Amines 23 1 Structure Classification u Amines are


- Slides: 44

Amines 23 -1

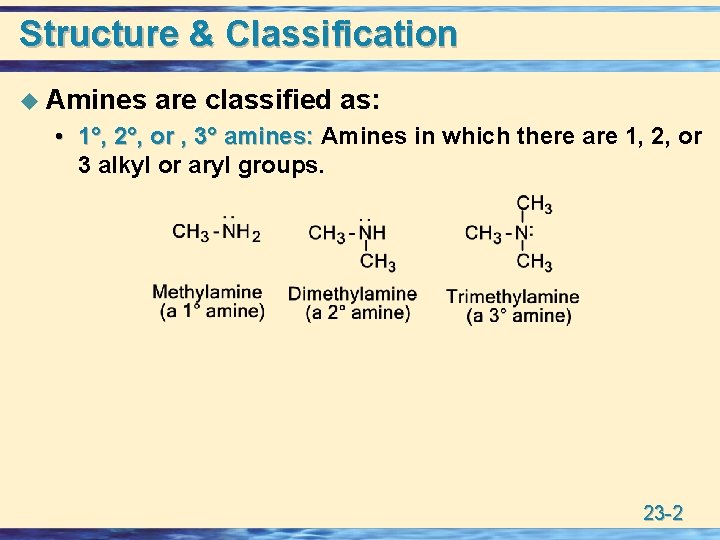
Structure & Classification u Amines are classified as: • 1°, 2°, or , 3° amines: Amines in which there are 1, 2, or 1°, 2°, or , 3° amines: 3 alkyl or aryl groups. 23 -2

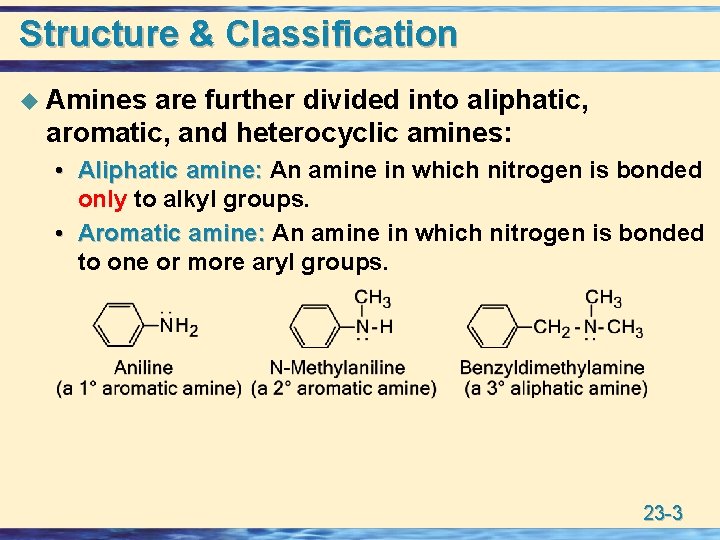
Structure & Classification u Amines are further divided into aliphatic, aromatic, and heterocyclic amines: • Aliphatic amine: An amine in which nitrogen is bonded amine: only to alkyl groups. • Aromatic amine: An amine in which nitrogen is bonded Aromatic amine: to one or more aryl groups. 23 -3

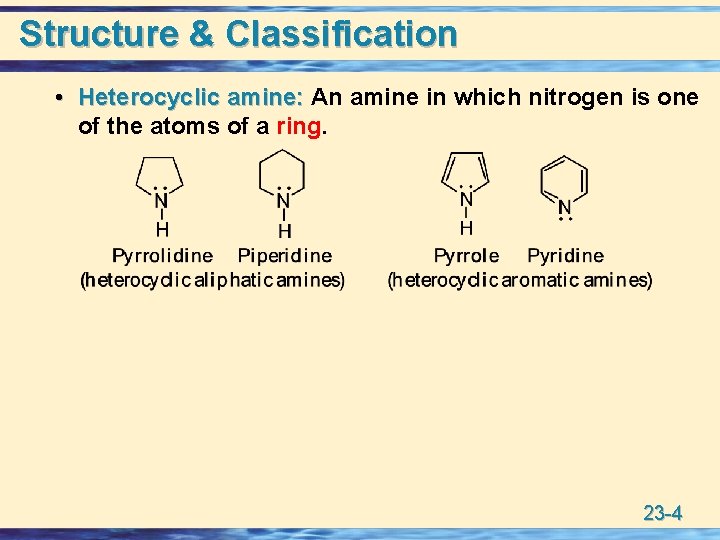
Structure & Classification • Heterocyclic amine: An amine in which nitrogen is one Heterocyclic amine: of the atoms of a ring. 23 -4

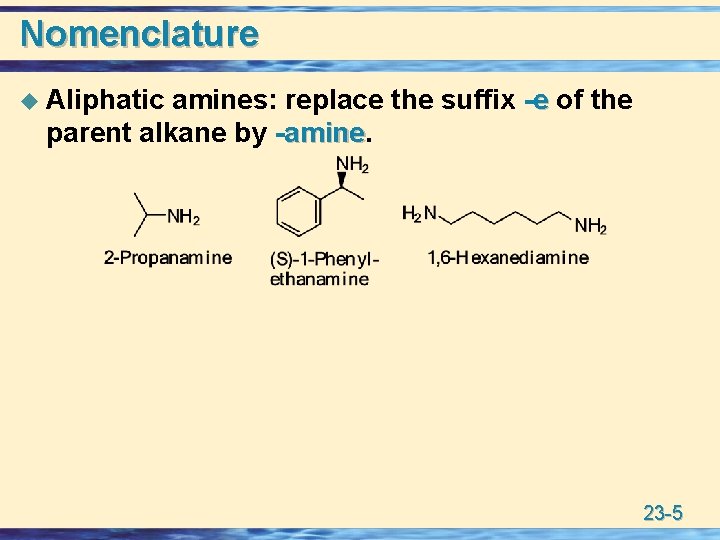
Nomenclature u Aliphatic amines: replace the suffix -e of the parent alkane by -amine 23 -5

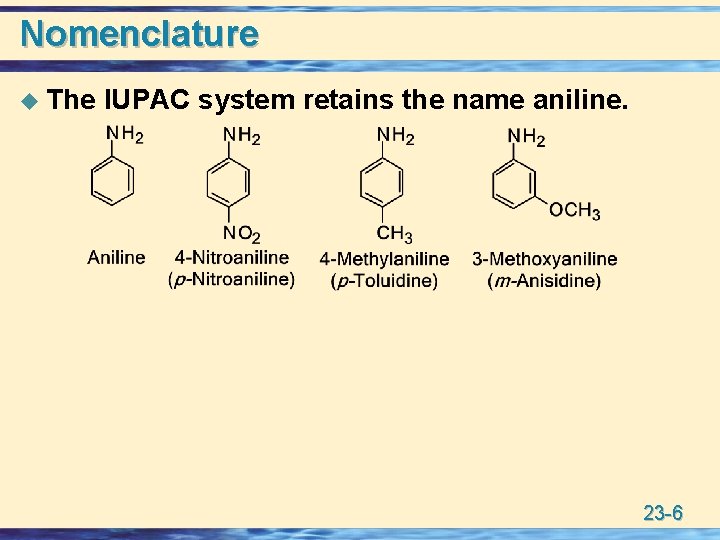
Nomenclature u The IUPAC system retains the name aniline. 23 -6

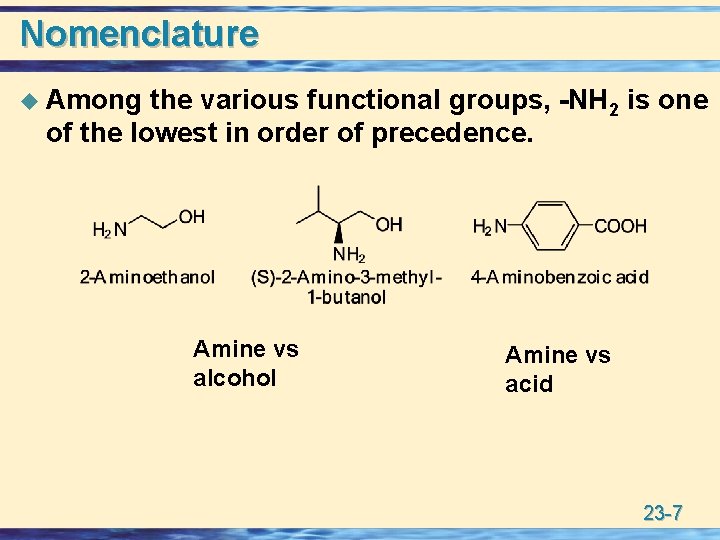
Nomenclature u Among the various functional groups, -NH 2 is one of the lowest in order of precedence. Amine vs alcohol Amine vs acid 23 -7

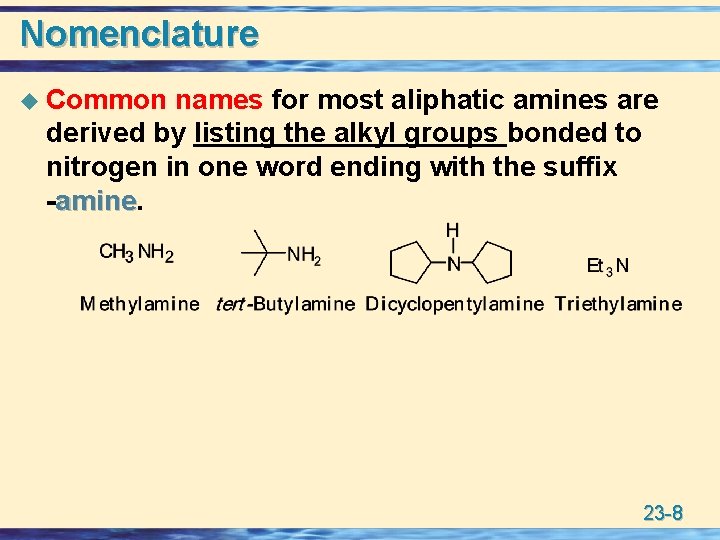
Nomenclature u Common names for most aliphatic amines are derived by listing the alkyl groups bonded to nitrogen in one word ending with the suffix -amine 23 -8

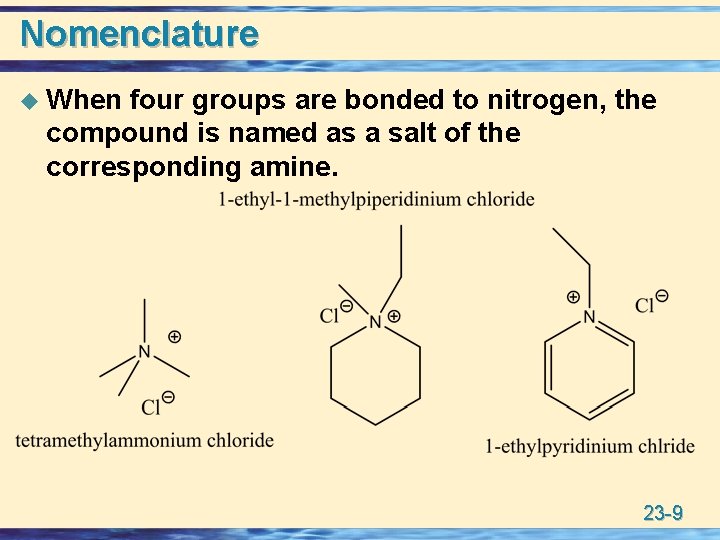
Nomenclature u When four groups are bonded to nitrogen, the compound is named as a salt of the corresponding amine. 23 -9

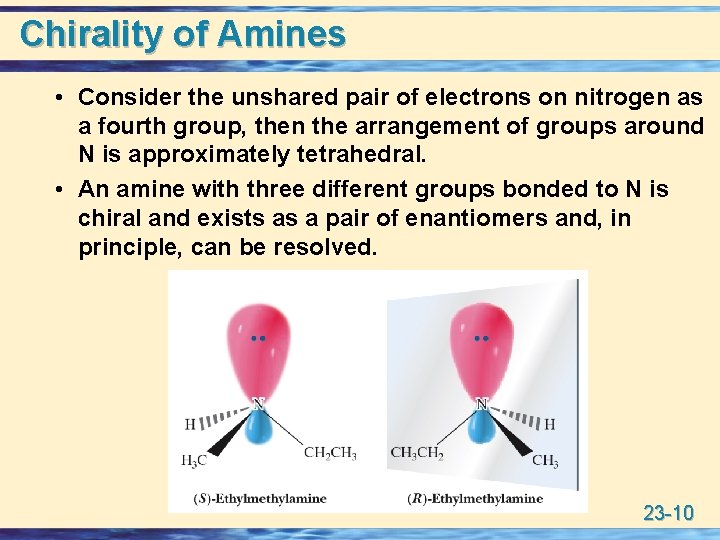
Chirality of Amines • Consider the unshared pair of electrons on nitrogen as a fourth group, then the arrangement of groups around N is approximately tetrahedral. • An amine with three different groups bonded to N is chiral and exists as a pair of enantiomers and, in principle, can be resolved. 23 -10

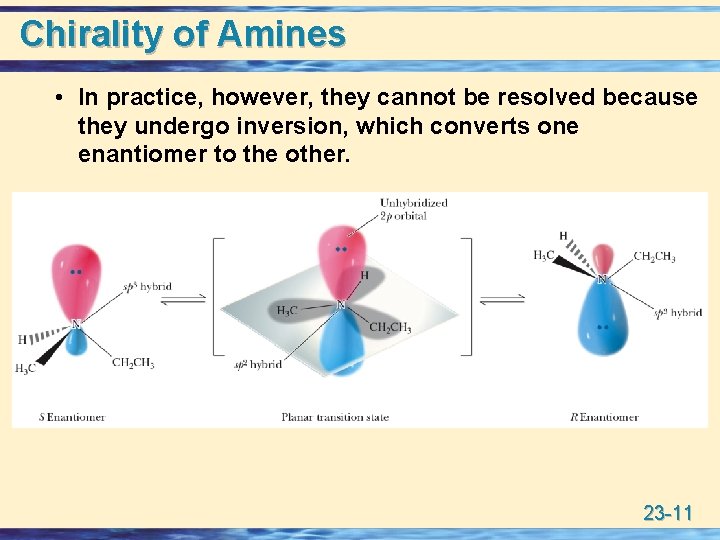
Chirality of Amines • In practice, however, they cannot be resolved because they undergo inversion, which converts one enantiomer to the other. 23 -11

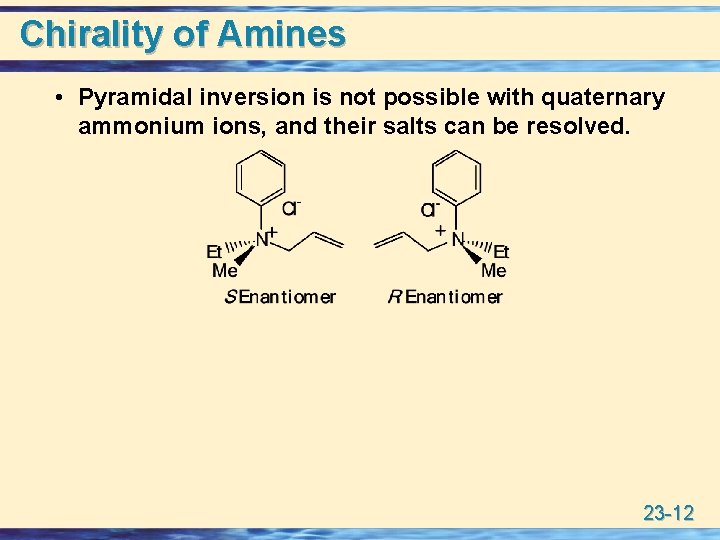
Chirality of Amines • Pyramidal inversion is not possible with quaternary ammonium ions, and their salts can be resolved. 23 -12

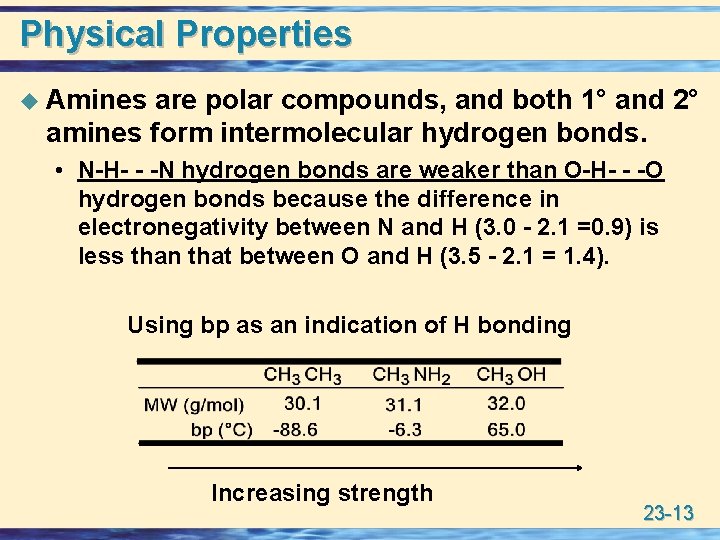
Physical Properties u Amines are polar compounds, and both 1° and 2° amines form intermolecular hydrogen bonds. • N-H- - -N hydrogen bonds are weaker than O-H- - -O hydrogen bonds because the difference in electronegativity between N and H (3. 0 - 2. 1 =0. 9) is less than that between O and H (3. 5 - 2. 1 = 1. 4). Using bp as an indication of H bonding Increasing strength 23 -13

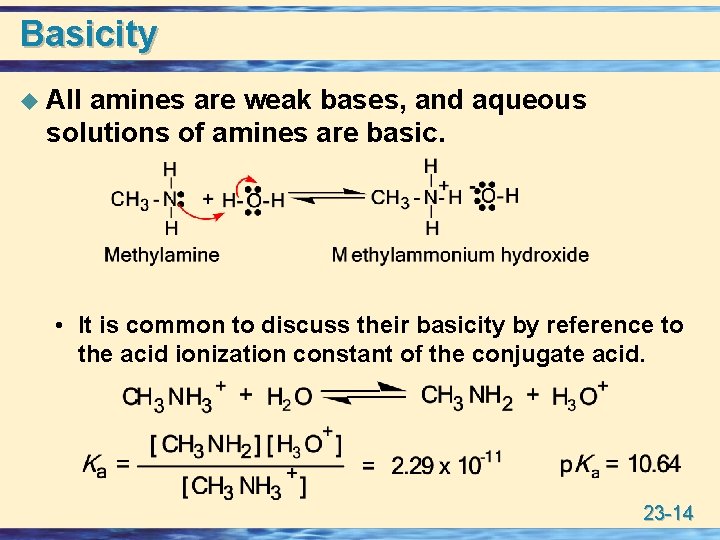
Basicity u All amines are weak bases, and aqueous solutions of amines are basic. • It is common to discuss their basicity by reference to the acid ionization constant of the conjugate acid. 23 -14

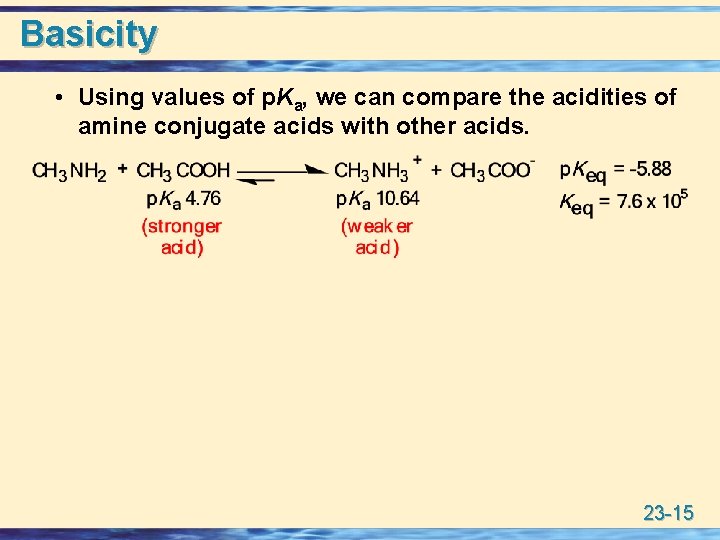
Basicity • Using values of p. Ka, we can compare the acidities of amine conjugate acids with other acids. 23 -15

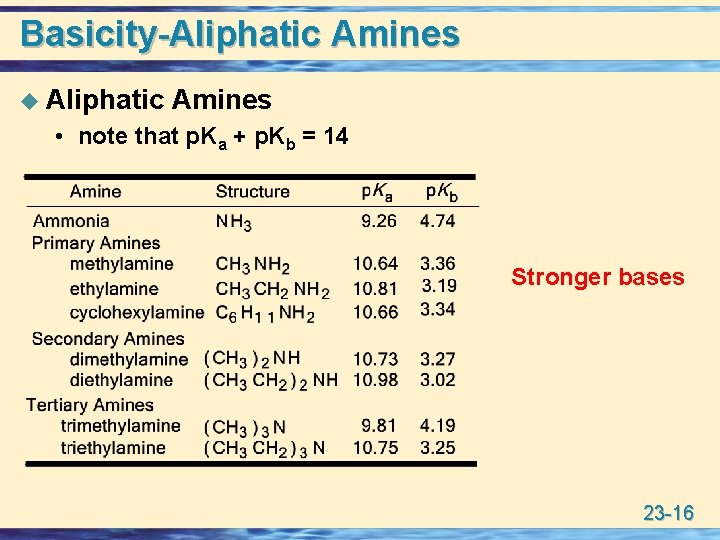
Basicity-Aliphatic Amines u Aliphatic Amines • note that p. Ka + p. Kb = 14 Stronger bases 23 -16

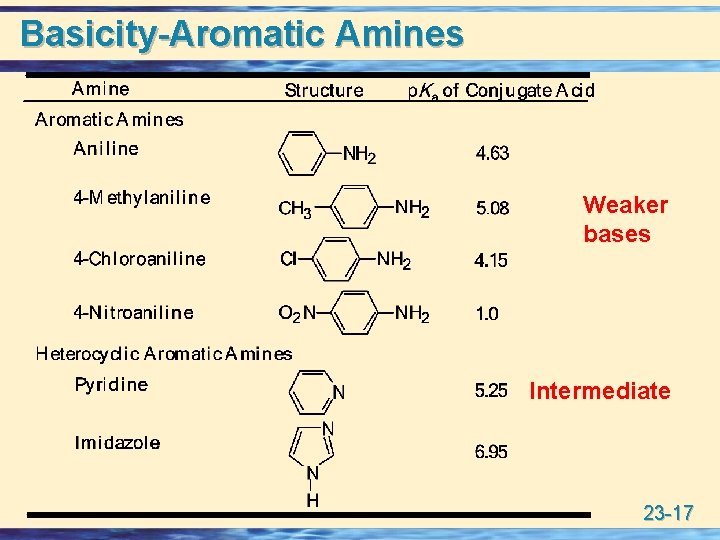
Basicity-Aromatic Amines Weaker bases Intermediate 23 -17

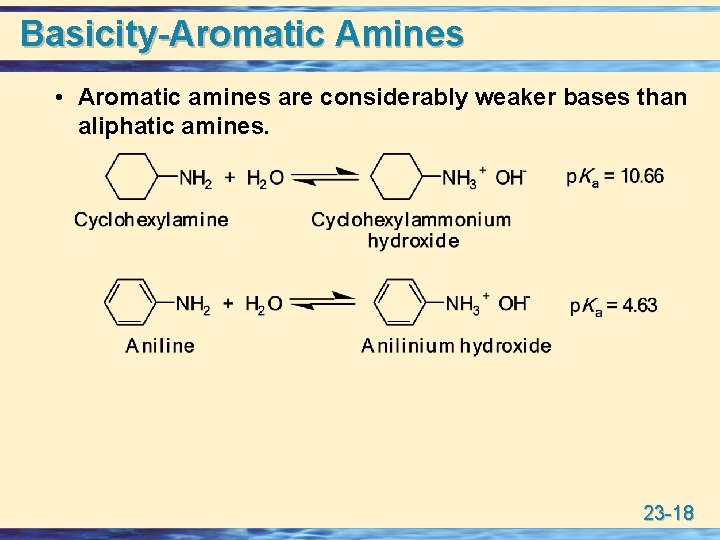
Basicity-Aromatic Amines • Aromatic amines are considerably weaker bases than aliphatic amines. 23 -18

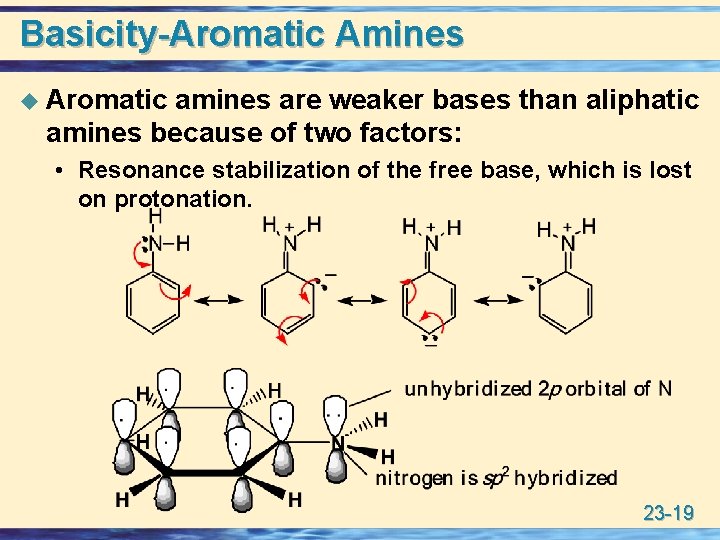
Basicity-Aromatic Amines u Aromatic amines are weaker bases than aliphatic amines because of two factors: • Resonance stabilization of the free base, which is lost on protonation. 23 -19

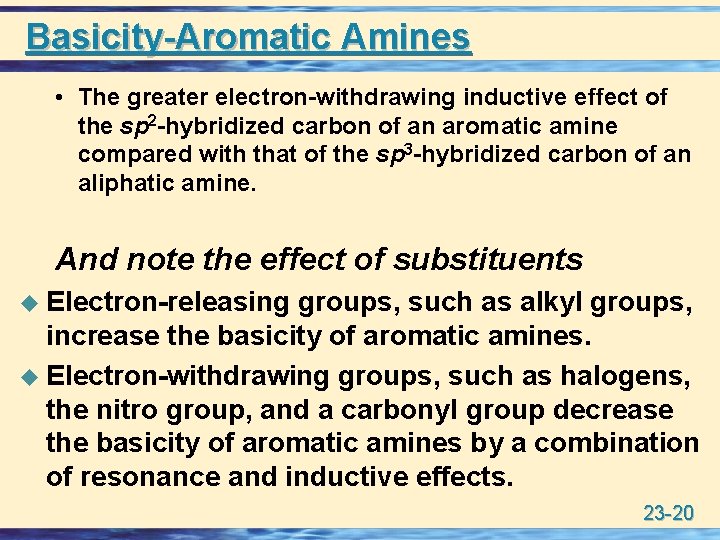
Basicity-Aromatic Amines • The greater electron-withdrawing inductive effect of the sp 2 -hybridized carbon of an aromatic amine compared with that of the sp 3 -hybridized carbon of an aliphatic amine. And note the effect of substituents u Electron-releasing groups, such as alkyl groups, increase the basicity of aromatic amines. u Electron-withdrawing groups, such as halogens, the nitro group, and a carbonyl group decrease the basicity of aromatic amines by a combination of resonance and inductive effects. 23 -20

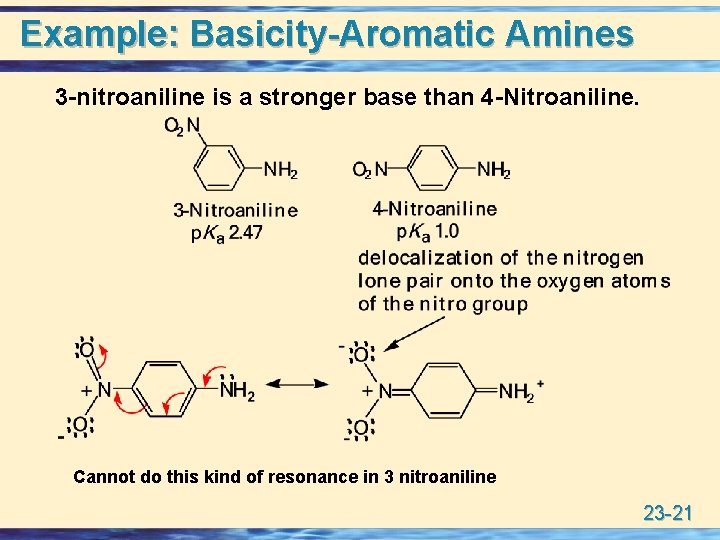
Example: Basicity-Aromatic Amines 3 -nitroaniline is a stronger base than 4 -Nitroaniline. Cannot do this kind of resonance in 3 nitroaniline 23 -21

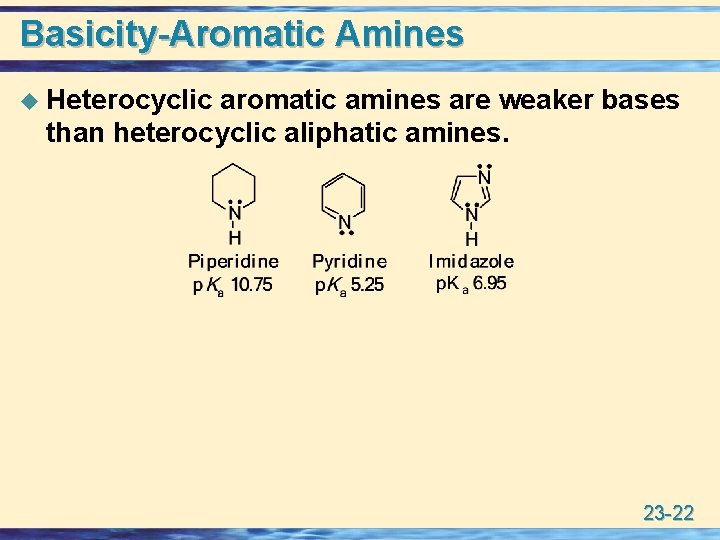
Basicity-Aromatic Amines u Heterocyclic aromatic amines are weaker bases than heterocyclic aliphatic amines. 23 -22

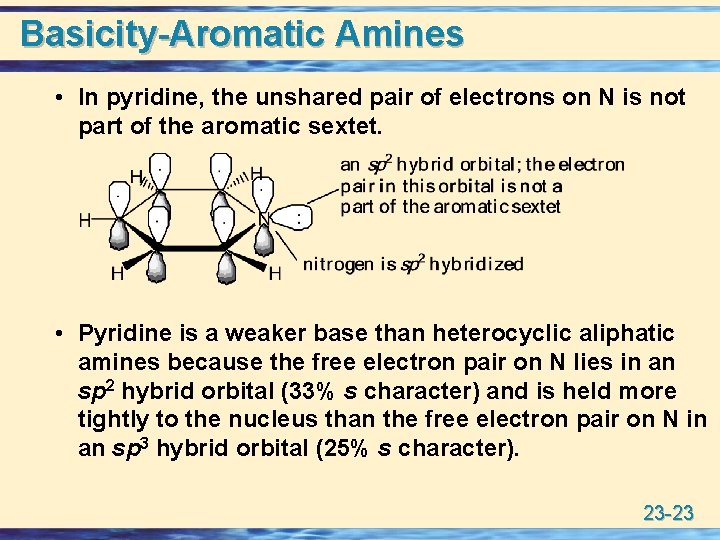
Basicity-Aromatic Amines • In pyridine, the unshared pair of electrons on N is not part of the aromatic sextet. • Pyridine is a weaker base than heterocyclic aliphatic amines because the free electron pair on N lies in an sp 2 hybrid orbital (33% s character) and is held more tightly to the nucleus than the free electron pair on N in an sp 3 hybrid orbital (25% s character). 23 -23

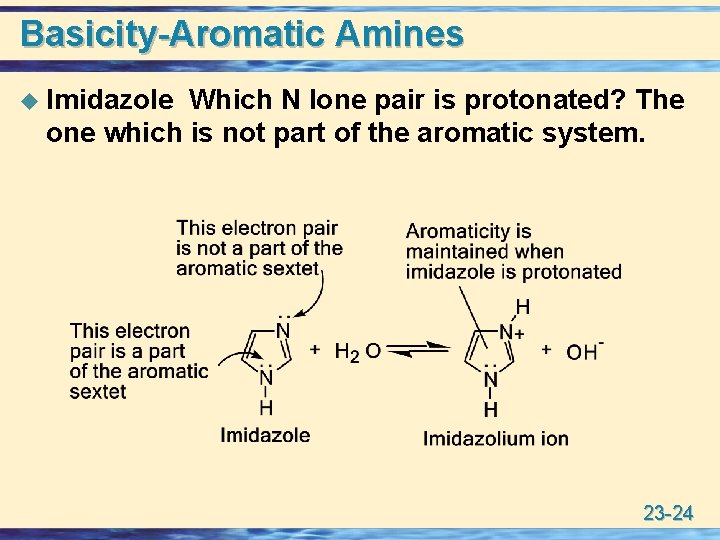
Basicity-Aromatic Amines u Imidazole Which N lone pair is protonated? The one which is not part of the aromatic system. 23 -24

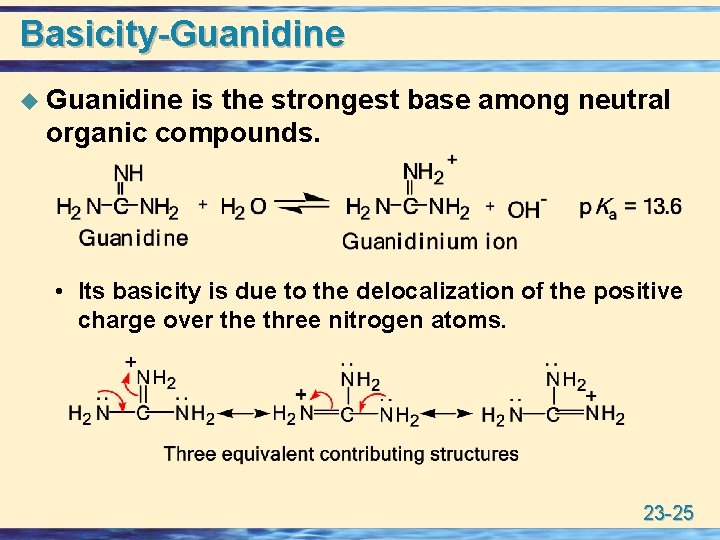
Basicity-Guanidine u Guanidine is the strongest base among neutral organic compounds. • Its basicity is due to the delocalization of the positive charge over the three nitrogen atoms. 23 -25

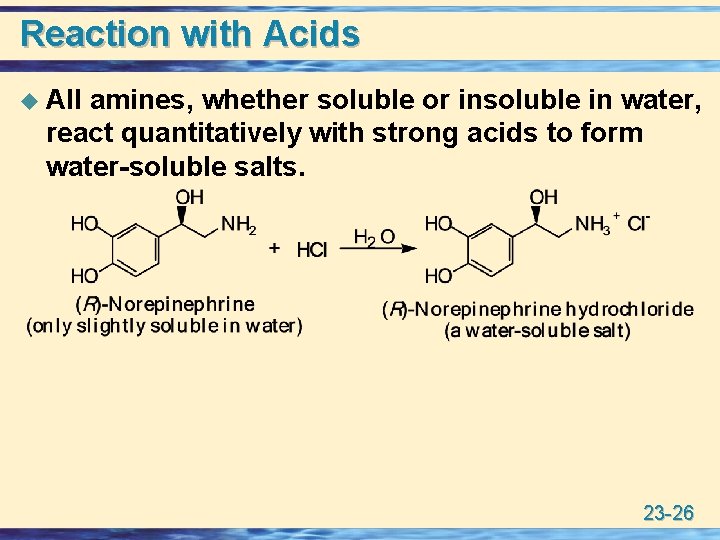
Reaction with Acids u All amines, whether soluble or insoluble in water, react quantitatively with strong acids to form water-soluble salts. 23 -26

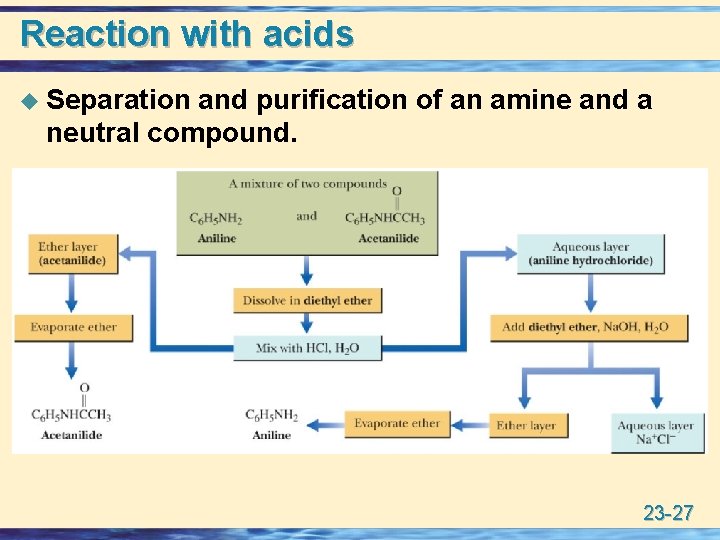
Reaction with acids u Separation and purification of an amine and a neutral compound. 23 -27

Preparation u We have already covered these methods • nucleophilic ring opening of epoxides by ammonia and amines. • addition of nitrogen nucleophiles to aldehydes and ketones to form imines • reduction of imines to amines • reduction of amides to amines by Li. Al. H 4 • reduction of nitriles to a 1° amine • nitration of arenes followed by reduction of the NO 2 group to a 1° amine 23 -28

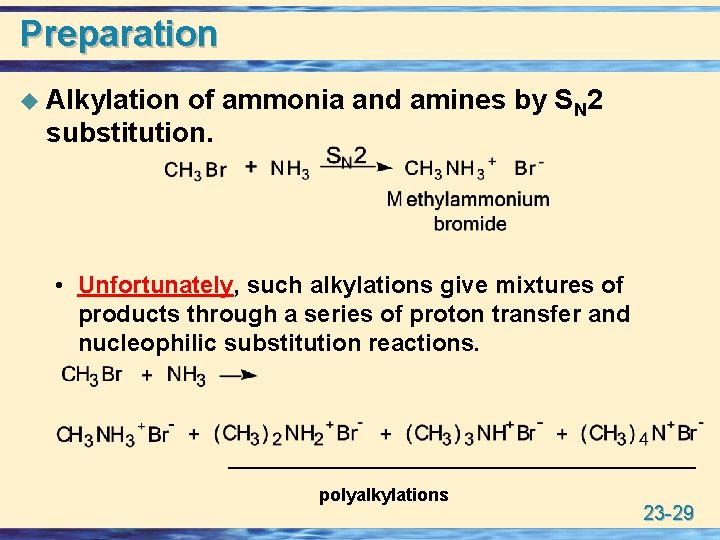
Preparation u Alkylation of ammonia and amines by SN 2 substitution. • Unfortunately, such alkylations give mixtures of products through a series of proton transfer and nucleophilic substitution reactions. polyalkylations 23 -29

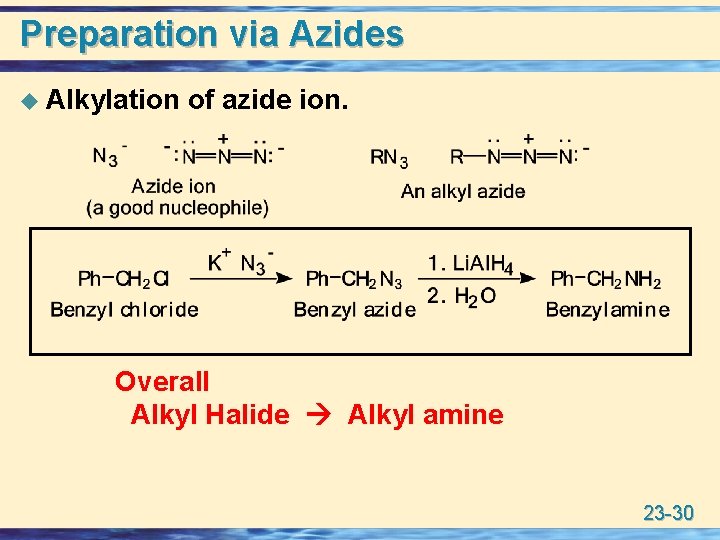
Preparation via Azides u Alkylation of azide ion. Overall Alkyl Halide Alkyl amine 23 -30

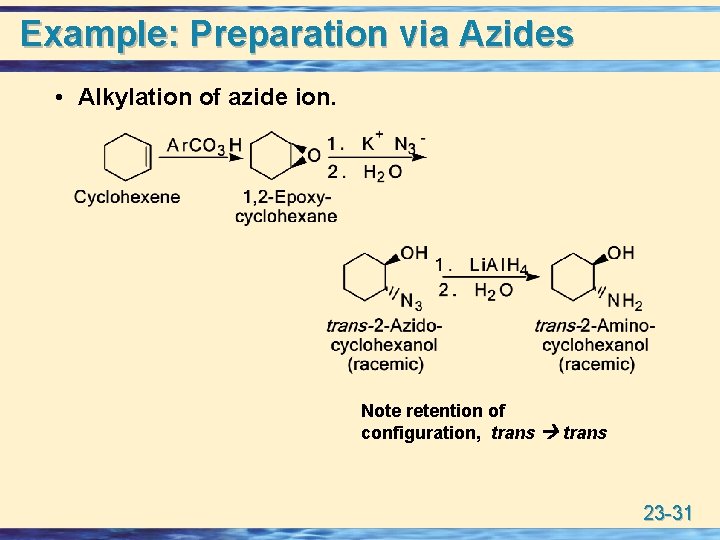
Example: Preparation via Azides • Alkylation of azide ion. Note retention of configuration, trans 23 -31

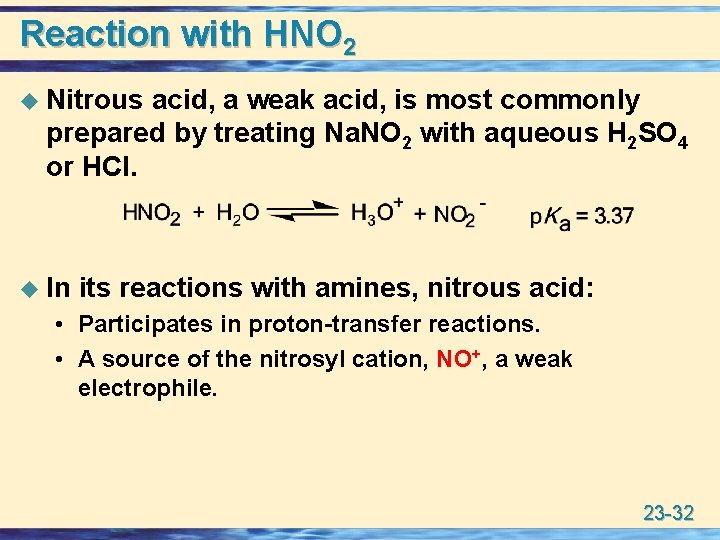
Reaction with HNO 2 u Nitrous acid, a weak acid, is most commonly prepared by treating Na. NO 2 with aqueous H 2 SO 4 or HCl. u In its reactions with amines, nitrous acid: • Participates in proton-transfer reactions. • A source of the nitrosyl cation, NO+, a weak electrophile. 23 -32

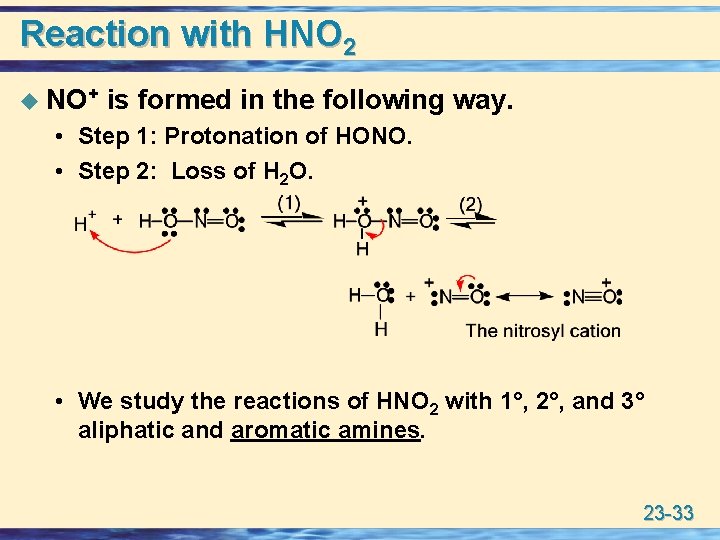
Reaction with HNO 2 u NO+ is formed in the following way. • Step 1: Protonation of HONO. • Step 2: Loss of H 2 O. • We study the reactions of HNO 2 with 1°, 2°, and 3° aliphatic and aromatic amines. 23 -33

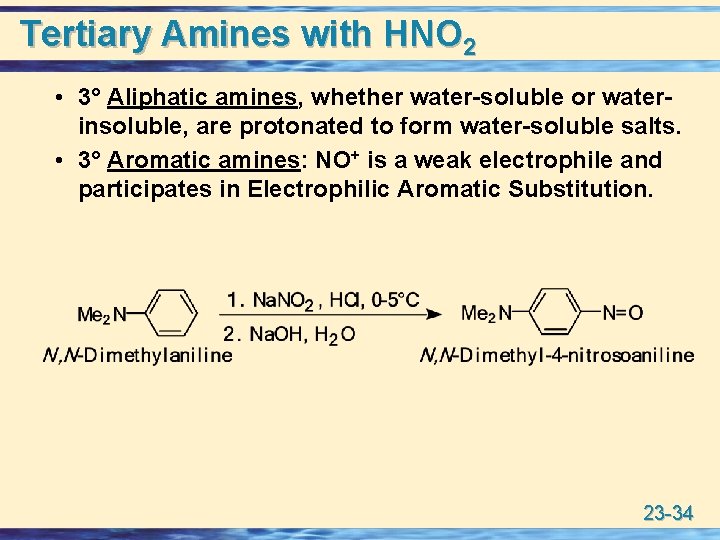
Tertiary Amines with HNO 2 • 3° Aliphatic amines, whether water-soluble or waterinsoluble, are protonated to form water-soluble salts. • 3° Aromatic amines: NO+ is a weak electrophile and participates in Electrophilic Aromatic Substitution. 23 -34

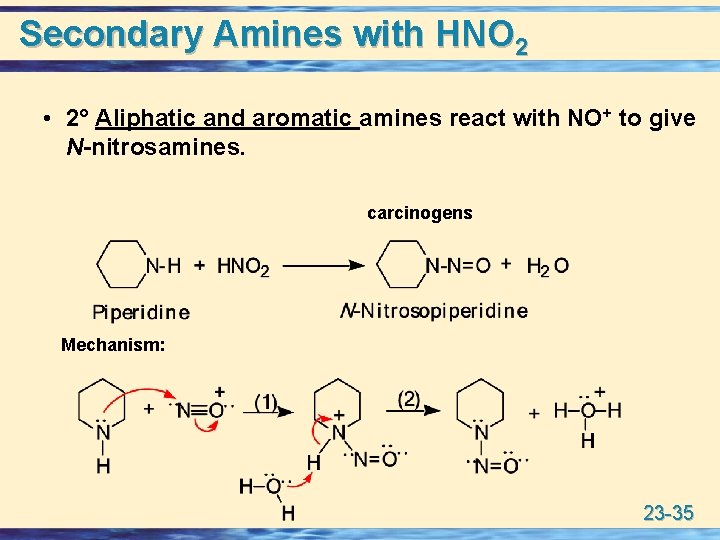
Secondary Amines with HNO 2 • 2° Aliphatic and aromatic amines react with NO+ to give N-nitrosamines. carcinogens Mechanism: 23 -35

RNH 2 with HNO 2 u 1° aliphatic amines give a mixture of unrearranged and rearranged substitution and elimination products, all of which are produced by way of a diazonium ion and its loss of N 2 to give a carbocation. + or Ar. N + ion u Diazonium ion: An RN Diazonium ion: 2 2 23 -36

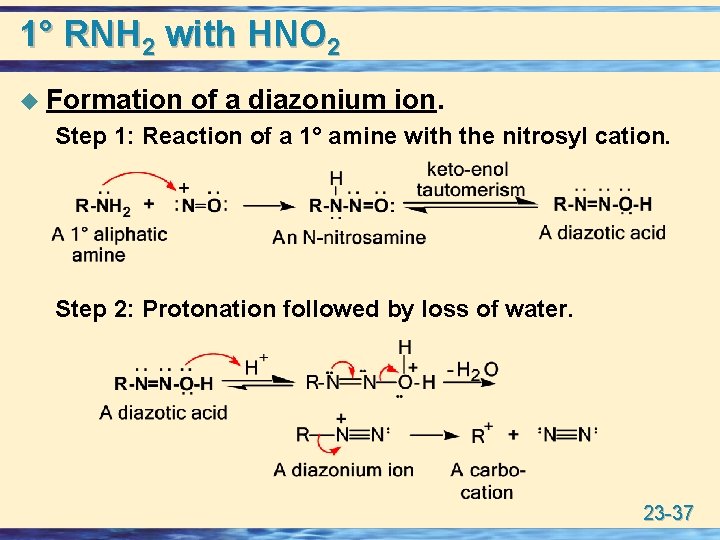
1° RNH 2 with HNO 2 u Formation of a diazonium ion. Step 1: Reaction of a 1° amine with the nitrosyl cation. Step 2: Protonation followed by loss of water. 23 -37

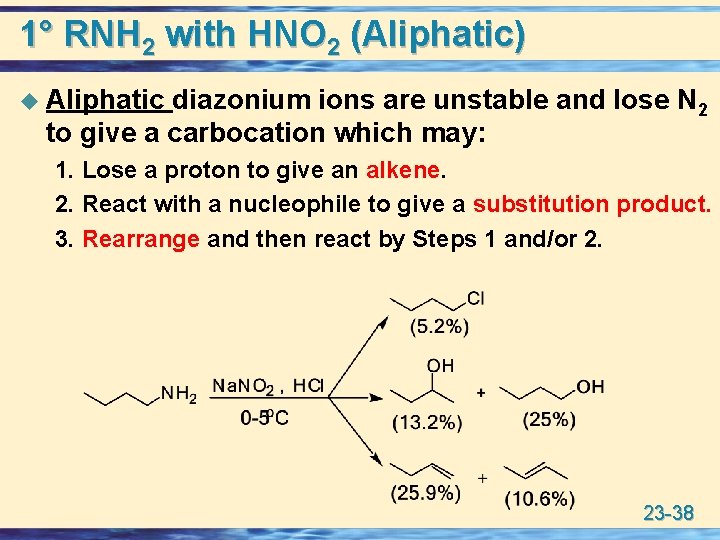
1° RNH 2 with HNO 2 (Aliphatic) u Aliphatic diazonium ions are unstable and lose N 2 to give a carbocation which may: 1. Lose a proton to give an alkene. 2. React with a nucleophile to give a substitution product. 3. Rearrange and then react by Steps 1 and/or 2. 23 -38

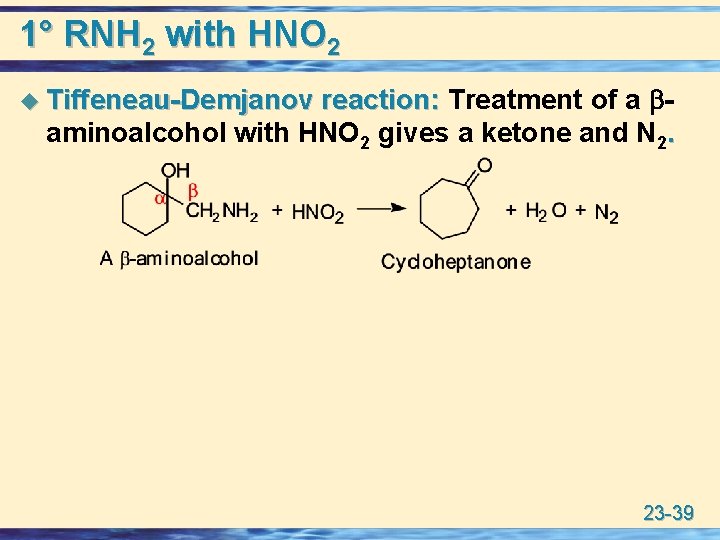
1° RNH 2 with HNO 2 u Tiffeneau-Demjanov reaction: Treatment of a Tiffeneau-Demjanov reaction: aminoalcohol with HNO 2 gives a ketone and N 2. 23 -39

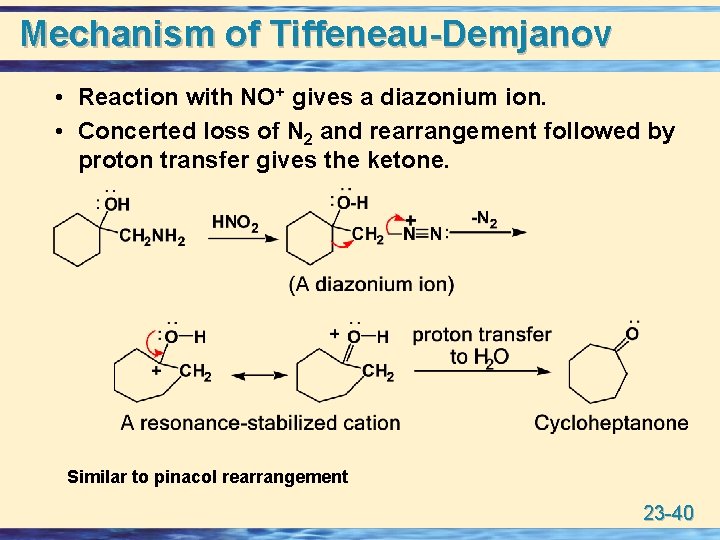
Mechanism of Tiffeneau-Demjanov • Reaction with NO+ gives a diazonium ion. • Concerted loss of N 2 and rearrangement followed by proton transfer gives the ketone. Similar to pinacol rearrangement 23 -40

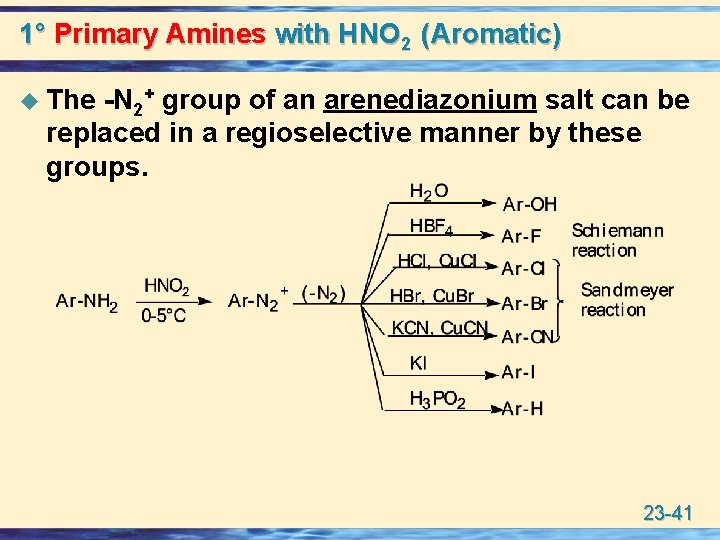
1° Primary Amines with HNO 2 (Aromatic) u The -N 2+ group of an arenediazonium salt can be replaced in a regioselective manner by these groups. 23 -41

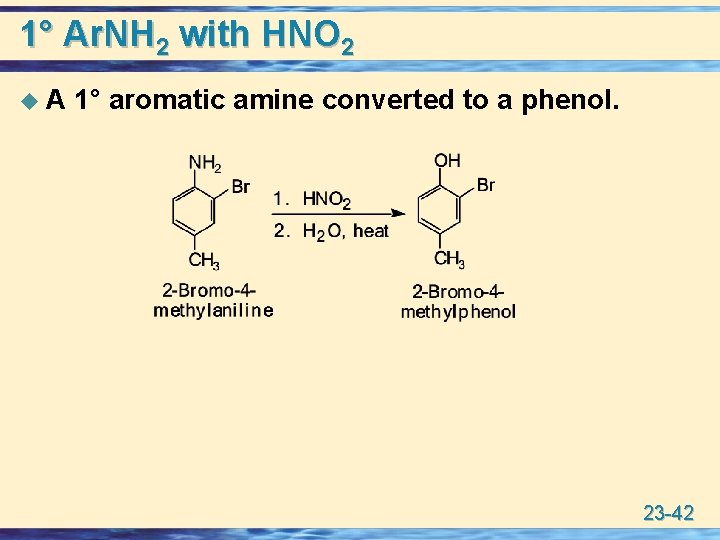
1° Ar. NH 2 with HNO 2 u A 1° aromatic amine converted to a phenol. 23 -42

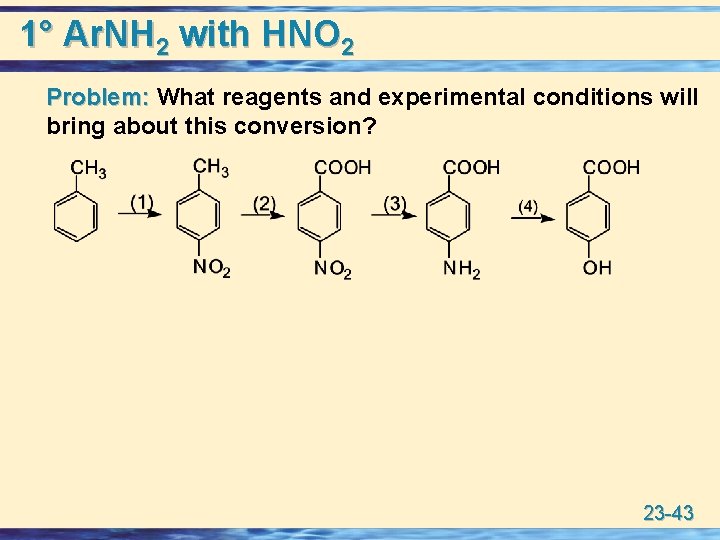
1° Ar. NH 2 with HNO 2 Problem: What reagents and experimental conditions will Problem: bring about this conversion? 23 -43

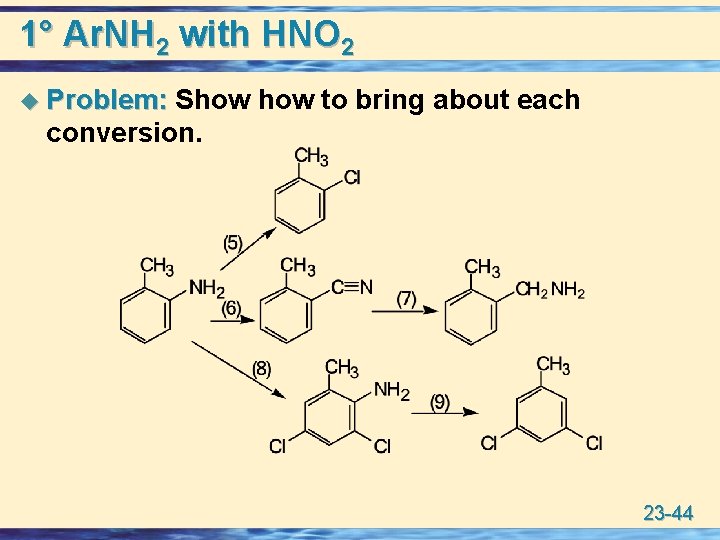
1° Ar. NH 2 with HNO 2 u Problem: Show to bring about each Problem: conversion. 23 -44