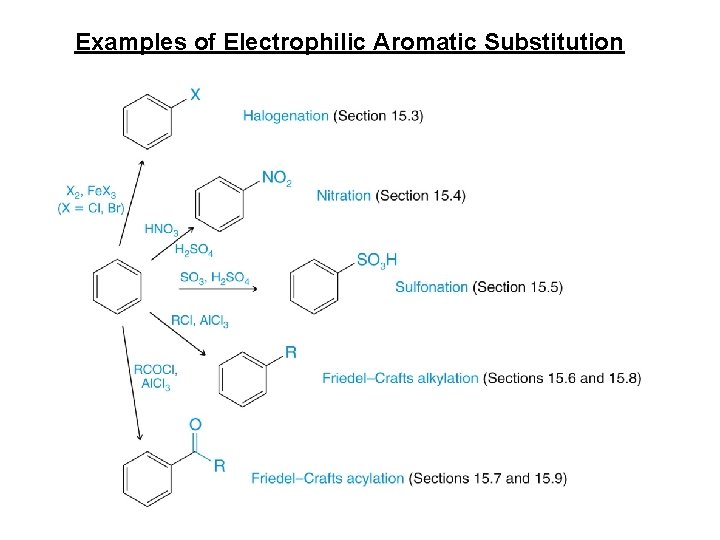
Examples of Electrophilic Aromatic Substitution
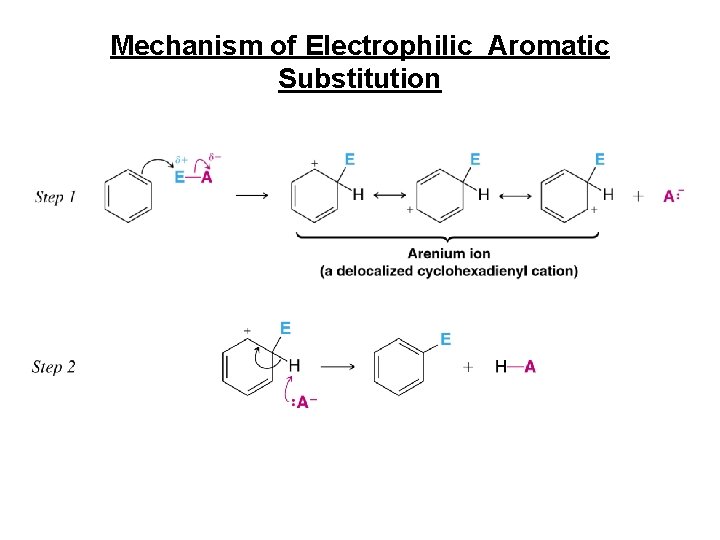
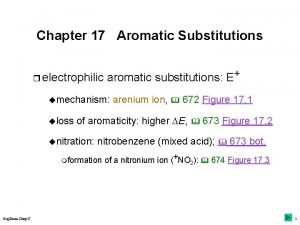
Mechanism of Electrophilic Aromatic Substitution
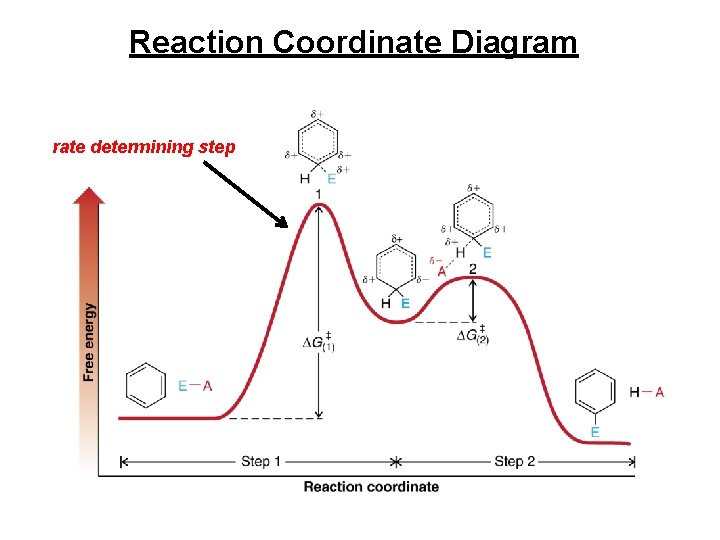
Reaction Coordinate Diagram rate determining step
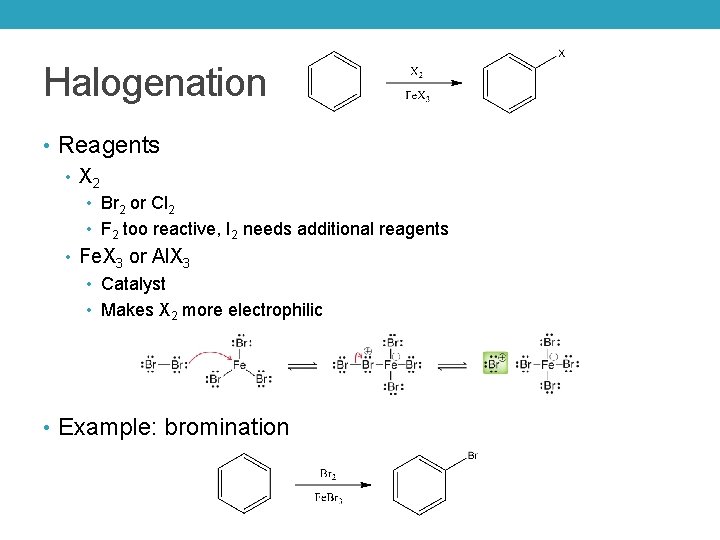
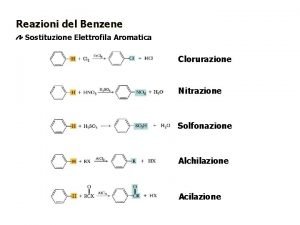
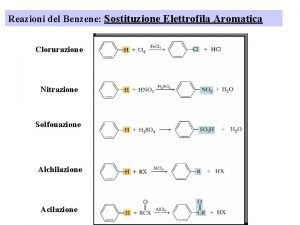
Halogenation • Reagents • X 2 • Br 2 or Cl 2 • F 2 too reactive, I 2 needs additional reagents • Fe. X 3 or Al. X 3 • Catalyst • Makes X 2 more electrophilic • Example: bromination
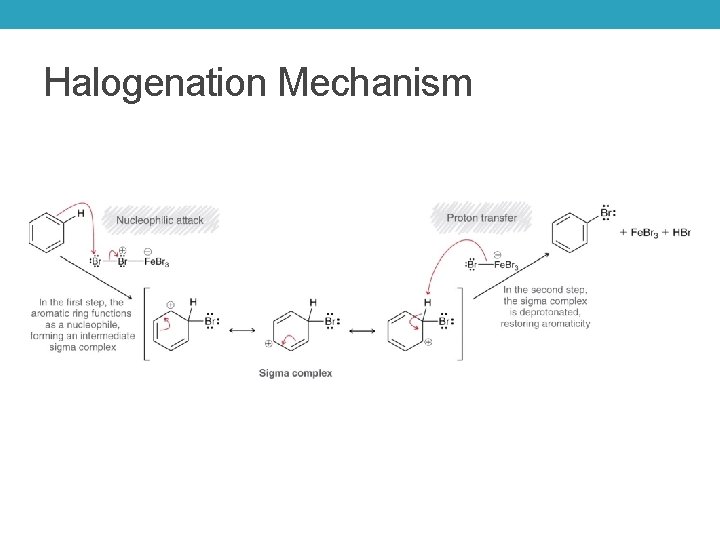
Halogenation Mechanism
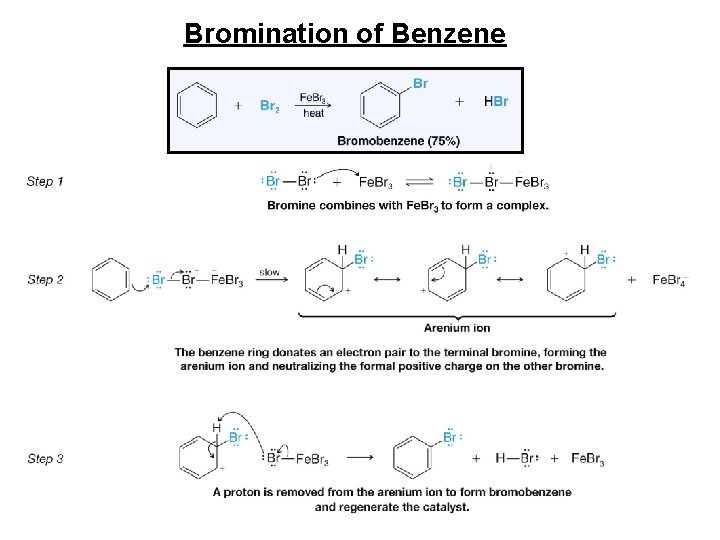
Bromination of Benzene
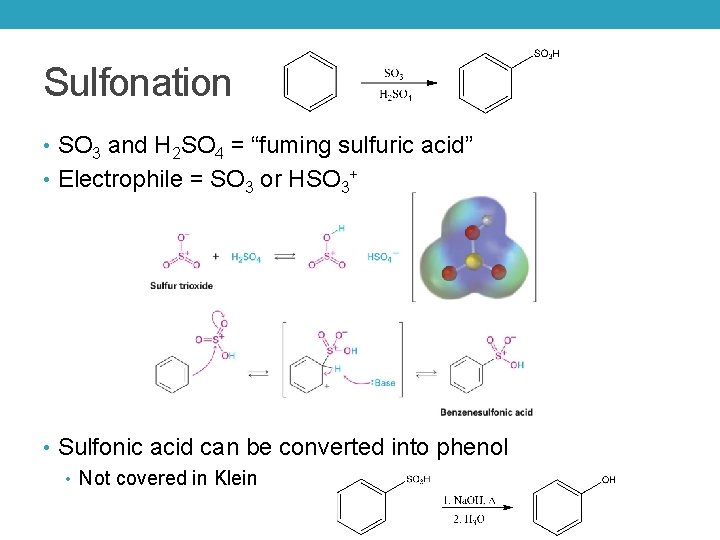
Sulfonation • SO 3 and H 2 SO 4 = “fuming sulfuric acid” • Electrophile = SO 3 or HSO 3+ • Sulfonic acid can be converted into phenol • Not covered in Klein
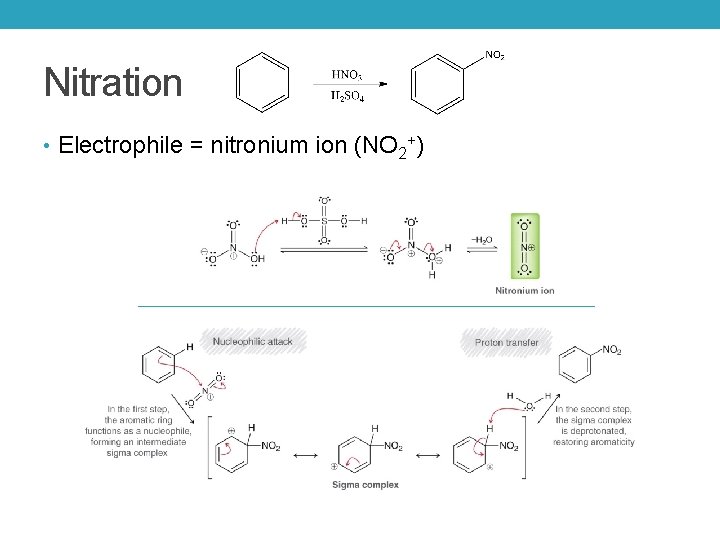
Nitration • Electrophile = nitronium ion (NO 2+)
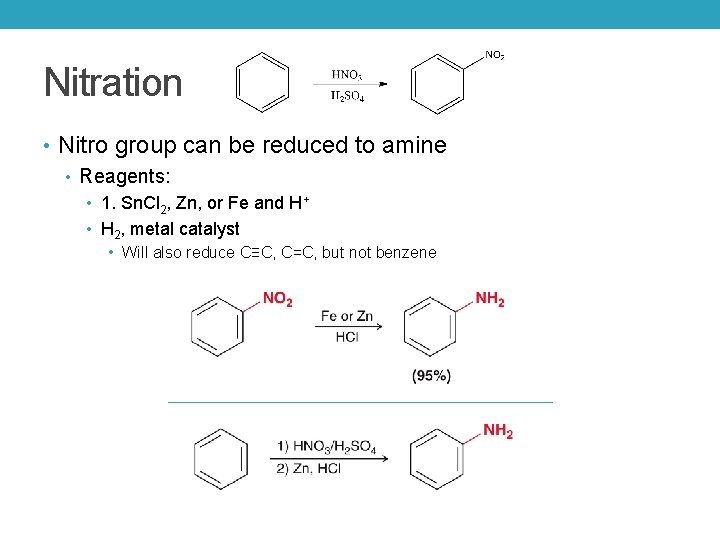
Nitration • Nitro group can be reduced to amine • Reagents: • 1. Sn. Cl 2, Zn, or Fe and H+ • H 2, metal catalyst • Will also reduce C≡C, C=C, but not benzene
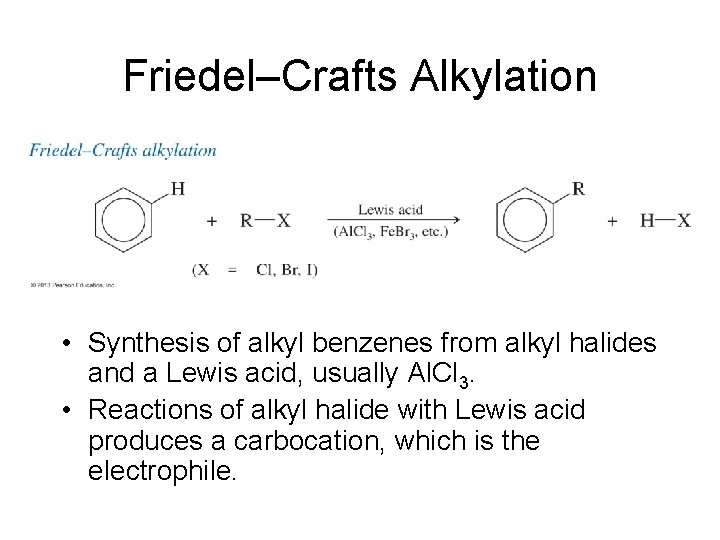
Friedel–Crafts Alkylation • Synthesis of alkyl benzenes from alkyl halides and a Lewis acid, usually Al. Cl 3. • Reactions of alkyl halide with Lewis acid produces a carbocation, which is the electrophile.
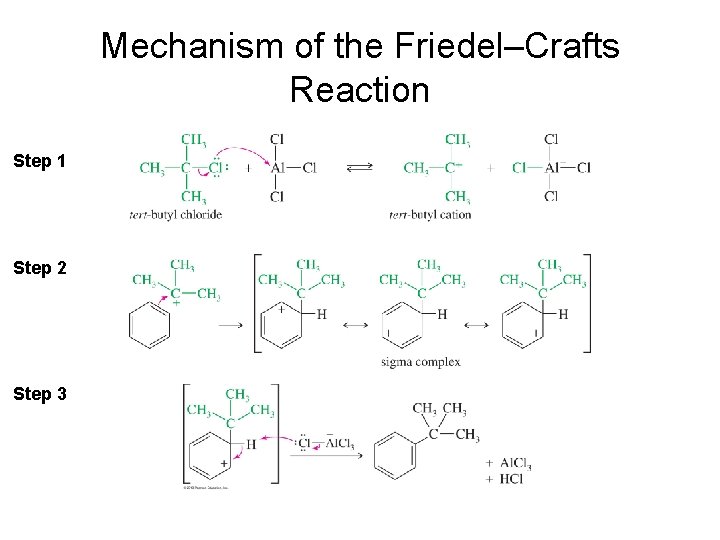
Mechanism of the Friedel–Crafts Reaction Step 1 Step 2 Step 3

Limitations of Friedel–Crafts • Reaction fails if benzene has a substituent that is more deactivating than halogens. • Rearrangements are possible. • The alkylbenzene product is more reactive than benzene, so polyalkylation occurs.
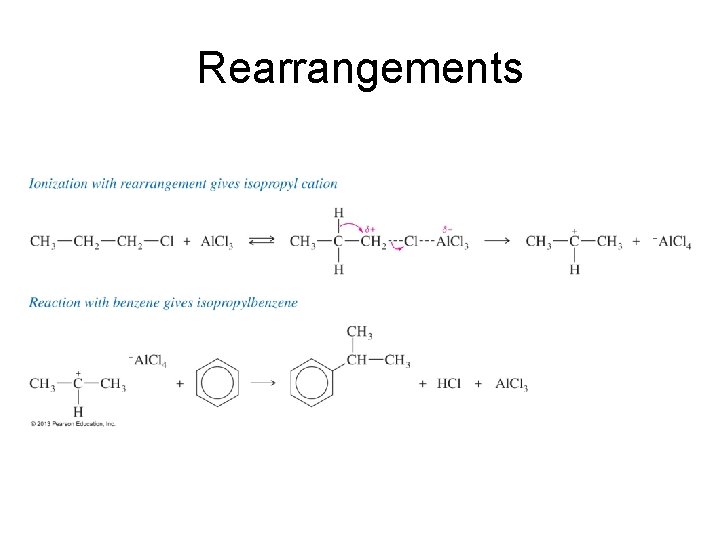
Rearrangements
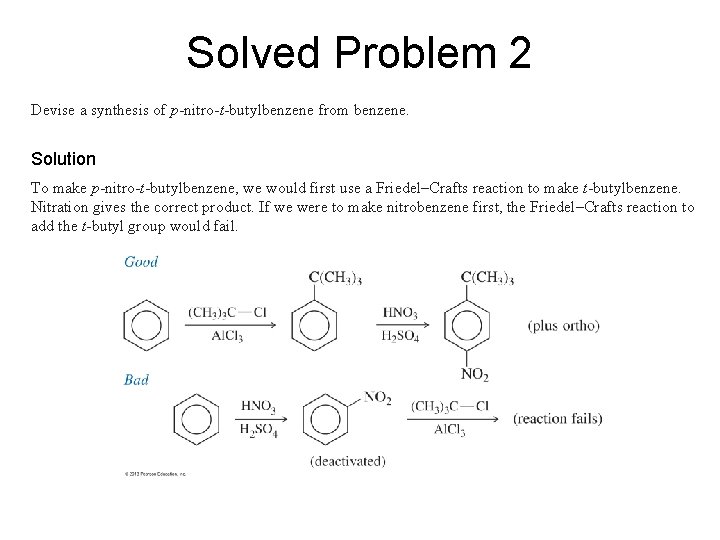
Solved Problem 2 Devise a synthesis of p-nitro-t-butylbenzene from benzene. Solution To make p-nitro-t-butylbenzene, we would first use a Friedel–Crafts reaction to make t-butylbenzene. Nitration gives the correct product. If we were to make nitrobenzene first, the Friedel–Crafts reaction to add the t-butyl group would fail.
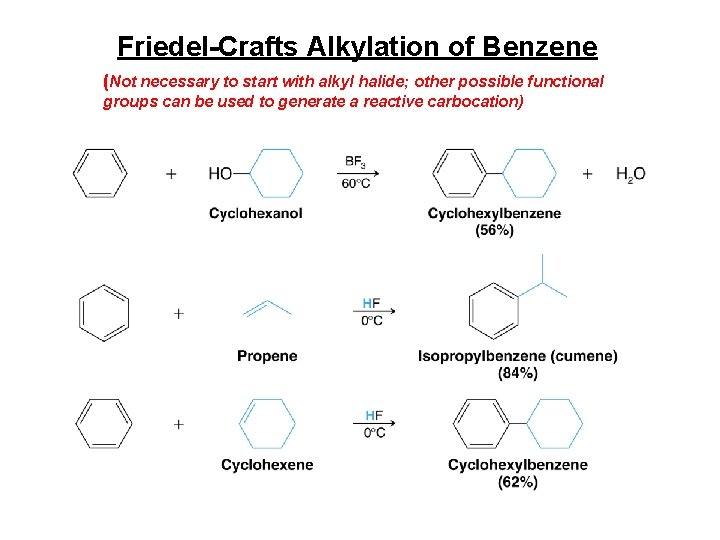
Friedel-Crafts Alkylation of Benzene (Not necessary to start with alkyl halide; other possible functional groups can be used to generate a reactive carbocation)
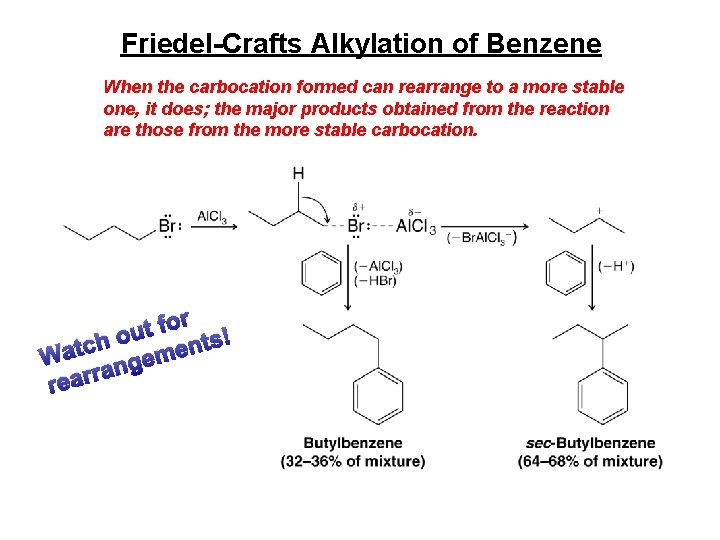
Friedel-Crafts Alkylation of Benzene When the carbocation formed can rearrange to a more stable one, it does; the major products obtained from the reaction are those from the more stable carbocation. r o f t ou h ts! c n t e a m W e g n ra r a e r
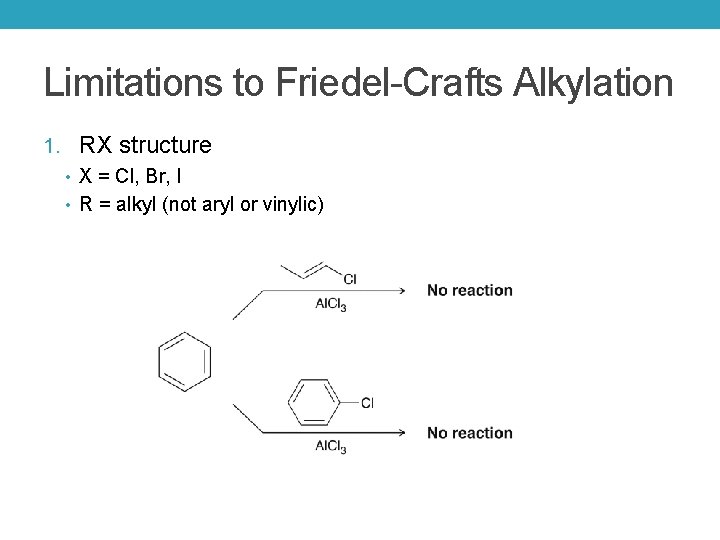
Limitations to Friedel-Crafts Alkylation 1. RX structure • X = Cl, Br, I • R = alkyl (not aryl or vinylic)
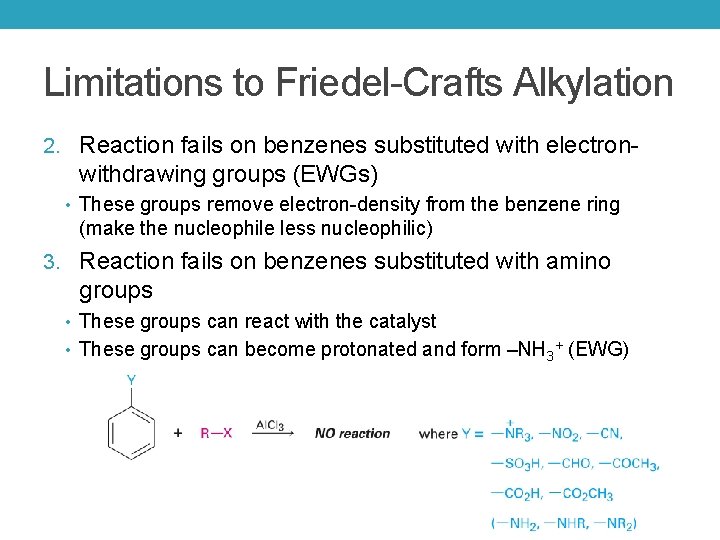
Limitations to Friedel-Crafts Alkylation 2. Reaction fails on benzenes substituted with electron- withdrawing groups (EWGs) • These groups remove electron-density from the benzene ring (make the nucleophile less nucleophilic) 3. Reaction fails on benzenes substituted with amino groups • These groups can react with the catalyst • These groups can become protonated and form –NH 3+ (EWG)
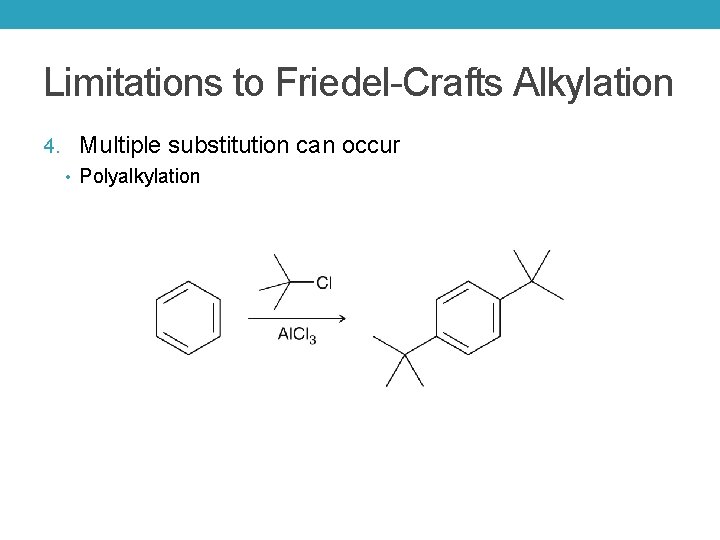
Limitations to Friedel-Crafts Alkylation 4. Multiple substitution can occur • Polyalkylation
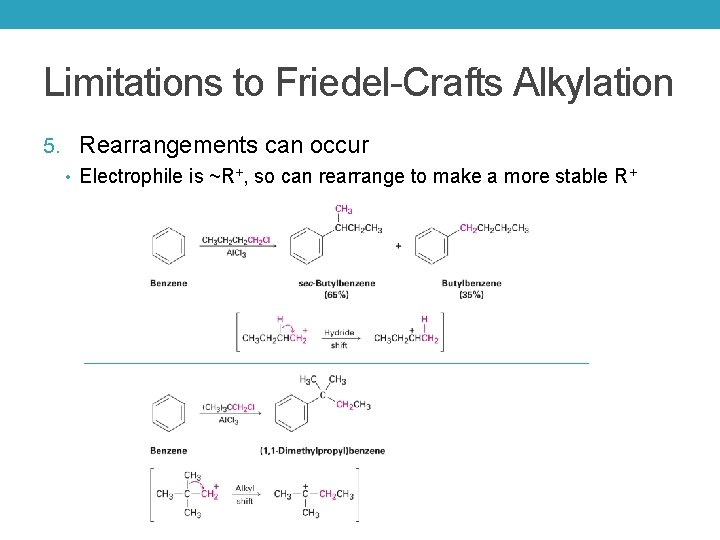
Limitations to Friedel-Crafts Alkylation 5. Rearrangements can occur • Electrophile is ~R+, so can rearrange to make a more stable R+
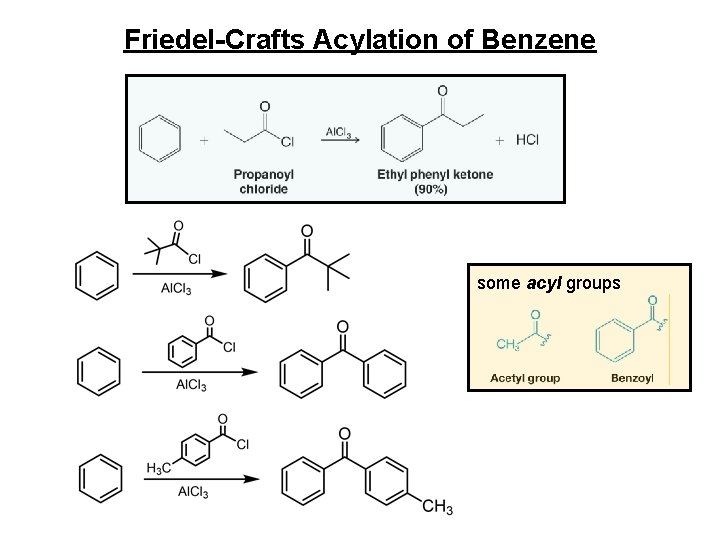
Friedel-Crafts Acylation of Benzene some acyl groups
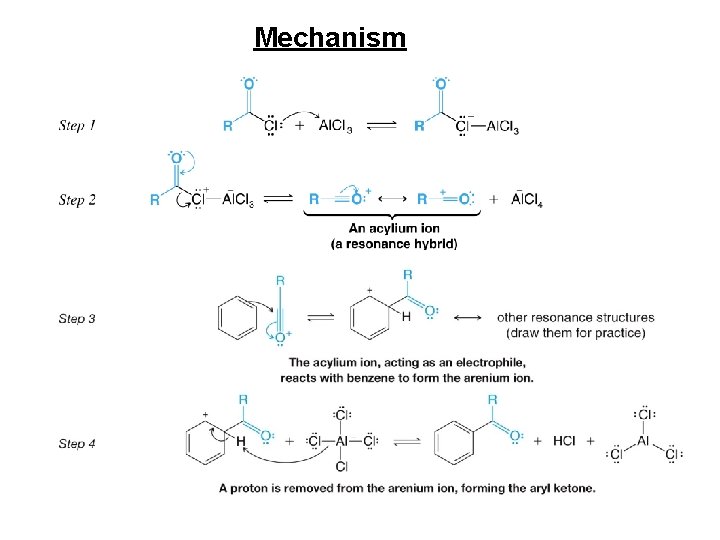
Mechanism
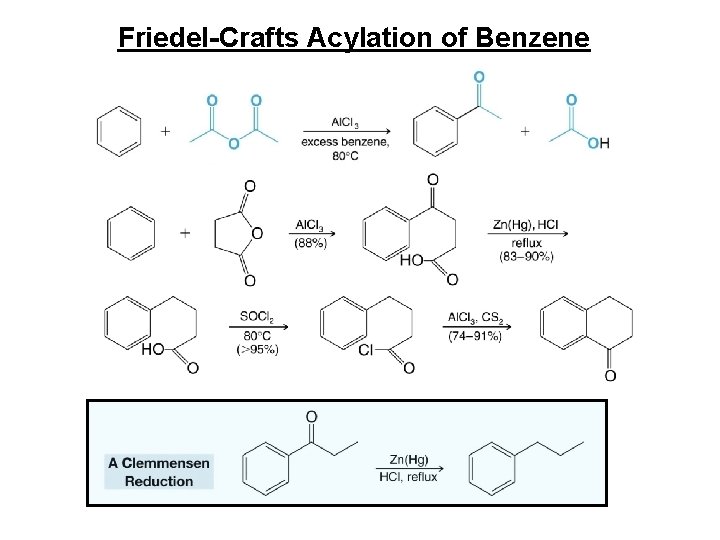
Friedel-Crafts Acylation of Benzene
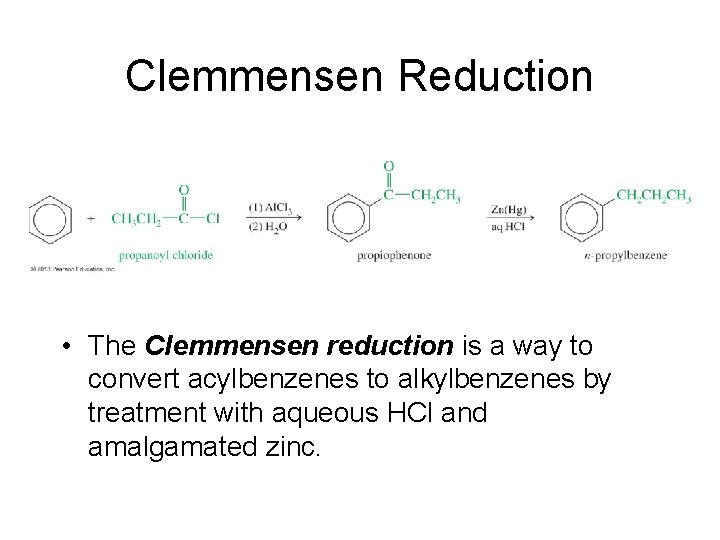
Clemmensen Reduction • The Clemmensen reduction is a way to convert acylbenzenes to alkylbenzenes by treatment with aqueous HCl and amalgamated zinc.
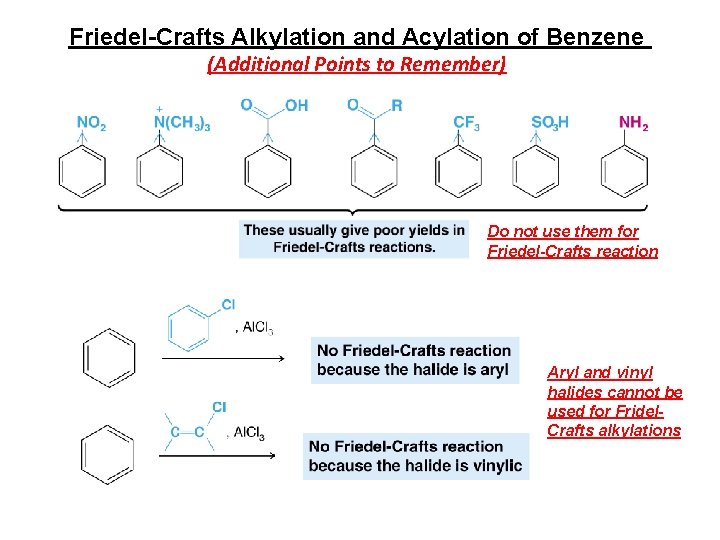
Friedel-Crafts Alkylation and Acylation of Benzene (Additional Points to Remember) Do not use them for Friedel-Crafts reaction Aryl and vinyl halides cannot be used for Fridel. Crafts alkylations
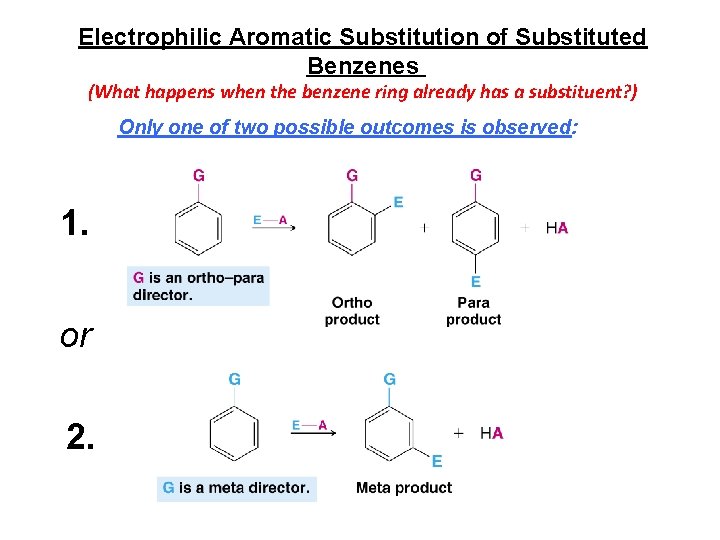
Electrophilic Aromatic Substitution of Substituted Benzenes (What happens when the benzene ring already has a substituent? ) Only one of two possible outcomes is observed: 1. or 2.
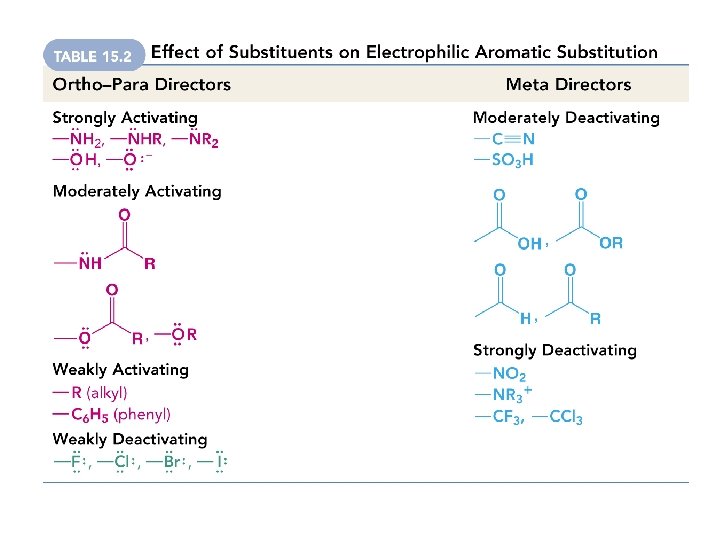
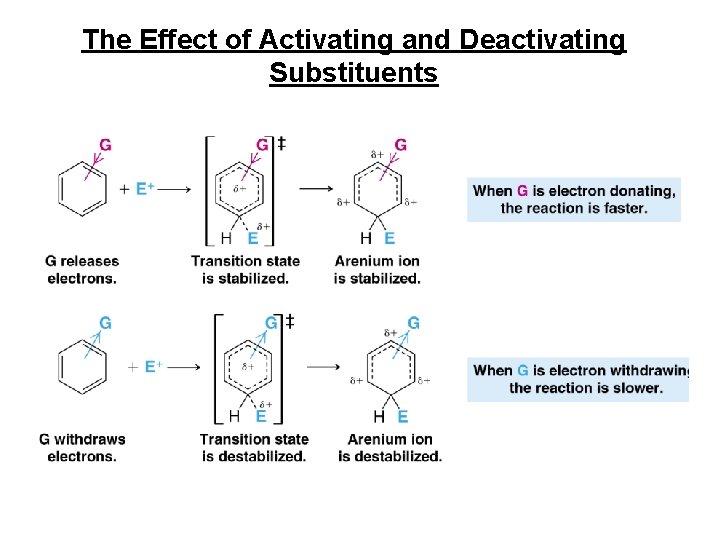
The Effect of Activating and Deactivating Substituents
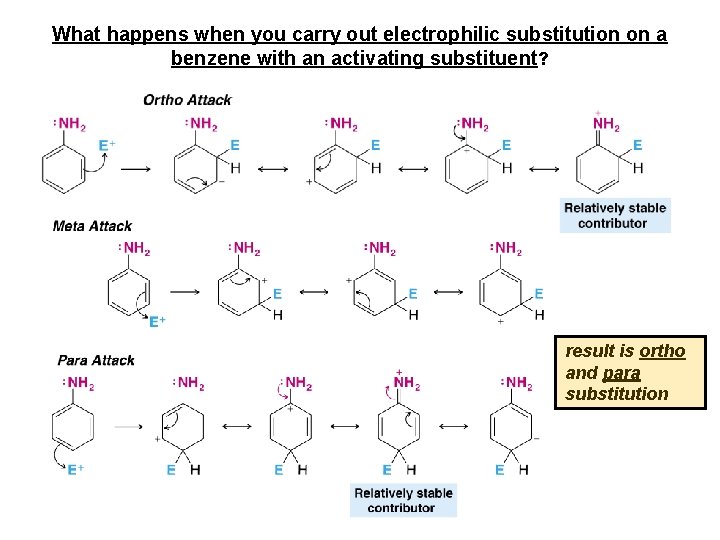
What happens when you carry out electrophilic substitution on a benzene with an activating substituent? result is ortho and para substitution
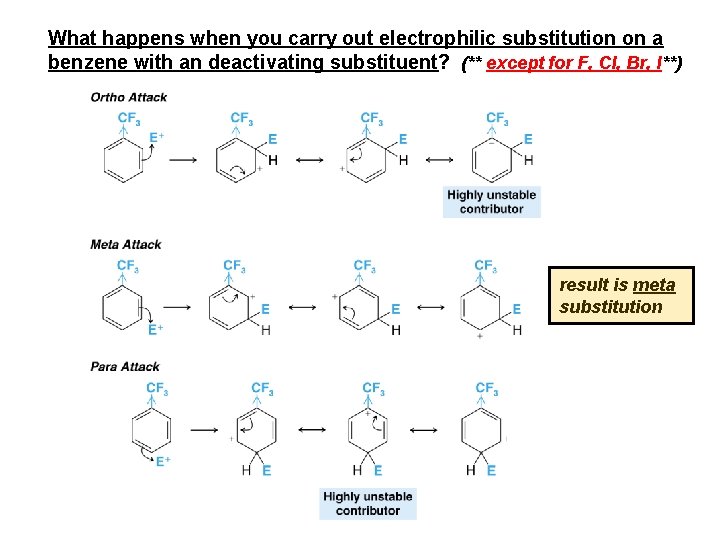
What happens when you carry out electrophilic substitution on a benzene with an deactivating substituent? (** except for F, Cl, Br, I**) result is meta substitution
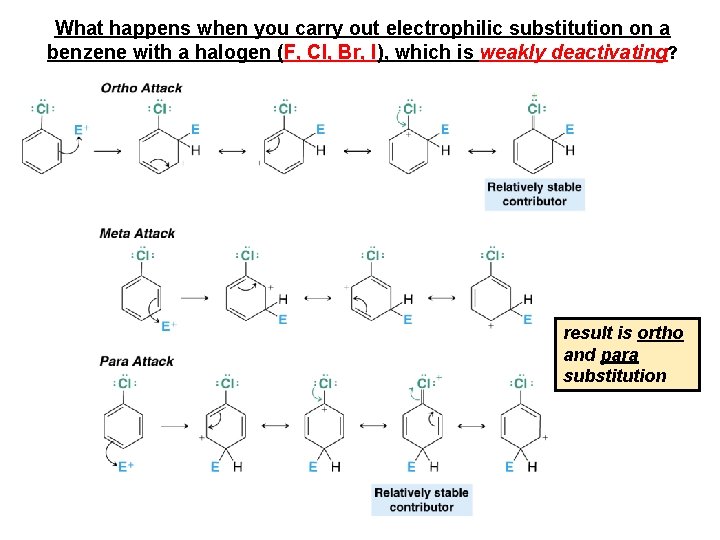
What happens when you carry out electrophilic substitution on a benzene with a halogen (F, Cl, Br, I), which is weakly deactivating? result is ortho and para substitution
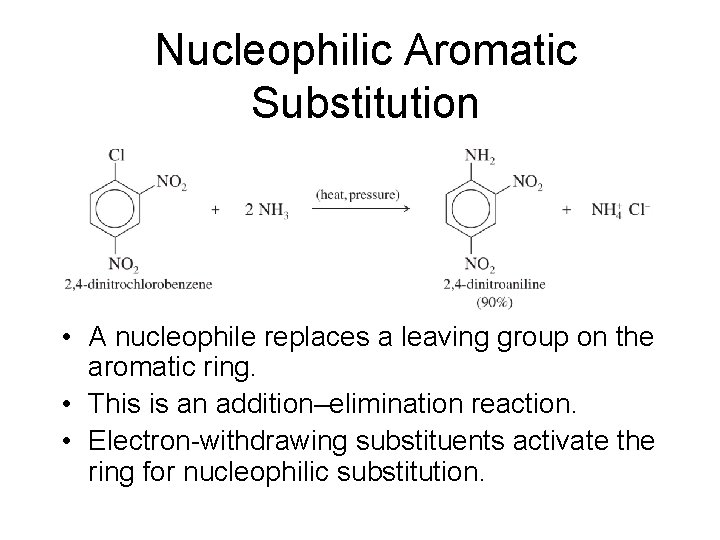
Nucleophilic Aromatic Substitution • A nucleophile replaces a leaving group on the aromatic ring. • This is an addition–elimination reaction. • Electron-withdrawing substituents activate the ring for nucleophilic substitution.
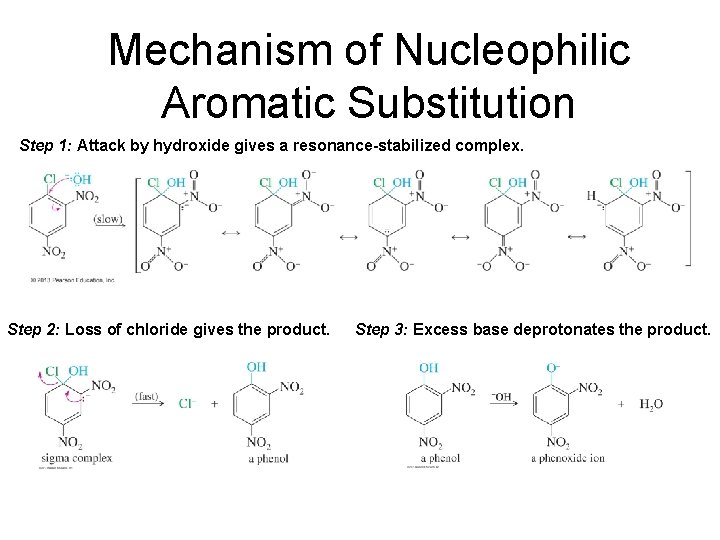
Mechanism of Nucleophilic Aromatic Substitution Step 1: Attack by hydroxide gives a resonance-stabilized complex. Step 2: Loss of chloride gives the product. Step 3: Excess base deprotonates the product.
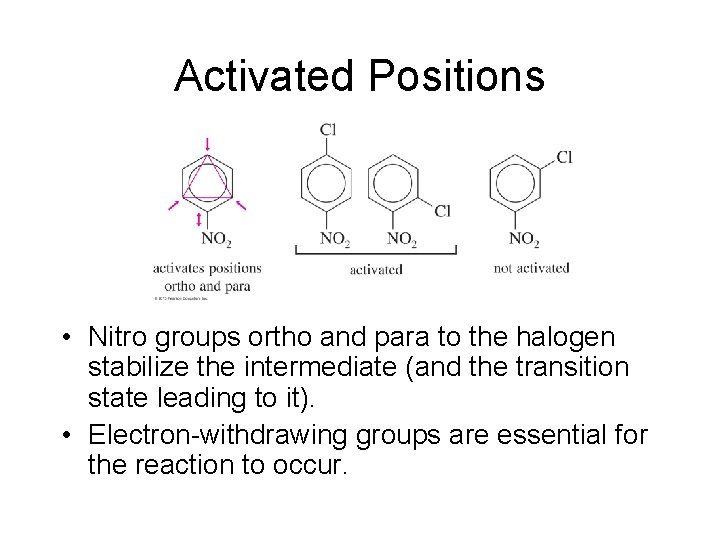
Activated Positions • Nitro groups ortho and para to the halogen stabilize the intermediate (and the transition state leading to it). • Electron-withdrawing groups are essential for the reaction to occur.
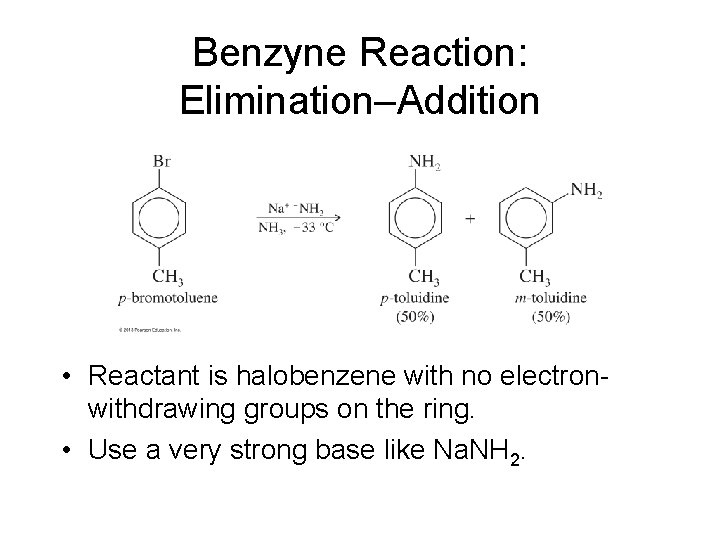
Benzyne Reaction: Elimination–Addition • Reactant is halobenzene with no electronwithdrawing groups on the ring. • Use a very strong base like Na. NH 2.
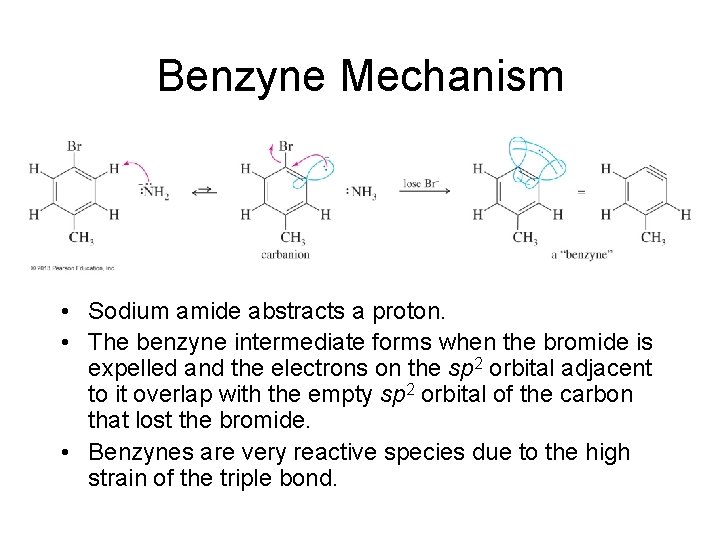
Benzyne Mechanism • Sodium amide abstracts a proton. • The benzyne intermediate forms when the bromide is expelled and the electrons on the sp 2 orbital adjacent to it overlap with the empty sp 2 orbital of the carbon that lost the bromide. • Benzynes are very reactive species due to the high strain of the triple bond.
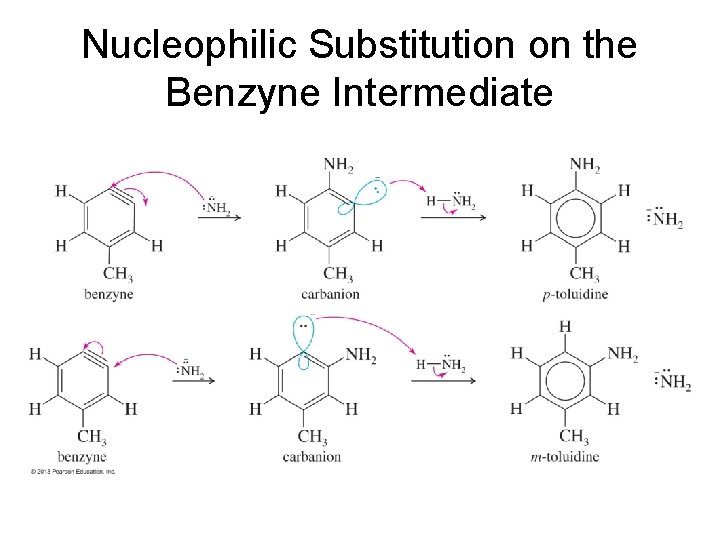
Nucleophilic Substitution on the Benzyne Intermediate

With strong electron-withdrawing groups ortho or para, the addition– elimination mechanism is more likely. Without these activating groups, stronger conditions are required, and the benzyne mechanism is likely.

Aromatic Substitutions Using Organometallic Reagents • Friedel–Craft reactions have limitations. – Rearrangements. – Multiple alkylations. – Cannot occur on deactivated rings. – Need strong electrophiles. • Organometallic reagents can add alkyl groups to the benzene without these limitations.
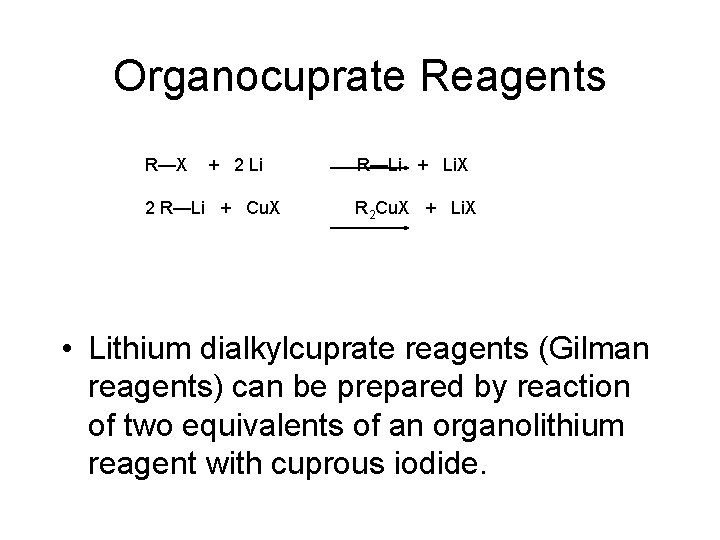
Organocuprate Reagents R—X + 2 Li 2 R—Li + Cu. X R—Li + Li. X R 2 Cu. X + Li. X • Lithium dialkylcuprate reagents (Gilman reagents) can be prepared by reaction of two equivalents of an organolithium reagent with cuprous iodide.
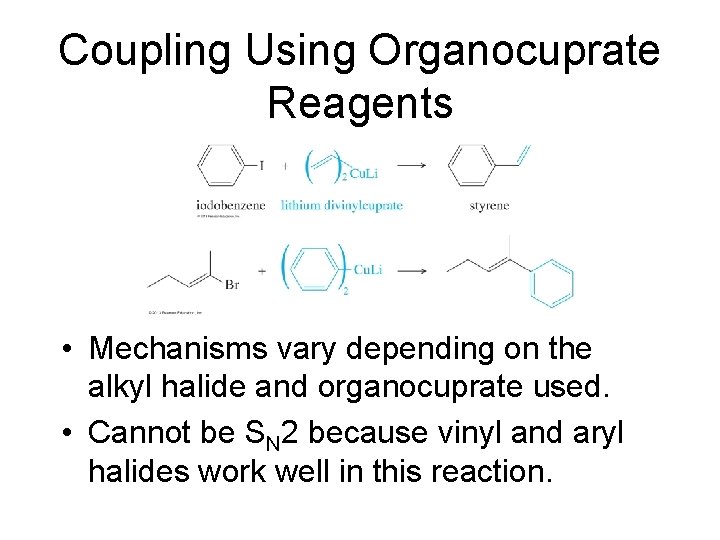
Coupling Using Organocuprate Reagents • Mechanisms vary depending on the alkyl halide and organocuprate used. • Cannot be SN 2 because vinyl and aryl halides work well in this reaction.
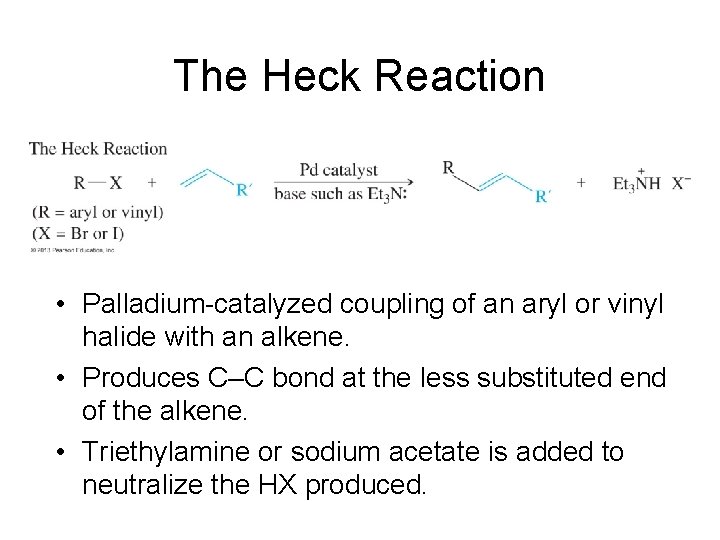
The Heck Reaction • Palladium-catalyzed coupling of an aryl or vinyl halide with an alkene. • Produces C–C bond at the less substituted end of the alkene. • Triethylamine or sodium acetate is added to neutralize the HX produced.
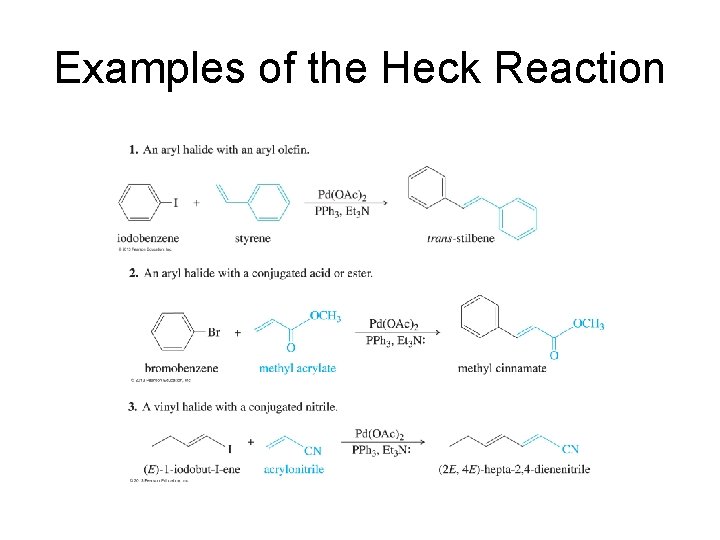
Examples of the Heck Reaction
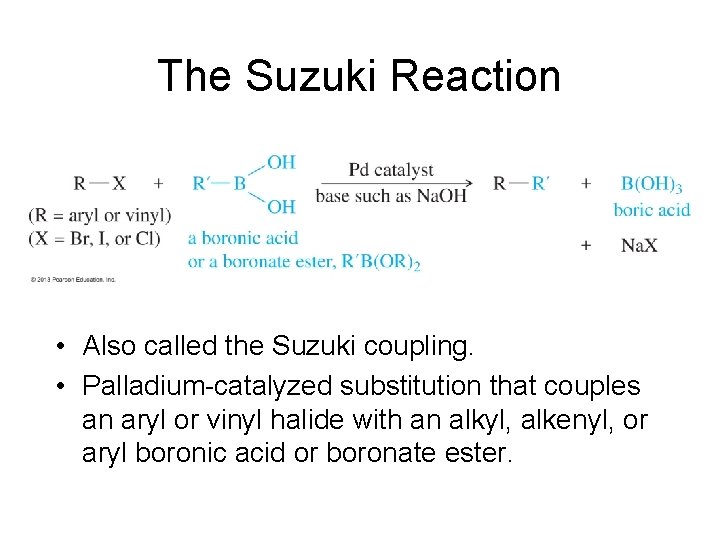
The Suzuki Reaction • Also called the Suzuki coupling. • Palladium-catalyzed substitution that couples an aryl or vinyl halide with an alkyl, alkenyl, or aryl boronic acid or boronate ester.
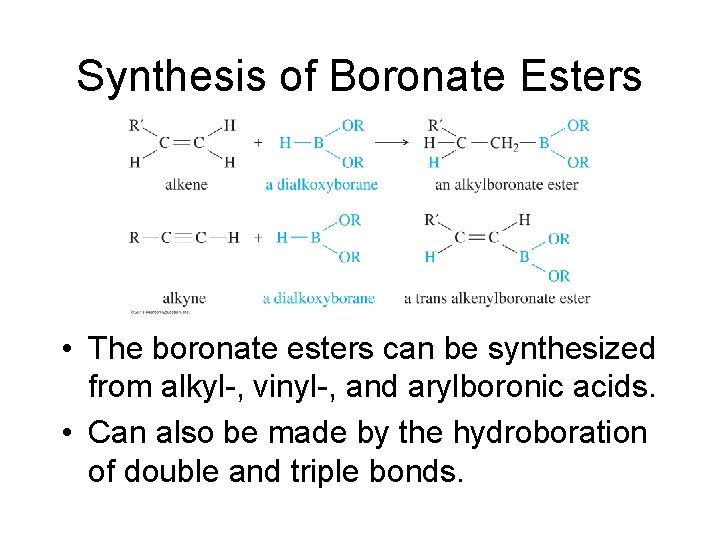
Synthesis of Boronate Esters • The boronate esters can be synthesized from alkyl-, vinyl-, and arylboronic acids. • Can also be made by the hydroboration of double and triple bonds.
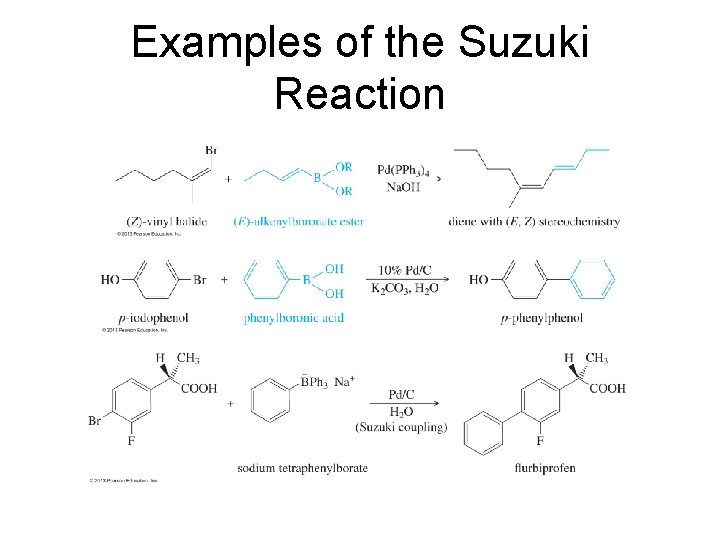
Examples of the Suzuki Reaction
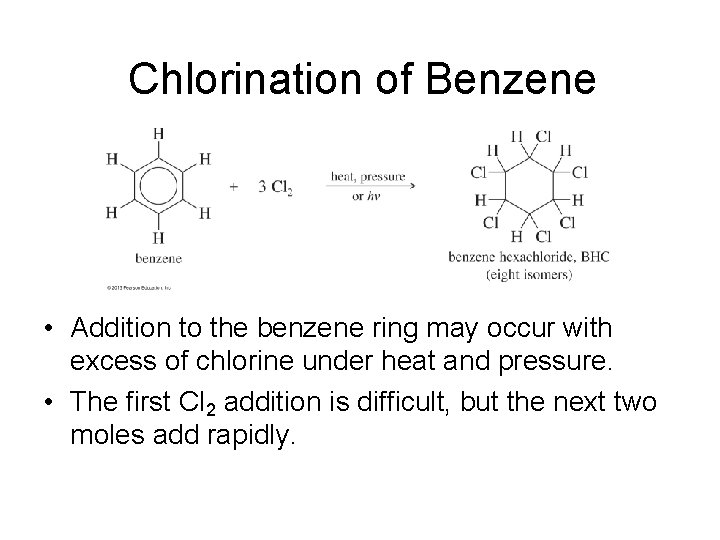
Chlorination of Benzene • Addition to the benzene ring may occur with excess of chlorine under heat and pressure. • The first Cl 2 addition is difficult, but the next two moles add rapidly.
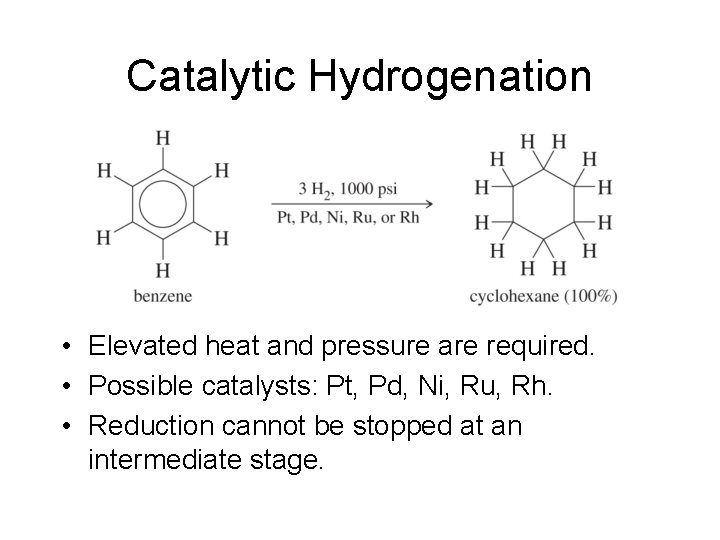
Catalytic Hydrogenation • Elevated heat and pressure are required. • Possible catalysts: Pt, Pd, Ni, Ru, Rh. • Reduction cannot be stopped at an intermediate stage.
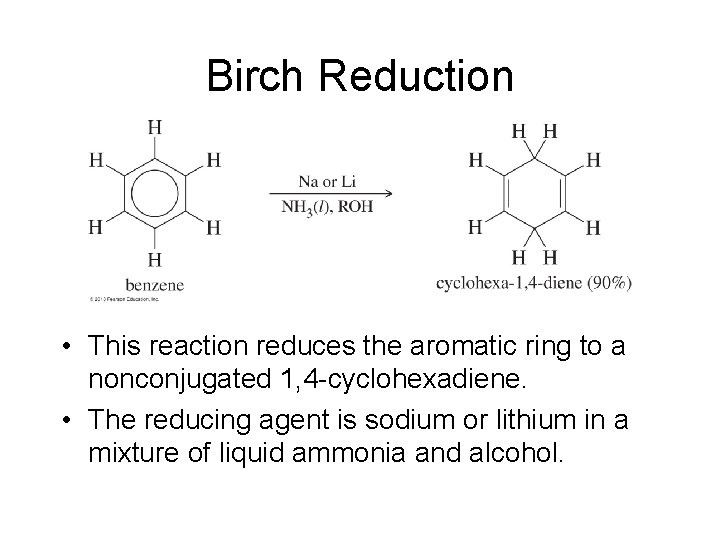
Birch Reduction • This reaction reduces the aromatic ring to a nonconjugated 1, 4 -cyclohexadiene. • The reducing agent is sodium or lithium in a mixture of liquid ammonia and alcohol.
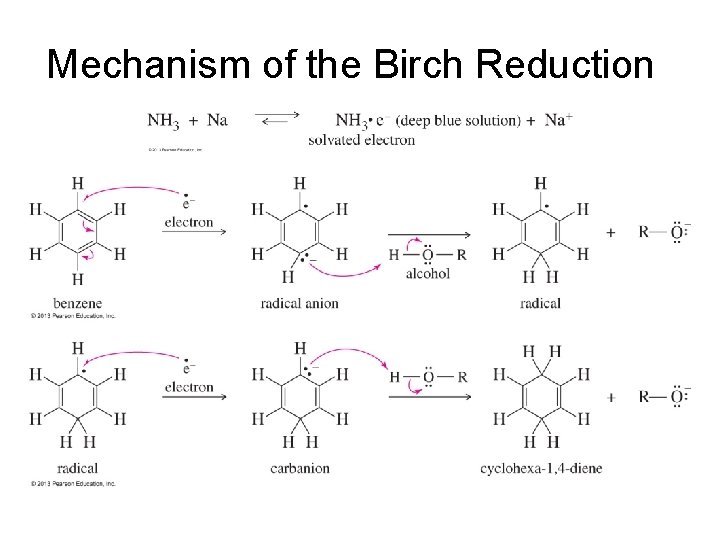
Mechanism of the Birch Reduction
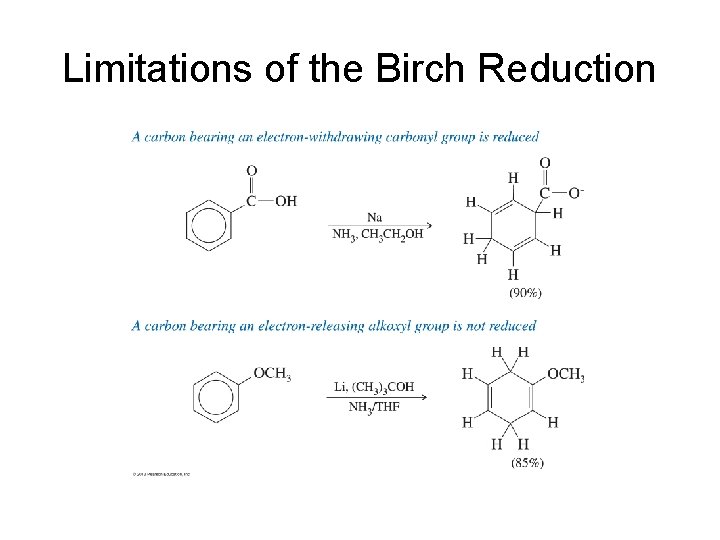
Limitations of the Birch Reduction
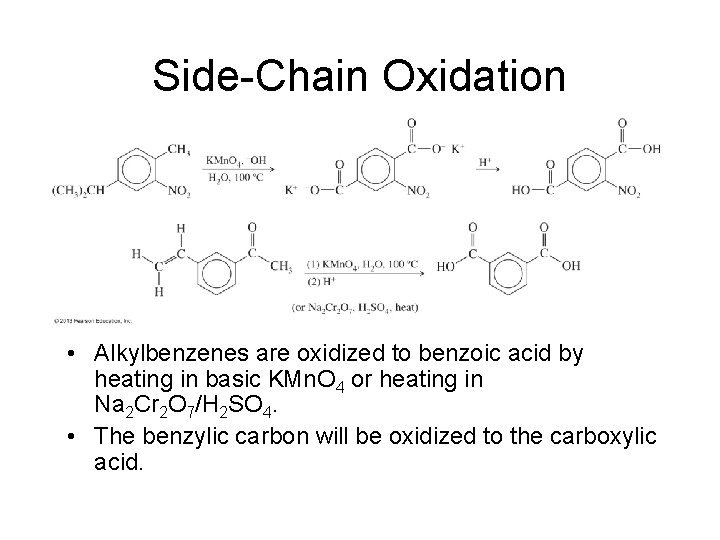
Side-Chain Oxidation • Alkylbenzenes are oxidized to benzoic acid by heating in basic KMn. O 4 or heating in Na 2 Cr 2 O 7/H 2 SO 4. • The benzylic carbon will be oxidized to the carboxylic acid.
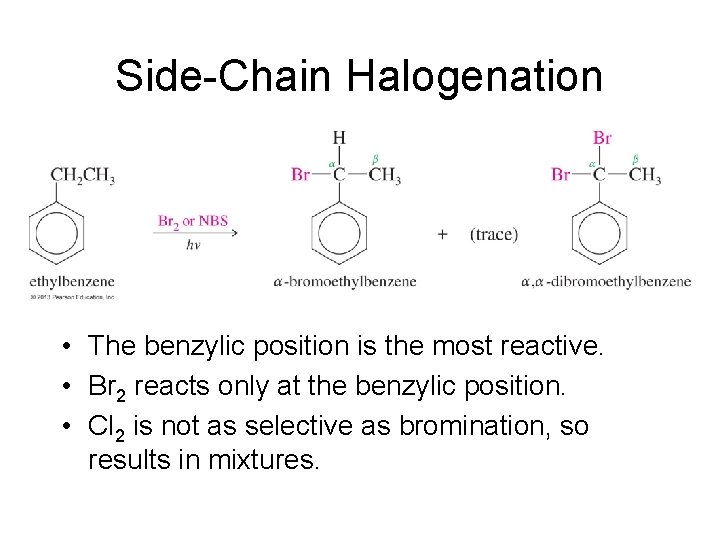
Side-Chain Halogenation • The benzylic position is the most reactive. • Br 2 reacts only at the benzylic position. • Cl 2 is not as selective as bromination, so results in mixtures.
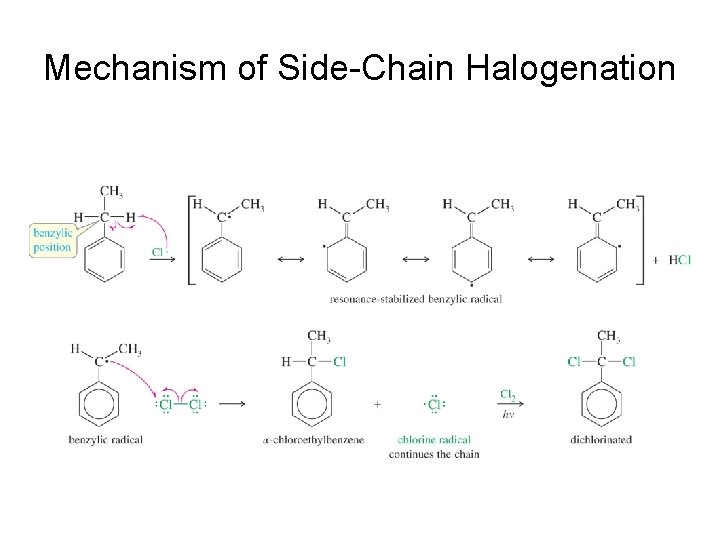
Mechanism of Side-Chain Halogenation

In predicting reactions on side chains of aromatic rings, consider resonance forms that delocalize a charge or a radical electron onto the ring.
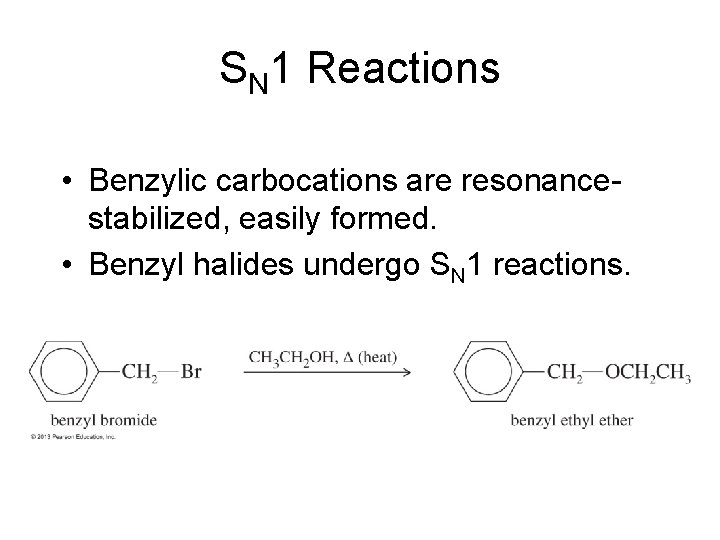
SN 1 Reactions • Benzylic carbocations are resonancestabilized, easily formed. • Benzyl halides undergo SN 1 reactions.
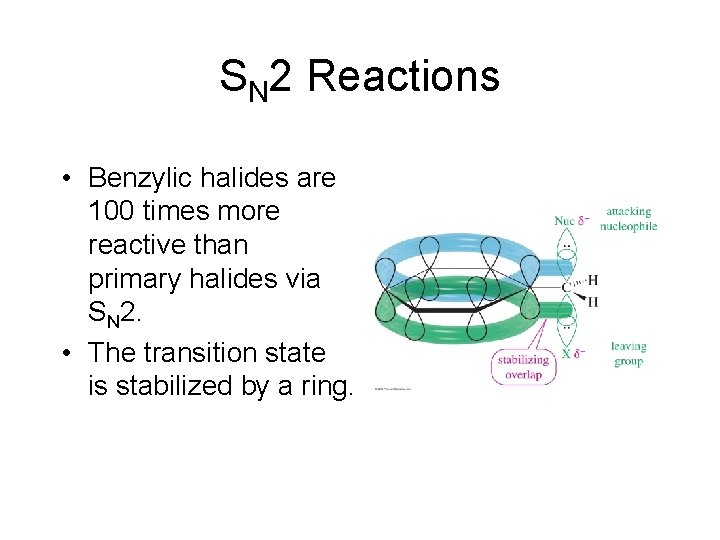
SN 2 Reactions • Benzylic halides are 100 times more reactive than primary halides via SN 2. • The transition state is stabilized by a ring.
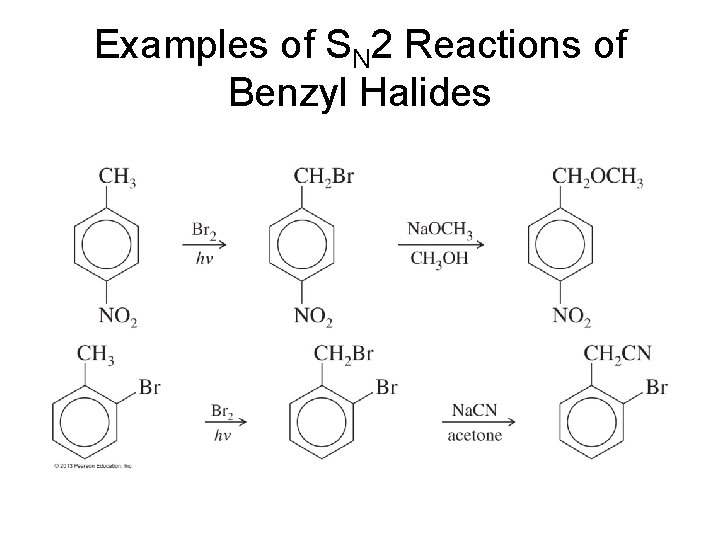
Examples of SN 2 Reactions of Benzyl Halides
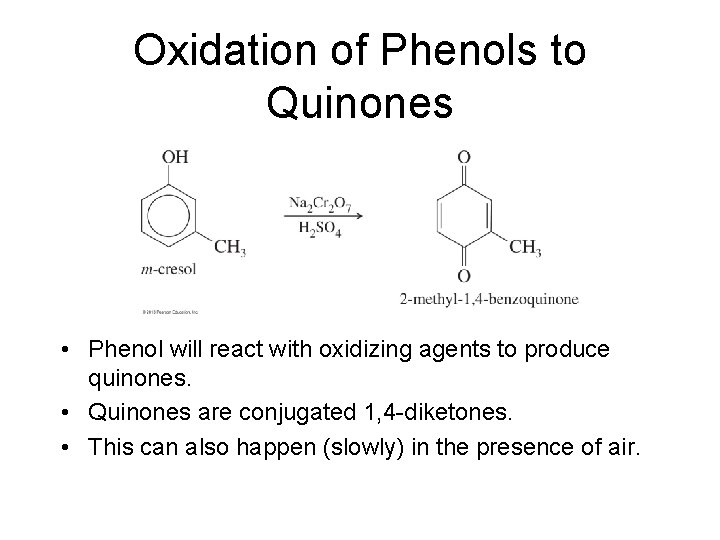
Oxidation of Phenols to Quinones • Phenol will react with oxidizing agents to produce quinones. • Quinones are conjugated 1, 4 -diketones. • This can also happen (slowly) in the presence of air.
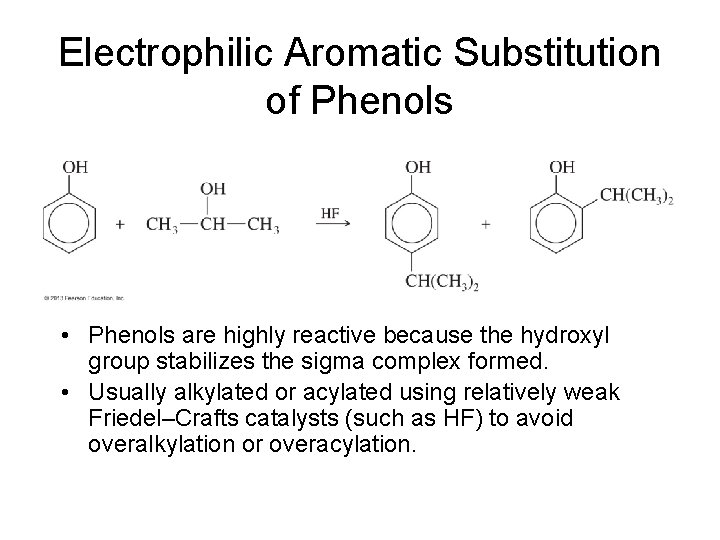
Electrophilic Aromatic Substitution of Phenols • Phenols are highly reactive because the hydroxyl group stabilizes the sigma complex formed. • Usually alkylated or acylated using relatively weak Friedel–Crafts catalysts (such as HF) to avoid overalkylation or overacylation.
- Slides: 67