CARBOXYLIC ACIDS Properties and Synthesis NOMENCLATURE IUPAC NOMENCLATURE

CARBOXYLIC ACIDS Properties and Synthesis
NOMENCLATURE
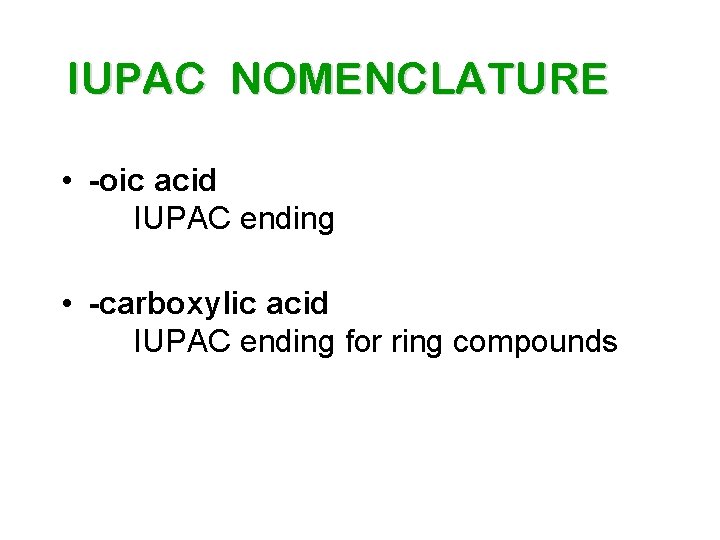
IUPAC NOMENCLATURE • -oic acid IUPAC ending • -carboxylic acid IUPAC ending for ring compounds
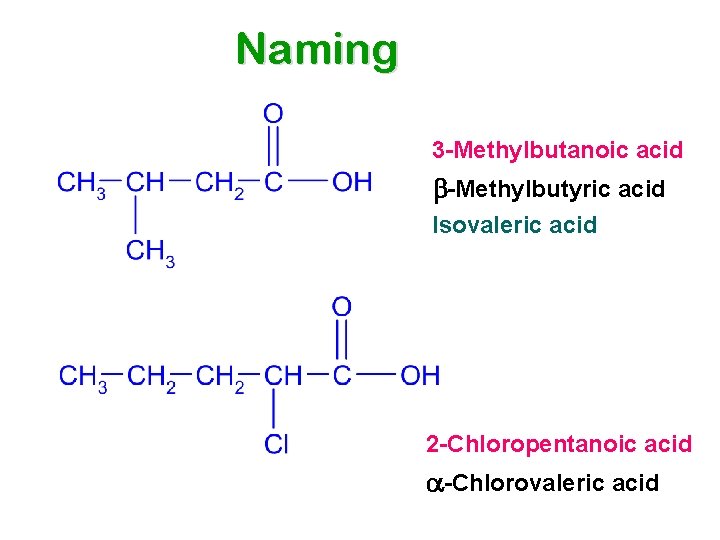
Naming 3 -Methylbutanoic acid b-Methylbutyric acid Isovaleric acid 2 -Chloropentanoic acid a-Chlorovaleric acid
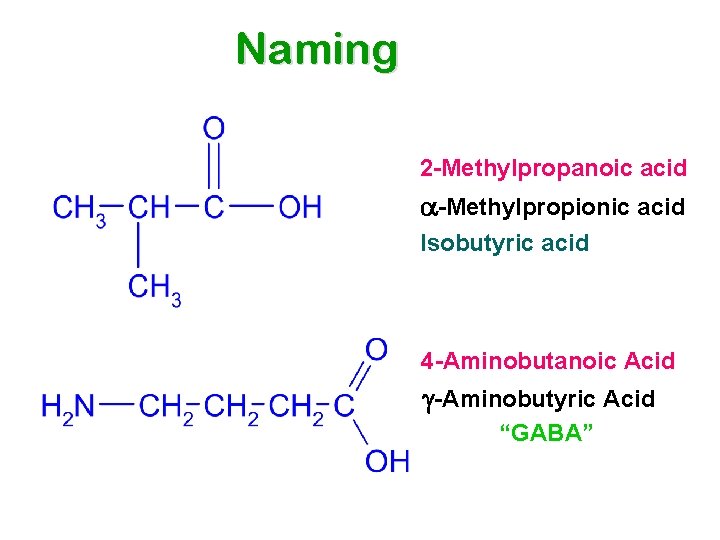
Naming 2 -Methylpropanoic acid a-Methylpropionic acid Isobutyric acid 4 -Aminobutanoic Acid -Aminobutyric Acid “GABA”
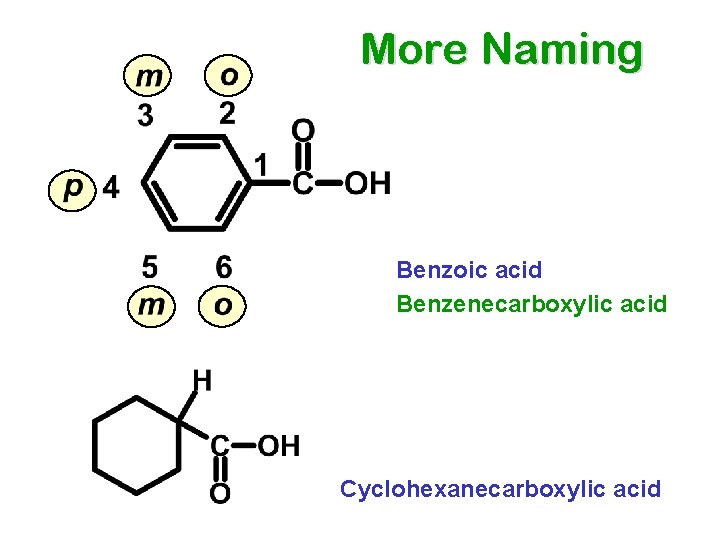
More Naming Benzoic acid Benzenecarboxylic acid Cyclohexanecarboxylic acid

Common Names
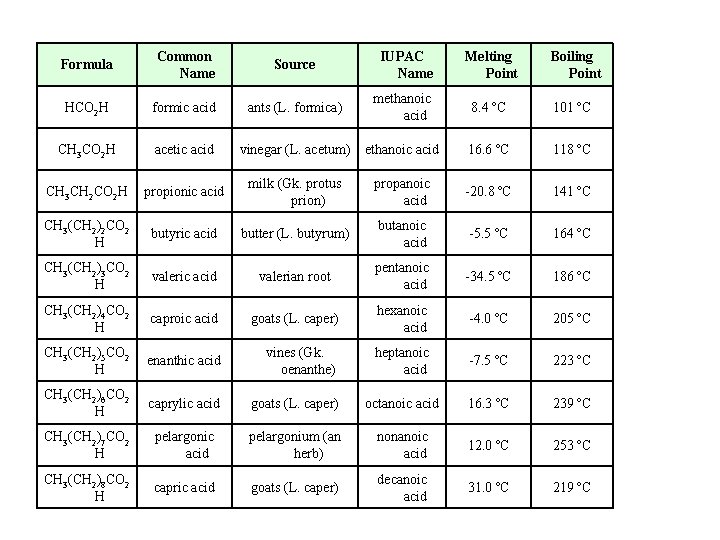
Formula Common Name Source HCO 2 H formic acid ants (L. formica) CH 3 CO 2 H acetic acid CH 3 CH 2 CO 2 H IUPAC Name Melting Point Boiling Point methanoic acid 8. 4 ºC 101 ºC vinegar (L. acetum) ethanoic acid 16. 6 ºC 118 ºC propionic acid milk (Gk. protus prion) propanoic acid -20. 8 ºC 141 ºC CH 3(CH 2)2 CO 2 H butyric acid butter (L. butyrum) butanoic acid -5. 5 ºC 164 ºC CH 3(CH 2)3 CO 2 H valeric acid valerian root pentanoic acid -34. 5 ºC 186 ºC CH 3(CH 2)4 CO 2 H caproic acid goats (L. caper) hexanoic acid -4. 0 ºC 205 ºC CH 3(CH 2)5 CO 2 H enanthic acid vines (Gk. oenanthe) heptanoic acid -7. 5 ºC 223 ºC CH 3(CH 2)6 CO 2 H caprylic acid goats (L. caper) octanoic acid 16. 3 ºC 239 ºC CH 3(CH 2)7 CO 2 H pelargonic acid pelargonium (an herb) nonanoic acid 12. 0 ºC 253 ºC CH 3(CH 2)8 CO 2 H capric acid goats (L. caper) decanoic acid 31. 0 ºC 219 ºC

SYNTHESIS OF CARBOXYLIC ACIDS
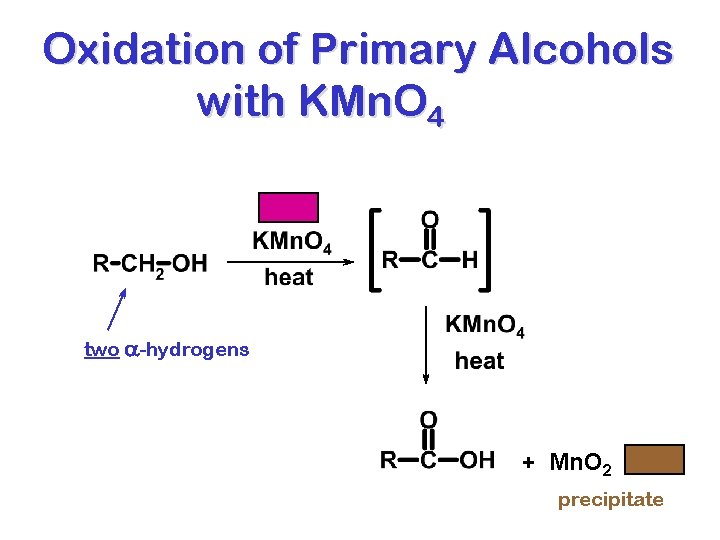
Oxidation of Primary Alcohols with KMn. O 4 two a-hydrogens + Mn. O 2 precipitate
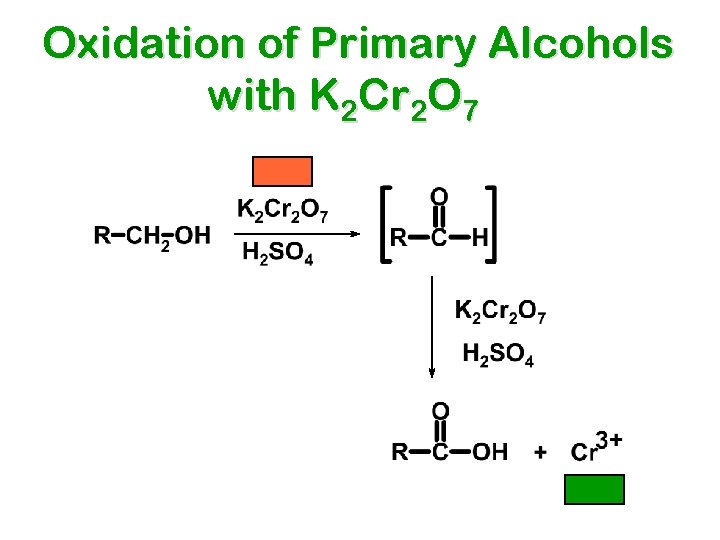
Oxidation of Primary Alcohols with K 2 Cr 2 O 7
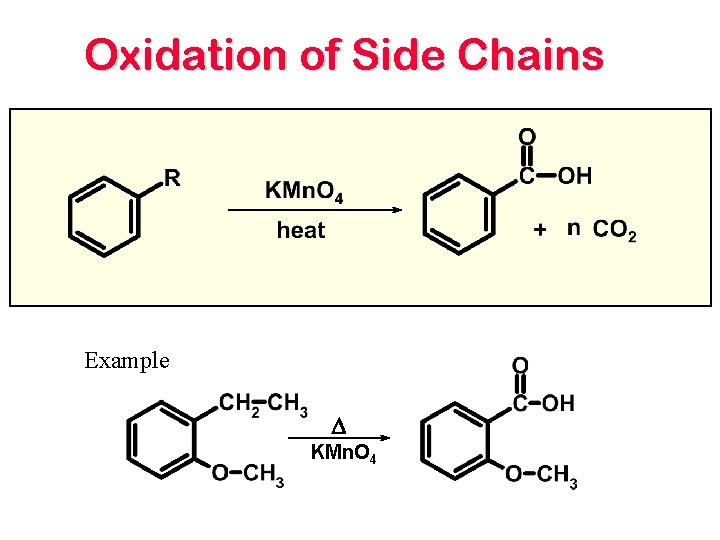
Oxidation of Side Chains Example D KMn. O 4
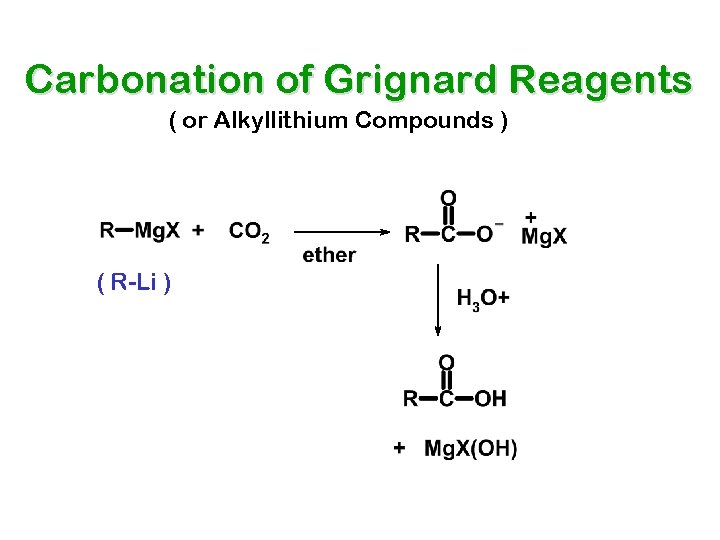
Carbonation of Grignard Reagents ( or Alkyllithium Compounds ) ( R-Li )
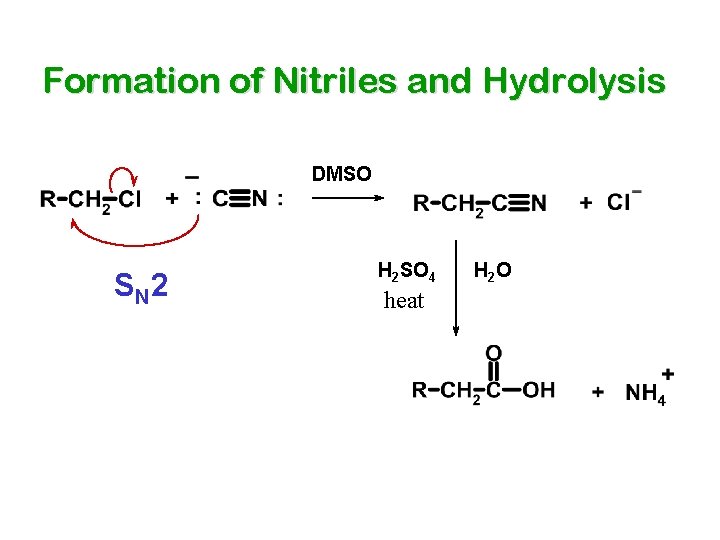
Formation of Nitriles and Hydrolysis DMSO S N 2 H 2 SO 4 heat H 2 O
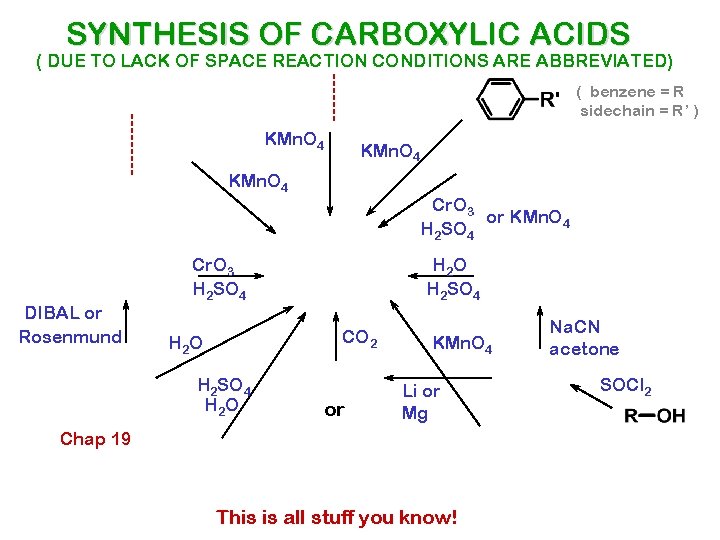
SYNTHESIS OF CARBOXYLIC ACIDS ( DUE TO LACK OF SPACE REACTION CONDITIONS ARE ABBREVIATED) ( benzene = R sidechain = R’ ) KMn. O 4 DIBAL or Rosenmund Cr. O 3 or KMn. O 4 H 2 SO 4 Cr. O 3 H 2 SO 4 H 2 O H 2 SO 4 CO 2 H 2 O H 2 SO 4 H 2 O or KMn. O 4 Li or Mg Chap 19 This is all stuff you know! Na. CN acetone SOCl 2

Physical Properties of Carboxylic Acids
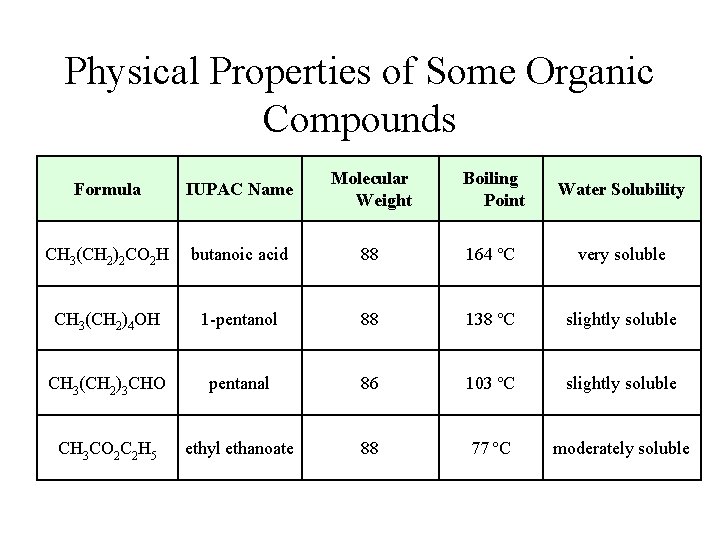
Physical Properties of Some Organic Compounds Formula IUPAC Name Molecular Weight Boiling Point Water Solubility CH 3(CH 2)2 CO 2 H butanoic acid 88 164 ºC very soluble CH 3(CH 2)4 OH 1 -pentanol 88 138 ºC slightly soluble CH 3(CH 2)3 CHO pentanal 86 103 ºC slightly soluble CH 3 CO 2 C 2 H 5 ethyl ethanoate 88 77 ºC moderately soluble
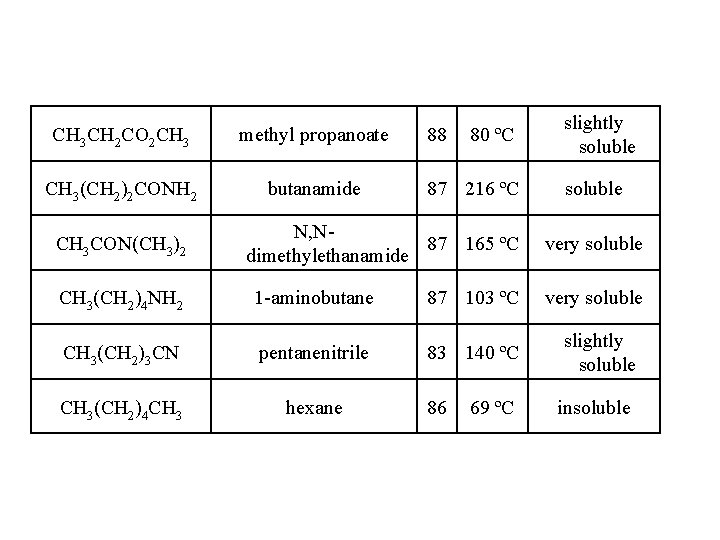
CH 3 CH 2 CO 2 CH 3 methyl propanoate CH 3(CH 2)2 CONH 2 butanamide CH 3 CON(CH 3)2 88 80 ºC 87 216 ºC N, N 87 165 ºC dimethylethanamide CH 3(CH 2)4 NH 2 1 -aminobutane 87 103 ºC CH 3(CH 2)3 CN pentanenitrile 83 140 ºC CH 3(CH 2)4 CH 3 hexane 86 69 ºC slightly soluble very soluble slightly soluble insoluble
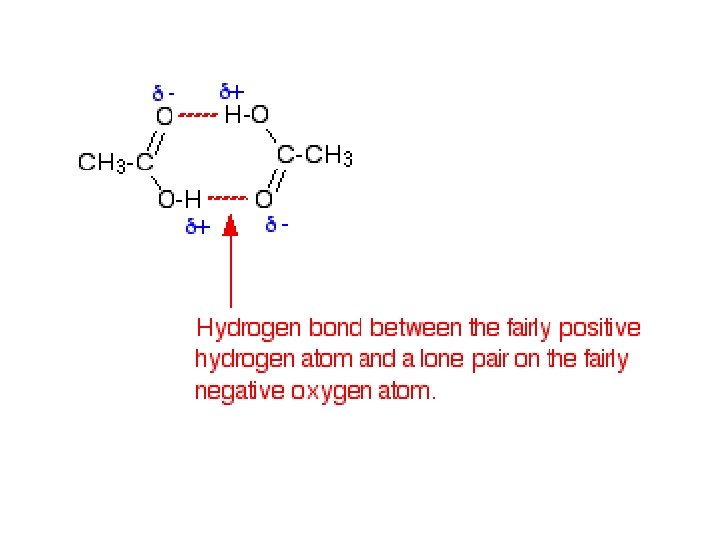
ACIDITY
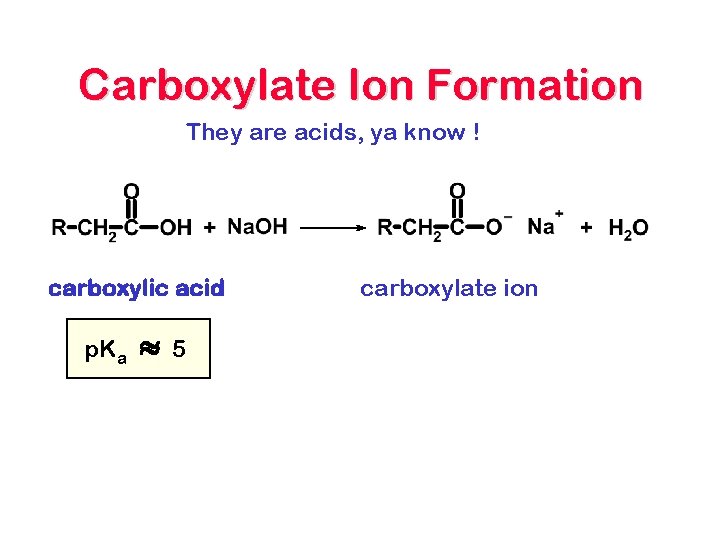
Carboxylate Ion Formation They are acids, ya know ! carboxylic acid p. Ka » 5 carboxylate ion
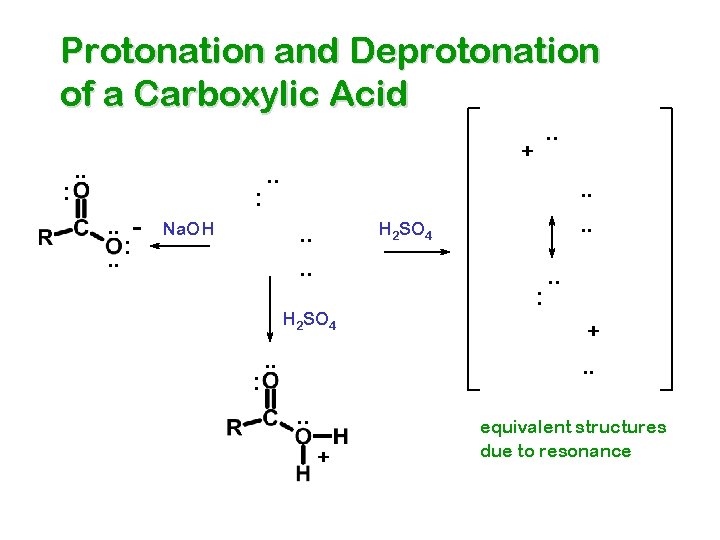
Protonation and Deprotonation of a Carboxylic Acid : . . : + . . Na. OH . . H 2 SO 4 . . H 2 SO 4 : . . + equivalent structures due to resonance
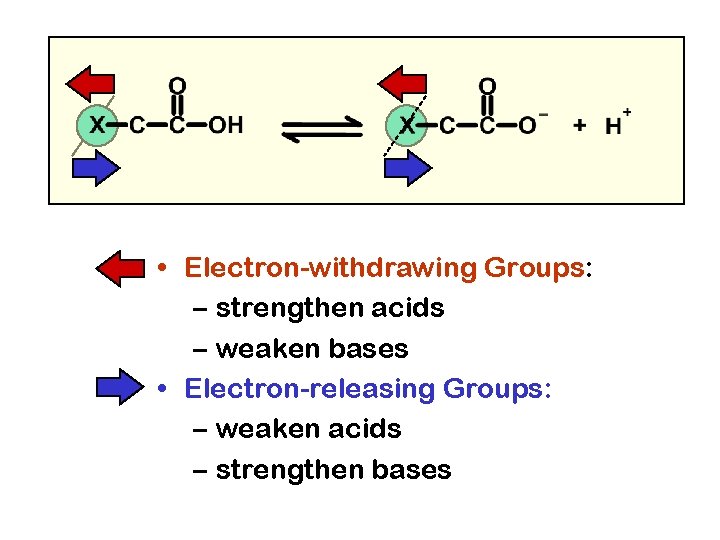
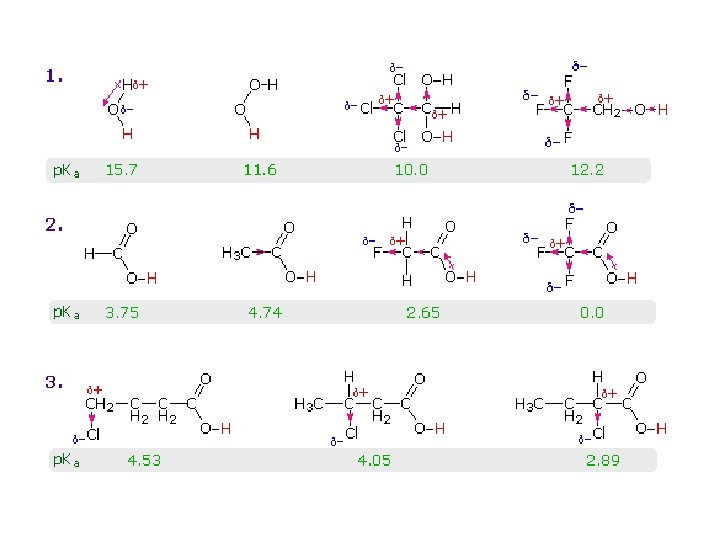
• Electron-withdrawing Groups: – strengthen acids – weaken bases • Electron-releasing Groups: – weaken acids – strengthen bases
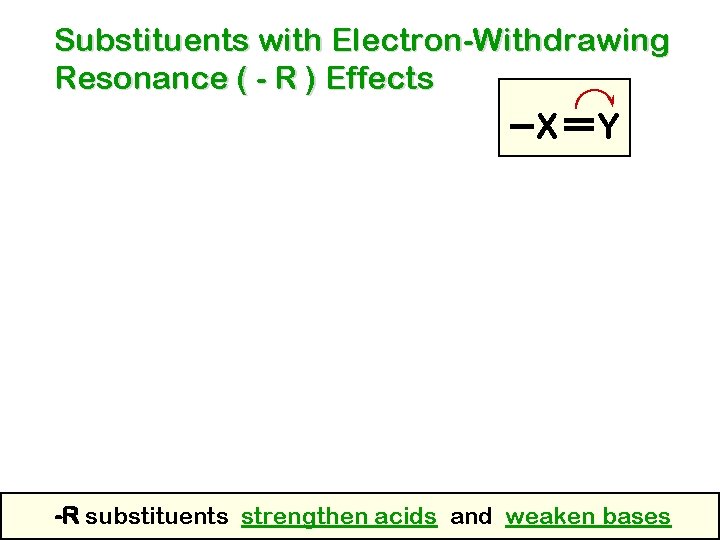
Substituents with Electron-Withdrawing Resonance ( - R ) Effects X Y -R substituents strengthen acids and weaken bases
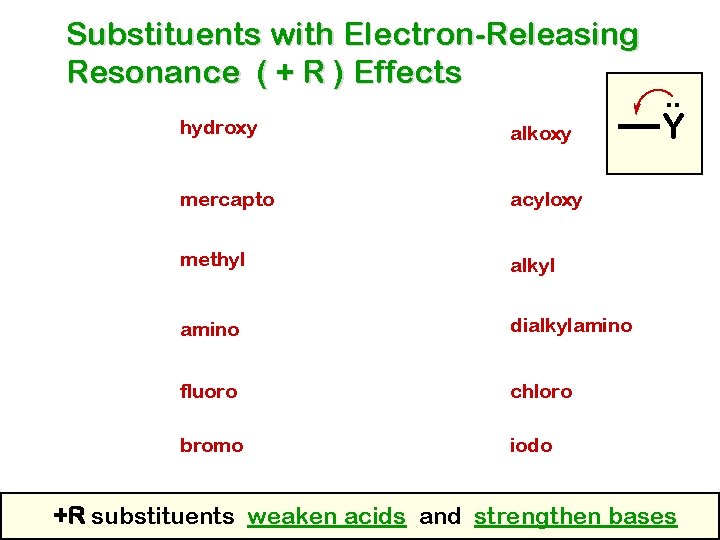
Substituents with Electron-Releasing Resonance ( + R ) Effects hydroxy alkoxy mercapto acyloxy methyl alkyl amino dialkylamino fluoro chloro bromo iodo . . Y +R substituents weaken acids and strengthen bases
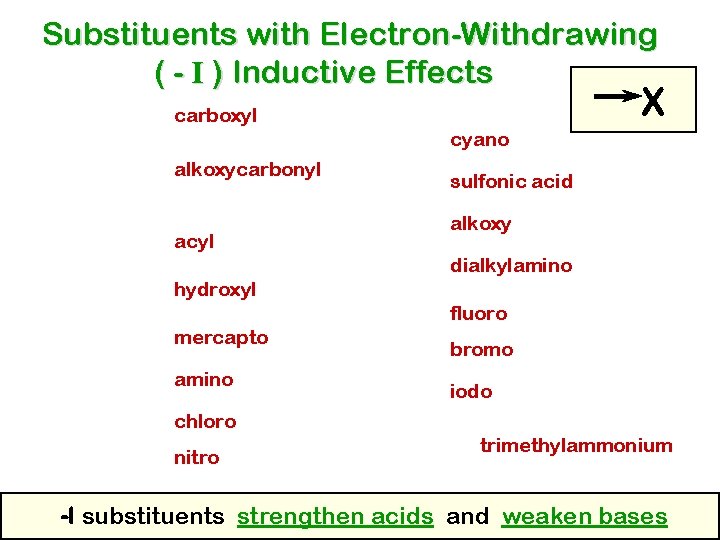
Substituents with Electron-Withdrawing ( - I ) Inductive Effects X carboxyl cyano alkoxycarbonyl acyl sulfonic acid alkoxy dialkylamino hydroxyl fluoro mercapto amino bromo iodo chloro nitro trimethylammonium -I substituents strengthen acids and weaken bases
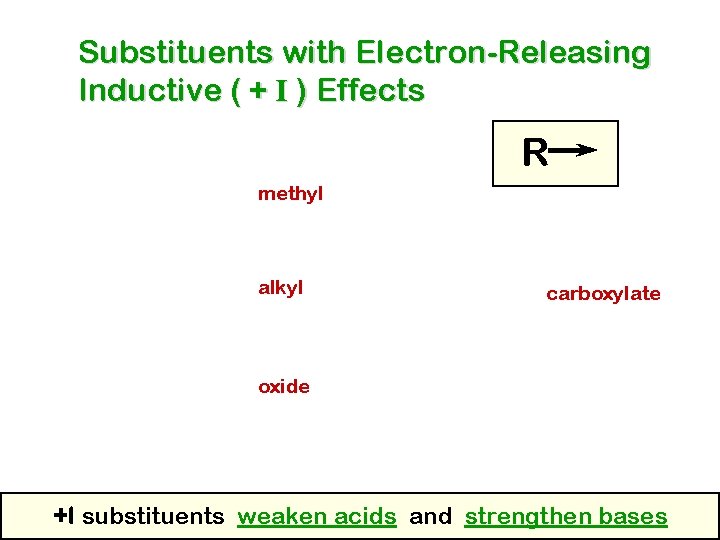
Substituents with Electron-Releasing Inductive ( + I ) Effects R methyl alkyl carboxylate oxide +I substituents weaken acids and strengthen bases
increasing acidity

ortho meta para Benzoic Acid: p. Ka = 4. 19

4. 08 4. 46 Benzoic Acid: p. Ka = 4. 19 4. 06 4. 48 2. 97
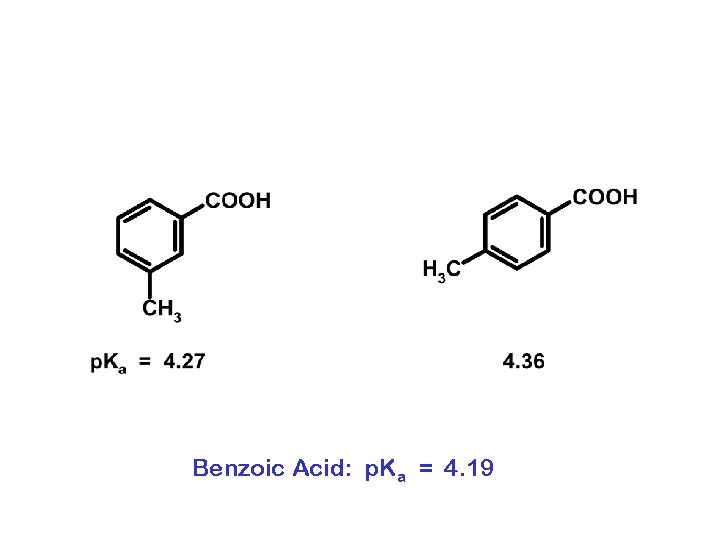
Benzoic Acid: p. Ka = 4. 19
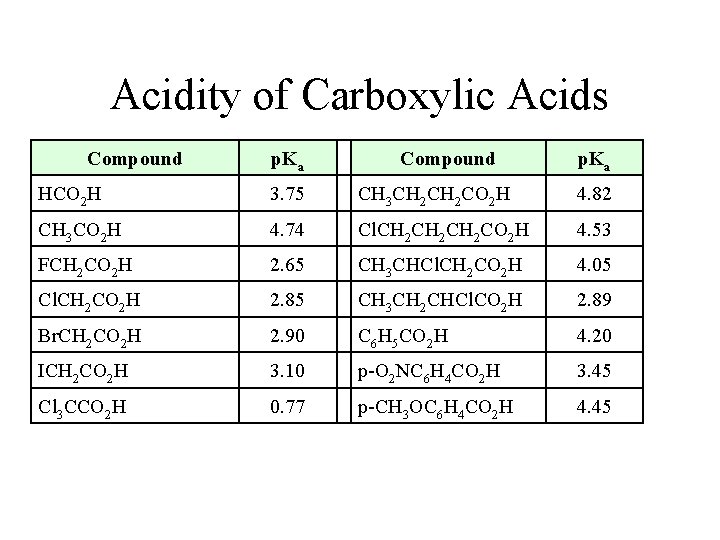
Acidity of Carboxylic Acids Compound p. Ka HCO 2 H 3. 75 CH 3 CH 2 CO 2 H 4. 82 CH 3 CO 2 H 4. 74 Cl. CH 2 CH 2 CO 2 H 4. 53 FCH 2 CO 2 H 2. 65 CH 3 CHCl. CH 2 CO 2 H 4. 05 Cl. CH 2 CO 2 H 2. 85 CH 3 CH 2 CHCl. CO 2 H 2. 89 Br. CH 2 CO 2 H 2. 90 C 6 H 5 CO 2 H 4. 20 ICH 2 CO 2 H 3. 10 p-O 2 NC 6 H 4 CO 2 H 3. 45 Cl 3 CCO 2 H 0. 77 p-CH 3 OC 6 H 4 CO 2 H 4. 45
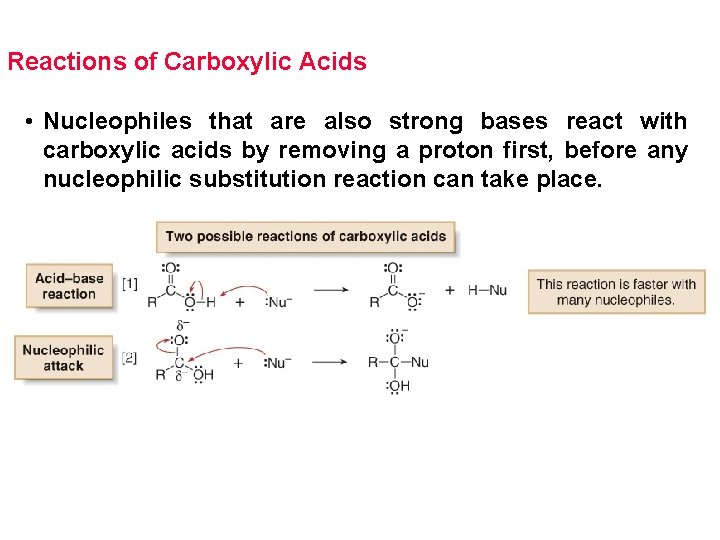
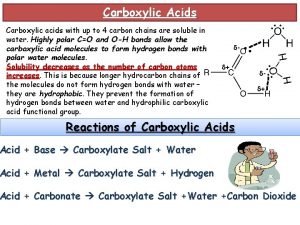
Reactions of Carboxylic Acids • Nucleophiles that are also strong bases react with carboxylic acids by removing a proton first, before any nucleophilic substitution reaction can take place.
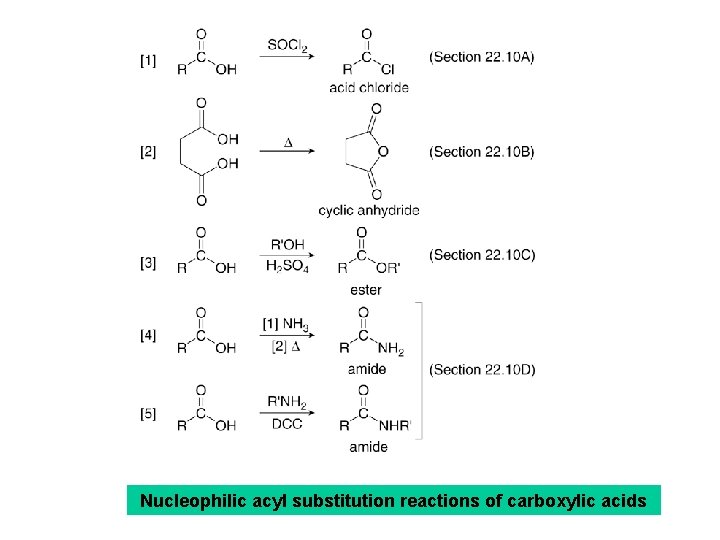
Nucleophilic acyl substitution reactions of carboxylic acids
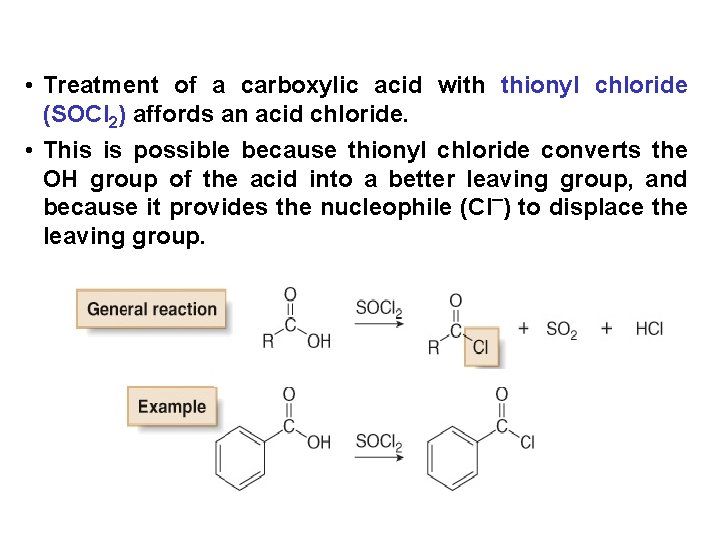
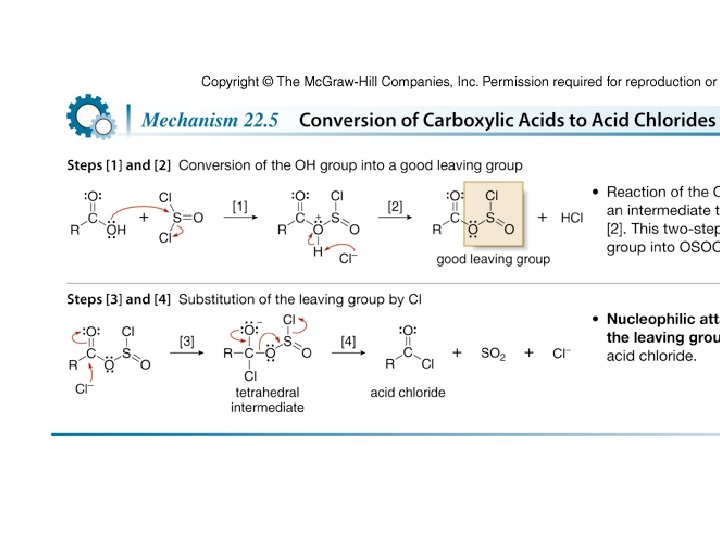
• Treatment of a carboxylic acid with thionyl chloride (SOCl 2) affords an acid chloride. • This is possible because thionyl chloride converts the OH group of the acid into a better leaving group, and because it provides the nucleophile (Cl¯) to displace the leaving group.
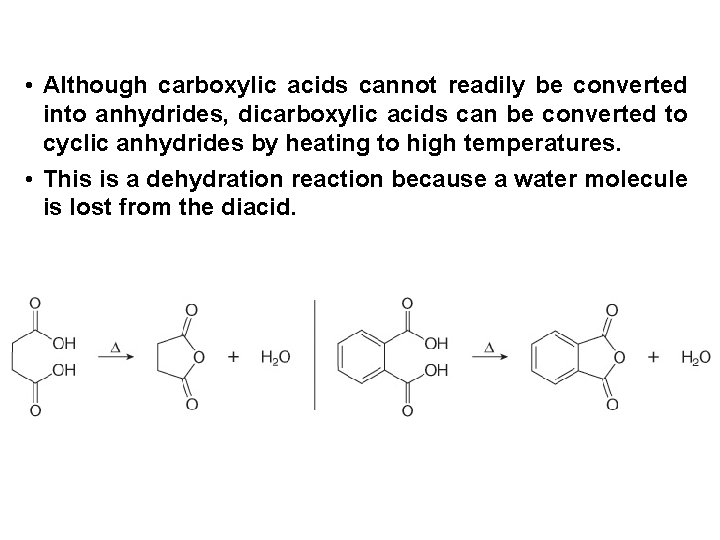
• Although carboxylic acids cannot readily be converted into anhydrides, dicarboxylic acids can be converted to cyclic anhydrides by heating to high temperatures. • This is a dehydration reaction because a water molecule is lost from the diacid.
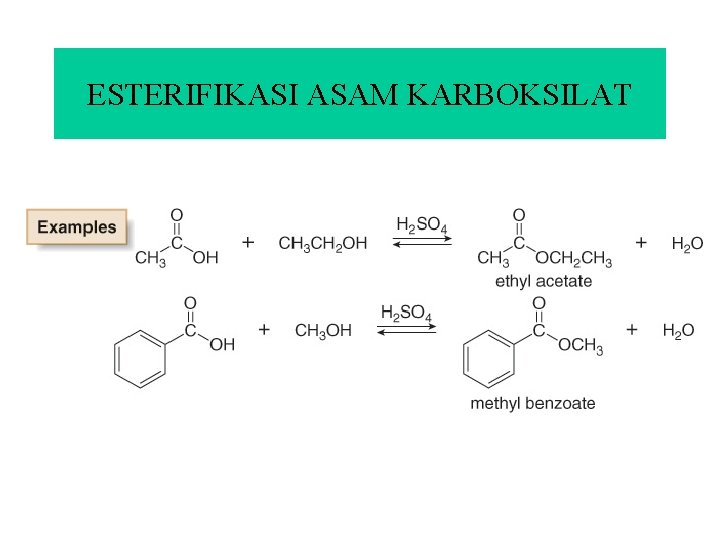
ESTERIFIKASI ASAM KARBOKSILAT
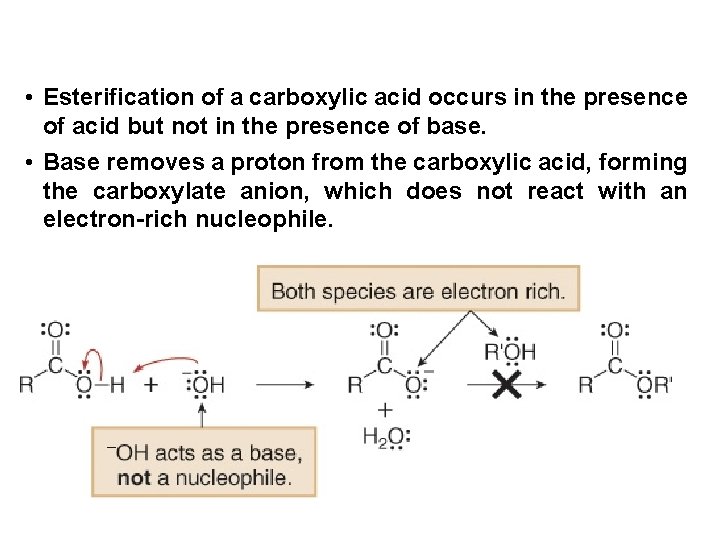
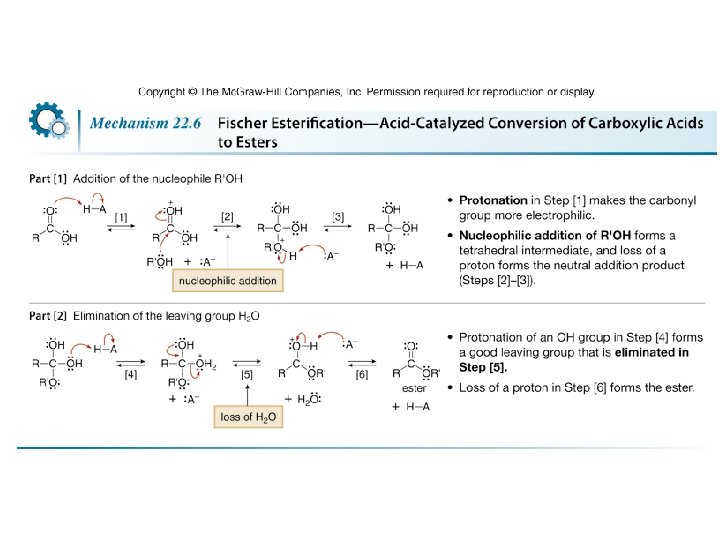
• Esterification of a carboxylic acid occurs in the presence of acid but not in the presence of base. • Base removes a proton from the carboxylic acid, forming the carboxylate anion, which does not react with an electron-rich nucleophile.
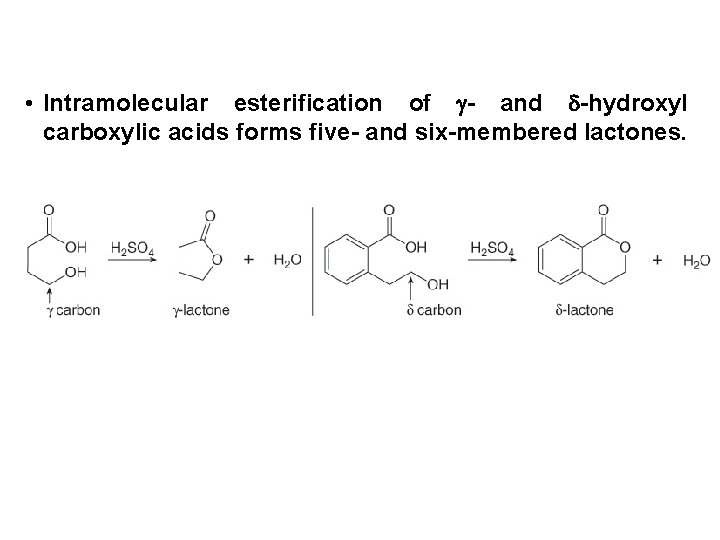
• Intramolecular esterification of - and -hydroxyl carboxylic acids forms five- and six-membered lactones.
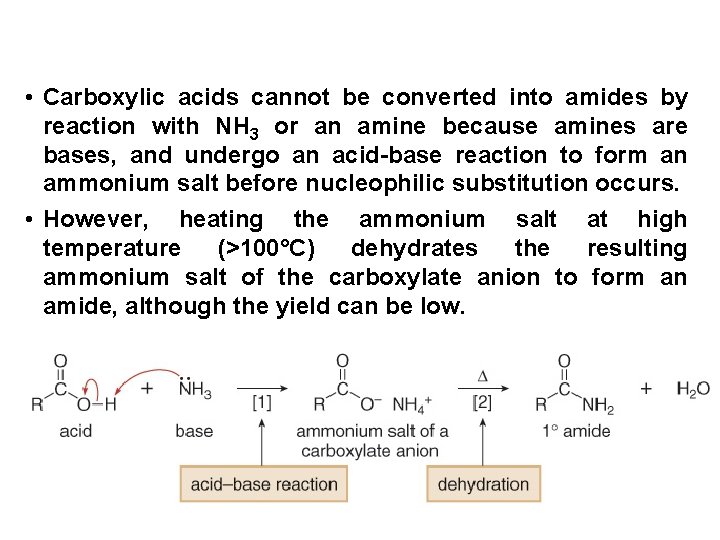
• Carboxylic acids cannot be converted into amides by reaction with NH 3 or an amine because amines are bases, and undergo an acid-base reaction to form an ammonium salt before nucleophilic substitution occurs. • However, heating the ammonium salt at high temperature (>100°C) dehydrates the resulting ammonium salt of the carboxylate anion to form an amide, although the yield can be low.
![• The overall conversion of RCOOH to RCONH 2 requires two steps: [1] • The overall conversion of RCOOH to RCONH 2 requires two steps: [1]](http://slidetodoc.com/presentation_image/3ee60a94a1aaf15ae403588eaa68ddef/image-45.jpg)
• The overall conversion of RCOOH to RCONH 2 requires two steps: [1] Acid-base reaction of RCOOH with NH 3 to form an ammonium salt. [2] Dehydration at high temperature (>100°C).
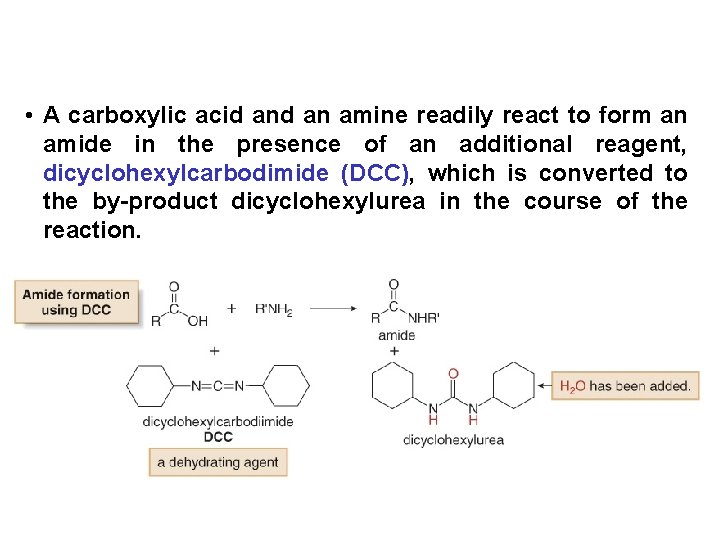
• A carboxylic acid an amine readily react to form an amide in the presence of an additional reagent, dicyclohexylcarbodimide (DCC), which is converted to the by-product dicyclohexylurea in the course of the reaction.
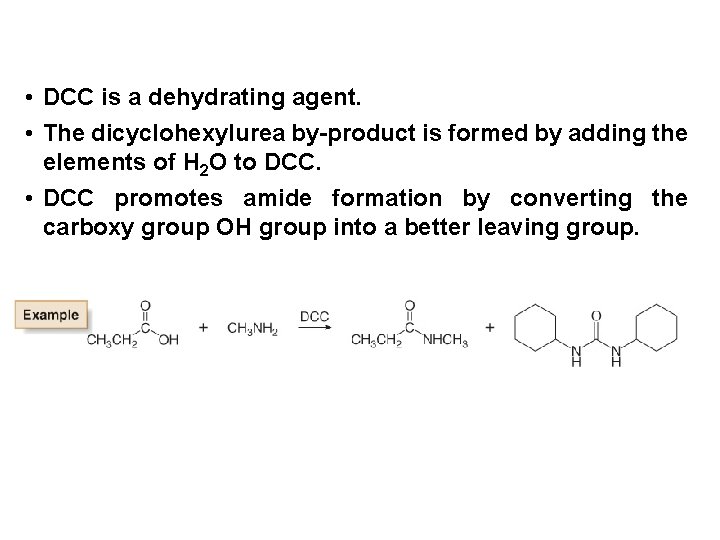
• DCC is a dehydrating agent. • The dicyclohexylurea by-product is formed by adding the elements of H 2 O to DCC. • DCC promotes amide formation by converting the carboxy group OH group into a better leaving group.
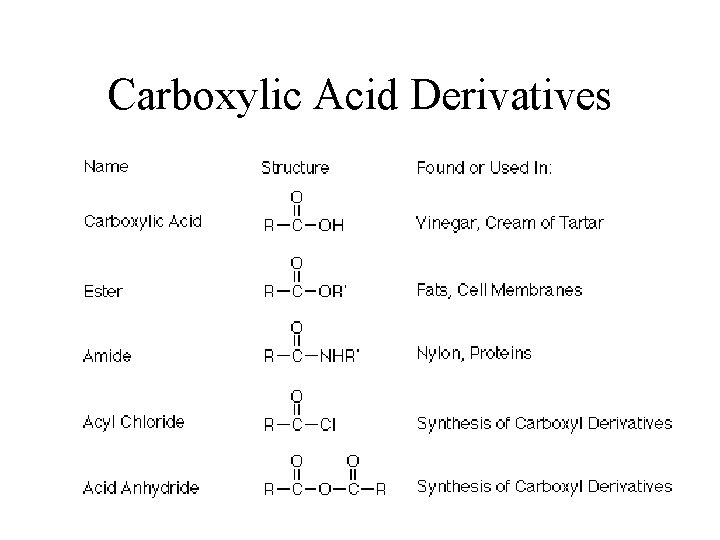
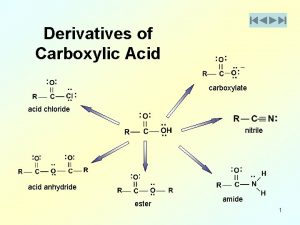
Carboxylic Acid Derivatives

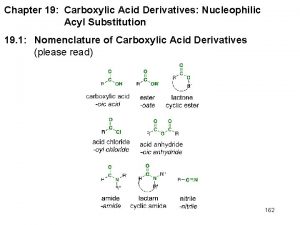
Related Carbonyl Derivatives
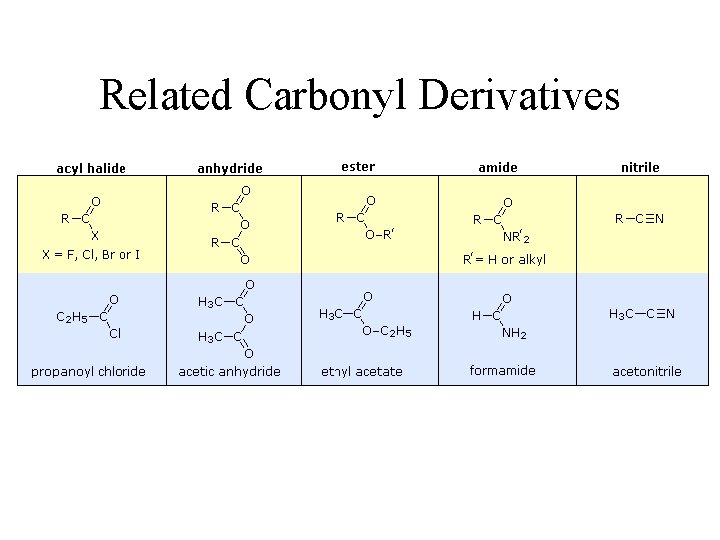
Related Carbonyl Derivatives
- Slides: 50