AMINES AMINES SALTS Aniline hydrochloride Aniline sulphate 1

- Slides: 7

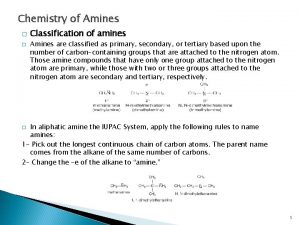
AMINES & AMINES SALTS

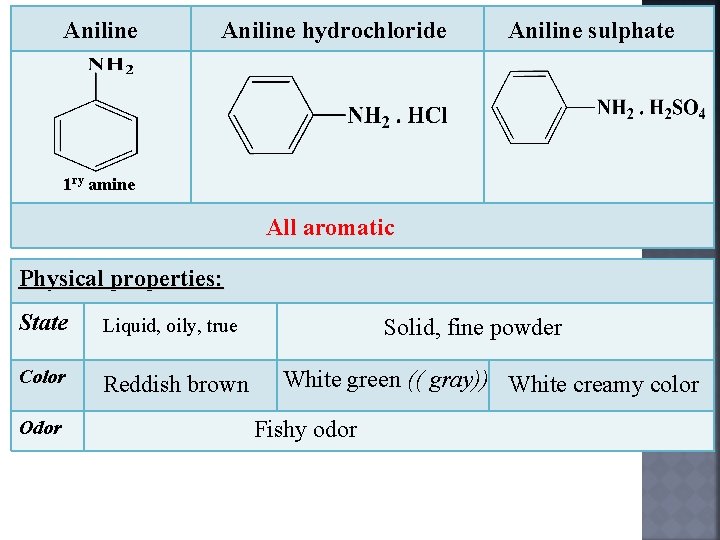
Aniline hydrochloride Aniline sulphate 1 ry amine All aromatic Physical properties: State Liquid, oily, true Color Reddish brown Odor Solid, fine powder White green (( gray)) White creamy color Fishy odor

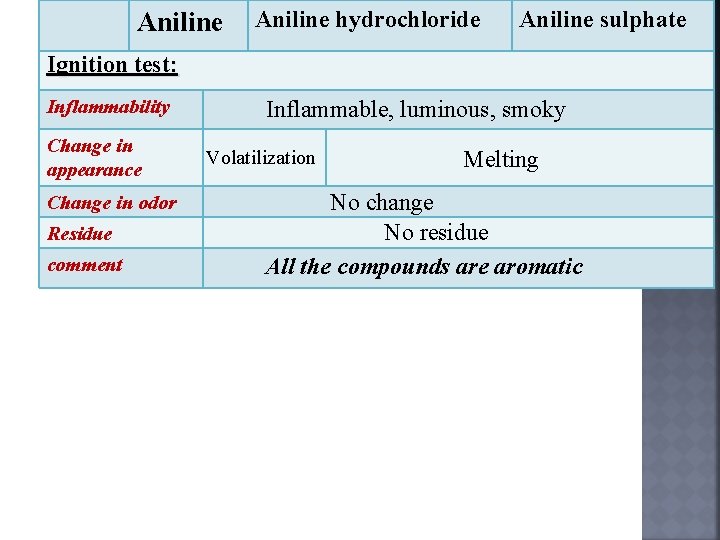
Aniline hydrochloride Aniline sulphate Ignition test: Inflammability Change in appearance Change in odor Residue comment Inflammable, luminous, smoky Volatilization Melting No change No residue All the compounds are aromatic

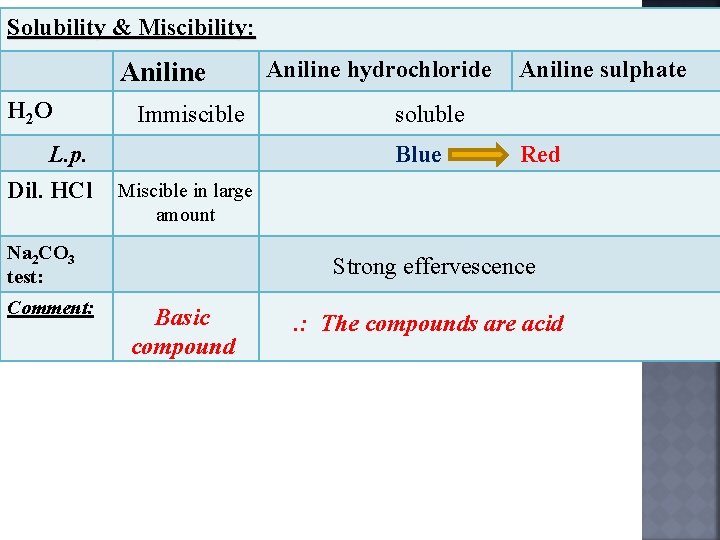
Solubility & Miscibility: Aniline H 2 O L. p. Dil. HCl Immiscible Aniline sulphate soluble Blue Red Miscible in large amount Na 2 CO 3 test: Comment: Aniline hydrochloride Strong effervescence Basic compound . : The compounds are acid

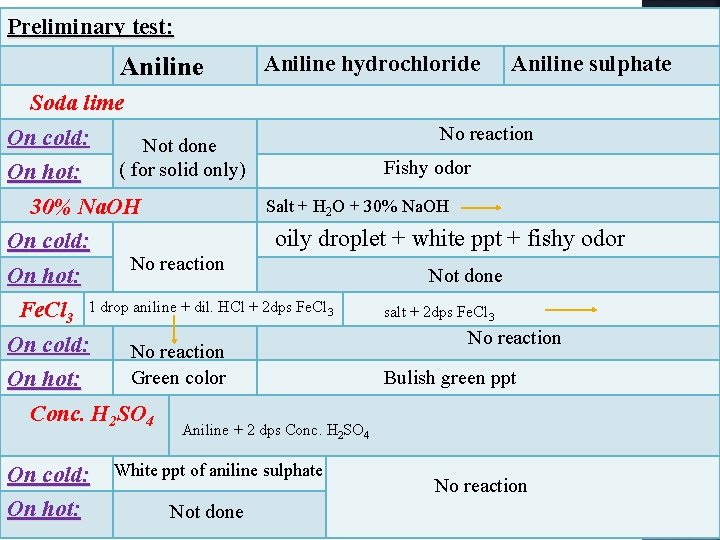
Preliminary test: Aniline Soda lime On cold: Not done ( for solid only) On hot: 30% Na. OH On cold: Aniline hydrochloride Aniline sulphate No reaction Fishy odor Salt + H 2 O + 30% Na. OH oily droplet + white ppt + fishy odor No reaction On hot: Fe. Cl 3 1 drop aniline + dil. HCl + 2 dps Fe. Cl 3 On cold: No reaction Green color On hot: Conc. H 2 SO 4 Not done salt + 2 dps Fe. Cl 3 No reaction Bulish green ppt Aniline + 2 dps Conc. H 2 SO 4 On cold: On hot: White ppt of aniline sulphate Not done No reaction

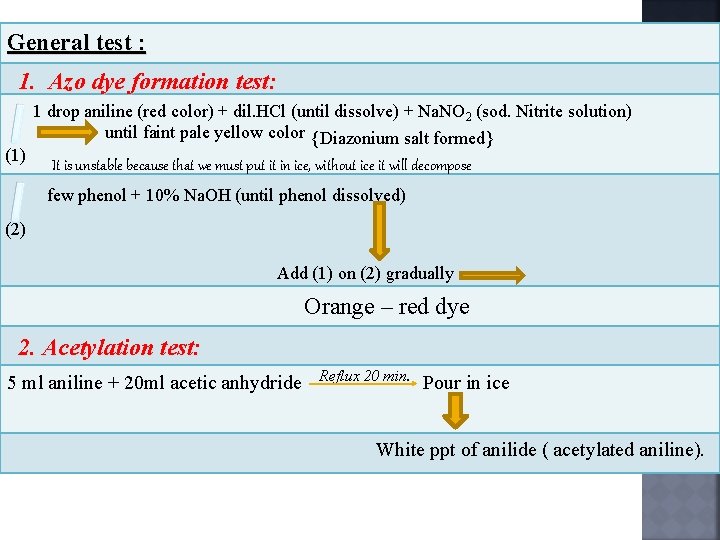
General test : 1. Azo dye formation test: (1) 1 drop aniline (red color) + dil. HCl (until dissolve) + Na. NO 2 (sod. Nitrite solution) until faint pale yellow color {Diazonium salt formed} It is unstable because that we must put it in ice, without ice it will decompose few phenol + 10% Na. OH (until phenol dissolved) (2) Add (1) on (2) gradually Orange – red dye 2. Acetylation test: 5 ml aniline + 20 ml acetic anhydride Reflux 20 min. Pour in ice White ppt of anilide ( acetylated aniline).

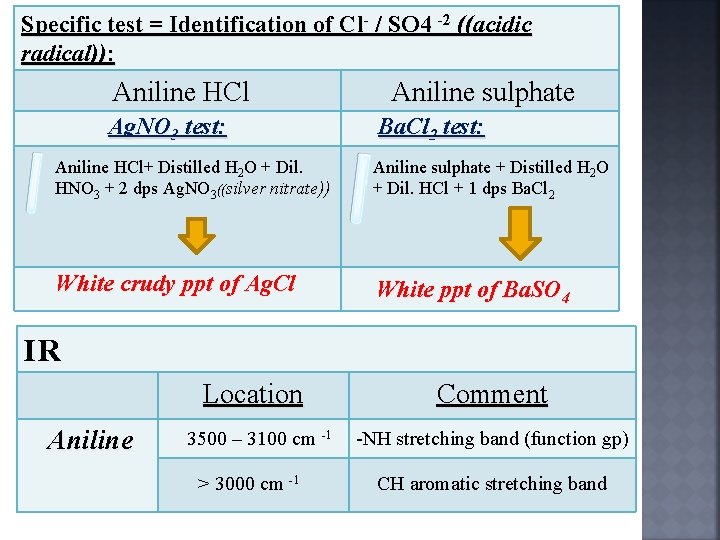
Specific test = Identification of Cl- / SO 4 -2 ((acidic radical)): Aniline HCl Ag. NO 3 test: Aniline sulphate Ba. Cl 2 test: Aniline HCl+ Distilled H 2 O + Dil. HNO 3 + 2 dps Ag. NO 3((silver nitrate)) Aniline sulphate + Distilled H 2 O + Dil. HCl + 1 dps Ba. Cl 2 White crudy ppt of Ag. Cl White ppt of Ba. SO 4 IR Location Aniline 3500 – 3100 cm -1 > 3000 cm -1 Comment -NH stretching band (function gp) CH aromatic stretching band